Abstract
Rhizophora mangle is commonly used in traditional medicine to treat infections, reduce inflammation, and promote healing. This study aimed to analyze the phytochemical profile of the methanolic extract of R. mangle leaves (MELRm) and evaluate its in vitro schistosomicidal activity against Schistosoma mansoni as well as its cytotoxicity. Plant material was collected in Itamaracá City, Pernambuco, Brazil. The extract was analyzed using UV/Vis spectrophotometry and high-performance liquid chromatography (HPLC). The motility, mortality, and cell viability of adult worms were assessed in a schistosomicidal assay, while cytotoxicity was evaluated through a colorimetric assay with MTT on RAW 264.7 cells. The primary compounds identified in MELRm were phenolic compounds. In the schistosomicidal assay, all concentrations of MELRs induced changes in the motility of adult worms. At a concentration of 400 μg/mL, MELRs resulted in 56.25% mortality after 72 h of incubation. After 120 h, mortality rates of 75%, 62.5%, and 50% were observed at MELRm concentrations of 400, 200, and 100 μg/mL, respectively. No eggs were detected at any MELRm concentration. MELRs did not show cytotoxicity towards RAW 264.7 cells at the concentrations tested. These results indicate that MELRs demonstrate schistosomicidal activity in vitro, suggesting they are promising candidates for in vivo studies.
1. Introduction
Schistosomiasis is a neglected parasitic disease caused by parasites of the genus Schistosoma spp., which has significant social and economic impacts [1]. The infection is prevalent among populations with low visibility and little political voice in areas with poor or non-existent basic sanitation, as well as limited access to health services and education [1,2,3,4]. Epidemiologically, around 779 million people live in areas at risk of infection, and 251.4 million are infected each year. Of those infected, 20 million develop the chronic phase of the disease, and 280,000 die annually [1,2].
For the past 50 years, praziquantel (PZQ) has been the only drug recommended by the World Health Organization (WHO) for the treatment of all types of human schistosomiasis. This measure has effectively reduced the prevalence and incidence of schistosomiasis worldwide, as there is currently no effective vaccine [3,4]. PZQ is administered orally, has low toxicity and acts only against adult worms. It is effective in treating various clinical forms of the infection [1,3,4]. These advantages, in part, contribute to the limited research efforts in the search for new schistosomal drug candidates. In endemic areas, there is commonly a shortage or even an absence of effective public health and sanitation policies for the prevention and treatment of schistosomiasis [4]. Furthermore, the number of available doses of PZQ is insufficient to meet the demand of those infected. According to the WHO, only 20% of those infected receive treatment [1].
The extensive use of PZQ in preventive and mass chemotherapy in endemic areas, the reliance on a single drug to treat this expanding disease, and reports of resistance and/or tolerance in strains of Schistosoma spp. to PZQ are concerns for the medical and scientific communities. These issues underscore the need for research into new pharmacological alternatives for schistosomiasis, including those derived from plants [3,5,6]. Mangrove ecosystems are attractive for prospecting new bioactive metabolites with biological and pharmacological properties and potential medicinal applications due to their biodiversity, and this is evidenced by the increasing number of publications in the literature [7,8,9,10].
Plants of the genus Rhizophora (family Rhizophoraceae) are widely distributed across tropical and subtropical regions where schistosomiasis is endemic, particularly in the mangrove areas of the Fiji, Tonga, and New Caledonia islands, as well as on the coasts of West Africa and Central and South America [11]. In South America, Brazil has the highest incidence and prevalence of schistosomiasis, with approximately 2 million people infected by S. mansoni [12]. The secondary metabolites of plants in the genus Rhizophora play a role in protection against predators and in adaptive mechanisms to their environment. Within this genus, R. mangle is a tree species dominant in Brazilian mangroves, with a latitudinal distribution extending approximately 3700 km along the Brazilian coast [13].
The leaves, stems, roots, and fruits of R. mangle have various uses in traditional medicine and exhibit activity against a range of conditions. They have shown efficacy in treating gastric ulcers [14], fatty liver disease and insulin resistance [15], and they have anti-inflammatory and analgesic effects [16]. Additionally, they possess properties for wound healing [17], antioxidant activity [14,18,19], antidiabetic effects [20], and fungicidal and bactericidal actions [19,21,22]. Extracts of R. mangle leaves have also demonstrated embryotoxic and embryostatic effects on Aedes aegypti eggs and larvae [23], as well as physiological and molluscicidal effects on Biomphalaria glabrata (the intermediate host of S. mansoni) [24].
The present study aimed to analyze the phytochemical profile of the methanolic extract of R. mangle leaves (MELRm) and evaluate its in vitro schistosomicidal activity against adult S. mansoni worm couples. This was evaluated through measurements of the mortality, motility, and cell viability of the worms, as well as an evaluation of the extract’s cytotoxicity.
2. Results
2.1. Chemical Composition of MELRm Extract
Phytochemical analysis of MELRm by UV/Vis spectrophotometry revealed its phenolic composition: flavonoids (215.5 mg QE/g of extract), flavonols (32.0 mg QE/g of extract), tannins (100.3 mg TAE/g of extract), and total phenolic content (423.5 mg GAE/g of extract). In our study, the extract yield was 9.12%.
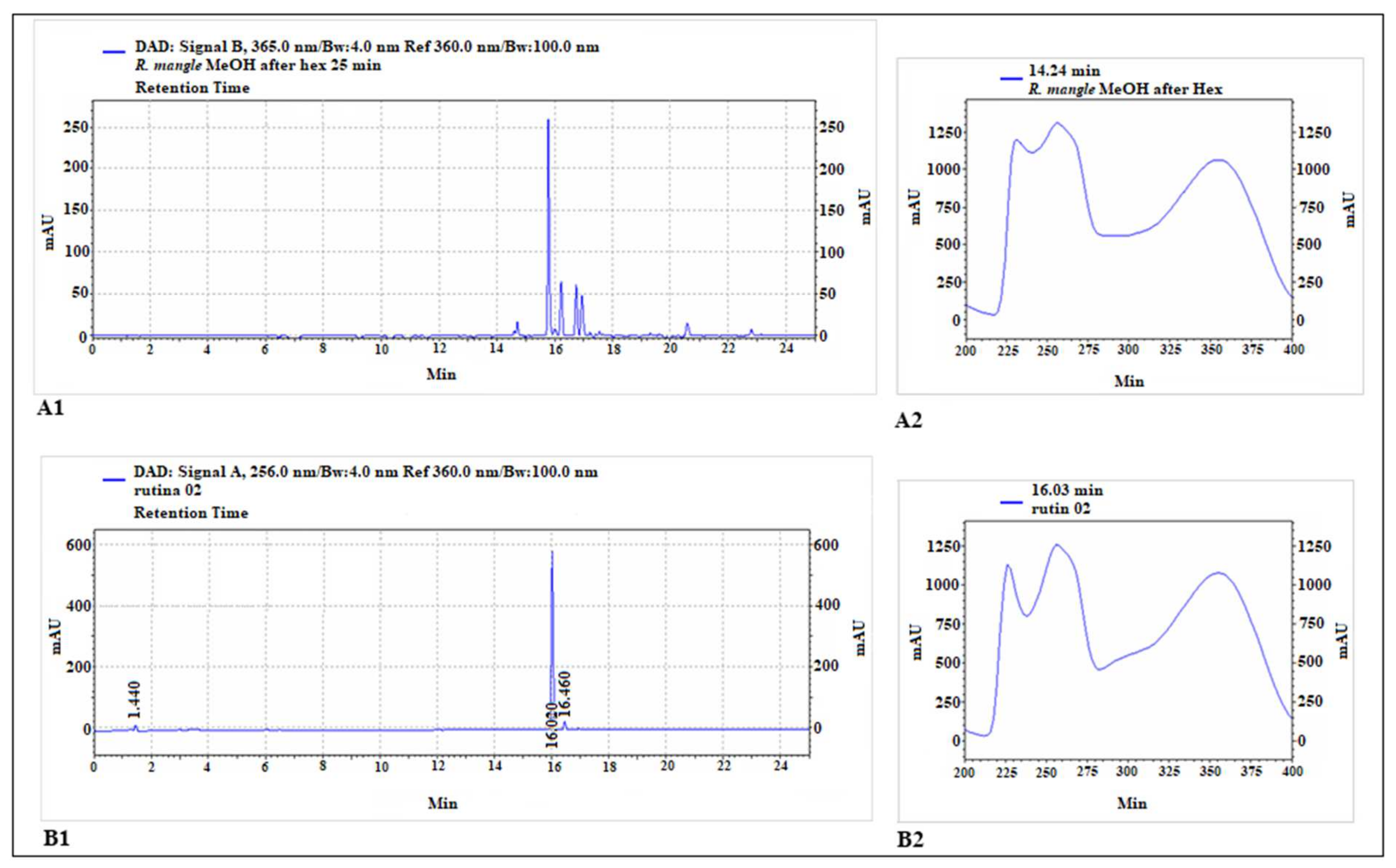
The HPLC chromatogram is shown in Figure 1. The constituents identified in MELRm were p-coumaric acid (14.7 min), rutin (15.8 min), ellagic acid (16.2 min), quercetin (16.6 min), apigenin (17.1 min), and geranium (20.6 min), respectively (Table 1). These results highlight that MELRm is predominantly phenolic and indicate the presence of a rutin-like chromophore.
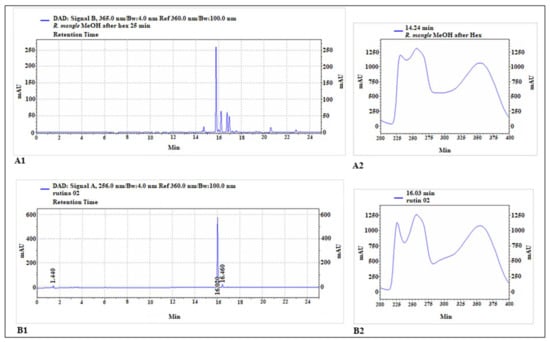
Figure 1.
Chromatographic analysis of methanolic extract of R. mangle and rutin standard. The main peak of the extract chromatogram (A1) exhibited a retention time close to that of rutin (B1). The chromophore of this main peak (A2) also showed great similarity to that of the rutin standard (B2).

Table 1.
MELRm extract constituents.
2.2. MELRm Induced Mortality, Altered Motility, and Caused Detachment of Adult S. mansoni Worms
Table 2 presents the motility scores of S. mansoni at observation intervals of 24, 48, 72, 96, and 120 h following exposure to MELRm. Throughout the observation period (24–120 h), pairs of adult worms incubated in supplemented RPMI 1640 medium (Control 1) or in RPMI 1640 medium with 1% DMSO (Control 2) exhibited normal movement throughout the body, peristalsis of internal organs, and suckers adhered to the bottom or sides of the culture plate, and they maintained the color and integrity of the integument (score 3), indicative of typical in vitro motility. After 24 h of incubation, PZQ (10 µM) resulted in 100% mortality. In this group, no peristaltic or bodily movements were observed (score 0), and mortality occurred while the worms remained paired. The results for the negative and positive control groups are consistent with those reported [25].

Table 2.
Motility scores of control adult worms treated with praziquantel (PZQ) or methanolic extract of Rhizophora mangle (MELRm).
MELRm altered motility and caused the separation of worm pairs in the early stages of the experiment. After just 24 h of incubation, the 400 µg/mL dose led to an 81.25% reduction in motor activity, with worms showing movement only in the extremities (anterior and/or posterior regions), the absence of peristalsis, non-adhering suckers (score 1), and the complete separation of worm couples. For this dose, mortality rates of 56.25% and 75% were observed at 72 and 120 h, respectively. At lower doses, in a dose-dependent manner, worms exhibited reduced peristaltic movement along the body, with occasional sucker adhesion (score 2) and the separation of worm couples after 48 h. At 200 and 100 µg/mL, after 72 h of incubation, all worms were uncoupled and showed score 1. Mortality rates at these doses were 62.5% and 50% within 120 h, respectively. At 50 µg/mL, worms did not mate, and changes in motility were observed after 48 h, with half of the worms showing score 1. By the end of the experiment (120 h), 50% of these worms were dead (score 0). At 25 µg/mL, worms maintained score 3 until 72 h of incubation, appearing more agitated than the control groups (Negative Control 1 and 2), with couples beginning to uncouple. By 120 h, at this dose, 100% of the worms had progressed to score 2, and all pairs were fully separated (both males and females).
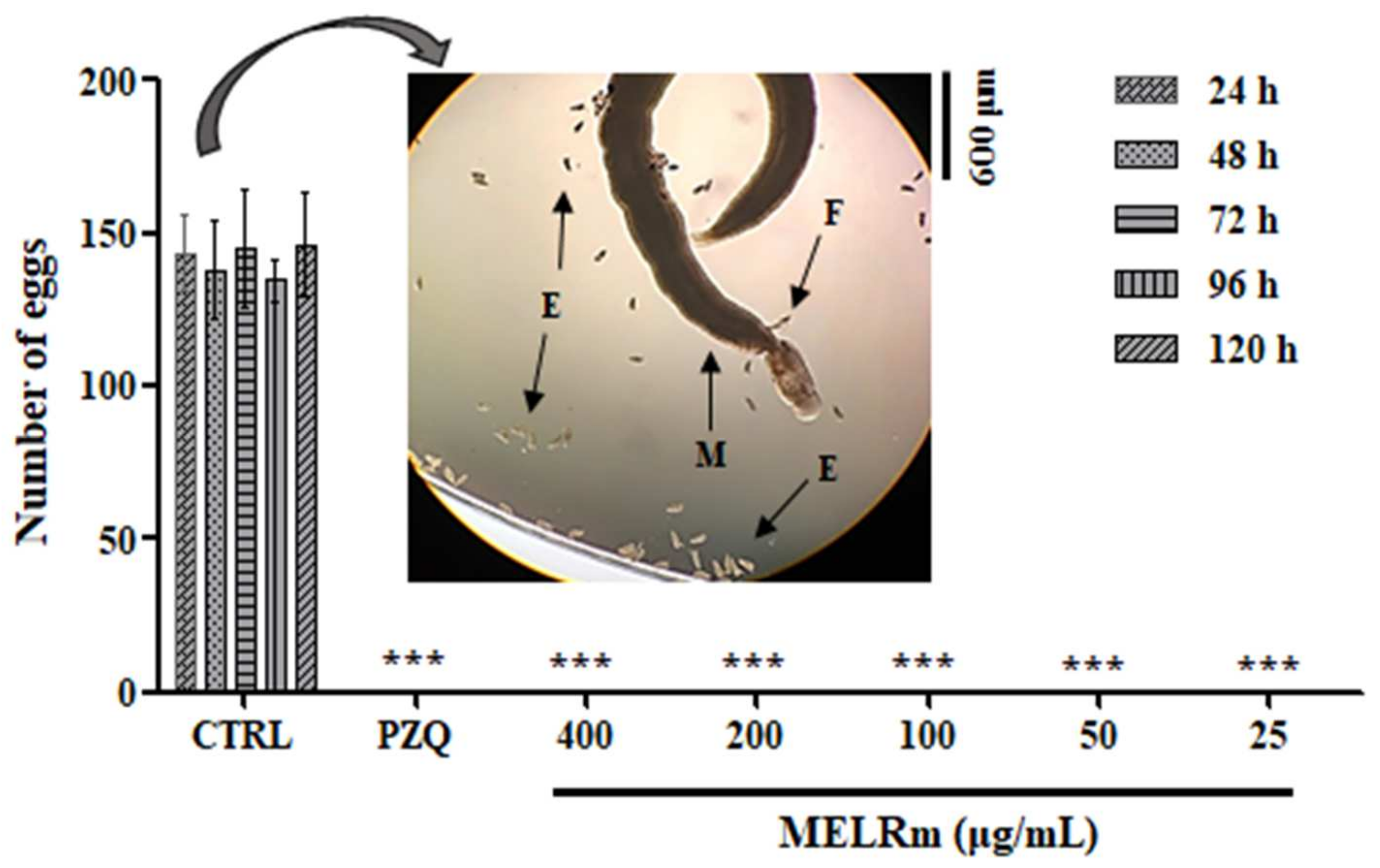
MELRm effectively inhibited S. mansoni oviposition in vitro. Throughout all observation intervals (24, 48, 72, 96, and 120 h) and at all concentrations (400–25 µg/mL), no S. mansoni eggs were observed in the culture plates, similar to the results seen with worms exposed to PZQ (Figure 2). In contrast, the worms in the negative control groups (Control 1 and Control 2) produced an average of 140.4 ± 7.33 eggs, with no statistically significant difference between the controls, indicating continued oviposition.
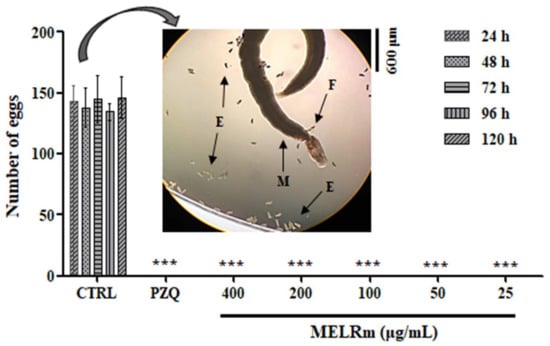
Figure 2.
In vitro effect of MELRm on egg production by S. mansoni couples. Pairs of parasites (n = 16 worms, male (M), female (F)) were incubated with MELRm at concentrations ranging from 400 to 25 µg/mL for 120 h. Egg (E) counts were assessed using an inverted microscope. Data are expressed as mean ± standard deviation. CTRL = Control 2, RPMI supplemented with 1% dimethyl sulfoxide (DMSO). Statistical significance was denoted as *** p < 0.0001 compared to the control.
2.3. MELRm Induced Cell Death in Adult S. mansoni Worms but Showed No Cytotoxicity towards RAW 264.7 Cells
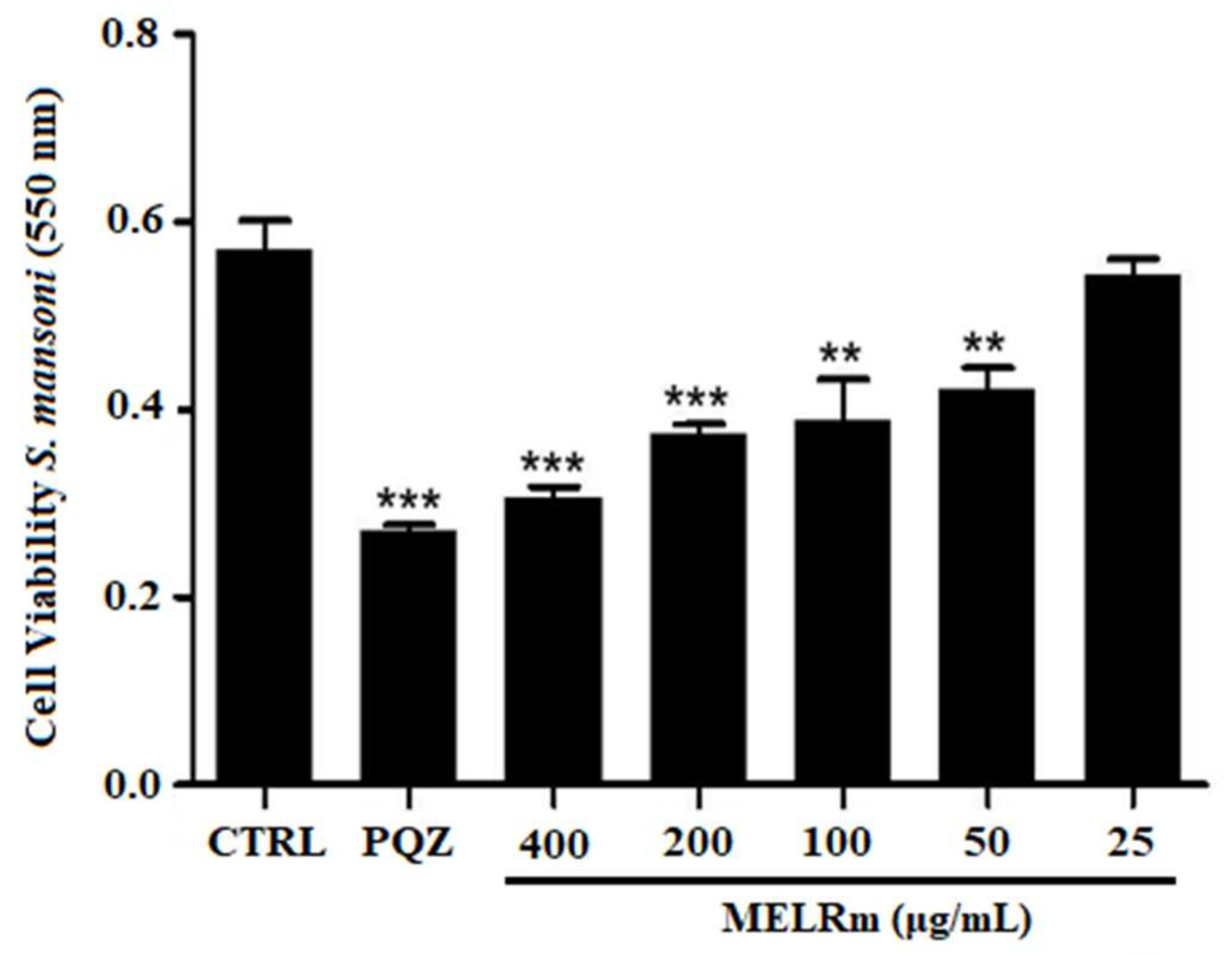
MELRm significantly reduced mitochondrial viability and, consequently, cell viability in S. mansoni couples, as evidenced by a decrease in formazan crystal formation (Figure 3). MELRm significantly reduced worm cell viability by 31.74% and 26.10% at concentrations of 100 and 50 µg/mL, respectively (p < 0.01), compared to the negative control groups. Additionally, concentrations of 200 and 400 µg/mL decreased worm cell viability by 34.55% and 46.45%, respectively (p < 0.001). The effects of 200 and 400 µg/mL of MELRm were not significantly different from those of PZQ (p > 0.5). DMSO did not affect the cell viability of S. mansoni couples, as formazan crystal formation was similar between Control 1 and Control 2.
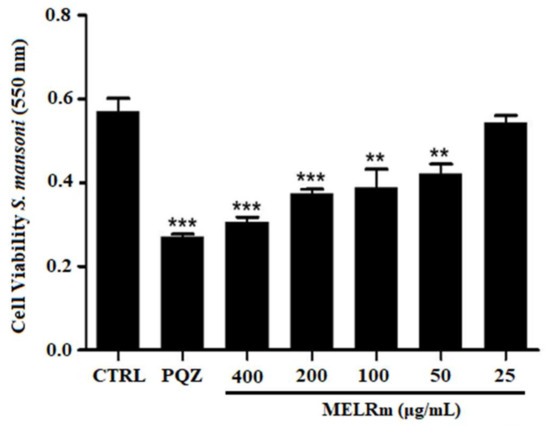
Figure 3.
In vitro effects of the methanolic extract of R. mangle leaves on the cell viability of S. mansoni adult worm couples. Worms in the negative control group (Control 2) were incubated in RPMI 1640 medium with 1.5% DMSO. Positive control worms were treated with praziquantel (PZQ, 10 µM). Cell viability was expressed as the mean ± standard deviation (SD) of absorbance values from four experiments. Statistical significance was denoted as ** p < 0.001 and *** p < 0.0001 compared to the control.
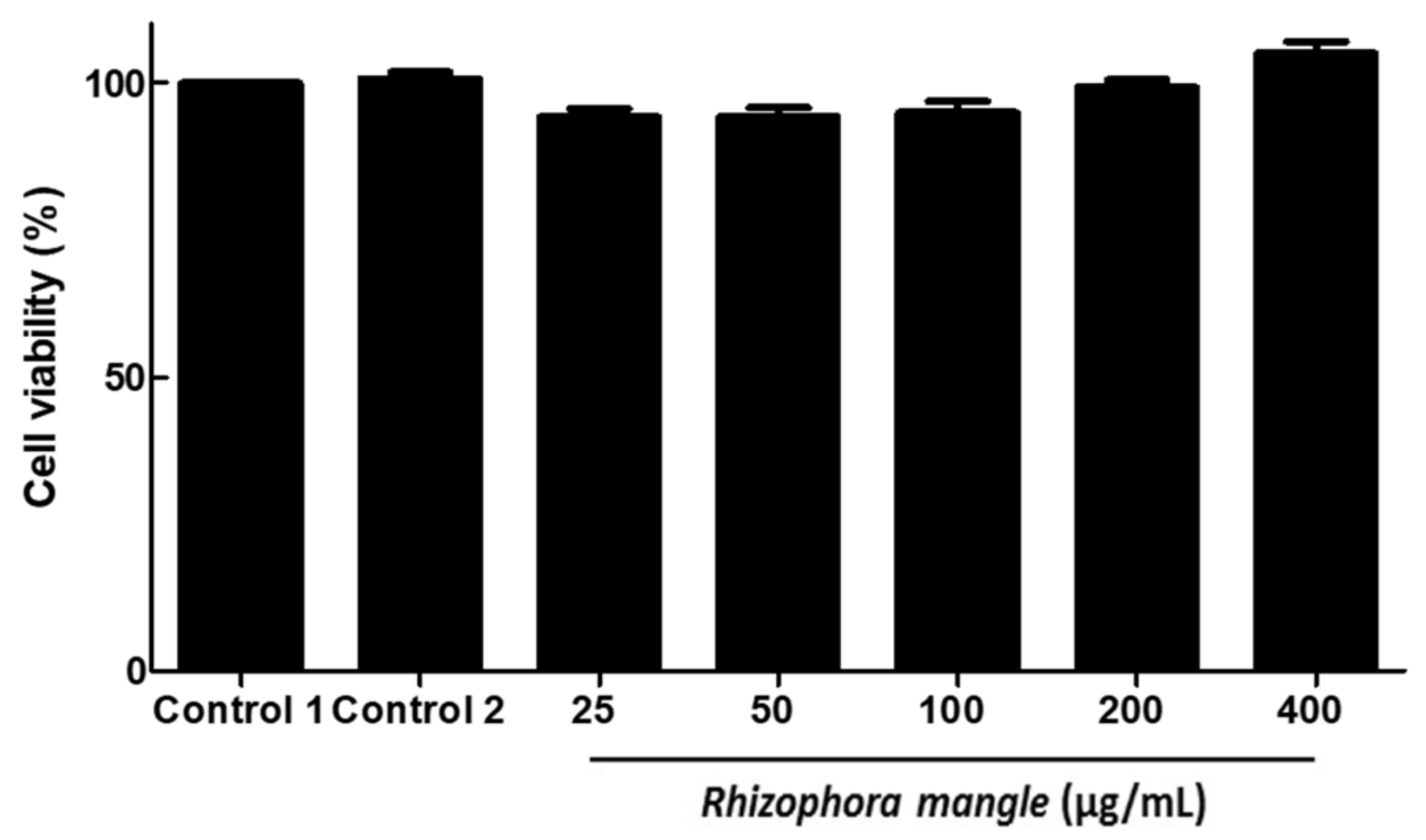
At the concentrations used in the schistosomicidal evaluation, MELRm did not exhibit cytotoxicity toward RAW cells, as demonstrated in Figure 4.
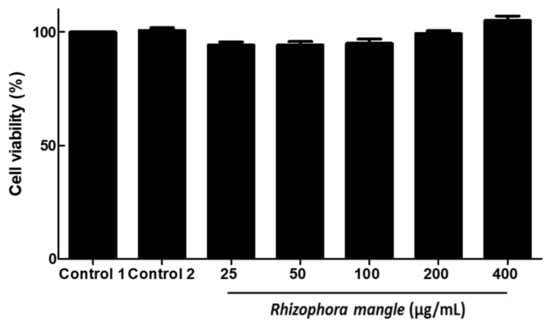
Figure 4.
In vitro effects of methanolic extract of R. mangle leaves on RAW cell viability.
3. Discussion
The emergence of resistance and/or tolerance of Schistosoma spp. strains to praziquantel (PZQ), coupled with the rising cost of PZQ and the lack of strategic initiatives for pharmacological interventions against schistosomiasis, has contributed to the inadequacy of current control programs [3]. This underscores the urgent need to develop cost-effective and therapeutic alternatives. In this context, plant-derived products have recently gained prominence as promising sources for new schistosomicidal agents [25,26]. Most studies focusing on screening natural compounds with biological and pharmacological properties have concentrated on terrestrial plants. However, research into marine plants, particularly mangroves, is sparse, despite Brazil’s extensive and underexplored mangrove resources. The authors of [27] emphasize the importance of investigating endemic plant species in regions where schistosomiasis is prevalent. To the best of our knowledge, this study is the first to present UV/Vis spectrophotometry and HPLC data for the methanolic extract of Rhizophora mangle (MELRm) collected in Northeast Brazil, along with its in vitro schistosomicidal activity.
Consistent with our findings, secondary metabolites such as terpenoids, tannins, polyphenols, steroids, alkaloids, flavonoids, and saponins have been identified as the primary classes of phytochemicals isolated from mangroves, and they have been characterized for their toxicological and pharmacological properties [8,28,29,30,31]. Our results align with studies reporting phenolic compounds as major constituents in Rhizophora mangle leaves collected from Twin Cays, Belize [32], and from Rio de Janeiro, Brazil [33]. An aqueous extract of R. mangle bark from Cuban mangroves also contained polyphenols, predominantly tannins, and highlighted the presence of ellagic acid and quercetin. However, unlike our study, additional compounds, such as epicatechin, catechin, chlorogenic acid, and gallic acid, were also identified in this extract [34]. Additionally, it was reported that ethanol, methanol, hexane, butanol, and chloroform extracts of R. apiculata leaves exhibited antimicrobial, antioxidant, and anticancer activities, attributed to the presence of phenols and flavonoids [10].
Mangroves are biochemically unique ecosystems with high productivity and diversity, offering a wealth of phytochemicals that are of significant interest to the pharmaceutical industry [35]. These plants exhibit a wide range of biological activities, including antibacterial, antioxidant, anticancer, antiseptic, anti-inflammatory, antiulcer, cytotoxic, antiproliferative, insecticidal, larvicidal, antifungal, antidiarrheal, central nervous system depressant, antimitotic, antileukemic, hypoglycemic, and antiplasmodial effects. The broad spectrum of these activities is largely attributed to their rich phytochemical composition, particularly phenolic compounds [28,35,36,37]. Mangrove extracts have been shown to effectively kill larvae of various mosquito species, including Anopheles stephensi (from Rhizophora apiculata), Culex tritaeniorhynchus (from Bruguiera cylindrica), Aedes aegypti (from Rhizophora mucronata), and Culex quinquefasciatus (from Excoecaria agallocha) [38]. Additionally, mangroves have commercial applications, serving as sources of wood for export, pigments for tanning leather, charcoal, paper materials, and food products [35,37].
The presence of a rutin-like chromophore in our study does not confirm that rutin is the primary compound in the methanolic extract of Rhizophora mangle; it merely indicates a similarity to flavonol-type rutin. Rutin (3′,4′,5,7-tetrahydroxyflavone-3-rutinoside), also known as quercetin-3-O-rutinoside and vitamin P, is a natural polyphenolic flavonoid widely distributed in the plant kingdom [35]. Kandil et al. [32] reported that rutin was the most abundant flavonol glycoside in R. mangle leaves from Belize, with significant changes observed during leaf development and senescence. Rutin is known for its extensive pharmacological properties, which have been utilized in human medicine and nutrition [39]. Although the chromatographic profile indicates that rutin is among the primary compounds in MELRm, further analyses are required to fully characterize the chemical profile of this plant and to isolate and investigate its individual constituents.
Extracts and metabolites from medicinal plants used in the treatment of neglected diseases such as leishmaniasis, trypanosomiasis, and schistosomiasis often contain quinones, phenolics, flavonoids, tannins, alkaloids, and terpenes [26,40,41]. Tasdemir et al. [42] investigated the antitrypanosomal and antileishmanial activities of flavonoids and their analogs, revealing that rutin effectively targets the amastigote form of Leishmania donovani. They identified rutin as a primary component among the flavonoids in the extract of Gonocytisus angulatus. These findings are consistent with [43], who evaluated the HPLC phenolic profile of Melissa officinalis and its activity against L. braziliensis, L. infantum, and Trypanosoma cruzi. Their HPLC analysis identified rutin and caffeic acid as principal compounds, and the extract effectively inhibited both promastigote and epimastigote forms, suggesting that these compounds are key to the extract’s antiprotozoal activity. Additionally, quercetin has been shown to exhibit a pro-oxidant effect, generating reactive oxygen species that disrupt the mitochondrial membrane of L. amazonensis amastigotes [44,45]. Furthermore, methanolic extracts of Achillea fragrantissima rich in flavonoids and tannins demonstrated notable efficacy against Trypanosoma evansi [40].
Alemán et al. [46] evaluated the anthelmintic activity of aqueous and methanolic extracts of R. mangle against larvae of Haemonchus spp. and Trichostrongylus spp., noting that 50 mg/mL of either extract induced over 60% mortality in the worms. Moreover, [47] demonstrated the in vitro schistosomicidal activity of the alkaloid saguinarine, which achieved total mortality and significantly reduced cell viability at low concentrations and in brief incubation periods. Recently, an ethanolic extract of Allium sativum (garlic) and rutin, a flavonoid derived from garlic, showed promising immunomodulatory and anti-inflammatory effects against S. mansoni in a murine model [41]. In this study, the extract reduced the overall worm load, decreased egg accumulation in liver tissue, and increased the elimination of eggs in feces.
In our research, we did not identify any in vitro or in vivo studies reporting the use of Rhizophora species against S. mansoni. However, the control of schistosomiasis through mangrove plants was documented by Mendes et al. [24]. They explored extracts of Avicennia schaueriana, Laguncularia racemosa, and R. mangle for their molluscicidal activity against Biomphalaria glabrata, the intermediate host of S. mansoni. Their results highlighted the presence of tannins, alkaloids, triterpenoids, steroids, and coumarins, with L. racemosa and R. mangle affecting snail motility, feeding, and oviposition, in addition to exhibiting molluscicidal activity. These findings are significant, as the transmission of S. mansoni relies on the presence of infected individuals and the contamination of aquatic environments with eggs, which sustain the life cycle of B. glabrata and contribute to the chronicity of the infection
Therefore, the development of new schistosomicides that also target oviposition suppression is highly desirable [25,47]. In our study, MELRm induced mortality and motility changes in a dose- and time-dependent manner, leading to the complete separation of worm pairs. Additionally, similar to the PZQ group, no eggs were observed in the culture medium of any groups treated with MELRm throughout the experiment (24–120 h), in contrast to the negative control groups (Control 1 and Control 2). During S. mansoni infection, eggs not expelled in feces become embolized and deposited in tissues, primarily in the liver and intestines. These eggs induce a granulomatous inflammatory response, which is a major cause of morbidity and mortality associated with schistosomiasis. While PZQ remains the drug of choice for schistosomiasis, its exact mechanism of action is not fully elucidated, though it is known to cause a rapid influx of calcium, leading to intense spasmodic contractions of the worms’ muscles and resulting in the death of even mated worms [3]. In the case of MELRm, mortality preceded by non-mating may have facilitated the complete exposure of the worms to the extract.
Studies indicate that over 50% of newly approved medications are derived directly or indirectly from plant extracts and/or secondary metabolites, underscoring the importance of identifying new compounds with potential therapeutic activity and low cytotoxicity [48,49,50]. In our study, MELRm did not exhibit cytotoxicity in RAW cells. The RAW 264.7 cell line, a monocyte-/macrophage-like cell line, has been widely used for over 40 years to assess cytotoxicity of various substances. While there is extensive research on the cytotoxicity of extracts and secondary metabolites from mangrove plants, including those of Rhizophora species [51,52], we found no prior publications specifically addressing the cytotoxicity of methanolic extracts from R. mangle leaves sourced from Northeast Brazil. An aqueous extract of R. mangle leaves, bark, and roots has demonstrated an in vitro proliferative effect on HeLa cells [53]. Conversely, the methanolic extract of R. mangle leaves, stems, and roots from the Yucatán Peninsula, Mexico, exhibited cytotoxicity in HeLa cells [54]. Compounds isolated from the methanolic extract of R. apiculata leaves showed no cytotoxicity against human lung (SK-LU-1), liver (HepG2), and breast (MCF7) cancer cell lines [51]. Additionally, the methanolic extract of R. mucronata leaves exhibited selectivity against tumor cell lines without showing cytotoxicity towards healthy cells [55]. It is known that the composition of plant-derived compounds is influenced by soil type, temperature, salinity, and environmental pollution. Furthermore, the choice of solvent for extraction affects the polarity of the metabolites, resulting in variations in biological activity.
Our results do not yet elucidate the potential therapeutic targets and the impact of MELRm on the pathophysiology of schistosomiasis, as this study is limited to in vitro analysis. Nonetheless, our research aligns with the guidelines for initial drug candidate screening, which is a crucial step before advancing to in vivo trials, in accordance with international ethical research standards. Future studies using in vivo experimental models developed by our research group will clarify the effects of MELRm and its fractions on the histopathological and histomorphometric aspects of liver and intestinal tissues, granulomas, cellular and humoral immunoregulation, and parasitological criteria related to S. mansoni infection.
4. Materials and Methods
4.1. Plant Material
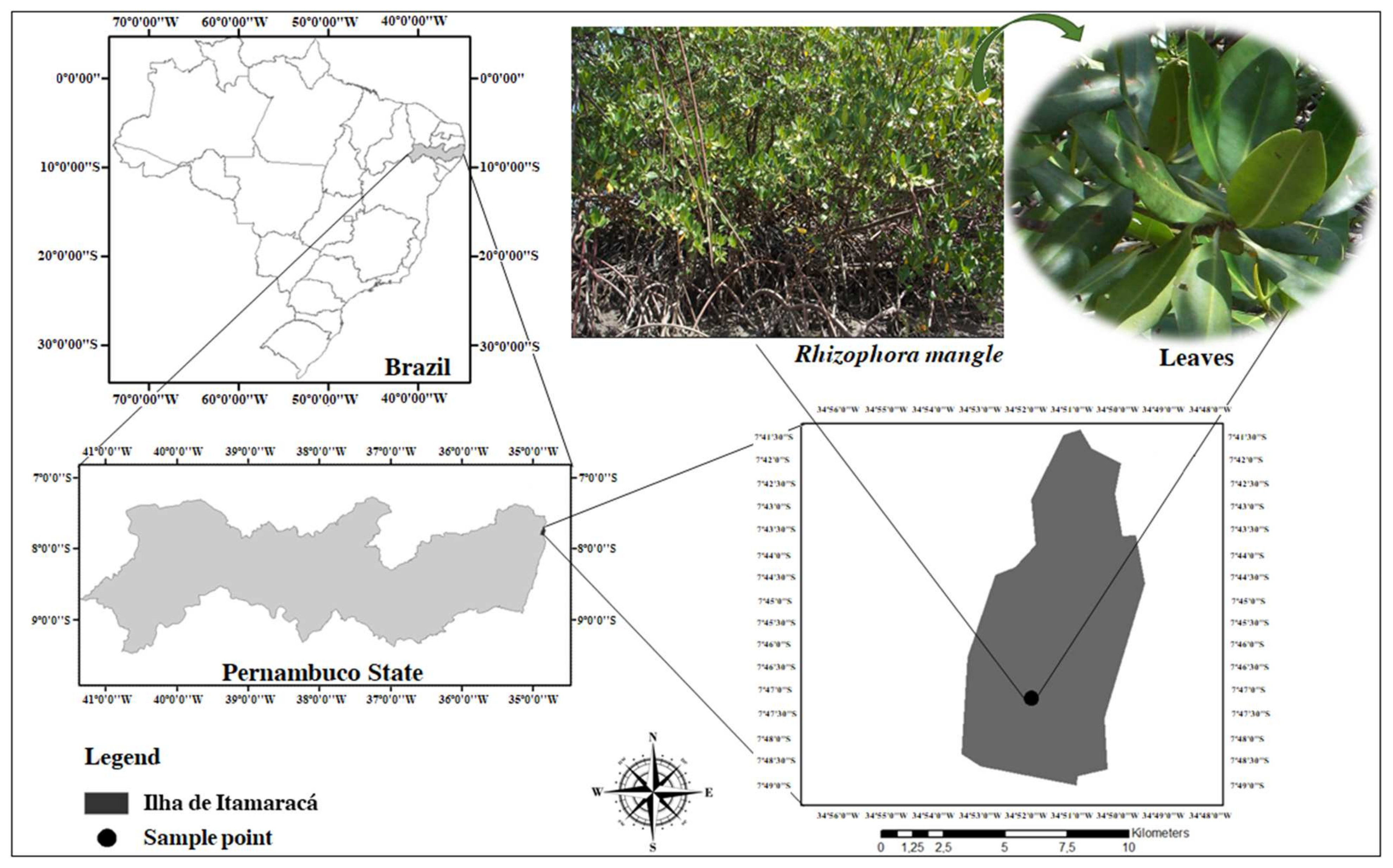
R. mangle leaves were collected from the mangrove forest in the city of Itamaracá, Vila Velha district, Pernambuco State, Brazil (7°40′ S latitude and 34°50′ W longitude). Only green leaves with a visually intact appearance, free of mechanical damage, pests, diseases, or discoloration, were selected (Figure 5). A voucher specimen, numbered 69,655, was identified by Prof. Dr. Marlene Barbosa, curator of the Herbarium at the Department of Botany, and is deposited in the Herbarium of the Federal University of Pernambuco (UFPE). Collection was authorized by the State Environmental Agency of Pernambuco, Brazil.
Figure 5.
Geographical location of Itamaracá City, Pernambuco, Brazil, indicating the site where R. mangle was collected.
4.2. Drugs and Reagents
Praziquantel (PZQ) [2 (Cyclohexylcarbonyl) 1,2,3,6,7 11b hexahydro 4H pyrazino [2,1 a]isoquinolin 4 one] (C19H24N2O2, molecular weight 312.41 and purity ≥98%), all analytical-grade solvents, reagents, and materials for cell culture and S. mansoni culture were obtained from Sigma Chemical Co., St. Louis, MO, USA.
4.3. Extract Preparation
Fresh leaves of R. mangle (1 kg) were ground to a particle size of 0.177 mm using a Pulverisette 14 Classic Line knife mill (Fritsch, Pittsboro, NC, USA). The extract was prepared through accelerated solvent extraction (ASE 350, Thermo Scientific, Waltham, MA, USA). Briefly, the crushed material was placed in duplicate into stainless-steel cells in the extractor, each containing 30 g of the material and 100 mL of methanol (Sigma-Aldrich, Darmstadt, Germany) as the mobile phase, under hydrogen pressure (H2). The collected R. mangle leaf extract (MELRm) was then concentrated under reduced pressure using a rotary evaporator (40.3 °C) (Buchi, New Castle, DE, USA; Vacuum Pump V-700) to remove the methanol. The yield of MELRm was calculated using Equation (1).
Phytochemical Characterization
The levels of constituents were determined using UV/Vis spectrophotometry based on the methodology proposed by Nerys et al. [56]. The total phenol content was measured using the Folin–Ciocalteau spectrophotometric method. For this assay, 1.0 mL of the extract at a concentration of 500 μg/mL was mixed with 1.0 mL of Folin–Ciocalteau reagent (diluted 1:10 v/v). After 5 min, 2.0 mL of a 7.5% sodium carbonate (Na2CO3) solution was added, and after 2 h, the absorbance was measured at 750 nm using a gallic acid standard curve (55 to 550 μg/mL). The results were expressed as milligrams of gallic acid equivalents (GAEs) per gram of extract.
The flavonoid content was determined using a 5 mL solution of 2% aluminum chloride in methanol, which was mixed with an equal volume of the extract solution (500 μg/mL). Absorbance was measured at 415 nm after 10 min. The standard curve was generated using quercetin, and the flavonoid content was expressed as milligrams of quercetin equivalents (QEs) per gram of extract.
The total flavonol content was measured by mixing 2 mL of the extract with 2 mL of 2% AlCl3 in ethanol and 3 mL of 50 g/L sodium acetate. The mixture was incubated for 2.5 h at 20 °C. Absorbance was measured at 440 nm, and the result was expressed as milligrams of quercetin equivalents (QEs) per gram of extract using a quercetin standard curve at concentrations ranging from 7.8 to 500 µg/mL. The tannin content was determined by mixing 2 mL of the extract with 3 mL of distilled water and 0.5 mL of Folin–Ciocalteau reagent, allowing it to react for 3 min. This was followed by the addition of 1.5 mL of 17% sodium carbonate (Na2CO3) and 3 mL of distilled water. The mixture was incubated for 2 h, and absorbance was measured at 725 nm. The equipment blank was prepared using the same proportions of distilled water. Tannin content was expressed as milligrams of tannic acid equivalents (TAEs) per gram of extract, based on a tannic acid standard curve with concentrations ranging from 7.8 to 500 µg/mL.
Characterization was performed using high-performance liquid chromatography (HPLC) with an Agilent 1200 Infinity Series system (Santa Clara, CA, USA), equipped with a quaternary pump, autosampler, column oven, and diode array detector. Separation was achieved on an RP-18 column (Zorbax SB-C18, Agilent, 4.6 × 250 mm, 5 µm). A 5 µL sample was analyzed using a linear gradient at a flow rate of 2.4 mL/min and a temperature of 30 °C, with the following gradient: 98% solvent A (0 min)–90% solvent A (10 min)–15% solvent A (27 min). Solvent A was 0.3% acetic acid in water, and solvent B was acetonitrile. Compound identification was carried out using standards of p-coumaric acid, rutin, ellagic acid, quercetin, apigenin, and geranium under the same experimental conditions used for characterizing the extract. All experiments were conducted in triplicate.
4.4. Animals and Ethical Considerations
All experimental procedures were approved by the Ethics Committee on the Use of Animals (CEUA) of the Federal University of Pernambuco (UFPE) under protocol number 0006/2016. Female Swiss Webster mice (Mus musculus, 30 ± 2 g) aged 30 days were supplied and housed in the vivarium of the Instituto Keizo Asami (iLIKA/UFPE) under standardized conditions (23 ± 2 °C, 40–50% humidity, and a 12 h light/dark cycle) with free access to water and Labina® food. Strains of Biomphalaria glabrata and S. mansoni (strain BH, Belo Horizonte, MG, Brazil) are maintained for successive generations in mollusks at the Academic Area of Tropical Medicine at UFPE.
4.5. Recovery of Adult Worm Couples
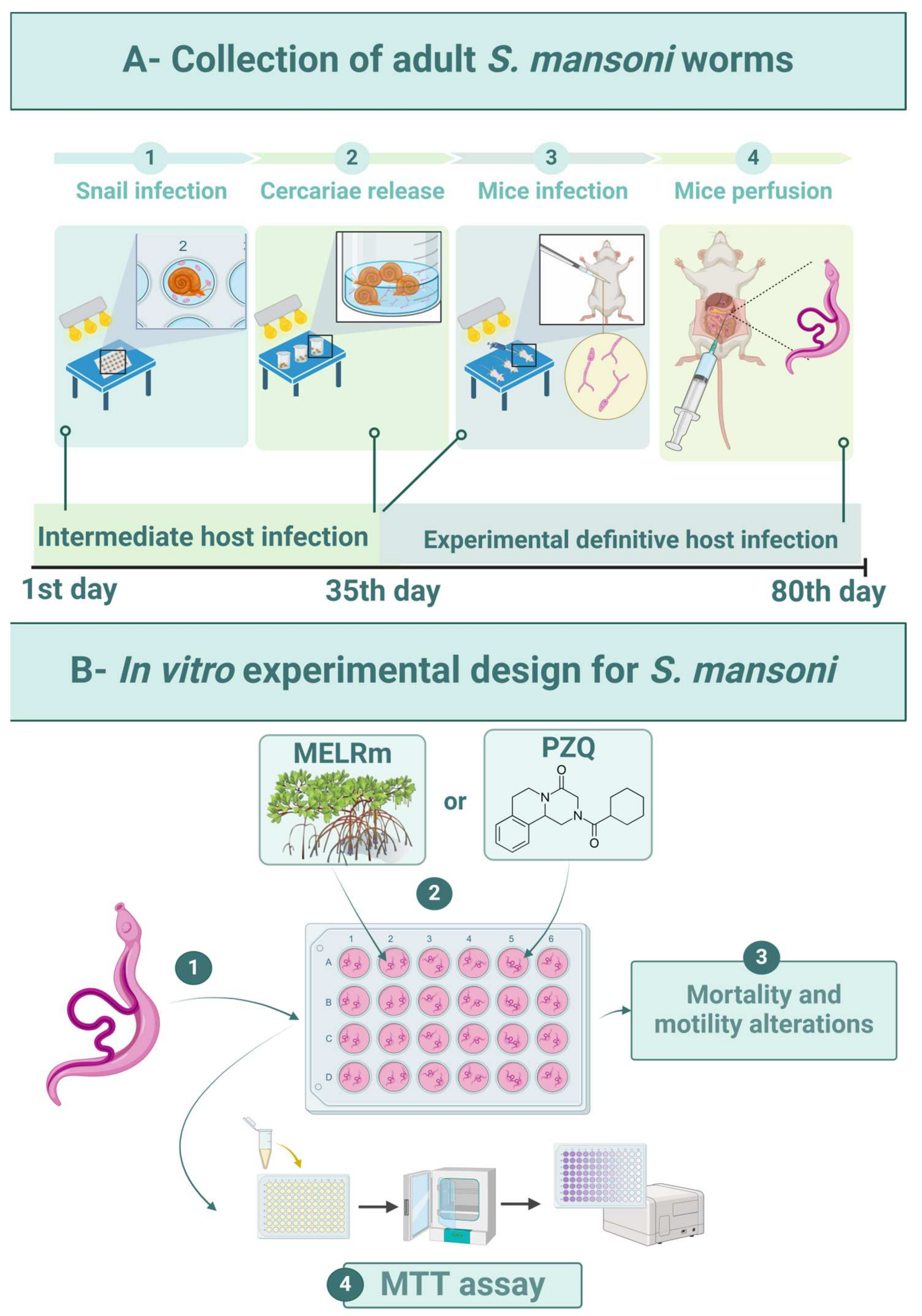
The intermediate hosts were maintained in plastic tanks (50 × 23 × 17 cm) under standardized conditions: filtered and dechlorinated water (20 L) was changed weekly, and the snails were fed daily with Lactuca sativa L. and kept at 25 ± 2 °C with a 12 h light/dark cycle. Feces from experimentally infected mice were processed by spontaneous sedimentation to isolate S. mansoni eggs. To obtain miracidia, the sedimented feces were exposed to artificial light (60 W, Lightex, Sofia, Bulgaria, model A5570). The infection of adult snails (n = 80) was carried out by placing five miracidia in each well of 24-well culture plates (TPP-Techno Plastic Products, Trasadingen, Switzerland) under artificial light for 4 h (Figure 6(A1)). Afterward, the snails were transferred to tanks and kept shielded from light. Infected snails were exposed to artificial light (60 W) for 2 h to release cercariae after 35 days of infection (Figure 6(A2)).
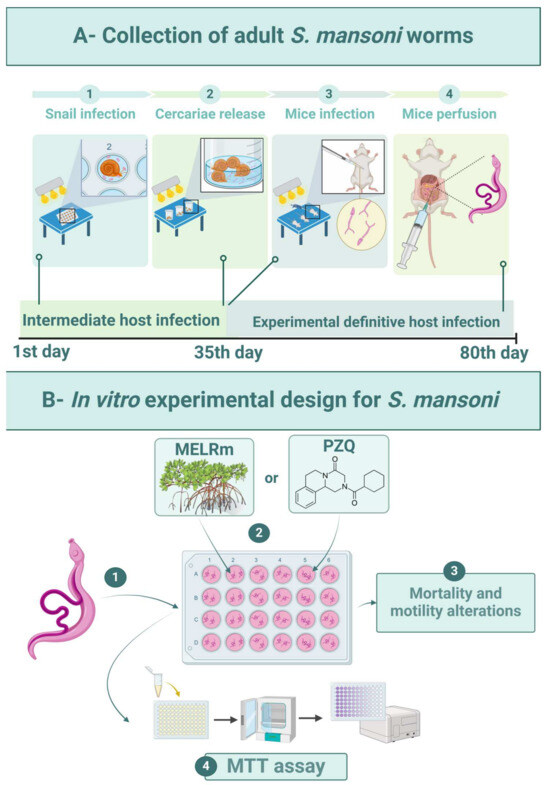
Figure 6.
Experimental design. (A1)—Experimental infection of B. glabrata snails with S. mansoni miracidia (BH strain). (A2)—Collection of S. mansoni cercariae. (A3)—Percutaneous infection of mice with cercarial suspension. (A4)—Perfusion of mice for worm recovery. (B1)—In vitro exposure of worms. (B2)—Distribution of MELRm or PZQ. (B3)—Evaluation of schistosomicidal activity. (B4)—Cell viability assay.
Mice (n = 10) were infected percutaneously with 120 S. mansoni cercariae [57] (Figure 6(A3)). Sixty days after infection, the mice were euthanized by cervical dislocation, and worms were aseptically recovered by perfusion of the hepatic portal system and mesenteric vessels with 0.9% NaCl (w/v) [58] (Figure 6(A4)). Only intact worm pairs were immediately transferred to RPMI 1640 medium supplemented with 20 mM HEPES, 100 μg/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum, and they were rinsed four times with this medium.
4.6. In Vitro Susceptibility against to S. mansoni
Worms were distributed (two pairs per well) into sterile 24-well culture plates containing 2 mL of supplemented RPMI 1640 medium (Figure 6(B1)). The worms were then incubated at 37 °C in a humidified atmosphere with 5% CO2. After two hours of incubation to allow for adaptation, MELRm was added at final concentrations of 25, 50, 100, 200, and 400 μg/mL (Figure 6(B2)). These concentrations were based on previous studies investigating natural compounds against S. mansoni [59]. The Negative Control 1 group consisted of worms incubated in supplemented RPMI 1640, while Negative Control 2 contained worms incubated in RPMI 1640 with 1% dimethyl sulfoxide (DMSO). PZQ at a concentration of 10 μM was used as a standard drug and positive control (Figure 6(B2)). PZQ (purity ≥ 98%) was purchased from Sigma Chemical Co. All experiments were performed in quadruplicate (n = 16 pairs of worms per concentration) and repeated at least twice.
4.6.1. Criteria of Schistosomicide Evaluation
Mortality and Changes in Motility
An inverted microscope (Leica Microsystems, DM IL, Wetzlar, Germany) was used to evaluate the general condition of the worms, including mortality and changes in motility (Figure 6(B3)). The worms were monitored for five consecutive days at intervals of 24, 48, 72, 96, and 120 h after incubation. The motility and survival of the worms were evaluated according to criteria established by Pica-Mattoccia et al. [60]. Briefly, worms were monitored based on decreasing viability using the following scoring system: score 3—worms exhibited typical movements, including peristalsis of internal organs and movement of suction cups, and were adhered to the bottom or sides of the culture plate (typical for negative control worms); score 2—reduction in overall body movements, peristalsis of internal organs, and suction cups; score 1—movements limited to the extremities or just one extremity (anterior and/or posterior regions), with an absence of peristalsis of internal organs and non-adherent suction cups; and score 0—complete absence of movements, with or without changes in color. The treatment was considered lethal when no parasite movements were observed for up to 2 min. The scoring evaluations were conducted by two double-blind evaluators.
Cell Viability Assay of Couples of S. mansoni Worms
The viability of S. mansoni after treatment was determined using the cytotoxicity assay based on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) (Figure 6(B4)) [61]. Briefly, two pairs of worms were placed in individual wells of 96-well plates containing 100 μL of MTT (5 mg/mL in phosphate-buffered saline—PBS) and incubated at 37 °C for 30 min. The MTT solution was then replaced with 200 μL of DMSO to dissolve the formazan crystals, and the optical density was measured at 550 nm using a microplate reader (M680, Bio-Rad Laboratories, Inc., Hercules, CA, USA). This procedure was performed with worms from the negative control (1 and 2) and positive control (PZQ) groups under the same experimental conditions. The assay was conducted in quadruplicate and repeated twice.
4.7. Cytotoxic Evaluation of MELRm
RAW 264.7 cells were cultured in RPMI 1640 medium supplemented with 2 mM L-glutamine, 1% penicillin-streptomycin, and 10% fetal bovine serum (FBS). The RAW 264.7 cell line (ATCC TIB-71) was obtained from the cell bank of Rio de Janeiro (Brazil) and is maintained in the Cell and Tissue Culture Laboratory of the Department of Histology and Embryology at UFPE. Briefly, cells were seeded into 96-well plates at a density of 1 × 106 cells/well and incubated in a humidified atmosphere of 5% CO2 at 37 °C for approximately 48 h, allowing the cells to reach 80–90% confluence. After the incubation period, the cells were washed twice with RPMI 1640 medium. Subsequently, the cells were incubated in RPMI-supplemented medium with different concentrations (25–400 μg/mL) of MELRm for 72 h in a final volume of 200 µL. Next, the culture medium containing MELRm was replaced with 100 µL of MTT solution (5 mg/mL in PBS), and the cells were incubated for 4 h at 37 °C, protected from light. The MTT solution was then removed, and 100 µL of DMSO was added to each well to dissolve the formed purple formazan crystals. Absorbance was measured at 550 nm using a microplate reader (M680, Bio-Rad Laboratories, Inc.). The blank was prepared following the same methodology, but the cells were cultured only in RPMI-supplemented medium [62]. The assays were performed in octuplicate in two independent experiments.
4.8. Statistical Analysis
The results were presented as the mean ± SD of the viability percentage from two independent experiments performed in quadruplicate. The median inhibitory concentration (IC50) was calculated from the dose–response curve using GraphPad Prism (San Diego, CA, USA, version 5).
5. Conclusions
MELRm is predominantly phenolic and exhibited a chromophore resembling rutin. The extract demonstrated promising schistosomicidal activity, inducing mortality, altering motility, and preventing mating in S. mansoni worm couples while also reducing worm cell viability. Importantly, MELRm was found to be non-cytotoxic, confirming its safety. This study underscores the potential of mangrove plants as a valuable natural resource in the search for new therapeutic agents, particularly for the control of S. mansoni.
Author Contributions
Conceptualization, S.D.M.F., A.L.A., R.G.L.N. and J.R.C.V.; methodology, S.D.M.F. and W.R.C.N.; validation, M.A.A.S.G., I.J.C.F. and W.W.M.F.; formal analysis, M.A.A.S.G.; investigation, M.T.V.G. and E.G.M.D.; resources, M.C.P.A.A.; data curation, M.A.A.S.G. and M.T.V.G.; writing—original draft preparation, R.G.L.N. and I.J.C.F.; writing—review and editing, M.A.A.S.G., S.D.M.F. and A.L.A.; visualization, W.W.M.F. and L.A.O.C.; supervision, M.C.P.A.A., H.D.A.A. and A.L.A.; project administration, J.R.C.V.; funding acquisition, J.R.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001 and the Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE). H.A.D. Araújo and I.J. Cruz Filho would like to thank FACEPE for the Regional Science Development Scholarship (Process DCR-0015-4.03/23) and Researcher Fixation Scholarships (Process BFP-0038-4.03/21) respectively. In addition, H.D.A. Araújo, and A.L. Aires would like to thank FACEPE Research Project Aid (APQ-0037-4.03/23 and Process APQ-Emergent 1181-4.03/22) respectively.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on Animal Use (CEUA) of the Federal University of Pernambuco (UFPE) on (protocol number 0006/2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank the Federal University of Pernambuco and the Pro-Rectories of Postgraduate Studies and Research and Innovation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Silva da Paz, W.; Dos Santos Reis, E.; Leal, I.B.; Barbosa, Y.M.; de Araújo, K.C.G.; de Jesus, A.R.; de Souza, C.D.F.; Dos Santos, A.D.; Bezerra-Santos, M. Basic and associated causes of schistosomiasis-related mortality in Brazil: A population-based study and a 20-year time series of a disease still neglected. J. Glob. Health 2021, 11, 04061. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 21 June 2024).
- Nogueira, R.A.; Lira, M.G.S.; Licá, I.C.L.; Frazão, G.C.C.G.; Dos Santos, V.A.F.; Filho, A.C.C.M.; Rodrigues, J.G.M.; Miranda, G.S.; Carvalho, R.C.; Nascimento, F.R.F. Praziquantel: An update on the mechanism of its action against schistosomiasis and new therapeutic perspectives. Mol. Biochem. Parasito 2022, 252, 111531. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.C.S.; da Silva, I.E.P.; de Araújo, H.D.A.; Barbosa, C.S. Malacological, socio-environmental evaluation, and evidence of local transmission and maintenance of schistosomiasis in an urban area of Northeast Brazil. Acta Trop. 2024, 252, 07145. [Google Scholar] [CrossRef]
- Ernould, J.C.; Ba, K.; Sellin, B. Increase of intestinal schistosomiasis after praziquantel treatment in a Schistosoma haematobium and Schistosoma mansoni mixed focus. Acta Trop. 1999, 73, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Pica-Mattoccia, L.; Doenhoff, M.J.; Valle, C.; Basso, A.; Troiani, A.R.; Liberti, P.; Festucci, A.; Guidi, A.; Cioli, D. Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni. Acta Trop. 2009, 111, 82–85. [Google Scholar] [CrossRef]
- Wu, M.J.; Xu, B.; Guo, Y.W. Unusual Secondary Metabolites from the Mangrove Ecosystems: Structures, Bioactivities, Chemical, and Bio-Syntheses. Mar. Drugs 2022, 20, 535. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955. [Google Scholar] [CrossRef]
- Ramasubburayan, R.; Prakash, S.; Pitchiah, S.; Dhanraj, G. Antifouling activity and biodegradable potential of the bioactive metabolites isolated from mangrove Avicennia officinalis L. Nat. Prod. Res. 2024, 38, 1680–1686. [Google Scholar] [CrossRef]
- Ramalingam, V.; Rajaram, R. Enhanced antimicrobial, antioxidant and anticancer activity of Rhizophora apiculata: An experimental report. 3 Biotech 2018, 8, 200. [Google Scholar] [CrossRef]
- He, Z.; Feng, X.; Chen, Q.; Li, L.; Li, S.; Han, K.; Guo, Z.; Wang, J.; Liu, M.; Shi, C.; et al. Evolution of coastal forests based on a full set of mangrove genomes. Nat. Ecol. Evol. 2022, 6, 738–749. [Google Scholar] [CrossRef]
- Pan American Health Organization. Available online: https://www.paho.org/en/topics/schistosomiasis (accessed on 19 June 2024).
- Lima, K.O.O.; Tognella, M.M.P.; Cunha, S.R.; Andrade, H.A. Growth models of Rhizophora mangle L. seedlings in tropical southwestern Atlantic. Estuar. Coast. Shelf Sci. 2018, 207, 154–163. [Google Scholar] [CrossRef]
- de-Faria, F.M.; Almeida, A.C.; Luiz-Ferreira, A.; Takayama, C.; Dunder, R.J.; da Silva, M.A.; Salvador, M.J.; Abdelnur, P.V.; Eberlin, M.N.; Vilegas, W.; et al. Antioxidant Action of Mangrove Polyphenols against Gastric Damage Induced by Absolute Ethanol and Ischemia-Reperfusion in the Rat. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Caria, C.R.E.P.; Santos, P.S.; Ruy, C.C.; da Silva Lima, N.; Moreira, D.K.T.; da Rocha, C.Q.; Murador, D.C.; de Rosso, V.V.; Gambero, A.; et al. Modulatory Effect of Polyphenolic Compounds from the Mangrove Tree Rhizophora mangle L. on Non-Alcoholic Fatty Liver Disease and Insulin Resistance in High-Fat Diet Obese Mice. Molecules 2018, 23, 2114. [Google Scholar] [CrossRef] [PubMed]
- de Souza Mesquita, L.M.; Rodrigues, C.F.B.; da Rocha, C.Q.; Bianchim, M.S.; Rodrigues, C.M.; de Oliveira, V.M.; Gaeta, H.H.; Belchor, M.N.; Toyama, M.H.; Vilegas, W. LC–ESI–IT-MS/MS and MALDI-TOF Approach: Identification of Natural Polymers from Rhizophora mangle Barks and Determination of Their Analgesic and Anti-inflammatory Properties. Nat. Prod. Bioprospect. 2019, 9, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.J.X.; Leal, L.B.; Sá, J.G.A.; Sabino, L.R.A.; Cavalcanti, I.M.F.S.D.; Silva, L.A.; Santana, E.S.; Fernandes, F.H.P.; C Filho, I.J.; Brandão, W.F.M.; et al. A preliminary study of cutaneous wound healing on the upper eyelid in a small Brazilian population using Rhizophora mangle-based cream. Anais da Academia Brasileira de Ciências 2024, 96, e20231143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Lin, Y.M.; Zhou, H.C.; Wei, S.D.; Chen, J.H. Condensed Tannins from Mangrove Species Kandelia candel and Rhizophora mangle and Their Antioxidant Activity. Molecules 2010, 15, 420–431. [Google Scholar] [CrossRef]
- Cruz, S.M.; Marroquín, N.; Alvarez, L.E.; Chang, D.E.; Cáceres, A. Evaluation of Mangrove (Rhizophora mangle L.) products as coloring, antimicrobial and antioxidant agents. Int. J. Phytocosmet. Nat. Ingred. 2015, 2, 12. [Google Scholar] [CrossRef]
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef]
- Sánchez Perera, L.M.; Varcalcel, L.; Escobar, A.; Noa, M. Polyphenol and Phytosterol Composition in an Antibacterial Extract from Rhizophora mangle L. Bark. J. Herb. Pharmacother. 2008, 7, 107–128. [Google Scholar] [CrossRef]
- Baker, S.; Bisht, N.; Bhat, P.; Karthik, R.N.; Prasad, A.; Prasad, H.; Prasad, M.N.N. Phytogenic synthesis of nanoparticles from Rhizophora mangle and their bactericidal potential with DNA damage activity. Nano-Struct. Nano-Objects 2017, 10, 112–115. [Google Scholar] [CrossRef]
- Rodrigues Neto, A.A.; Gomes Júnior, P.P.; Silva, M.C.; Lima, C.S.A.; Yara, R.; Guimarães, E.B.; Santana, E.S.; Silva, L.A.D.; Lira, E.J.R.V.; Vieira, J.R.C. Evaluation of embryotoxic and embryostatic effects of the aqueous extract of Rhizophora mangle and tannic acid on eggs and larvae of Aedes aegypti. Anais da Academia Brasileira de Ciências 2018, 90, 2141–2148. [Google Scholar] [CrossRef]
- Mendes, R.J.A.; Pereira Filho, A.A.; Nogueira, A.J.L.; Araújo, K.R.F.; França, C.R.C.; Carvalho, I.B.; Silva, N.M.L.D.; Azevedo, A.S.; Rosa, I.G. Evaluation of molluscicidal activity of three mangrove species (Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle) and their effects on the bioactivity of Biomphalaria glabrata Say, 1818. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e7. [Google Scholar] [CrossRef]
- Silva, L.M.N.; França, W.W.M.; Santos, V.H.B.; Souza, R.A.F.; Silva, A.M.; Diniz, E.G.M.; Aguiar, T.W.A.; Rocha, J.V.R.; Souza, M.A.A.; Nascimento, W.R.C.; et al. Plumbagin: A Promising In Vivo Antiparasitic Candidate against Schistosoma mansoni and In Silico Pharmacokinetic Properties (ADMET). Biomedicines 2023, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Simoben, C.V.; Ntie-Kang, F.; Akone, S.H.; Sippl, W. Compounds from African Medicinal Plants with Activities Against Selected Parasitic Diseases: Schistosomiasis, Trypanosomiasis and Leishmaniasis. Nat. Prod. Bioprospect 2018, 8, 151–169. [Google Scholar] [CrossRef]
- Duarte Galhardo de Albuquerque, R.D.; Mahomoodally, M.F.; Lobine, D.; Suroowan, S.; Rengasamy, K.R. Botanical Products in the Treatment and Control of Schistosomiasis: Recent Studies and Distribution of Active Plant Resources According to Affected Regions. Biology 2020, 9, 223. [Google Scholar] [CrossRef]
- Patra, J.K.; Thatoi, H.N. Metabolic diversity and bioactivity screening of mangrove plants: A review. Acta Physiol. Plant 2011, 33, 1051–1061. [Google Scholar] [CrossRef]
- Costa, F.N.; da Silva, M.D.; Borges, R.M.; Leitão, G.G. Isolation of phenolics from Rhizophora mangle by combined counter-current chromatography and gel-filtration. Nat. Prod. Commun. 2014, 9, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Nabeelah Bibi, S.; Fawzi, M.M.; Gokhan, Z.; Rajesh, J.; Nadeem, N.; RR, R.K.; RDDG, A.; Pandian, S.K. Ethnopharmacology, Phytochemistry, and Global Distribution of Mangroves—A Comprehensive Review. Mar. Drugs 2019, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Willian, N.; Syukri, S.; Zulhadjri, Z.; Arief, S. Marine plant mediated green synthesis of silver nanoparticles using mangrove Rhizophora stylosa: Effect of variable process and their antibacterial activity. F1000Research 2022, 10, 768. [Google Scholar] [CrossRef]
- Kandil, F.; Grace, M.; Seigler, D.; Cheeseman, J. Polyphenolics in Rhizophora mangle L. leaves and their changes during leaf development and senescence. Trees 2004, 18, 5. [Google Scholar] [CrossRef]
- da Silva Pontes, A.L.; Monteiro Leal, C.; Pereira Lucas, M.; Caamaño Muiño da Silva, G.; Braga Alves Peixoto, J.V.; Barbosa Succar, J.; Ribeiro Flores, V.; Neves Direito, I.C.; Ribeiro da Silva, A.J.; de Oliveira Chaves, F.; et al. Dereplication Tools for Rhizophora mangle Extracts from Different Mangrove Areas and their Potential Against Staphylococcus aureus. Chem. Biodivers. 2024, 21, e202400687. [Google Scholar] [CrossRef]
- Sánchez, J.; Melchor, G.; Martínez, G.; Escobar, A.; Faure, R. Antioxidant activity of Rhizophora mangle bark. Fitoterapia 2006, 77, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Kalasuba, K.; Miranti, M.; Rahayuningsih, S.R.; Safriansyah, W.; Syamsuri, R.R.P.; Farabi, K.; Oktavia, D.; Alhasnawi, A.N.; Doni, F. Red Mangrove (Rhizophora stylosa Griff.)—A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Prospects. Plants 2023, 12, 2196. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Escandón-Rivera, S.M.; Torres-Valle, G.M.; Quijano, L. Phytochemical composition and chronic hypoglycemic effect of Rhizophora mangle cortex on STZ-NA-induced diabetic rats. Rev. Bras. Farmacogn. 2017, 27, 744–750. [Google Scholar] [CrossRef]
- Sormin, R.B.D.; Nendissa, D.M.; Mailoa, M.N.; Rieuwpassa, F.; Wenno, M.R. Antibacterial activity of Rhizophora apiculata extract originated from Inner Ambon Bay against selected pathogen bacteria. IOP Conf. Ser. Earth Environ. Sci. 2021, 797, 012017. [Google Scholar] [CrossRef]
- Kathiresan, K. A review of studies on Pichavaram mangrove, southeast India. Hydrobiologia 2000, 430, 185–205. [Google Scholar] [CrossRef]
- Tobar-Delgado, E.; Mejía-España, D.; Osorio-Mora, O.; Serna-Cock, L. Rutin: Family Farming Products’ Extraction Sources, Industrial Applications and Current Trends in Biological Activity Protection. Molecules 2023, 28, 5864. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, I.M.; Al-Wabel, N.A.; Bayad, A.E. Achillea fragrantissima, rich in flavonoids and tannins, potentiates the activity of diminazine aceturate against Trypanosoma evansi in rats. Asian Pac. J. Trop. Med. 2016, 9, 228–234. [Google Scholar] [CrossRef]
- Hamad, R.S. Rutin, a Flavonoid Compound Derived from Garlic, as a Potential Immunomodulatory and Anti-Inflammatory Agent against Murine Schistosomiasis mansoni. Nutrients 2023, 15, 1206. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Cunha, F.; Tintino, S.R.; Figueredo, F.; Barros, L.; Duarte, A.E.; Vega Gomez, M.C.; Coronel, C.C.; Rolón, M.; Leite, N.; Sobral-Souza, C.E.; et al. HPLC-DAD phenolic profile, cytotoxic and anti-kinetoplastidae activity of Melissa officinalis. Pharm. Biol. 2016, 54, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, F.; Inacio, J.D.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive Oxygen Species Production by Quercetin Causes the Death of Leishmania amazonensis Intracellular Amastigotes. J. Nat. Prod. 2013, 76, 1505–1508. [Google Scholar] [CrossRef]
- Silva, E.R.; Brogi, S.; Lucon-Júnior, J.F.; Campiani, G.; Gemma, S.; Maquiaveli, C. Dietary polyphenols rutin, taxifolin and quercetin related compounds target Leishmania amazonensis arginase. Food Funct. 2019, 10, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Alemán Gainza, Y.; Sánchez Perera, L.; Tania, P.; Rodriguez Perdomo, Y.; Olivares, J.L.; Rodríguez, J.G. Actividad larvicida de extractos de Rhizophora mangle l. contra estrongílidos gastrointestinales de ovinos larvicidal activity of extracts from Rhizophora mangle l. against gastrointestinal strongylid of sheep. Rev. Salud Anim. 2011, 33, 111–115. [Google Scholar]
- de Souza Silva, M.S.; Dos Santos, M.L.M.F.; da Silva, A.M.; França, W.W.M.; Araújo, S.B.; da Silva, R.L.; do Nascimento, W.R.C.; da Silva Santos, N.P.; da Cruz Filho, I.J.; de Azevedo Albuquerque, M.C.P.; et al. Sanguinarine: An alkaloid with promising in vitro and in vivo antiparasitic activity against different developmental stages of Schistosoma mansoni and in silico pharmacokinetic properties (ADMET). Parasitol. Res. 2024, 123, 2. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hussain, K.; Abbas, R.; Abbas, A.; Samiullah, K.; Ahmed, T.; Siddique, F.; Mohsin, M.; Rehman, A.; Rahman, A.; Waqas, M. Anticoccidial potential of Ageratum conyzoides and its effect on Blood parameters of experimentally infected Broiler Chickens. J. Hell. Vet. Med. Soc. 2021, 72, 3085–3090. [Google Scholar] [CrossRef]
- Mohsin, M.; Aleem, M.T.; Goraya, M.U.; Aguilar-Marcelino, L.; Abbas, R.Z.; Abbas, A. Editorial: Natural products and pseudo-natural products against veterinary disease-causing microorganisms. Front. Vet. Sci. 2024, 11, 1429587. [Google Scholar] [CrossRef]
- Thao, N.; Linh, K.; Quan, N.; Trung, V.; Binh, P.; Nguyen, C.; Nam, N.; Van Thanh, N. Cytotoxic metabolites from the leaves of the mangrove Rhizophora apiculata. Phytochem. Lett. 2022, 47, 51–55. [Google Scholar] [CrossRef]
- Santana, E.; Leal, L.; da Silva, L.; Barbosa, I.; Melo, C.; Santos, D.; Vieira, J. Association of Rhizophora mangle and ascorbic acid in hydrogels: Evaluation of cytotoxic and immunomodulatory effects. Braz. J. Pharm. Sci. 2023, 59, e20179. [Google Scholar] [CrossRef]
- Sá, J.; Brandão, W.; Santana, M.; da Silva, L.; Santana, E.; Silva, E.; de Carvalho, E.; Vieira, J. Avaliação da citotoxicidade e caracterização do perfil fitoquímico de Rhizophora mangle l. Do mangue brasileiro. In Ciências da Saúde e suas Descobertas Científicas; Seven Editora: São José dos Pinhais, Brazil, 2023. [Google Scholar] [CrossRef]
- Mena-Rejon, G.; Caamal-Fuentes, E.; Cantillo-Ciau, Z.; Cedillo-Rivera, R.; Flores-Guido, J.; Moo-Puc, R. In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J. Ethnopharmacol. 2009, 121, 462–465. [Google Scholar] [CrossRef]
- Youssef, A.M.M.; Maaty, D.A.M.; Al-Saraireh, Y.M. Phytochemistry and Anticancer Effects of Mangrove (Rhizophora mucronata Lam.) Leaves and Stems Extract against Different Cancer Cell Lines. Pharmaceuticals 2022, 16, 4. [Google Scholar] [CrossRef]
- Nerys, L.; Jacob, I.; Silva, P.; da Silva, A.; Macário, A.; Rocha, W.; Pereira, D.; Abreu, A.; Silva, R.; Filho, I.; et al. Photoprotective, biological activities and chemical composition of the non-toxic hydroalcoholic extract of Clarisia racemosa with cosmetic and pharmaceutical applications. Ind. Crops Prod. 2022, 180, 114762. [Google Scholar] [CrossRef]
- Olivier, L.; Stirewalt, M.A. An efficient method for exposure of mice to cercariae of Schistosoma mansoni. J. Parasitol. 1952, 38, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Smithers, S.R.; Terry, R.J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 1965, 55, 695–700. [Google Scholar] [CrossRef]
- Araújo, H.D.A.; Aires, A.L.; Soares, C.L.R.; Brito, T.G.S.; Nascimento, W.M.; Martins, M.C.B.; Silva, T.G.; Brayner, F.A.; Alves, L.C.; Silva, N.H.; et al. Usnic acid potassium salt from Cladonia substellata (Lichen): Synthesis, cytotoxicity and in vitro anthelmintic activity and ultrastructural analysis against adult worms of Schistosoma mansoni. Acta Trop. 2019, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, N.H.; Albuquerque, M.C.P.A.; Aires, A.L.; Lima, V.L.M. Potassium usnate, a water-soluble usnic acid salt, shows enhanced activity against Schistosoma mansoni in vitro. Exp. Parasitol. 2020, 208, 107779. [Google Scholar] [CrossRef]
- Aires, A.L.; Ximenes, E.C.; Silva, R.A.; Barbosa, V.X.; Góes, A.J.; Peixoto, C.A.; Souza, V.M.; Albuquerque, M.C. Ultrastructural analysis of β-lapachone-induced surface membrane damage in male adult Schistosoma mansoni BH strain worms. Exp. Parasitol. 2014, 142, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari Nejad, A.S.; Fotouhi, F.; Mehrbod, P.; Keshavarz, M.; Alikhani, M.Y.; Ghaemi, A. Oncolytic effects of Hitchner B1 strain of newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways. Microb. Pathog. 2020, 147, 104438. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).