Comparison of the In Vivo Efficacy of Cuban (Raydel®) and Chinese (BOC Science) Policosanol in Alleviating Dyslipidemia and Inflammation via Safeguarding Major Organs and Reproductive Health in Hyperlipidemic Zebrafish: A Twelve-Week Consumption Study
Abstract
1. Introduction
2. Results
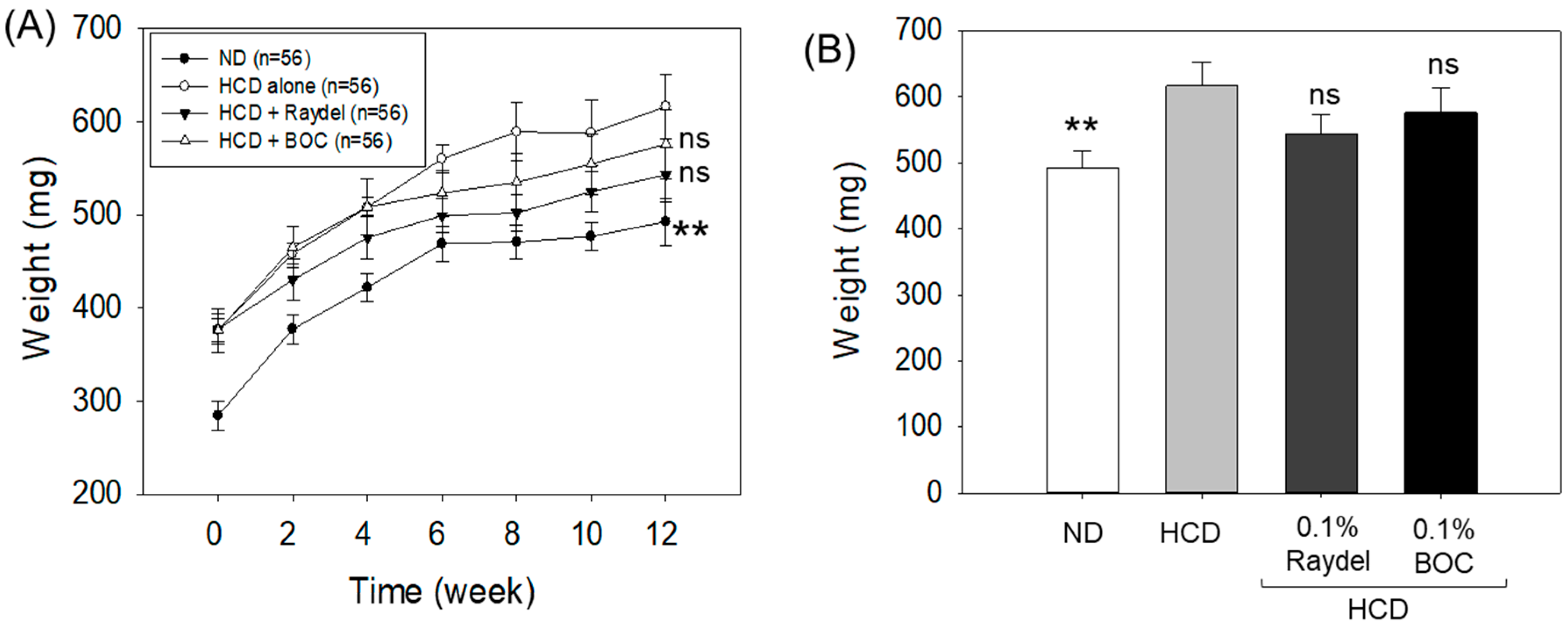
2.1. Zebrafish Survivability and Change in Body Mass
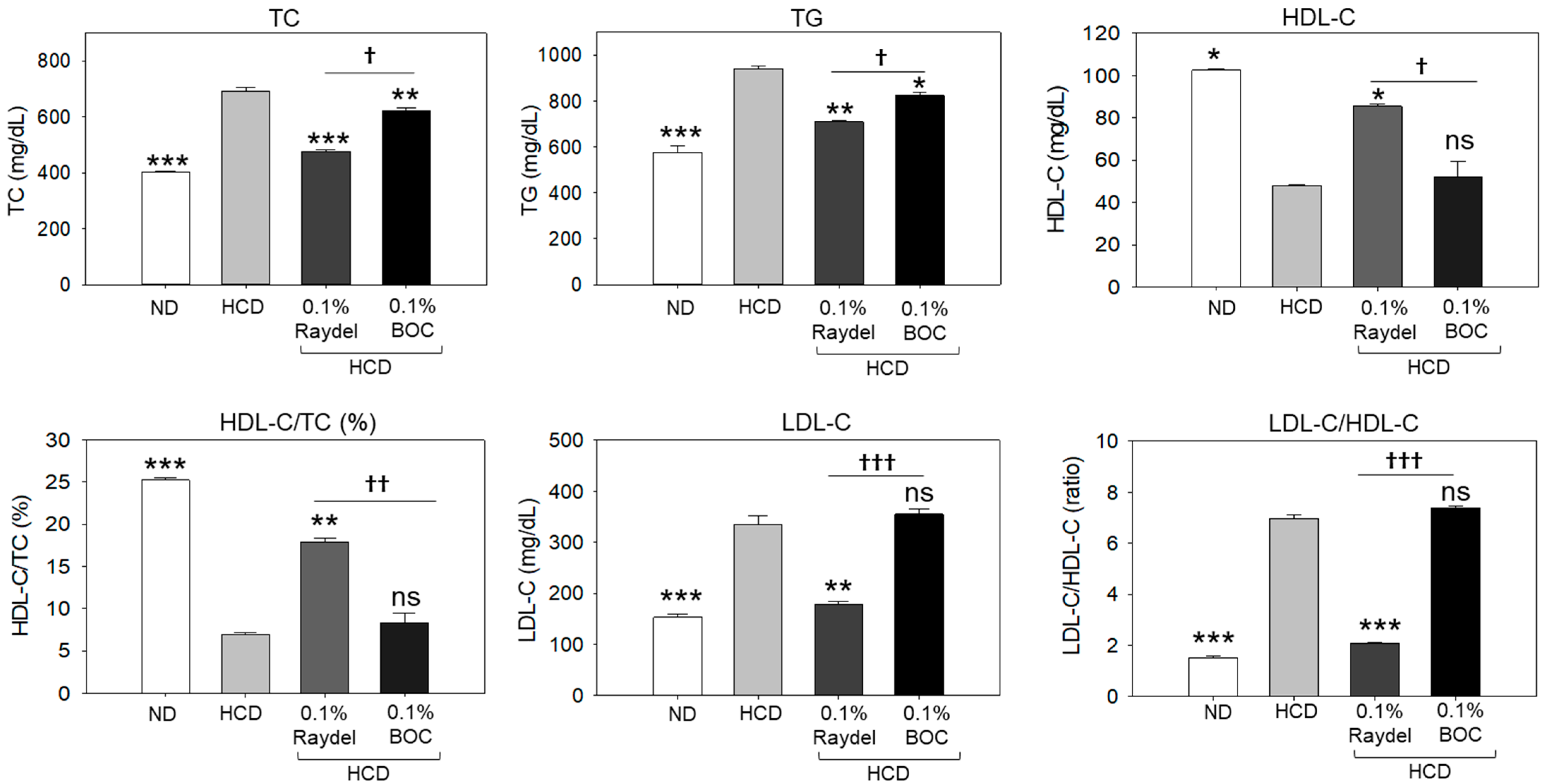
2.2. Assessment of Plasma Lipid Profile
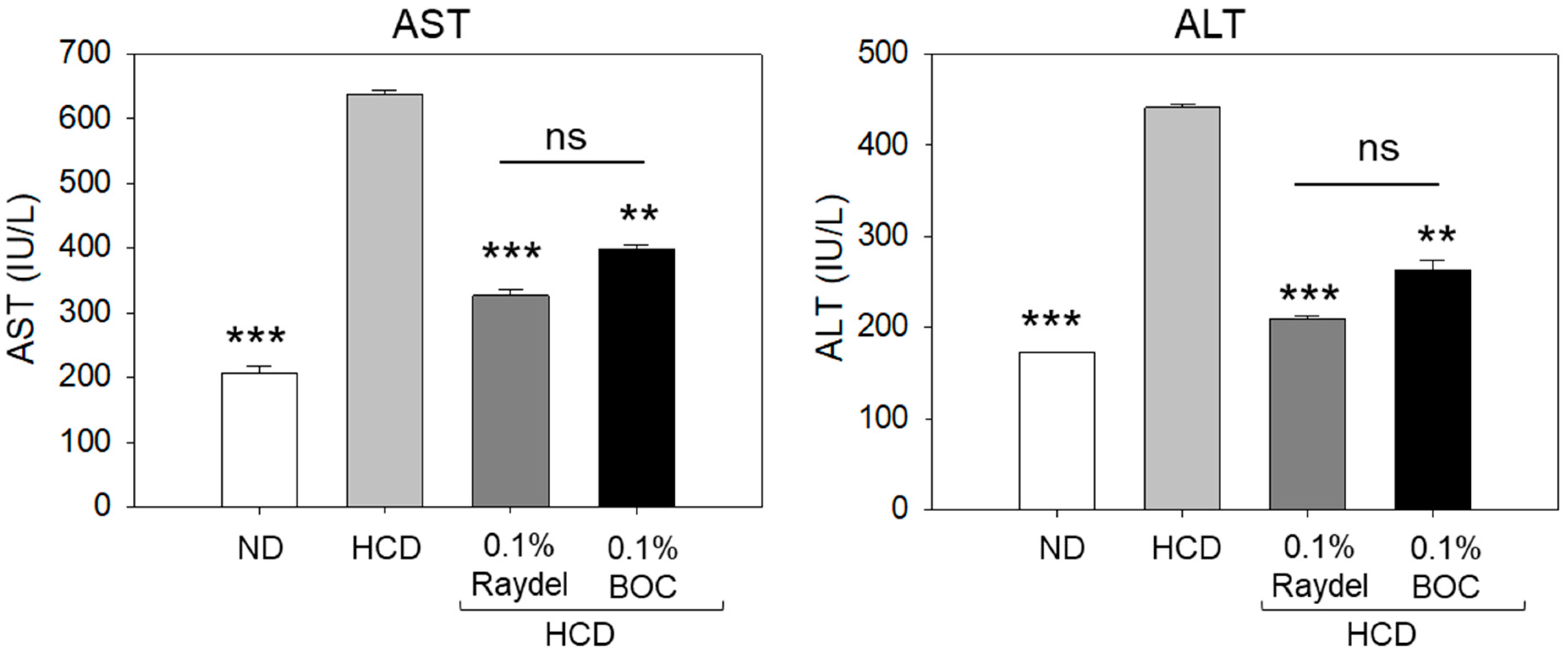
2.3. Plasma Hepatic Function Biomarkers
2.4. Liver Histology
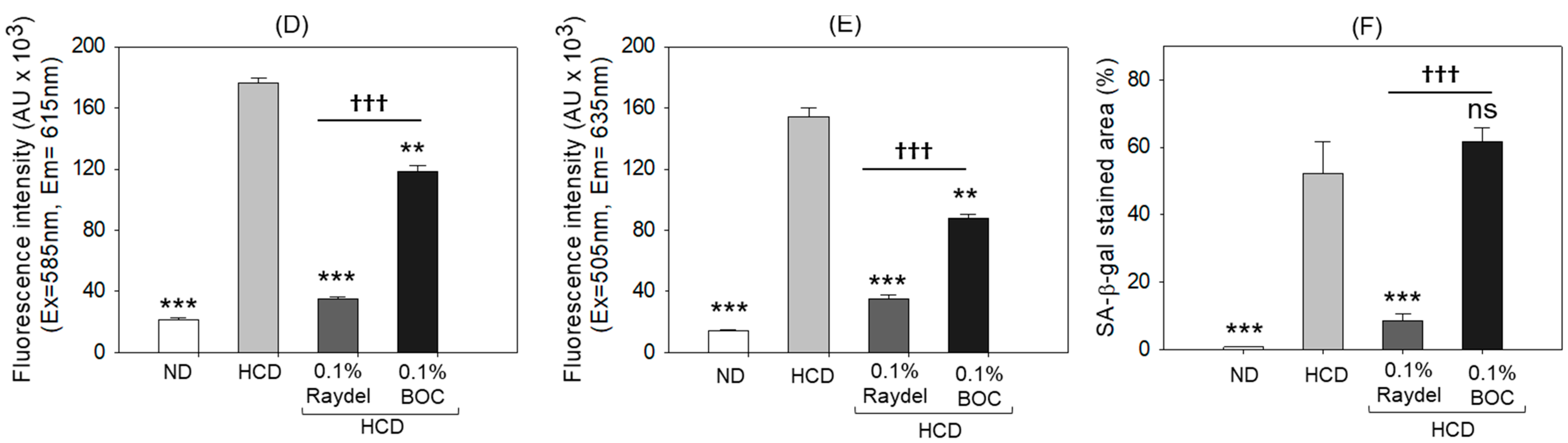
2.5. Extent of Reactive Oxygen Species (ROS) Production, Apoptosis, and Senescence in the Liver
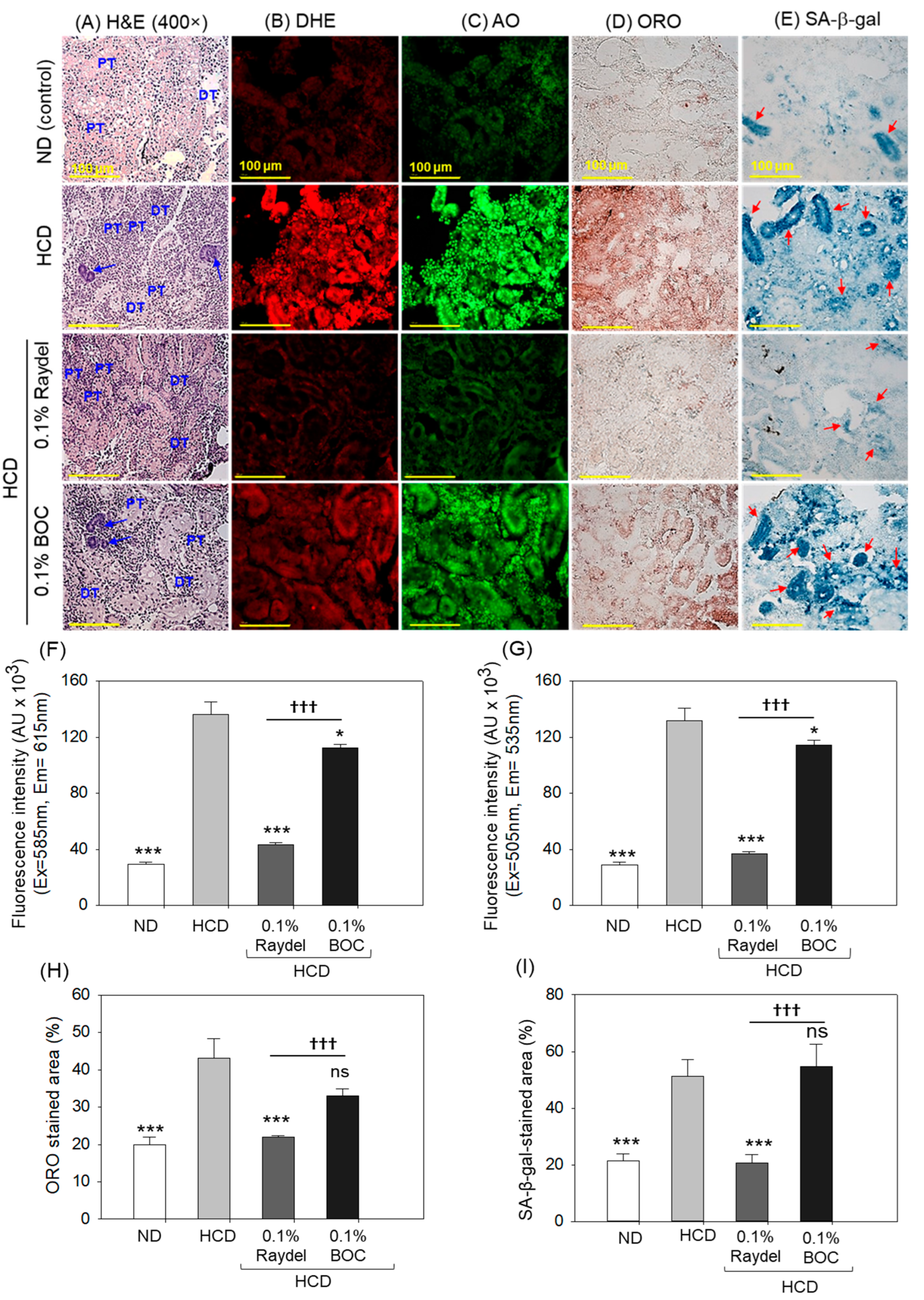
2.6. Kidney Histology
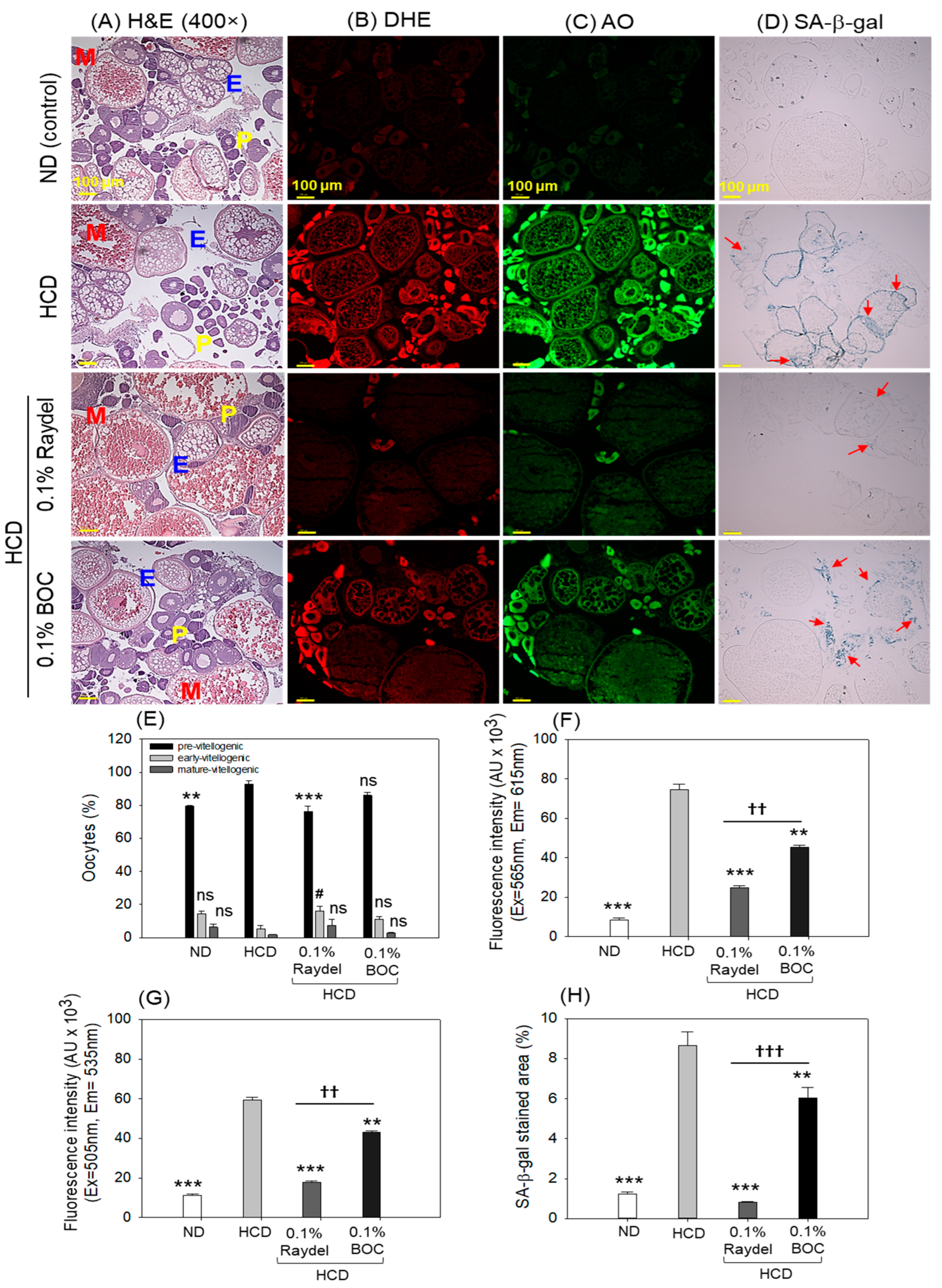
2.7. Ovary Section Evaluation
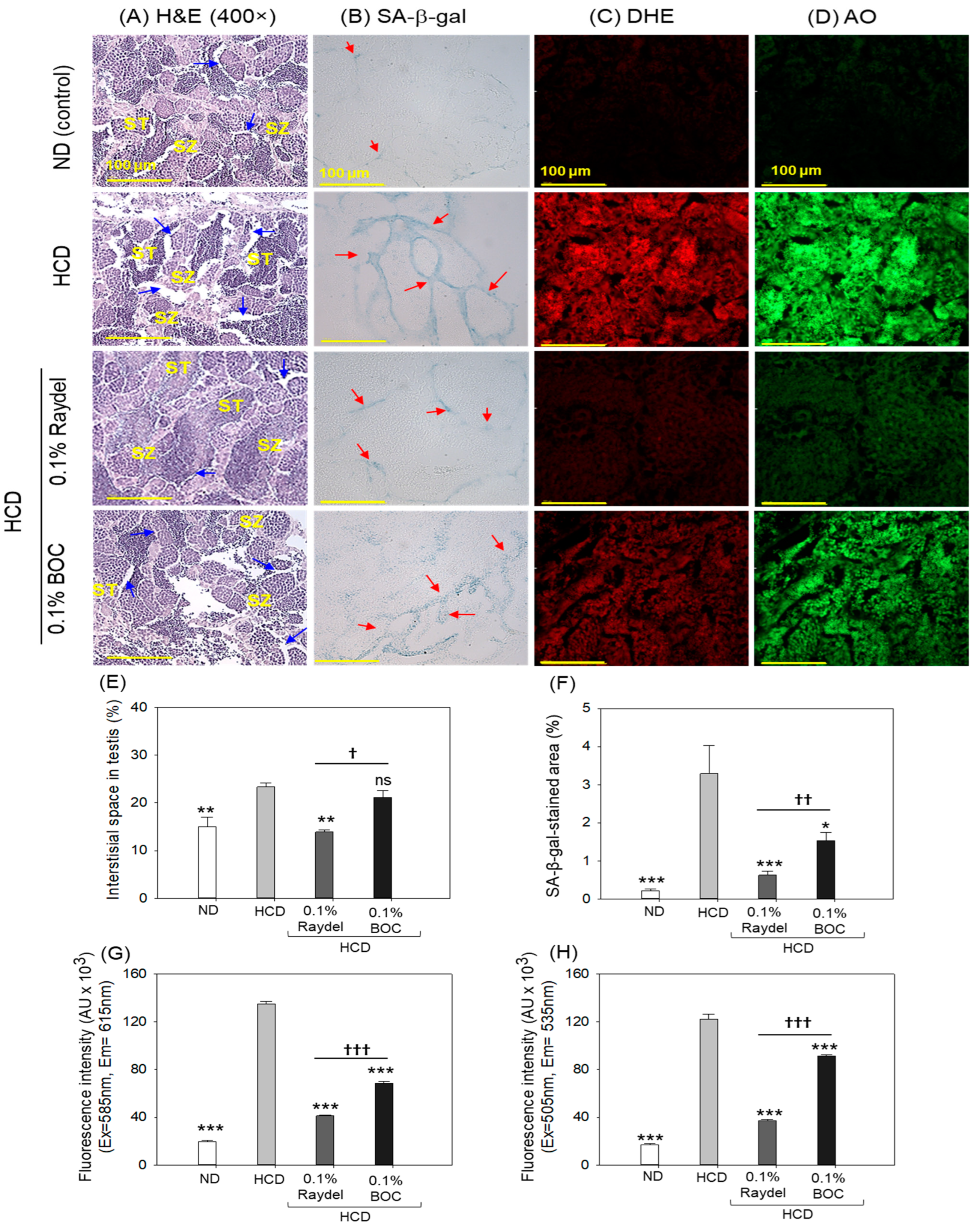
2.8. Testicular Tissue Evaluation
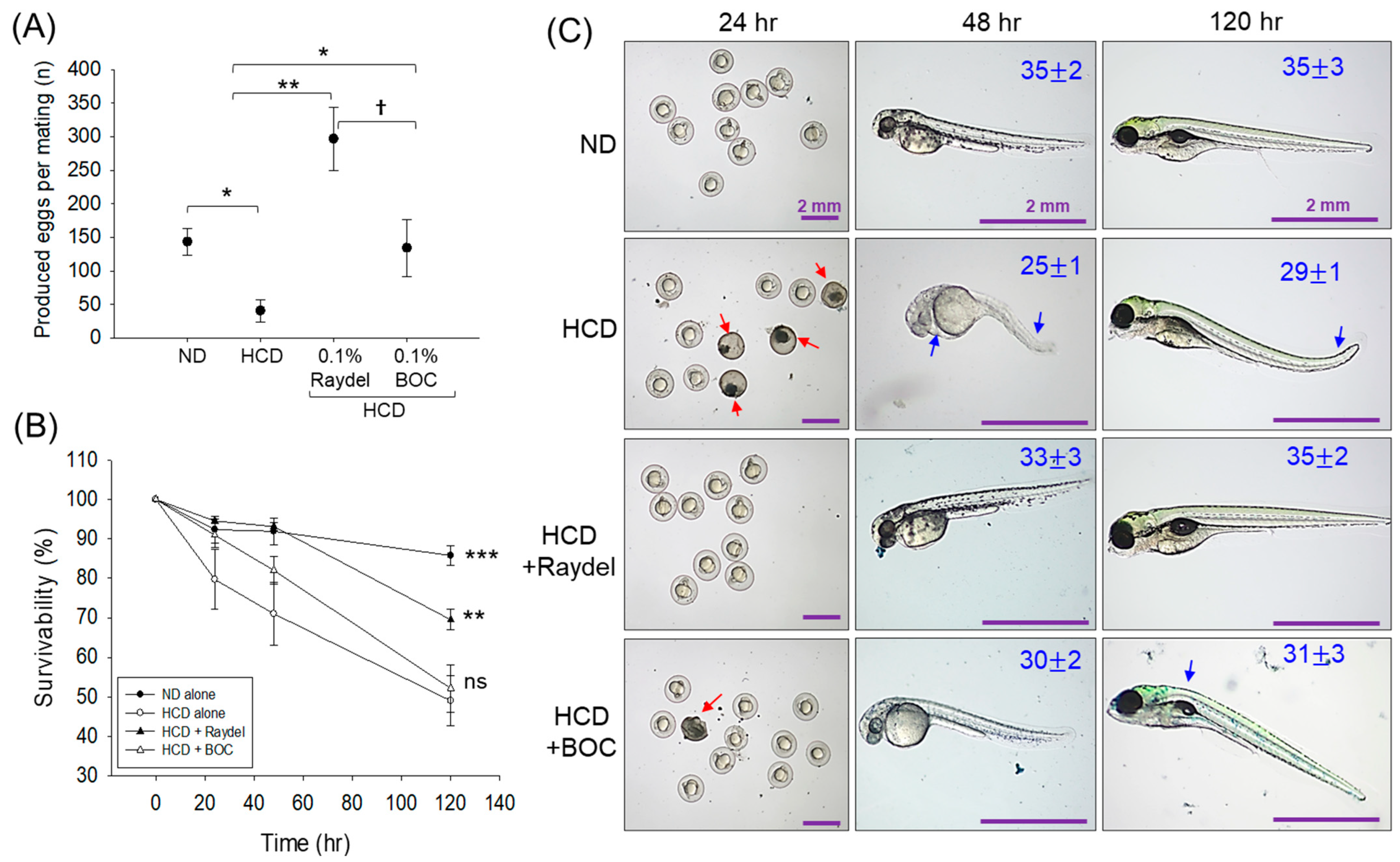
2.9. Egg-Laying Ability in Response to Policosanol Consumption
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Formulation of High-Cholesterol Diet (HCD) Enriched with Policosanol
4.3. Zebrafish Culture and Feeding with High-Cholesterol Diet (HCD)
4.4. Zebrafish Feeding with High-Cholesterol Diet (HCD) Enriched with Policosanol
4.5. Blood Analysis
4.6. Histological Analysis
4.7. Detection of Interleukin (IL-6) by Immunohistochemistry (IHC)
4.8. Fluorescent Staining for ROS Production and Apoptosis
4.9. Senescent-Associated β-Galactose Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olatunji, L.K.; Jimoh, A.O.; Tukur, U.M.; Imam, M.U. A review of the effects of policosanol on metabolic syndrome. Clin. Complement. Med. Pharmacol. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Nam, D.-E.; Yun, J.-M.; Kim, D.; Kim, O.-K. Policosanol attenuates cholesterol synthesis via AMPK activation in hypercholesterolemic rats. J. Med. Food 2019, 22, 1110–1117. [Google Scholar] [CrossRef]
- Cho, K.-H.; Bahuguna, A.; Kim, J.-E.; Lee, S.H. Efficacy Assessment of Five Policosanol Brands and Damage to Vital Organs in Hyperlipidemic Zebrafish by Six-Week Supplementation: Highlighting the Toxicity of Red Yeast Rice and Safety of Cuban Policosanol (Raydel®). Pharmaceuticals 2024, 17, 714. [Google Scholar] [CrossRef] [PubMed]
- Canavaciolo, V.L.G.; Gómez, C.V. “Copycat-policosanols” versus genuine policosanol. Rev. CENIC Cienc. Quím. 2007, 38, 207–213. [Google Scholar]
- Cho, K.-H.; Baek, S.H.; Nam, H.-S.; Kim, J.-E.; Kang, D.-J.; Na, H.; Zee, S. Cuban Sugar Cane Wax Alcohol Exhibited Enhanced Antioxidant, Anti-Glycation and Anti-Inflammatory Activity in Reconstituted High-Density Lipoprotein (rHDL) with Improved Structural and Functional Correlations: Comparison of Various Policosanols. Int. J. Mol. Sci. 2023, 24, 3186. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Nam, H.-S.; Baek, S.-H.; Bahuguna, A. Consumption of Policosanol (Raydel®) Improves Hepatic, Renal, and Reproductive Functions in Zebrafish: In Vivo Comparison Study among Cuban, Chinese, and American Policosanol. Pharmaceuticals 2024, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62, 1700280. [Google Scholar] [CrossRef]
- Askarpour, M.; Ghaedi, E.; Roshanravan, N.; Hadi, A.; Mohammadi, H.; Symonds, M.E.; Miraghajani, M. Policosanol supplementation significantly improves blood pressure among adults: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, S.-J.; Yadav, D.; Kim, J.-Y.; Kim, J.-R. Consumption of Cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxidative Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef]
- Lee, E.-Y.; Yoo, J.-A.; Lim, S.-M.; Cho, K.-H. Anti-aging and tissue regeneration ability of policosanol along with lipid-lowering effect in hyperlipidemic zebrafish via enhancement of high-density lipoprotein functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.-M.; Kim, S.-J.; Lee, E.-Y.; Kim, J.-R.; Cho, K.-H. Consumption of policosanol enhances HDL functionality via CETP inhibition and reduces blood pressure and visceral fat in young and middle-aged subjects. Int. J. Mol. Med. 2017, 39, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Crespo, N.; Illnait, J.; Mas, R.; Fernandez, L.; Fernandez, J.; Castano, G. Comparative study of the efficacy and tolerability of policosanol and lovastatin in patients with hypercholesterolemia and noninsulin dependent diabetes mellitus. Int. J. Clin. Pharmacol. Res. 1999, 29, 117–127. [Google Scholar]
- Mirkin, A.; Mas, R.; Martinto, M.; Boccanera, R.; Robertis, A.; Poudes, R.; Fuster, A.; Lastreto, E.; Yañez, M.; Irico, G.; et al. Efficacy and tolerability of policosanol in hypercholesterolemic postmenopausal women. Int. J. Clin. Pharmacol. Res. 2001, 21, 31–41. [Google Scholar] [PubMed]
- Castano, G.; Mas, R.; Fernandez, J.C.; Fernández, L.; Alvarez, E.; Lezcay, M. Efficacy and tolerability of policosanol compared with lovastatin in patients with type II hypercholesterolemia and concomitant coronary risk factors. Curr. Ther. Res. Clin. Exp. 2000, 61, 137–146. [Google Scholar] [CrossRef]
- Castano, G.; Mas, R.; Fernandez, L.; Illnait, J.; Mesa, M.; Alvarez, E.; Lezcay, M. Comparison of the efficacy and tolerability of policosanol with atorvastatin in elderly patients with type II hypercholesterolaemia. Drugs Aging 2003, 20, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ortensi, G.; Gladstein, J.; Valli, H.; Tesone, P.A. A comparative study of policosanol versus simvastatin in elderly patients with hypercholesterolemia. Curr. Ther. Res. Clin. Exp. 1997, 58, 390–401. [Google Scholar] [CrossRef]
- Illnait, J.; Castano, G.; Mas, R.; Fernandez, J.C. A comparative study on the efficacy and tolerability of policosanol and simvastatin for treating type II hypercholesterolemia. Can. J. Cardiol. 1997, 13, 342B. [Google Scholar]
- Benitez, M.; Romero, C.; Mas, R.; Fernández, L.; Fernández, J.C. A comparative study of policosanol versus pravastatin in patients with type II hypercholesterolemia. Curr. Ther. Res. Clin. Exp. 1997, 58, 859–867. [Google Scholar] [CrossRef]
- Castano, G.; Mas, R.; Arruzazabala, M.D.L.; Noa, M.; Illnait, J.; Fernández, J.C.; Molina, V.; Menéndez, A. Effects of policosanol and pravastatin on lipid profile, platelet aggregation and endothelemia in older hypercholesterolemic patients. Int. J. Clin. Pharmacol. Res. 1999, 29, 105–116. [Google Scholar] [CrossRef]
- Alcocer, L.; Fernandez, L.; Compos, E.; Mas, R.A. comparative study of policosanol versus acipimox in patients with type II hypercholesterolemia. Int. J. Tissue React. 1999, 21, 85–92. [Google Scholar]
- Pons, P.; Illnait, J.; Más, R.; Rodríguez, M.; Alemán, C.; Fernaández, J.C.; Fernández, L.; Martin, M. A comparative study of policosanol versus probucol in patients with hypercholesterolemia. Curr. Ther. Res. Clin. Exp. 1997, 58, 26–35. [Google Scholar] [CrossRef]
- Guo, Y.-L.; Xu, R.-X.; Zhu, C.-G.; Wu, N.-Q.; Cui, Z.-P.; Li, J.-J. Policosanol attenuates statin-induced increases in serum proprotein convertase subtilisin/kexin type 9 when combined with atorvastatin. Evid. Based Complement. Altern. Med. 2014, 2014, 926087. [Google Scholar] [CrossRef]
- Al-Miahy, A.J.; Alkalby, J.M. Effect of Policosanol Extract and Simvastatin on Some Liver Enzymes and Histopathology In Hypercholestrolemic Female Rats During Lactation. J. Educ. Pure Sci. 2018, 8, 43–56. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Jiao, Q.-P.; Chen, S.-Y.; Sheng, J.; Jiang, H.; Lu, J.; Zheng, S.-B.; Fang, N.-Y. Efficacy and safety of Policosanol plus Fenofibrate combination therapy in elderly patients with mixed Dyslipidemia: A randomized, controlled clinical study. Am. J. Med. Sci. 2018, 356, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Gloviczki, P.; Antignani, P.L.; Comerota, A.J.; Dardik, A.; Davies, A.H.; Eckstein, H.-H.; Faggioli, G.; Fernandes, J.F.E.; Fraedrich, G. Benefits and drawbacks of statins and non-statin lipid lowering agents in carotid artery disease. Prog. Cardiovasc. Dis. 2022, 73, 41–47. [Google Scholar] [CrossRef]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.T.; Gao, Z.Y.; Shi, D.Z. Effects of coenzyme Q10 on statin-induced myopathy: An updated meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Mikhailidis, D.P. Statin intolerance: Some practical hints. Cardiol. Clin. 2018, 36, 225–231. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V. Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2015, 11, 1–23. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Kim, N.-Y.; Lee, M.-S.; Kang, D.-J. Combination Therapy of Cuban Policosanol (Raydel®, 20 mg) and Intensive Exercise for 12 Weeks Resulted in Improvements in Obesity, Hypertension, and Dyslipidemia without a Decrease in Serum Coenzyme Q10: Enhancement of Lipoproteins Quality and Antioxidant Functionality in Obese Participants. Pharmaceuticals 2024, 17, 132. [Google Scholar] [CrossRef]
- Yanai, H.; Katsuyama, H.; Hamasaki, H.; Abe, S.; Tada, N.; Sako, A. Effects of dietary fat intake on HDL metabolism. J. Clin. Med. Res. 2015, 7, 145–149. [Google Scholar] [CrossRef]
- Chow, S.L.; Bozkurt, B.; Baker, W.L.; Bleske, B.E.; Breathett, K.; Fonarow, G.C.; Greenberg, B.; Khazanie, P.; Leclerc, J.; Morris, A.A. Complementary and Alternative Medicines in the Management of Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2023, 147, e4–e30. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sphericalinsights.com/reports/policosanol-market (accessed on 16 May 2024).
- Harrabi, S.; Ferchichi, A.; Bacheli, A.; Fellah, H. Policosanol composition, antioxidant and anti-arthritic activities of milk thistle (Silybium marianum L.) oil at different seed maturity stages. Lipids Health Dis. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Baek, S.H. Cuban policosanol (Raydel®) potently protects the liver, ovary, and testis with an improvement in dyslipidemia in hyperlipidemic zebrafish: A comparative study with three Chinese policosanols. Molecules 2023, 28, 6609. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Lee, M.-S.; Bahuguna, A. Cuban Policosanol (Raydel®) Exerts Higher Antioxidant and Anti-Glycation Activities than Chinese Policosanol (BOC Sciences) in Reconstituted High-Density Lipoproteins: In Vivo Anti-Inflammatory Activities in Zebrafish and Its Embryos. Pharmaceuticals 2024, 17, 406. [Google Scholar] [CrossRef]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef]
- Juan-García, A.; Bind, M.-A.; Engert, F. Larval zebrafish as an in vitro model for evaluating toxicological effects of mycotoxins. Ecotoxicol. Environ. Saf. 2020, 202, 110909. [Google Scholar] [CrossRef]
- Gupta, H.R.; Patil, Y.; Singh, D.; Thakur, M. Embryonic Zebrafish Model—A Well-Established Method for Rapidly Assessing the Toxicity of Homeopathic Drugs–Toxicity Evaluation of Homeopathic Drugs Using Zebrafish Embryo Model. J. Pharmacopunct. 2016, 19, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, C.; Miller, Y.I. Zebrafish models of dyslipidemia: Relevance to atherosclerosis and angiogenesis. Transl. Res. 2014, 163, 99–108. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choi, H.-Y.; Kang, Y.-R.; Chang, H.-B.; Chun, H.-S.; Lee, M.-S.; Kwon, Y.-I. Effects of long-term supplementation of policosanol on blood cholesterol/glucose levels and 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in a rat model fed high cholesterol diets. Food Sci. Biotechnol. 2016, 25, 899–904. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Cao, F.L.; Luo, F.J.; Li, Q.L. Octacosanol and health benefits: Biological functions and mechanisms of action. Food Biosci. 2022, 47, 101632. [Google Scholar] [CrossRef]
- Bai, J.; Yang, T.; Zhou, Y.; Xu, W.; Han, S.; Guo, T.; Zhu, L.; Qin, D.; Luo, Y.; Hu, Z.; et al. Octacosanol modifies obesity, expression profile and inflammation response of hepatic tissues in high-fat diet mice. Foods 2022, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Li, L.; Porter, T.D. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J. Pharmacol. Exp. Ther. 2006, 318, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jia, Y.; Thach, T.T.; Han, Y.; Kim, B.; Wu, C.; Kim, Y.; Seo, W.D.; Lee, S.J. Hexacosanol reduces plasma and hepatic cholesterol by activation of AMP-activated protein kinase and suppression of sterol regulatory element-binding protein-2 in HepG2 and C57BL/6J mice. Nutr. Res. 2017, 43, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Arteche-Hidalgo, L.; Fernandez-Travieso, J.; Suarez-Camejo, N.; Marin-Preval, J.; Alvarez-Acosta, V.; Chaviano-Pereira, J.; Garcia-Sanchez, M.; Esquivel-Moinelo, I.; Diaz-Gonzalez, M.; Matos-Reyes, O.; et al. Effects of Policosanol in Patients with Metabolic Syndrome: A Six-Month Study. J. Endocrinol. Metab. 2020, 10, 36–44. [Google Scholar] [CrossRef]
- Park, H.-J.; Yadav, D.; Jeong, D.-J.; Kim, S.-J.; Bae, M.-A.; Kim, J.-R.; Cho, K.-H. Short-term consumption of Cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy Korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health 2019, 16, 809. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Zein, N.; Aldhamy, S.E.; Elsawy, M.M.; Saeid, S.A. Policosanol is a new inhibitor candidate for vascular calcification in diabetic hyperlipidemic rats. Exp. Biol. Med. 2016, 241, 1943–1949. [Google Scholar] [CrossRef]
- Teratani, T.; Tomita, K.; Suzuki, T.; Oshikawa, T.; Yokoyama, H.; Shimamura, K.; Tominaga, S.; Hiroi, S.; Irie, R.; Okada, Y. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology 2012, 142, 152–164.e10. [Google Scholar] [CrossRef]
- Christian, P.; Sacco, J.; Adeli, K. Autophagy: Emerging roles in lipid homeostasis and metabolic control. Biochim. Biophys. Acta 2013, 1831, 819–824. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, X.-L.; Yang, L.; Shi, F.; Gao, L.-B.; Zhong, Y.-J.; Sun, H.; He, F.; Lin, Y.; Wang, X. Reactive oxygen species-mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin-induced cytotoxicity in cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 159. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Flensted-Jensen, M.; Oró, D.; Rørbeck, E.A.; Zhang, C.; Madsen, M.R.; Madsen, A.N.; Norlin, J.; Feigh, M.; Larsen, S.; Hansen, H.H. Dietary intervention reverses molecular markers of hepatocellular senescence in the GAN diet-induced obese and biopsy-confirmed mouse model of NASH. BMC Gastroenterol. 2024, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Sahadevan, M.; Kasiske, B.L. Hyperlipidemia in kidney disease causes and consequences. Curr. Opin. Nephrol. Hypertens. 2002, 11, 323–329. [Google Scholar] [CrossRef]
- Çiftci, G.; Tuna, E. Effects of cholesterol and Lactobacillus acidophilus on testicular function. Clin. Exp. Reprod. Med. 2021, 48, 229. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chang, T.-C.; Lin, S.-H.; Wu, S.-T.; Cha, T.-L.; Tsao, C.-W. Metformin ameliorates testicular function and spermatogenesis in male mice with high-fat and high-cholesterol diet-induced obesity. Nutrients 2020, 12, 1932. [Google Scholar] [CrossRef]
- Elnagar, G.M.; Elseweidy, M.M.M.; Elkomy, N.M.; Keshawy, M.M.; Fathy, O.M.; Sobh, M.S.; Mahmoud, Y.K. Policosanol ameliorates renal inflammation and pyroptosis in hypercholesterolemic rabbits via modulation of HMGB1/PI3K/mTOR/NLRP3/Caspase-1 pathway. J. Funct. Foods 2022, 97, 105250. [Google Scholar] [CrossRef]
- Okada, S.; Saito, M.; Kazuyama, E.; Hanada, T.; Kawaba, Y.; Hayashi, A.; Satoh, K.; Kanzaki, S. Effects of N-hexacosanol on nitric oxide synthase system in diabetic rat nephropathy. Mol. Cell. Biochem. 2008, 315, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Kinoshita, Y.; Satoh, I.; Shinbori, C.; Kono, T.; Hanada, T.; Uemasu, J.; Suzuki, H.; Yamada, M.; Satoh, K. N-hexacosanol ameliorates streptozotocin-induced diabetic rat nephropathy. Eur. J. Pharmacol. 2006, 544, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.D.; García, H. Teratogenic and reproductive studies of policosanol in the rat and rabbit. Teratog. Carcinog. Mutagen. 1994, 14, 107–113. [Google Scholar] [CrossRef]
- Long, L.; Wu, S.G.; Yuan, F.; Zhang, H.J.; Wang, J.; Qi, G.H. Effects of dietary octacosanol supplementation on laying performance, egg quality, serum hormone levels, and expression of genes related to the reproductive axis in laying hens. Poult. Sci. 2017, 96, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-H.; Kim, J.-E.; Bahuguna, A.; Kang, D.-J. Long-term supplementation of ozonated sunflower oil improves dyslipidemia and hepatic inflammation in hyperlipidemic zebrafish: Suppression of oxidative stress and inflammation against carboxymethyllysine toxicity. Antioxidants 2023, 12, 1240. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef] [PubMed]
- Shull, L.C.; Sen, R.; Menzel, J.; Goyama, S.; Kurokawa, M.; Artinger, K.B. The conserved and divergent roles of Prdm3 and Prdm16 in zebrafish and mouse craniofacial development. Dev. Biol. 2020, 461, 132–144. [Google Scholar] [PubMed]
- Cho, K.-H.; Bahuguna, A.; Kang, D.-J.; Kim, J.-E. Prolonged Supplementation of Ozonated Sunflower Oil Bestows an Antiaging Effect, Improves Blood Lipid Profile and Spinal Deformities, and Protects Vital Organs of Zebrafish (Danio rerio) against Age-Related Degeneration: Two-Years Consumption Study. Antioxidants 2024, 13, 123. [Google Scholar] [CrossRef]



| Description/Composition of Long-Chain Aliphatic Alcohols | Raydel Policosanol | BOC Policosanol |
|---|---|---|
| Source material and country of origin | Sugarcane wax from Cuba | Sugarcane wax from China |
| Manufacturer | CNIC, Cuba | BOC Sciences, USA |
| Average molecular weight | 418 | 411 |
| Composition of long-chain aliphatic alcohols (LCAAs) (mg/g) (%) | ||
| 1-tetracosanol (C24H50O) | 0.3 (0.0) | 4.0 (0.5) |
| 1-hexacosanol (C26H54O) | 38.0 (3.9) | 11.0 (1.2) |
| 1-heptacosanol (C27H56O) | 9.0 (0.9) | 21.0 (2.3) |
| 1-octacosanol (C28H58O) | 692.0 (70.5) | 819.0 (90.5) |
| 1-nonacosanol (C29H60O) | 6.0 (0.6) | 12.0 (1.4) |
| 1-triacontanol (C30H62O) | 139.0 (14.2) | 24.0 (2.7) |
| 1-dotriacontanol (C32H66O) | 78.0 (7.9) | 2.0 (0.2) |
| 1-tetratriacontanol (C34H70O) | 20.0 (2.0) | 0.0 (0.0) |
| Total amount | 982.0 (100) | 902.0 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Lee, Y.; Lee, S.H.; Kim, J.-E.; Bahuguna, A. Comparison of the In Vivo Efficacy of Cuban (Raydel®) and Chinese (BOC Science) Policosanol in Alleviating Dyslipidemia and Inflammation via Safeguarding Major Organs and Reproductive Health in Hyperlipidemic Zebrafish: A Twelve-Week Consumption Study. Pharmaceuticals 2024, 17, 1103. https://doi.org/10.3390/ph17081103
Cho K-H, Lee Y, Lee SH, Kim J-E, Bahuguna A. Comparison of the In Vivo Efficacy of Cuban (Raydel®) and Chinese (BOC Science) Policosanol in Alleviating Dyslipidemia and Inflammation via Safeguarding Major Organs and Reproductive Health in Hyperlipidemic Zebrafish: A Twelve-Week Consumption Study. Pharmaceuticals. 2024; 17(8):1103. https://doi.org/10.3390/ph17081103
Chicago/Turabian StyleCho, Kyung-Hyun, Yunki Lee, Sang Hyuk Lee, Ji-Eun Kim, and Ashutosh Bahuguna. 2024. "Comparison of the In Vivo Efficacy of Cuban (Raydel®) and Chinese (BOC Science) Policosanol in Alleviating Dyslipidemia and Inflammation via Safeguarding Major Organs and Reproductive Health in Hyperlipidemic Zebrafish: A Twelve-Week Consumption Study" Pharmaceuticals 17, no. 8: 1103. https://doi.org/10.3390/ph17081103
APA StyleCho, K.-H., Lee, Y., Lee, S. H., Kim, J.-E., & Bahuguna, A. (2024). Comparison of the In Vivo Efficacy of Cuban (Raydel®) and Chinese (BOC Science) Policosanol in Alleviating Dyslipidemia and Inflammation via Safeguarding Major Organs and Reproductive Health in Hyperlipidemic Zebrafish: A Twelve-Week Consumption Study. Pharmaceuticals, 17(8), 1103. https://doi.org/10.3390/ph17081103







