A Comprehensive Review of Silver and Gold Nanoparticles as Effective Antibacterial Agents
Abstract
1. Introduction
2. Literature Compilation and Analysis
Data Features
3. Data Preprocessing
4. NPs Synthesis Methods
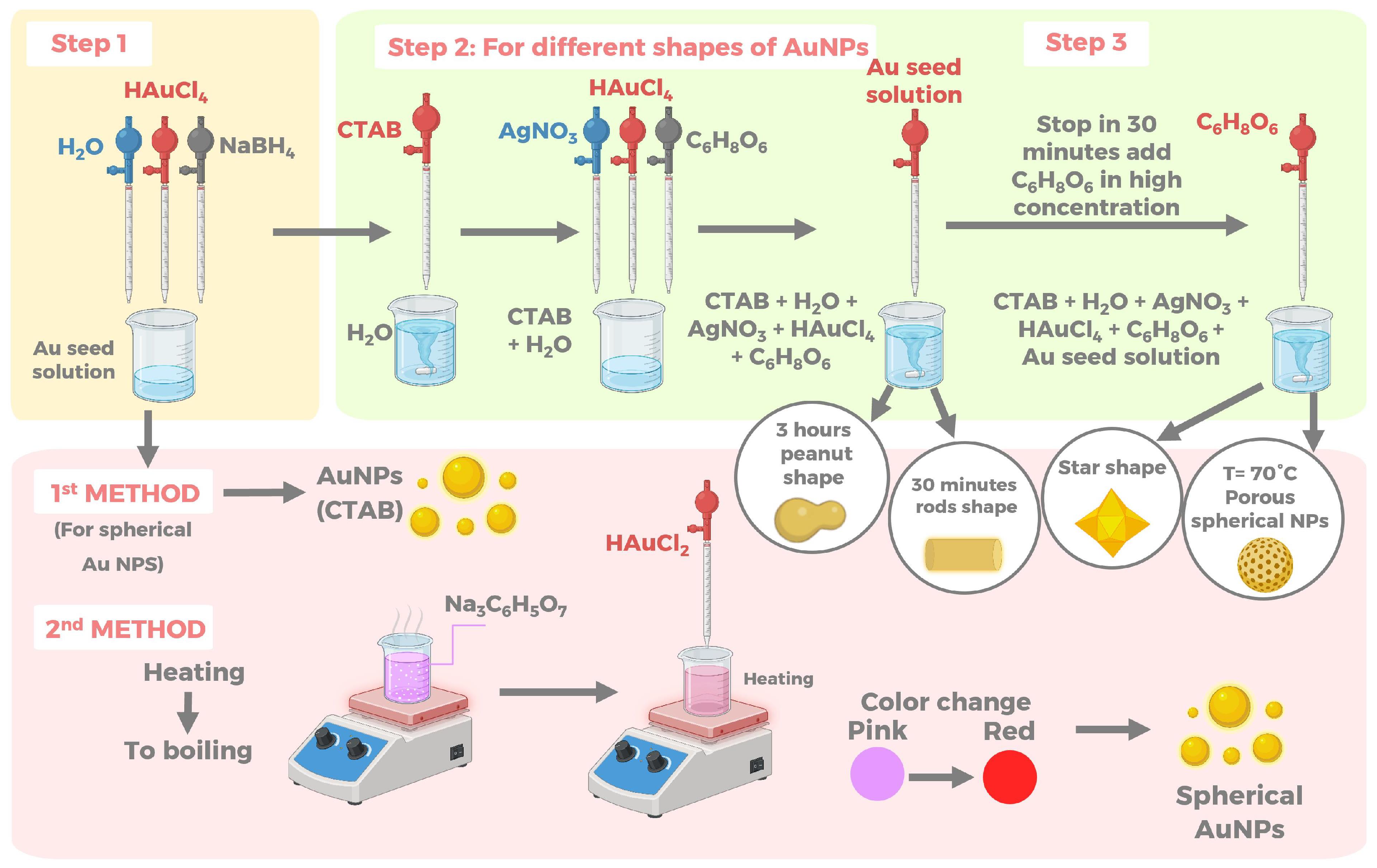
4.1. Chemical Methods
4.2. Physical Methods
4.3. Green Methods
4.4. Comparison of Methods
5. NPs Antibacterial Properties
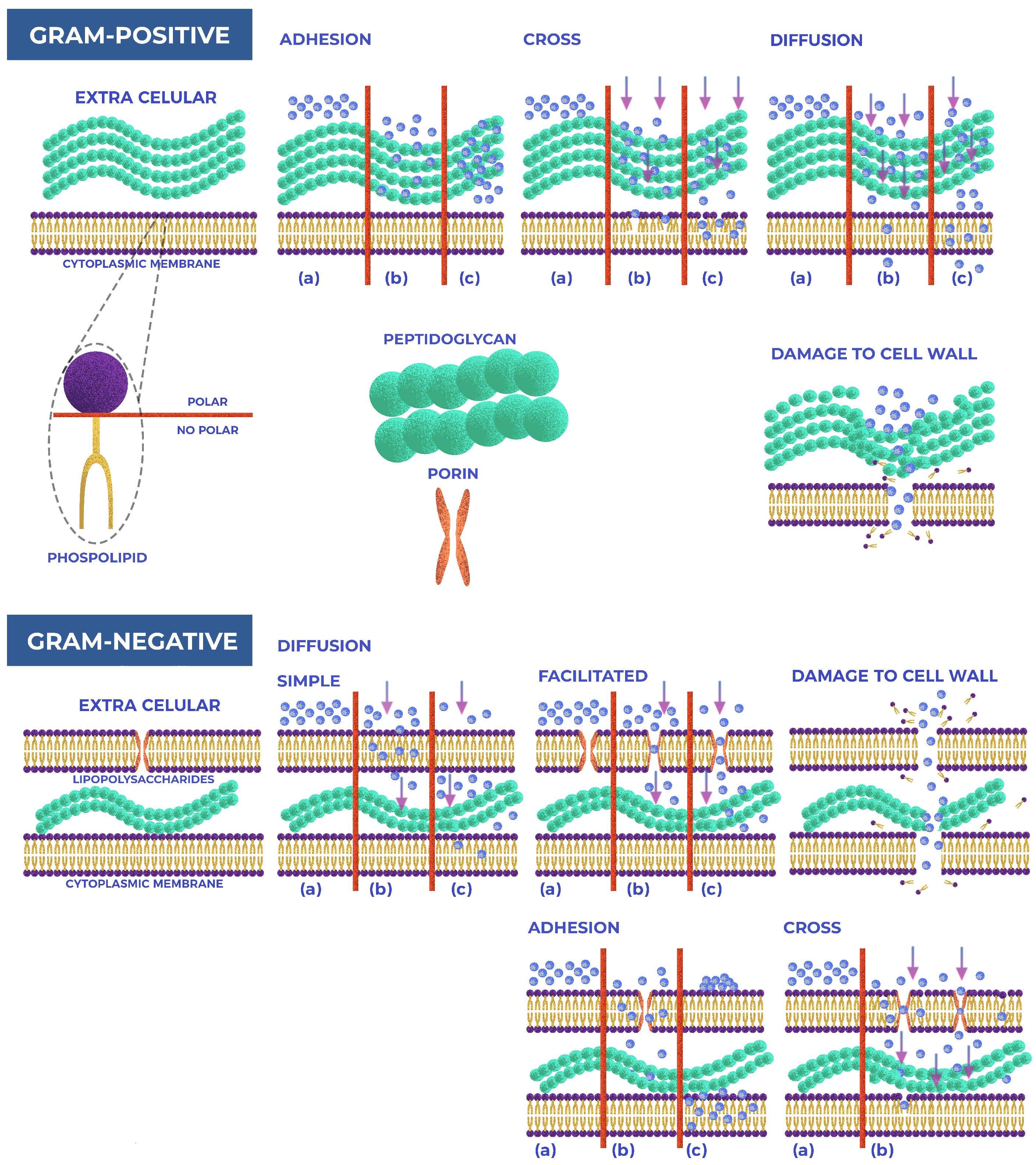
5.1. Interaction Gram(+/−) Bacteria and NPs
5.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
6. Effect of NPs Based on Morphology
6.1. Effect of Size
6.2. Effect of Shape
6.3. Comparison between Au and Ag
7. Challenges and Need for Standardization
7.1. Variability in Testing Methods and Techniques
7.2. Need for Standardized Protocols
8. Importance of NPs against Antibiotic Resistance
9. Future Directions and Potential Applications
10. Patents and Clinical Trials
10.1. Patents on Nanoparticles
10.2. Clinical Trials Involving Nanoparticles
10.2.1. Clinical Trials
10.2.2. Drug Delivery
10.2.3. Multidrug-Resistant
10.2.4. Wound Healing and Infections
11. Regulatory Status of Nanoparticles
11.1. Toxicity of Nanoparticles
11.1.1. In Vitro and In Vivo Studies
11.1.2. Comparative Toxicity
11.2. Regulatory Framework
11.2.1. United States (FDA)
11.2.2. European Union (EMA)
11.2.3. Health Canada
11.2.4. International Standards
11.3. Forward-Looking View
11.3.1. Standardization
11.3.2. Long-Term Studies
11.3.3. Regulatory Pathways
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPs | Nanoparticle |
| Au NPs | Gold Nanoparticles |
| Ag NPs | Silver Nanoparticles |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| CTAB | Cetrimonium Bromide |
| STEM | Scanning Transmission Electron Microscopy |
| ATCC | American Type Culture Collection |
| PVP | Polyvinylpyrrolidone |
| PEG | Polyethylene Glycol |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| MDR | multidrug-resistant |
References
- Walker, B.; Barrett, S.; Polasky, S.; Galaz, V.; Folke, C.; Engström, G.; Ackerman, F.; Arrow, K.; Carpenter, S.; Chopra, K.; et al. Looming Global-Scale Failures and Missing Institutions. Science 2009, 325, 1345–1346. [Google Scholar] [CrossRef]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Serwecińska, L. Antimicrobials and antibiotic-resistant bacteria: A risk to the environment and to public health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- World Health Organization. WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 17 July 2024).
- World Health Organization. 2023 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis. Available online: https://www.who.int/publications/i/item/9789240094000 (accessed on 17 July 2024).
- Asokan, G.V.; Ramadhan, T.; Ahmed, E.; Sanad, H. WHO global priority pathogens list: A bibliometric analysis of Medline-PubMed for knowledge mobilization to infection prevention and control practices in Bahrain. Oman Med. J. 2019, 34, 184–193. [Google Scholar] [CrossRef]
- Santos, C.A.D.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-antibiotics strategies to control salmonella infection in poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Size-controlled silver nanoparticles synthesized over the range 5-100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014, 4, 3974–3983. [Google Scholar] [CrossRef]
- Ng, C.T.; Baeg, G.H.; Yu, L.E.; Ong, C.N.; Bay, B.H. Biomedical Applications of Nanomaterials as Therapeutics. Curr. Med. Chem. 2017, 25, 1409–1419. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 00341. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Perelshtein, I.; Lipovsky, A.; Perkas, N.; Gedanken, A.; Moschini, E.; Mantecca, P. The influence of the crystalline nature of nano-metal oxides on their antibacterial and toxicity properties. Nano Res. 2015, 8, 695–707. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Hsieh, Y.K.; Wang, C.F.; Chen, I.C.; Huang, Y.J. Trojan-Horse Mechanism in the Cellular Uptake of Silver Nanoparticles Verified by Direct Intra- and Extracellular Silver Speciation Analysis. Environ. Sci. Technol. 2015, 49, 3813–3821. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Effects of silver nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa from mastitis-infected goats: An alternative approach for antimicrobial therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Abadi, A.J.N.; Abasi, E.; Akbarzadeh, A. A Review on Potential Role of Silver Nanoparticles and Possible Mechanisms of their Actions on Bacteria. Drug Res. 2017, 67, 70–76. [Google Scholar] [CrossRef]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim. Biophys. Acta BBA Gen. Subj. 2015, 1850, 299–306. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 2015, 720654. [Google Scholar] [CrossRef]
- de Lacerda Coriolano, D.; de Souza, J.B.; Bueno, E.V.; de Fátima, S.M.; dos Santos Medeiros, R.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Antibacterial and antibiofilm potential of silver nanoparticles against antibiotic-sensitive and multidrug-resistant Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2021, 52, 267–278. [Google Scholar] [CrossRef]
- You, C.; Han, C.; Wang, X.; Zheng, Y.; Li, Q.; Hu, X.; Sun, H. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol. Biol. Rep. 2012, 39, 9193–9201. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: A case study on Staphylococcus aureus and Escherichia coli as models for gram-positive and gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, M.; Li, Y.; Sun, M.; Kong, W.; Wang, L.; Song, S.; Fan, C.; Jia, L.; Qiu, S.; et al. Stable Nanocomposite Based on PEGylated and Silver Nanoparticles Loaded Graphene Oxide for Long-Term Antibacterial Activity. ACS Appl. Mater. Interfaces 2017, 9, 15328–15341. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. In Vitro 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Lee, W.; Kim, K.J.; Lee, D.G. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli. BioMetals 2014, 27, 1191–1201. [Google Scholar] [CrossRef]
- Jin, J.C.; Wu, X.J.; Xu, J.; Wang, B.B.; Jiang, F.L.; Liu, Y. Ultrasmall silver nanoclusters: Highly efficient antibacterial activity and their mechanisms. Biomater. Sci. 2017, 5, 247–257. [Google Scholar] [CrossRef]
- Lombardo, P.C.; Poli, A.L.; Castro, L.F.; Perussi, J.R.; Schmitt, C.C. Photochemical Deposition of Silver Nanoparticles on Clays and Exploring Their Antibacterial Activity. ACS Appl. Mater. Interfaces 2016, 8, 21640–21647. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Braun, G.B.; She, Z.G.; Hussain, S.; Ruoslahti, E.; Sailor, M.J. Composite Porous Silicon-Silver Nanoparticles as Theranostic Antibacterial Agents. ACS Appl. Mater. Interfaces 2016, 8, 30449–30457. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Kądzioła, K.; Felczak, A.; Wrońska, N.; Piwoński, I.; Kisielewska, A.; Lisowska, K. Surface area or diameter—Which factor really determines the antibacterial activity of silver nanoparticles grown on TiO2 coatings? New J. Chem. 2014, 38, 3275–3281. [Google Scholar] [CrossRef]
- Klueh, U.; Wagner, V.; Kelly, S.; Johnson, A.; Bryers, J.D. Efficacy of Silver-Coated Fabric to Prevent Bacterial Colonization and Subsequent Device-Based Biofilm Formation. J. Biomed. Mater. Res. 2000, 53, 621–631. [Google Scholar]
- Holt, K.B.; Bard, A.J. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: An electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Long, Y.M.; Hu, L.G.; Yan, X.T.; Zhao, X.C.; Zhou, Q.F.; Cai, Y.; Jiang, G.B. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. Int. J. Nanomed. 2017, 12, 3193–3206. [Google Scholar] [CrossRef]
- Siritongsuk, P.; Hongsing, N.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Tuanyok, A.; Patramanon, R. Two-phase bactericidal mechanism of silver nanoparticles against Burkholderia pseudomallei. PLoS ONE 2016, 11, e168098. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Natan, M.; Banin, E. From Nano to Micro: Using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Y.T.; Wamer, W.G.; Hu, X.; Wu, X.; Zheng, Z.; Boudreau, M.D.; Yin, J.J. Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging. Biomaterials 2013, 34, 765–773. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Gold Nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef] [PubMed]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold nanoparticles: An efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and Gold Nanoparticles for Antimicrobial Purposes against Multi-Drug Resistance Bacteria. Materials 2022, 15, 1799. [Google Scholar] [CrossRef]
- Elsupikhe, R.F.; Shameli, K.; Ahmad, M.B.; Ibrahim, N.A.; Zainudin, N. Green sonochemical synthesis of silver nanoparticles at varying concentrations of κ-carrageenan. Nanoscale Res. Lett. 2015, 10, 302. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001, Corrigendum in Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 049501.. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef]
- Das, B.; Dash, S.K.; Mandal, D.; Ghosh, T.; Chattopadhyay, S.; Tripathy, S.; Das, S.; Dey, S.K.; Das, D.; Roy, S. Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab. J. Chem. 2017, 10, 862–876. [Google Scholar] [CrossRef]
- Lu, Z.; Rong, K.; Li, J.; Yang, H.; Chen, R. Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J. Mater. Sci. Mater. Med. 2013, 24, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Paredes, D.; Ortiz, C.; Torres, R. Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin-resistant staphylococcus aureus (MRSA). Int. J. Nanomed. 2014, 9, 1717–1729. [Google Scholar] [CrossRef][Green Version]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef]
- Rao, Y.; Inwati, G.K.; Singh, M. Green synthesis of capped gold nanoparticles and their effect on Gram-positive and Gram-negative bacteria. Future Sci. OA 2017, 3, FSO239. [Google Scholar] [CrossRef] [PubMed]
- Lavaee, F.; Ranjbar, Z.; Modaresi, F.; Keshavarz, F. The Effect of Gold Nano Particles with Different Sizes on Streptococcus Species. J. Dent. 2021, 22, 235–242. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef]
- Timoszyk, A.; Grochowalska, R. Mechanism and Antibacterial Activity of Gold Nanoparticles (AuNPs) Functionalized with Natural Compounds from Plants. Pharmaceutics 2022, 14, 2599. [Google Scholar] [CrossRef]
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomed. 2017, 12, 2457–2468. [Google Scholar] [CrossRef]
- Al-Dbass, A.M.; Daihan, S.A.; Al-Nasser, A.A.; Al-Suhaibani, L.S.; Almusallam, J.; Alnwisser, B.I.; Saloum, S.; Alotaibi, R.S.; Alessa, L.A.; Bhat, R.S. Biogenic Silver Nanoparticles from Two Varieties of Agaricus bisporus and Their Antibacterial Activity. Molecules 2022, 27, 7656. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar]
- Perito, B.; Giorgetti, E.; Marsili, P.; Muniz-Miranda, M. Antibacterial activity of silver nanoparticles obtained by pulsed laser ablation in pure water and in chloride solution. Beilstein J. Nanotechnol. 2016, 7, 465–473. [Google Scholar] [CrossRef]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Mittelman, A.M.; Fortner, J.D.; Pennell, K.D. Effects of ultraviolet light on silver nanoparticle mobility and dissolution. Environ. Sci. Nano 2015, 2, 683–691. [Google Scholar] [CrossRef]
- Pandey, J.K.; Swarnkar, R.K.; Soumya, K.K.; Dwivedi, P.; Singh, M.K.; Sundaram, S.; Gopal, R. Silver Nanoparticles Synthesized by Pulsed Laser Ablation: As a Potent Antibacterial Agent for Human Enteropathogenic Gram-Positive and Gram-Negative Bacterial Strains. Appl. Biochem. Biotechnol. 2014, 174, 1021–1031. [Google Scholar] [CrossRef]
- Fanoro, O.T.; Oluwafemi, O.S. Bactericidal antibacterial mechanism of plant synthesized silver, gold and bimetallic nanoparticles. Pharmaceutics 2020, 12, 1044. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Naghizadeh, A.; Amiri, O.; Shirzadi-Ahodashti, M.; Mortazavi-Derazkola, S. Green and facile synthesis of Ag nanoparticles using Crataegus pentagyna fruit extract (CP-AgNPs) for organic pollution dyes degradation and antibacterial application. Bioorganic Chem. 2020, 94, 103425. [Google Scholar] [CrossRef]
- Bakht Dalir, S.J.; Djahaniani, H.; Nabati, F.; Hekmati, M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon 2020, 6, e03624. [Google Scholar] [CrossRef]
- Singhal, M.; Chatterjee, S.; Kumar, A.; Syed, A.; Bahkali, A.H.; Gupta, N.; Nimesh, S. Exploring the antibacterial and antibiofilm efficacy of silver nanoparticles biosynthesized using Punica granatum leaves. Molecules 2021, 26, 5762. [Google Scholar] [CrossRef]
- Balakumaran, M.D.; Ramachandran, R.; Balashanmugam, P.; Mukeshkumar, D.J.; Kalaichelvan, P.T. Mycosynthesis of silver and gold nanoparticles: Optimization, characterization and antimicrobial activity against human pathogens. Microbiol. Res. 2016, 182, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Golińska, P.; Wypij, M.; Rathod, D.; Tikar, S.; Dahm, H.; Rai, M. Synthesis of silver nanoparticles from two acidophilic strains of Pilimelia columellifera subsp. pallida and their antibacterial activities. J. Basic Microbiol. 2016, 56, 541–556. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Goda, D.A.; Al-Zaban, M.I. Lethal mechanisms of nostoc-synthesized silver nanoparticles against different pathogenic bacteria. Int. J. Nanomed. 2020, 15, 10499–10517. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Green synthesis and antibacterial applications of gold and silver nanoparticles from Ligustrum vulgare berries. Sci. Rep. 2022, 12, 7902. [Google Scholar] [CrossRef]
- Qamer, S.; Romli, M.H.; Che-Hamzah, F.; Misni, N.; Joseph, N.M.; Al-Haj, N.A.; Amin-Nordin, S. Systematic review on biosynthesis of silver nanoparticles and antibacterial activities: Application and theoretical perspectives. Molecules 2021, 26, 5057. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Duval, R.E.; Gouyau, J.; Lamouroux, E. Limitations of recent studies dealing with the antibacterial properties of silver nanoparticles: Fact and opinion. Nanomaterials 2019, 9, 1775. [Google Scholar] [CrossRef]
- Cavassin, E.D.; de Figueiredo, L.F.P.; Otoch, J.P.; Seckler, M.M.; de Oliveira, R.A.; Franco, F.F.; Marangoni, V.S.; Zucolotto, V.; Levin, A.S.S.; Costa, S.F. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Khubchandani, M.; Thosar, N.R.; Dangore-Khasbage, S.; Srivastava, R. Applications of Silver Nanoparticles in Pediatric Dentistry: An Overview. Cureus 2022, 14, e26956. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Bhagat, N.; Kumari, M.; Choudhury, A.R.; Sarkar, B.; Ghosh, B.D. Insight study on synthesis and antibacterial mechanism of silver nanoparticles prepared from indigenous plant source of Jharkhand. J. Genet. Eng. Biotechnol. 2023, 21, 30. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef]
- Okkeh, M.; Bloise, N.; Restivo, E.; Vita, L.D.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the Next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, J.; Tang, H.; Xia, D.; Lin, H. Antibacterial Properties of Gold Nanoparticles in the Modification of Medical Implants: A Systematic Review. Pharmaceutics 2022, 14, 2654. [Google Scholar] [CrossRef]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo; Darmayanti, R.; Puspitasari, B.; Cheng, T.-M.; Kuo, T.-R. Gold-Based Nanostructures for Antibacterial Application. Int. J. Mol. Sci. 2023, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-based green synthesis of metallic nanoparticles: Applications beyond fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Katas, H.; Lim, C.S.; Azlan, A.Y.H.N.; Buang, F.; Busra, M.F.M. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotis and chitosan. Saudi Pharm. J. 2019, 27, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Ștefan Vasile, B.; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wei, M.; Wu, H.; Li, F.; Ling, D. Antibacterial metal nanoclusters. J. Nanobiotechnol. 2022, 20, 328. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial activity of silver nanoparticles of different particle size against Vibrio natriegens. PLoS ONE 2019, 14, e222322. [Google Scholar] [CrossRef]
- Gimenez-Ingalaturre, A.C.; Rubio, E.; Chueca, P.; Laborda, F.; Goñi, P. Contribution to optimization and standardization of antibacterial assays with silver nanoparticles: The culture medium and their aggregation. J. Microbiol. Methods 2022, 203, 106618. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Lavaee, F.; Faez, K.; Faez, K.; Hadi, N.; Modaresi, F. Antimicrobial and antibiofilm activity of silver, titanium dioxide and iron nano particles. Am. J. Dent. 2016, 29, 315–320. [Google Scholar] [PubMed]
- Li, Y.; Zhen, J.; Tian, Q.; Shen, C.; Zhang, L.; Yang, K.; Shang, L. One step synthesis of positively charged gold nanoclusters as effective antimicrobial nanoagents against multidrug-resistant bacteria and biofilms. J. Colloid Interface Sci. 2020, 569, 235–243. [Google Scholar] [CrossRef]
- Lagha, R.; Abdallah, F.B.; Mezni, A.; Alzahrani, O.M. Effect of plasmonic gold nanoprisms on biofilm formation and heat shock proteins expression in human pathogenic bacteria. Pharmaceuticals 2021, 14, 1335. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.B.; Lim, J.J.; Shameli, K.; Ibrahim, N.A.; Tay, M.Y.; Chieng, B.W. Antibacterial activity of silver bionanocomposites synthesized by chemical reduction route. Chem. Cent. J. 2012, 6, 101. [Google Scholar] [CrossRef]
- Platania, V.; Kaldeli-Kerou, A.; Karamanidou, T.; Kouki, M.; Tsouknidas, A.; Chatzinikolaidou, M. Antibacterial effect of colloidal suspensions varying in silver nanoparticles and ions concentrations. Nanomaterials 2022, 12, 31. [Google Scholar] [CrossRef]
- Prihapsara, F.; Artanti, A.N.; Ni’mah, L.F. Characterization and antimicrobial activity of gold nanoparticles fruit infusion of Medinilla speciosa. J. Phys. Conf. Ser. 2022, 2190, 012030. [Google Scholar] [CrossRef]
- Zhang, Y.; Dasari, T.P.S.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Fatima, F. In vitro antimicrobicidal and cytotoxicity efficacy of gold nanoparticles synthesized from Alternaria brassicae (KF934409). SOJ Pharm. Pharm. Sci. 2016, 3, 1–6. [Google Scholar] [CrossRef]
- B S, S.; Keerthiraj, D.N.; Byrappa, K. Eco-friendly synthesis of gold nanoparticles by gold mine bacteria Brevibacillus formosus and their antibacterial and biocompatible studies. J. Pharm. 2017, 7, 53–60. [Google Scholar]
- Athanasopoulou, K.; Daneva, G.N.; Adamopoulos, P.G.; Scorilas, A. Artificial Intelligence: The Milestone in Modern Biomedical Research. BioMedInformatics 2022, 2, 727–744. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakayama, K.I. Artificial intelligence in oncology. Cancer Sci. 2020, 111, 1452–1460. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Bakhrebah, M.A.; AlSaihati, H.; Alhumaid, S.; Alsubki, R.A.; Turkistani, S.A.; Al-Abdulhadi, S.; Aldawood, Y.; Alsaleh, A.A.; Alhashem, Y.N.; et al. Artificial Intelligence for Clinical Diagnosis and Treatment of Prostate Cancer. Cancers 2022, 14, 5595. [Google Scholar] [CrossRef]
- Popa, S.L.; Pop, C.; Dita, M.O.; Brata, V.D.; Bolchis, R.; Czako, Z.; Saadani, M.M.; Ismaiel, A.; Dumitrascu, D.I.; Grad, S.; et al. Deep Learning and Antibiotic Resistance. Antibiotics 2022, 11, 1674. [Google Scholar] [CrossRef]
- Huber, D.L.; Watt, J.D.; Chavez, J.; Ammerman, L.M. Direct Formation of Gold Nanoparticles Using Ultrasound. 2019. Available online: https://lens.org/195-643-896-374-225 (accessed on 17 July 2024).
- Karandikar, B.M.; Gibbins, B.L.; Cornell, K.A. Method of Preparing Silver Nanoparticles. 2014. Available online: https://lens.org/192-417-549-788-306 (accessed on 17 July 2024).
- Hendi, A.A.; Awad, M.A.; Eisa, N.E.; Ortashi, K.M. Method for Preparing Noble Metal Nanoparticles. 2018. Available online: https://lens.org/011-627-397-147-949 (accessed on 17 July 2024).
- Zhong, C.J.; Njoki, P.N.; Luo, J. Controlled Synthesis of Highly Monodispersed Gold Nanoparticles. 2009. Available online: https://lens.org/022-970-675-621-856 (accessed on 17 July 2024).
- Height, M.J. Method for Producing Nanoparticle Loaded Powders Using Flame Spray Pyrolysis and Applications Thereof. 2008. Available online: https://lens.org/045-389-273-453-154 (accessed on 17 July 2024).
- Katti, K.V.; Katti, K.K. Au, Ag and Rich Phytochemical Payload Nanomaterials, Antiviral/Antibacterial Products and Synthesis Methods. 2022. Available online: https://lens.org/162-576-589-007-746 (accessed on 17 July 2024).
- Chen, Q.; Qi, Z.; Xin, D.; Lv, J.; Tan, X. Methods and Compositions for Increasing Protein and/or Oil Content and Modifying Oil Profile in a Plant. 2023. Available online: https://lens.org/175-196-710-651-363 (accessed on 17 July 2024).
- Krutyakov, Y.; Klimov, A.; Violin, B.; Kuzmin, V.; Ryzhikh, V.; Gusev, A.; Zakharova, O.; Lisichkin, G. Benzyldimethyl[3-(miristoylamino)-propyl]ammonium chloride stabilized silver nanoparticles (Argumistin™) in medicine: Results of clinical trials for treatment of infectious diseases of dogs and perspectives for humans. Eur. J. Nanomed. 2016, 8, 185–194. [Google Scholar] [CrossRef]
- Yao, L.; Bojic, D.; Liu, M. Applications and safety of gold nanoparticles as therapeutic devices in clinical trials. J. Pharm. Anal. 2023, 13, 960–967. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Aflakian, F.; Mirzavi, F.; Aiyelabegan, H.T.; Soleimani, A.; Gholizadeh Navashenaq, J.; Karimi-Sani, I.; Rafati Zomorodi, A.; Vakili-Ghartavol, R. Nanoparticles-based therapeutics for the management of bacterial infections: A special emphasis on FDA approved products and clinical trials. Eur. J. Pharm. Sci. 2023, 188, 106515. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa; Vidya, S.M.; Mutalik, S.; Bhat, K.U.; Huilgol, P.; Avadhani, K. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 2016, 153, 171–179. [Google Scholar] [CrossRef]
- Kamarehei, F. Newly designed nanoparticle-drug delivery systems against Staphylococcus aureus infection: A systematic review. Int. J. Clin. Exp. Pathol. 2024, 17, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Desouky, E.; Shalaby, M.; Gohar, M.; Gerges, M. Evaluation of antibacterial activity of silver nanoparticles against multidrug-resistant Gram negative bacilli clinical isolates from Zagazig University Hospitals. Microbes Infect. Dis. 2020, 1, 15–23. [Google Scholar] [CrossRef][Green Version]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Chang, L.; Huang, C.C.; Chang, H.T. Dual-functional gold nanoparticles with antimicrobial and proangiogenic activities improve the healing of multidrug-resistant bacteria-infected wounds in diabetic mice. Biomater. Sci. 2019, 7, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Boroumand, Z.; Golmakani, N.; Boroumand, S. Clinical trials on silver nanoparticles for wound healing. Nanomed. J. 2018, 5, 186–191. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef]
- Miyayama, T.; Arai, Y.; Hirano, S. Health Effects of Silver Nanoparticles and Silver Ions. In Biological Effects of Fibrous and Particulate Substances; Otsuki, T., Yoshioka, Y., Holian, A., Eds.; Springer: Tokyo, Japan, 2016; pp. 137–147. [Google Scholar] [CrossRef]
- Cazzola, M.; Barberi, J.; Ferraris, S.; Cochis, A.; Cempura, G.; Czyrska-Filemonowicz, A.; Rimondini, L.; Spriano, S. Bioactive Titanium Surfaces Enriched with Silver Nanoparticles Through an In Situ Reduction: Looking for a Balance between Cytocompatibility and Antibacterial Activity. Adv. Eng. Mater. 2023, 25, 2200883. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Docket Number: FDA-2010-D-0530. 2014. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considering-whether-fda-regulated-product-involves-application-nanotechnology (accessed on 17 July 2024).
- Hansen, S.F.; Baun, A. European Regulation Affecting Nanomaterials—Review of Limitations and Future Recommendations. Dose-Response 2012, 10, 364–383. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2018/1881 of 3 December 2018 Amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Annexes I, III, VI, VII, VIII, IX, X, XI, and XII to Address Nanoforms of Substances. Regulation Number: 2018/1881. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32018R1881 (accessed on 17 July 2024).
- Health Canada. Policy Statement on Health Canada’s Working Definition for Nanomaterial. 2011. Available online: https://www.canada.ca/en/health-canada/services/science-research/reports-publications/nanomaterial/policy-statement-health-canada-working-definition.html (accessed on 25 July 2024).
- Rasmussen, K.; Rauscher, H.; Kearns, P.; González, M.; Riego Sintes, J. Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data. Regul. Toxicol. Pharmacol. 2019, 104, 74–83. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef]
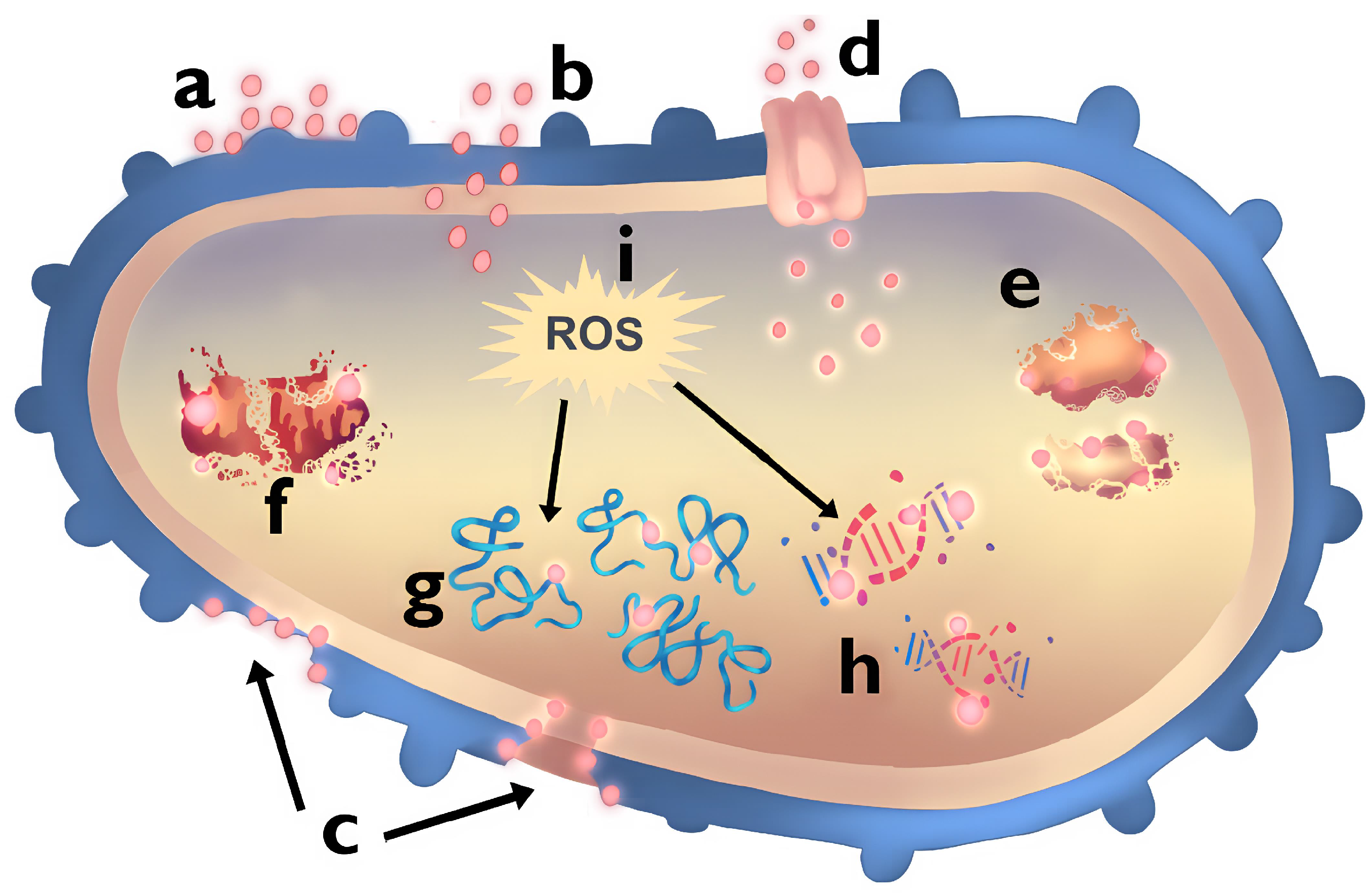
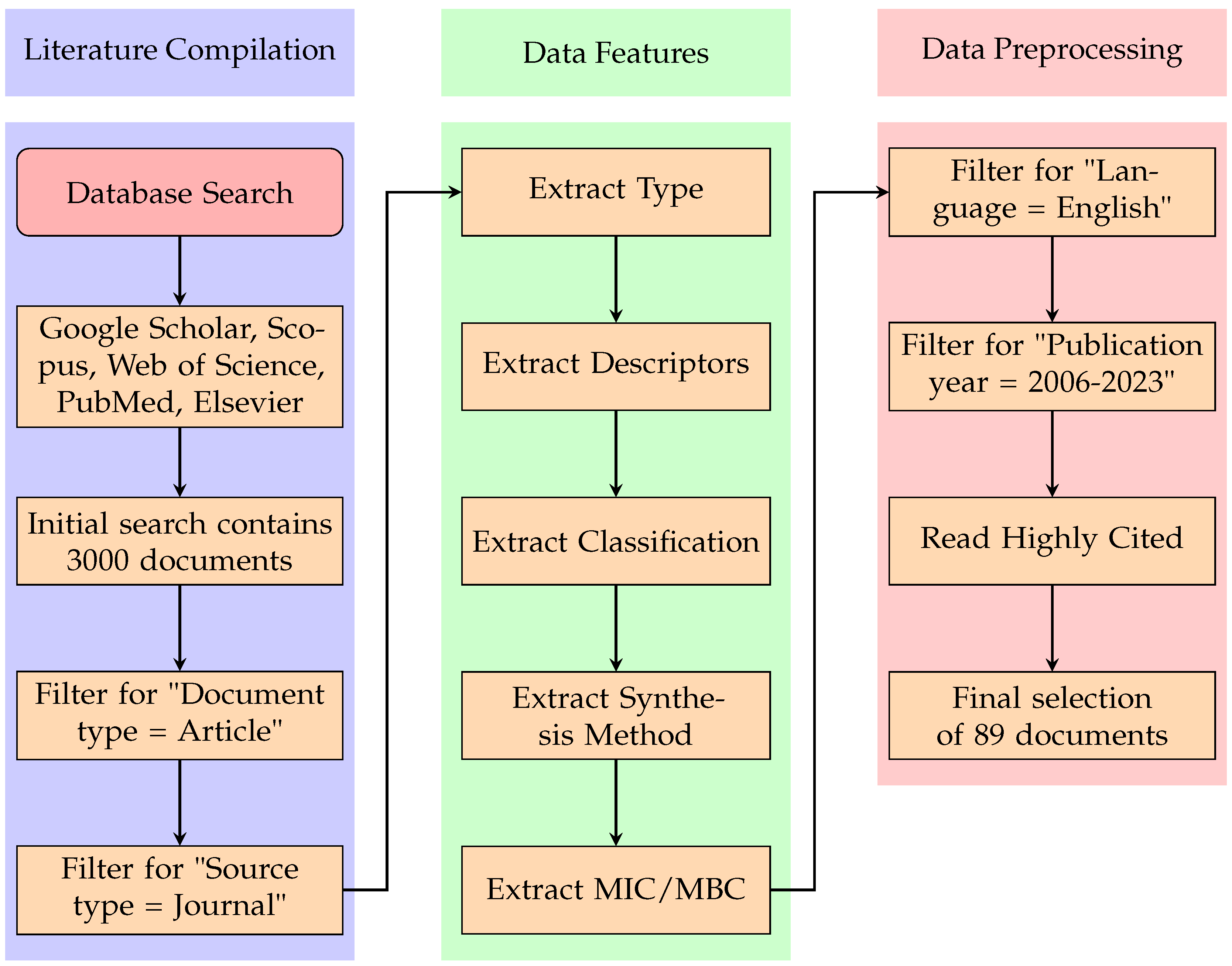
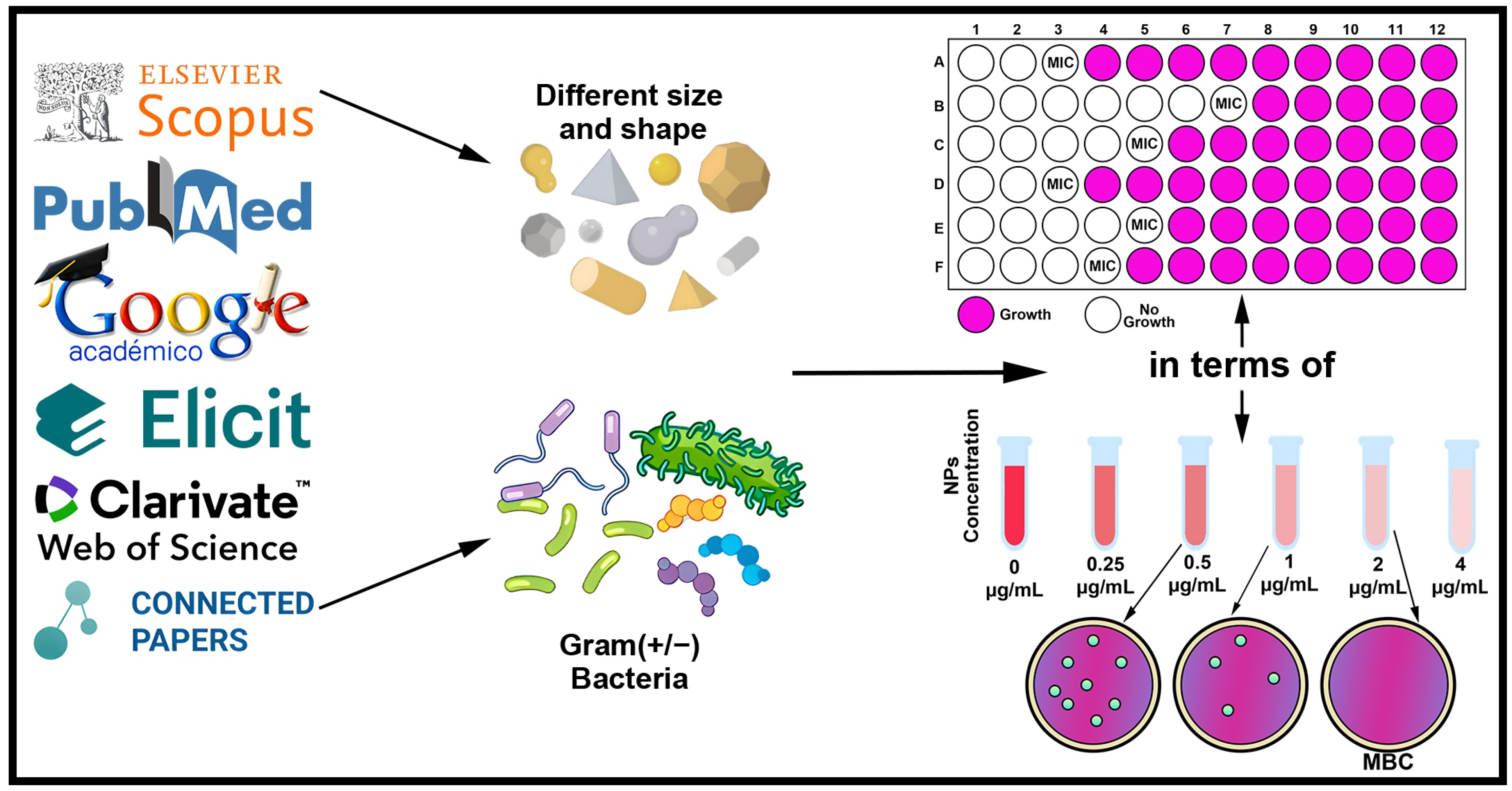
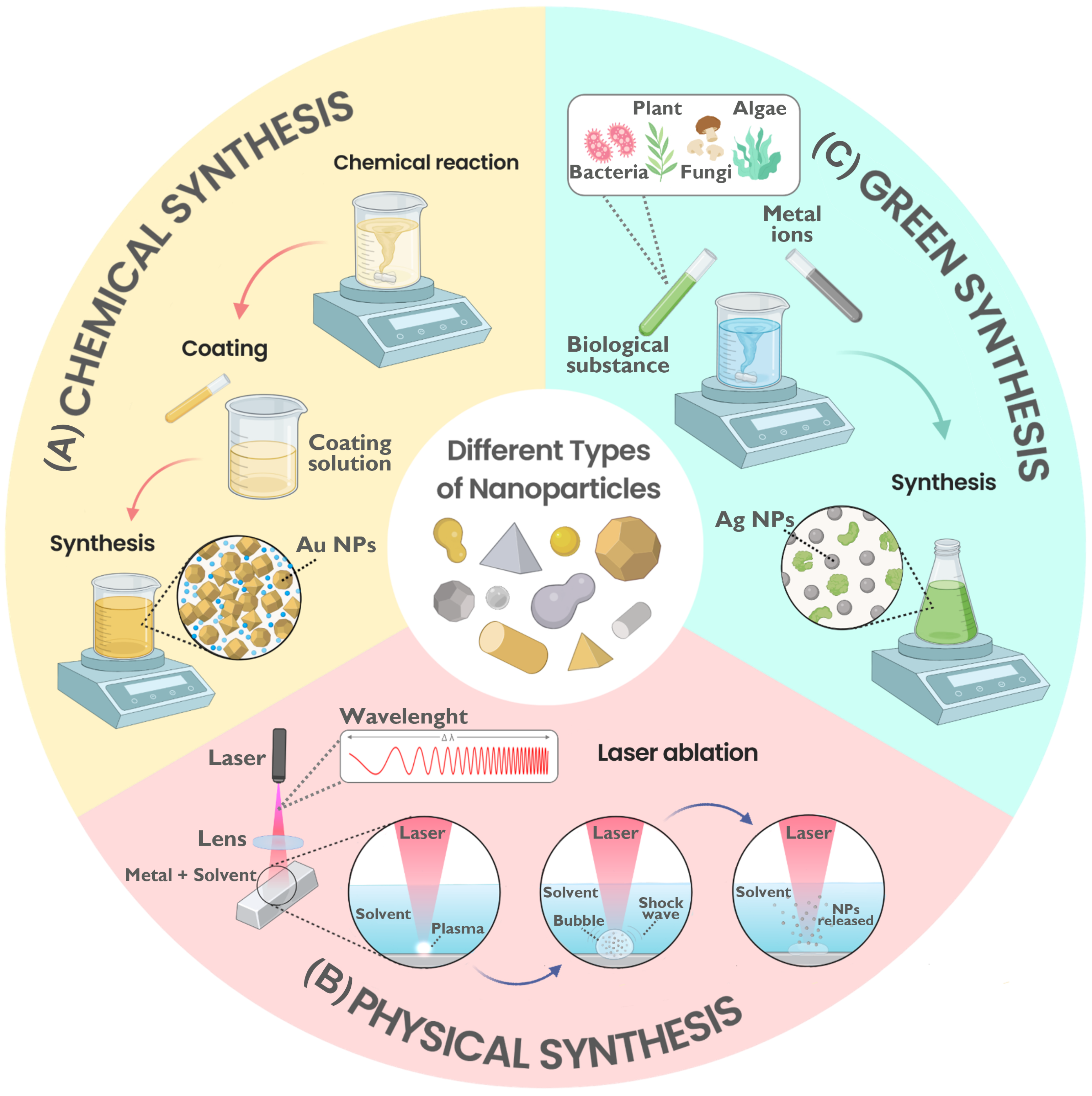
| Priority | Bacterium | Resistant Antibiotics |
|---|---|---|
| Critical | Acinetobacter baumannii | Carbapenems |
| Critical | Pseudomonas aeruginosa | Carbapenems |
| Critical | Enterobacteriaceae | Carbapenems, Cephalosporins |
| Critical | Mycobacterium tuberculosis | Rifampicin |
| High | Enterococcus faecium | Vancomycin |
| High | Staphylococcus aureus | Methicillin, Vancomycin |
| High | Helicobacter pylori | Clarithromycin |
| High | Campylobacter spp. | Fluoroquinolones |
| High | Salmonellae | Fluoroquinolones |
| High | Neisseria gonorrhoeae | Cephalosporins, Fluoroquinolones |
| High | Pseudomonas aeruginosa | Carbapenems |
| Medium | Streptococcus pneumoniae | Penicillin, Macrolides |
| Medium | Haemophilus influenzae | Ampicillin |
| Medium | Shigella spp. | Fluoroquinolones |
| Synthesis Method | Advantages | Disadvantages | Impact on NPs Features and Antibacterial Activity | References |
|---|---|---|---|---|
| Physical | High purity NPs. Large quantities. | High energy requirement. Special equipment. Specific conditions needed. | Homogeneous size and shape. High interaction with bacteria. Suitable for high-tech applications. | [41,45] |
| Chemical | Scalable. Cost-effective. | Potentially toxic reagents. Residual solvents. | Controllable size and shape. Variable surface modifications. Effective in various antibacterial assays. | [47,48] |
| Green | Eco-friendly. Low toxicity. Cost-effective. | Complexity due to biological components. Variability NP properties. | High solubility and stability. High effectiveness against a broad range of Gram(+/−) bacteria. | [13,45,49] |
| Nanoparticle | Data Collected | MIC (g/mL) | MBC (g/mL) |
|---|---|---|---|
| Ag | Total: 458; MIC: 318, MBC: 140 | 0.11–1200 | 0.22–1500 |
| Au | Total: 300; MIC: 200, MBC: 100 | 0.00008–8000 | 0.00008–16,000 |
| Nanoparticle | Size (nm) | Shape | Synthesis Method | Bacterial Strain | MIC (g/mL) | MBC (g/mL) | Reference |
|---|---|---|---|---|---|---|---|
| Ag | 5 | Spherical | Chemical | A. actinomycetemcomitans | 6–50 | - | [51] |
| 5 | Spherical | Chemical | E. coli MTCC 443 | 20–110 | - | [10] | |
| 7 | E. coli MTCC 739 | 60–160 | - | ||||
| 10 | B. subtilis MTCC 441 | 30–120 | - | ||||
| 15 | S. aureus NCIM 5021 | 70–200 | - | ||||
| 15 | Spherical | Chemical | F. nucleatum | 12–50 | - | [51] | |
| 55 | Spherical | Chemical | S. sanguis | 100–200 | - | ||
| S. mutans | - | ||||||
| S. mitis | - | ||||||
| E. coli | - | ||||||
| Au | 25 | Spherical/Stars | Chemical | S. mutans | 0.97–3.17 | 1.95–6.46 | [55] |
| S. sanguinis | |||||||
| S. salivarius | |||||||
| 60 | Spherical/Stars | Chemical | S. mutans | 91.61–148.21 | 125–289.28 | ||
| S. sanguinis | |||||||
| S. salivarius | |||||||
| 90 | Spherical/Stars | Chemical | S. mutans | 232.95–500 | 217.26–1000 | ||
| S. sanguinis | |||||||
| S. salivarius |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Garay, R.; Lara-Ortiz, L.F.; Campos-López, M.; Gonzalez-Rodriguez, D.E.; Gamboa-Lugo, M.M.; Mendoza-Pérez, J.A.; Anzueto-Ríos, Á.; Nicolás-Álvarez, D.E. A Comprehensive Review of Silver and Gold Nanoparticles as Effective Antibacterial Agents. Pharmaceuticals 2024, 17, 1134. https://doi.org/10.3390/ph17091134
Aguilar-Garay R, Lara-Ortiz LF, Campos-López M, Gonzalez-Rodriguez DE, Gamboa-Lugo MM, Mendoza-Pérez JA, Anzueto-Ríos Á, Nicolás-Álvarez DE. A Comprehensive Review of Silver and Gold Nanoparticles as Effective Antibacterial Agents. Pharmaceuticals. 2024; 17(9):1134. https://doi.org/10.3390/ph17091134
Chicago/Turabian StyleAguilar-Garay, Ricardo, Luis F. Lara-Ortiz, Maximiliano Campos-López, Dafne E. Gonzalez-Rodriguez, Margoth M. Gamboa-Lugo, Jorge A. Mendoza-Pérez, Álvaro Anzueto-Ríos, and Dulce E. Nicolás-Álvarez. 2024. "A Comprehensive Review of Silver and Gold Nanoparticles as Effective Antibacterial Agents" Pharmaceuticals 17, no. 9: 1134. https://doi.org/10.3390/ph17091134
APA StyleAguilar-Garay, R., Lara-Ortiz, L. F., Campos-López, M., Gonzalez-Rodriguez, D. E., Gamboa-Lugo, M. M., Mendoza-Pérez, J. A., Anzueto-Ríos, Á., & Nicolás-Álvarez, D. E. (2024). A Comprehensive Review of Silver and Gold Nanoparticles as Effective Antibacterial Agents. Pharmaceuticals, 17(9), 1134. https://doi.org/10.3390/ph17091134











