Insights from Traditional Chinese Medicine for Restoring Skin Barrier Functions
Abstract
:1. Introduction
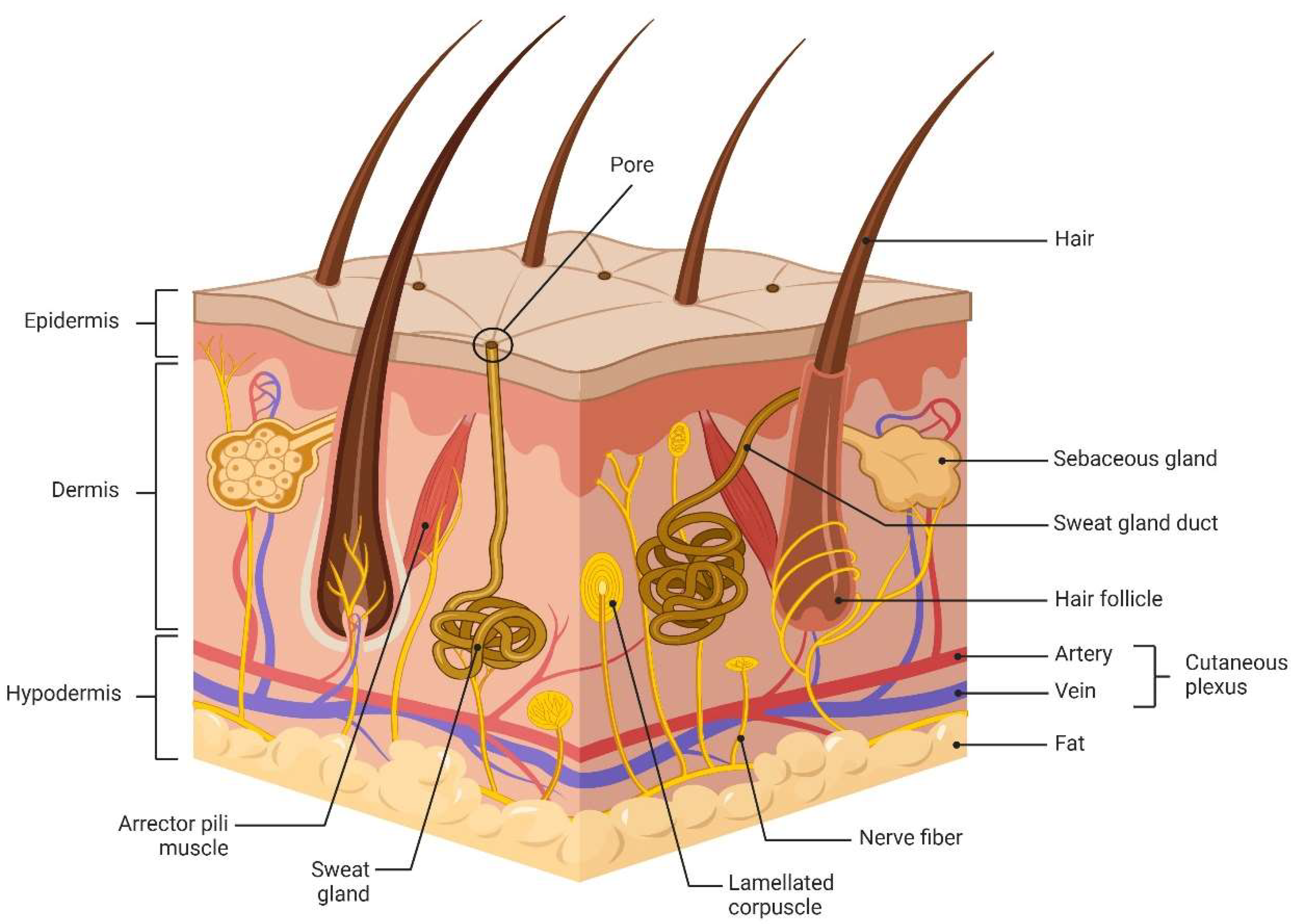
2. Physiological Properties and Functions of Skin Barrier
3. Skin-Related Diseases
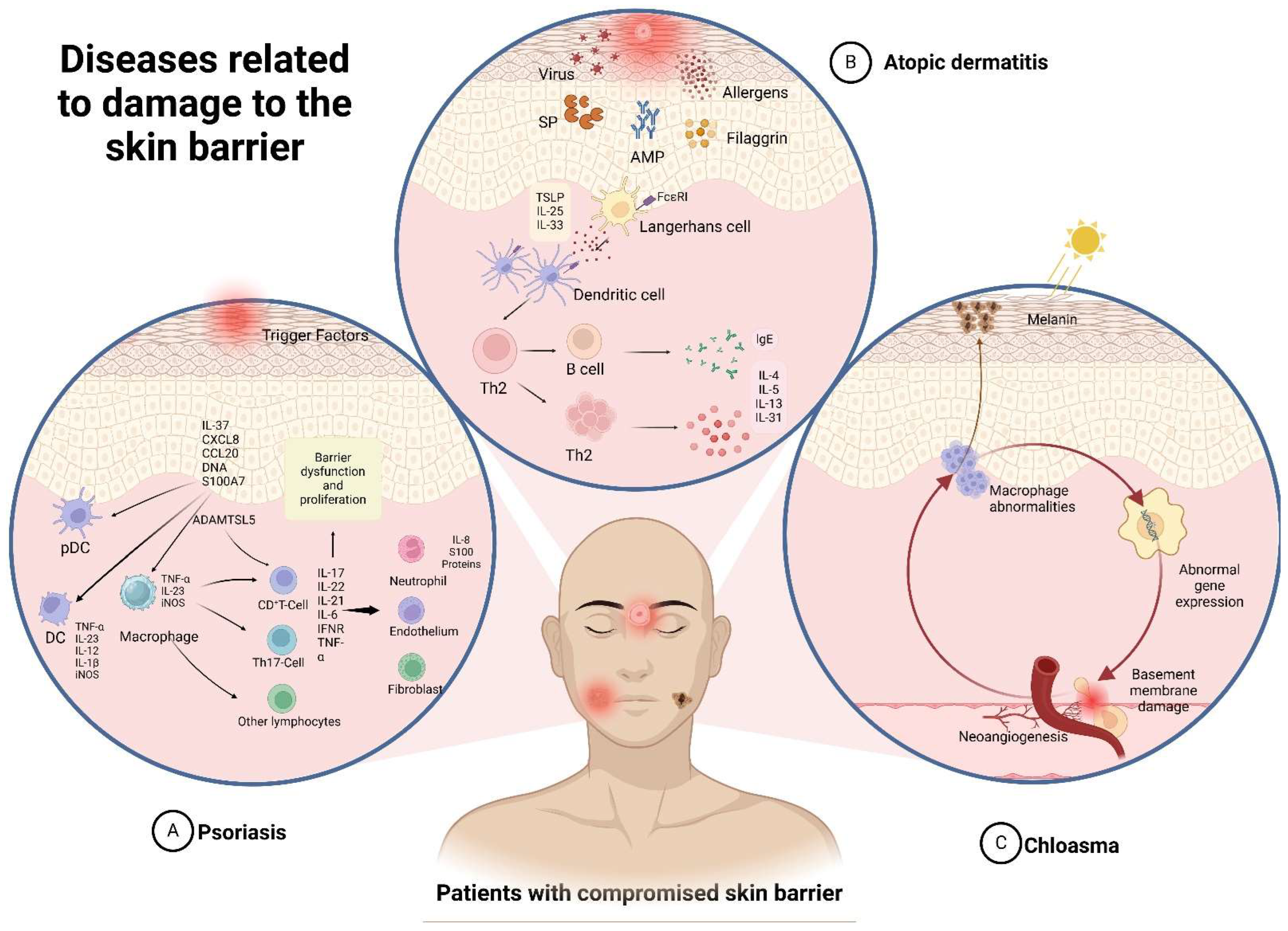
3.1. Atopic Dermatitis
3.2. Psoriasis
3.3. Chloasma
3.4. Other Related Diseases
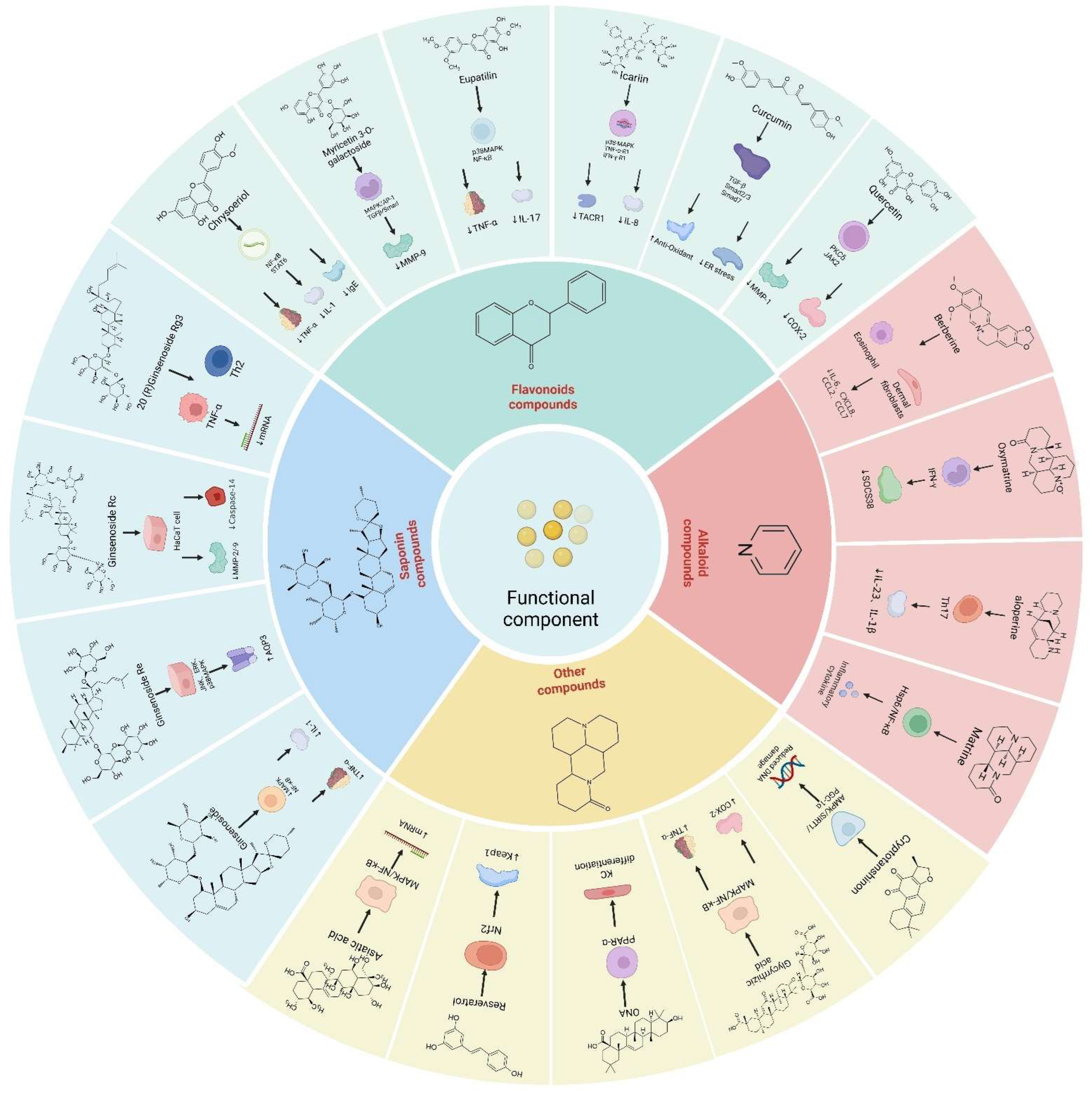
4. The Mechanism and Targets of Traditional Chinese Medicine in Restoring Skin Barrier
4.1. Ginsenoside
4.2. Flavonoids
4.3. Alkaloid
4.4. Carbohydrates
4.5. Other Compounds
5. State-of-the-Art Research on Traditional Chinese Medicine
6. Summary and Outlook
Funding
Conflicts of Interest
Abbreviations
| TCM | Traditional Chinese Medicine | IL-13 | interleukin-13 |
| TEWL | transepidermal water loss | TCS | topical corticosteroids |
| TJs | tight junctions | TCI | calcineurin inhibitors |
| TLR | toll-like receptor | pDCs | plasmacytoid dendritic cells |
| NMFs | natural moisturizing factors | IFN | interferon |
| SC | stratum corneum | mDCs | myeloid dendritic cells |
| UV | ultraviolet | IL-17 | interleukin-17 |
| KLK | kallikrein-related peptidase | IL-1 | interleukin-1 |
| SG | stratum granulosum | IL-6 | interleukin-6 |
| AMPs | antimicrobial peptides | CXCL1 | chemokine (C-X-C motif) ligand 1 |
| hBDs | human beta-defensins | CCL20 | chemokine ligand 20 |
| LCs | langerhans cells | PASI | Psoriasis Area and Severity Index |
| DCs | dermal dendritic cells | TH2 | T helper cell 2 |
| IL-7 | interleukin-7 | TH17 | T helper cell 17 |
| IL-15 | interleukin-15 | TH9 | T helper cell 9 |
| TGF-β | transforming growth factor b | TNF-α | tumor necrosis factor-α |
| IL-34 | interleukin-34 | TNCB | 2,4,6-trinitrochlorobenzene |
| PAMPs | pathogen-associated molecular patterns | DNCB | 1-Chloro-2,4-dinitrobenzene |
| DAMPs | damage-associated molecular patterns | CK2α | casein kinase 2 |
| NF-κB | nuclear factor kappa-B | GSH | glutathione, reduced |
| AD | atopic dermatitis | BALB/c | laboratory-bred strain |
| TSLP | thymic stromal lymphopoietin | IVL | involucrin |
| IgE | immunoglobulin E | AQP3 | aquaporin 3 |
| IL-4 | interleukin-4 | ATP | adenosine triphosphate |
| ELISA | enzyme-linked immunosorbent assay | COX-7 | cyclooxygenase-7 |
| HaCaT | human keratinocyte cells | COX-2 | cyclooxygenase-2 |
| UVB | ultraviolet B | GAL | galangin |
| SOD | superoxide dismutase | GST | glutathione S-transferase |
| FLG | filaggrin | GR | glutathione reductase |
| Cldn-1 | claudin-1 | LPS | lipopolysaccharides |
| NHDF | human dermal fibroblasts | GL | glycyrrhizic acid |
| AP-1 | activator protein-1 | MCP-1 | monocyte chemotactic protein-1 |
| HO-1 | heme oxygenase-1-IN-1 | TLR | toll-like receptors |
| NQO-1 | NAD (P)H quinone dehydrogenase 1 | SOCS1 | suppressor of cytokine signaling 1 |
| iNOS | inducible nitric oxide sythase | PCR | polymerase chain reaction |
| ROS | reactive oxygen species | SPINK5 | serine protease inhibitor Kazal type-5 |
| IL-1β | interleukin-1β | KLK7 | kallikrein-related peptidase 7 |
| IMQ | imiquimod | LBP | lycium barbarum polysaccharide |
| CAT | catalase | APS | astragalus polysaccharide |
| GSH | glutathione, reduced | AP | aloe polysaccharide |
| Vit-C | vitamin C | FCM | flow cytometry |
| ICAM-1 | intercellular cell adhesion molecule-1 | Bcl-2 | B-cell lymphoma-2 |
| IFN-γ | interferon-γ | GSTP1 | glutathione S-transferase Pi |
| TACR1 | tachykinin receptor 1 | UA | ursolic acid |
| UVA | ultraviolet A | SREBP-1 | sterol regulatory element-binding protein-1 |
| PKCδ | protein kinase C-δ | RANTES | recombinant human C-C motif chemokine 5 |
| CD177 | CD177 Molecule | IP-10 | recombinant human C-X-C motif chemokine 10 |
| MDC | monodansylcadaverine | MIP-3a | macrophage Inflammatory Protein-3 |
| NFAT | nuclear factor of activated T cells | PI3K | phosphatidylin-ositol-3-kinase |
| PMA | phorbol 12-myristate 13-acetate | SIRT1 | silent information regulator family protein 1 |
| GSO | ginseng oligosaccharide | HBD-2 | human β-defensin-2 |
| KLK5 | kallikrein-related peptidase 5 | SCORAD | SCORing Atopic Dermatitis |
| DSG1 | desmoglein 1 | INM-A | Indigo Naturalis |
| NFG | nerve growth factor | PCNA | proliferating cell nuclear antigen |
| MTT | thiazolyl blue | TUNEL | terminal deoxynucleotidyl transferase-mediated dUTP nick labeling |
| PI | propidium iodide | PGC-1α | peroxisome proliferators-activated receptor γ coactivator lalpha |
| Bax | BCL2-associated X | OXPHOS | oxidative phosphorylation |
| GCLC | glutamate cysteine ligase catalysis | Ki-67 | large (395 kDa) nuclear protein |
| NFG | nerve growth factor | ITK | interleukin-2-inducible T-cell kinase |
| ONA | oleanolic acid | CD4(+) | cluster of differentiation 4 receptors |
| TARC | thymic and activating regulatory chemokine | MCP-1 | monocyte chemotactic protein-1 |
References
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Chae, Y.J.; Choi, J.W.; Chang, J.E. Potential Therapeutic Approaches through Modulating the Autophagy Process for Skin Barrier Dysfunction. Int. J. Mol. Sci. 2021, 22, 7869. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Misery, L.; Proksch, E.; Metz, M.; Ständer, S.; Schmelz, M. Skin Barrier Damage and Itch: Review of Mechanisms, Topical Management and Future Directions. Acta Derm. -Venereol. 2019, 99, 1201–1209. [Google Scholar] [CrossRef]
- Lu, H.F.; Zhou, Y.C.; Yang, L.T.; Zhou, Q.; Wang, X.J.; Qiu, S.Q.; Cheng, B.H.; Zeng, X.H. Involvement and repair of epithelial barrier dysfunction in allergic diseases. Front. Immunol. 2024, 15, 1348272. [Google Scholar] [CrossRef]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 1088–1096. [Google Scholar] [CrossRef]
- Grennan, D.; Wang, S. Steroid Side Effects. JAMA 2019, 322, 282. [Google Scholar] [CrossRef] [PubMed]
- Forte, W.C.; Sumita, J.M.; Rodrigues, A.G.; Liuson, D.; Tanaka, E. Rebound phenomenon to systemic corticosteroid in atopic dermatitis. Allergol. Immunopathol. 2005, 33, 307–311. [Google Scholar] [CrossRef]
- Davies, E.J.; Reijers, S.J.M.; Van Akkooi, A.C.J.; Van Houdt, W.J.; Hayes, A.J. Isolated limb perfusion for locally advanced melanoma in the immunotherapy era. Eur. J. Surg. Oncol. 2022, 48, 1288–1292. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Paichitrojjana, A. Oral Isotretinoin and Its Uses in Dermatology: A Review. Drug Des. Dev. Ther. 2023, 17, 2573–2591. [Google Scholar] [CrossRef]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible light. Part I: Properties and cutaneous effects of visible light. J. Am. Acad. Dermatol. 2021, 84, 1219–1231. [Google Scholar] [CrossRef]
- Yan, F.; Li, F.; Liu, J.; Ye, S.; Zhang, Y.; Jia, J.; Li, H.; Chen, D.; Mo, X. The formulae and biologically active ingredients of Chinese herbal medicines for the treatment of atopic dermatitis. Biomed. Pharmacother. 2020, 127, 110142. [Google Scholar] [CrossRef] [PubMed]
- Sahle, F.F.; Gebre-Mariam, T.; Dobner, B.; Wohlrab, J.; Neubert, R.H. Skin diseases associated with the depletion of stratum corneum lipids and stratum corneum lipid substitution therapy. Skin Pharmacol. Physiol. 2015, 28, 42–55. [Google Scholar] [CrossRef]
- Strugar, T.L.; Kuo, A.; Seité, S.; Lin, M.; Lio, P. Connecting the Dots: From Skin Barrier Dysfunction to Allergic Sensitization, and the Role of Moisturizers in Repairing the Skin Barrier. J. Drugs Dermatol. 2019, 18, 581. [Google Scholar]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The Skin Barrier and Moisturization: Function, Disruption, and Mechanisms of Repair. Skin. Pharmacol. Physiol. 2023, 36, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kiatsurayanon, C.; Ogawa, H.; Niyonsaba, F. The Role of Host Defense Peptide Human β-defensins in the Maintenance of Skin Barriers. Curr. Pharm. Des. 2018, 24, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The mechanism of skin lipids influencing skin status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Cheong, K.A.; Lee, T.R.; Lee, A.Y. Complementary effect of hydroquinone and retinoic acid on corneocyte desquamation with their combination use. J. Dermatol. Sci. 2017, 87, 192–200. [Google Scholar] [CrossRef]
- Boer, D.E.C.; van Smeden, J.; Al-Khakany, H.; Melnik, E.; van Dijk, R.; Absalah, S.; Vreeken, R.J.; Haenen, C.C.P.; Lavrijsen, A.P.M.; Overkleeft, H.S.; et al. Skin of atopic dermatitis patients shows disturbed β-glucocerebrosidase and acid sphingomyelinase activity that relates to changes in stratum corneum lipid composition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158673. [Google Scholar] [CrossRef]
- van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef]
- Gibbs, N.K. l-Histidine Supplementation in Adults and Young Children with Atopic Dermatitis (Eczema). J. Nutr. 2020, 150, 2576s–2579s. [Google Scholar] [CrossRef] [PubMed]
- Spada, F.; Barnes, T.M.; Greive, K.A. Skin hydration is significantly increased by a cream formulated to mimic the skin’s own natural moisturizing systems. Clin. Cosmet. Investig. Dermatol. 2018, 11, 491–497. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Leung, D.Y.M.; Guttman-Yassky, E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2018, 120, 34–41. [Google Scholar] [CrossRef]
- Bäsler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control Release 2016, 242, 105–118. [Google Scholar] [CrossRef]
- Eyerich, S.; Eyerich, K.; Traidl-Hoffmann, C.; Biedermann, T. Cutaneous Barriers and Skin Immunity: Differentiating A Connected Network. Trends Immunol. 2018, 39, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Naik, S.; Nagao, K. Choreographing Immunity in the Skin Epithelial Barrier. Immunity 2019, 50, 552–565. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The diverse contribution of keratinocytes to immune responses in skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Leung, D.Y. Atopic dermatitis: A disease of altered skin barrier and immune dysregulation. Immunol. Rev. 2011, 242, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Sum, C.H.; Ching, J.; Zhang, H.; Loo, S.; Lo, C.W.; Lai, M.K.; Cheong, P.K.; Yu, C.L.; Lin, Z.X. Integrated Chinese and western medicine interventions for atopic dermatitis: A systematic review and meta-analysis. Chin. Med. 2021, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Sabat, R.; Philipp, S.; Höflich, C.; Kreutzer, S.; Wallace, E.; Asadullah, K.; Volk, H.D.; Sterry, W.; Wolk, K. Immunopathogenesis of psoriasis. Exp. Dermatol. 2007, 16, 779–798. [Google Scholar] [CrossRef]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef]
- Elkhawaga, O.Y.; Ellety, M.M.; Mofty, S.O.; Ghanem, M.S.; Mohamed, A.O. Review of natural compounds for potential psoriasis treatment. Inflammopharmacology 2023, 31, 1183–1198. [Google Scholar] [CrossRef]
- Wu, I.B.; Lambert, C.; Lotti, T.M.; Hercogová, J.; Sintim-Damoa, A.; Schwartz, R.A. Melasma. G. Ital. Di Dermatol. E Venereol. Organo Uff. Soc. Ital. Di Dermatol. E Sifilogr. 2012, 147, 413–418. [Google Scholar]
- Liu, W.; Chen, Q.; Xia, Y. New Mechanistic Insights of Melasma. Clin. Cosmet. Investig. Dermatol. 2023, 16, 429–442. [Google Scholar] [CrossRef]
- Piętowska, Z.; Nowicka, D.; Szepietowski, J.C. Understanding Melasma—How Can Pharmacology and Cosmetology Procedures and Prevention Help to Achieve Optimal Treatment Results? A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 12084. [Google Scholar] [CrossRef]
- Marenholz, I.; Esparza-Gordillo, J.; Lee, Y.A. The genetics of the skin barrier in eczema and other allergic disorders. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 426–434. [Google Scholar] [CrossRef]
- Kumari, V.; Timm, K.; Kühl, A.A.; Heine, G.; Worm, M. Impact of systemic alitretinoin treatment on skin barrier gene and protein expression in patients with chronic hand eczema. Br. J. Dermatol. 2016, 175, 1243–1250. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.Z.; Geliebter, J.; Tiwari, R.; Li, X.M. Traditional Chinese medicine for food allergy and eczema. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2021, 126, 639–654. [Google Scholar] [CrossRef]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 2717–2744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xie, H.; Cheng, L.; Li, J. Clinical characteristics and epidermal barrier function of papulopustular rosacea: A comparison study with acne vulgaris. Pak. J. Med. Sci. 2016, 32, 1344–1348. [Google Scholar] [CrossRef]
- Dréno, B.; Bettoli, V.; Araviiskaia, E.; Sanchez Viera, M.; Bouloc, A. The influence of exposome on acne. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef]
- Huston, D.P.; Bressler, R.B. Urticaria and angioedema. Med. Clin. N. Am. 1992, 76, 805–840. [Google Scholar] [CrossRef] [PubMed]
- Jan, H.J.H. DNA Damage, Aging, and Cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Klapp, V.; Álvarez-Abril, B.; Leuzzi, G.; Kroemer, G.; Ciccia, A.; Galluzzi, L. The DNA Damage Response and Inflammation in Cancer. Cancer Discov. 2023, 13, 1521–1545. [Google Scholar] [CrossRef]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tetzlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Wang, X.; Zou, Y.; Xiang, W.; Lu, N. Evaluation of the Composite Skin Patch Loaded with Bioactive Functional Factors Derived from Multicellular Spheres of EMSCs for Regeneration of Full-thickness Skin Defects in Rats. Curr. Stem Cell Res. Ther. 2024, 19, 1142–1152. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, R.; Wang, M.; Zhai, L.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J. Ginseng Res. 2022, 46, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, L.; Fan, D. Insights into Recent Studies on Biotransformation and Pharmacological Activities of Ginsenoside Rd. Biomolecules 2022, 12, 512. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Ko, S.G.; Moon, P.D.; Park, H.J. Ginsenoside Rg3 attenuates skin disorders via down-regulation of MDM2/HIF1α signaling pathway. J. Ginseng Res. 2021, 45, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, D.H.; Kim, B.K.; Yoon, S.K.; Kim, M.H.; Lee, J.Y.; Kim, H.O.; Park, Y.M. Effects of topically applied Korean red ginseng and its genuine constituents on atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 2011, 11, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.Y.; Jeon, Y.D.; Kim, D.S.; Han, Y.H.; Park, J.; Youn, D.H.; Kim, S.J.; Ahn, K.S.; Um, J.Y.; Hong, S.H. Korean Red Ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J. Ginseng Res. 2017, 41, 134–143. [Google Scholar] [CrossRef]
- Sohn, E.H.; Jang, S.A.; Lee, C.H.; Jang, K.H.; Kang, S.C.; Park, H.J.; Pyo, S. Effects of korean red ginseng extract for the treatment of atopic dermatitis-like skin lesions in mice. J. Ginseng Res. 2011, 35, 479–486. [Google Scholar] [CrossRef]
- Oh, Y.; Lim, H.W.; Park, K.H.; Huang, Y.H.; Yoon, J.Y.; Kim, K.; Lim, C.J. Ginsenoside Rc protects against UVB-induced photooxidative damage in epidermal keratinocytes. Mol. Med. Rep. 2017, 16, 2907–2914. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, K.; Lim, C.J. Protective properties of ginsenoside Rb1 against UV-B radiation-induced oxidative stress in human dermal keratinocytes. Die Pharm. 2015, 70, 381–387. [Google Scholar]
- Liu, X.Y.; Hwang, E.; Park, B.; Ngo, H.T.T.; Xiao, Y.K.; Yi, T.H. Ginsenoside C-Mx Isolated from Notoginseng Stem-leaf Ginsenosides Attenuates Ultraviolet B-mediated Photoaging in Human Dermal Fibroblasts. Photochem. Photobiol. 2018, 94, 1040–1048. [Google Scholar] [CrossRef]
- Huang, W.C.; Huang, T.H.; Yeh, K.W.; Chen, Y.L.; Shen, S.C.; Liou, C.J. Ginsenoside Rg3 ameliorates allergic airway inflammation and oxidative stress in mice. J. Ginseng Res. 2021, 45, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Choi, W.Y.; Jung, H.J. Stereoselective skin anti-photoaging properties of ginsenoside Rg3 in UV-B-irradiated keratinocytes. Biol. Pharm. Bull. 2014, 37, 1583–1590. [Google Scholar] [CrossRef]
- Shi, Q.; He, Q.; Chen, W.; Long, J.; Zhang, B. Ginsenoside Rg1 abolish imiquimod-induced psoriasis-like dermatitis in BALB/c mice via downregulating NF-κB signaling pathway. J. Food Biochem. 2019, 43, e13032. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Choo, M.K.; Han, M.J.; Kim, D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int. Arch. Allergy Immunol. 2004, 133, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Park, K.I.; Heo, J.D.; Kim, H.W.; Kim, G.S. Flavones: Six Selected Flavones and Their Related Signaling Pathways That Induce Apoptosis in Cancer. Int. J. Mol. Sci. 2022, 23, 10965. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Chen, Y.J.; Bai, L.; Liu, Y.X.; Fu, X.Q.; Zhu, P.L.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Wang, Y.P.; et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine Int. J. Phytother. Phytopharm. 2020, 68, 153173. [Google Scholar] [CrossRef]
- Sangaraju, R.; Alavala, S.; Nalban, N.; Jerald, M.K.; Sistla, R. Galangin ameliorates Imiquimod-Induced psoriasis-like skin inflammation in BALB/c mice via down regulating NF-κB and activation of Nrf2 signaling pathways. Int. Immunopharmacol. 2021, 96, 107754. [Google Scholar] [CrossRef]
- Jo, B.G.; Park, N.J.; Jegal, J.; Choi, S.; Lee, S.W.; Yi, L.W.; Kim, S.N.; Yang, M.H. Stellera chamaejasme and Its Main Compound Luteolin 7-O-Glucoside Alleviates Skin Lesions in Oxazolone- and 2,4-Dinitrochlorobenzene-Stimulated Murine Models of Atopic Dermatitis. Planta Medica 2019, 85, 583–590. [Google Scholar] [CrossRef]
- Bai, D.; Cheng, X.; Li, Q.; Zhang, B.; Zhang, Y.; Lu, F.; Sun, T.; Hao, J. Eupatilin inhibits keratinocyte proliferation and ameliorates imiquimod-induced psoriasis-like skin lesions in mice via the p38 MAPK/NF-κB signaling pathway. Immunopharmacol. Immunotoxicol. 2023, 45, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhao, W.; Zhang, B.; Tu, Y.; Wang, Q.; Li, J. Cimifugin ameliorates imiquimod-induced psoriasis by inhibiting oxidative stress and inflammation via NF-κB/MAPK pathway. Biosci. Rep. 2020, 40, BSR20200471. [Google Scholar] [CrossRef]
- Xiong, H.; Xu, Y.; Tan, G.; Han, Y.; Tang, Z.; Xu, W.; Zeng, F.; Guo, Q. Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-α-induced ICAM-1 expression via NF-κB/MAPK in HaCaT cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 1335–1346. [Google Scholar] [CrossRef]
- Kong, L.; Liu, J.; Wang, J.; Luo, Q.; Zhang, H.; Liu, B.; Xu, F.; Pang, Q.; Liu, Y.; Dong, J. Icariin inhibits TNF-α/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int. Immunopharmacol. 2015, 29, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Park, N.J.; Jo, B.G.; Paik, J.H.; Choi, S.; Kim, S.N.; Yang, M.H. 7-O-Methylluteolin Suppresses the 2,4-Dinitrochlorobenzene-Induced Nrf2/HO-1 Pathway and Atopic Dermatitis-like Lesions. Antioxidants 2022, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Wu, N.L.; Pu, C.M.; Hsiao, C.Y.; Chang, D.C.; Hung, C.F. Chrysin alleviates imiquimod-induced psoriasis-like skin inflammation and reduces the release of CCL20 and antimicrobial peptides. Sci. Rep. 2020, 10, 2932. [Google Scholar] [CrossRef]
- Svobodová, A.; Zdarilová, A.; Walterová, D.; Vostálová, J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J. Dermatol. Sci. 2007, 48, 213–224. [Google Scholar] [CrossRef]
- Ren, X.; Shi, Y.; Zhao, D.; Xu, M.; Li, X.; Dang, Y.; Ye, X. Naringin protects ultraviolet B-induced skin damage by regulating p38 MAPK signal pathway. J. Dermatol. Sci. 2016, 82, 106–114. [Google Scholar] [CrossRef]
- Sherwani, M.A.; Yang, K.; Jani, A.; Abed, R.A.; Taufique, A.K.; Dosunmu, T.G.; Yusuf, N. Protective Effect of Baicalin Against TLR4-mediated UVA-induced Skin Inflammation. Photochem. Photobiol. 2019, 95, 605–611. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Ai, Y.; Xu, X.; Zhu, S.; Zhang, B.; Tang, M.; Zhang, L.; He, T. Glabridin Liposome Ameliorating UVB-Induced Erythema and Lethery Skin by Suppressing Inflammatory Cytokine Production. J. Microbiol. Biotechnol. 2021, 31, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Lee, H.J.; Im, A.R.; Kim, S.M.; Kang, H.S.; Lee, J.D.; Chae, S. The flavonoid hesperidin exerts anti-photoaging effect by downregulating matrix metalloproteinase (MMP)-9 expression via mitogen activated protein kinase (MAPK)-dependent signaling pathways. BMC Complement. Altern. Med. 2018, 18, 39. [Google Scholar] [CrossRef]
- Nisar, M.F.; Liu, T.; Wang, M.; Chen, S.; Chang, L.; Karisma, V.W.; Weixu; Diao, Q.; Xue, M.; Tang, X.; et al. Eriodictyol protects skin cells from UVA irradiation-induced photodamage by inhibition of the MAPK signaling pathway. J. Photochem. Photobiol. B Biol. 2022, 226, 112350. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Park, S.Y.; Seo, Y.; Kong, C.S. Anticatabolic and Anti-Inflammatory Effects of Myricetin 3-O-β-d-Galactopyranoside in UVA-Irradiated Dermal Cells via Repression of MAPK/AP-1 and Activation of TGFβ/Smad. Molecules 2020, 25, 1331. [Google Scholar] [CrossRef]
- Huang, K.F.; Ma, K.H.; Chang, Y.J.; Lo, L.C.; Jhap, T.Y.; Su, Y.H.; Liu, P.S.; Chueh, S.H. Baicalein inhibits matrix metalloproteinase 1 expression via activation of TRPV1-Ca-ERK pathway in ultraviolet B-irradiated human dermal fibroblasts. Exp. Dermatol. 2019, 28, 568–575. [Google Scholar] [CrossRef]
- Jung, S.K.; Ha, S.J.; Jung, C.H.; Kim, Y.T.; Lee, H.K.; Kim, M.O.; Lee, M.H.; Mottamal, M.; Bode, A.M.; Lee, K.W.; et al. Naringenin targets ERK2 and suppresses UVB-induced photoaging. J. Cell. Mol. Med. 2016, 20, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Mo, X.; Lin, Y.; Liu, J.; Ye, S.; Zhang, Y.; Fan, X.; Chen, D.; Yan, F. Inhibitory effects of isoliquiritin on an atopic dermatitis model through the CD177/JAK2/STAT pathway in vitro and in vivo. Ann. Transl. Med. 2022, 10, 980. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.J.; Ding, P.J.; Yang, L.L.; He, X.F.; Chen, M.J.; Wang, D.M.; Tian, Y.X.; Zhang, H.M. Oxymatrine Sensitizes the HaCaT Cells to the IFN-γ Pathway and Downregulates MDC, ICAM-1, and SOCS1 by Activating p38, JNK, and Akt. Inflammation 2018, 41, 606–613. [Google Scholar] [CrossRef]
- Chan, T.C.; Lee, M.S.; Huang, W.C.; Chang, W.Y.; Krueger, J.G.; Tsai, T.F. Capsaicin attenuates imiquimod-induced epidermal hyperplasia and cutaneous inflammation in a murine model of psoriasis. Biomed. Pharmacother. 2021, 141, 111950. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Hu, F.; Yang, Z.B.; Pan, Y.; Zhou, R.; Yan, Y.N.; Wang, H.Z.; Wang, C. Matrine regulates Th1/Th2 inflammatory responses by inhibiting the Hsp90/NF-κB signaling axis to alleviate atopic dermatitis. Kaohsiung J. Med. Sci. 2023, 39, 501–510. [Google Scholar] [CrossRef]
- Tsang, M.S.; Jiao, D.; Chan, B.C.; Hon, K.L.; Leung, P.C.; Lau, C.B.; Wong, E.C.; Cheng, L.; Chan, C.K.; Lam, C.W.; et al. Anti-Inflammatory Activities of Pentaherbs Formula, Berberine, Gallic Acid and Chlorogenic Acid in Atopic Dermatitis-Like Skin Inflammation. Molecules 2016, 21, 519. [Google Scholar] [CrossRef]
- Gao, S.; Li, W.; Lin, G.; Liu, G.; Deng, W.; Zhai, C.; Bian, C.; He, G.; Hu, Z. Norisoboldine, an alkaloid from Radix linderae, inhibits NFAT activation and attenuates 2,4-dinitrofluorobenzene-induced dermatitis in mice. Immunopharmacol. Immunotoxicol. 2016, 38, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Wang, F.X.; Sun, F.; Liu, X.; Rong, S.J.; Luo, J.H.; Yue, T.T.; Xiao, J.; Yang, C.L.; Lu, W.Y.; et al. Aloperine Ameliorates IMQ-Induced Psoriasis by Attenuating Th17 Differentiation and Facilitating Their Conversion to Treg. Front. Pharmacol. 2022, 13, 778755. [Google Scholar] [CrossRef] [PubMed]
- Lone, A.N.; Malik, A.T.; Naikoo, H.S.; Raghu, R.S.; S, A.T. Trigonelline, a naturally occurring alkaloidal agent protects ultraviolet-B (UV-B) irradiation induced apoptotic cell death in human skin fibroblasts via attenuation of oxidative stress, restoration of cellular calcium homeostasis and prevention of endoplasmic reticulum (ER) stress. J. Photochem. Photobiol. B Biol. 2020, 202, 111720. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Progress. Mol. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar] [CrossRef]
- Liu, J.; Bai, R.; Liu, Y.; Zhang, X.; Kan, J.; Jin, C. Isolation, structural characterization and bioactivities of naturally occurring polysaccharide-polyphenolic conjugates from medicinal plants-A reivew. Int. J. Biol. Macromol. 2018, 107, 2242–2250. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, R.; Jing, C.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Protective effect of oligosaccharides isolated from Panax ginseng C. A. Meyer against UVB-induced skin barrier damage in BALB/c hairless mice and human keratinocytes. J. Ethnopharmacol. 2022, 283, 114677. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef]
- Chen, R.X.; Zheng, S.; Guo, C.Y.; Zhang, Q. Effects of Astragalus polysaccharide on imiquimod-induced psoriasiform dermatitis in mice and its mechanisms. Chin. J. Appl. Physiol. 2022, 38, 154–159. [Google Scholar] [CrossRef]
- Yuan, L.; Duan, X.; Zhang, R.; Zhang, Y.; Qu, M. Aloe polysaccharide protects skin cells from UVB irradiation through Keap1/Nrf2/ARE signal pathway. J. Dermatol. Treat. 2020, 31, 300–308. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.W.; Yi, T.H. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother. Res. PTR 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Wei, J.; Chen, H.; Lu, Y.; Li, L.; Han, L.; Lu, C. Gallic acid inhibits the expression of keratin 16 and keratin 17 through Nrf2 in psoriasis-like skin disease. Int. Immunopharmacol. 2018, 65, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhou, X. Gallic Acid Ameliorates Atopic Dermatitis-Like Skin Inflammation Through Immune Regulation in a Mouse Model. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Lim, S.W.; Hong, S.P.; Jeong, S.W.; Kim, B.; Bak, H.; Ryoo, H.C.; Lee, S.H.; Ahn, S.K. Simultaneous effect of ursolic acid and oleanolic acid on epidermal permeability barrier function and epidermal keratinocyte differentiation via peroxisome proliferator-activated receptor-alpha. J. Dermatol. 2007, 34, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Song, H.K.; Park, S.H.; Jang, S.; Park, K.S.; Song, K.H.; Lee, S.K.; Kim, T. Terminalia chebula Retz. extract ameliorates the symptoms of atopic dermatitis by regulating anti-inflammatory factors in vivo and suppressing STAT1/3 and NF-ĸB signaling in vitro. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 104, 154318. [Google Scholar] [CrossRef]
- Song, H.K.; Park, S.H.; Kim, H.J.; Jang, S.; Kim, T. Alpinia officinarum water extract inhibits the atopic dermatitis-like responses in NC/Nga mice by regulation of inflammatory chemokine production. Biomed. Pharmacother. 2021, 144, 112322. [Google Scholar] [CrossRef]
- Min, G.Y.; Kim, T.I.; Kim, J.H.; Cho, W.K.; Yang, J.H.; Ma, J.Y. Anti-Atopic Effect of Isatidis Folium Water Extract in TNF-α/IFN-γ-Induced HaCaT Cells and DNCB-Induced Atopic Dermatitis Mouse Model. Molecules 2023, 28, 3960. [Google Scholar] [CrossRef]
- Han, X.; Chen, Z.; Yuan, J.; Wang, G.; Han, X.; Wu, H.; Shi, H.; Chou, G.; Yang, L.; Wu, X. Artemisia annua water extract attenuates DNCB-induced atopic dermatitis by restraining Th2 cell mediated inflammatory responses in BALB/c mice. J. Ethnopharmacol. 2022, 291, 115160. [Google Scholar] [CrossRef]
- Li, H.; Jiang, N.; Liang, B.; Liu, Q.; Zhang, E.; Peng, L.; Deng, H.; Li, R.; Li, Z.; Zhu, H. Pterostilbene protects against UVB-induced photo-damage through a phosphatidylinositol-3-kinase-dependent Nrf2/ARE pathway in human keratinocytes. Redox Rep. Commun. Free Radic. Res. 2017, 22, 501–507. [Google Scholar] [CrossRef]
- Guo, K.; Liu, R.; Jing, R.; Wang, L.; Li, X.; Zhang, K.; Fu, M.; Ye, J.; Hu, Z.; Zhao, W.; et al. Cryptotanshinone protects skin cells from ultraviolet radiation-induced photoaging via its antioxidant effect and by reducing mitochondrial dysfunction and inhibiting apoptosis. Front. Pharmacol. 2022, 13, 1036013. [Google Scholar] [CrossRef]
- Farrukh, M.R.; Nissar, U.A.; Kaiser, P.J.; Afnan, Q.; Sharma, P.R.; Bhushan, S.; Tasduq, S.A. Glycyrrhizic acid (GA) inhibits reactive oxygen Species mediated photodamage by blocking ER stress and MAPK pathway in UV-B irradiated human skin fibroblasts. J. Photochem. Photobiol. B Biol. 2015, 148, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Fu, M.; Zhao, Q.; Wang, Y.; Li, T.; Feng, B.; Li, E.; Nishijima, Y.; Sun, Z.; Hu, Z. α-ionone promotes keratinocyte functions and accelerates epidermal barrier recovery. Ann. Transl. Med. 2023, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, H.; Sun, L.; Cheng, K.; Lv, Z.; Chen, K.; Qian, F.; Li, Y. (-)-α-Bisabolol Alleviates Atopic Dermatitis by Inhibiting MAPK and NF-κB Signaling in Mast Cell. Molecules 2022, 27, 3985. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective effect of curcumin against ultraviolet A irradiation-induced photoaging in human dermal fibroblasts. Mol. Med. Rep. 2018, 17, 7227–7237. [Google Scholar] [CrossRef]
- Liu, Y.; Chan, F.; Sun, H.; Yan, J.; Fan, D.; Zhao, D.; An, J.; Zhou, D. Resveratrol protects human keratinocytes HaCaT cells from UVA-induced oxidative stress damage by downregulating Keap1 expression. Eur. J. Pharmacol. 2011, 650, 130–137. [Google Scholar] [CrossRef]
- Wang, J.; Ke, J.; Wu, X.; Yan, Y. Astragaloside prevents UV-induced keratinocyte injury by regulating TLR4/NF-κB pathway. J. Cosmet. Dermatol. 2022, 21, 1163–1170. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.W.; Cho, S.H. The Beneficial Effect of Korean Red Ginseng Extract on Atopic Dermatitis Patients: An 8 Weeks Open, Noncomparative Clinical Study. Ann. Dermatol. 2018, 30, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Son, S.W. Efficacy of korean red ginseng in the treatment of atopic dermatitis. J. Ginseng Res. 2011, 35, 149–154. [Google Scholar] [CrossRef]
- Kim, D.Y.; Jung, J.A.; Kim, T.H.; Seo, S.W.; Jung, S.K.; Park, C.S. Oral administration of Uncariae rhynchophylla inhibits the development of DNFB-induced atopic dermatitis-like skin lesions via IFN-gamma down-regulation in NC/Nga mice. J. Ethnopharmacol. 2009, 122, 567–572. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Stewart, J.M.; Tsilioni, I. Tolerability and benefit of a tetramethoxyluteolin-containing skin lotion. Int. J. Immunopathol. Pharmacol. 2017, 30, 146–151. [Google Scholar] [CrossRef]
- Lee, C.L.; Wang, C.M.; Song, Y.C.; Liu, C.T.; Chu, M.Y.; Yen, H.R. An alkaloid-rich phytopharmaceutical prepared from Qing Dai against IL-17A-induced psoriasis. J. Ethnopharmacol. 2024, 318, 116924. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.J.; Zhou, H.; Ma, A.L.; Wang, L.; Gao, Q.; Zhang, N.; Song, H.B.; Bo, K.P.; Ma, W. Oxymatrine therapy inhibited epidermal cell proliferation and apoptosis in severe plaque psoriasis. Br. J. Dermatol. 2019, 181, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, H.J.; Yang, J.; Chen, W.G.; Xia, L.; Song, H.B.; Bo, K.P.; Ma, W. Efficacy of oxymatrine for treatment and relapse suppression of severe plaque psoriasis: Results from a single-blinded randomized controlled clinical trial. Br. J. Dermatol. 2017, 176, 1446–1455. [Google Scholar] [CrossRef]
- Jang, A.H.; Kim, T.H.; Kim, G.D.; Kim, J.E.; Kim, H.J.; Kim, S.S.; Jin, Y.H.; Park, Y.S.; Park, C.S. Rosmarinic acid attenuates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol. 2011, 11, 1271–1277. [Google Scholar] [CrossRef]
- Lu, J.; Cong, T.; Wen, X.; Li, X.; Du, D.; He, G.; Jiang, X. Salicylic acid treats acne vulgaris by suppressing AMPK/SREBP1 pathway in sebocytes. Exp. Dermatol. 2019, 28, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.H.; Lee, Y.; Kim, E.K.; Chung, K.H.; Lee, K.J.; An, J.H. Immunomodulatory and Anti-inflammatory Effects of Asiatic Acid in a DNCB-Induced Atopic Dermatitis Animal Model. Nutrients 2021, 13, 2448. [Google Scholar] [CrossRef]
- Oh, J.H.; Joo, Y.H.; Karadeniz, F.; Ko, J.; Kong, C.S. Syringaresinol Inhibits UVA-Induced MMP-1 Expression by Suppression of MAPK/AP-1 Signaling in HaCaT Keratinocytes and Human Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3981. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Korivi, M.; Lin, F.Y.; Li, M.L.; Lin, R.W.; Wu, J.J.; Yang, H.L. Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblasts. J. Dermatol. Sci. 2018, 90, 123–134. [Google Scholar] [CrossRef]
- Sun, Z.; Du, J.; Hwang, E.; Yi, T.H. Paeonol extracted from Paeonia suffruticosa Andr. ameliorated UVB-induced skin photoaging via DLD/Nrf2/ARE and MAPK/AP-1 pathway. Phytother. Res. PTR 2018, 32, 1741–1749. [Google Scholar] [CrossRef]
- Chen, J.; Hong, X.; Duan, Y.; Zhang, Y.; Han, Y. Effects of osthole on skin barrier and chronic pruritus in mice with specific dermatitis. Chin. Tradit. Pat. Med. 2021, 43, 3489–3492. [Google Scholar]
- Yang, R.; Wang, L.Q.; Yuan, B.C.; Liu, Y. The Pharmacological Activities of Licorice. Planta Medica 2015, 81, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Hu, M.; Zang, X.; Liu, Q.; Du, J.; Hu, J.; Zhang, L.; Du, Z.; Xiang, Z. Luteolin attenuates imiquimod-induced psoriasis-like skin lesions in BALB/c mice via suppression of inflammation response. Biomed. Pharmacother. 2020, 131, 110696. [Google Scholar] [CrossRef] [PubMed]
- Gaid, M.; Haas, P.; Beuerle, T.; Scholl, S.; Beerhues, L. Hyperforin production in Hypericum perforatum root cultures. J. Biotechnol. 2016, 222, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, J.; Yu, J.; Chen, X.; Zhang, F.; Wei, W.; Zhang, L.; Chen, W.; Lin, N.; Wu, Y. Hyperforin Ameliorates Imiquimod-Induced Psoriasis-Like Murine Skin Inflammation by Modulating IL-17A-Producing γδ T Cells. Front. Immunol. 2021, 12, 635076. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Wang, P.W.; Lin, T.Y.; Yang, P.M.; Fang, J.Y.; Li, W.T.; Pan, T.L. Therapeutic efficacy of Scutellaria baicalensis Georgi against psoriasis-like lesions via regulating the responses of keratinocyte and macrophage. Biomed. Pharmacother. 2022, 155, 113798. [Google Scholar] [CrossRef]
- Saleh, M.A.; Shabaan, A.A.; May, M.; Ali, Y.M. Topical application of indigo-plant leaves extract enhances healing of skin lesion in an excision wound model in rats. J. Appl. Biomed. 2022, 20, 124–129. [Google Scholar] [CrossRef]
- Xie, X.J.; Di, T.T.; Wang, Y.; Wang, M.X.; Meng, Y.J.; Lin, Y.; Xu, X.L.; Li, P.; Zhao, J.X. Indirubin ameliorates imiquimod-induced psoriasis-like skin lesions in mice by inhibiting inflammatory responses mediated by IL-17A-producing γδ T cells. Mol. Immunol. 2018, 101, 386–395. [Google Scholar] [CrossRef]
- Tian, J.; Qin, S.; Han, J.; Meng, J.; Liang, A. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J. Ethnopharmacol. 2022, 289, 114984. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, M.; Xie, X.; Di, T.; Zhao, J.; Lin, Y.; Xu, X.; Li, N.; Zhai, Y.; Wang, Y.; et al. Paeonol ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice by inhibiting the maturation and activation of dendritic cells. Int. J. Mol. Med. 2017, 39, 1101–1110. [Google Scholar] [CrossRef]
- Rafiq, S.; Hao, H.; Ijaz, M.; Raza, A. Pharmacological Effects of Houttuynia cordata Thunb (H. cordata): A Comprehensive Review. Pharmaceuticals 2022, 15, 1079. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef] [PubMed]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Schmid, D.; Gruber, M.; Woehs, F.; Prinz, S.; Etzlstorfer, B.; Prucker, C.; Fuzzati, N.; Kopp, B.; Moeslinger, T. Inhibition of inducible nitric oxide synthesis by Cimicifuga racemosa (Actaea racemosa, black cohosh) extracts in LPS-stimulated RAW 264.7 macrophages. J. Pharm. Pharmacol. 2009, 61, 1089–1096. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, C.Z.; Mohammadi, S.; Sawadogo, W.R.; Ma, Q.; Yuan, C.S. Pharmacological Effects of Ginseng: Multiple Constituents and Multiple Actions on Humans. Am. J. Chin. Med. 2023, 51, 1085–1104. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, K.; Jiang, K.; Tao, S.; Li, Y.; Chen, W.; Kou, S.; Gu, C.; Li, Z.; Guo, L.; et al. A Review of Flavonoids from Cassia Species and their Biological Activity. Curr. Pharm. Biotechnol. 2016, 17, 1134–1146. [Google Scholar] [CrossRef]
- Lim, Y.H.; Kim, I.H.; Seo, J.J. In vitro activity of kaempferol isolated from the Impatiens balsamina alone and in combination with erythromycin or clindamycin against Propionibacterium acnes. J. Microbiol. 2007, 45, 473–477. [Google Scholar]
- Sun, W.; Shahrajabian, M.H.; Cheng, Q. Fenugreek Cultivation with Emphasis on Historical Aspects and its uses in Traditional Medicine and Modern Pharmaceutical Science. Mini Rev. Med. Chem. 2021, 21, 724–730. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, G.D.; Ahn, H.J.; Cho, J.J.; Park, Y.S.; Park, C.S. The inhibitory effect of naringenin on atopic dermatitis induced by DNFB in NC/Nga mice. Life Sci. 2013, 93, 516–524. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef]
- Zhao, T.; Li, C.; Wang, S.; Song, X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules 2022, 27, 3909. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–s243. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.; Fatemeh, K.; Leila, N.; Mona, P.; Mohammad, Z.; Mozafar, K. Pharmacological and therapeutic properties of the Red Clover (Trifolium pratense L.): An overview of the new finding. J. Tradit. Chin. Med. 2021, 41, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Liu, J.; Yang, Y.A.; Zhu, H.L. A Review: The Anti-inflammatory, Anticancer and Antibacterial Properties of Four Kinds of Licorice Flavonoids Isolated from Licorice. Curr. Med. Chem. 2020, 27, 1997–2011. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E.G. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine Int. J. Phytother. Phytopharm. 2000, 7, 427–448. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, W.; Tao, G.; Li, Q.; Wang, L.; Huang, W.; Gao, L.; Yin, L.; Ye, Y. Comparison of Chemical Compositions and Antioxidant Activities for the Immature Fruits of Citrus changshan-huyou Y.B. Chang and Citrus aurantium L. Molecules 2023, 28, 5057. [Google Scholar] [CrossRef]
- Lee, J.; Song, K.M.; Jung, C.H. Diosmin restores the skin barrier by targeting the aryl hydrocarbon receptor in atopic dermatitis. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 81, 153418. [Google Scholar] [CrossRef]
- Xiao-Yan, L.; Yuan, Z.; Long-Bo, Z.; Da-Hui, L.; Xian-Zhang, H.; Li, Z.; Li-Ping, K. Research progress on chemical constituents from Artemisiae argyi Folium and their pharmacological activities and quality control. China J. Chin. Mater. Medica 2020, 45, 4017–4030. [Google Scholar] [CrossRef]
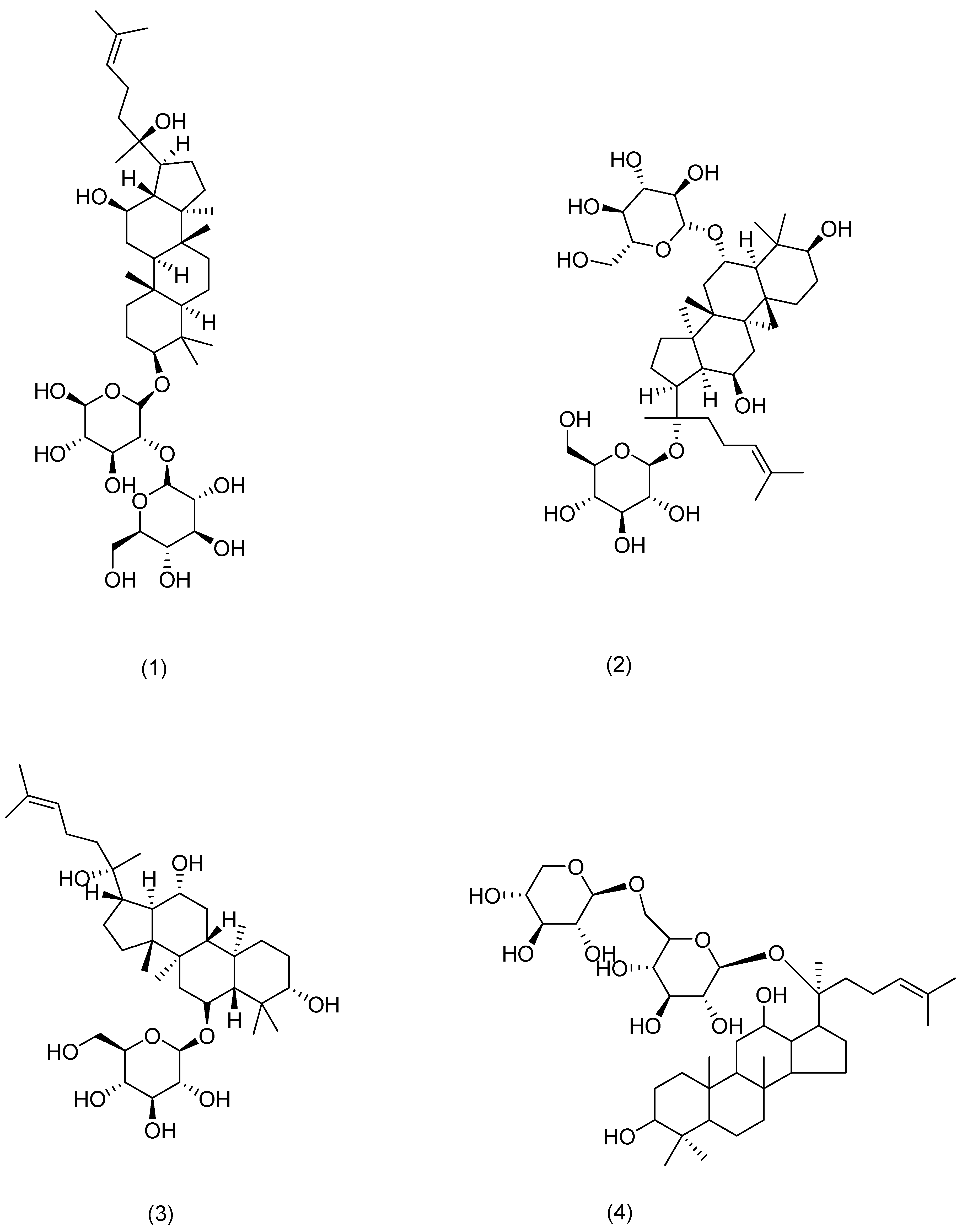
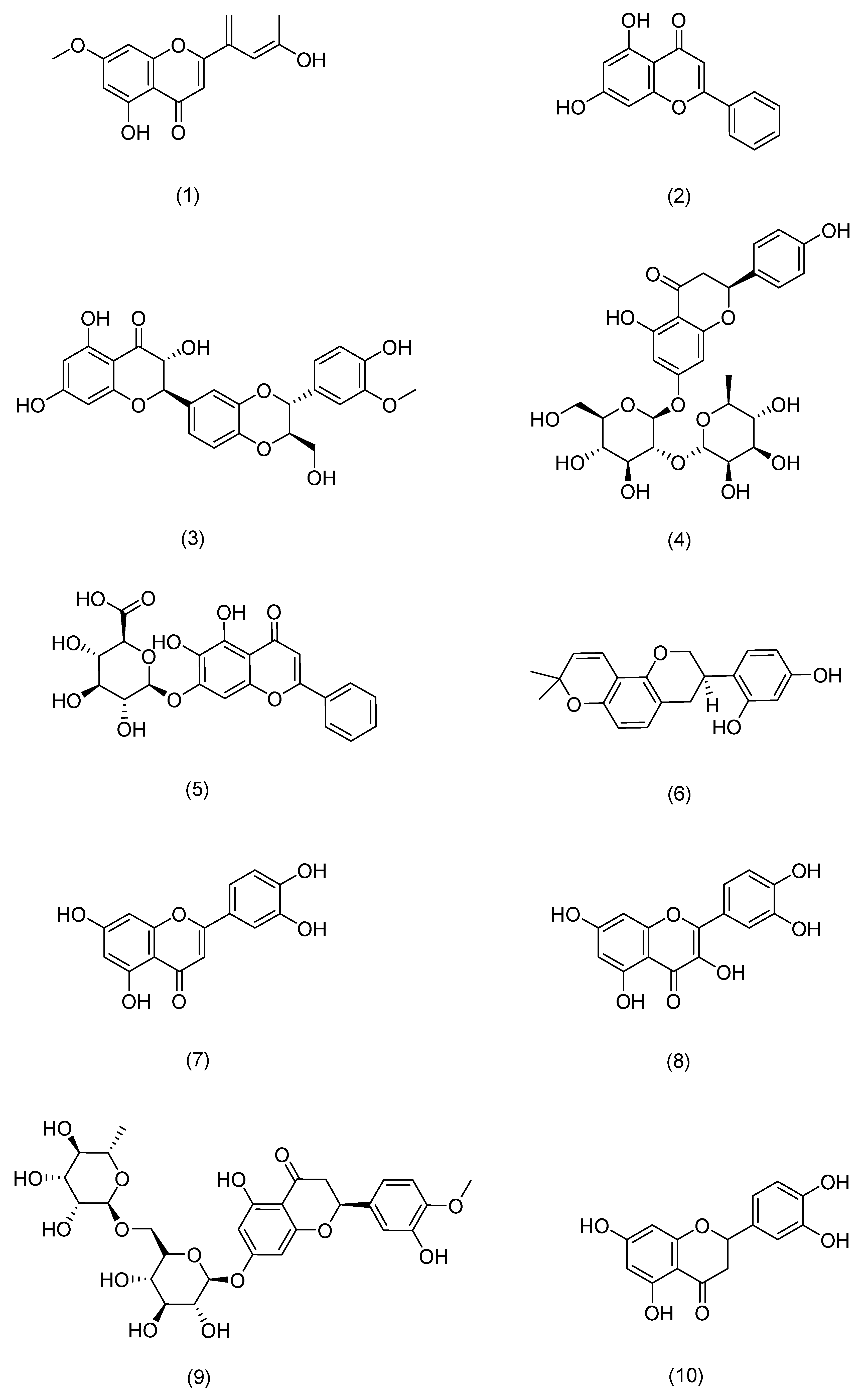

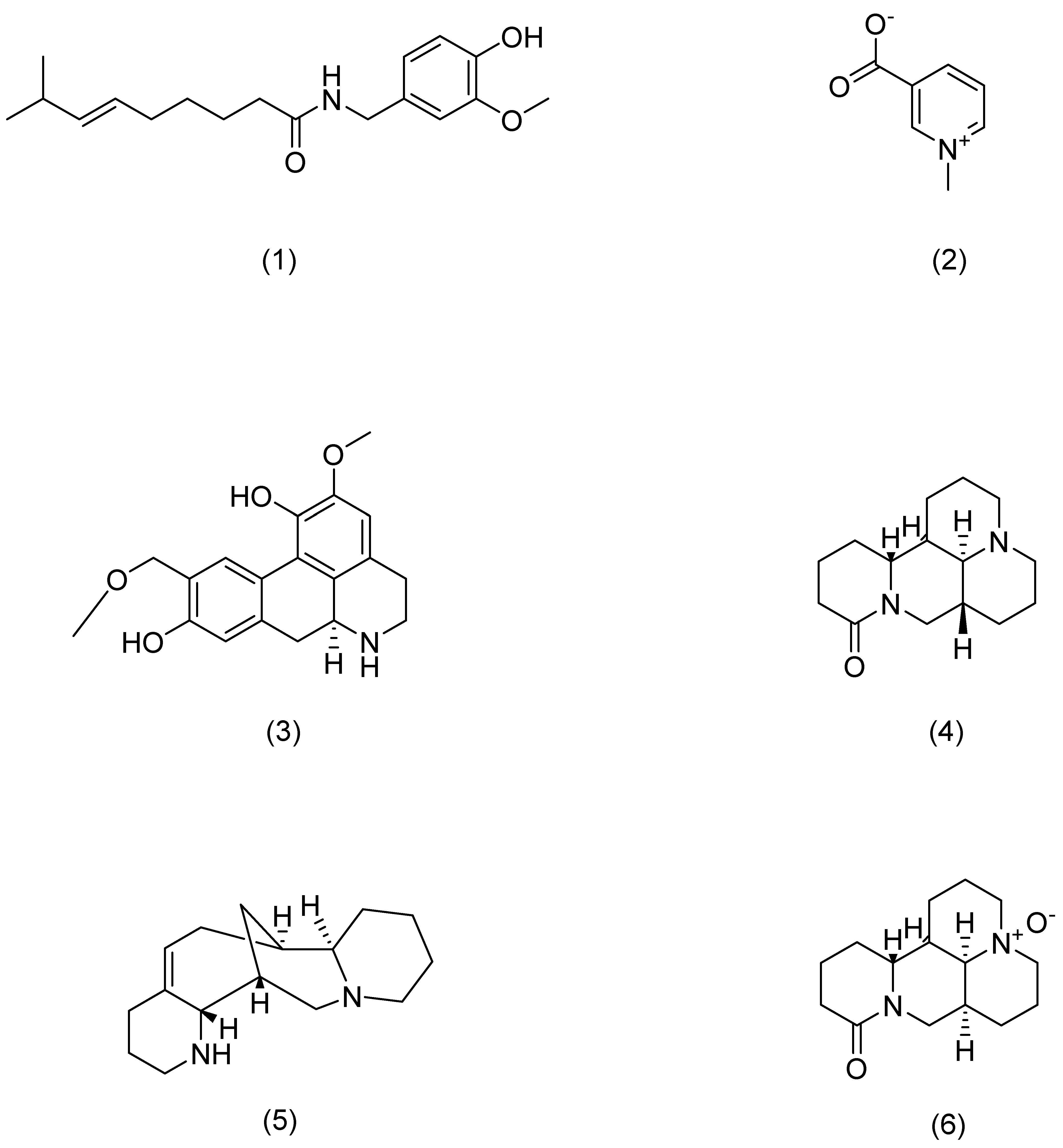

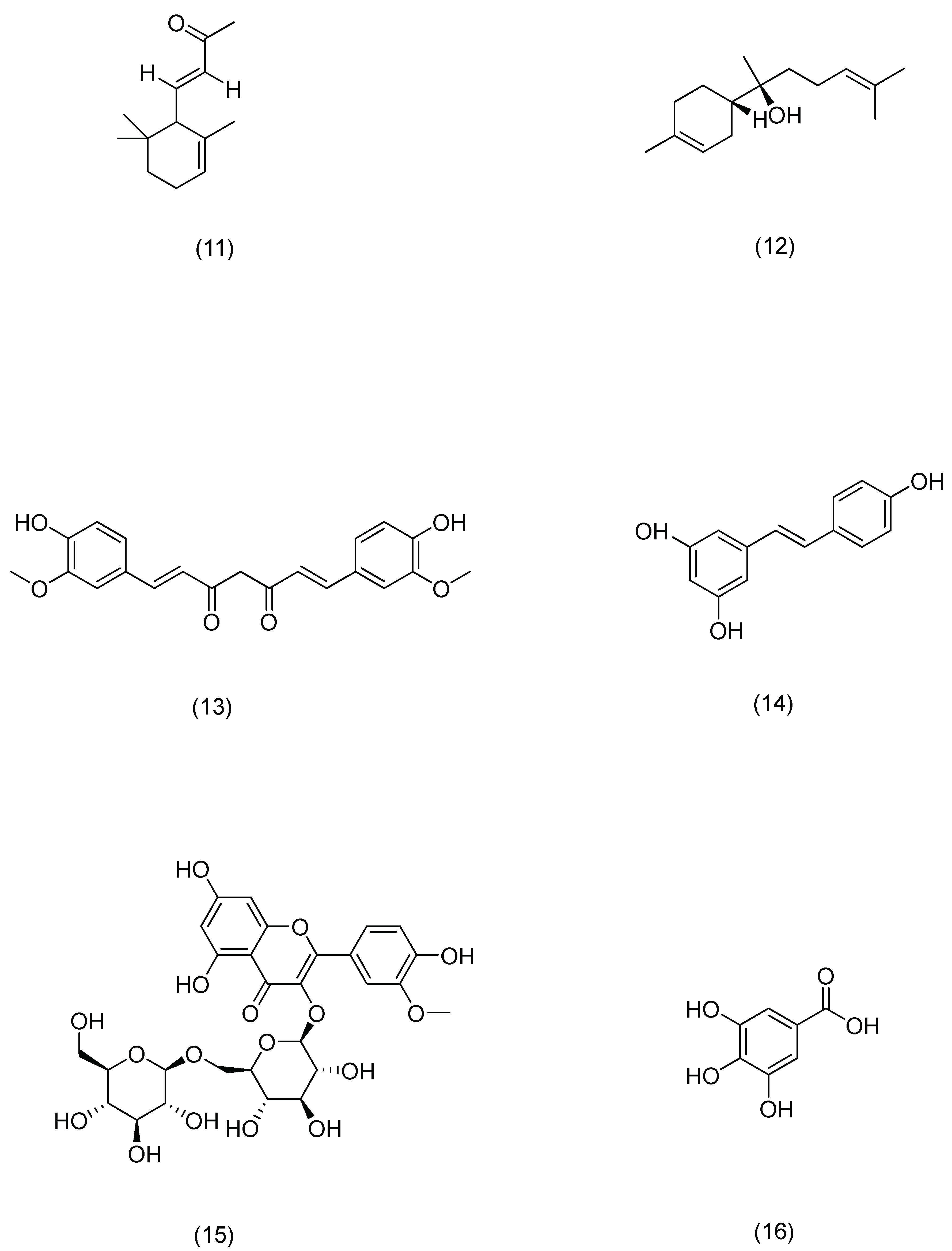
| Serial Number | Ginsenosides | Source | Latin Name | Structural Formula | Pathway | Pharmacological Effects | Mechanism | Literature |
|---|---|---|---|---|---|---|---|---|
| 1 | Ginsenoside Rg3 | Ginseng | Panax ginseng C. A. Mey. | Compound (1) in Figure 4 | MDM2/HIF1α | anti-inflammatory, antitumor, anti-photoaging [60,61] | Restoration of mitochondrial ATP and membrane potential inhibited the production and mRNA expression levels of TSLP and VEGF in activated HMC-1 cells. Rg3 downregulates MDM2 expression levels. | HanN R [53] |
| 2 | Ginsenoside Rg1 | Compound (2) in Figure 4 | NF-κB | anti-inflammatory [62] | Downregulation of NF-κB signaling pathway eliminates psoriasis-like dermatitis. | Shi Q and Park E K [62,63] | ||
| 3 | Ginsenoside Rh1 | Compound (3) in Figure 4 | NF-κB | anti-anaphylaxis, anti-inflammatory, antitumor [63] | Inhibition of the protein expression of iNOS and COX-7. | Park E K [63] | ||
| 4 | Ginsenoside C-Mx | Notoginseng | Panax notoginseng (Burkill) F. H. Chen ex C. H. Chow | Compound (4) in Figure 4 | TGF-β, Smad, AP-1 | anti-inflammatory, antioxidant, anti-photoaging [59] | Inhibition of intracellular ROS, MMP-1, and IL-6 expression, acceleration of the secretion of TGF-β and type I procollagen. | Liu X Y [59] |
| Serial Number | Flavonoids | Source | Latin Name | Structural Formula | Pathway | Pharmacological Effects | Mechanism | Literature |
|---|---|---|---|---|---|---|---|---|
| 1 | 7-O-methylluteolin | Hawthorn berry | Crataegus pinnatifida Bunge | Compound (1) in Figure 5 | Nrf2,HO-1 | anti-inflammatory, antioxidant [72] | Reduces serum immunoglobulin E (IgE) and interleukin-4 (IL-4) levels. | Kim T Y [72] |
| 2 | Chrysin | Passiflora caerulea | Passiflora caerulea L. | Compound (2) in Figure 5 | MAPK,JAK-STAT, NF-κB | anti-inflammatory, antioxidant [73] | Reduces TNF-α-, IL-17A-, and IL-22-induced release of CCL20 and antimicrobial peptides from epidermal keratinocytes. | Li H J [73] |
| 3 | silibinin | Milk thistle | Silybum marianum (L.) Gaertn. | Compound (3) in Figure 5 | NF-κB | antioxidant [74] | Inhibits intracellular ATP and GSH consumption, ROS production, and membrane lipid peroxidation. | Svobodová A [74] |
| 4 | Naringin | Tangerine | Citrus reticulata Blanco | Compound (4) in Figure 5 | MAPK,p38 | antioxidant, anti-inflammatory [75] | Inhibits ROS production, COX-2 overexpression, and strong inflammatory response. | Ren X [75] |
| 5 | Baicalin | Skullcap | Scutellaria baicalensis Georgi | Compound (5) in Figure 5 | TLR4 | antioxidant, anti-inflammatory, anti-photoaging [76] | Protective effects on UVA-induced oxidative damage and inflammation in mouse skin by up-regulating IL-12 and IL-23 cytokines. | Sherwani M A [76] |
| 6 | glabridin | Licorice | Glycyrrhiza uralensis Fisch. | Compound (6) in Figure 5 | MAPK, NF-κB | anti-inflammatory, anti-photoaging [77] | Inhibits the production of inflammatory cytokines such as TNF-α, IL-6, and IL-10. | Zhang C [77] |
| 7 | Luteolin | Callicarpa nudiflora | Callicarpa nudiflora Hook. & Arn. | Compound (7) in Figure 5 | NF-κB,JAK-STAT,TLR | antioxidant, anti-inflammatory, anti-photoaging [78] | Inhibits pro-inflammatory mediators IL-1β, IL-6, IL-8, IL-17, IL-22, TNF-α, and COX-2. | Gendrisch F [78] |
| 8 | Quercetin | Honeysuckle | Lonicera japonica Thunb. | Compound (8) in Figure 5 | PKCδ,JAK2 | antioxidant, against cancer, anti-inflammatory, antidiabetic [79] | Inhibition of UV-induced MMP-1 and COX-2 expression. | Shin E J [79] |
| 9 | Hesperidin | Tangerine | Citrus reticulata Blanco | Compound (9) in Figure 5 | MAPK | antioxidant, anti-inflammatory, immunomodulatory [80] | Reduces expression of MMP-9 and pro-inflammatory cytokines. | Lee H J [80] |
| 10 | eriodictyol | Lemon | Citrus × limon (L.) Osbeck | Compound (10) in Figure 5 | MAPK | anti-inflammatory, anti-photoaging [81] | Enhances cell proliferation, reduces intracellular ROS production, downregulates the expression of inflammatory factors and MMP-1, and upregulates the expression of Timp1 and Col1. | Nisar M F [81] |
| 11 | Myricetin 3-O-β-d-galactopyranoside | Lemons | Citrus × limon (L.) Osbeck | Compound (11) in Figure 5 | MAPK,AP-1,TGFβ/Smad | anti-inflammatory, anti-photoaging [82] | Downregulates the expression of MMP-1, but also reduces the protein levels of MMP-9 and MMP-3. | Oh J H [82] |
| 12 | Baicalein | Skullcap | Scutellaria baicalensis Georgi | Compound (12) in Figure 5 | TRPV1-Ca-ERK | antioxidant, anti-photoaging [83] | Inhibits MMP-1 expression. | Huang K F [83] |
| 13 | Naringenin | Tangerine | Citrus reticulata Blanco | Compound (13) in Figure 5 | ERK2 | anti-photoaging [84] | Downregulates AP-1 transactivation and MMP-1 expression. | Jung S K [84] |
| 14 | Cimifugin | Cimicifuga | Actaea cimicifuga L. | Compound (14) in Figure 5 | NF-κB (IκB, p65), MAPK (JNK, ERK, p38) | anti-inflammatory, antioxidant [69] | Inactivates the NF-κB/MAPK signaling pathway to prevent oxidative stress and inflammation in psoriasis-like pathogenesis. | Liu A [69] |
| 15 | galangin | Galangal | Alpinia officinarum Hance | Compound (15) in Figure 5 | NF-κB,Nrf2 | anti-inflammatory, against cancer [66] | Downregulates NF-κB and activates the Nrf2 signaling pathway to improve skin inflammation. | Sangaraju R [66] |
| 16 | Isoliquiritigenin | Licorice | Glycyrrhiza uralensis Fisch. | Compound (16) in Figure 5 | CD177, JAK2, STAT | anti-inflammatory [85] | Downregulates the expression of IL-4, IL-6, IgE, and TSLP. | Wu Q [85] |
| 17 | Chrysoeriol | Cardiospermum halicacabum | Cardiospermum halicacabum L. | Compound (17) in Figure 5 | NF-Κb, STAT3 | antioxidant, anti-inflammatory [65] | Reduces protein levels of iNOS, COX-2, IL-6, IL-1β, and TNF-α. | Wu J Y [65] |
| 18 | Eupatilin | Mugwort leaves | Artemisia argyi H. Lév. & Vaniot | Compound (18) in Figure 5 | P38MAPK, NF-κB | anti-inflammatory [68] | Inhibits the excessive proliferation of LPS-stimulated HaCaT cells and reduces the levels of TNF-α, IL-6, IL-23, and IL-17 in serum. | Bai D [68] |
| Serial Number | Alkaloids | Source | Latin Name | Structural Formula | Pathway | Pharmacological Effects | Mechanism | Literature |
|---|---|---|---|---|---|---|---|---|
| 1 | Capsaicin | Chili pepper | Capsicum annuum L. | Compound (1) in Figure 6 | TRPV1 | anti-inflammatory [88] | Blocks activation of IL-23/IL-17. | Chan T C [88] |
| 2 | Trigonelline | Fenugreek | Trigonella foenum-graecum L. | Compound (2) in Figure 6 | PERK | anti-inflammatory, antioxidant, anti-photoaging [93] | Attenuates oxidative stress-mediated ER-stress and restores Ca2+ homeostasis. | Lone A N [93] |
| 3 | Norisoboldine | Lindera aggregata | Lindera aggregata (Sims) Kosterm. | Compound (3) in Figure 6 | NFAT | anti-inflammatory [91] | Reduces mRNA levels of INF-γ, TNF-α, IL-4, and IL-6. | Gao S [91] |
| 4 | Aloperine | Sophora | Sophora alopecuroides L. | Compound (4) in Figure 6 | STAT3 | anti-inflammatory [92] | Inhibits Th17 differentiation and dendritic cell activation, and reduces the expression and secretion of pro-inflammatory cytokines. | Zhou H F [92] |
| 5 | Matrine | Sophora flavescens | Sophora flavescens Aiton | Compound (5) in Figure 6 | Hsp6,NF-κB | anti-inflammatory [89] | Inhibits inflammatory cytokine secretion. | Huang P [89] |
| 6 | Oxymatrine | Sophora flavescens | Sophora flavescens Aiton | Compound (6) in Figure 6 | IFN-γ | anti-inflammatory [87] | Activates p1, JNK, and Akt and downregulates MDC, ICAM-1, and SOCS38 to repair skin barrier. | Gao C J [87] |
| Serial Number | Carbohydrates | Source | Latin Name | Pathway | Pharmacological Effects | Mechanism | Literature |
|---|---|---|---|---|---|---|---|
| 1 | Lycium barbarum polysaccharide | Lycium chinense | Lycium chinense Mill. | Nrf2/ARE, p38 MAPK | antioxidant, anti-inflammatory, anti-photoaging [97] | Scavenging ROS and reducing DNA damage, inhibiting caspase-3 activation and MMP-9 expression. | Li H [97] |
| 2 | Aloe polysaccharide | Aloe vera | Aloe vera (L.) Burm. f. | Keap1/Nrf2/ARE | antioxidant, anti-photoaging [99] | Improving cell antioxidant capacity to improve cell viability and proliferation to protect cells | Yuan L [99] |
| Serial Number | Compound | Source | Latin Name | Structural Formula | Pathway | Pharmacological Effects | Mechanism | Literature |
|---|---|---|---|---|---|---|---|---|
| 1 | Rosmarinic acid | Rosemary | Rosmarinus officinalis L. | Compound (1) in Figure 7 | PLC-γ1,ITK | anti-inflammatory, antioxidant, antibacterial [109] | Activates CD4(+) T cells and significantly inhibits IFN-γ and IL-4 production. | Jang A H [109] |
| 2 | Salicylic acid | Red clover | Trifolium pratense L. | Compound (2) in Figure 7 | SREBP-1,NF-κB | anti-inflammatory [110] | Reduces lipogenesis in sebocytes and suppresses inflammation in cells. | Lu J [110] |
| 3 | Asiatic acid | Centella | Centella asiatica (L.) Urb. | Compound (3) in Figure 7 | NF-κB,MAPK | anti-inflammatory, immunomodulatory [111] | Downregulates the mRNA expression levels of AD-related cytokines. | Moon G H [111] |
| 4 | Syringaresinol | Tricyrtis pilosa | Tricyrtis pilosa Wall. | Compound (4) in Figure 7 | MAPK,AP-1 | anti-inflammatory, anti-photoaging [112] | Inhibits MMP-1 upregulation. | Oh J H [112] |
| 5 | Trans-cinnamic acid | Cinnamon | Cinnamomum cassia (L.) D. Don | Compound (5) in Figure 7 | AP-1,Nrf2 | antioxidant, anti-photoaging [113] | Inhibits MMP-1/-3 activation. | Hseu Y C [113] |
| 6 | Paeonol | Peony | Paeonia × suffruticosa Andrews | Compound (6) in Figure 7 | DLD,Nrf2,ARE,MAPK,AP-1 | anti-photoaging [114] | Inhibits the phosphorylation of mitogen-activated protein kinase and activator protein 1, resulting in the degradation of type I procollagen. | Sun Z [114] |
| 7 | Osthole | Cnidium monnieri | Cnidium monnieri (L.) Spreng. | Compound (7) in Figure 7 | PI3K,Akt | anti-inflammatory, anti-viral, anti-anaphylaxis [115] | Controls the expression of tight-junction proteins in the skin, and can improve skin barrier damage. | Chen J R [115] |
| 8 | Pterostilbene | Pterocarpus indicus | Pterocarpus indicus Willd. | Compound (8) in Figure 7 | Nrf2,ARE | antioxidant, anti-inflammatory, anti-cancer [116] | Induces the expression of antioxidant enzymes, thereby preventing UVB-induced oxidative stress. | Li H [116] |
| 9 | Cryptotanshinone | Salvia | Salvia miltiorrhiza Bunge | Compound (9) in Figure 7 | AMPK,SIRT1,PGC-1α | anti-inflammatory, antioxidant, anti-tumor [117] | Inhibits ROS production and reduces DNA damage, reduces mitochondrial dysfunction, and promotes mitochondrial biogenesis. | Guo K [117] |
| 10 | Glycyrrhizinate | Licorice | Glycyrrhiza uralensis Fisch. | Compound (10) in Figure 7 | MAPK,NF-κB | anti-photoaging, antioxidant [118] | Prevents epidermal hyperplasia, lymphocyte infiltration, and the expression of several inflammatory proteins: p38, JNK, COX-2, NF-κB, and ICAM-1. | Farrukh M R [118] |
| 11 | α-ionone | Raspberry | Rubus idaeus L. | Compound (11) in Figure 7 | cAMP | anti-photoaging [119] | Improves cell proliferation and migration, as well as HA and HBD-2 production in HaCaT cells. | Yang D [119] |
| 12 | (-)-α-bisabolol | Chamomile | Matricaria chamomilla L. | Compound (12) in Figure 7 | MAPK,NF-κB | anti-inflammatory [120] | Reduces levels of beta-hexosaminidase, histamine, and TNF-alpha. | Li G [120] |
| 13 | Curcumin | Turmeric | Curcuma longa L. | Compound (13) in Figure 7 | TGF-β, Smad2/3,Smad7 | anti-photoaging, antioxidant [121] | Restores the activity of antioxidant enzymes and attenuates ER stress, inflammation, and apoptosis signals. | Liu X [121] |
| 14 | Resveratrol | Knotweed | Reynoutria japonica Houtt. | Compound (14) in Figure 7 | Nrf2 | anti-photoaging, antioxidant [122] | Degrades Keap1 protein and promotes Nrf2 accumulation in the nucleus. | Liu Y [122] |
| 15 | Astragaloside | Astragalus | Astragalus membranaceus (Fisch.) Bunge | Compound (15) in Figure 7 | TLR4,NF-κB | anti-photoaging, antioxidant [123] | Inhibits the production of pro-inflammatory cytokines and the expression of TLR4 and its downstream signaling molecules, NF-κB, iNOS, and COX-2 proteins. | Wang J [123] |
| 16 | Gallic acid | Cornus officinalis | Cornus officinalis Siebold & Zucc. | Compound (16) in Figure 7 | Nrf2 | anti-photoaging [101] | Decreases the mRNA and protein expression of keratin 16 and keratin 17, which are the markers of psoriasis. | Zhang J [101] |
| Serial Number | Compound | Source | Pharmacological Effects | Application |
|---|---|---|---|---|
| 1 | Luteolin | Licorice | Anti-tumor, anti-inflammatory, anti-viral, immunomodulatory, hepatoprotective, memory enhancement, and neuroprotective effects [131] | Psoriasis [132] |
| 2 | Hypericin | Hypericum perforatum | Anti-inflammatory, antidepressant, antibacterial [133] | Psoriasis [134] |
| 3 | Baicalin | Skullcap | Liver protection, anti-tumor, antibacterial, antiviral, antioxidant effects [135] | Psoriasis [136] |
| 4 | Indigo | Indigo | Antioxidant, anti-inflammatory [137] | Psoriasis [138] |
| 5 | Paeoniflorin | Wood Dan | Hepatoprotective, choleretic, anti-inflammatory, antioxidant, neuroprotective, anti-diabetic, anti-apoptotic, and anti-tumor [139] | Psoriasis [140] |
| 6 | Quercetin | Houttuynia cordata | Anti-inflammatory, antibacterial, antiviral, antioxidant, and antitumor [141] | Psoriasis [142], atopic dermatitis [143] |
| 7 | Galangin | Plantago | Anti-ulcer, anti-cancer, immune modulation, anti-infection, anti-inflammatory, and antioxidant [144] | Psoriasis [66] |
| 8 | Cimicithin | Cimicifuga | Anti-inflammatory [145] | Psoriasis [69] |
| 9 | Ginsenoside Rg1 | Ginseng | Anti-inflammation, anti-fatigue, and immune regulation [146] | Psoriasis [62] |
| 10 | Kaempferol | Cassia | Antibacterial, anti-inflammatory, antioxidant, antimalarial, and antimutagenic activity [147] | Acne [148] |
| 11 | Naringenin | Fenugreek | Anti-cholesterol, anti-tumor, and anti-inflammatory [149] | Atopic dermatitis [150] |
| 12 | Rosmarinic acid | Rosemary | Antibacterial, anti-inflammatory, antioxidant, anti-apoptotic, anti-tumorigenic, anti-nociceptive, and neuroprotective properties [151] | Atopic dermatitis [109] |
| 13 | Epigallocatechin gallate | Green tea | Antioxidant, anticancer, hypoglycemic, antibacterial, antiviral, and neuroprotective [152] | Acne [153] |
| 14 | Capsaicin | Chili | Anti-inflammatory, analgesic, anticonvulsant,a and neuroprotective effects [154] | Psoriasis [88] |
| 15 | Salicylic acid | Red clover | Antioxidant, anticancer, and blood sugar regulation [155] | Acne [110] |
| 16 | Isoliquiritigenin | Licorice | Anti-inflammatory, anti-ulcer, antibacterial, and anti-cancer [156] | Atopic dermatitis [85] |
| 17 | Licorice acid | Licorice | Antioxidant, anticancer, and diuretic [157] | Psoriasis [70] |
| 18 | Asiatic acid | Centella | Sedative, analgesic, antidepressant, antibacterial, antiviral, and immunomodulatory effects [158] | Atopic dermatitis [111] |
| 19 | Chioku Shiaki | Citrus aurantium | Antioxidant [159] | Atopic dermatitis [160] |
| 20 | Isozoranthin | Mugwort leaves | Antibacterial, antiviral, hemostatic, antitumor, hepatoprotective, analgesic, anti-inflammatory, and antioxidant [161] | Psoriasis [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Guo, J.; Tang, P.; Yan, S.; Wang, X.; Li, H.; Xie, J.; Deng, J.; Hou, X.; Du, Z.; et al. Insights from Traditional Chinese Medicine for Restoring Skin Barrier Functions. Pharmaceuticals 2024, 17, 1176. https://doi.org/10.3390/ph17091176
Yang J, Guo J, Tang P, Yan S, Wang X, Li H, Xie J, Deng J, Hou X, Du Z, et al. Insights from Traditional Chinese Medicine for Restoring Skin Barrier Functions. Pharmaceuticals. 2024; 17(9):1176. https://doi.org/10.3390/ph17091176
Chicago/Turabian StyleYang, Jieyi, Jiageng Guo, Peiling Tang, Shidu Yan, Xiaodong Wang, Huaying Li, Jinling Xie, Jiagang Deng, Xiaotao Hou, Zhengcai Du, and et al. 2024. "Insights from Traditional Chinese Medicine for Restoring Skin Barrier Functions" Pharmaceuticals 17, no. 9: 1176. https://doi.org/10.3390/ph17091176
APA StyleYang, J., Guo, J., Tang, P., Yan, S., Wang, X., Li, H., Xie, J., Deng, J., Hou, X., Du, Z., & Hao, E. (2024). Insights from Traditional Chinese Medicine for Restoring Skin Barrier Functions. Pharmaceuticals, 17(9), 1176. https://doi.org/10.3390/ph17091176






