Biologically Synthesized Gold Nanoparticles with Enhanced Antioxidant and Catalytic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the b-AuNPs
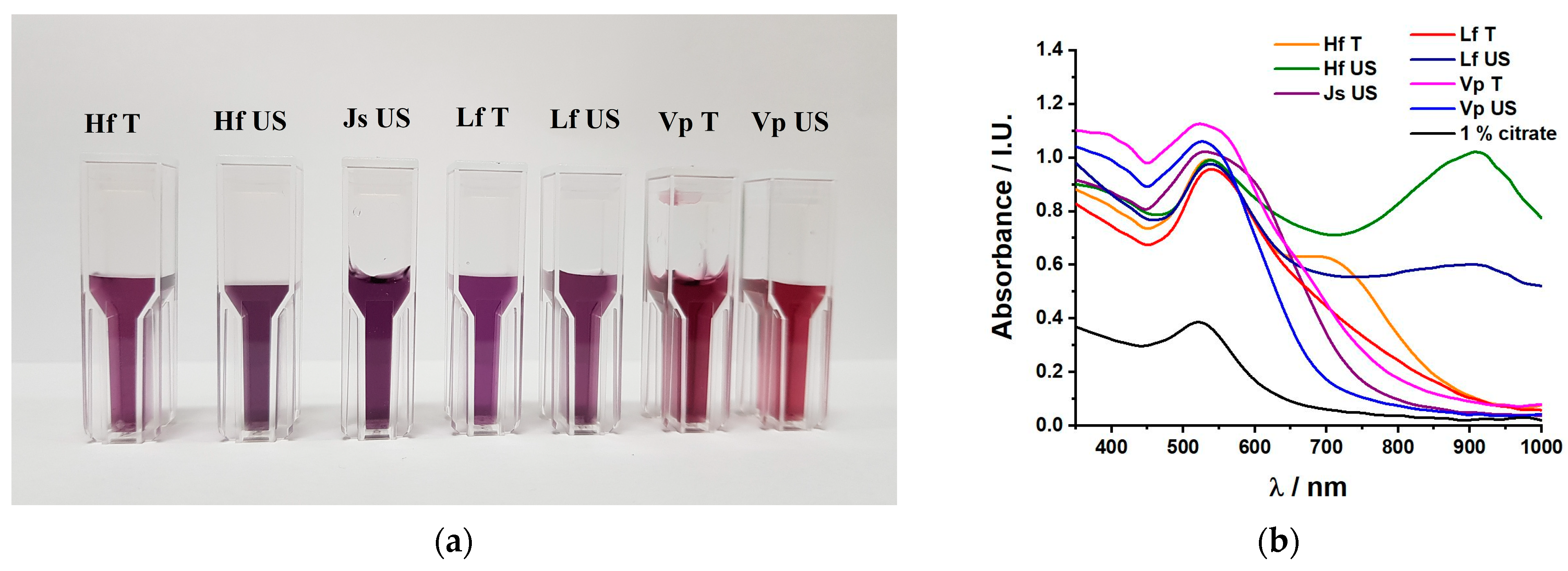
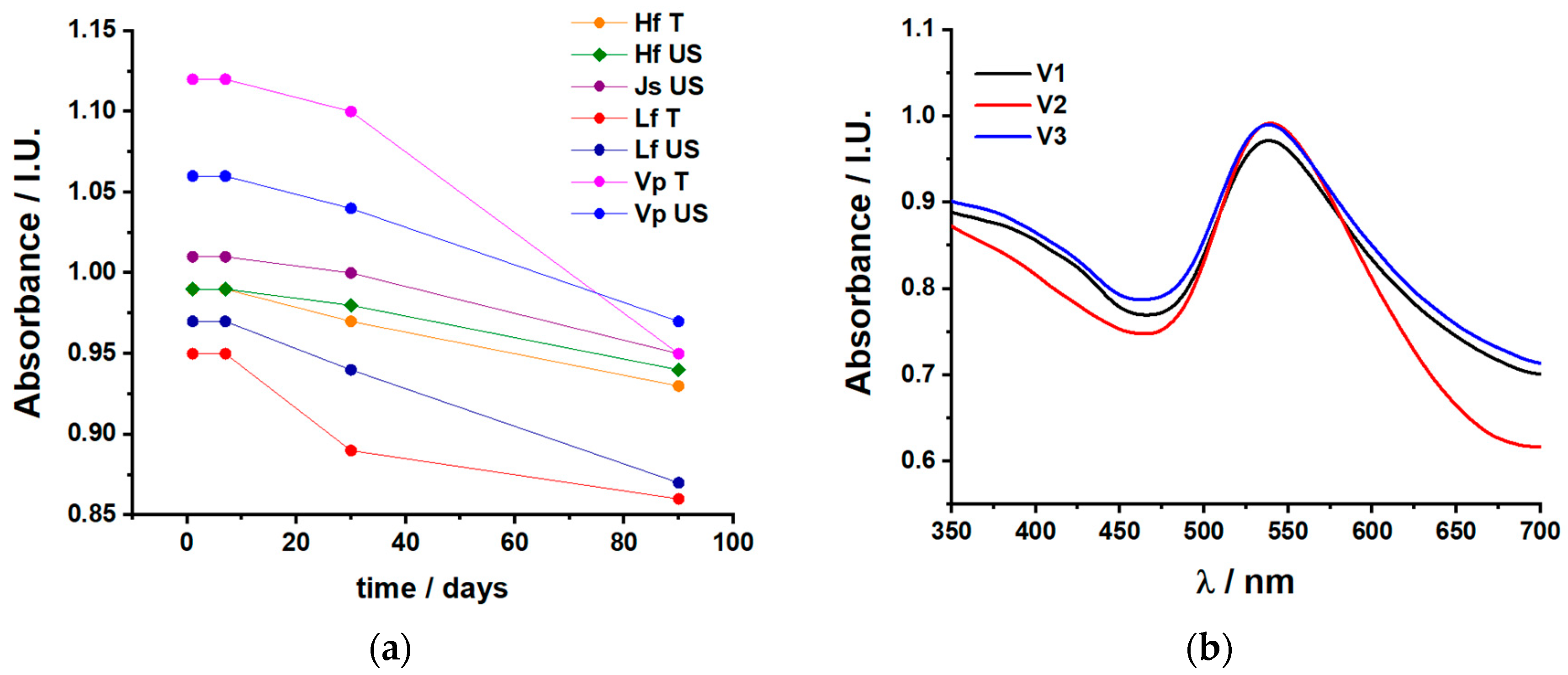
2.1.1. UV–Vis Spectroscopy
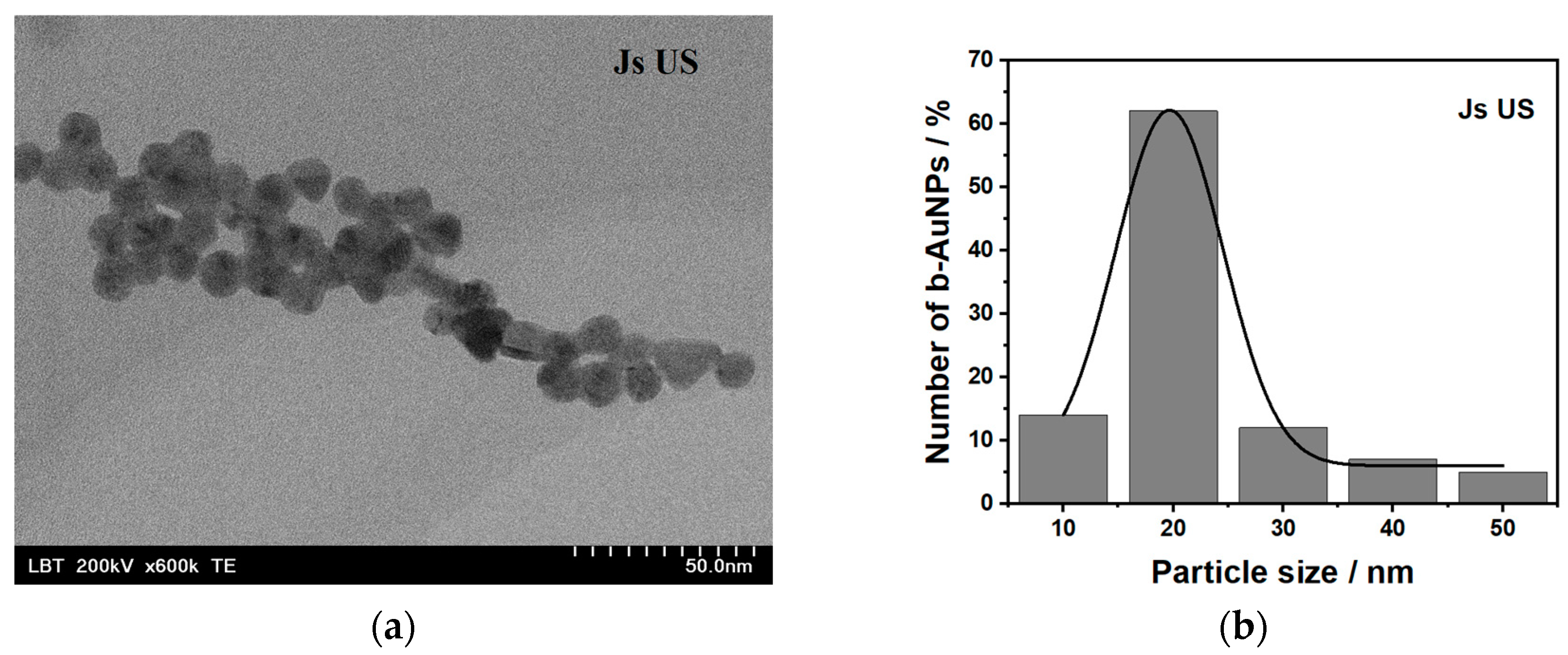
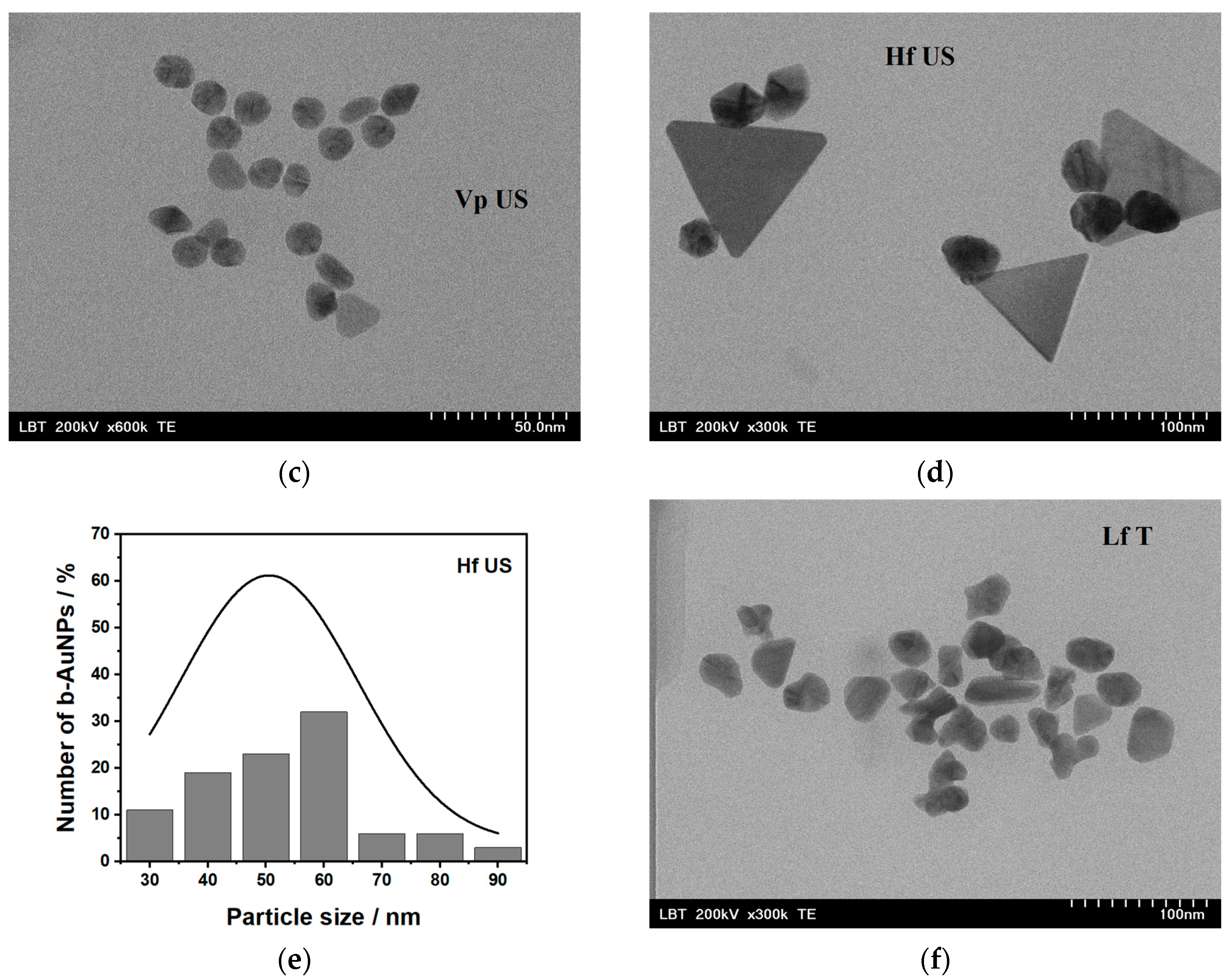
2.1.2. Transmission Electron Microscopy
2.1.3. Fourier Transform Infrared Analysis
2.2. Catalytic and Antioxidant Properties of the b-AuNPs
2.2.1. Catalytic Properties
2.2.2. Antioxidant Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of Plant Extracts
3.3. Colloidal AuNP Solution Synthesis
3.4. Formation of Liposomes for In Vitro Evaluation of the H2O2 Scavenging Activity
3.5. In Vitro Validation of the Antioxidant Activity
3.6. Characterization Techniques
3.6.1. UV–Vis Spectroscopy
3.6.2. Transmission Electron Microscopy
3.6.3. Fourier Transform Infrared Spectroscopy
3.6.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K.; Rodrigues, J. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Shahri, M.M.; Rahman, H.S.; Rahim, R.A.; Rasedee, A.; Mohamad, R. Green synthesis palladium nanoparticles mediated by white tea (Camellia sinensis) extract with antioxidant, antibacterial, and antiproliferative activities toward the human leukemia (MOLT-4) cell line. Int. J. Nanomed. 2017, 12, 8841–8853. [Google Scholar] [CrossRef]
- Shreyash, N.; Bajpai, S.; Khan, M.A.; Vijay, Y.; Tiwary, S.K.; Sonker, M. Green Synthesis of Nanoparticles and Their Biomedical Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 11428–11457. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. RETRACTED: Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.; Akhtar, K.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Biosynthesis of silver nanoparticles: A colorimetric optical sensor for detection of hexavalent chromium and ammonia in aqueous solution. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 103, 367–376. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sripriya, N.; Shanmugavel, M.; Manikandan, E.; Gnanamani, A.; Senthilkumar, P. Surface active gold nanoparticles biosynthesis by new approach for bionanocatalytic activity. J. Photochem. Photobiol. B Biol. 2018, 179, 119–125. [Google Scholar] [CrossRef]
- Dudhane, A.A.; Waghmode, S.R.; Dama, L.B.; Mhaindarkar, V.P.; Sonawane, A.; Katariya, S. Synthesis and characterization of gold nanoparticles using plant extract of Terminalia arjuna with antibacterial activity. Int. J. Nanosci. Nanotechnol. 2019, 15, 75–82. [Google Scholar]
- Abolghasemi, R.; Haghighi, M.; Solgi, M.; Mobinikhaledi, A. Rapid synthesis of ZnO nanoparticles by waste thyme (Thymus vulgaris L.). Int. J. Environ. Sci. Technol. 2018, 16, 6985–6990. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2015, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, C.; Stihi, C.; Ilie, M.; Lazurcă, D.; Gruia, R.; Olaru, O.T.; Bute, O.C.; Dulama, I.D.; Stirbescu, R.M.; Teodorescu, S.; et al. Characterization of Phenolics in Lavandula angustifolia. Anal. Lett. 2017, 50, 2839–2850. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Lee, J.; Hwang, B.H. Evaluation of the Effects, Causes, and Risks of Gold Nanorods Promoting Cell Proliferation. Biotechnol. Bioprocess Eng. 2022, 27, 213–220. [Google Scholar] [CrossRef]
- David, M.; Serban, A.; Radulescu, C.; Danet, A.F.; Florescu, M. Bioelectrochemical evaluation of plant extracts and gold nanozyme-based sensors for total antioxidant capacity determination. Bioelectrochemistry 2019, 129, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Shao, S.; Liu, M.; Shi, Y.; Zhang, H.; Chen, D. Cell membrane-coated nanoparticles as peroxidase mimetics for cancer cell targeted detection and therapy. Talanta 2021, 238, 123071. [Google Scholar] [CrossRef]
- Chen, S.; Lu, J.; You, T.; Sun, D. Metal-organic frameworks for improving wound healing. Co-ord. Chem. Rev. 2021, 439, 213929. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Bhagat, E.; Sadras, S.R. Green synthesis of silver and gold nanoparticles from Gymnema sylvestre leaf extract: Study of antioxidant and anticancer activities. J. Nanoparticle Res. 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Devi, P.R.; Kumar, C.S.; Selvamani, P.; Subramanian, N.; Ruckmani, K. Synthesis and characterization of Arabic gum capped gold nanoparticles for tumor-targeted drug delivery. Mater. Lett. 2015, 139, 241–244. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 2022, 9, 1036295. [Google Scholar] [CrossRef] [PubMed]
- Kaka, G.; Yaghoobi, K.; Davoodi, S.; Hosseini, S.R.; Sadraie, S.H.; Mansouri, K. Assessment of the Neuroprotective Effects of Lavandula angustifolia Extract on the Contusive Model of Spinal Cord Injury in Wistar Rats. Front. Neurosci. 2016, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Sochorova, L.; Prusova, B.; Cebova, M.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Nedomova, S.; Baron, M.; Sochor, J. Health Effects of Grape Seed and Skin Extracts and Their Influence on Biochemical Markers. Molecules 2020, 25, 5311. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, V. Beneficial Effects of Walnuts on Cognition and Brain Health. Nutrients 2020, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, S.; Ali, S.; Esa, M.; Khan, A.; Yan, H. Recent Advancements and Unexplored Biomedical Applications of Green Synthesized Ag and Au Nanoparticles: A Review. Int. J. Nanomed. 2024, 19, 3187–3215. [Google Scholar] [CrossRef]
- David, M.; Şerban, A.; Popa, C.V.; Florescu, M. A Nanoparticle-Based Label-Free Sensor for Screening the Relative Antioxidant Capacity of Hydrosoluble Plant Extracts. Sensors 2019, 19, 590. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Xu, Q.; Tariq, M.; Song, M.; Liu, C.; Yan, H. Assessing the Potential of Aconitum Laeve Extract for Biogenic Silver and Gold Nanoparticle Synthesis and Their Biological and Catalytic Applications. Molecules 2024, 29, 2640. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, X.; Gu, Q.; Zhang, J.; Ding, Y.; Xue, L.; Chen, M.; Wang, J.; Wu, S.; Yang, X.; et al. Cascade amplification based on PEI-functionalized metal-organic framework supported gold nanoparticles/nitrogen-doped graphene quantum dots for amperometric biosensing applications. Electrochim. Acta 2022, 405, 139803. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Foo, Y.Y.; Periasamy, V.; Kiew, L.V.; Kumar, G.G.; Malek, S.N.A. Curcuma mangga-Mediated Synthesis of Gold Nanoparticles: Characterization, Stability, Cytotoxicity, and Blood Compatibility. Nanomaterials 2017, 7, 123. [Google Scholar] [CrossRef]
- Pandey, S.; Oza, G.; Mewada, A.; Sharon, M. Green synthesis of highly stable gold nanoparticles using Momordica charantia as nano fabricator. Arch. Appl. Sci. Res. 2012, 4, 1135–1141. [Google Scholar]
- Anuradha, J.; Abbasi, T.; Abbasi, S. An eco-friendly method of synthesizing gold nanoparticles using an otherwise worthless weed pistia (Pistia stratiotes L.). J. Adv. Res. 2014, 6, 711–720. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process. Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Chen, L.; Ji, F.; Xu, Y.; He, L.; Mi, Y.; Bao, F.; Sun, B.; Zhang, X.; Zhang, Q. High-Yield Seedless Synthesis of Triangular Gold Nanoplates through Oxidative Etching. Nano Lett. 2014, 14, 7201–7206. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological synthesis of triangular gold nanoprisms. Nat. Mater. 2004, 3, 482–488. [Google Scholar] [CrossRef]
- Radulescu, C.; Olteanu, R.L.; Stihi, C.; Florescu, M.; Stirbescu, R.M.; Stanescu, S.G.; Nicolescu, C.M.; Bumbac, M. Chemometrics-based vibrational spectroscopy for Juglandis semen extracts investigation. J. Chemom. 2020, 34, e3234. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Xu, Q.; Khan, I.; Cao, X.; Yang, R.; Yan, H. Green synthesis of gold and silver nanoparticles using crude extract of Aconitum violaceum and evaluation of their antibacterial, antioxidant and photocatalytic activities. Front. Bioeng. Biotechnol. 2024, 11, 1320739. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef]
- Gupta, B.S.; Jelle, B.P.; Gao, T. In vitro cell composition identification of wood decay fungi by Fourier transform infrared spectroscopy. R. Soc. Open Sci. 2022, 9, 201935. [Google Scholar] [CrossRef]
- Patil, T.P.; Vibhute, A.A.; Patil, S.L.; Dongale, T.D.; Tiwari, A.P. Green synthesis of gold nanoparticles via Capsicum annum fruit extract: Characterization, antiangiogenic, antioxidant and anti-inflammatory activities. Appl. Surf. Sci. Adv. 2023, 13, 100372. [Google Scholar] [CrossRef]
- Gerlache, M.; Senturk, Z.; Quarin, G.; Kauffmann, J. Electrochemical behavior of H2O2 on gold. Electroanalysis 1997, 9, 1088–1092. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Chen, Z.; Zheng, Y. Penetration of Lipid Membranes by Gold Nanoparticles: Insights into Cellular Uptake, Cytotoxicity, and Their Relationship. ACS Nano 2010, 4, 5421–5429. [Google Scholar] [CrossRef]
- Ouchi, Y.; Unoura, K.; Nabika, H. Role of Oxidized Lipids in Permeation of H2O2 Through a Lipid Membrane: Molecular Mechanism of an Inhibitor to Promoter Switch. Sci. Rep. 2019, 9, 12497. [Google Scholar] [CrossRef]
- Sanz, C.G.; Serrano, S.H.P.; Brett, C.M.A. Electroanalysis of Cefadroxil Antibiotic at Carbon Nanotube/Gold Nanoparticle Modified Glassy Carbon Electrodes. ChemElectroChem 2020, 7, 2151–2158. [Google Scholar] [CrossRef]
- Kluczyk, D.; Matwijczuk, A.; Górecki, A.; Karpińska, M.M.; Szymanek, M.; Niewiadomy, A.; Gagoś, M. Molecular Organization of Dipalmitoylphosphatidylcholine Bilayers Containing Bioactive Compounds 4-(5-Heptyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diol and 4-(5-Methyl-1,3,4-thiadiazol-2-yl) Benzene-1,3-diols. J. Phys. Chem. B 2016, 120, 12047–12063. [Google Scholar] [CrossRef]
- Boomi, P.; Ganesan, R.; Prabu Poorani, G.; Jegatheeswaran, S.; Balakumar, C.; Gurumallesh Prabu, H.; Anand, K.; Marimuthu Prabhu, N.; Jeyakanthan, J.; Saravanan, M. Phyto-Engineered Gold Nanoparticles (AuNPs) with Potential Antibacterial, Antioxidant, and Wound Healing Activities Under in vitro and in vivo Conditions. Int. J. Nanomed. 2020, 15, 7553–7568. [Google Scholar] [CrossRef]

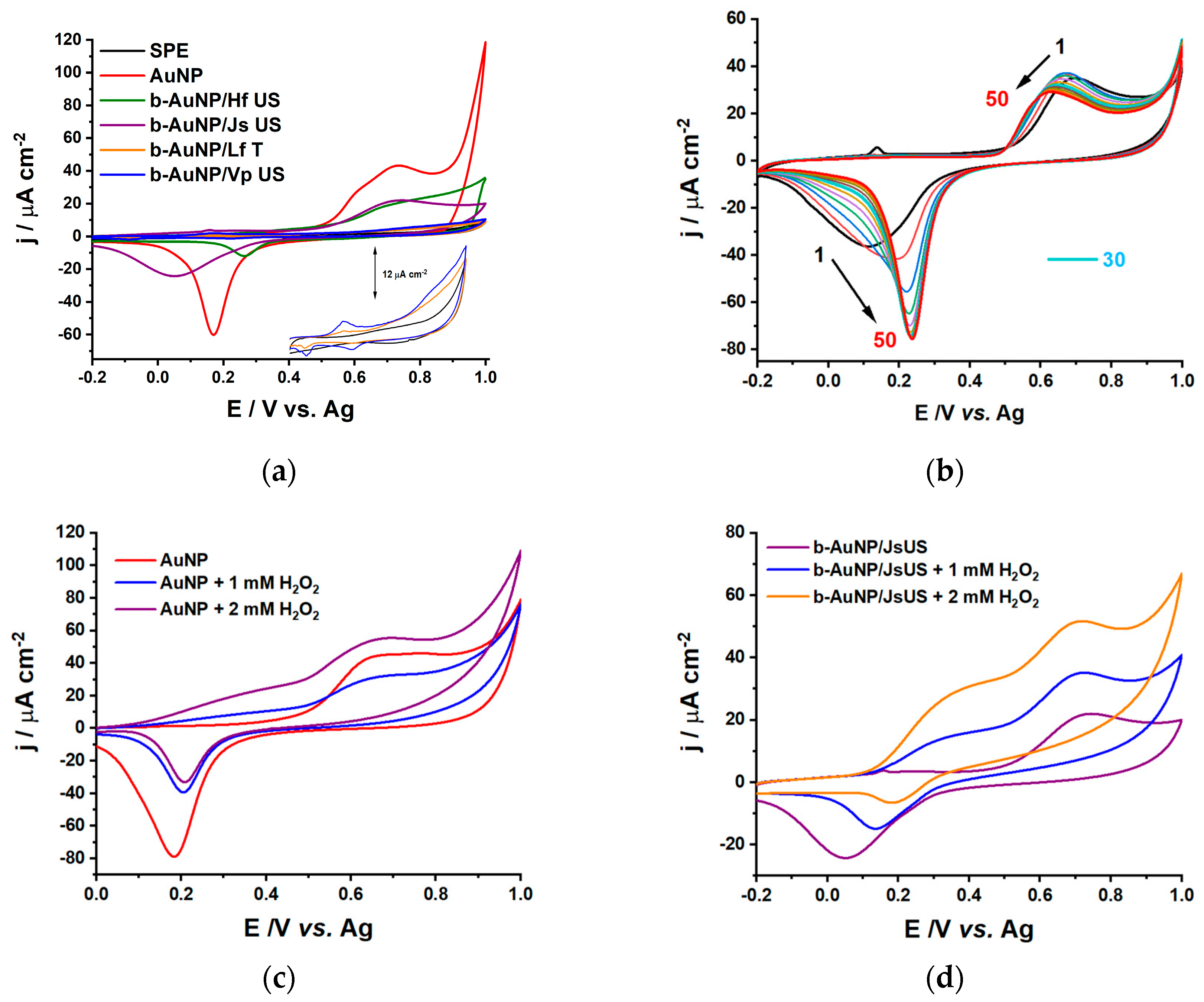
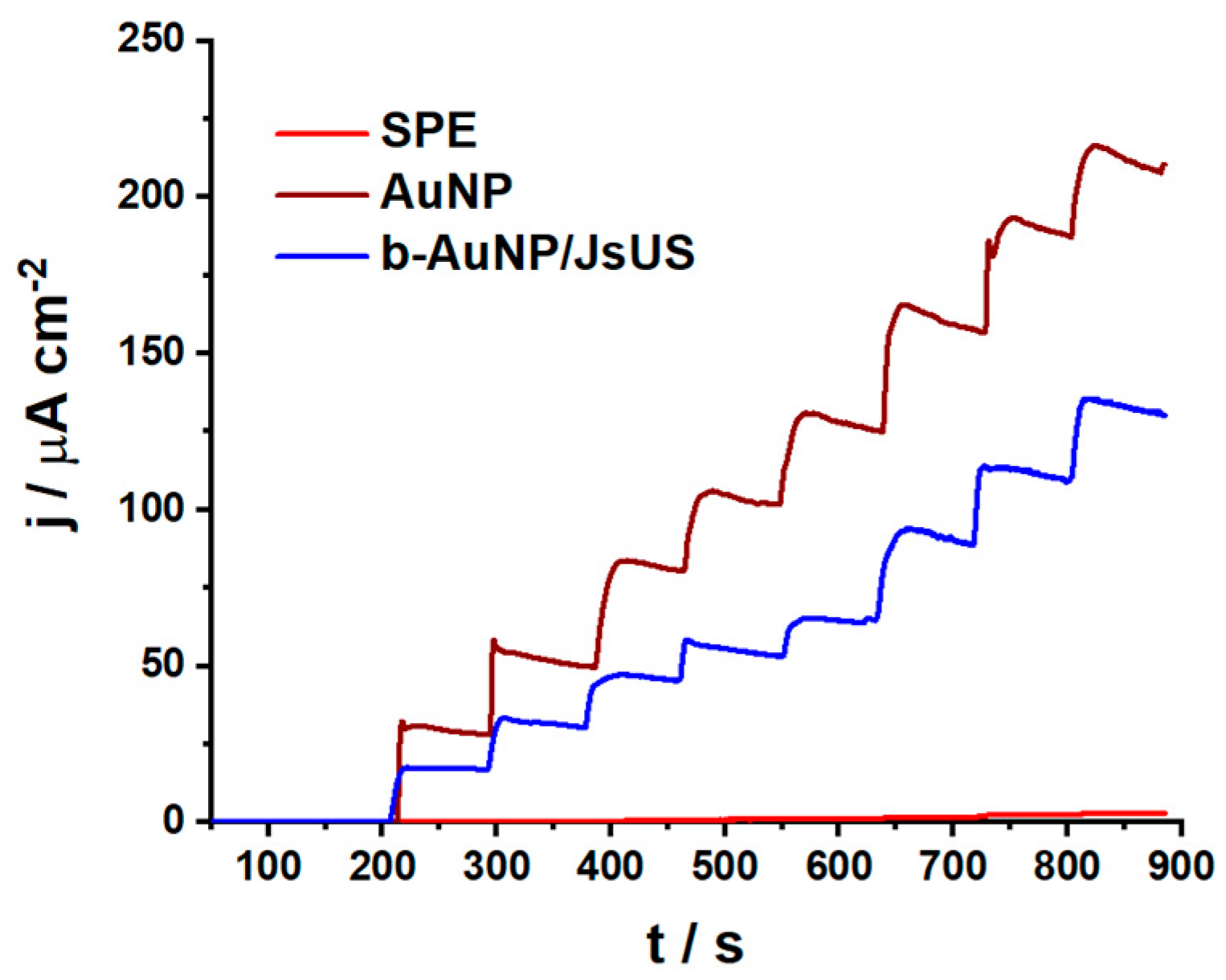
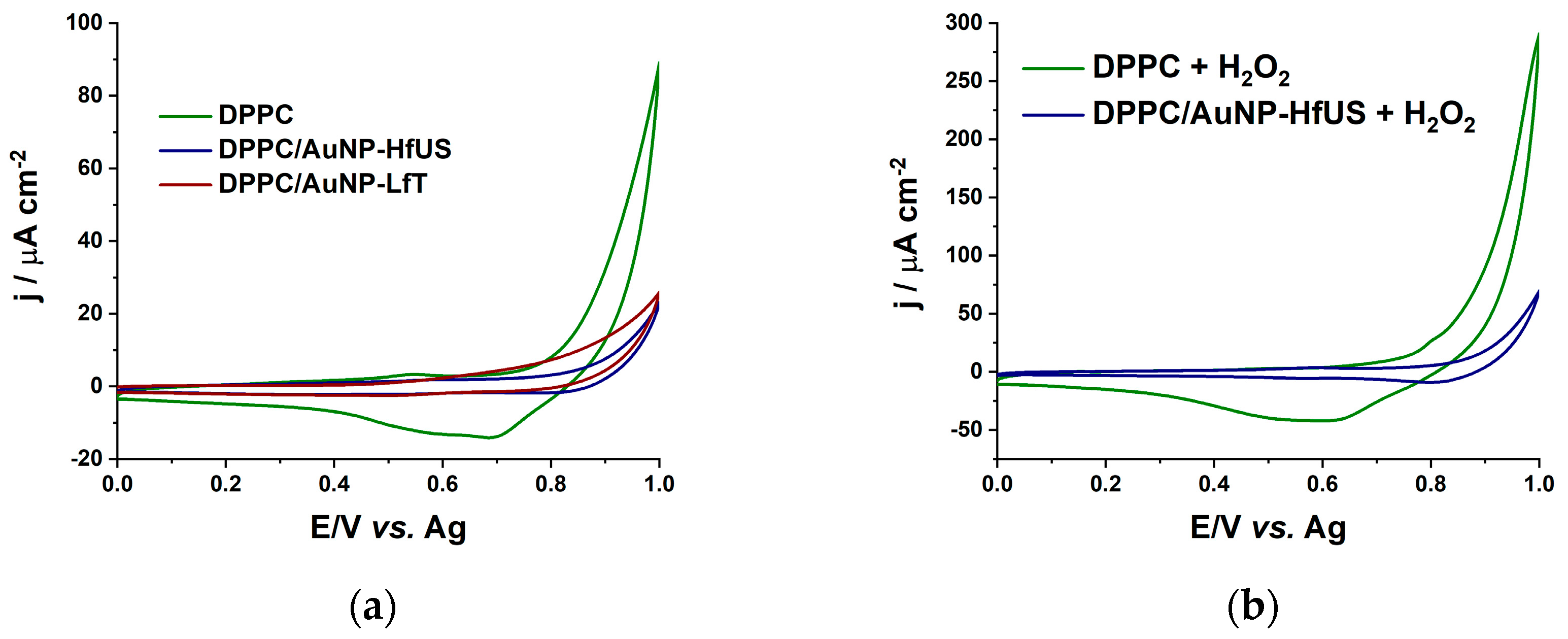

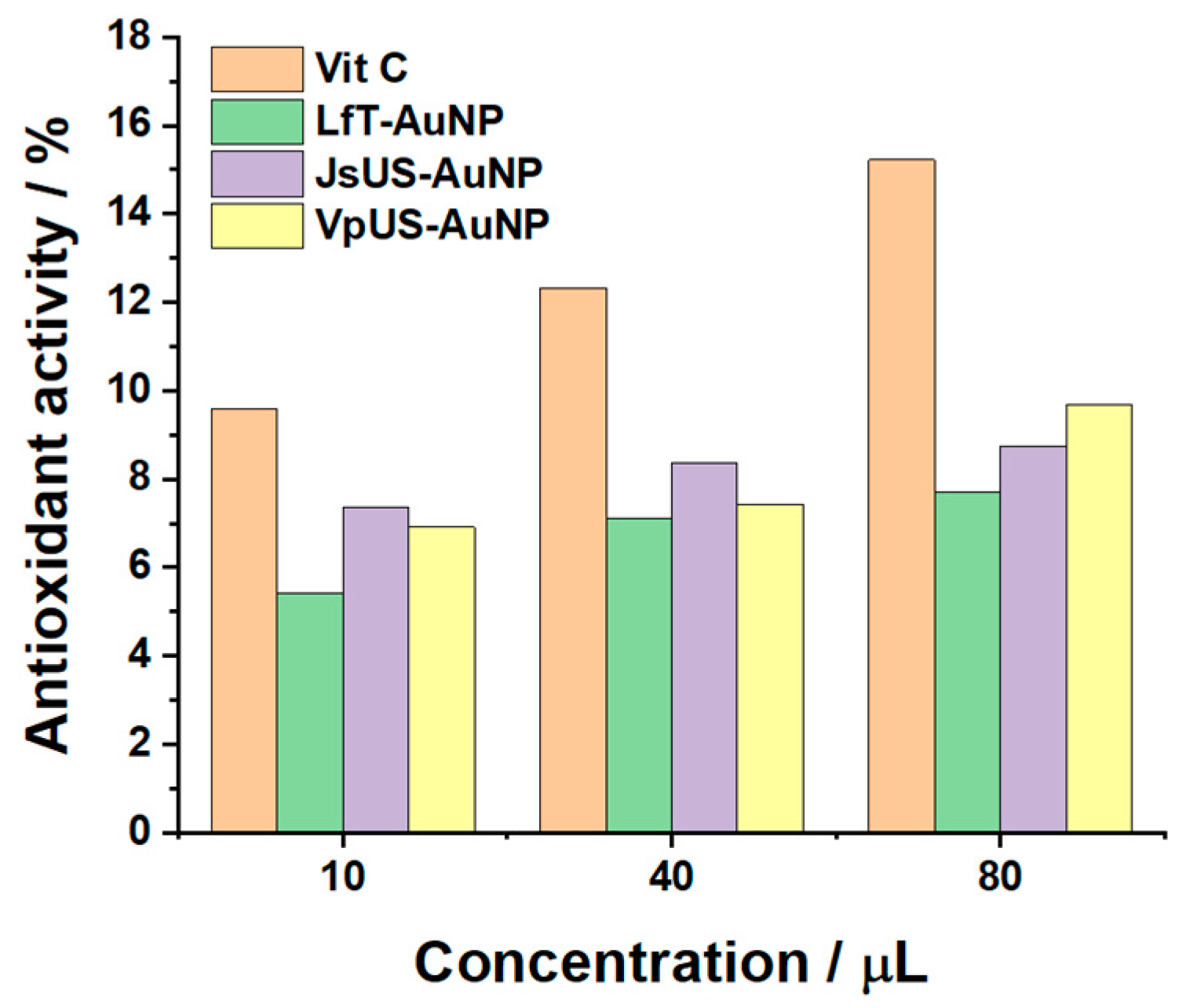
| Sensor | Oxidation Peak Current/ μA cm−2 | Reduction Peak Current/μA cm−2 |
|---|---|---|
| SPE | - | - |
| AuNP | 44.6 | −61.5 |
| b-AuNP/Hf US | 20.7 | −12.7 |
| b-AuNP/Js US | 22.1 | −24.4 |
| b-AuNP/Lf T | 4.1 | −0.6 |
| b-AuNP/Vp US | 5.2 | −1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, M.; Enache, T.A.; Barbu-Tudoran, L.; Bala, C.; Florescu, M. Biologically Synthesized Gold Nanoparticles with Enhanced Antioxidant and Catalytic Properties. Pharmaceuticals 2024, 17, 1105. https://doi.org/10.3390/ph17091105
David M, Enache TA, Barbu-Tudoran L, Bala C, Florescu M. Biologically Synthesized Gold Nanoparticles with Enhanced Antioxidant and Catalytic Properties. Pharmaceuticals. 2024; 17(9):1105. https://doi.org/10.3390/ph17091105
Chicago/Turabian StyleDavid, Melinda, Teodor A. Enache, Lucian Barbu-Tudoran, Camelia Bala, and Monica Florescu. 2024. "Biologically Synthesized Gold Nanoparticles with Enhanced Antioxidant and Catalytic Properties" Pharmaceuticals 17, no. 9: 1105. https://doi.org/10.3390/ph17091105
APA StyleDavid, M., Enache, T. A., Barbu-Tudoran, L., Bala, C., & Florescu, M. (2024). Biologically Synthesized Gold Nanoparticles with Enhanced Antioxidant and Catalytic Properties. Pharmaceuticals, 17(9), 1105. https://doi.org/10.3390/ph17091105












