The Role of Endogenous Beta-Endorphin and Enkephalins in the Crosstalk Between Ethanol and Morphine
Abstract
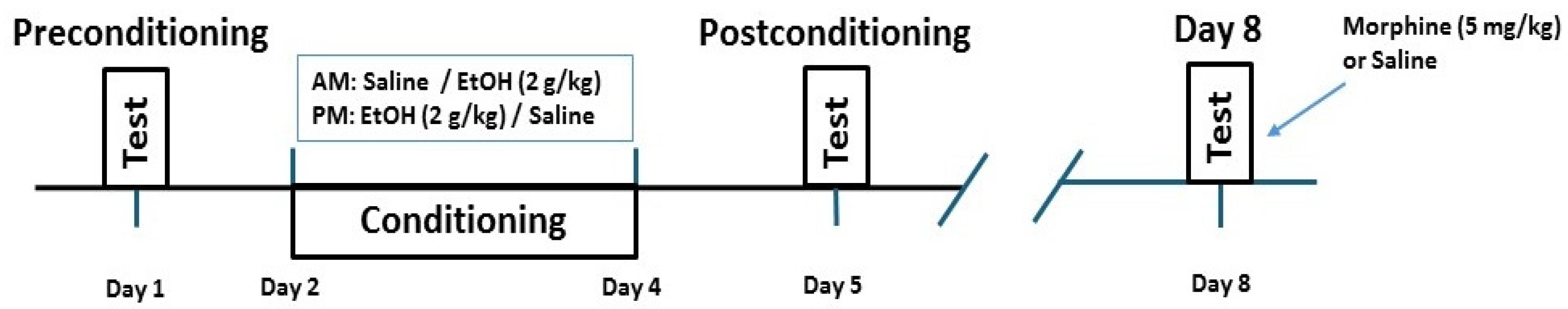
:1. Introduction
2. Results
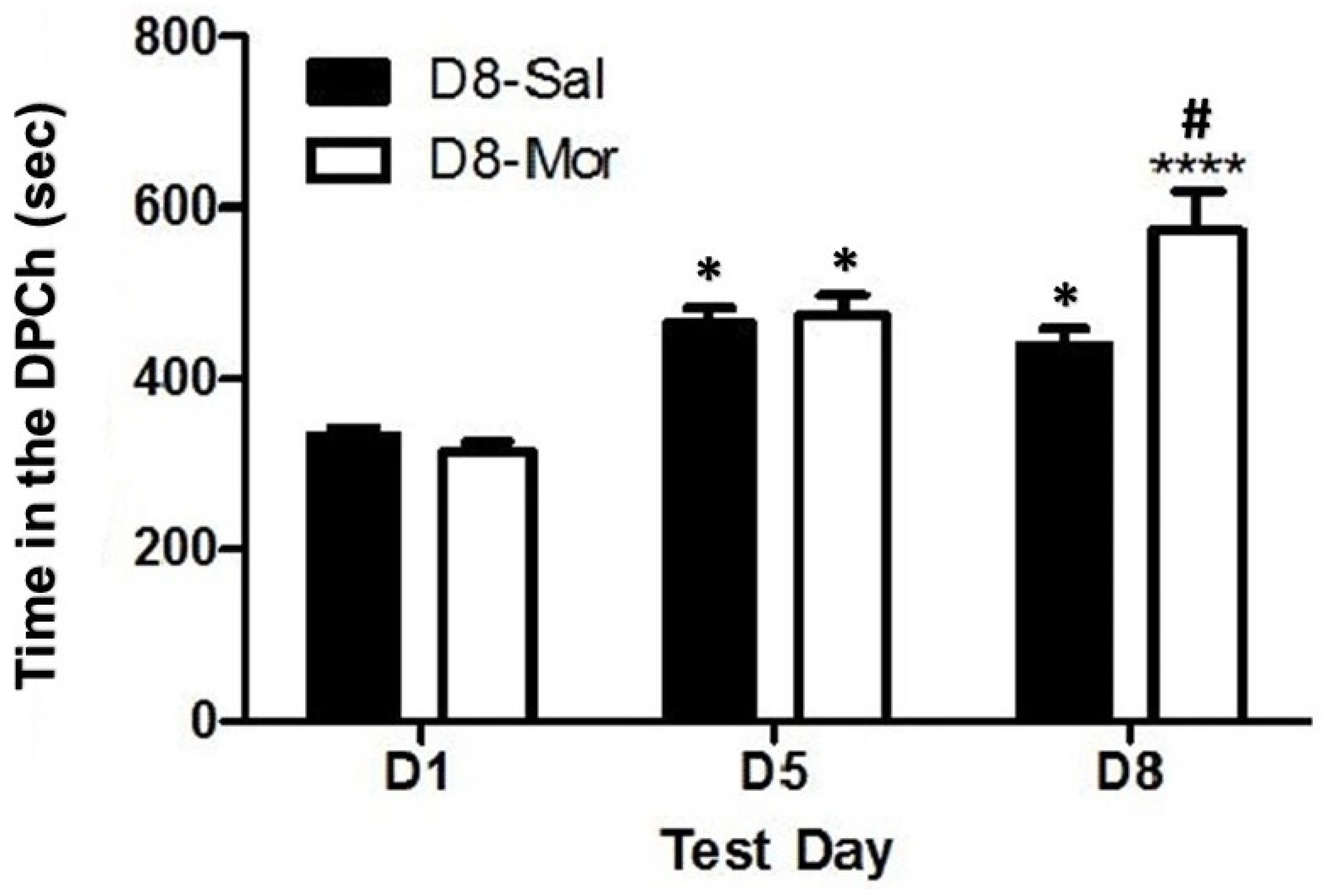
2.1. Ethanol-Induced CPP in C57BL/6J Mice, and This Response Was Potentiated by a Morphine Challenge
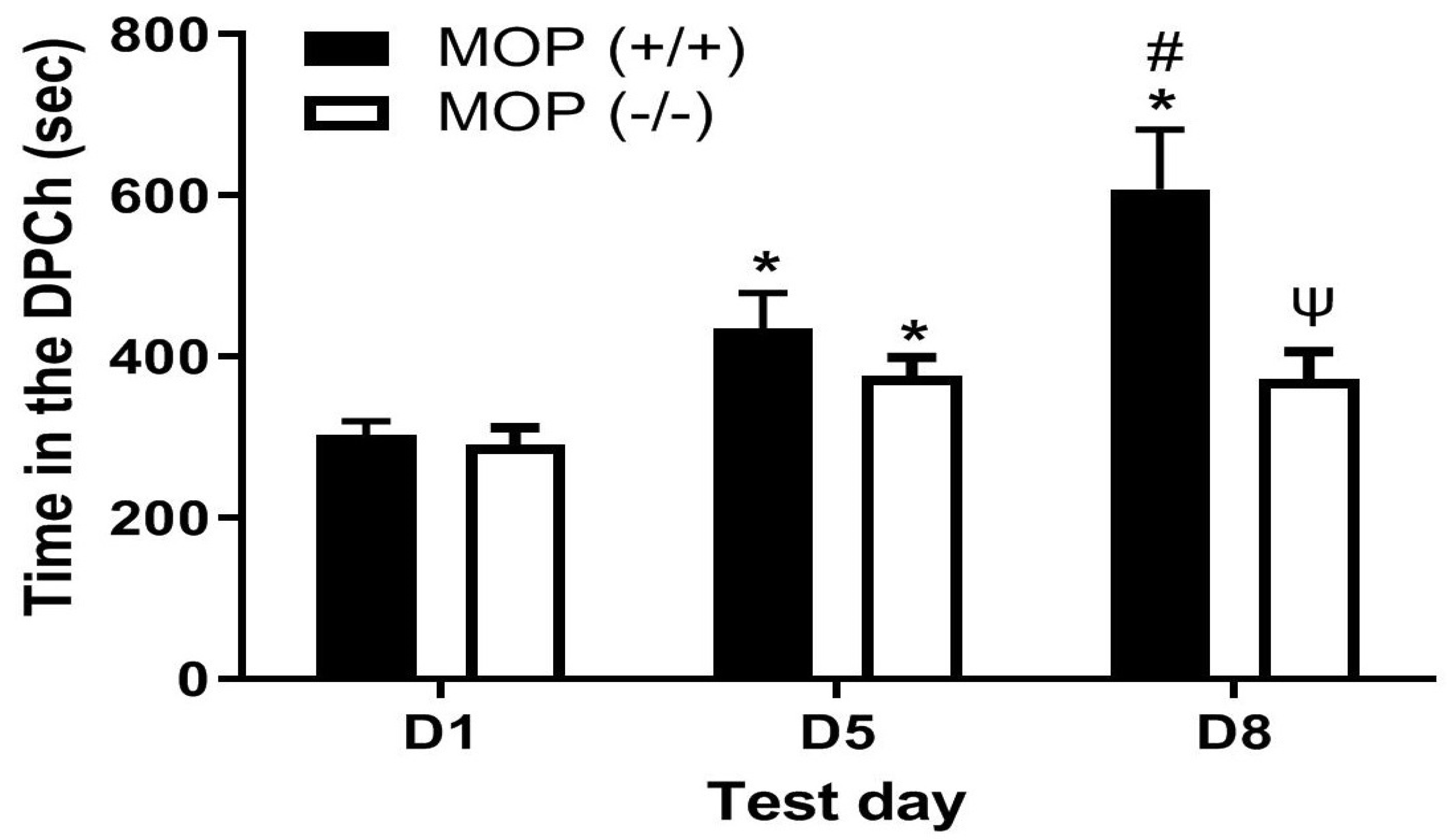
2.2. Morphine Potentiated Ethanol-Induced CPP in Wild-Type but Not MOP-Null Mice
2.3. Morphine-Induced Potentiation of Ethanol CPP Was Not Altered in Mice Lacking β-END Compared to Wild-Type Controls
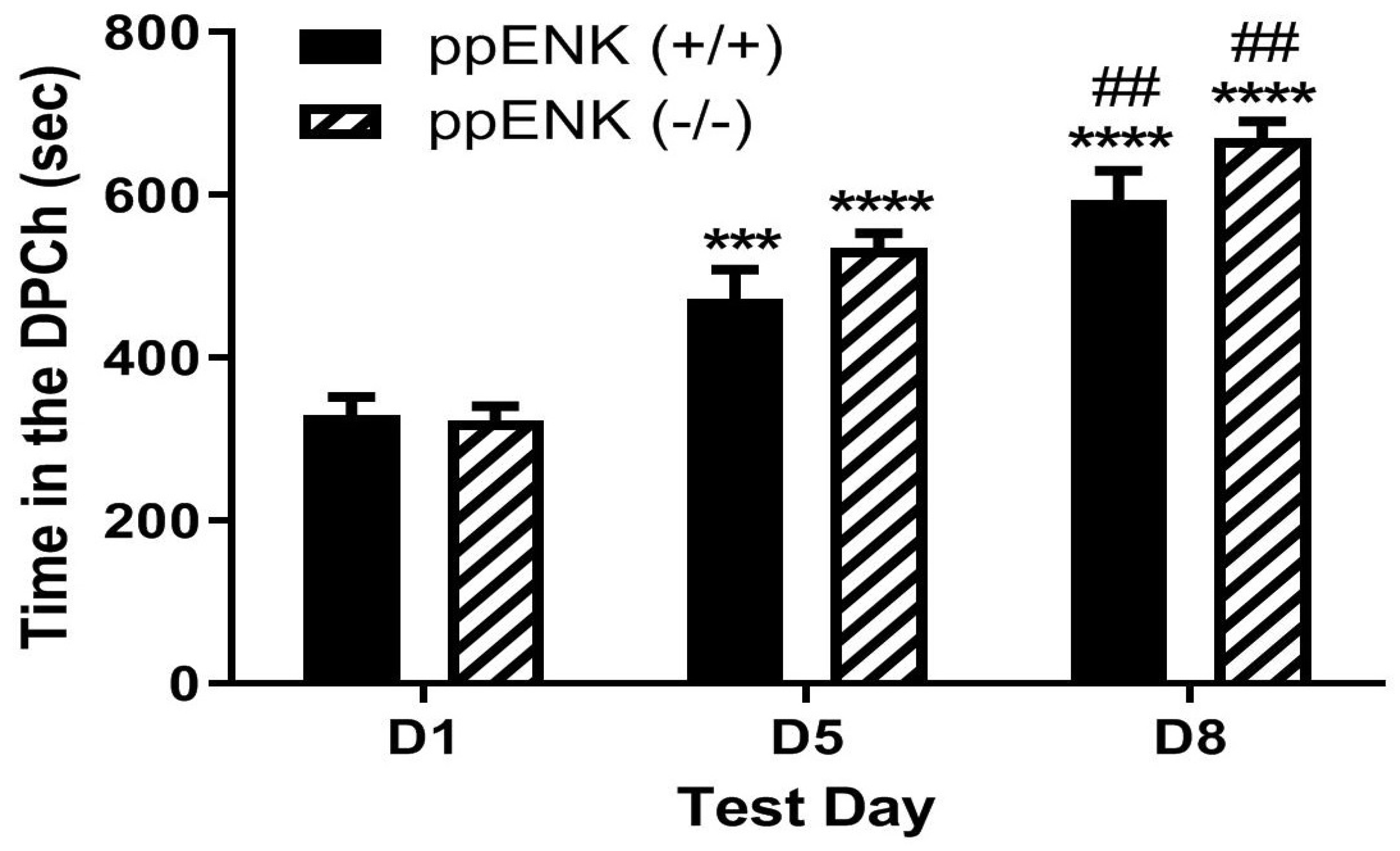
2.4. Morphine-Induced Potentiation of Ethanol CPP Was Not Altered in Mice Lacking Enkephalins Compared to Wild-Type Controls
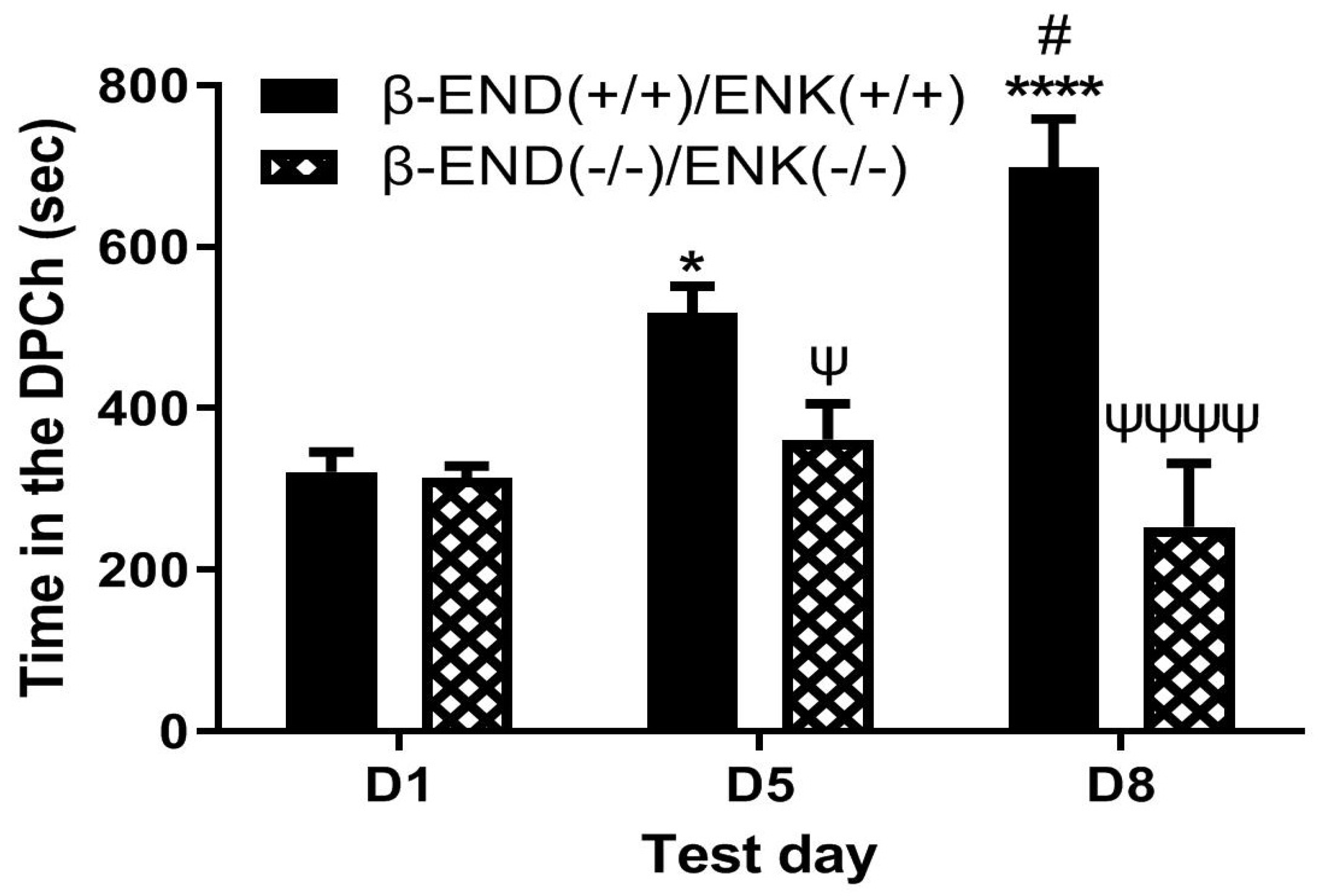
2.5. Morphine-Induced Potentiation of Ethanol CPP Was Abolished in Mice Lacking Both β-END and Enkephalins
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Drugs
4.3. Place Conditioning Paradigm
4.3.1. Apparatus
4.3.2. Experimental Procedure
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altshuler, H.L.; Phillips, P.E.; Feinhandler, D.A. Alteration of ethanol self-administration by naltrexone. Life Sci. 1980, 26, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, J.C.; Harts, J.; Lumeng, L.; Li, T.K. Naloxone attenuates voluntary ethanol intake in rats selectively bred for high ethanol preference. Pharmacol. Biochem. Behav. 1990, 35, 385–390. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, S.S.; Krishnan-Sarin, S.; Farren, C.; Sinha, R.; Kreek, M.J. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology 2002, 160, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, J.R.; Alterman, A.I.; Hayashida, M.; O’Brien, C.P. Naltrexone in the treatment of alcohol dependence. Arch. Gen. Psychiatry 1992, 49, 876–880. [Google Scholar] [CrossRef]
- Zhou, Y.; Kreek, M.J. Clinically utilized kappa-opioid receptor agonist nalfurafine combined with low-dose naltrexone prevents alcohol relapse-like drinking in male and female mice. Brain Res. 2019, 1724, 146410. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.P.; Marinelli, P.W.; Bai, L.; Gianoulakis, C. Effects of acute ethanol on opioid peptide release in the central amygdala: An in vivo microdialysis study. Psychopharmacology 2008, 201, 261–271. [Google Scholar] [CrossRef]
- Lam, M.P.; Nurmi, H.; Rouvinen, N.; Kiianmaa, K.; Gianoulakis, C. Effects of acute ethanol on beta-endorphin release in the nucleus accumbens of selectively bred lines of alcohol-preferring AA and alcohol-avoiding ANA rats. Psychopharmacology 2010, 208, 121–130. [Google Scholar] [CrossRef]
- Marinelli, P.W.; Bai, L.; Quirion, R.; Gianoulakis, C. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol. Clin. Exp. Res. 2005, 29, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, P.W.; Quirion, R.; Gianoulakis, C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology 2003, 169, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Olive, M.F.; Koenig, H.N.; Nannini, M.A.; Hodge, C.W. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J. Neurosci. 2001, 21, RC184. [Google Scholar] [CrossRef]
- Li, X.W.; Li, T.K.; Froehlich, J.C. Enhanced sensitivity of the nucleus accumbens proenkephalin system to alcohol in rats selectively bred for alcohol preference. Brain Res. 1998, 794, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Zale, E.L.; Maisto, S.A.; Ditre, J.W. Interrelations between pain and alcohol: An integrative review. Clin. Psychol. Rev. 2015, 37, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Esser, M.B.; Guy, G.P., Jr.; Zhang, K.; Brewer, R.D. Binge Drinking and Prescription Opioid Misuse in the U.S., 2012–2014. Am. J. Prev. Med. 2019, 57, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Boissoneault, J.; Lewis, B.; Nixon, S.J. Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol 2019, 75, 47–54. [Google Scholar] [CrossRef]
- Ferguson, E.; Lewis, B.; Teitelbaum, S.; Reisfield, G.; Robinson, M.; Boissoneault, J. Longitudinal associations between pain and substance use disorder treatment outcomes. J. Subst. Abus. Treat. 2022, 143, 108892. [Google Scholar] [CrossRef] [PubMed]
- Weathermon, R.; Crabb, D.W. Alcohol and medication interactions. Alcohol. Res. Health J. Natl. Inst. Alcohol. Abus. Alcohol. 1999, 23, 40–54. [Google Scholar]
- Ruttenber, A.J.; Kalter, H.D.; Santinga, P. The role of ethanol abuse in the etiology of heroin-related death. J. Forensic Sci. 1990, 35, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Paulozzi, L.J.; Mack, K.A. Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths—United States, 2010. Morb. Mortal. Wkly. Rep. 2014, 63, 881–885. [Google Scholar]
- Hood, L.E.; Leyrer-Jackson, J.M.; Olive, M.F. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin. Pharmacother. 2020, 21, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Fidecka, S.; Tamborska, E.; Malec, D.; Langwinski, R. The development of cross tolerance between ethanol and morphine. Pol. J. Pharmacol. Pharm. 1986, 38, 277–284. [Google Scholar]
- Reid, L.D.; Hunter, G.A. Morphine and naloxone modulate intake of ethanol. Alcohol 1984, 1, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.M.; Shen, D.D.; Shireman, L.; Saxon, A.J.; Simpson, T.; Men, A.; Kooner, P.; Terman, G.W. Elevated customary alcohol consumption attenuates opioid effects. Pharmacol. Biochem. Behav. 2021, 211, 173295. [Google Scholar] [CrossRef]
- Tseng, A.; Nguyen, K.; Hamid, A.; Garg, M.; Marquez, P.; Lutfy, K. The role of endogenous beta-endorphin and enkephalins in ethanol reward. Neuropharmacology 2013, 73, 290–300. [Google Scholar] [CrossRef]
- Hubbell, C.L.; Czirr, S.A.; Hunter, G.A.; Beaman, C.M.; LeCann, N.C.; Reid, L.D. Consumption of ethanol solution is potentiated by morphine and attenuated by naloxone persistently across repeated daily administrations. Alcohol 1986, 3, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.S.; Morley, J.E.; Gosnell, B.A.; Billington, C.J.; Bartness, T.J. Opioids and consummatory behavior. Brain Res. Bull. 1985, 14, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Bardo, M.T.; Bevins, R.A. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology 2000, 153, 31–43. [Google Scholar] [CrossRef]
- Jarjour, S.; Bai, L.; Gianoulakis, C. Effect of acute ethanol administration on the release of opioid peptides from the midbrain including the ventral tegmental area. Alcohol. Clin. Exp. Res. 2009, 33, 1033–1043. [Google Scholar] [CrossRef]
- Blum, K.; Wallace, J.E.; Schwerter, H.A.; Eubanks, J.D. Morphine suppression of ethanol withdrawal in mice. Experientia 1976, 32, 79–82. [Google Scholar] [CrossRef]
- Venho, I.; Eerola, R.; Venho, E.V.; Vartiainen, O. Sensitisation to morphine by experimentally induced alcoholism in white mice. Ann. Med. Exp. Biol. Fenn. 1955, 33, 249–252. [Google Scholar]
- Nguyen, K.; Tseng, A.; Marquez, P.; Hamid, A.; Lutfy, K. The role of endogenous dynorphin in ethanol-induced state-dependent CPP. Behav. Brain Res. 2012, 227, 58–63. [Google Scholar] [CrossRef]
- Zaveri, N.T.; Marquez, P.V.; Meyer, M.E.; Polgar, W.E.; Hamid, A.; Lutfy, K. A Novel and Selective Nociceptin Receptor (NOP) Agonist (1-(1-((cis)-4-isopropylcyclohexyl)piperidin-4-yl)-1H-indol-2-yl)methanol (AT-312) Decreases Acquisition of Ethanol-Induced Conditioned Place Preference in Mice. Alcohol. Clin. Exp. Res. 2018, 42, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.L.; Bakner, L.; Schuette, L.M.; Young, E.A. Morphine and ethanol pretreatment effects on expression and extinction of ethanol-induced conditioned place preference and aversion in mice. Psychopharmacology 2021, 238, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, M.; Watanabe, K.; Takeda, K.; Itoh, T.; Tsuyuki, T.; Narita, M.; Mori, T.; Suzuki, T. Effect of chronic ethanol treatment on μ-opioid receptor function, interacting proteins and morphine-induced place preference. Psychopharmacology 2013, 228, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Huang, W.; Han, H.; Sariyer, I.K. Binge-Like Exposure to Ethanol Enhances Morphine’s Anti-nociception in B6 Mice. Front. Psychiatry 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.; Leriche, M.; Calva, J.C. Acute ethanol administration differentially modulates mu opioid receptors in the rat meso-accumbens and mesocortical pathways. Mol. Brain Res. 2001, 94, 148–156. [Google Scholar] [CrossRef]
- Cowen, M.S.; Rezvani, A.H.; Jarrott, B.; Lawrence, A.J. Ethanol consumption by Fawn-Hooded rats following abstinence: Effect of naltrexone and changes in mu-opioid receptor density. Alcohol. Clin. Exp. Res. 1999, 23, 1008–1014. [Google Scholar] [CrossRef]
- Morzorati, S.L.; Marunde, R.L.; Downey, D. Limited access to ethanol increases the number of spontaneously active dopamine neurons in the posterior ventral tegmental area of nondependent P rats. Alcohol 2010, 44, 257–264. [Google Scholar] [CrossRef]
- Johnson, S.W.; North, R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992, 12, 483–488. [Google Scholar] [CrossRef]
- Vezina, P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: An in vivo microdialysis study in the rat. Brain Res. 1993, 605, 332–337. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Abhold, R. Enkephalin release into the ventral tegmental area in response to stress: Modulation of mesocorticolimbic dopamine. Brain Res. 1987, 414, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Marquez, P.; Bebawy, D.; Lelievre, V.; Coute, A.C.; Evans, C.J.; Waschek, J.A.; Lutfy, K. The role of endogenous PACAP in motor stimulation and conditioned place preference induced by morphine in mice. Psychopharmacology 2009, 204, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, R.M.; Brissett, D.I.; Whistler, J.L. Distinctive modulation of ethanol place preference by delta opioid receptor-selective agonists. Drug Alcohol. Depend. 2012, 122, 156–159. [Google Scholar] [CrossRef]
- Roberts, A.J.; Gold, L.H.; Polis, I.; McDonald, J.S.; Filliol, D.; Kieffer, B.L.; Koob, G.F. Increased ethanol self-administration in delta-opioid receptor knockout mice. Alcohol. Clin. Exp. Res. 2001, 25, 1249–1256. [Google Scholar] [PubMed]
- Hayward, M.D.; Hansen, S.T.; Pintar, J.E.; Low, M.J. Operant self-administration of ethanol in C57BL/6 mice lacking beta-endorphin and enkephalin. Pharmacol. Biochem. Behav. 2004, 79, 171–181. [Google Scholar] [CrossRef]
- Koenig, H.N.; Olive, M.F. Ethanol consumption patterns and conditioned place preference in mice lacking preproenkephalin. Neurosci. Lett. 2002, 325, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Gupta, A.; Filipovska, J.; Szeto, H.H.; Pintar, J.E.; Devi, L.A. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. USA 2004, 101, 5135–5139. [Google Scholar] [CrossRef]
- Matthes, H.W.; Maldonado, R.; Simonin, F.; Valverde, O.; Slowe, S.; Kitchen, I.; Befort, K.; Dierich, A.; Le Meur, M.; Dolle, P.; et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 1996, 383, 819–823. [Google Scholar] [CrossRef]
- Rubinstein, M.; Mogil, J.S.; Japon, M.; Chan, E.C.; Allen, R.G.; Low, M.J. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 3995–4000. [Google Scholar] [CrossRef]
- Konig, M.; Zimmer, A.M.; Steiner, H.; Holmes, P.V.; Crawley, J.N.; Brownstein, M.J.; Zimmer, A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature 1996, 383, 535–538. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Whistler, J.L. Chronic ethanol consumption in rats produces opioid antinociceptive tolerance through inhibition of mu opioid receptor endocytosis. PLoS ONE 2008, 6, e19372. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, A.; Ahmad, S.M.; Hamid, A.; Lutfy, K. The Role of Endogenous Beta-Endorphin and Enkephalins in the Crosstalk Between Ethanol and Morphine. Pharmaceuticals 2025, 18, 107. https://doi.org/10.3390/ph18010107
Tseng A, Ahmad SM, Hamid A, Lutfy K. The Role of Endogenous Beta-Endorphin and Enkephalins in the Crosstalk Between Ethanol and Morphine. Pharmaceuticals. 2025; 18(1):107. https://doi.org/10.3390/ph18010107
Chicago/Turabian StyleTseng, Andy, Syed Muzzammil Ahmad, Abdul Hamid, and Kabirullah Lutfy. 2025. "The Role of Endogenous Beta-Endorphin and Enkephalins in the Crosstalk Between Ethanol and Morphine" Pharmaceuticals 18, no. 1: 107. https://doi.org/10.3390/ph18010107
APA StyleTseng, A., Ahmad, S. M., Hamid, A., & Lutfy, K. (2025). The Role of Endogenous Beta-Endorphin and Enkephalins in the Crosstalk Between Ethanol and Morphine. Pharmaceuticals, 18(1), 107. https://doi.org/10.3390/ph18010107







