Therapeutic Potential of Clove Oil in Mitigating Cadmium-Induced Hepatorenal Toxicity Through Antioxidant, Anti-Inflammatory, and Antiapoptotic Mechanisms
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis and Antioxidant Activity of Clove Oil
2.2. CLO Analysis Using Gas Chromatography–Mass Spectroscopy (GC–MS)
2.3. Hematological Result
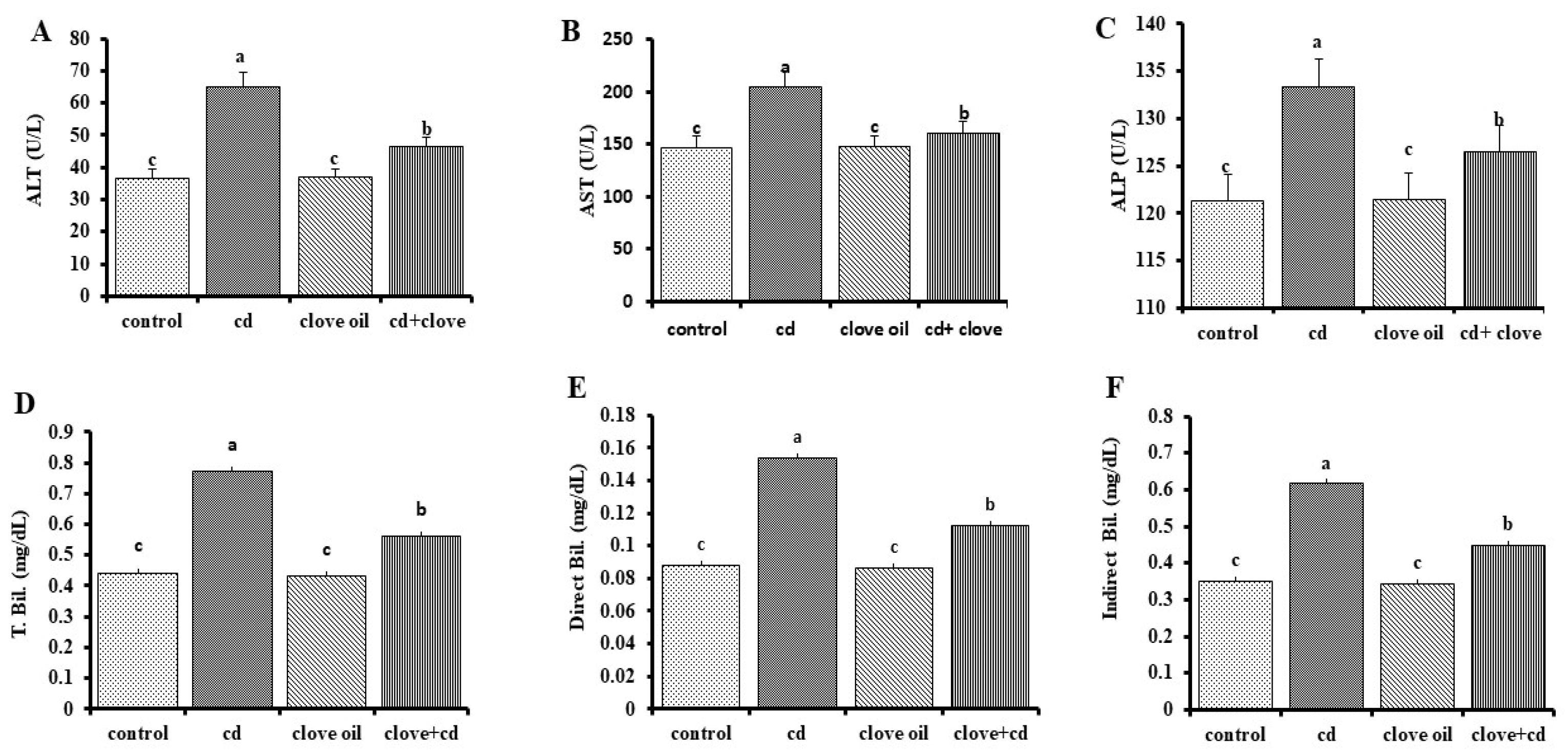
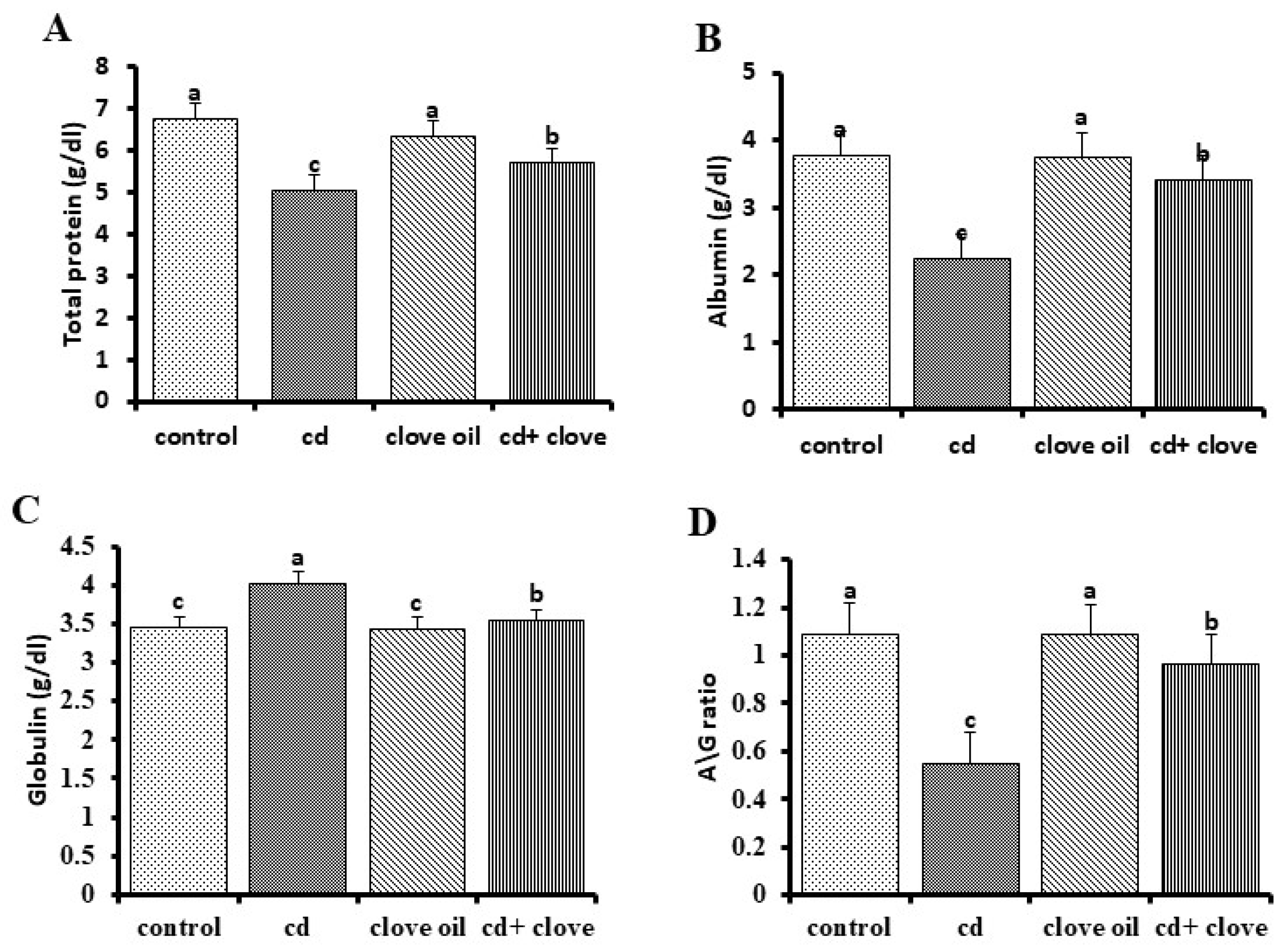
2.4. Liver and Kidney Function Biomarkers
2.5. Hepatic Antioxidant and Oxidative Markers
2.6. Cadmium Residues in Experimental Rat Liver
2.7. Histopathological Investigation
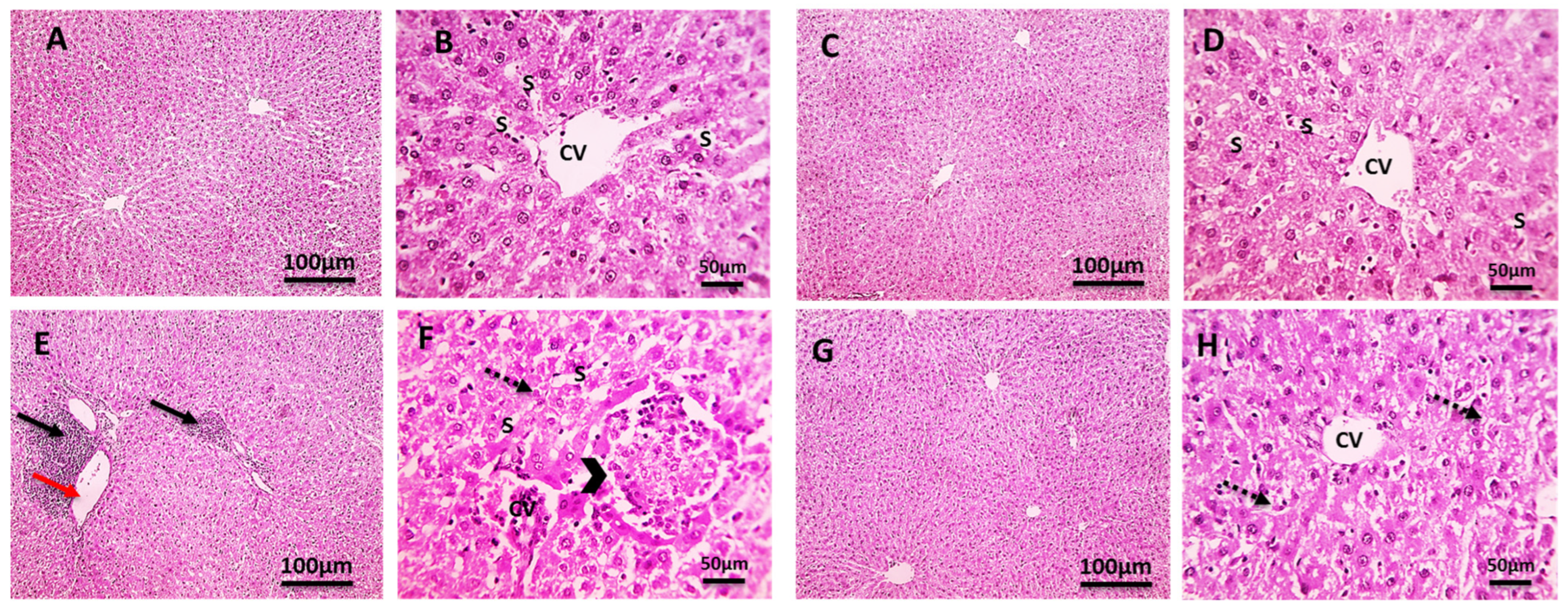
2.7.1. Liver
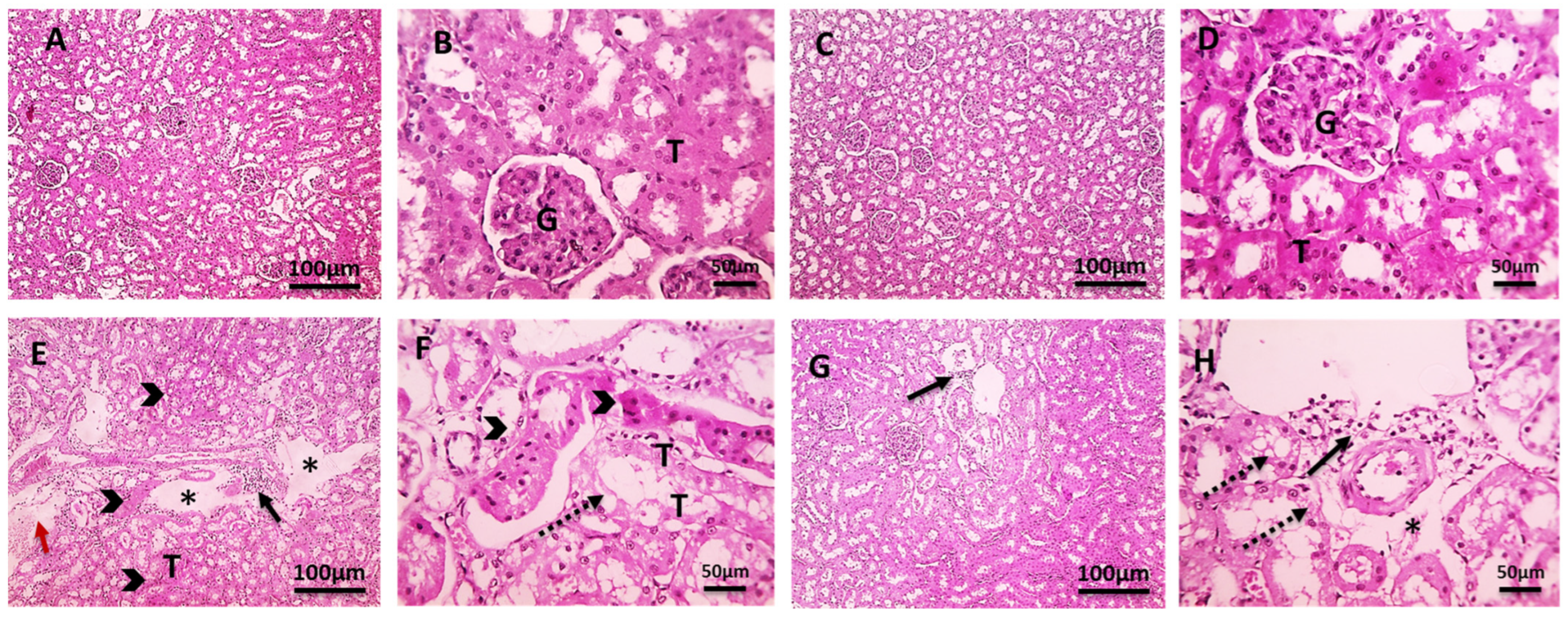
2.7.2. Kidney
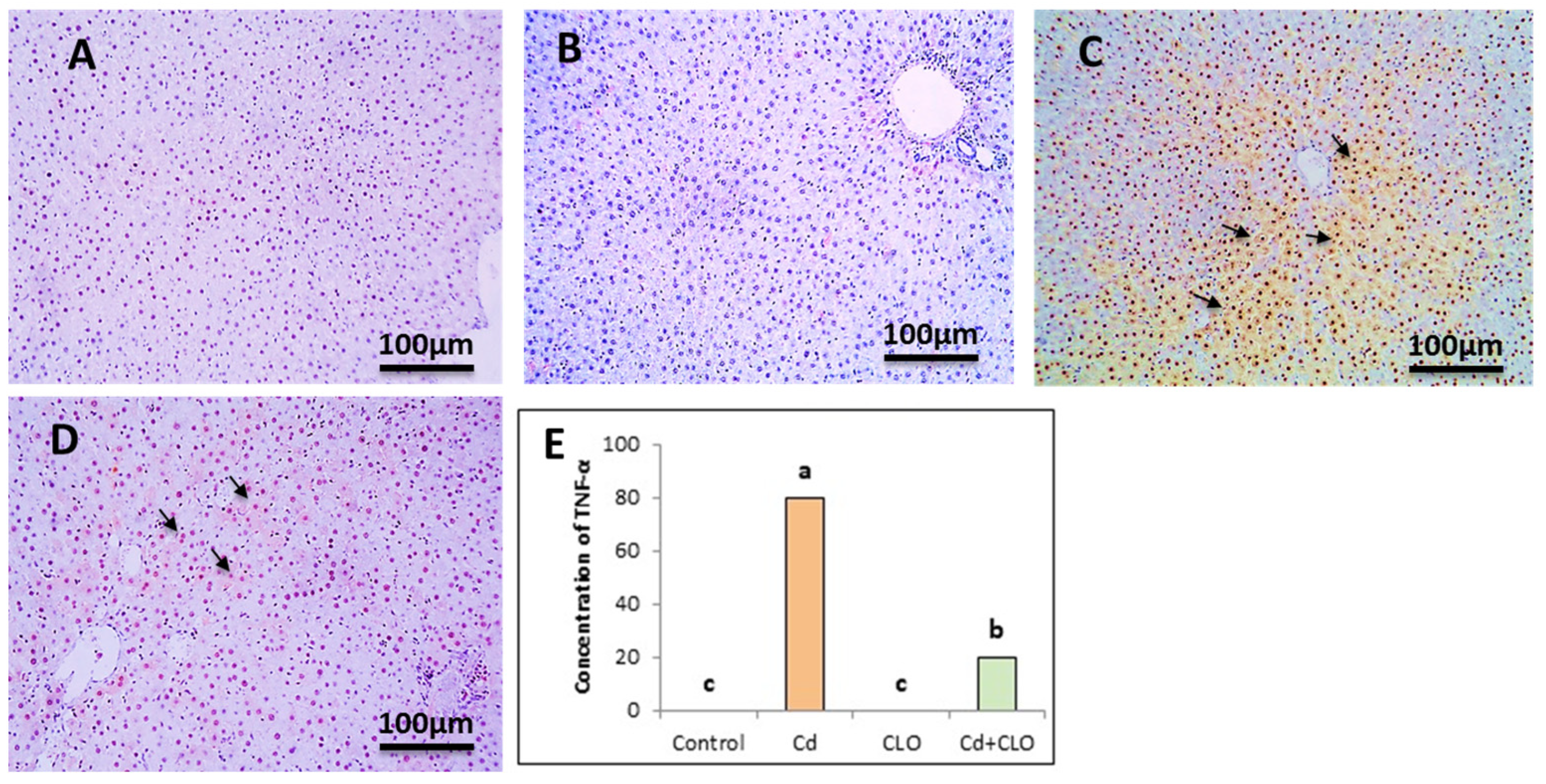
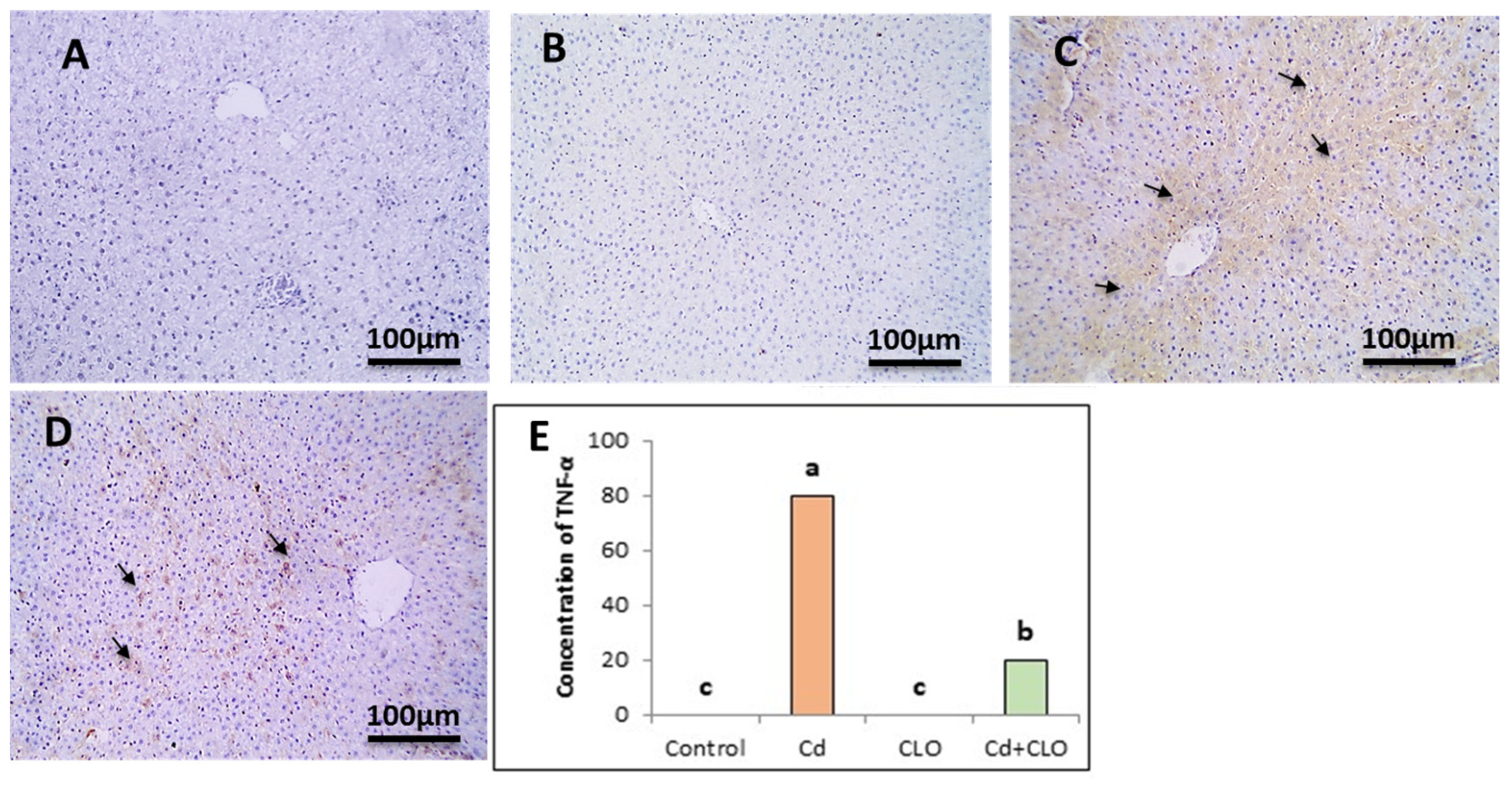
2.8. Immunohistochemical Expression of Caspase-3 and TNF-α
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Phytochemical Composition of Clove Oil
4.2.1. Total Amount of Phenolics
4.2.2. Total Amount of Flavonoids
4.2.3. Assay for Antioxidant Activity 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
4.2.4. Gas Chromatography–Mass Spectroscopy (GC–MS) of Clove Oil Samples
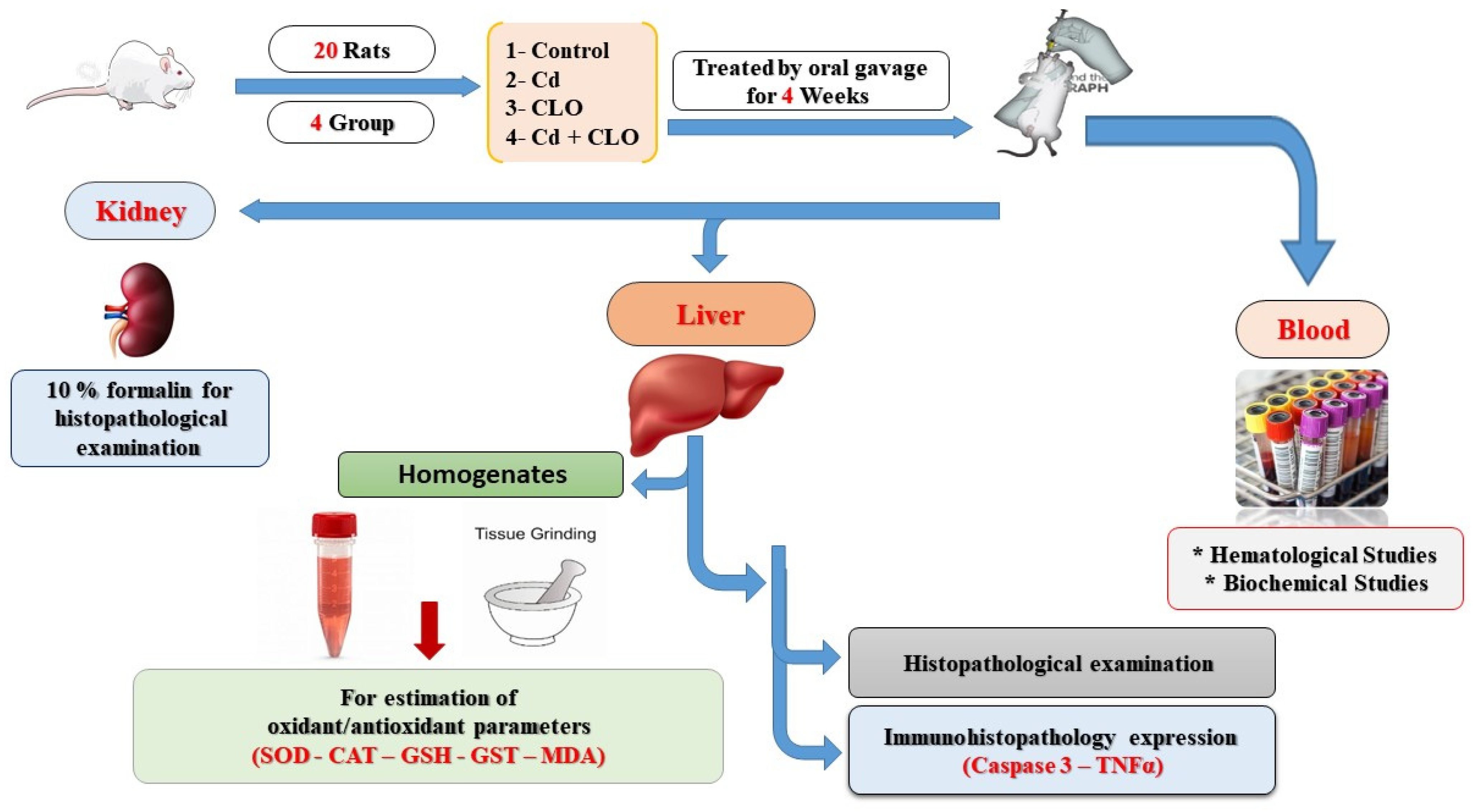
4.3. Animals and Experimental Protocol
4.4. Sampling
4.5. Evaluation of Hematological Parameters
4.6. Evaluation of Serum Biochemical Parameters
4.7. Assessment of Hepatic Lipid Peroxidation Indicators and Antioxidants in Hepatic Homogenates
4.8. Cadmium Accumulation in Liver
4.9. Evaluation of Liver Histopathology
4.10. Expression of TNF-α and Caspase-3 via Immunohistochemistry
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ögütçü, G.; Kocamaz, G.; Edebal, O.; Pamuk, S.; Kükner, A. The Effect of L-Carnitine on Cadmium Toxicity in Liver and Kidney Tissue in Prepubertal Female Rats. Int. J. Morphol. 2024, 42, 819–825. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Yao, W.; Ba, Q.; Wang, H. Effects of Cadmium Exposure on the Immune System and Immunoregulation. Front. Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef] [PubMed]
- Atere, T.G.; Akinloye, O.A. High dose of standardised extract of Costus afer leaves potentiates cadmium reproductive toxicity in Wistar rats. Andrologia 2019, 51, e13360. [Google Scholar] [CrossRef]
- Okutu Jackson, B.; Enebrayi, O.N. Ameliorative effect of allium sativum and justicia carnea extracts co-administration on acute cadmium chloride-induced changes on liver function parameters of albino rats. World J. Pharm. Life Sci. 2022, 4, 11–24. [Google Scholar]
- Hossein-Khannazer, N.; Azizi, G.; Eslami, S.; Alhassan Mohammed, H.; Fayyaz, F.; Hosseinzadeh, R.; Usman, A.B.; Kamali, A.N.; Mohammadi, H.; Jadidi-Niaragh, F.; et al. The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Pacini, A.; Gulisano, M.; Taddei, N.; Fiorillo, C.; Becatti, M. Cadmium-Induced Cytotoxicity: Effects on Mitochondrial Electron Transport Chain. Front. Cell Dev. Biol. 2020, 8, 604377. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Buha Djordjevic, A.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Mirkov, I.; Popov Aleksandrov, A.; Ninkov, M.; Tucovic, D.; Kulas, J.; Zeljkovic, M.; Popovic, D.; Kataranovski, M. Immunotoxicology of cadmium: Cells of the immune system as targets and effectors of cadmium toxicity. Food Chem. Toxicol. 2021, 149, 112026. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; Zheng, Y.; Liu, Q.; Shi, G.; Zhang, Z. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazek, H.M.A.; Helmy, S.A.; Elsayed, D.H.; Ebaid, H.M.; Mohamed, R.M. Ameliorating effects of green tea extract on cadmium induced reproductive injury in male Wistar rats with respect to androgen receptors and caspase-3. Reprod. Biol. 2016, 16, 300–308. [Google Scholar] [CrossRef]
- Saeed, M.; Khan, M.S.; Alagawany, M.; Farag, M.R.; Alqaisi, O.; Aqib, A.I.; Qumar, M.; Siddique, F.; Ramadan, M.F. Clove (Syzygium aromaticum) and its phytochemicals in ruminant feed: An updated review. Rend. Lincei Sci. Fis. Nat. 2021, 32, 273–285. [Google Scholar] [CrossRef]
- Fiqardina, A.; Yusrini Djabir, Y.; Santoso, A.; Nurul Salsabil, S.; Ismail, I. The Nephroprotective Effect of Clove Oil (Oleum Caryophylli) Against Levofloxacin Toxicity in Rats. Iran. J. Toxicol. 2022, 16, 27–34. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Turhan, S. Performance of mechanically deboned chicken meat protein coatings containing thyme or clove essential oil for storage quality improvement of beef sucuks. Meat Sci. 2019, 158, 107912. [Google Scholar] [CrossRef]
- Soliman, W.; Ibrahim, M.; El Baz, H. In Vitro evaluation of Syzygium aromaticum L. ethanol extract as biocontrol agent against postharvest tomato and potato diseases. Egypt. J. Bot. 2019, 59, 81–94. [Google Scholar]
- Hemalatha, R.; Nivetha, P.; Mohanapriya, C.; Sharmila, G.; Muthukumaran, C.; Gopinath, M. Phytochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J. Food Sci. Technol. 2016, 53, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, X.; He, W.-H.; Xu, H.-G.; Yuan, F.; Gao, Y.-X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, G.; Xia, Y.; Wen, C.; Fan, Y. Fast analysis of principal volatile compounds in crude and processed Atractylodes macrocephala by an automated static headspace gas chromatography-mass spectrometry. Pharmacogn. Mag. 2014, 10, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Bakour, M.; Soulo, N.; Hammas, N.; Fatemi, H.E.; Aboulghazi, A.; Taroq, A.; Abdellaoui, A.; Al-Waili, N.; Lyoussi, B. The Antioxidant Content and Protective Effect of Argan Oil and Syzygium aromaticum Essential Oil in Hydrogen Peroxide-Induced Biochemical and Histological Changes. Int. J. Mol. Sci. 2018, 19, 610. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.d.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid. Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef] [PubMed]
- El Ghallab, Y.; Al Jahid, A.; Jamal Eddine, J.; Ait Haj Said, A.; Zarayby, L.; Derfoufi, S. Syzygium aromaticum L.: Phytochemical investigation and comparison of the scavenging activity of essential oil, extracts and eugenol. Adv. Tradit. Med. 2019, 20, 153–158. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Durai, L.; Gopakumar, V.; Nagarajan, R. Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int. J. Nanomed. 2019, 14, 6439–6450. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Doghish, A.S.; El-Dakroury, W.A.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Hashem, A.H. Antimicrobial, Antibiofilm, and Anticancer Activities of Syzygium aromaticum Essential Oil Nanoemulsion. Molecules 2023, 28, 5812. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz-Oral, D.; Onder, A.; Gur, S.; Carbonell-Barrachina, Á.A.; Kaya-Sezginer, E.; Oztekin, C.V.; Zor, M. The beneficial effect of clove essential oil and its major component, eugenol, on erectile function in diabetic rats. Andrologia 2020, 52, e13606. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Rana, K.; Verma, Y.; Rana, S.V.S. Possible Mechanisms of Liver Injury Induced by Cadmium Sulfide Nanoparticles in Rat. Biol. Trace Elem. Res. 2020, 199, 216–226. [Google Scholar] [CrossRef]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology-Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef]
- Hong, D.; Min, J.-Y.; Min, K.-B. Association Between Cadmium Exposure and Liver Function in Adults in the United States: A Cross-sectional Study. J. Prev. Med. Public Health 2021, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Djokic, J.; Ninkov, M.; Mirkov, I.; Popov Aleksandrov, A.; Zolotarevski, L.; Kataranovski, D.; Kataranovski, M. Differential effects of cadmium administration on peripheral blood granulocytes in rats. Environ. Toxicol. Pharmacol. 2014, 37, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Rafati Rahimzadeh, M.; Rafati Rahimzadeh, M.; Kazemi, S.; Moghadamnia, A.R.; Ghaemi Amiri, M.; Moghadamnia, A.A. Iron; Benefits or threatens (with emphasis on mechanism and treatment of its poisoning). Hum. Exp. Toxicol. 2023, 42, 09603271231192361. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef] [PubMed]
- Katsaros, M.; Paschos, P.; Giouleme, O. Red cell distribution width as a marker of activity in inflammatory bowel disease: A narrative review. Ann. Gastroenterol. 2020, 33, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2020, 10, 1534. [Google Scholar] [CrossRef]
- Al-Baqami, N.; Hamza, R. Protective Effect of Resveratrol against Hepatotoxicity of Cadmium in Male Rats: Antioxidant and Histopathological Approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- Hamza, R.Z.; El-Megharbel, S.M.; Altalhi, T.; Gobouri, A.A.; Alrogi, A.A. Hypolipidemic and hepatoprotective synergistic effects of selenium nanoparticles and vitamin. E against acrylamide-Induced hepatic alterations in male albino mice. Appl. Organomet. Chem. 2020, 34, e5458. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Fathi, G.E.; Salem, H.A.; Akram, N.H.; Gamil, S.A. Protective role of pectin against cadmium-induced testicular toxicity and oxidative stress in rats. Toxicol. Mech. Methods 2013, 23, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Nayila, I. Effect of Ascorbic Acid Supplementation on Liver Function Tests in Hepatitis C Patients. Open J. Intern. Med. 2020, 10, 263–279. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Malki, N.A.; Alharthi, S.; Alharthy, S.A.; Albogami, B.; El-Megharbel, S.M. Chemical Characterization of Taif Rose (Rosa damascena) Methanolic Extract and Its Physiological Effect on Liver Functions, Blood Indices, Antioxidant Capacity, and Heart Vitality against Cadmium Chloride Toxicity. Antioxidants 2022, 11, 1229. [Google Scholar] [CrossRef]
- Mladenović, J.; Ognjanović, B.; Đorđević, N.; Matić, M.; Knežević, V.; Štajn, A.; Saičić, Z. Protective effects of oestradiol against cadmium-induced changes in blood parameters and oxidative damage in rats. Arch. Ind. Hyg. Toxicol. 2014, 65, 37–46. [Google Scholar] [CrossRef]
- Ude, C.; Achikanu, C.; Nwobodo, F. Effects of cadmium ion on the structure and function of rat hepatocyte. Sci. Afr. 2014, 13, 349–357. [Google Scholar]
- Alshehri, A.S.; El-kott, A.F.; El-Gerbed, M.S.A.; El-Kenawy, A.E.; Albadrani, G.M.; Khalifa, H.S. Kaempferol prevents cadmium chloride-induced liver damage by upregulating Nrf2 and suppressing NF-κB and keap1. Environ. Sci. Pollut. Res. 2021, 29, 13917–13929. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.N.; Tangpong, J.; Rahman, M.M. Toxicodynamics of Lead, Cadmium, Mercury and Arsenic-induced kidney toxicity and treatment strategy: A mini review. Toxicol. Rep. 2018, 5, 704–713. [Google Scholar] [CrossRef]
- Gabr, S.A.; Alghadir, A.H.; Ghoniem, G.A. Biological activities of ginger against cadmium-induced renal toxicity. Saudi J. Biol. Sci. 2019, 26, 382–389. [Google Scholar] [CrossRef]
- Yang, H.; Shu, Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int. J. Mol. Sci. 2015, 16, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, B.; Hay, E.B.; Nebert, D.W. Discovery of ZIP transporters that participate in cadmium damage to testis and kidney. Toxicol. Appl. Pharmacol. 2009, 238, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Hiramatsu, N. The oxidative stress: Endoplasmic reticulum stress axis in cadmium toxicity. BioMetals 2010, 23, 941–950. [Google Scholar] [CrossRef]
- Hussein, R.; Khalaf, M.; Mohamed, W. Hesperidin and eugenol attenuate cadmium-induced nephrotoxicity via regulation of oxidative stress, Bax/Bcl2 and cleaved caspase 3 expression. Turk. J. Biochem. 2020, 45, 767–775. [Google Scholar] [CrossRef]
- Sembiring, F.; Unitly, A.J.A.; Eddy, L. Effects of clove syrup therapy on creatinine, urea, and kidney histology of diabetes mellitus rat. J. Biotechnol. Conserv. Wallacea 2021, 1, 31–41. [Google Scholar]
- Wang, J.; Wang, K.; Ding, L.; Zhao, P.; Zhang, C.; Wang, H.; Yang, Z.; Liu, Z. Alleviating effect of quercetin on cadmium-induced oxidative damage and apoptosis by activating the Nrf2-keap1 pathway in BRL-3A cells. Front. Pharmacol. 2022, 13, 969892. [Google Scholar] [CrossRef]
- Aja, P.M.; Ekpono, E.U.; Awoke, J.N.; Famurewa, A.C.; Izekwe, F.I.; Okoro, E.J.; Okorie, C.F.; Orji, C.L.; Nwite, F.; Ale, B.A.; et al. Hesperidin ameliorates hepatic dysfunction and dyslipidemia in male Wistar rats exposed to cadmium chloride. Toxicol. Rep. 2020, 7, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, F.M.; Caglayan, C.; Darendelioğlu, E.; Küçükler, S.; İzol, E.; Kandemir, Ö. Modulatory effects of carvacrol against cadmium-induced hepatotoxicity and nephrotoxicity by molecular targeting regulation. Life Sci. 2021, 277, 119610. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, A.; Abdelkader, A.; Elgazzar, D.; Aboubakr, M.; Abdulah, O.A.; Shoghy, K.; Abdel-Daim, M.; El-Serehy, H.A.; Najda, A.; El-Mleeh, A. Coenzyme Q10 supplementation mitigates piroxicam-induced oxidative injury and apoptotic pathways in the stomach, liver, and kidney. Biomed. Pharmacother. 2020, 130, 110627. [Google Scholar] [CrossRef] [PubMed]
- Askari, E.; Fallah, A.A.; Habibian Dehkordi, S.; Bahadoran, S.; Mohebbi, A.; Mohamadi, S. Effect of Dietary Clove (Syzygium Aromaticum) Essential Oil on Growth Performance, Oxidative Indices, Lipid Profile, and Cadmium Accumulation in Cd-exposed Quails. J. Environ. Health Sustain. Dev. 2022, 7. [Google Scholar] [CrossRef]
- Rumahlatu, D. Effect of cadmium on the concentration and expression of TNF-α protein in sea urchin Diadema setosum (Leske, 1778). Hidrobiológica 2019, 29, 181–188. [Google Scholar] [CrossRef]
- Antar, S.A.; El-Gammal, M.A.; Hazem, R.M.; Moustafa, Y.M. Etanercept Mitigates Cadmium Chloride-induced Testicular Damage in Rats “An Insight into Autophagy, Apoptosis, Oxidative Stress and Inflammation”. Environ. Sci. Pollut. Res. 2022, 29, 28194–28207. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- Portt, L.; Norman, G.; Clapp, C.; Greenwood, M.; Greenwood, M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 238–259. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Acilan, C.; Yilmaz, Y. Apoptosis: Why and how does it occur in biology? Cell Biochem. Funct. 2011, 29, 468–480. [Google Scholar] [CrossRef]
- Lavrik, I.N. Systems biology of apoptosis signaling networks. Curr. Opin. Biotechnol. 2010, 21, 551–555. [Google Scholar] [CrossRef]
- Pathak, N.; Mitra, S.; Khandelwal, S. Cadmium Induces Thymocyte Apoptosis via Caspase—Dependent and Caspase—Independent Pathways. J. Biochem. Mol. Toxicol. 2013, 27, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.I.; Yan, Y.; Wang, J.; Gu, J.; Chun, B.; Liu, Z. Investigation of cadmium-induced apoptosis and the protective effect of N-acetylcysteine in BRL 3A cells. Mol. Med. Rep. 2016, 14, 373–379. [Google Scholar] [CrossRef]
- Abadi, A.V.M.; Karimi, E.; Oskoueian, E.; Mohammad, G.R.K.S.; Shafaei, N. Chemical investigation and screening of anti-cancer potential of Syzygium aromaticum L. bud (clove) essential oil nanoemulsion. 3 Biotech 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Kona, P.; Ajay, B.C.; Gangadhara, K.; Kumar, N.; Choudhary, R.R.; Mahatma, M.K.; Singh, S.; Reddy, K.K.; Bera, S.K.; Sangh, C.; et al. AMMI and GGE biplot analysis of genotype by environment interaction for yield and yield contributing traits in confectionery groundnut. Sci. Rep. 2024, 14, 2943. [Google Scholar] [CrossRef] [PubMed]
- Mekky, A.E.; Emam, A.E.; Selim, M.N.; Abdelmouty, E.S.; Khedr, M. Antibacterial and antineoplastic MCF-7 and HePG-2 characteristics of the methanolic (80%) clove (Syzygium aromaticum L.) extract. Biomass Convers. Biorefinery 2023, 14, 16787–16798. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Kitts, D.D.; Wijewickreme, A.N.; Hu, C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000, 203, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amelia, B.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.; Sulistyoningrum, A.S.; Haib, J. GC-MS analysis of clove (Syzygium aromaticum) bud essential oil from Java and Manado. AIP Conf. Proc. 2017, 1862, 030082. [Google Scholar]
- Ognjanović, B.I.; Marković, S.D.; Pavlović, S.Z.; Žikić, R.V.; Štajn, A.; Saičić, Z.S. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: Protective effect of selenium. Physiol. Res. 2008, 57, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, M. Neuroprotective Effects of Clove Oil in Acrylamide Induced Neurotoxicity in Rats. Pak. Vet. J. 2019, 39, 111–115. [Google Scholar] [CrossRef]
- Fernandez-Botran, R.; Gorantla, V.; Sun, X.; Ren, X.; Perez-Abadia, G.; Crespo, F.A.; Oliver, R.; Orhun, H.I.; Quan, E.E.; Maldonado, C.; et al. Targeting of glycosaminoglycan-cytokine interactions as a novel therapeutic approach in allotransplantation1. Transplantation 2002, 74, 623–629. [Google Scholar] [CrossRef]
- Feldman, B. Practical Transfusion Medicine for the Small Animal Practitioner; Teton NewMedia: Jackson, WY, USA, 2004. [Google Scholar] [CrossRef]
- Kaneko, J. Clinical Biochemistry of Domestic Animals; Academic Press: New York, NY, USA, 2008; Volume 273. [Google Scholar]
- Kei, S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Goldbeg, D.M.; Spooner, R.J. Assay of Glutathione reductase. Methods Enzym. Anal. 1983, 3, 258–265. [Google Scholar]
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Philadelphia, PA, USA, 2008. [Google Scholar]
- Elshopakey, G.E.; Elazab, S.T. Cinnamon Aqueous Extract Attenuates Diclofenac Sodium and Oxytetracycline Mediated Hepato-Renal Toxicity and Modulates Oxidative Stress, Cell Apoptosis, and Inflammation in Male Albino Rats. Vet. Sci. 2021, 8, 9. [Google Scholar] [CrossRef]
| Oil sample | Phytochemical Analysis | |
| Phenolic Contents “mg gallic acid equivalent/g. oil” | Flavonoid Contents “mg catechin equivalent/g. oil” | |
| 172.38 | 54.358 | |
| Compound Name | Compound Class | Structure | Effects | Retention Time | Focus |
|---|---|---|---|---|---|
| Eugenol | Phenylpropanoid | C10H12O2 | Anti-inflammatory, antioxidant, and antimicrobial properties | 16 min | 76.8% |
| Beta-caryophyllene | Sesquiterpene (Terpenes) | C15H24 | Anti-inflammatory, analgesic, antioxidant, neuroprotective, anticancer, and antimicrobial properties | 18 min | 17.4% |
| Alpha-caryophyllene | Sesquiterpene (Terpenes) | C15H24 | Anti-inflammatory, analgesic, and anticancer properties | 19 min | 2.1% |
| Eugenol acetate | Phenylpropanoid | C10H12O2 | Anti-inflammatory, antioxidant, analgesic, and antimicrobial properties | 21 min | 1.2% |
| Group | RBCs (×106/µL) | Hb (g/dL) | PCV (%) | MCV (fl) | MCH (pg) | MCHC (%) | WBCs (×103/µL) |
|---|---|---|---|---|---|---|---|
| Control | 6.74 ± 0.25 a | 14.76 ± 0.23 a | 40.60 ± 0.65 a | 59.46 ± 2.29 a | 21.96 ± 0.71 a | 36.97 ± 0.35 a | 17.33 ± 0.30 c |
| Cd | 5.42 ± 0.07 c | 13.02 ± 0.21 c | 34.86 ± 0.41 c | 59.60 ± 1.00 b | 22.26 ± 0.54 b | 37.34 ± 0.36 c | 20.29 ± 0.08 a |
| CLO | 6.86 ± 0.24 a | 14.57 ± 0.14 a | 40.92 ± 0.76 a | 59.26 ± 2.00 a | 20.56 ± 0.64 a | 34.74 ± 0.74 a | 17.15 ± 0.34 c |
| Cd+CLO | 6.08 ± 0.06 b | 13.59 ± 0.16 b | 39.37 ± 1.08 b | 60.56 ± 1.11 b | 21.83 ± 0.33 b | 36.06 ± 0.47 b | 19.57 ± 0.04 b |
| Group | Urea (mg/dL) | Uric Acid (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|
| Control | 40.27 ± 0.64 c | 0.80 ± 0.02 c | 0.44 ± 0.006 c |
| Cd | 62.17 ± 0.61 a | 1.18 ± 0.04 a | 0.84 ± 0.011 a |
| CLO | 40.22 ± 0.64 c | 0.81 ± 0.015 c | 0.43 ± 0.0.007 c |
| Cd+CLO | 45.17 ± 0.30 b | 0.92 ± 0.011 b | 0.49 ± 0.014 b |
| Group | MDA (nmol/g.Tissue) | SOD (U/g.Tissue) | CAT (U/g.Tissue) | GSH (mg/g.Tissue) | GST (U/g.Tissue) |
|---|---|---|---|---|---|
| Control | 24.54 ± 2.87 c | 611.60 ± 0.92 a | 19.3 ± 0.07 a | 19.54 ± 1.42 a | 24.49 ± 0.59 b |
| Cd | 72.93 ± 6.19 a | 320.24 ± 44.09 c | 10.7 ± 0.16 c | 9.68 ± 0.55 c | 18.67 ± 0.56 d |
| CLO | 17.97 ± 2.92 c | 607.60 ± 2.24 a | 19.7 ± 0.007 a | 19.02 ± 1.56 a | 26.24 ± 0.54 a |
| Cd+CLO | 37.62 ± 3.62 b | 513.60 ± 12.34 b | 17.6 ± 0.03 b | 14.56 ± 0.68 b | 20.38 ± 0.26 c |
| Group | Cadmium Residues in Liver (mg/kg Liver) |
|---|---|
| Control | 0.00013 ± 0.000042 c |
| Cd | 0.024496 ± 0.0036 a |
| CLO | 0.000346 ± 0.0001 c |
| Cd+CLO | 0.0333 ± 0.0051 b |
| Sample Conc. (mg/mL) | % Remaining DPPH | IC50 (mg/mL) | |
|---|---|---|---|
| Oil sample | 0.168 | 49.72 | 0.1572 |
| 0.084 | 59.94 | ||
| 0.042 | 75.71 | ||
| 0.021 | 85.23 | ||
| Ascorbic acid | 0.062 | 15.267 | 0.0222 |
| 0.031 | 39.084 | ||
| 0.016 | 61.069 | ||
| 0.008 | 74.809 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgharib, I.M.; Abdelhamid, F.M.; Elshopakey, G.E.; Sembawa, H.; Albukhari, T.A.; Filimban, W.A.; Bagadood, R.M.; El-Boshy, M.E.; Risha, E.F. Therapeutic Potential of Clove Oil in Mitigating Cadmium-Induced Hepatorenal Toxicity Through Antioxidant, Anti-Inflammatory, and Antiapoptotic Mechanisms. Pharmaceuticals 2025, 18, 94. https://doi.org/10.3390/ph18010094
Elgharib IM, Abdelhamid FM, Elshopakey GE, Sembawa H, Albukhari TA, Filimban WA, Bagadood RM, El-Boshy ME, Risha EF. Therapeutic Potential of Clove Oil in Mitigating Cadmium-Induced Hepatorenal Toxicity Through Antioxidant, Anti-Inflammatory, and Antiapoptotic Mechanisms. Pharmaceuticals. 2025; 18(1):94. https://doi.org/10.3390/ph18010094
Chicago/Turabian StyleElgharib, Inas M., Fatma M. Abdelhamid, Gehad E. Elshopakey, Hatem Sembawa, Talat A. Albukhari, Waheed A. Filimban, Rehab M. Bagadood, Mohamed E. El-Boshy, and Engy F. Risha. 2025. "Therapeutic Potential of Clove Oil in Mitigating Cadmium-Induced Hepatorenal Toxicity Through Antioxidant, Anti-Inflammatory, and Antiapoptotic Mechanisms" Pharmaceuticals 18, no. 1: 94. https://doi.org/10.3390/ph18010094
APA StyleElgharib, I. M., Abdelhamid, F. M., Elshopakey, G. E., Sembawa, H., Albukhari, T. A., Filimban, W. A., Bagadood, R. M., El-Boshy, M. E., & Risha, E. F. (2025). Therapeutic Potential of Clove Oil in Mitigating Cadmium-Induced Hepatorenal Toxicity Through Antioxidant, Anti-Inflammatory, and Antiapoptotic Mechanisms. Pharmaceuticals, 18(1), 94. https://doi.org/10.3390/ph18010094







