New Evidence for Cotinus coggygria Scop. Extracts Application in Gastrointestinal Ailments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
2.2. Antifungal and Antibacterial Activities of Tested Extracts
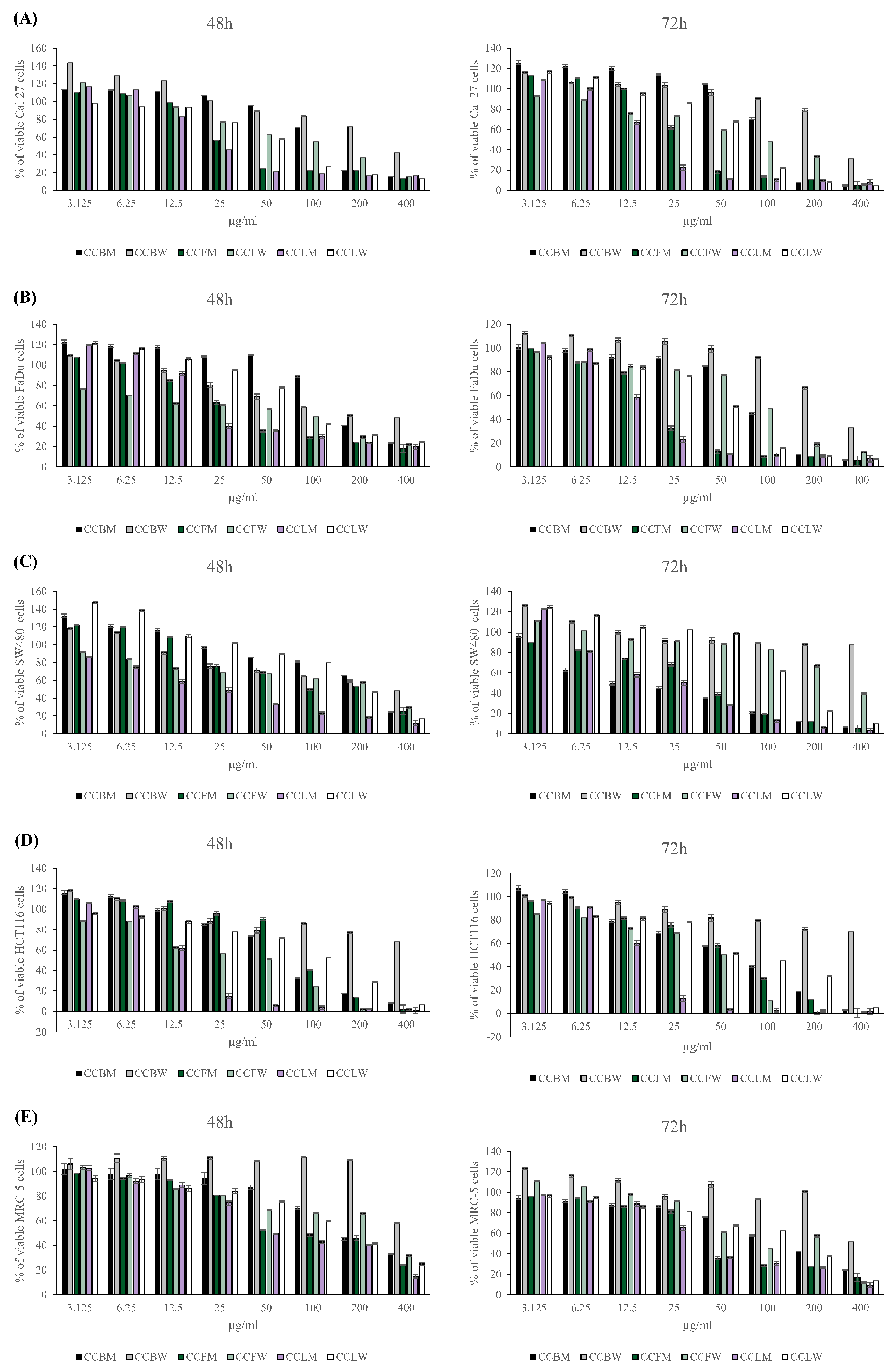
2.3. Cytotoxic Capacity of C. coggygria Extracts
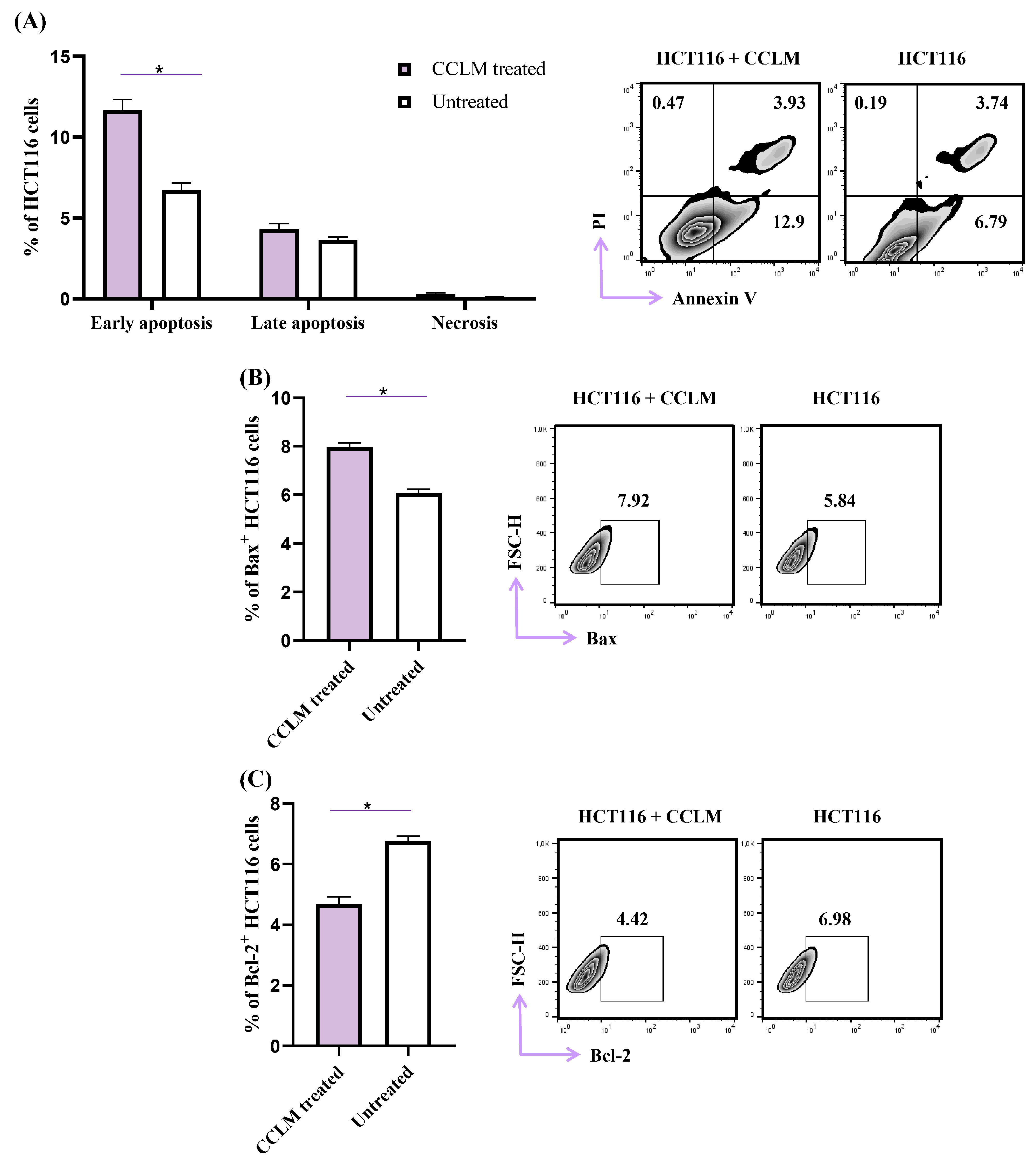
2.4. CCLM Facilitates Apoptotic Cell Death in Human Colon Cancer Cells (HCT116)
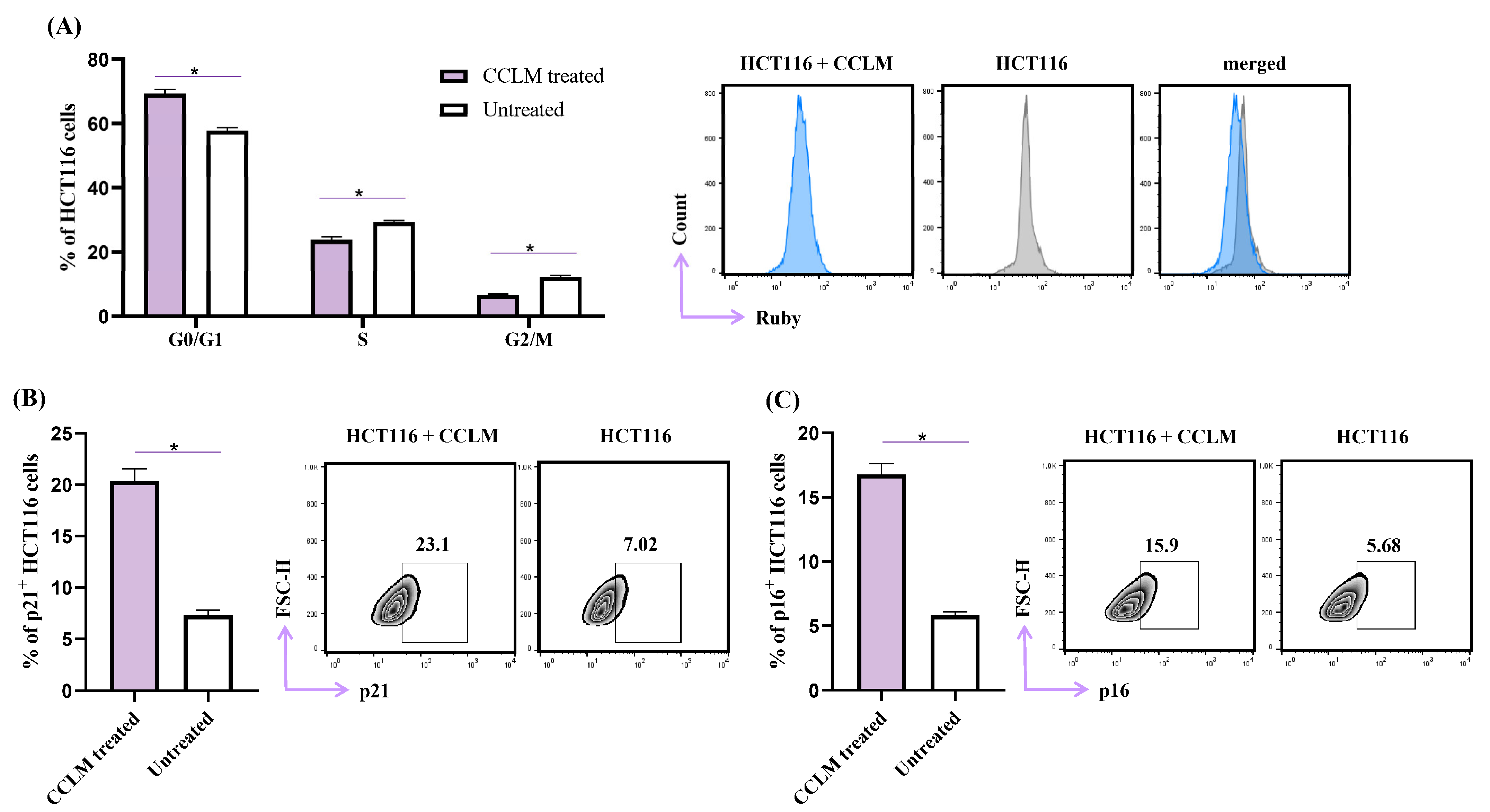
2.5. CCLM Induced Cell Cycle Arrest in G0/G1 Phase
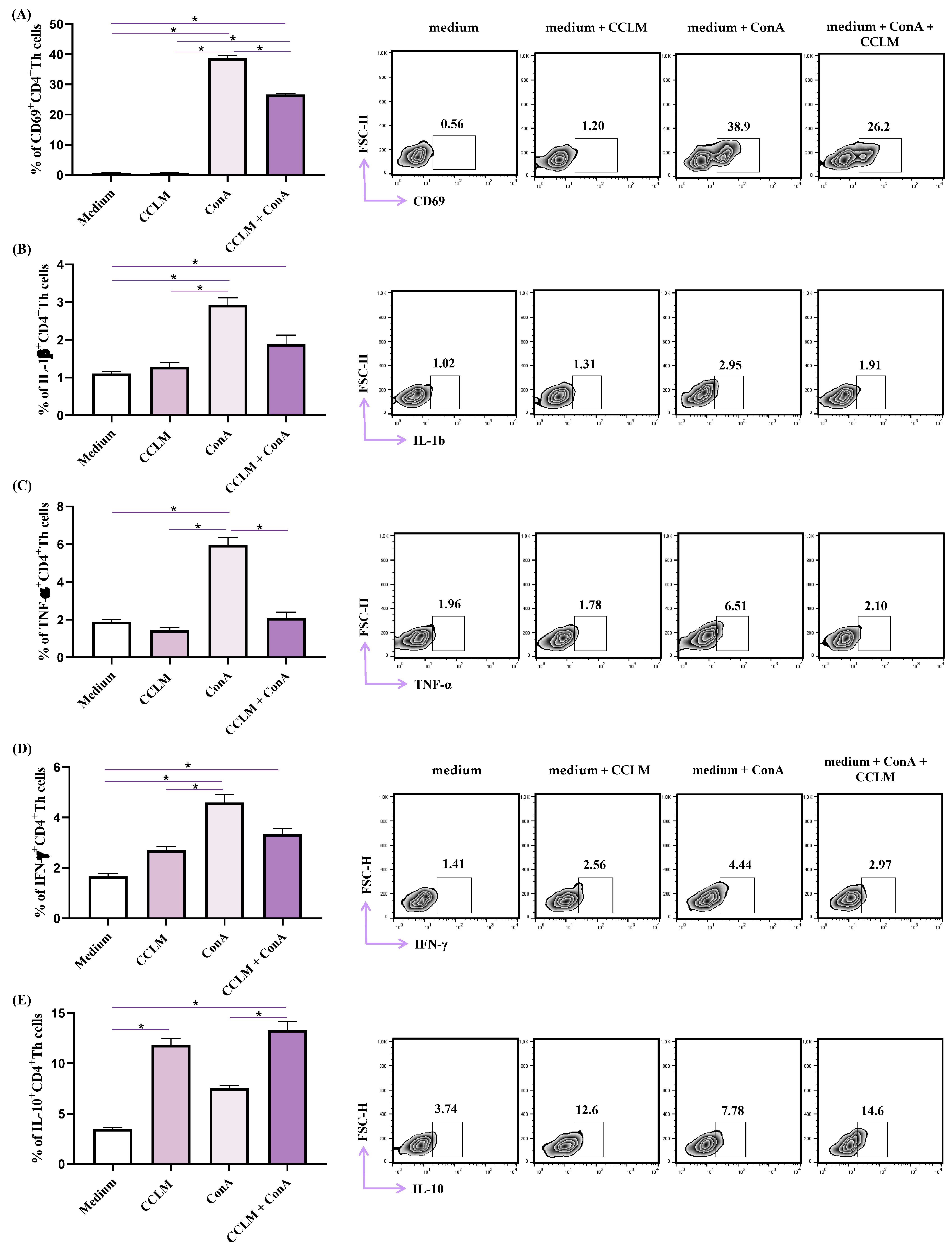
2.6. Effects of CCLM on Functional Phenotype of Th Cells
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Extracts for Chemical and Biological Activity
3.3. HPLC Analysis
3.4. Microorganisms and Culture Conditions
3.5. Anticandidal Activity
3.6. Antibacterial Activity
3.7. Cell Culture
3.8. In Vitro Cytotoxic Assay
3.9. Analysis of Cell Death
3.10. Cell Cycle Distribution
3.11. Flow Cytometry Analysis of Bax, Bcl-2, p16 and p21 Expression
3.12. Analysis of Functional Phenotype of Th Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dodington, D.W.; Nowak, K.M.; Chetty, R. Infections in the Gastrointestinal Tract That Can Mimic Malignancy. Diagn. Histopathol. 2022, 28, 435–448. [Google Scholar] [CrossRef]
- Chocolatewala, N.; Chaturvedi, P.; Desale, R. The Role of Bacteria in Oral Cancer. Indian J. Med. Paediatr. Oncol. 2010, 31, 126. [Google Scholar] [CrossRef]
- Meurman, J.H. Oral Microbiota and Cancer. J. Oral Microbiol. 2010, 2, 5195. [Google Scholar] [CrossRef]
- Selgrad, M.; Malfertheiner, P.; Fini, L.; Goel, A.; Boland, C.R.; Ricciardiello, L. The Role of Viral and Bacterial Pathogens in Gastrointestinal Cancer. J. Cell Physiol. 2008, 216, 378. [Google Scholar] [CrossRef] [PubMed]
- Kouzu, K.; Tsujimoto, H.; Kishi, Y.; Ueno, H.; Shinomiya, N. Bacterial Translocation in Gastrointestinal Cancers and Cancer Treatment. Biomedicines 2022, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, M.; Lu, X.; Zhu, X.; Deng, J. Gut Microbes in Gastrointestinal Cancers. Semin. Cancer Biol. 2022, 86, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Shaboyan, N.K.; Moghrovyan, A.V.; Dumanyan, K.H.; Ghukasyan, N.H.; Altunyan, A.A.; Arshakyan, N.I.; Ghazaryan, A.M.; Ulikhanyan, G.R.; Ginosyan, A.L.; Dadayan, A.S.; et al. Phytochemical Analysis and Antioxidant Activity of Cotinus Coggygria Scop. from Armenian Flora. Pharmacogn. J. 2021, 13, 933–941. [Google Scholar] [CrossRef]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and Antioxidant Capacity of Bulgarian Medicinal Plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef]
- Matić, S.; Stanić, S.; Solujić, S.; Milošević, T.; Niciforović, N. Biological Properties of the Cotinus Coggygria Methanol Extract. Period. Biol. 2011, 113, 87–92. [Google Scholar]
- Matić, S.; Stanić, S.; Mihailović, M.; Bogojević, D. Cotinus Coggygria Scop.: An Overview of Its Chemical Constituents, Pharmacological and Toxicological Potential. Saudi J. Biol. Sci. 2016, 23, 452–461. [Google Scholar] [CrossRef]
- Antal, D.S.; Schwaiger, S.; Ellmerer-Müller, E.P.; Stuppner, H. Cotinus Coggygria Wood: Novel Flavanone Dimer and Development of an HPLC/UV/MS Method for the Simultaneous Determination of Fourteen Phenolic Constituents. Planta Med. 2010, 76, 1765. [Google Scholar] [CrossRef]
- Šavikin, K.; Zdunic, G.; Jankovic, T.; Stanojkovic, T.; Juranic, Z.; Menkovic, N. In Vitro Cytotoxic and Antioxidative Activity of Cornus Mas and Cotinus Coggygria. Nat. Prod. Res. 2009, 23, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Elsalem, L.; Jum’ah, A.A.; Alfaqih, M.A.; Aloudat, O. The Bacterial Microbiota of Gastrointestinal Cancers: Role in Cancer Pathogenesis and Therapeutic Perspectives. Clin. Exp. Gastroenterol. 2020, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Tateda, M.; Shiga, K.; Saijo, S.; Sone, M.; Hori, T.; Yokoyama, J.; Matsuura, K.; Takasaka, T.; Miyagi, T. Streptococcus Anginosus in Head and Neck Squamous Cell Carcinoma: Implication in Carcinogenesis. Int. J. Mol. Med. 2000, 6, 699–703. [Google Scholar] [CrossRef]
- Pham, P.T.T.; Danovitch, G.M.; Pham, P.C.T. Medical Management of the Kidney Transplant Recipient: Infections and Malignant Neoplasms. Compr. Clin. Nephrol. 2010, 2010, 1177–1188. [Google Scholar] [CrossRef]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, E.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida Albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. mBio 2022, 13, e03144-21. [Google Scholar] [CrossRef] [PubMed]
- Zuza-Alves, D.L.; Silva-Rocha, W.P.; Chaves, G.M. An Update on Candida Tropicalis Based on Basic and Clinical Approaches. Front. Microbiol. 2017, 8, 1927. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.M.; Mansano, E.S.B.; Miazima, E.S.; Rodrigues, F.A.V.; Hernandes, L.; Svidzinski, T.I.E. Radiation Used for Head and Neck Cancer Increases Virulence in Candida Tropicalis Isolated from a Cancer Patient. BMC Infect. Dis. 2017, 17, 783. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Gowen, R.; Lionakis, M.S.; Ghannoum, M. Update on the Pathogenesis, Virulence, and Treatment of Candida Auris. Pathog. Immun. 2022, 7, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Şen, A.; Birteksöz Tan, A.S.; Kültür, Ş.; Bitiş, L. Isolation and Characterization of Antimicrobial Compounds from Cotinus Coggygria Scop. Ethyl Acetate Extract. İstanbul J. Pharm. 2020, 50, 272–276. [Google Scholar] [CrossRef]
- Yang, J.; Kwon, Y.S.; Kim, M.J. Antimicrobial Activity and Active Compounds of a Rhus Verniciflua Stokes Extract. Z. Naturforschung—Sect. C J. Biosci. 2018, 73, 457–463. [Google Scholar] [CrossRef]
- Tunc, K.; Hos, A.; Gunes, B. Investigation of Antibacterial Properties of Cotinus Coggygria from Turkey. Pol. J. Environ. Stud. 2013, 22, 1559–1561. [Google Scholar]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial Activity of Gallic Acid against Food-Related Pseudomonas Strains and Its Use as Biocontrol Tool to Improve the Shelf Life of Fresh Black Truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, J.; Liu, Y.; Zhang, M. Fisetin inhibits the growth of gastric carcinoma cells by suppressing ERK 1/2 activation. J. Cell Physiol. 2018, 233, 5276–5284. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, M. Fisetin inhibits tumor growth and metastasis in a mouse model of gastric cancer. Cancer Sci. 2018, 109, 2424–2432. [Google Scholar] [CrossRef]
- Poudel, S.; Song, J.; Jin, E.J.; Song, K. Sulfuretin-induced miR-30C selectively downregulates cyclin D1 and D2 and triggers cell death in human cancer cell lines. Biochem. Biophys. Res. Commun. 2013, 431, 572–578. [Google Scholar] [CrossRef]
- Kim, J.; Noh, E.; Kwon, K.; Kim, J.; You, Y.; Hwang, J.; Hwang, B.; Kim, M.; Lee, S.; Jung, S.; et al. Suppression of TPA-induced tumor cell invasion by sulfuretin via inhibition of NF-κB-dependent MMP-9 expression. Oncol. Rep. 2013, 29, 1231–1237. [Google Scholar] [CrossRef]
- Gospodinova, Z.; Bózsity, N.; Nikolova, M.; Krasteva, M.; Zupkó, I. Antiproliferative Properties Against Human Breast, Cervical and Ovarian Cancer Cell Lines, and Antioxidant Capacity of Leaf Aqueous Ethanolic Extract from Cotinus Coggygria Scop. Acta Medica Bulg. 2017, 44, 20–25. [Google Scholar] [CrossRef]
- Gospodinova, Z.I.; Zupkó, I.; Bózsity, N.; Manova, V.I.; Georgieva, M.S.; Todinova, S.J.; Taneva, S.G.; Ocsovszki, I.; Krasteva, M.E. Cotinus Coggygria Scop. Induces Cell Cycle Arrest, Apoptosis, Genotoxic Effects, Thermodynamic and Epigenetic Events in MCF7 Breast Cancer Cells. Z. Naturforsch C J. Biosci. 2020, 76, 129–140. [Google Scholar] [CrossRef]
- Danjolli-Hashani, D.; Selen-Isbilir, S. Cytotoxic Effect of Cotinus Coggygria Extract on Hep3B Cancer Cell Line. Nat. Prod. Res. 2023, 37, 4004–4007. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, S.; Dai, W. Fisetin Modulates Human Oral Squamous Cell Carcinoma Proliferation by Blocking PAK4 Signaling Pathways. Drug Des. Devel Ther. 2020, 14, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Farsad-Naeimi, A.; Alizadeh, M.; Esfahani, A.; Darvish Aminabad, E. Effect of Fisetin Supplementation on Inflammatory Factors and Matrix Metalloproteinase Enzymes in Colorectal Cancer Patients. Food Funct. 2018, 9, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, H.; Ismail, I.; Suppian, R.; Zakaria, N.M. Natural Gallic Acid and Methyl Gallate Induces Apoptosis in Hela Cells through Regulation of Intrinsic and Extrinsic Protein Expression. Int. J. Mol. Sci. 2023, 24, 8495. [Google Scholar] [CrossRef]
- Yang, C.; Xie, X.; Tang, H.; Dong, X.; Zhang, X.; Huang, F. Transcriptome analysis reveals GA induced apoptosis in HCT116 human colon cancer cells through calcium and p53 signal pathways. RSC Adv. 2018, 8, 12449–12458. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Li, L.; Kong, R.; Pan, S.; Ji, L.; Liu, H.; Chen, H.; Sun, B. Hyperoside induces apoptosis and inhibits growth in pancreatic cancer via Bcl-2 family and NF-κB signaling pathway both in vitro and in vivo. Tumour Biol. 2016, 37, 7345–7355. [Google Scholar] [CrossRef] [PubMed]
- Khafif, A.; Schantz, S.P.; Chou, T.C.; Edelstein, D.; Sacks, P.G. Quantitation of chemopreventive synergism between (-)-epigallocatechin-3-gallate and curcumin in normal, premalignant and malignant human oral epithelial cells. Carcinogenesis 1998, 19, 419–424. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The Role of BCL-2 Family Proteins in Regulating Apoptosis and Cancer Therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Han, H.; Dong, L.; Wang, Z.; Qin, Y. Function of P21 and Its Therapeutic Effects in Esophageal Cancer (Review). Oncol. Lett. 2021, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Pu, S.; Lin, C.; He, L.; Zhao, H.; Yang, C.; Guo, Z.; Xu, S.; Zhou, Z. Curcumin Selectively Induces Colon Cancer Cell Apoptosis and S Cell Cycle Arrest by Regulates Rb/E2F/P53 Pathway. J. Mol. Struct. 2022, 1263, 133180. [Google Scholar] [CrossRef]
- Choi, Y.J.; Fan, M.; Wedamulla, N.E.; Tang, Y.; Kim, E.K. Alleviatory effect of isoquercetin on benign prostatic hyperplasia via IGF-1/PI3K/Akt/mTOR pathway. Food Sci. Hum. Wellness. 2024, 13, 1698–1710. [Google Scholar] [CrossRef]
- Ding, L.; Cao, J.; Lin, W.; Chen, H.; Xiong, X.; Ao, H.; Yu, M.; Lin, J.; Cui, Q. The Roles of Cyclin-Dependent Kinases in Cell-Cycle Progression and Therapeutic Strategies in Human Breast Cancer. Int. J. Mol. Sci. 2020, 21, 1960. [Google Scholar] [CrossRef] [PubMed]
- Law, M.E.; Corsino, P.E.; Narayan, S.; Law, B.K. Cyclin-Dependent Kinase Inhibitors as Anticancer Therapeutics. Mol. Pharmacol. 2015, 88, 846–852. [Google Scholar] [CrossRef]
- Pennycook, B.R.; Barr, A.R. Palbociclib-Mediated Cell Cycle Arrest Can Occur in the Absence of the CDK Inhibitors P21 and P27. Open Biol. 2021, 11, 210125. [Google Scholar] [CrossRef]
- Pollio, A.; Zarrelli, A.; Romanucci, V.; Di Mauro, A.; Barra, F.; Pinto, G.; Crescenzi, E.; Roscetto, E.; Palumbo, G. Polyphenolic Profile and Targeted Bioactivity of Methanolic Extracts from Mediterranean Ethnomedicinal Plants on Human Cancer Cell Lines. Molecules 2016, 21, 395. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Du, L.; Li, F. Effect and Mechanism of Total Flavonoids Extracted from Cotinus Coggygria against Glioblastoma Cancer In Vitro and In Vivo. Biomed. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Du, L.; Fei, L.; To, S.S.T. Inhibitory Kinetics and Mechanism of Flavonoids Extracted from Cotinus Coggygria Scop. Against Glioblastoma Cancer. Nutr. Cancer 2016, 68, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 Family Isoforms in Apoptosis and Cancer. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 Protein Family: Attractive Targets for Cancer Therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef]
- Marčetić, M.; Božić, D.; Milenković, M.; Malešević, N.; Radulović, S.; Kovačević, N. Antimicrobial, Antioxidant and Anti-Inflammatory Activity of Young Shoots of the Smoke Tree, Cotinus Coggygria Scop. Phytother. Res. 2013, 27, 1658–1663. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Kannan, A.; Stojković, D.; Glamočlija, J.; Grdadolnik, S.G.; Sanglard, D.; Soković, M. Revealing the Astragalin Mode of Anticandidal Action. EXCLI J. 2020, 19, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Ivanov, M.; Fernandes, Â.; Pinela, J.; Calhelha, R.C.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Soković, M.; Ćirić, A. Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity. Molecules 2020, 25, 4336. [Google Scholar] [CrossRef] [PubMed]
- Jurisevic, M.; Arsenijevic, A.; Pantic, J.; Gajovic, N.; Milovanovic, J.; Milovanovic, M.; Poljarevic, J.; Sabo, T.; Vojvodic, D.; Radosavljevic, G.D.; et al. The Organic Ester O,O’-Diethyl-(S,S)-Ethylenediamine-N,N’-Di-2-(3-Cyclohexyl)Propanoate Dihydrochloride Attenuates Murine Breast Cancer Growth and Metastasis. Oncotarget 2018, 9, 28195–28212. [Google Scholar] [CrossRef] [PubMed]
- Konovalov, B.; Franich, A.A.; Jovanović, M.; Jurisević, M.; Gajović, N.; Jovanović, M.; Arsenijević, N.; Maric, V.; Jovanović, I.; Živković, M.D.; et al. Synthesis, DNA-/Bovine Serum Albumin-Binding Affinity, and Cytotoxicity of Dinuclear Platinum(II) Complexes with 1,6-Naphthyridine-Bridging Ligand. Appl. Organomet. Chem. 2021, 35, e6112. [Google Scholar] [CrossRef]
- Gajovic, N.; Jurisevic, M.; Pantic, J.; Radosavljevic, G.; Arsenijevic, N.; Lukic, M.L.; Jovanovic, I. Attenuation of NK Cells Facilitates Mammary Tumor Growth in Streptozotocin-Induced Diabetes in Mice. Endocr. Relat. Cancer 2018, 25, 493–507. [Google Scholar] [CrossRef] [PubMed]
| Compound | Extract | |||||
|---|---|---|---|---|---|---|
| CCBM | CCBW | CCLM | CCLW | CCFM | CCFW | |
| (mg/g dw) | ||||||
| Sulfuretin | 164.90 | 13.58 | 0.76 | 0.84 | Nd | Nd |
| Fisetin | 22.74 | 1.98 | 0.10 | 0.02 | Nd | Nd |
| Gallic acid | 3.17 | 19.98 | 2.53 | 43.29 | 7.47 | 77.63 |
| Isoquercetin | 5.75 | 2.73 | 3.02 | 6.23 | 4.71 | 3.99 |
| Hyperoside | 5.67 | 1.73 | 1.36 | 0.63 | 4.87 | 2.24 |
| Chlorogenic acid | 0.60 | 0.32 | 0.38 | 2.94 | 0.52 | 1.82 |
| Quercetin | 1.18 | 0.01 | Tr | Tr | 0.01 | Tr |
| Strains | CCBM | CCBW | CCFM | CCFW | CCLM | CCLW | Ketoconazole | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| C. albicans ATCC 10231 | 1 | 2 | 2 | 4 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0.0016 | 0.0064 |
| C. glabrata 4/6/15 | 0.5 | 1 | 1 | 2 | 0.5 | 1 | 1 | 2 | 2 | 4 | 0.5 | 1 | 0.0016 | 0.0064 |
| C. krusei H1/16 | 0.5 | 1 | 0.5 | 1 | 0.5 | 1 | 0.25 | 0.5 | 1 | 2 | 0.25 | 0.5 | 0.0016 | 0.0032 |
| C. tropicalis ATCC 750 | 0.12 | 0.25 | 0.5 | 1 | 0.5 | 1 | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.0016 | 0.0064 |
| C. auris CDC B 11903 | 1 | 2 | 2 | 4 | 1 | 2 | 8 | >8 | 8 | >8 | 1 | 2 | - | - |
| CCBM | CCBW | CCFM | CCFW | CCLM | CCLW | Streptomycin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Micrococcus luteus (dT_9/2) | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.12 | 0.25 | 0.12 | 0.25 | 0.5 | 1 | 0.006 | 0.012 |
| Rothia mucilaginosa (oT_22/2) | 0.5 | 1 | 1 | 2 | 0.5 | 1 | 0.12 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 0.012 | 0.025 |
| Streptococcus anginosus (oT_26) | 0.5 | 1 | 0.25 | 0.5 | 1 | 2 | 0.5 | 1 | 0.12 | 0.25 | 1 | 2 | 0.003 | 0.006 |
| Streptococcus dysgalactiae (oT_21/2) | 0.12 | 0.25 | 0.25 | 0.5 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.25 | 0.5 | 0.006 | 0.012 |
| Streptococcus oralis (oT_5) | 0.5 | 1 | 0.5 | 1 | 1 | 2 | 1 | 2 | 0.5 | 1 | 1 | 2 | 0.012 | 0.025 |
| Streptococcus parasanguinis (oT_3) | 0.12 | 0.25 | 0.25 | 0.5 | 0.5 | 1 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.003 | 0.006 |
| Streptococcus pyogenes (dT_14) | 0.25 | 0.5 | 0.25 | 0.5 | 2 | 4 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.003 | 0.006 |
| Streptococcus salivarius (dT_12) | 0.5 | 1 | 0.5 | 1 | 2 | 4 | 0.12 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.006 | 0.012 |
| Staphylococcus hominis (oT_14/2) | 0.5 | 1 | 0.25 | 0.5 | 1 | 2 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.038 | 0.075 |
| Enterobacter cloacae (oT_18) | 2 | 4 | 2 | 4 | 2 | 4 | 8 | >8 | 8 | >8 | 0.25 | 0.5 | 0.038 | 0.075 |
| Stenotrophomonas maltophilia (A_12) | 4 | 8 | 0.25 | 0.5 | 1 | 2 | 0.12 | 0.25 | 0.12 | 0.25 | 0.25 | 0.5 | 0.038 | 0.075 |
| Extract | IC50 (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cal 27 | FADU | SW480 | HCT116 | MRC-5 | ||||||
| 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | |
| CCBM | 147.3 ± 9.1 $ | 122.3 ± 4.5 $ | 191.2 ± 9.9 | 75.1 ± 1.1 $ | 225.5 ± 6.15 | 20.7 ± 0.2 | 73.2 ± 1.5 | 55.8 ± 2.7 | 196.5 ± 9.1 | 144.9 ± 8.1 |
| CCBW | 325.1 ± 3.8 | 322.1 ± 12.7 | 212.4 ± 6.6 | 306.7 ± 12.4 | >400 | >400 | >400 | >400 | >400 | >400 |
| CCFM | 33.3 ± 1.7 $ | 41.1 ± 0.3 $ | 37.1 ± 1.4 $ | 24.3 ± 0.6 $ | 135.0 ± 9.9 | 33.15 ± 1.2 $ | 83.2 ± 4.1 $ | 45.9 ± 2.1 $ | 83.7 ± 4.9 $ | 51.3 ± 3.4 $ |
| CCFW | 96.2 ± 3.9 | 95.8 ± 3.1 | 72.6 ± 1.5 | 75.4 ± 2.4 | 196.9 ± 3.6 | 337.1 ± 7.7 | 31.5 ± 2.3 | 29.7 ± 0.7 | 272.0 ± 8.1 | 117.7 ± 7.5 |
| CCLM | 28.1 ± 0.9 $ | 23.9 ± 1.3 $ | 20.9 ± 0.6 $ | 22.7 ± 0.7 | 25.9 ± 1.4 $ | 28.6 ± 1.5 $ | 20.9 ± 0.9 $ | 17.0 ± 0.9 $ | 63.4 ± 3.6 $ | 49.4 ± 1.8 $ |
| CCLW | 57.5 ± 3.1 | 54.6 ± 0.9 | 95.8 ± 2.5 | 47.8 ± 0.2 | 185.0 ± 5.8 | 122.8 ± 2.0 | 96.3 ± 3.6 | 59.3 ± 3.6 | 149.3 ± 4.6 | 158.7 ± 13.1 |
| Extract | Cal 27 | FADU | SW480 | HCT116 | ||||
|---|---|---|---|---|---|---|---|---|
| 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | 48 h | 72 h | |
| CCBM | 1.33 | 1.18 | 1.03 | 1.93 | 0.87 | 7 | 2.68 | 2.60 |
| CCBW | / | / | / | / | / | / | / | / |
| CCFM | 2.51 | 1.25 | 2.26 | 2.11 | 0.62 | 1.55 | 1.01 | 1.12 |
| CCFW | 2.83 | 1.23 | 3.75 | 1.56 | 1.38 | 0.35 | 8.63 | 3.96 |
| CCLM | 2.26 | 2.07 | 3.03 | 2.18 | 2.45 | 1.73 | 3.03 | 2.91 |
| CCLW | 2.60 | 2.91 | 1.56 | 3.32 | 0.81 | 1.29 | 1.55 | 2.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojković, D.; Dragičević, N.; Ivanov, M.; Gajović, N.; Jurišević, M.; Jovanović, I.; Tomović, M.; Živković, J. New Evidence for Cotinus coggygria Scop. Extracts Application in Gastrointestinal Ailments. Pharmaceuticals 2025, 18, 98. https://doi.org/10.3390/ph18010098
Stojković D, Dragičević N, Ivanov M, Gajović N, Jurišević M, Jovanović I, Tomović M, Živković J. New Evidence for Cotinus coggygria Scop. Extracts Application in Gastrointestinal Ailments. Pharmaceuticals. 2025; 18(1):98. https://doi.org/10.3390/ph18010098
Chicago/Turabian StyleStojković, Dejan, Nina Dragičević, Marija Ivanov, Nevena Gajović, Milena Jurišević, Ivan Jovanović, Marina Tomović, and Jelena Živković. 2025. "New Evidence for Cotinus coggygria Scop. Extracts Application in Gastrointestinal Ailments" Pharmaceuticals 18, no. 1: 98. https://doi.org/10.3390/ph18010098
APA StyleStojković, D., Dragičević, N., Ivanov, M., Gajović, N., Jurišević, M., Jovanović, I., Tomović, M., & Živković, J. (2025). New Evidence for Cotinus coggygria Scop. Extracts Application in Gastrointestinal Ailments. Pharmaceuticals, 18(1), 98. https://doi.org/10.3390/ph18010098








