Role of Metformin in Preventing New-Onset Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus
Abstract
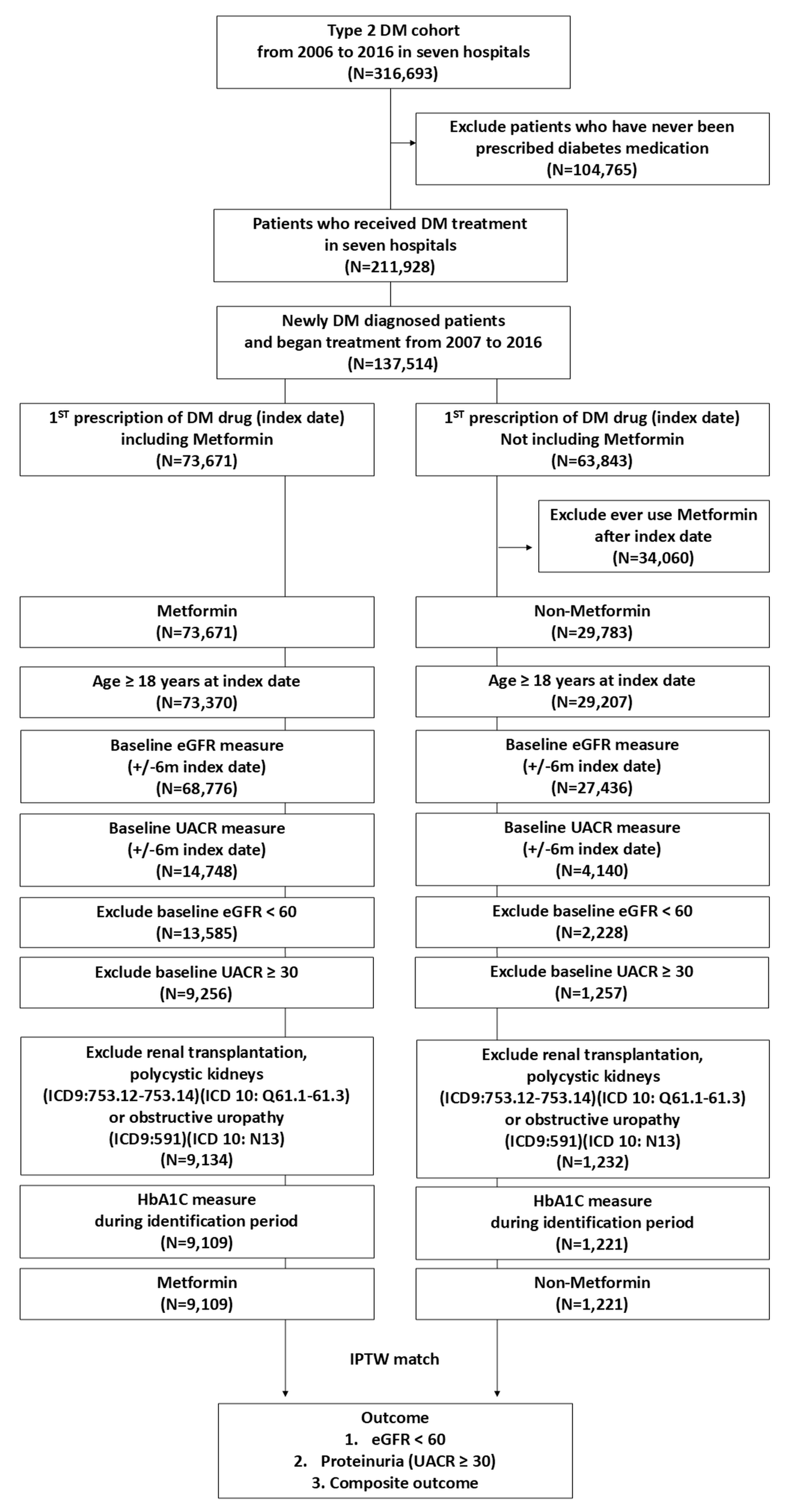
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Incidence of eGFR Persistently Below 60 mL/min/1.73 m2
2.3. Incidence of Sustained UACR Levels of ≥30 mg/g
2.4. Incidence of New-Onset CKD
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Database and Study Sample
4.3. Matching
4.4. Data Collection
4.5. Outcome
4.6. Duration of Follow-Up/Censor Date
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grzybowska, M.; Bober, J.; Olszewska, M. Metformin-mechanisms of action and use for the treatment of type 2 diabetes mellitus. Postepy Hig. Med. Dosw. 2011, 65, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Matthaei, S.; Greten, H. Evidence that metformin ameliorates cellular insulin-resistance by potentiating insulin-induced translocation of glucose transporters to the plasma membrane. Diabete Metab. 1991, 17, 150–158. [Google Scholar] [PubMed]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Hsiao, P.J.; Lin, P.C.; Chen, S.C.; Lee, M.Y.; Shin, S.J. Effect of metformin on kidney function in patients with type 2 diabetes mellitus and moderate chronic kidney disease. Oncotarget 2018, 9, 5416–5423. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.M.; Fujihara, C.K.; Malheiros, D.; de Avila, V.F.; Formigari, G.P.; de Faria, J.B.L. Metformin arrests the progression of established kidney disease in the subtotal nephrectomy model of chronic kidney disease. Am. J. Physiol.-Renal Physiol. 2020, 318, F1229–F1236. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Yu, X.; Wu, Y.; Sui, D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp. Ther. Med. 2017, 14, 383–390. [Google Scholar] [CrossRef]
- Kim, D.I.; Park, M.J.; Heo, Y.R.; Park, S.H. Metformin ameliorates lipotoxicity-induced mesangial cell apoptosis partly via upregulation of glucagon like peptide-1 receptor (GLP-1R). Arch. Biochem. Biophys. 2015, 584, 90–97. [Google Scholar] [CrossRef]
- Polianskyte-Prause, Z.; Tolvanen, T.A.; Lindfors, S.; Dumont, V.; Van, M.; Wang, H.; Dash, S.N.; Berg, M.; Naams, J.B.; Hautala, L.C.; et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J. 2019, 33, 2858–2869. [Google Scholar] [CrossRef]
- Lee, M.; Katerelos, M.; Gleich, K.; Galic, S.; Kemp, B.E.; Mount, P.F.; Power, D.A. Phosphorylation of Acetyl-CoA Carboxylase by AMPK Reduces Renal Fibrosis and Is Essential for the Anti-Fibrotic Effect of Metformin. J. Am. Soc. Nephrol. 2018, 29, 2326–2336. [Google Scholar] [CrossRef]
- Takiyama, Y.; Harumi, T.; Watanabe, J.; Fujita, Y.; Honjo, J.; Shimizu, N.; Makino, Y.; Haneda, M. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: A possible role of HIF-1alpha expression and oxygen metabolism. Diabetes 2011, 60, 981–992. [Google Scholar] [CrossRef]
- Jiang, X.; Ruan, X.L.; Xue, Y.X.; Yang, S.; Shi, M.; Wang, L.N. Metformin Reduces the Senescence of Renal Tubular Epithelial Cells in Diabetic Nephropathy via the MBNL1/miR-130a-3p/STAT3 Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 8708236. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Norgard, M.O.; Jensen, M.S.; Moller, B.K.; Norregaard, R. Metformin modulates immune cell infiltration into the kidney during unilateral ureteral obstruction in mice. Physiol. Rep. 2019, 7, e14141. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Schiffer, T.A.; Gustafsson, H.; Krag, S.P.; Norregaard, R.; Palm, F. Metformin attenuates renal medullary hypoxia in diabetic nephropathy through inhibition uncoupling protein-2. Diabetes/Metab. Res. Rev. 2019, 35, e3091. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, L.Q.; Xu, L.L.; Xing, Y.; Ye, S. Metformin alleviates renal injury in diabetic rats by inducing Sirt1/FoxO1 autophagic signal axis. Clin. Exp. Pharmacol. Physiol. 2020, 47, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Shao, Y.; Wu, C.; Ma, X.; Lv, C.; Wang, Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol. Cell Endocrinol. 2020, 500, 110628. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, S.; Zhang, Y.; Xiao, H. Metformin attenuates renal fibrosis in both AMPKα2-dependent and independent manners. Clin. Exp. Pharmacol. Physiol. 2017, 44, 648–655. [Google Scholar] [CrossRef]
- Hashimoto, H.; Nomura, N.; Shoda, W.; Isobe, K.; Kikuchi, H.; Yamamoto, K.; Fujimaru, T.; Ando, F.; Mori, T.; Okado, T.; et al. Metformin increases urinary sodium excretion by reducing phosphorylation of the sodium-chloride cotransporter. Metabolism 2018, 85, 23–31. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- De Jager, J.; Kooy, A.; Lehert, P.; Bets, D.; Wulffele, M.G.; Teerlink, T.; Scheffer, P.G.; Schalkwijk, C.G.; Donker, A.J.; Stehouwer, C.D. Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: A randomized, placebo-controlled trial. J. Intern. Med. 2005, 257, 100–109. [Google Scholar] [CrossRef]
- Amador-Licona, N.; Guizar-Mendoza, J.; Vargas, E.; Sanchez-Camargo, G.; Zamora-Mata, L. The short-term effect of a switch from glibenclamide to metformin on blood pressure and microalbuminuria in patients with type 2 diabetes mellitus. Arch. Med. Res. 2000, 31, 571–575. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The Long-term Effects of Metformin on Patients With Type 2 Diabetic Kidney Disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef] [PubMed]
- de Jager, J.; Kooy, A.; Schalkwijk, C.; van der Kolk, J.; Lehert, P.; Bets, D.; Wulffele, M.G.; Donker, A.J.; Stehouwer, C.D. Long-term effects of metformin on endothelial function in type 2 diabetes: A randomized controlled trial. J. Intern. Med. 2014, 275, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lachin, J.M.; Viberti, G.; Zinman, B.; Haffner, S.M.; Aftring, R.P.; Paul, G.; Kravitz, B.G.; Herman, W.H.; Holman, R.R.; Kahn, S.E.; et al. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Kwon, E.J.; Yun, G.; Park, S.; Jeong, J.C.; Na, K.Y.; Chin, H.J.; Yoo, S.; Kim, S.; Oh, T.J.; et al. Impact of metformin on cardiovascular and kidney outcome based on kidney function status in type 2 diabetic patients: A multicentric, retrospective cohort study. Sci. Rep. 2024, 14, 2081. [Google Scholar] [CrossRef]
- El-Damanawi, R.; Stanley, I.K.; Staatz, C.; Pascoe, E.M.; Craig, J.C.; Johnson, D.W.; Mallett, A.J.; Hawley, C.M.; Milanzi, E.; Hiemstra, T.F.; et al. Metformin for preventing the progression of chronic kidney disease. Cochrane Database Syst. Rev. 2024, 2024, CD013414. [Google Scholar] [CrossRef]
- Wang, H.H.; Lin, S.H.; Hung, S.Y.; Chiou, Y.Y.; Hsu, W.C.; Chang, C.M.; Liou, H.H.; Chang, M.Y.; Ho, L.C.; Wu, C.F.; et al. Renal protective effect of metformin in type 2 diabetes patients. J. Clin. Endocrinol. Metab. 2024, dgae477. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- de Boer, I.H.; Khunti, K.; Sadusky, T.; Tuttle, K.R.; Neumiller, J.J.; Rhee, C.M.; Rosas, S.E.; Rossing, P.; Bakris, G. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global Outcomes (KDIGO). Diabetes Care 2022, 45, 3075–3090. [Google Scholar] [CrossRef]
- Corremans, R.; Vervaet, B.A.; Dams, G.; D’Haese, P.C.; Verhulst, A. Metformin and Canagliflozin Are Equally Renoprotective in Diabetic Kidney Disease but Have No Synergistic Effect. Int. J. Mol. Sci. 2023, 24, 9043. [Google Scholar] [CrossRef]
- Corremans, R.; Neven, E.; Maudsley, S.; Leysen, H.; De Broe, M.E.; D’Haese, P.C.; Vervaet, B.A.; Verhulst, A. Progression of established non-diabetic chronic kidney disease is halted by metformin treatment in rats. Kidney Int. 2022, 101, 929–944. [Google Scholar] [CrossRef]
- Kang, Z.; Zeng, J.; Zhang, T.; Lin, S.; Gao, J.; Jiang, C.; Fan, R.; Yin, D. Hyperglycemia induces NF-κB activation and MCP-1 expression via downregulating GLP-1R expression in rat mesangial cells: Inhibition by metformin. Cell Biol. Int. 2019, 43, 940–953. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.K.; Jeong, J.U.; Chang, J.W.; Yang, W.S.; Kim, S.B.; Park, S.K.; Park, J.S.; Lee, S.K. Activation of AMP-activated protein kinase inhibits albumin-induced endoplasmic reticulum stress and apoptosis through inhibition of reactive oxygen species. Nephron Exp. Nephrol. 2012, 121, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Allouch, S.; Munusamy, S. Metformin attenuates albumin-induced alterations in renal tubular cells in vitro. J. Cell. Physiol. 2017, 232, 3652–3663. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Beneficial effects of metformin and irbesartan on advanced glycation end products (AGEs)-RAGE-induced proximal tubular cell injury. Pharmacol. Res. 2012, 65, 297–302. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm. Metab. Res. 2012, 44, 891–895. [Google Scholar] [CrossRef]
- Georgianos, P.I.; Agarwal, R. Hypertension in chronic kidney disease—Treatment standard 2023. Nephrol Dial Transplant 2023, 38, 2694–2703. [Google Scholar] [CrossRef]
- Lee, S.; Oh, H.J.; Lee, E.K.; Lee, O.; Ha, E.; Kim, S.J.; Kang, D.H.; Choi, K.B.; Ryu, D.R. Blood Pressure Control During Chronic Kidney Disease Progression. Am. J. Hypertens. 2017, 30, 610–616. [Google Scholar] [CrossRef]
- Cases, A.; Coll, E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int. Suppl. 2005, 68, S87–S93. [Google Scholar] [CrossRef]
- Wang, Y.; Dalbeth, N.; Terkeltaub, R.; Zhang, Y.; Li, X.; Zeng, C.; Lei, G.; Wei, J. Target Serum Urate Achievement and Chronic Kidney Disease Progression in Patients With Gout and Kidney Disease. JAMA Intern. Med. 2024, 185, 74–82. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef]
- Kelly, D.M.; Ademi, Z.; Doehner, W.; Lip, G.Y.H.; Mark, P.; Toyoda, K.; Wong, C.X.; Sarnak, M.; Cheung, M.; Herzog, C.A.; et al. Chronic Kidney Disease and Cerebrovascular Disease: Consensus and Guidance From a KDIGO Controversies Conference. Stroke 2021, 52, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Gueutin, V.; Cardineau, A.; Mathian, A.; Lanot, A.; Comoz, F.; Brocheriou, I.; Izzedine, H. Renal involvement in solid cancers: Epidemiological, clinical and histological characteristics study of 154 onconephrology patients. BMC Nephrol. 2024, 25, 367. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H. Systemic autoimmune diseases and the kidney. Kidney Int. 2020, 97, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Chacko, E.C.; Surrun, S.K.; Mubarack Sani, T.P.; Pappachan, J.M. Chronic viral hepatitis and chronic kidney disease. Postgrad. Med. J. 2010, 86, 486–492. [Google Scholar] [CrossRef]
- Szlagor, M.; Dybiec, J.; Mlynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]

| Variables | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| Metformin (N = 9109) | Non-Metformin (N = 1221) | aSMD | Metformin | Non-Metformin | aSMD | |
| n (%)/Median (IQR) | n (%)/Median (IQR) | n (%)/Median (IQR) | n (%)/Median (IQR) | |||
| Baseline eGFR, mL/min/1.73 m2 | 0.157 | 0.032 | ||||
| 60–<70 | 633 (6.95) | 135 (11.06) | 670 (7.30) | 61 (7.33) | ||
| 70–<80 | 1057 (11.60) | 147 (12.04) | 1062 (11.58) | 91 (10.90) | ||
| 80–<90 | 1321 (14.50) | 149 (12.20) | 1306 (14.24) | 120 (14.43) | ||
| ≥90 | 6098 (66.94) | 790 (64.70) | 6133 (66.88) | 560 (67.34) | ||
| Median (IQR) | 100.97 [84.81,120.00] | 100.74 [81.33,120.00] | 0.036 | 101.30 [84.68,120.00] | 100.85 [84.25,120.00] | 0.001 |
| Baseline UACR, mg/dL | 9.20 [6.00,15.00] | 9.90 [6.20,16.70] | 0.106 | 9.30 [6.00,15.20] | 9.80 [6.30,16.40] | 0.077 |
| Age | 0.203 | 0.070 | ||||
| <45 | 1407 (15.45) | 233 (19.08) | 1437 (15.67) | 120 (14.42) | ||
| 45–<55 | 2323 (25.50) | 251 (20.56) | 2297 (25.05) | 200 (24.08) | ||
| 55–<65 | 3126 (34.32) | 357 (29.24) | 3102 (33.83) | 289 (34.80) | ||
| ≥65 | 2253 (24.73) | 380 (31.12) | 2334 (25.45) | 222 (26.71) | ||
| Median (IQR) | 57.56 [49.81,64.90] | 58.98 [48.81,67.75] | 0.038 | 57.67 [49.79,65.13] | 58.68 [50.36,65.93] | 0.086 |
| DM duration (months) | 0.23 [0.00,5.88] | 0.62 [0.00,11.96] | 0.114 | 0.23 [0.00,6.01] | 0.49 [0.00,9.92] | 0.038 |
| Follow-up duration (years) | 2.35 [1.25,3.64] | 1.96 [1.03,3.16] | 0.228 | 2.32 [1.23,3.60] | 1.95 [1.00,3.21] | 0.231 |
| Male | 5099 (55.98) | 685 (56.10) | 0.002 | 5158 (56.25) | 481 (57.89) | 0.033 |
| Comorbidity | ||||||
| Hypertension | 3196 (35.09) | 399 (32.68) | 0.051 | 3215 (35.06) | 287 (34.55) | 0.011 |
| Hyperlipidemia | 2869 (31.50) | 322 (26.37) | 0.113 | 2846 (31.04) | 243 (29.23) | 0.039 |
| Coronary artery disease | 757 (8.31) | 93 (7.62) | 0.026 | 772 (8.42) | 73 (8.73) | 0.011 |
| Cerebrovascular disease | 547 (6.01) | 104 (8.52) | 0.097 | 586 (6.39) | 59 (7.09) | 0.028 |
| Congestive heart failure | 196 (2.15) | 33 (2.70) | 0.036 | 204 (2.22) | 20 (2.40) | 0.012 |
| Gout | 366 (4.02) | 44 (3.60) | 0.022 | 370 (4.03) | 32 (3.90) | 0.007 |
| Nephrolithiasis | 147 (1.61) | 12 (0.98) | 0.056 | 139 (1.52) | 11 (1.36) | 0.014 |
| Chronic hepatitis and liver cirrhosis | 1406 (15.44) | 195 (15.97) | 0.015 | 1405 (15.33) | 117 (14.08) | 0.035 |
| Autoimmune disorders | 76 (0.83) | 15 (1.23) | 0.039 | 80 (0.87) | 7 (0.86) | 0.001 |
| Chronic lung disease | 462 (5.07) | 57 (4.67) | 0.019 | 448 (4.89) | 35 (4.26) | 0.030 |
| Malignant | 1109 (12.17) | 161 (13.19) | 0.030 | 1118 (12.19) | 97 (11.64) | 0.017 |
| Laboratory data | ||||||
| SBP, ≥140 mmHg | 3691 (41.01) | 409 (34.08) | 0.143 | 3690 (40.73) | 317 (38.71) | 0.041 |
| DBP, ≥90 mmHg | 1389 (15.43) | 117 (9.75) | 0.172 | 1365 (15.06) | 88 (10.70) | 0.131 |
| TC, ≥200 mg/dL | 3287 (36.51) | 335 (27.99) | 0.183 | 3250 (35.87) | 235 (28.96) | 0.148 |
| LDL, ≥100 mg/dL | 5784 (64.19) | 629 (52.68) | 0.235 | 5770 (63.60) | 440 (54.27) | 0.190 |
| HDL, ≥40 mg/dL | 6257 (69.92) | 809 (68.27) | 0.036 | 6227 (69.11) | 555 (68.81) | 0.007 |
| TG, ≥150 mg/Dl | 3520 (39.14) | 348 (29.22) | 0.210 | 3541 (39.11) | 267 (32.89) | 0.130 |
| Hb, ≥12 g/dL | 6589 (86.99) | 751 (74.88) | 0.312 | 6585 (86.15) | 545 (80.24) | 0.159 |
| HbA1C | 0.150 | 0.100 | ||||
| ≥9% | 2562 (28.13) | 396 (32.43) | 2730 (29.77) | 268 (32.26) | ||
| 8%–<9% | 1228 (13.48) | 198 (16.22) | 1248 (13.61) | 133 (16.02) | ||
| 7%–<8% | 2673 (29.34) | 296 (24.24) | 2631 (28.69) | 209 (25.14) | ||
| <7% | 2646 (29.05) | 331 (27.11) | 2561 (27.93) | 221 (26.59) | ||
| Initial medications in the index data | ||||||
| Sulfonylurea, yes | 1215 (13.34) | 259 (21.21) | 0.209 | 1395 (15.21) | 274 (32.95) | 0.424 |
| Meglitinide, yes | 66 (0.72) | 80 (6.55) | 0.315 | 146 (1.59) | 20 (2.42) | 0.059 |
| AGIs, yes | 181 (1.99) | 187 (15.32) | 0.488 | 381 (4.16) | 53 (6.40) | 0.100 |
| TZD, yes | 144 (1.58) | 46 (3.77) | 0.136 | 195 (2.13) | 48 (5.82) | 0.190 |
| DPP4 inhibitor, yes | 1906 (20.92) | 456 (37.35) | 0.367 | 2263 (24.68) | 391 (47.05) | 0.480 |
| GLP1, yes | 2 (0.02) | 3 (0.25) | 0.061 | 3 (0.03) | 4 (0.48) | 0.088 |
| SGLT2 inhibitor, yes | 1 (0.01) | 9 (0.74) | 0.119 | 1 (0.01) | 24 (2.91) | 0.243 |
| Insulin, yes | 126 (1.38) | 338 (27.68) | 0.804 | 401 (4.37) | 58 (6.96) | 0.112 |
| ACEI, yes | 491 (5.39) | 49 (4.01) | 0.065 | 487 (5.31) | 41 (4.95) | 0.016 |
| ARB, yes | 3170 (34.80) | 425 (34.81) | 0 | 3209 (35.00) | 310 (37.34) | 0.049 |
| Renin inhibitor, yes | 30 (0.33) | 4 (0.33) | 0 | 31 (0.34) | 2 (0.22) | 0.023 |
| Statin, yes | 3085 (33.87) | 322 (26.37) | 0.164 | 3051 (33.27) | 270 (32.46) | 0.017 |
| Anti-gout, yes | 264 (2.90) | 38 (3.11) | 0.013 | 265 (2.88) | 22 (2.68) | 0.012 |
| CC blockers, yes | 1658 (18.20) | 191 (15.64) | 0.068 | 1644 (17.93) | 141 (16.95) | 0.026 |
| Beta blockers, yes | 1608 (17.65) | 206 (16.87) | 0.021 | 1601 (17.46) | 137 (16.54) | 0.024 |
| Diuretics, yes | 1115 (12.24) | 162 (13.27) | −0.031 | 1127 (12.29) | 95 (11.43) | 0.026 |
| Aspirin, yes | 1420 (15.59) | 205 (16.79) | 0.033 | 1481 (16.15) | 159 (19.10) | 0.078 |
| NSAID, yes | 800 (8.78) | 85 (6.96) | 0.068 | 783 (8.54) | 59 (7.10) | 0.053 |
| Type of Treatment (No. of Patients) | No. of Events | Incidence Rate per 100 Patient-Years | Study Outcome, HR (95% CI) |
|---|---|---|---|
| eGFR < 60 mL/min/1.73 m2 | |||
| Nonuser (n = 1221) | 72 | 2.89 | |
| Metformin user (n = 9109) | 383 | 1.72 | |
| Model 1 a | 0.68 (0.50–0.91) | ||
| Model 2 b | 0.64 (0.47–0.86) | ||
| Model 3 c | 0.70 (0.51–0.95) | ||
| Model 4 d | 0.71 (0.51–0.98) | ||
| Model 5 e | 0.71 (0.56–0.90) | ||
| Proteinuria (UACR ≥ 30) | |||
| Nonuser (n = 1221) | 50 | 1.93 | |
| Metformin user (n = 9109) | 355 | 1.56 | |
| Model 1 a | 0.82 (0.57–1.17) | ||
| Model 2 b | 0.95 (0.64–1.42) | ||
| Model 3 c | 1.00 (0.67–1.51) | ||
| Model 4 d | 1.03 (0.69–1.55) | ||
| Model 5 e | 0.77 (0.59–1.01) | ||
| Composite outcome | |||
| Nonuser (n = 1221) | 117 | 4.82 | |
| Metformin user (n = 9109) | 716 | 3.27 | |
| Model 1 a | 0.76 (0.60–0.97) | ||
| Model 2 b | 0.82 (0.64–1.05) | ||
| Model 3 c | 0.90 (0.69–1.16) | ||
| Model 4 d | 0.91 (0.69–1.20) | ||
| Model 5 e | 0.78 (0.65–0.94) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-L.; Lin, S.-H.; Wang, H.-H.; Hsu, W.-C.; Hung, S.-Y.; Chiou, Y.-Y.; Liou, H.-H.; Chang, M.-Y.; Ho, L.-C.; Wu, C.-F.; et al. Role of Metformin in Preventing New-Onset Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus. Pharmaceuticals 2025, 18, 95. https://doi.org/10.3390/ph18010095
Lin Y-L, Lin S-H, Wang H-H, Hsu W-C, Hung S-Y, Chiou Y-Y, Liou H-H, Chang M-Y, Ho L-C, Wu C-F, et al. Role of Metformin in Preventing New-Onset Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus. Pharmaceuticals. 2025; 18(1):95. https://doi.org/10.3390/ph18010095
Chicago/Turabian StyleLin, Yu-Ling, Sheng-Hsiang Lin, Hsi-Hao Wang, Wan-Chia Hsu, Shih-Yuan Hung, Yuan-Yow Chiou, Hung-Hsiang Liou, Min-Yu Chang, Li-Chun Ho, Ching-Fang Wu, and et al. 2025. "Role of Metformin in Preventing New-Onset Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus" Pharmaceuticals 18, no. 1: 95. https://doi.org/10.3390/ph18010095
APA StyleLin, Y.-L., Lin, S.-H., Wang, H.-H., Hsu, W.-C., Hung, S.-Y., Chiou, Y.-Y., Liou, H.-H., Chang, M.-Y., Ho, L.-C., Wu, C.-F., & Lee, Y.-C. (2025). Role of Metformin in Preventing New-Onset Chronic Kidney Disease in Patients with Type 2 Diabetes Mellitus. Pharmaceuticals, 18(1), 95. https://doi.org/10.3390/ph18010095






