Development and Optimization of Nasal Composition of a Neuroprotective Agent for Use in Neonatology after Prenatal Hypoxia
Abstract
1. Introduction
2. Results
2.1. Formulation Design
2.2. Organoleptic Characteristics
2.3. Hydrogen Index (pH)
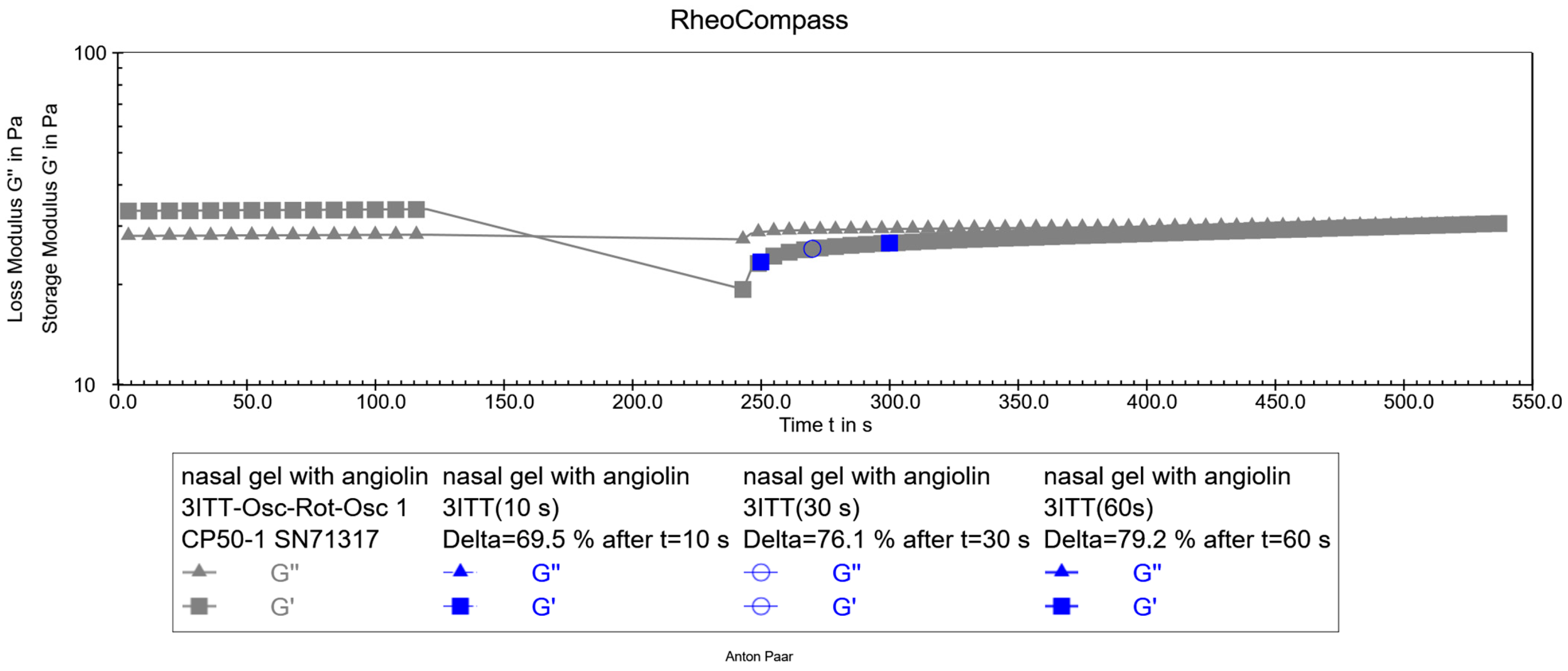
2.4. Rheological Characteristics
2.5. Pharmaco-Toxicological Research
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Design of the Experiment
4.3. Characteristics of the Obtained Gel Samples
4.3.1. pH Test
4.3.2. Biopharmaceutical Study of the Kinetics of Substance Release through a Semipermeable Membrane
4.3.3. Rheological Studies
4.3.4. Viscosity Test of Sample Gels
4.3.5. Thixotropy Test
4.4. Pharmaco-Toxicological Methods of Research
4.4.1. Acute Toxicity Definition
4.4.2. Evaluation of Allergizing and Skin-Resorptive Activity by the Method of Skin Applications
4.4.3. Examination of Local Irritant Effect (Conjunctival Test)
4.4.4. Examination of Active Dermal Anaphylaxis
4.5. Statistical Methods
5. Conclusions
Perspectives for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gomez, D.; Martinez, J.A.; Hanson, L.R.; Frey, W.H.; Toth, C.C. Intranasal treatment of neurodegenerative diseases and stroke. Front. Biosci. 2012, 4, 74–89. [Google Scholar] [CrossRef]
- Madden, S.; Carrazana, E.; Rabinowicz, A.L. Optimizing Absorption for Intranasal Delivery of Drugs Targeting the Central Nervous System Using Alkylsaccharide Permeation Enhancers. Pharmaceutics 2023, 15, 2119. [Google Scholar] [CrossRef]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef]
- Veronesi, M.C.; Alhamami, M.; Miedema, S.B.; Yun, Y.; Ruiz-Cardozo, M.; Vannier, M.W. Imaging of intranasal drug delivery to the brain. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 1–31. [Google Scholar]
- Dwibhashyam, V.S.; Nagappa, A.N. Strategies for enhanced drug delivery to the central nervous system. Indian J. Pharm. Sci. 2008, 70, 145–153. [Google Scholar]
- Trevino, J.T.; Quispe, R.C.; Khan, F.; Novak, V. Non-Invasive Strategies for Nose-to-Brain Drug Delivery. J. Clin. Trials 2020, 10, 439. [Google Scholar]
- Kousalya, S.; Kuppusamy, G.; Veera, V.S.R.K. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2088–2095. [Google Scholar]
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef]
- Chapman, C.D.; Frey, W.H.; Craft, S.; Danielyan, L.; Hallschmid, M.; Schiöth, H.B.; Benedict, C. Intranasal treatment of central nervous system dysfunction in humans. Pharm. Res. 2013, 30, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Peters, J.M.; Detyniecki, K.; Tatum, W.; Rabinowicz, A.L.; Carrazana, E. The nose has it: Opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav. Rep. 2022, 21, 100581. [Google Scholar] [CrossRef] [PubMed]
- Shrewsbury, S.B. The Upper Nasal Space: Option for Systemic Drug Delivery, Mucosal Vaccines and “Nose-to-Brain”. Pharmaceutics 2023, 15, 1720. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tao, J.; Wang, J. Design and Application in Delivery System of Intranasal Antidepressants. Front. Bioeng. Biotechnol. 2020, 8, 626882. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A. In Situ-Based Gels for Nose to Brain Delivery for the Treatment of Neurological Diseases. Pharmaceutics 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Belenichev, I.F.; Aliyeva, O.G.; Popazova, O.O.; Bukhtiyarova, N.V. Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: The prospect of using HSP70 modulators. Front. Cell. Neurosci. 2023, 17, 1131683. [Google Scholar] [CrossRef] [PubMed]
- Cerio, F.G.; Lara-Celador, I.; Alvarez, A.; Hilario, E. Neuroprotective therapies after perinatal hypoxic-ischemic brain injury. Brain Sci. 2013, 3, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Andelius, T.C.K.; Bøgh, N.; Pedersen, M.V.; Omann, C.; Andersen, M.; Andersen, H.B.; Hjortdal, V.E.; Pedersen, M.; Rasmussen, M.B.; Kyng, K.J.; et al. Early changes in cerebral metabolism after perinatal hypoxia-ischemia: A study in normothermic and hypothermic piglets. Front. Pediatr. 2023, 11, 1167396. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.J.; Ling, E.A.; Li, F. Immunomodulatory Mechanism and Potential Therapies for Perinatal Hypoxic-Ischemic Brain Damage. Front. Pharmacol. 2020, 11, 580428. [Google Scholar] [CrossRef] [PubMed]
- Gorbacheva, S.V. Nitrosative stress restriction in vitro conditions using modulators of thiol-disul-fide system. Bull. Prob. Boil. Med. 2015, 4, 133–135. [Google Scholar]
- Aliyeva, O.; Belenichev, I.; Bukhtiyarova, N.; Semenov, D.; Voloshchuk, S. Comparative Assessment of the Effectiveness of HSP70/HIF-1α System Modulators after Prenatal Hypoxia. Biomed. Pharmacol. J. 2024, 17, 223–233. [Google Scholar] [CrossRef]
- Chekman, I.S.; Kazakova, O.A.; Mazur, I.A.; Nagornaya, E.A.; Belenichev, I.F.; Gorchakova, N.A. New original metabolitotropic endothelioprotector “Angiolin”: Quantum-chemical parameters and peculiarities of pharmacological action. Rep. NAS Ukraine 2017, 8, 86–93. [Google Scholar]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Savchenko, D.; Oksenych, V.; Kamyshnyi, O. Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection. Antioxidants 2024, 13, 504. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, Y.M. Report on Preclinical Study of Specific Biological Activity (Anti-Ischemic, Endothelioprotective) of the Drug Lisinium (Angiolin) at Parenteral Administration, Zaporozhye, Ukraine. Unpublished internal report, 2018; 11, 2584. [Google Scholar]
- Kucherenko, L.I.; Bidnenko, O.S.; Tkachenko, G.I. Regarding L-lysine 3-methyl-1.2.4-triazole-5-thioacetate Standardization. Pharm. Rev. 2017, 1, 28–31. [Google Scholar] [CrossRef][Green Version]
- Keller, T.; Körber, F.; Oberthuer, A.; Schafmeyer, L.; Mehler, K.; Kuhr, K.; Kribs, A. Intranasal breast milk for premature infants with severe intraventricular hemorrhage-an observation. Eur. J. Pediatr. 2019, 178, 199–206. [Google Scholar] [CrossRef]
- Scafidi, J.; Hammond, T.R.; Scafidi, S.; Ritter, J.; Jablonska, B.; Roncal, M.; Szigeti-Buck, K.; Coman, D.; Huang, Y.; McCarter, R.J., Jr.; et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature 2014, 506, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J. ANOVA and the analysis of drug combination experiments. Nat. Methods 2015, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54, e01006. [Google Scholar] [CrossRef]
- Adams, S.Y.; Tucker, R.; Clark, M.A.; Lechner, B.E. “Quality of life”: Parent and neonatologist perspectives. J. Perinatol. 2020, 40, 1809–1820. [Google Scholar] [CrossRef]
- Piešová, M.; Mach, M. Impact of perinatal hypoxia on the developing brain. Physiol. Res. 2020, 69, 199–213. [Google Scholar] [CrossRef]
- Millar, L.J.; Shi, L.; Hoerder-Suabedissen, A.; Molnár, Z. Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges. Front. Cell. Neurosci. 2017, 11, 78. [Google Scholar] [CrossRef]
- Wang, B.; Zeng, H.; Liu, J.; Sun, M. Effects of Prenatal Hypoxia on Nervous System Development and Related Diseases. Front. Neurosci. 2021, 15, 755554. [Google Scholar] [CrossRef]
- Mielecki, D.; Godlewski, J.; Salinska, E. Hyperbaric oxygen therapy for the treatment of hypoxic/ischemic injury upon perinatal asphyxia—Are we there yet? Front. Neurol. 2024, 15, 1386695. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Gulati, A. Advances in Therapies to Treat Neonatal Hypoxic-Ischemic Encephalopathy. J. Clin. Med. 2023, 12, 6653. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Danielyan, L.; Schäfer, R.; von Ameln-Mayerhofer, A.; Buadze, M.; Geisler, J.; Klopfer, T.; Burkhardt, U.; Proksch, B.; Verleysdonk, S.; Ayturan, M.; et al. Intranasal delivery of cells to the brain. Eur. J. Cell Biol. 2009, 88, 315–324. [Google Scholar] [CrossRef]
- Semenenko, S.I.; Miedviedieva, K.P.; Vasiuk, S.O.; Burlaka, B.S. Development of a spectrophotometric technique for the quantitative determination of ademol. Curr. Issues Pharm. Med. Sci. Pract. 2023, 16, 28–32. [Google Scholar] [CrossRef]
- Dagda, R.K.; Dagda, R.Y.; Vazquez-Mayorga, E.; Martinez, B.; Gallahue, A. Intranasal Administration of Forskolin and Noopept Reverses Parkinsonian Pathology in PINK1 Knockout Rats. Int. J. Mol. Sci. 2023, 24, 690. [Google Scholar] [CrossRef]
- Burlaka, B.S.; Belenichev, I.F.; Ryzhenko, O.I.; Ryzhenko, V.P.; Aliyeva, O.G.; Makyeyeva, L.V.; Popazova, O.O.; Bak, P.G. The effect of intranasal administration of an IL-1b antagonist (RAIL) on the state of the nitroxydergic system of the brain during modeling of acute cerebrovascular accident. Pharmacia 2021, 68, 665–670. [Google Scholar] [CrossRef]
- Council Directive 86/609/EEC of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes. Off. J. Eur. Union 1986, 358, 1–28.
- Buzek, J.; Chastel, O. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]


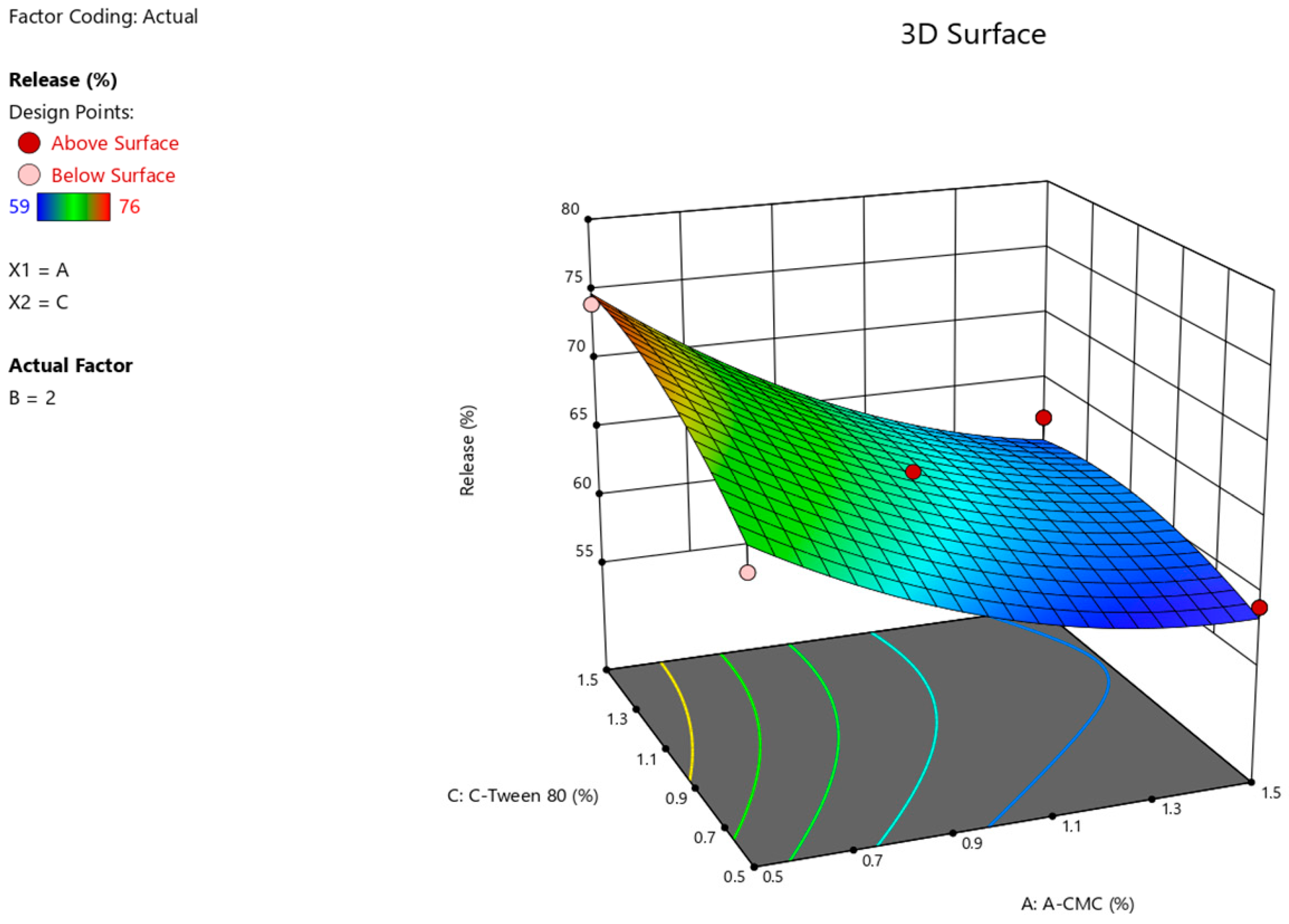

| Run | Factor 1 A: CMC % | Factor 2 B: D-Panthenol, % | Factor 3 C: Tween 80, % | Response 1, pH | Response 2, Viscosity, mPa × s | Response 3 Release, % |
|---|---|---|---|---|---|---|
| 1 | 1.5 | 3 | 1 | 6.41 | 782.15 | 60 |
| 2 | 0.5 | 2 | 1.5 | 6.41 | 80.01 | 74 |
| 3 | 1 | 2 | 1 | 6.41 | 432.57 | 65 |
| 4 | 1.5 | 2 | 0.5 | 6.41 | 1657.2 | 59 |
| 5 | 1 | 3 | 0.5 | 6.41 | 258.08 | 63 |
| 6 | 1 | 3 | 1.5 | 6.42 | 392.88 | 66 |
| 7 | 1 | 1 | 0.5 | 6.41 | 573.06 | 62 |
| 8 | 0.5 | 3 | 1 | 6.41 | 88.28 | 76 |
| 9 | 0.5 | 1 | 1 | 6.42 | 547.31 | 75 |
| 10 | 0.5 | 2 | 0.5 | 6.41 | 29.74 | 66 |
| 11 | 1.5 | 2 | 1.5 | 6.42 | 1698.5 | 62 |
| 12 | 1.5 | 1 | 1 | 6.41 | 604.28 | 62 |
| 13 | 1 | 1 | 1.5 | 6.41 | 443.38 | 66 |
| 14 | 1 | 2 | 1 | 6.41 | 161.23 | 64 |
| 15 | 1 | 2 | 1 | 6.42 | 439.65 | 64 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Test Result |
|---|---|---|---|---|---|---|
| Model | 0.0000 | 0 | ||||
| Residual | 0.0003 | 14 | 0.0000 | |||
| Lack of Fit | 0.0002 | 12 | 0.0000 | 0.5667 | 0.7871 | not significant |
| Pure Error | 0.0001 | 2 | 0.0000 | |||
| Cor Total | 0.0003 | 14 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Test Result |
|---|---|---|---|---|---|---|
| Model | 1.997 × 106 | 1 | 1.997 × 106 | 16.14 | 0.0015 | significant |
| A-A-CMC | 1.997 × 106 | 1 | 1.997 × 106 | 16.14 | 0.0015 | |
| Residual | 1.608 × 106 | 13 | 1.237 × 106 | |||
| Lack of Fit | 1.558 × 106 | 11 | 1.416 × 106 | 5.62 | 0.1606 | not significant |
| Pure Error | 50,397.74 | 2 | 25,198.87 | |||
| Cor Total | 3.605 × 106 | 14 | 16.14 | 0.0015 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Test Result |
|---|---|---|---|---|---|---|
| Model | 377.93 | 9 | 41.99 | 13.40 | 0.0053 | significant |
| A-A-CMC | 288.00 | 1 | 288.00 | 91.91 | 0.0002 | |
| B-D-Panthenol | 5.684 × 10−14 | 1 | 5.684 × 10−14 | 1.814 × 10−14 | 1.0000 | |
| C-C-Tween 80 | 40.50 | 1 | 40.50 | 12.93 | 0.0156 | |
| AB | 2.25 | 1 | 2.25 | 0.7181 | 0.4354 | |
| AC | 6.25 | 1 | 6.25 | 1.99 | 0.2170 | |
| BC | 0.2500 | 1 | 0.2500 | 0.0798 | 0.7889 | |
| A2 | 22.31 | 1 | 22.31 | 7.12 | 0.0444 | |
| B2 | 7.85 | 1 | 7.85 | 2.51 | 0.1743 | |
| C2 | 8.78 | 1 | 8.78 | 2.80 | 0.1551 | |
| Residual | 15.67 | 5 | 3.13 | |||
| Lack of Fit | 15.00 | 3 | 5.00 | 15.00 | 0.0631 | not significant |
| Pure Error | 0.6667 | 2 | 0.3333 | |||
| Cor Total | 393.60 | 14 |
| Number | A-CMC, % | D-Panthenol, % | C-Tween 80, % | pH | Viscosity, mPa × s | Release, % | Desirability | Selected |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.500 | 1.000 | 1.203 | 6.413 | 1045.480 | 63.253 | 0.390 | Selected |
| 2 | 1.500 | 1.000 | 1.199 | 6.413 | 1045.483 | 63.252 | 0.390 | |
| 3 | 1.500 | 1.000 | 1.156 | 6.413 | 1045.484 | 63.240 | 0.390 | |
| 4 | 1.500 | 1.000 | 1.267 | 6.413 | 1045.485 | 63.228 | 0.389 | |
| 5 | 1.500 | 1.000 | 1.317 | 6.413 | 1045.485 | 63.173 | 0.387 | |
| 6 | 1.147 | 1.000 | 1.346 | 6.413 | 693.050 | 65.195 | 0.381 | |
| 7 | 1.147 | 1.000 | 1.347 | 6.413 | 693.083 | 65.195 | 0.381 | |
| 8 | 1.145 | 1.000 | 1.351 | 6.413 | 690.815 | 65.216 | 0.381 | |
| 9 | 1.149 | 1.000 | 1.352 | 6.413 | 695.111 | 65.176 | 0.381 | |
| 10 | 1.141 | 1.000 | 1.343 | 6.413 | 686.560 | 65.256 | 0.381 | |
| 11 | 1.158 | 1.000 | 1.337 | 6.413 | 703.316 | 65.101 | 0.381 | |
| 12 | 1.128 | 1.000 | 1.364 | 6.413 | 673.421 | 65.382 | 0.381 | |
| 13 | 1.111 | 1.000 | 1.356 | 6.413 | 656.933 | 65.547 | 0.380 | |
| 14 | 1.173 | 1.000 | 1.364 | 6.413 | 718.592 | 64.959 | 0.380 | |
| 15 | 1.097 | 1.000 | 1.361 | 6.413 | 642.599 | 65.694 | 0.380 | |
| 16 | 1.098 | 1.000 | 1.377 | 6.413 | 643.747 | 65.682 | 0.380 | |
| 17 | 1.221 | 1.000 | 1.318 | 6.413 | 766.358 | 64.569 | 0.380 | |
| 18 | 1.321 | 1.000 | 1.289 | 6.413 | 867.085 | 63.898 | 0.380 | |
| 19 | 1.238 | 1.000 | 1.295 | 6.413 | 783.634 | 64.437 | 0.380 | |
| 20 | 1.071 | 1.000 | 1.371 | 6.413 | 616.924 | 65.969 | 0.380 | |
| 21 | 0.982 | 3.000 | 1.331 | 6.413 | 527.538 | 66.722 | 0.368 | |
| 22 | 0.982 | 3.000 | 1.337 | 6.413 | 527.577 | 66.721 | 0.368 | |
| 23 | 0.978 | 3.000 | 1.334 | 6.413 | 523.881 | 66.779 | 0.368 | |
| 24 | 0.980 | 3.000 | 1.327 | 6.413 | 525.567 | 66.752 | 0.368 | |
| 25 | 0.983 | 3.000 | 1.327 | 6.413 | 528.520 | 66.706 | 0.368 | |
| 26 | 0.978 | 3.000 | 1.339 | 6.413 | 523.890 | 66.778 | 0.368 | |
| 27 | 0.982 | 3.000 | 1.315 | 6.413 | 528.122 | 66.711 | 0.368 | |
| 28 | 0.981 | 3.000 | 1.351 | 6.413 | 526.517 | 66.735 | 0.368 | |
| 29 | 0.994 | 3.000 | 1.355 | 6.413 | 539.887 | 66.527 | 0.368 | |
| 30 | 0.974 | 3.000 | 1.383 | 6.413 | 519.721 | 66.829 | 0.368 | |
| 31 | 0.959 | 3.000 | 1.286 | 6.413 | 505.317 | 67.054 | 0.367 | |
| 32 | 0.982 | 3.000 | 1.428 | 6.413 | 528.014 | 66.657 | 0.367 | |
| 33 | 1.027 | 3.000 | 1.260 | 6.413 | 572.949 | 66.021 | 0.367 | |
| 34 | 0.970 | 3.000 | 1.451 | 6.413 | 516.307 | 66.816 | 0.366 | |
| 35 | 0.970 | 3.000 | 1.109 | 6.413 | 516.073 | 66.581 | 0.361 | |
| 36 | 0.975 | 3.000 | 1.067 | 6.413 | 521.297 | 66.378 | 0.358 |
| Name | Quantity (g) |
|---|---|
| Angiolin | 1.0 |
| Sodium CMC | 1.5 |
| D-panthenol | 1 |
| Benzalkonium chloride | 0.02 |
| Tween-80 | 1.2 |
| Purified water | Up to 100 |
| Volume, mL/100 g | Dose, mg/kg | Number of Rats | Lethality, % | ||
|---|---|---|---|---|---|
| Total | Dead | Survived | |||
| 0.4 | 4 | 6 | 0 | 6 | 0 |
| Number of Animals | Period of the Study, Day | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Control | Angiolin Gel | Control | Angiolin Gel | Control | Angiolin Gel | |
| 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Number of Animals | Period of the Study, Hours | |||||
|---|---|---|---|---|---|---|
| 6 | 12 | 24 | ||||
| Control | Angiolin Gel | Control | Angiolin Gel | Control | Angiolin Gel | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| Factor | Parameter | Levels | ||
|---|---|---|---|---|
| Low (−) | Medium (0) | High (+) | ||
| x1 | Carboxymethylcellulose sodium salt, Na CMC, % | 0.5 | 1 | 1.5 |
| x2 | D-panthenol % | 1 | 2 | 3 |
| x3 | Tween-80, % | 0.5 | 1 | 1.5 |
| Reaction Designation | Symbols | Reaction Description |
|---|---|---|
| negative | - | no change in the skin |
| doubtful | ± | small erythema without edema |
| weak-positive | + | erythema and edema at the application site |
| positive | ++ | erythema, edema, papules |
| strong positive | +++ | erythema, edema, papules, isolated vesicles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belenichev, I.; Aliyeva, O.; Burlaka, B.; Burlaka, K.; Kuchkovskyi, O.; Savchenko, D.; Oksenych, V.; Kamyshnyi, O. Development and Optimization of Nasal Composition of a Neuroprotective Agent for Use in Neonatology after Prenatal Hypoxia. Pharmaceuticals 2024, 17, 990. https://doi.org/10.3390/ph17080990
Belenichev I, Aliyeva O, Burlaka B, Burlaka K, Kuchkovskyi O, Savchenko D, Oksenych V, Kamyshnyi O. Development and Optimization of Nasal Composition of a Neuroprotective Agent for Use in Neonatology after Prenatal Hypoxia. Pharmaceuticals. 2024; 17(8):990. https://doi.org/10.3390/ph17080990
Chicago/Turabian StyleBelenichev, Igor, Olena Aliyeva, Bogdan Burlaka, Kristina Burlaka, Oleh Kuchkovskyi, Dmytro Savchenko, Valentyn Oksenych, and Oleksandr Kamyshnyi. 2024. "Development and Optimization of Nasal Composition of a Neuroprotective Agent for Use in Neonatology after Prenatal Hypoxia" Pharmaceuticals 17, no. 8: 990. https://doi.org/10.3390/ph17080990
APA StyleBelenichev, I., Aliyeva, O., Burlaka, B., Burlaka, K., Kuchkovskyi, O., Savchenko, D., Oksenych, V., & Kamyshnyi, O. (2024). Development and Optimization of Nasal Composition of a Neuroprotective Agent for Use in Neonatology after Prenatal Hypoxia. Pharmaceuticals, 17(8), 990. https://doi.org/10.3390/ph17080990










