Reduction of Multispecies Biofilms on an Acrylic Denture Base Model by Antimicrobial Photodynamic Therapy Mediated by Natural Photosensitizers
Abstract
1. Introduction
2. Results
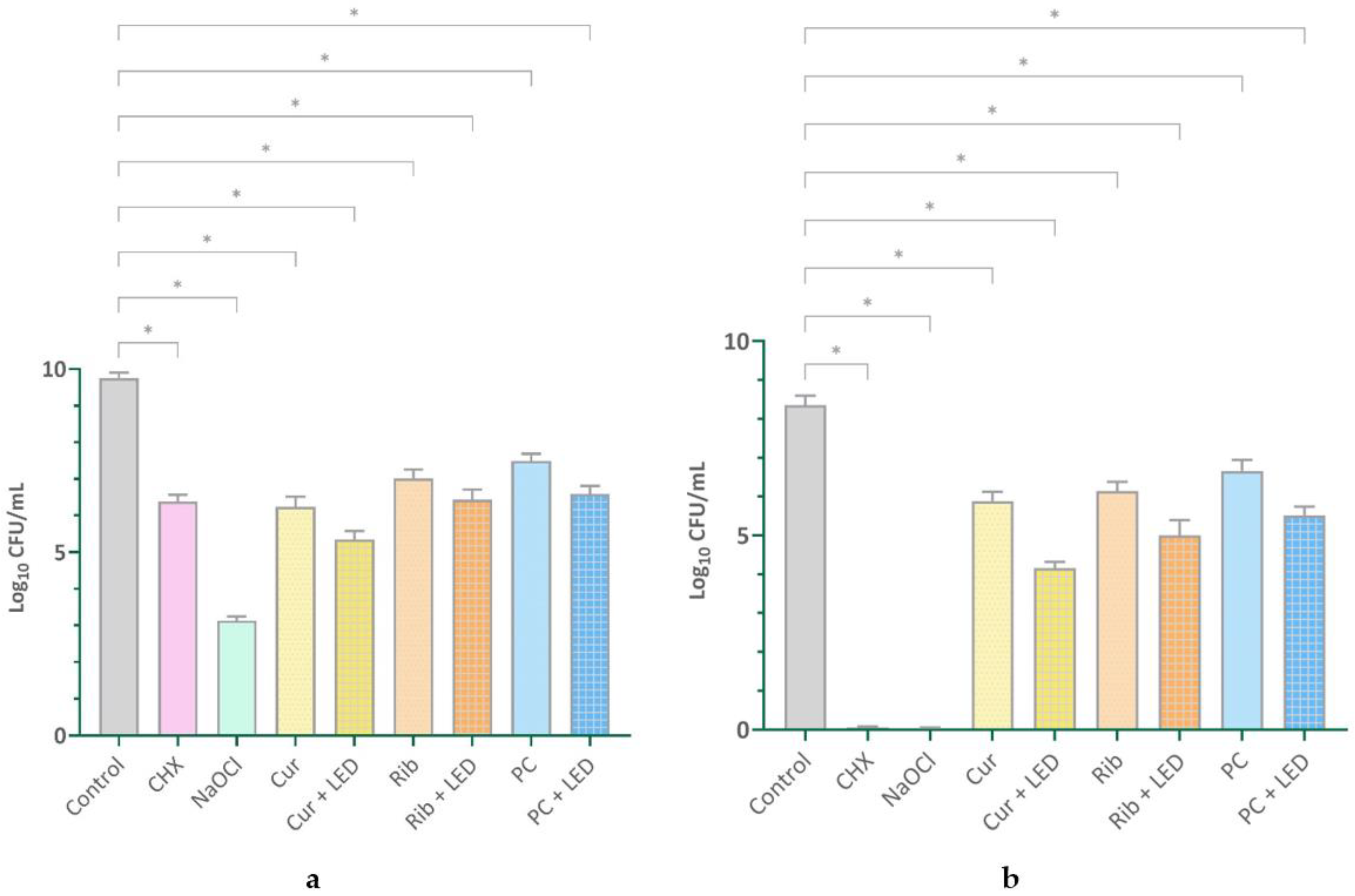
2.1. Quantifying Viable Cells
2.2. Biofilm Degradation Assay Results
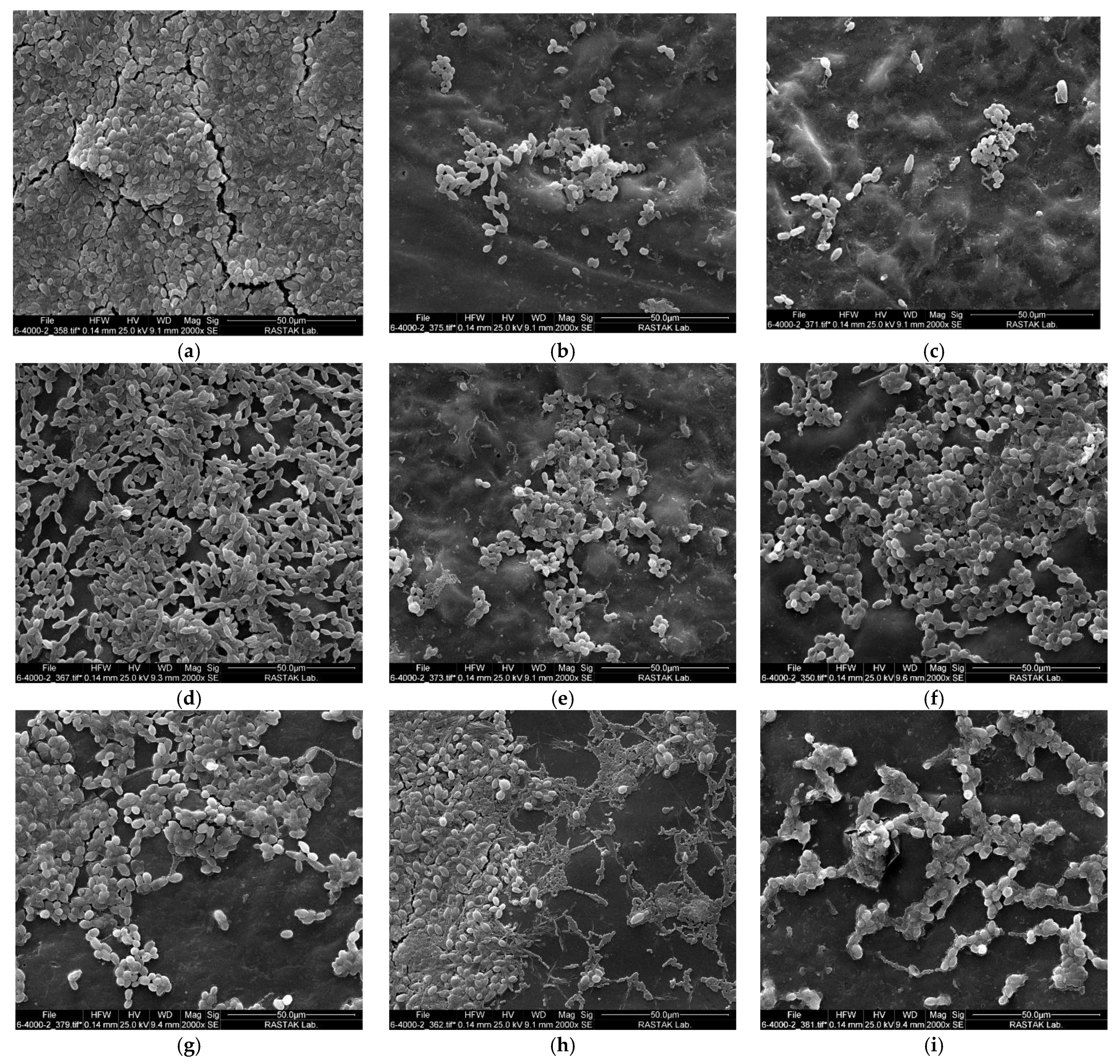
2.3. Scanning Electron Microscopy Results
3. Discussion
4. Materials and Methods
4.1. Fabrication of Acrylic Resin Specimens
4.2. Microorganisms and Biofilm Formation
4.3. Photosensitizers and Light Sources
4.4. Study Groups
4.5. Sampling and Counting of Viable Cells
4.6. Assessment of Biofilm Degradation by a Colorimetric Assay
4.7. Assessment of Biofilm Degradation by a SEM
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AlHamdan, E.M. Influence of contemporary photoactivated disinfection on the mechanical properties and antimicrobial activity of PMMA denture base: A systematic review and meta analysis. Photodiagnosis Photodyn. Ther. 2023, 42, 103523. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; Lopez-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hannah, V.E.; O’Donnell, L.; Robertson, D.; Ramage, G. Denture Stomatitis: Causes, Cures and Prevention. Prim. Dent. J. 2017, 6, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sartawi, S.Y.; Abu-Hammad, S.; Salim, N.A.; Al-Omoush, S. Denture Stomatitis Revisited: A Summary of Systematic Reviews in the Past Decade and Two Case Reports of Papillary Hyperplasia of Unusual Locations. Int. J. Dent. 2021, 2021, 7338143. [Google Scholar] [CrossRef]
- McReynolds, D.E.; Moorthy, A.; Moneley, J.O.; Jabra-Rizk, M.A.; Sultan, A.S. Denture stomatitis-An interdisciplinary clinical review. J. Prosthodont. 2023, 32, 560–570. [Google Scholar] [CrossRef]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust. Dent. J. 1998, 43, 45–50. [Google Scholar] [CrossRef]
- Aguayo, S.; Marshall, H.; Pratten, J.; Bradshaw, D.; Brown, J.S.; Porter, S.R.; Spratt, D.; Bozec, L. Early Adhesion of Candida albicans onto Dental Acrylic Surfaces. J. Dent. Res. 2017, 96, 917–923. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Figueiral, M.H.; Sousa-Rodrigues, P.; Fernandes, M.H.; Scully, C. The effect of denture adhesives on Candida albicans growth in vitro. Gerodontology 2012, 29, e348–e356. [Google Scholar] [CrossRef]
- Pereira, C.A.; Domingues, N.; Araujo, M.I.; Junqueira, J.C.; Back-Brito, G.N.; Jorge, A.O. Production of virulence factors in Candida strains isolated from patients with denture stomatitis and control individuals. Diagn. Microbiol. Infect. Dis. 2016, 85, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Fidel, P.L., Jr.; Vazquez, J.A.; Sobel, J.D. Candida glabrata: Review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 1999, 12, 80–96. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Freitas, L.S.; Rossoni, R.D.; Jorge, A.O.C.; Junqueira, J.C. Repeated applications of photodynamic therapy on Candida glabrata biofilms formed in acrylic resin polymerized. Lasers Med. Sci. 2017, 32, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Satala, D.; Gonzalez-Gonzalez, M.; Smolarz, M.; Surowiec, M.; Kulig, K.; Wronowska, E.; Zawrotniak, M.; Kozik, A.; Rapala-Kozik, M.; Karkowska-Kuleta, J. The Role of Candida albicans Virulence Factors in the Formation of Multispecies Biofilms with Bacterial Periodontal Pathogens. Front. Cell Infect. Microbiol. 2021, 11, 765942. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Romeiro, R.L.; Costa, A.C.; Machado, A.K.; Junqueira, J.C.; Jorge, A.O. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: An in vitro study. Lasers Med. Sci. 2011, 26, 341–348. [Google Scholar] [CrossRef]
- Al-Qahtani, M.A. Efficacy of antimicrobial photodynamic therapy in disinfection of Candida biofilms on acrylic dentures: A systematic review. Photodiagnosis Photodyn. Ther. 2022, 40, 102980. [Google Scholar] [CrossRef]
- Abuhajar, E.; Ali, K.; Zulfiqar, G.; Al Ansari, K.; Raja, H.Z.; Bishti, S.; Anweigi, L. Management of Chronic Atrophic Candidiasis (Denture Stomatitis)-A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3029. [Google Scholar] [CrossRef]
- Pires, C.W.; Fraga, S.; Beck, A.C.; Braun, K.O.; Peres, P.E. Chemical Methods for Cleaning Conventional Dentures: What is the Best Antimicrobial Option? An In Vitro Study. Oral. Health Prev. Dent. 2017, 15, 73–77. [Google Scholar] [CrossRef]
- Amaya Arbelaez, M.I.; Vergani, C.E.; Barbugli, P.A.; Pavarina, A.C.; Sanita, P.V.; Jorge, J.H. Long-Term Effect of Daily Chemical Disinfection on Surface Topography and Candida albicans Biofilm Formation on Denture Base and Reline Acrylic Resins. Oral. Health Prev. Dent. 2020, 18, 999–1010. [Google Scholar] [CrossRef]
- Santos Sousa, T.M.; Rodrigues de Farias, O.; Dantas Batista, A.U.; Souto de Medeiros, E.; Santiago, B.M.; Cavalcanti, Y.W. Effectiveness of denture microwave disinfection for treatment of denture stomatitis: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2021, 19, 62–77. [Google Scholar] [CrossRef]
- Sartori, E.A.; Schmidt, C.B.; Walber, L.F.; Shinkai, R.S. Effect of microwave disinfection on denture base adaptation and resin surface roughness. Braz. Dent. J. 2006, 17, 195–200. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.M.B.; Venante, H.S.; Pordeus, M.D.; Chappuis-Chocano, A.P.; Neppelenbroek, K.H.; Santiago Junior, J.F.; Porto, V.C. Does microwave disinfection affect the dimensional stability of denture base acrylic resins? A systematic review. Gerodontology 2022, 39, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Shahabi, S.; Jazaeri, M.; Hadilou, M.; Fekrazad, R. Clinical applications of antimicrobial photodynamic therapy in dentistry. Front. Microbiol. 2022, 13, 1020995. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Fuchs, B.B.; Coleman, J.J.; Prates, R.A.; Astrakas, C.; St Denis, T.G.; Ribeiro, M.S.; Mylonakis, E.; Hamblin, M.R.; Tegos, G.P. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front. Microbiol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Jao, Y.; Ding, S.J.; Chen, C.C. Antimicrobial photodynamic therapy for the treatment of oral infections: A systematic review. J. Dent. Sci. 2023, 18, 1453–1466. [Google Scholar] [CrossRef]
- Li, Y.; Sun, G.; Xie, J.; Xiao, S.; Lin, C. Antimicrobial photodynamic therapy against oral biofilm: Influencing factors, mechanisms, and combined actions with other strategies. Front. Microbiol. 2023, 14, 1192955. [Google Scholar] [CrossRef]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Chignell, C.F.; Bilski, P.; Reszka, K.J.; Motten, A.G.; Sik, R.H.; Dahl, T.A. Spectral and photochemical properties of curcumin. Photochem. Photobiol. 1994, 59, 295–302. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef]
- Farah, N.; Chin, V.K.; Chong, P.P.; Lim, W.F.; Lim, C.W.; Basir, R.; Chang, S.K.; Lee, T.Y. Riboflavin as a promising antimicrobial agent? A multi-perspective review. Curr. Res. Microb. Sci. 2022, 3, 100111. [Google Scholar] [CrossRef] [PubMed]
- Bartzatt, R.; Follis, M. Detection and Assay of Riboflavin (Vitamin B2) Utilizing UV/VIS Spectrophotometer and Citric Acid Buffer. J. Sci. Res. Rep. 2014, 3, 799. [Google Scholar] [CrossRef]
- Bharathiraja, S.; Seo, H.; Manivasagan, P.; Santha Moorthy, M.; Park, S.; Oh, J. In vitro photodynamic effect of phycocyanin against breast cancer cells. Molecules 2016, 21, 1470. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Pourhajibagher, M.; Chiniforush, N.; Aminian, M.; Rasi Varaei, S.S.; Bahador, A. Effects of sub-lethal dose of antimicrobial photodynamic therapy on major virulence traits of Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2020, 32, 102044. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.V.; Karibasappa, G.N.; Dodamani, A.; Prashanth, V.K. Microbial contamination of removable dental prosthesis at different interval of usage: An in vitro study. J. Indian. Prosthodont. Soc. 2016, 16, 346–351. [Google Scholar] [CrossRef]
- Jainkittivong, A.; Aneksuk, V.; Langlais, R.P. Oral mucosal lesions in denture wearers. Gerodontology 2010, 27, 26–32. [Google Scholar] [CrossRef]
- Kim, D.; Liu, Y.; Benhamou, R.I.; Sanchez, H.; Simon-Soro, A.; Li, Y.; Hwang, G.; Fridman, M.; Andes, D.R.; Koo, H. Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. ISME J. 2018, 12, 1427–1442. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An In Vitro Model for Oral Mixed Biofilms of Candida albicans and Streptococcus gordonii in Synthetic Saliva. Front. Microbiol. 2016, 7, 686. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Pizziolo, P.G.; Clemente, L.M.; Aguiar, H.C.; Poker, B.C.; Silva, A.; Makrakis, L.R.; Fifolato, M.A.; Souza, G.C.; Oliveira, V.C.; et al. Strategies for Preventing and Treating Oral Mucosal Infections Associated with Removable Dentures: A Scoping Review. Antibiotics 2024, 13, 273. [Google Scholar] [CrossRef]
- Peric, M.; Milicic, B.; Kuzmanovic Pficer, J.; Zivkovic, R.; Arsic Arsenijevic, V. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida spp. Distribution. J. Fungi 2024, 10, 328. [Google Scholar] [CrossRef]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef] [PubMed]
- Vale-Silva, L.A.; Sanglard, D. Tipping the balance both ways: Drug resistance and virulence in Candida glabrata. FEMS Yeast Res. 2015, 15, fov025. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Sharma, S.; Shrivastva, P. Multi-species biofilm of Candida albicans and non-Candida albicans Candida species on acrylic substrate. J. Appl. Oral. Sci. 2012, 20, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Alhenaki, A.M.; Alqarawi, F.K.; Tanveer, S.A.; Alshahrani, F.A.; Alshahrani, A.; AlHamdan, E.M.; Alzahrani, K.M.; Aldahiyan, N.; Naseem, M.; Vohra, F.; et al. Disinfection of acrylic denture resin polymer with Rose Bengal, Methylene blue and Porphyrin derivative in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 35, 102362. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Rogoz, K.; Mysliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieslar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The use of photodynamic therapy in medical practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef]
- Konopka, K.; Goslinski, T. Photodynamic therapy in dentistry. J. Dent. Res. 2007, 86, 694–707. [Google Scholar] [CrossRef]
- Alshehri, A.H. Mechanical and antimicrobial effects of riboflavin-mediated photosensitization of in vitro C. albicans formed on polymethyl methacrylate resin. Photodiagnosis Photodyn. Ther. 2021, 36, 102488. [Google Scholar] [CrossRef]
- Farah, N.; Lim, C.W.; Chin, V.K.; Chong, P.P.; Basir, R.; Yeo, W.W.Y.; Tay, S.T.; Choo, S.; Lee, T.Y. Photoactivated riboflavin inhibits planktonic and biofilm growth of Candida albicans and non-albicans Candida species. Microb. Pathog. 2024, 191, 106665. [Google Scholar] [CrossRef]
- Mansoorabadi, S.O.; Thibodeaux, C.J.; Liu, H.W. The diverse roles of flavin coenzymes–nature’s most versatile thespians. J. Org. Chem. 2007, 72, 6329–6342. [Google Scholar] [CrossRef]
- Etemadi, A.; Hamidain, M.; Parker, S.; Chiniforush, N. Blue Light Photodynamic Therapy with Curcumin and Riboflavin in the Management of Periodontitis: A Systematic Review. J. Lasers Med. Sci. 2021, 12, e15. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Pavarina, A.C.; Ribeiro, A.P.; Brunetti, I.L.; Costa, C.A.; Jacomassi, D.P.; Bagnato, V.S.; Kurachi, C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem. Photobiol. 2011, 87, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Pileggi, G.; Wataha, J.C.; Girard, M.; Grad, I.; Schrenzel, J.; Lange, N.; Bouillaguet, S. Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagnosis Photodyn. Ther. 2013, 10, 134–140. [Google Scholar] [CrossRef]
- Araujo, N.C.; Fontana, C.R.; Bagnato, V.S.; Gerbi, M.E. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers Med. Sci. 2014, 29, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Quishida, C.C.; De Oliveira Mima, E.G.; Jorge, J.H.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic inactivation of a multispecies biofilm using curcumin and LED light. Lasers Med. Sci. 2016, 31, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Labban, N.; Taweel, S.M.A.; ALRabiah, M.A.; Alfouzan, A.F.; Alshiddi, I.F.; Assery, M.K. Efficacy of Rose Bengal and Curcumin mediated photodynamic therapy for the treatment of denture stomatitis in patients with habitual cigarette smoking: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2021, 35, 102380. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Eftekhari Bayati, S.; Pourhajibagher, M.; Chiniforush, N. In vitro effect of antimicrobial photodynamic therapy with phycocyanin on Aggregatibacter actinomycetemcomitans biofilm on SLA titanium discs. Photodiagnosis Photodyn. Ther. 2020, 32, 102062. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, A.S.; Jafari, Z.; Chiniforush, N.; Afrasiabi, S. In vitro antibiofilm effect of different irradiation doses in infected root canal model. Photodiagnosis Photodyn. Ther. 2024, 46, 104053. [Google Scholar] [CrossRef]
- Moradi, Z.; Baghbani, F.; Kermanshah, H.; Chiniforush, N.; Afrasiabi, S. Destruction of cariogenic biofilms by antimicrobial photodynamic therapy mediated by phycocyanin and toluidine blue along with sodium fluoride varnish or titanium tetrafluoride. Photodiagnosis Photodyn. Ther. 2024, 49, 104296. [Google Scholar] [CrossRef]
- Yun, S.H.; Kwok, S.J.J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 2017, 1, 0008. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Sorbellini, E.; Rucco, M.; Rinaldi, F. Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: An update. Lasers Med. Sci. 2018, 33, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Mitsuoka, T.; Torikai, A.; Fueki, K. Wavelength sensitivity of the photodegradation of poly(methyl methacrylate). J. Appl. Polym. Sci. 1993, 47, 1027–1032. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.X.; Xu, H.H. Effects of quaternary ammonium chain length on the antibacterial and remineralizing effects of a calcium phosphate nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahi Ardakani, A.; Benedicenti, S.; Solimei, L.; Shahabi, S.; Afrasiabi, S. Reduction of Multispecies Biofilms on an Acrylic Denture Base Model by Antimicrobial Photodynamic Therapy Mediated by Natural Photosensitizers. Pharmaceuticals 2024, 17, 1232. https://doi.org/10.3390/ph17091232
Shahi Ardakani A, Benedicenti S, Solimei L, Shahabi S, Afrasiabi S. Reduction of Multispecies Biofilms on an Acrylic Denture Base Model by Antimicrobial Photodynamic Therapy Mediated by Natural Photosensitizers. Pharmaceuticals. 2024; 17(9):1232. https://doi.org/10.3390/ph17091232
Chicago/Turabian StyleShahi Ardakani, Ali, Stefano Benedicenti, Luca Solimei, Sima Shahabi, and Shima Afrasiabi. 2024. "Reduction of Multispecies Biofilms on an Acrylic Denture Base Model by Antimicrobial Photodynamic Therapy Mediated by Natural Photosensitizers" Pharmaceuticals 17, no. 9: 1232. https://doi.org/10.3390/ph17091232
APA StyleShahi Ardakani, A., Benedicenti, S., Solimei, L., Shahabi, S., & Afrasiabi, S. (2024). Reduction of Multispecies Biofilms on an Acrylic Denture Base Model by Antimicrobial Photodynamic Therapy Mediated by Natural Photosensitizers. Pharmaceuticals, 17(9), 1232. https://doi.org/10.3390/ph17091232







