Anxiolytic and Antidepressant Effects of Tribulus terrestris Ethanolic Extract in Scopolamine-Induced Amnesia in Zebrafish: Supported by Molecular Docking Investigation Targeting Monoamine Oxidase A
Abstract
1. Introduction
2. Results
2.1. In Vivo Bioassays
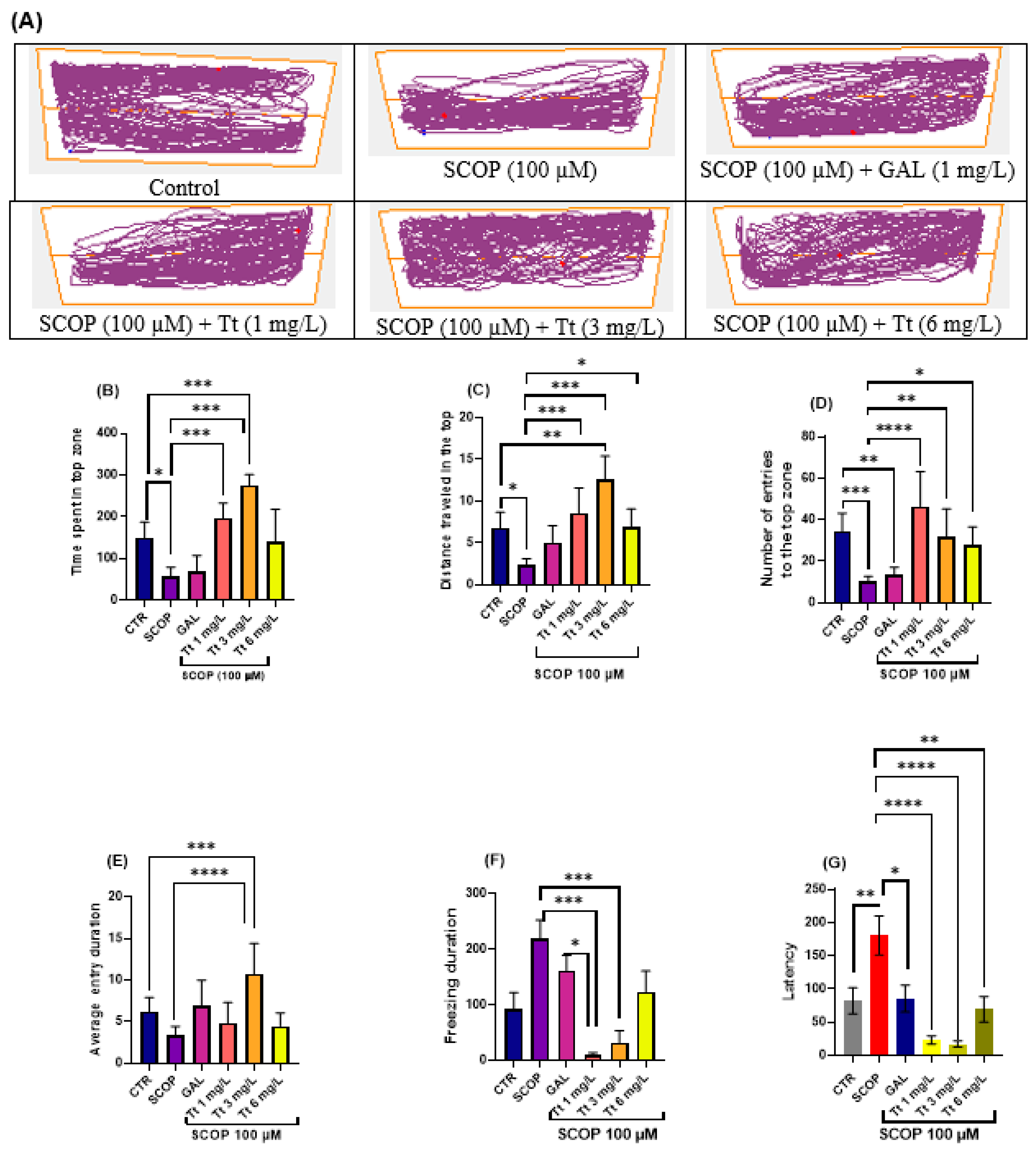
2.2. Effects on the Zebrafish NTT Response
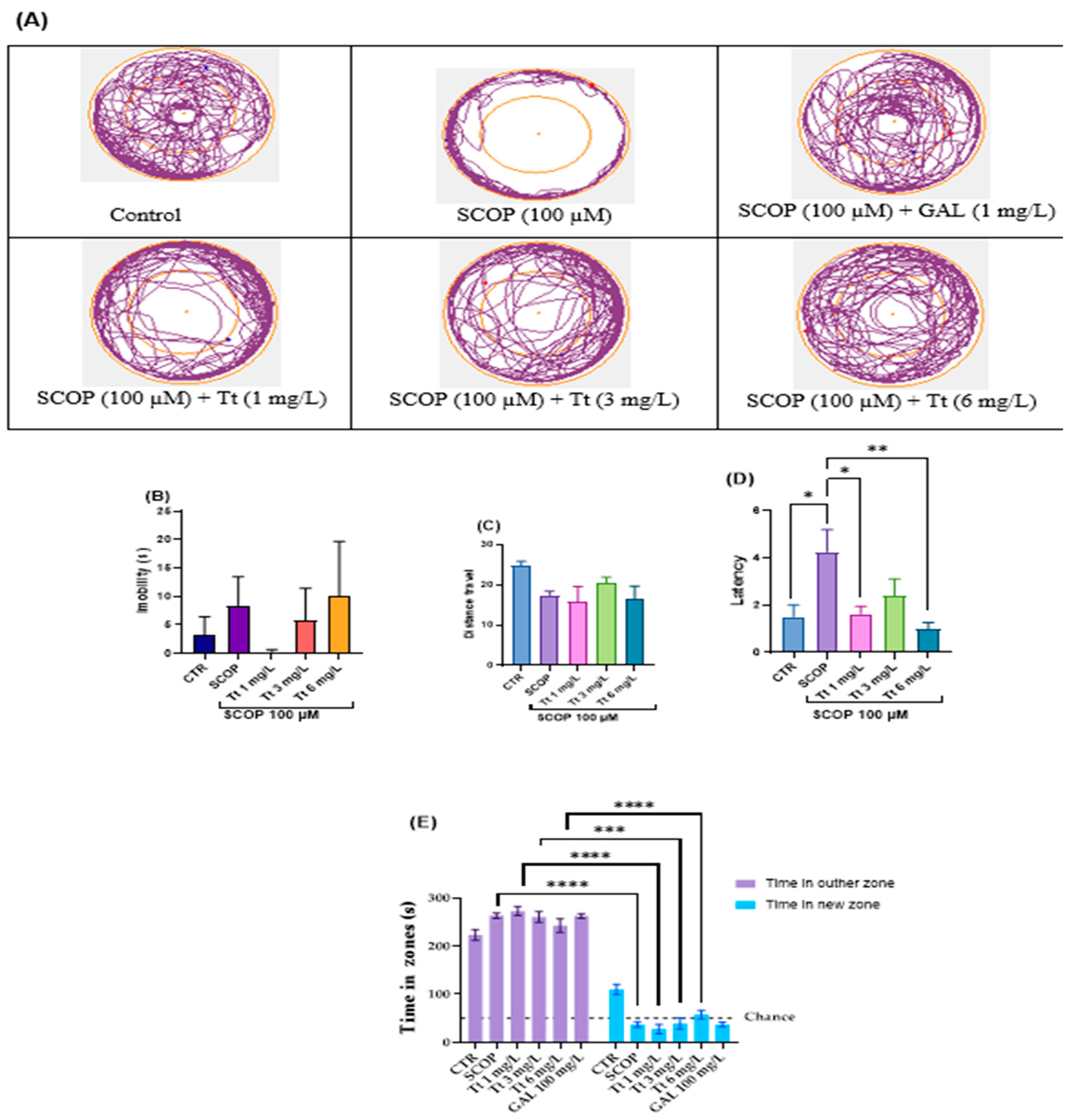
2.3. Effects on Zebrafish Novel Approach Test Response
2.4. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Conventional Heat Reflux Extraction
4.3. Fish Care and Maintenance
4.4. Animals and Drug Treatment
4.5. Behavioral Evaluation
4.6. Novel Tank Diving Test (NTT)
4.7. Novel Approach Test (NAT Test)
4.8. In Silico Docking Experiments
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef] [PubMed]
- Newby, J.M.; McKinnon, A.; Kuyken, W.; Gilbody, S.; Dalgleish, T. Systematic review and meta-analysis of transdiagnostic psychological treatments for anxiety and depressive disorders in adulthood. Clin. Psychol. Rev. 2015, 40, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; Goodspeed, J.; et al. Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology 2012, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Blaser, R.E.; Rosemberg, D.B. Measures of anxiety in zebrafish (Danio rerio): Dissociation of black/white preference and novel tank test. PLoS ONE 2012, 7, e36931. [Google Scholar] [CrossRef] [PubMed]
- Lenz, R.A.; Maxwell, A.M.; Freeman, K.B.; Mills, S.M.; Morozova, E.; Etminan, N.; Bowker, A.T.; Burch, A.E.; Shornstein, T.; Kase, S.; et al. The scopolamine model as a pharmacodynamic marker in early drug development. Psychopharmacology 2012, 220, 97–107. [Google Scholar] [CrossRef]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Peixoto, T.C.; Grosser, K.; Bogo, M.R.; Bonan, C.D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2011, 217, 10–15. [Google Scholar] [CrossRef]
- Stewart, A.; Maximino, C.; Marques de Brito, T.; Herculano, A.M.; Gouveia, A.; Morato, S.; Cachat, J.M.; Gaikwad, S.; Elegante, M.F.; Hart, P.C.; et al. Neurophenotyping of adult zebrafish using the light/dark box paradigm. In Zebrafish Neurobehavioral Protocols; Kaluef, A.V., Cachat, J.M., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 157–167. [Google Scholar]
- Kim, Y.H.; Lee, Y.; Kim, D.; Jung, M.W.; Lee, C.J. Scopolamine-induced learning impairment reversed by physostigmine in zebrafish. Neurosci. Res. 2010, 67, 156–161. [Google Scholar] [CrossRef]
- Cho, H.; Lee, C.-J.; Choi, J.; Hwang, J.; Lee, Y. Anxiolytic effects of an acetylcholinesterase inhibitor, physostigmine, in the adult zebrafish. Anim. Cells Syst. 2012, 16, 198–206. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Kalueff, A.V. Principles of modeling brain diseases and their therapy based on zebrafish studies. Rev. Clin. Pharmacol. Drug Ther. 2022, 20, 119–122. [Google Scholar] [CrossRef]
- Kolesnikova, T.O.; Demin, K.A.; Costa, F.V.; Zabegalov, K.N.; de Abreu, M.S.; Gerasimova, E.V.; Kalueff, A.V. Towards zebrafish models of CNS channelopathies. Int. J. Mol. Sci. 2022, 23, 13979. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Ebrahimie, E.; Lardelli, M. Using the zebrafish model for Alzheimer’s disease research. Front. Genet. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Chen, Y.-C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.; Rico, E.P.; Burgos, J. Can zebrafish be used as animal model to study Alzheimer’s disease? Am. J. Neurodegener. Dis. 2012, 1, 32–48. [Google Scholar]
- Papke, R.L. Studying the pharmacology of nicotinic receptors with drugs and toxins: New methods for an old receptor. Neuropharmacology 2012, 63, 868–878. [Google Scholar]
- Kysil, E.V.; Meshalkina, D.A.; Frick, E.E.; Echevarria, D.J.; Rosemberg, D.B.; Maximino, C.; Kalueff, A.V. Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 2017, 14, 197–208. [Google Scholar] [CrossRef]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in animal model of cognitive deficit-like condition of Alzheimer’s disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef]
- Zhang, Z.J. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004, 75, 1659–1699. [Google Scholar] [CrossRef]
- Houghton, P.J.; Howes, M.J. Natural products and derivatives affecting neurotransmission relevant to Alzheimer’s and Parkinson’s disease. Neurosignals 2005, 14, 6–22. [Google Scholar] [CrossRef]
- Hritcu, L.; Foyet, H.S.; Stefan, M.; Mihasan, M.; Asongalem, A.E.; Kamtchouing, P. Neuroprotective effect of the methanolic extract of Hibiscus asper leaves in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. J. Ethnopharmacol. 2011, 137, 585–591. [Google Scholar] [CrossRef]
- Batista, F.L.A.; Lima, L.M.; Abrante, I.A.; De Araújo, J.I.F.; Batista, F.L.A.; Abrante, I.A.; Magalhães, F.E.A.; De Lima, D.R.; Lima, M.D.C.L.; Prado, B.S.D.; et al. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed. Pharmacother. 2018, 108, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Todorov, I.N.; Zaikov, G.E.; Degterev, I.A. Bioactive Compounds: Biotransformation of Biological Action, Mechanism of Antistress and Anabolic Action of Eleutherococcus; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 1993. [Google Scholar]
- Moreira, E.G.; Rial, D.; Aguiar, A., Jr.; Figueiredo, C.; Siqueira, J.; DalBó, S.; Horst, H.; Oliveira, J.; Mancini, G.; dos Santos, T.; et al. Proanthocyanidin-rich fraction from Croton celtidifolius Baill confers neuroprotection in the intranasal 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine rat model of Parkinson’s disease. J. Neural Transm. 2010, 117, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-X.; Sze, S.C.-W.; Ng, T.-B.; Lee, C.K.-F.; Leung, G.P.H.; Shaw, P.-C.; Tong, Y.; Zhang, Y.-B. Anti-parkinsonian drug discovery from herbal medicines: What have we got from neurotoxic models? J. Ethnopharmacol. 2012, 139, 698–711. [Google Scholar] [CrossRef]
- Orhan, I.; Daglia, M.; Nabavi, S.; Loizzo, M.; Sobarzo-Sánchez, E.; Nabavi, S. Flavonoids and dementia: An update. Curr. Med. Chem. 2015, 22, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.R.; Greenamyre, J.T. Neurotoxic in vivo models of Parkinson’s disease: Recent advances. Prog. Brain Res. 2010, 184, 17–33. [Google Scholar]
- Zhang, J.; Yao, W.; Hashimoto, K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Akram, M.; Asif, H.M.; Akhtar, N.; Shah, P.A.; Uzair, M.; Shaheen, G.; Shamim, T. Tribulus terrestris Linn.: A review article. J. Med. Plants Res. 2011, 5, 3601–3605. [Google Scholar]
- Adaikan, P.G. Effect of Tribulus terrestris on nicotinamide adenine dinucleotide phosphate-diaphorase activity and androgen receptors in rat brain. J. Ethnopharmacol. 2005, 96, 127–133. [Google Scholar]
- Sharifi, A.M. Study of antihypertensive mechanism of Tribulus terrestris in 2K1C hypertensive rats: Role of tissue ACE activity. Life Sci. 2003, 73, 2963–2971. [Google Scholar] [CrossRef]
- Gauthaman, K. Sexual effects of puncture vine (Tribulus terrestris) extract (Protodioscin): An evaluation using a rat model. J. Altern. Complement. Med. 2003, 9, 257–265. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Sgheier, R.M.; Salmi, S.; Khalifi, D.; Laouni, D.; Ben-Attia, M. Current Approaches and Challenges for Chemical Characterization of inhibitory effect against cancer Cell line isolated from Gokshur Extract. J. Chromatogr. B 2016, 1026, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bouabdallah, S.; Laouini, D.; Bouzouita, N.; El Bok, S.; Sghaier, R.M.; Selmi, S.; Ben-Attia, M. Separation and evaluation of natural antileishmanial potential against Leishmania major and infantum isolated from the Tunisia strains. Bangladesh J. Pharmacol. 2018, 13, 74–81. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Brinza, I.; Boiangiu, R.S.; Ibrahim, M.H.; Honceriu, I.; Al-Maktoum, A.; Cioanca, O.; Hancianu, M.; Amin, A.; Ben-Attia, M.; et al. The effect of a Tribulus-based formulation in alleviating cholinergic system impairment and scopolamine-induced memory loss in zebrafish (Danio rerio): Insights from molecular docking and in vitro/in vivo approaches. Pharmaceuticals 2024, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Samad, N.; Saleem, A.; Yasmin, F.; Shehzad, M.A. Quercetin Protects against Stress-Induced Anxiety-and Depression-Like Behavior and Improves Memory in Male Mice. Physiol. Res. 2018, 67, 795–808. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Delarami Far, A.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G. Anticancer and Apoptosis-inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Islam, M.S.; Quispe, C.; Hossain, R.; Islam, M.T.; Al-Harrasi, A.; Al-Rawahi, A.; Martorell, M.; Mamurova, A.; Seilkhan, A.; Altybaeva, N. Neuropharmacological Effects of Quercetin: A Literature-Based Review. Front. Pharmacol. 2021, 12, 665031. [Google Scholar] [CrossRef]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Mesgari-Abbasi, M.; Salari, A.A. Quercetin Mitigates Anxiety-like Behavior and Normalizes Hypothalamus–Pituitary–Adrenal Axis Function in a Mouse Model of Mild Traumatic Brain Injury. Behav. Pharmacol. 2019, 30, 282–289. [Google Scholar] [CrossRef]
- Miller, R.L.; Siani, A.; Smith, J.D.; Hoyer, D. Brain Monoamine Oxidase: Localization and Role in Neurotransmission. J. Neurosci. Res. 2008, 86, 1219–1227. [Google Scholar] [CrossRef]
- Moghbelinejad, S.; Alizadeh, S.; Mohammadi, G.; Khodabandehloo, F.; Rashvand, Z.; Najafipour, R.; Nassiri-Asl, M. The effects of quercetin on the gene expression of the GABAA receptor A5 subunit gene in a mouse model of kainic acid-induced seizure. J. Physiol. Sci. 2017, 67, 339–343. [Google Scholar] [CrossRef]
- Parker, M.O.; Millington, M.E.; Combe, F.J.; Brennan, C.H. Development and implementation of a three-choice serial reaction time task for zebrafish (Danio rerio). Behav. Brain Res. 2012, 227, 73–80. [Google Scholar] [CrossRef]
- Zaki, J.; Weber, J.; Bolger, N.; Ochsner, K.N. The neural bases of empathic concern for others’ suffering. Proc. Natl. Acad. Sci. USA 2009, 106, 8073–8078. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef]
- Blaser, R.E.; Penalosa, Y.M. Stimuli affecting zebrafish (Danio rerio) behavior in the light/dark preference test. Physiol. Behav. 2011, 104, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Kannan, R.R. Zebrafish: An emerging real-time model system to study Alzheimer’s disease and neurospecific drug discovery. Cell Death Discov. 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.R.; Crawley, J.N. Anxiety-related behaviors in mice. In Methods of Behavior Analysis in Neuroscience, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009. [Google Scholar]
- McGown, A.; Shaw, D.P.; Ramesh, T. ZNStress: A high-throughput drug screening protocol for identification of compounds modulating neuronal stress in the transgenic mutant sod1G93R zebrafish model of amyotrophic lateral sclerosis. Mol. Neurodegener. 2016, 11, 56. [Google Scholar] [CrossRef]
- Kafeel, H.; Rukh, R. Anxiolytic activity of ethanolic extract of aerial parts of Tribulus terrestris in mice. J. Phytopharmacol. 2015, 4, 17–21. [Google Scholar] [CrossRef]
- Gaweska, H.; Fitzpatrick, P.F. Structures and mechanism of the monoamine oxidase family. Biomol. Concepts 2011, 2, 163–171. [Google Scholar] [CrossRef]
- Shalaby, R.; Petzer, J.P.; Petzer, A.; Ashraf, U.M.; Atari, E.; Alasmari, F.; Kumarasamy, Y.; Sari, Y.; Khalil, A. SAR and molecular mechanism studies of monoamine oxidase inhibition by selected chalcone analogs. J. Enzyme Inhib. Med. Chem. 2019, 34, 1166–1180. [Google Scholar] [CrossRef]
- Drevets, W.C.; Savitz, J. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008, 13, 663–681. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y.; Wang, Y.; Qi, S.; Wang, Y.; Ma, C.; Li, S.; Jiang, B.; Cheng, X.; Wang, Z. Anti-amnesic effect of extract and alkaloid fraction from aerial parts of Peganum harmala on scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2017, 204, 95–106. [Google Scholar] [CrossRef]
- Fontana, B.D.; Alnassar, N.; Parker, M.O. The Zebrafish (Danio rerio) Anxiety Test Battery: Comparison of Behavioral Responses in the Novel Tank Diving and Light–Dark Tasks Following Exposure to Anxiogenic and Anxiolytic Compounds. Psychopharmacology 2022, 239, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Bouabdallah, S.; Azzouz, R.; Al-Khafaji, K.; Ben-Attia, M. Investigating flavonoids derived from Tribulus terrestris L. as prospective candidates for Alzheimer’s disease treatment: Molecular docking modelingfuel of their interactions with physiological system receptors. J. Bioresour. Environ. Sci. 2024, 3, 93–100. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci. Rep. 2017, 7, 15374. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Hamilton, T.J. Modafinil decreases anxiety-like behaviour in zebrafish. PeerJ 2017, 5, e2994. [Google Scholar] [CrossRef]
- Maximino, C.; Lima, M.G.; Costa, C.C.; Guedes, I.M.; Herculano, A.M. Fluoxetine and WAY 100,635 dissociate increases in scototaxis and analgesia induced by conspecific alarm substance in zebrafish (Danio rerio Hamilton 1822). Pharmacol. Biochem. Behav. 2014, 124, 425–433. [Google Scholar] [CrossRef]
- Son, S.Y.; Ma, J.; Kondou, Y.; Yoshimura, M.; Yamashita, E.; Tsukihara, T. Structure of human monoamine oxidase A at 2.2-Å resolution: The control of opening the entry for substrates/inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 8017–8022. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- BIOVIA Discovery Studio Visualizer, v21.1.0.20298; BIOVIA, Dassault Systèmes: Vélizy-Villacoublay, France, 2005.
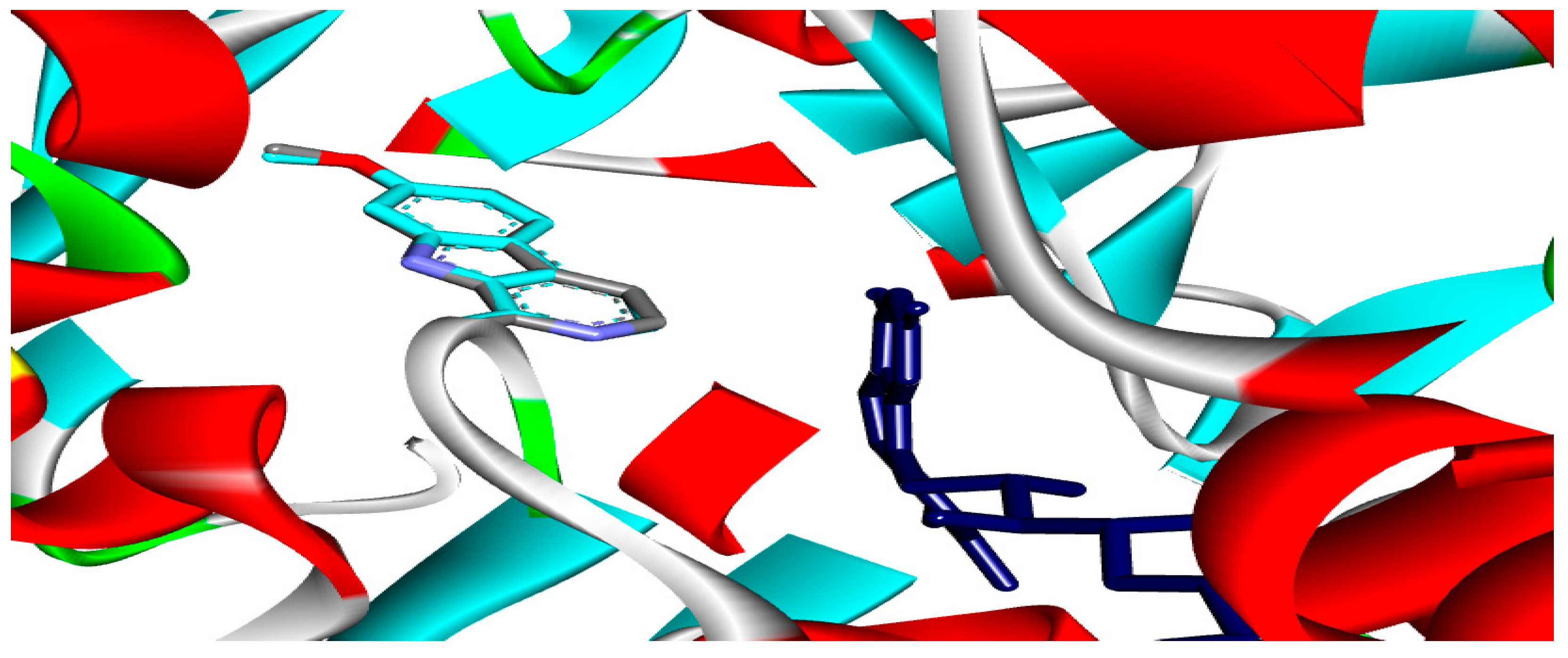

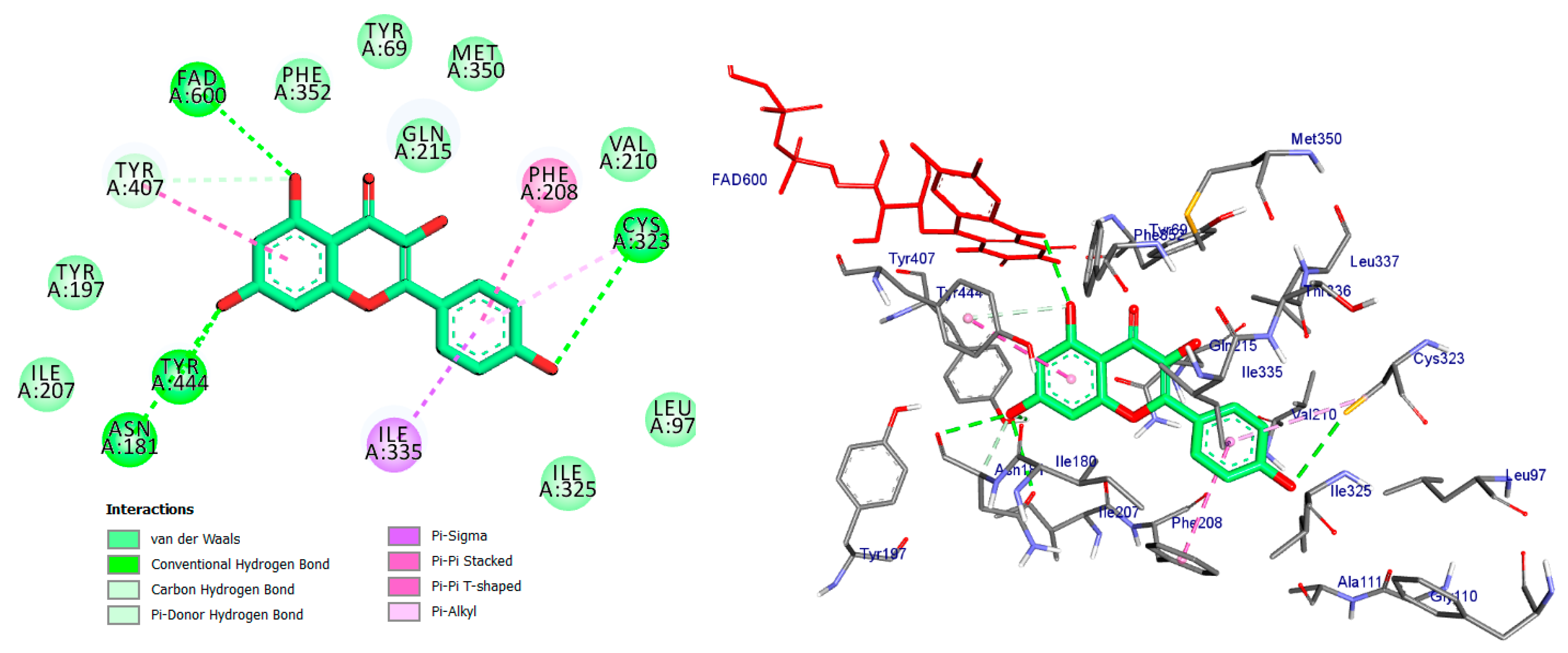
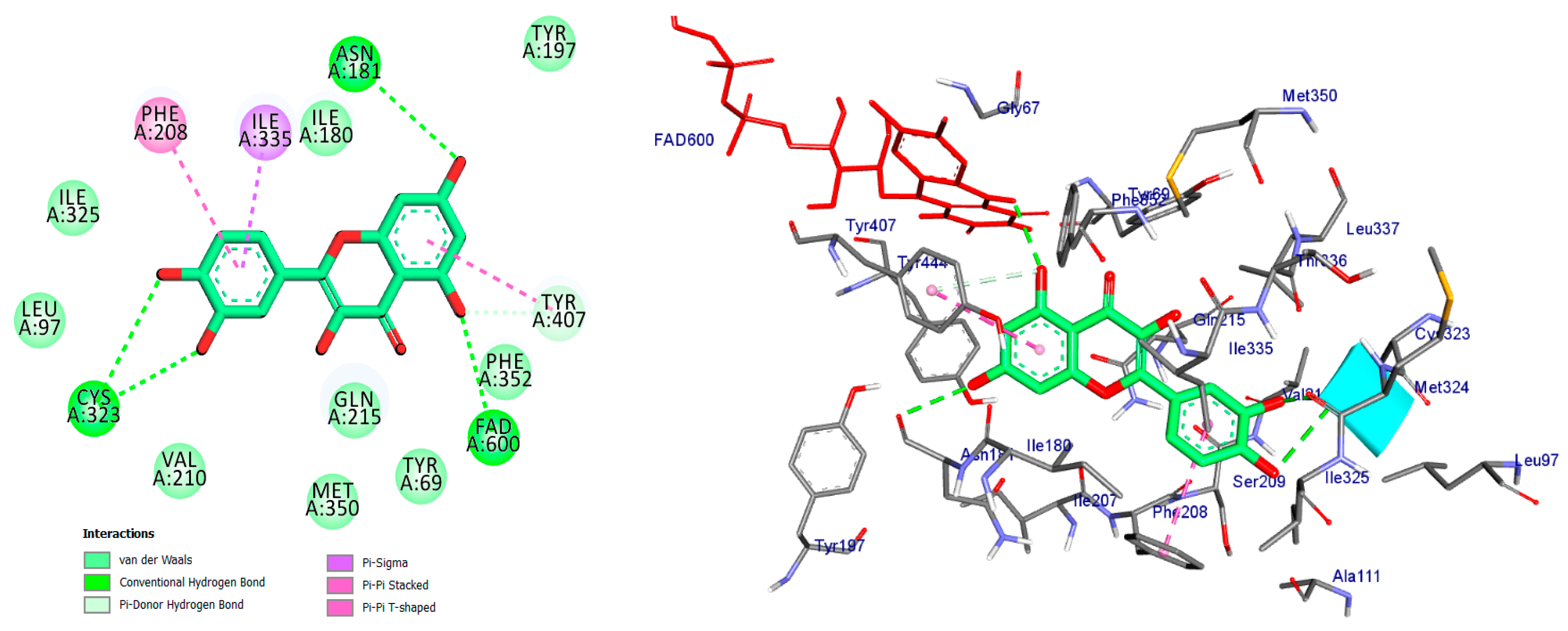


| Compound Name | Interaction/Amino Acid/Distance Å | Docking Energy Scores in kcal/mol |
|---|---|---|
| Co-crystal ligand (Harmine) | Pi-Sigma/Tyr407/3.74 Pi-Sigma/Tyr444/3.65 Pi-Sigma/FAD600/3.66 Pi-Sigma/FAD600/3.72 Pi-Pi Stacked/Tyr407/4.29 Pi-Alkyl/Ile335/4.46 Pi-Alkyl/Leu337/5.40 | −8.7 |
| Apigetrin | Conv. H-Bond/Val210/2.11 Conv. H-Bond/Cys321/3.43 Conv. H-Bond/Cys323/3.74 Conv. H-Bond/Thr336/2.57 Conv. H-Bond/Ala111/2.71 Car H-Bond/Ser209/3.42 Pi-Donor H-Bond/Tyr407/3.57 Pi-Donor H-Bond/Tyr444/3.90 Pi-Donor H-Bond/FAD600/4.05 Pi-Donor H-Bond/FAD600/4.12 Pi-Sigma/Ile335/3.50 Pi-Sulfur/Cys323/4.93 Pi-Pi Stacked/Tyr407/4.29 Pi-Alkyl/Leu337/5.23 | −7.2 |
| Kaempferol | Conv. H-Bond/Cys323/3.53 Conv. H-Bond/Tyr444/2.45 Conv. H-Bond/FAD600/2.95 Conv. H-Bond/Asn181/3.31 Conv. H-Bond/Asn181/3.18 Car H-Bond/Asn181/3.58 Pi-Donor H-Bond/Tyr407/4.12 Pi-Sigma/Ile335/3.54 Pi-Pi Stacked/Tyr407/4.78 Pi-Pi T-shaped/Phe208/4.71 Pi-Alkyl/Cys323/5.30 | −9.7 |
| Quercetin | Conv. H-Bond/Cys323/3.04 Conv. H-Bond/Cys323/3.70 Conv. H-Bond/FAD600/2.70 Conv. H-Bond/Asn181/3.30 Pi-Donor H-Bond/Tyr407/4.16 Pi-Sigma/Ile335/3.51 Pi-Pi Stacked/Tyr407/4.59 Pi-Pi T-shaped/Phe208/4.60 | −8.7 |
| Luteoline | Conv. H-Bond/Cys323/3.00 Conv. H-Bond/Cys323/3.55 Conv. H-Bond/Tyr444/2.47 Conv. H-Bond/FAD600/3.01 Conv. H-Bond/Asn181/3.38 Conv. H-Bond/Asn181/3.36 Pi-Sigma/Ile335/3.53 Pi-Pi Stacked/Tyr407/4.84 Pi-Pi T-shaped/Phe208/4.55 Pi-Alkyl/Cys323/5.39 | −8.8 |
| Epigallocatechin | Conv. H-Bond/Cys323/3.58 Conv. H-Bond/Tyr444/2.28 Conv. H-Bond/FAD600/2.55 Conv. H-Bond/Thr336/2.27 Conv. H-Bond/Phe208/2.84 Conv. H-Bond/Asn181/2.64 Pi-Donor H-Bond/Tyr407/3.78 Pi-Donor H-Bond/Tyr444/3.74 Pi-Donor H-Bond/Tyr407/3.70 Pi-Sigma/Ile335/3.70 Pi-Sulfur/Cys323/4.65 Pi-Pi Stacked/Tyr407/4.40 Pi-Alkyl/Leu337/4.81 | −7.2 |
| No. | Formula | Transformations | Error (ppm) | [M-H]−1 Experimental | [M-H]−1 Theoretical | Identification |
|---|---|---|---|---|---|---|
| 1 | C9H10O7 | Hydration, Oxidation | −0.03814 | 180.04225 | 179.03498 | Caffeic acid |
| 2 | C9H8O3 | Hydration, Oxidation | −0.02587 | 198.0402 | 199.0606 | Hydroxycinnamic acid |
| 3 | C21H20O11 | Reduction | −0.38036 | 196.05823 | 195.05095 | Cynarosie |
| 4 | C15H10O7 | Reduction | 0.17143 | 301.0353 | 302.24 | Quercetin |
| 5 | C15H10O6 | Nitro Reduction | −0.21529 | 285.0409 | 286.24 | Kaempferol |
| 6 | C15H10O6 | Nitro Reduction | 0.28338 | 285.04120 | 286.24 | Luteoline |
| 7 | C15H14O7 | Hydration, Oxidation | 0.45039 | 356.07451 | 306.27 | Epigallocatechin |
| 8 | C21H20O10 | Reduction | 0.18654 | 435.5 | 432.4 | Apigetrin |
| 9 | C27H30O16 | Hydratation | −1.45637 | 609.1482 | 610.1084 | Rutin |
| 10 | C45H72O17 | Reduction | −4.4 | 915.4550 | 915.4590 | Terreside A |
| 11 | C27H42O4 | Reduction | −0.38036 | 255.08642 | 430.30764 | Hecogenine |
| 12 | C39H62O14 | Desaturation, Nitro Reduction | 0.3610 | 755.262 | 754.901 | Terreside B |
| 13 | C45H12O15 | Desaturation, Nitro Reduction | −0.38036 | 918.4232 | 915.4590 | Terrestrosin C |
| 14 | C39H62O13 | Desaturation, Nitro Reduction | 0.95112 | 738.05805 | 738.05077 | Trillarin |
| 15 | C51H84O22 | Oxidation, Nitro Reduction | −0.10032 | 1047.5413 | 1049.2 | Protodioscin |
| 16 | C33H52O8 | Desaturation, Nitro Reduction | 2.37949 | 560.22 | 576.761 | Disogluside–Trillin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouabdallah, S.; Ibrahim, M.H.; Brinza, I.; Boiangiu, R.S.; Honceriu, I.; Amin, A.; Ben-Attia, M.; Hritcu, L. Anxiolytic and Antidepressant Effects of Tribulus terrestris Ethanolic Extract in Scopolamine-Induced Amnesia in Zebrafish: Supported by Molecular Docking Investigation Targeting Monoamine Oxidase A. Pharmaceuticals 2024, 17, 1208. https://doi.org/10.3390/ph17091208
Bouabdallah S, Ibrahim MH, Brinza I, Boiangiu RS, Honceriu I, Amin A, Ben-Attia M, Hritcu L. Anxiolytic and Antidepressant Effects of Tribulus terrestris Ethanolic Extract in Scopolamine-Induced Amnesia in Zebrafish: Supported by Molecular Docking Investigation Targeting Monoamine Oxidase A. Pharmaceuticals. 2024; 17(9):1208. https://doi.org/10.3390/ph17091208
Chicago/Turabian StyleBouabdallah, Salwa, Mona H. Ibrahim, Ion Brinza, Razvan Stefan Boiangiu, Iasmina Honceriu, Amr Amin, Mossadok Ben-Attia, and Lucian Hritcu. 2024. "Anxiolytic and Antidepressant Effects of Tribulus terrestris Ethanolic Extract in Scopolamine-Induced Amnesia in Zebrafish: Supported by Molecular Docking Investigation Targeting Monoamine Oxidase A" Pharmaceuticals 17, no. 9: 1208. https://doi.org/10.3390/ph17091208
APA StyleBouabdallah, S., Ibrahim, M. H., Brinza, I., Boiangiu, R. S., Honceriu, I., Amin, A., Ben-Attia, M., & Hritcu, L. (2024). Anxiolytic and Antidepressant Effects of Tribulus terrestris Ethanolic Extract in Scopolamine-Induced Amnesia in Zebrafish: Supported by Molecular Docking Investigation Targeting Monoamine Oxidase A. Pharmaceuticals, 17(9), 1208. https://doi.org/10.3390/ph17091208











