Mechanisms of Copper-Induced Autophagy and Links with Human Diseases
Abstract
1. Introduction
2. Effects of Copper on Autophagy
2.1. Copper Ions
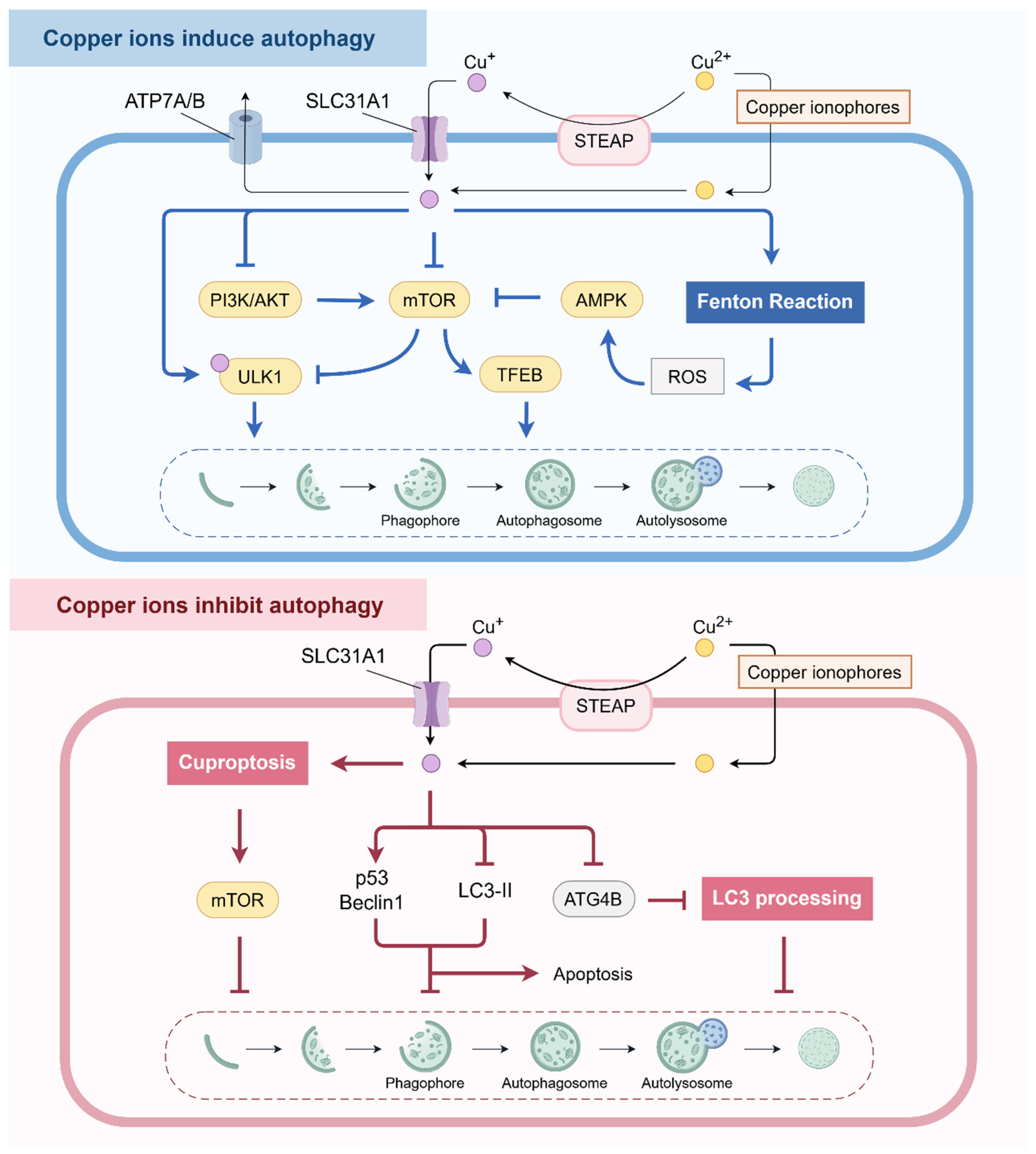
2.1.1. Copper Ions Induce Autophagy
2.1.2. Copper Ions Inhibit Autophagy
2.2. Copper Complexes
2.2.1. Disulfiram (DSF)/Copper Complex
2.2.2. Quinoline Ligand-Based Copper Complexes
2.2.3. Schiff Base-Ligated Copper Complexes
2.2.4. Mononuclear/Polynuclear Copper Complexes
2.2.5. Other Copper Complexes
2.3. Copper-Based NPs
2.3.1. Copper-Based NPs Affect the Lysosomes
2.3.2. Copper-Based NPs Regulate ROS
2.3.3. Copper-Based NPs Regulate mTOR-Related Signaling Pathways
3. Copper-Induced Autophagy and Cancer
3.1. Copper Ions
3.2. Copper Complexes
3.2.1. Inhibiting Cancer Cell Proliferation and Angiogenesis
3.2.2. Exhibiting Cytotoxicity and Inducing Cancer Cell Death
3.2.3. Overcoming Drug Resistance
3.2.4. Improving the Efficacy of Chemodynamic Therapy
3.2.5. Promoting Cancer Cell Survival
3.3. Copper-Based NPs
4. Copper-Induced Autophagy and Other Human Diseases
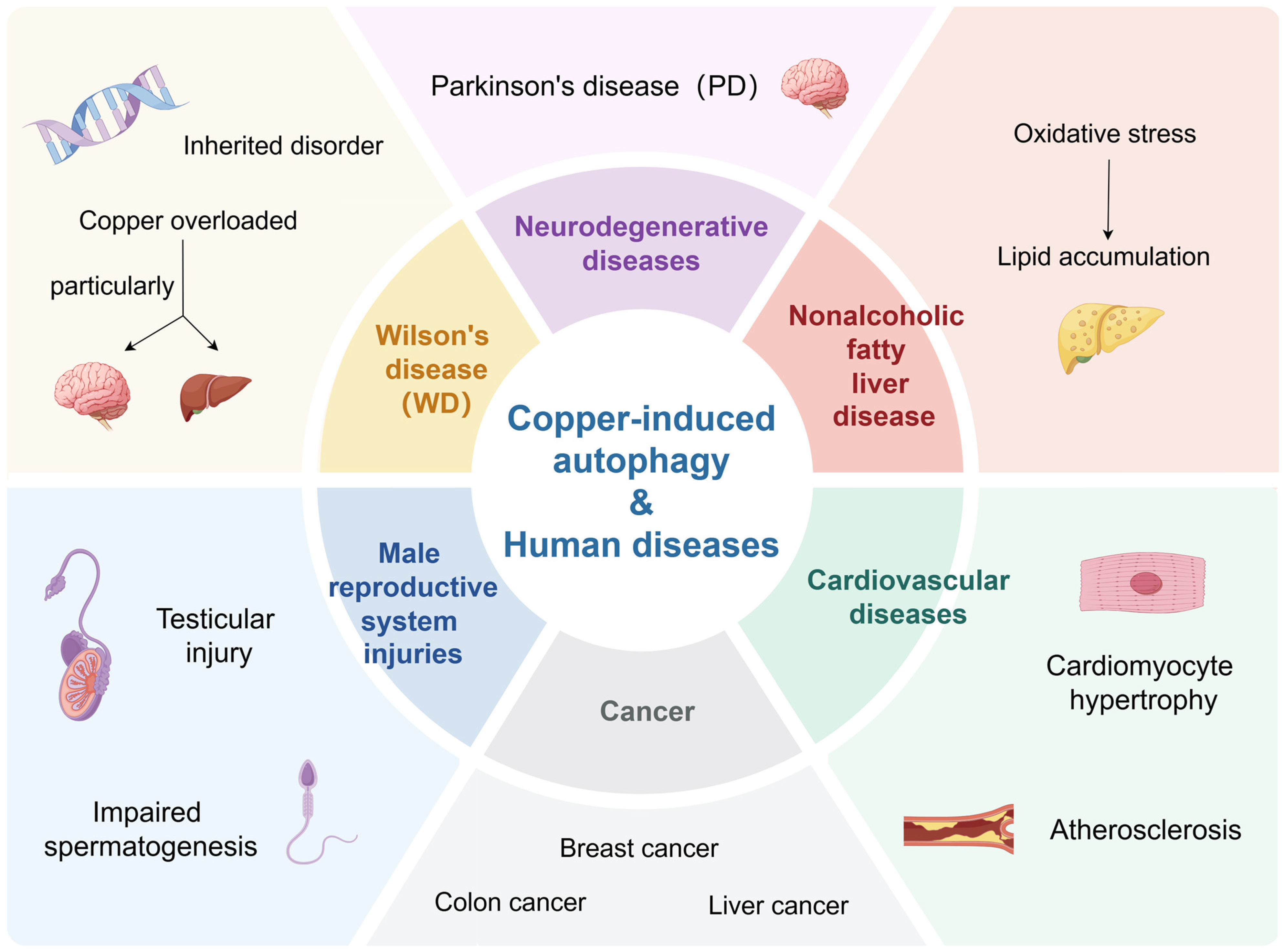
4.1. Wilson’s Disease
4.2. Neurodegenerative Diseases
4.3. Cardiovascular Diseases
4.4. Male Reproductive System Injuries
4.5. Nonalcoholic Fatty Liver Disease (NAFLD)
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell. Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- Kimmelman, A.C.; White, E. Autophagy and Tumor Metabolism. Cell Metab. 2017, 25, 1037–1043. [Google Scholar] [CrossRef]
- Ozpolat, B.; Benbrook, D.M. Targeting autophagy in cancer management-strategies and developments. Cancer Manag. Res. 2015, 7, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Husain, N.; Ali, S.N.; Arif, H.; Khan, A.A.; Mahmood, R. Oral Administration of Copper Chloride Damages DNA, Lowers Antioxidant Defense, Alters Metabolic Status, and Inhibits Membrane Bound Enzymes in Rat Kidney. Biol. Trace Elem. Res. 2023, 201, 3367–3380. [Google Scholar] [CrossRef]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases-Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luo, C.; Shan, C.; You, Q.; Lu, J.; Elf, S.; Zhou, Y.; Wen, Y.; Vinkenborg, J.L.; Fan, J.; et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem. 2015, 7, 968–979. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, D.; Chen, X.; Li, X.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Chen, X.; Tang, D.; Liu, J. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy 2023, 19, 1982–1996. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, J.D. Wilson disease. Gastroenterology 2003, 125, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.A.; Schilsky, M.L. Diagnosis and treatment of Wilson disease: An update. Hepatology 2008, 47, 2089–2111. [Google Scholar] [CrossRef]
- Xue, Q.; Kang, R.; Klionsky, D.J.; Tang, D.; Liu, J.; Chen, X. Copper metabolism in cell death and autophagy. Autophagy 2023, 19, 2175–2195. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Li, X.; Gao, J.; Wang, Y.; Xiao, L.; Chang, X.; Liu, F.; Feng, Z.; Zhang, X. Transcription factor p8 regulates autophagy in response to disulfiram via PI3K/mTOR/p70S6K signaling pathway in pancreatic cancer cells. Hum. Cell 2022, 35, 1464–1474. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, Z.; Wang, B.; Xu, G.; Wu, Q.; Zhang, Y.; Yuan, Z.; Yang, X.; Yu, C. Lysosomal deposition of copper oxide nanoparticles triggers HUVEC cells death. Biomaterials 2018, 161, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Qu, C.; Wang, Z.; Gao, H.; Liu, W.; Wang, H.; Sun, H.; Gu, J.; Yang, Z.; Wang, X. Cuproptosis enhances docetaxel chemosensitivity by inhibiting autophagy via the DLAT/mTOR pathway in prostate cancer. FASEB J. 2023, 37, e23145. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, M.A.; Abdel Malak, C.A.; El-Shafey, E.S. Influence of copper (I) nicotinate complex and autophagy modulation on doxorubicin-induced cytotoxicity in HCC1806 breast cancer cells. Adv. Med. Sci. 2019, 64, 202–209. [Google Scholar] [CrossRef]
- Gonzalez-Alcocer, A.; Gopar-Cuevas, Y.; Soto-Dominguez, A.; Castillo-Velazquez, U.; de Jesus Loera-Arias, M.; Saucedo-Cardenas, O.; de Oca-Luna, R.M.; Garcia-Garcia, A.; Rodriguez-Rocha, H. Combined chronic copper exposure and aging lead to neurotoxicity in vivo. Neurotoxicology 2023, 95, 181–192. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of Autophagy, Observed in Liver Tissues From Patients With Wilson Disease and From ATP7B-Deficient Animals, Protects Hepatocytes From Copper-Induced Apoptosis. Gastroenterology 2019, 156, 1173–1189.e5. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Fu, Y.; Xie, H.; Chen, Y.; Fang, D.; Zhang, W.; Liu, P.; Li, M. Suppression of ATG4B by copper inhibits autophagy and involves in Mallory body formation. Redox Biol. 2022, 52, 102284. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, Y.; Cai, M.; Zhao, Y.; Cao, W.; Liu, Z.; Cui, G.; Tang, B. Copper sulfide nanoparticles as a photothermal switch for TRPV1 signaling to attenuate atherosclerosis. Nat. Commun. 2018, 9, 231. [Google Scholar] [CrossRef]
- Li, N.; Du, H.; Mao, L.; Xu, G.; Zhang, M.; Fan, Y.; Dong, X.; Zheng, L.; Wang, B.; Qin, X.; et al. Reciprocal regulation of NRF2 by autophagy and ubiquitin-proteasome modulates vascular endothelial injury induced by copper oxide nanoparticles. J. Nanobiotechnol. 2022, 20, 270. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem Rev 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Shu, Y.; Wang, J. Metal-Organic Frameworks Encapsulating Carbon Dots Enable Fast Speciation of Mono- and Divalent Copper. Anal. Chem. 2022, 94, 2255–2262. [Google Scholar] [CrossRef]
- Zhang, S.; Mei, Y.; Liu, J.; Liu, Z.; Tian, Y. Alkyne-tagged SERS nanoprobe for understanding Cu+ and Cu2+ conversion in cuproptosis processes. Nat. Commun. 2024, 15, 3246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huo, Z.; Qi, X.; Zuo, T.; Wu, Z. Copper-induced tumor cell death mechanisms and antitumor theragnostic applications of copper complexes. Nanomedicine 2022, 17, 303–324. [Google Scholar] [CrossRef]
- Zhu, L.; He, S.; Huang, L.; Ren, D.; Nie, T.; Tao, K.; Xia, L.; Lu, F.; Mao, Z.; Yang, Q. Chaperone-mediated autophagy degrades Keap1 and promotes Nrf2-mediated antioxidative response. Aging Cell 2022, 21, e13616. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Shao, Y.; Li, X.; Wu, T.; Yu, L.; Liang, J.; Zhang, Y.; Wang, J.; Sun, T.; Zhu, Y.; et al. NR3C1/Glucocorticoid receptor activation promotes pancreatic β-cell autophagy overload in response to glucolipotoxicity. Autophagy 2023, 19, 2538–2557. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 2020, 22, 412–424. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; Zhao, H.; Mu, M.; Yang, X.; Fei, D.; Liu, Y.; Zong, H.; Xing, M. Oxidative damage under As3+ and/or Cu2+ stress leads to apoptosis and autophagy and may be cross-talking with mitochondrial disorders in bursa of Fabricius. J. Inorg. Biochem. 2020, 205, 110989. [Google Scholar] [CrossRef]
- Liu, H.; Deng, H.; Cui, H.; Jian, Z.; Guo, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; et al. Copper induces hepatocyte autophagy via the mammalian targets of the rapamycin signaling pathway in mice. Ecotoxicol. Environ. Saf. 2021, 208, 111656. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, S.; Dwivedi, S.; Trivedi, A.; Dubey, I.; Trivedi, S.P. Copper-induced Genotoxicity, Oxidative Stress, and Alteration in Transcriptional Level of Autophagy-associated Genes in Snakehead Fish Channa punctatus. Biol. Trace Elem. Res. 2023, 201, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Cho, Y.S.; Huh, Y.D.; Park, H. Copper Ion from Cu2O Crystal Induces AMPK-Mediated Autophagy via Superoxide in Endothelial Cells. Mol. Cells 2016, 39, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, Y.Y.; Chen, H.; Wu, Y.C.; Zhang, L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, H.; Wang, Y.; Shao, Y.; Wang, B.; Wang, Y.; Xing, M. Regulation of autophagy factors by oxidative stress and cardiac enzymes imbalance during arsenic or/and copper induced cardiotoxicity in Gallus gallus. Ecotoxicol. Environ. Saf. 2018, 148, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, J.; Zhao, H.; Wang, Y.; Liu, J.; Shao, Y.; Xue, Y.; Xing, M. Synergistic effect of copper and arsenic upon oxidative stress, inflammation and autophagy alterations in brain tissues of Gallus gallus. J. Inorg. Biochem. 2018, 178, 54–62. [Google Scholar] [CrossRef]
- Guo, H.; Ouyang, Y.; Yin, H.; Cui, H.; Deng, H.; Liu, H.; Jian, Z.; Fang, J.; Zuo, Z.; Wang, X.; et al. Induction of autophagy via the ROS-dependent AMPK-mTOR pathway protects copper-induced spermatogenesis disorder. Redox Biol. 2022, 49, 102227. [Google Scholar] [CrossRef]
- Bo, L.Y.; Li, T.J.; Zhao, X.H. Copper or Manganese Supplementation Endows the Peptic Hydrolysate from Bovine Lactoferrin with Enhanced Activity to Human Gastric Cancer AGS Cells. Biol. Trace Elem. Res. 2019, 189, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Nie, K.; Song, Y.; Liu, H.; Zhou, Y.; Yuan, Y.; Chen, D.; Peng, X.; Yan, W.; Song, J.; et al. Monitoring the Cellular Delivery of Doxorubicin-Cu Complexes in Cells by Fluorescence Lifetime Imaging Microscopy. J. Phys. Chem. 2020, 124, 4235–4240. [Google Scholar] [CrossRef]
- Lu, X.; Lin, B.; Xu, N.; Huang, H.; Wang, Y.; Lin, J.M. Evaluation of the accumulation of disulfiram and its copper complex in A549 cells using mass spectrometry. Talanta 2020, 211, 120732. [Google Scholar] [CrossRef] [PubMed]
- Musci, G.; Di Marco, S.; Bellenchi, G.C.; Calabrese, L. Reconstitution of ceruloplasmin by the Cu(I)-glutathione complex. Evidence for a role of Mg2+ and ATP. J. Biol. Chem. 1996, 271, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Man, X.; Li, S.; Xu, G.; Li, W.; Zhu, M.; Zhang, Z.; Liang, H.; Yang, F. Developing a Copper(II) Isopropyl 2-Pyridyl Ketone Thiosemicarbazone Compound Based on the IB Subdomain of Human Serum Albumin-Indomethacin Complex: Inhibiting Tumor Growth by Remodeling the Tumor Microenvironment. J. Med. Chem. 2024, 67, 5744–5757. [Google Scholar] [CrossRef]
- Sequeira, D.; Baptista, P.V.; Valente, R.; Piedade, M.F.M.; Garcia, M.H.; Morais, T.S.; Fernandes, A.R. Cu(I) complexes as new antiproliferative agents against sensitive and doxorubicin resistant colorectal cancer cells: Synthesis, characterization, and mechanisms of action. Dalton Trans. 2021, 50, 1845–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, P.; Ding, S.Y.; Sun, T.; Liu, L.; Han, S.; DeLeo, A.B.; Sadagopan, A.; Guo, W.; Wang, X. Induction of autophagy-dependent apoptosis in cancer cells through activation of ER stress: An uncovered anti-cancer mechanism by anti-alcoholism drug disulfiram. Am. J. Cancer Res. 2019, 9, 1266–1281. [Google Scholar] [PubMed]
- Hu, Y.; Qian, Y.; Wei, J.; Jin, T.; Kong, X.; Cao, H.; Ding, K. The Disulfiram/Copper Complex Induces Autophagic Cell Death in Colorectal Cancer by Targeting ULK1. Front. Pharmacol. 2021, 12, 752825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, Y.; Fuchs, B.C.; Guo, W.; Drum, D.L.; Erstad, D.J.; Shi, B.; DeLeo, A.B.; Zheng, H.; Cai, L.; et al. Improving the Therapeutic Efficacy of Sorafenib for Hepatocellular Carcinoma by Repurposing Disulfiram. Front. Oncol. 2022, 12, 913736. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Lin, L.; Wang, H.; He, E.; Wang, G.; Zhao, Q. The disulfiram/copper complex induces apoptosis and inhibits tumour growth in human osteosarcoma by activating the ROS/JNK signalling pathway. J. Biochem. 2021, 170, 275–287. [Google Scholar] [CrossRef]
- Zha, J.; Bi, S.; Deng, M.; Chen, K.; Shi, P.; Feng, L.; He, J.; Pu, X.; Guo, C.; Zhao, H.; et al. Disulfiram/copper shows potent cytotoxic effects on myelodysplastic syndromes via inducing Bip-mediated apoptosis and suppressing autophagy. Eur. J. Pharmacol. 2021, 902, 174107. [Google Scholar] [CrossRef]
- Shen, W.Y.; Jia, C.P.; Liao, L.Y.; Chen, L.L.; Yuan, C.C.; Gu, Y.Q.; Liu, Y.H.; Liang, H.; Chen, Z.F. Copper(II) complex enhanced chemodynamic therapy through GSH depletion and autophagy flow blockade. Dalton Trans. 2023, 52, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.C.; Tsai, M.T.; Hsu, M.H.; Wang, S.H.; Way, T.D.; Huang, C.H.; Lin, H.Y.; Qian, K.; Dong, Y.; Lee, K.H.; et al. Design, synthesis, and preclinical evaluation of new 5,6- (or 6,7-) disubstituted-2-(fluorophenyl)quinolin-4-one derivatives as potent antitumor agents. J. Med. Chem. 2010, 53, 8047–8058. [Google Scholar] [CrossRef]
- Raju, C.E.; Balasubramanian, S.; Karunakar, G.V. Copper(I)-Catalyzed Formation of Isoquinoline and Quinoline Substituted Isobenzofurans. Org. Lett. 2022, 24, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.Y.; Jia, C.P.; Liao, L.Y.; Chen, L.L.; Hou, C.; Liu, Y.H.; Liang, H.; Chen, Z.F. Copper(II) Complexes of Halogenated Quinoline Schiff Base Derivatives Enabled Cancer Therapy through Glutathione-Assisted Chemodynamic Therapy and Inhibition of Autophagy Flux. J. Med. Chem. 2022, 65, 5134–5148. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Machura, B.; Szlapa-Kula, A.; Malecki, J.G.; Raposo, L.; Roma-Rodrigues, C.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Square planar Au(III), Pt(II) and Cu(II) complexes with quinoline-substituted 2,2’:6’,2″-terpyridine ligands: From in vitro to in vivo biological properties. Eur. J. Med. Chem. 2021, 218, 113404. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Deng, C.X.; Zhou, W.F.; Zhou, L.Y.; Cao, Q.Q.; Shen, W.Y.; Liang, H.; Chen, Z.F. Synthesis and in vitro antitumor activity evaluation of copper(II) complexes with 5-pyridin-2-yl-[1,3]dioxolo[4,5-g]isoquinoline derivatives. J. Inorg. Biochem. 2019, 201, 110820. [Google Scholar] [CrossRef]
- Gul, N.S.; Khan, T.M.; Chen, M.; Huang, K.B.; Hou, C.; Choudhary, M.I.; Liang, H.; Chen, Z.F. New copper complexes inducing bimodal death through apoptosis and autophagy in A549 cancer cells. J. Inorg. Biochem. 2020, 213, 111260. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, C.; Wambang, N.; Bousquet, T.; Vercoutter-Edouart, A.S.; Pélinski, L.; Cailliau, K.; Martoriati, A. A Novel Copper(II) Indenoisoquinoline Complex Inhibits Topoisomerase I, Induces G2 Phase Arrest, and Autophagy in Three Adenocarcinomas. Front. Oncol. 2022, 12, 837373. [Google Scholar] [CrossRef]
- Molinaro, C.; Wambang, N.; Pellegrini, S.; Henry, N.; Lensink, M.F.; Germain, E.; Bousquet, T.; de Ruyck, J.; Cailliau, K.; Pélinski, L.; et al. Synthesis and Biological Activity of a New Indenoisoquinoline Copper Derivative as a Topoisomerase I Inhibitor. Int. J. Mol. Sci. 2023, 24, 14590. [Google Scholar] [CrossRef] [PubMed]
- Hajrezaie, M.; Paydar, M.; Moghadamtousi, S.Z.; Hassandarvish, P.; Gwaram, N.S.; Zahedifard, M.; Rouhollahi, E.; Karimian, H.; Looi, C.Y.; Ali, H.M.; et al. A Schiff base-derived copper (II) complex is a potent inducer of apoptosis in colon cancer cells by activating the intrinsic pathway. Sci. World J. 2014, 2014, 540463. [Google Scholar] [CrossRef]
- Koňariková, K.; Perdikaris, G.A.; Gbelcová, H.; Andrezálová, L.; Švéda, M.; Ruml, T.; Laubertová, L.; Režnáková, S.; Žitňanová, I. Autophagy in MCF-7 cancer cells induced by copper complexes. Pharmacol. Rep. 2016, 68, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Kordestani, N.; Amiri Rudbari, H.; Fernandes, A.R.; Raposo, L.R.; Luz, A.; Baptista, P.V.; Bruno, G.; Scopelliti, R.; Fateminia, Z.; Micale, N.; et al. Copper(ii) complexes with tridentate halogen-substituted Schiff base ligands: Synthesis, crystal structures and investigating the effect of halogenation, leaving groups and ligand flexibility on antiproliferative activities. Dalton Trans. 2021, 50, 3990–4007. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zhang, S.N.; Wang, Z.F.; Wei, Q.M.; Zhang, S.H. Discovery of thirteen cobalt(II) and copper(II) salicylaldehyde Schiff base complexes that induce apoptosis and autophagy in human lung adenocarcinoma A549/DDP cells and that can overcome cisplatin resistance in vitro and in vivo. Dalton Trans. 2022, 51, 4068–4078. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, X.; Zhang, L.; Zhang, J.; Li, C.; Zhang, N.; Xu, H.; Li, Y. A new Schiff base coordinated copper(II) compound induces apoptosis and inhibits tumor growth in gastric cancer. Cancer Cell Int. 2019, 19, 81. [Google Scholar] [CrossRef]
- Illán-Cabeza, N.A.; Jiménez-Pulido, S.B.; Hueso-Ureña, F.; Ramírez-Expósito, M.J.; Martínez-Martos, J.M.; Moreno-Carretero, M.N. Relationship between the antiproliferative properties of Cu(II) complexes with the Schiff base derived from pyridine-2-carboxaldehyde and 5,6-diamino-1,3-dimethyluracil and the redox status mediated by antioxidant defense systems on glioma tumoral cells. J. Inorg. Biochem. 2020, 207, 111053. [Google Scholar] [CrossRef] [PubMed]
- Manzur, J.; Mora, H.; Vega, A.; Venegas-Yazigi, D.; Novak, M.A.; Sabino, J.R.; Paredes-García, V.; Spodine, E. Mononuclear and polynuclear copper(II) complexes derived from pyridylalkylaminomethylphenol polypodal ligands. Inorg. Chem. 2009, 48, 8845–8855. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Li, S.; Xu, G.; Zhang, Z.; Yang, F. Novel mono-, bi-, tri- and tetra-nuclear copper complexes that inhibit tumor growth through apoptosis and anti-angiogenesis. J. Inorg. Biochem. 2024, 250, 112403. [Google Scholar] [CrossRef] [PubMed]
- Trávníček, Z.; Vančo, J.; Belza, J.; Zoppellaro, G.; Dvořák, Z. Dinuclear copper(II) complexes with a bridging bis(chalcone) ligand reveal considerable in vitro cytotoxicity on human cancer cells and enhanced selectivity. J. Inorg. Biochem. 2024, 252, 112481. [Google Scholar] [CrossRef]
- Ribeiro, N.; Bulut, I.; Cevatemre, B.; Teixeira, C.; Yildizhan, Y.; André, V.; Adão, P.; Pessoa, J.C.; Acilan, C.; Correia, I. Cu(ii) and V(iv)O complexes with tri- or tetradentate ligands based on (2-hydroxybenzyl)-l-alanines reveal promising anticancer therapeutic potential. Dalton Trans. 2021, 50, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.L.; Hui, C.N.; Wei, Z.; Kong, L.Y.; Long, J.Y.; Qiao, C.; Chen, Z.F. Copper(II) and iron(III) complexes of chiral dehydroabietic acid derived from natural rosin: Metal effect on structure and cytotoxicity. Met. Integr. Biometal Sci. 2021, 13, mfab014. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.L.; Tu, S.; Wei, Z.; Wang, P.; Long, J.Y.; Qiao, C.; Chen, Z.F. Biological evaluation of optically pure chiral binuclear copper(ii) complexes based on a rosin derivative as highly potential anticancer agents. Dalton Trans. 2019, 48, 15646–15656. [Google Scholar] [CrossRef]
- Fei, B.L.; Tu, S.; Wei, Z.; Wang, P.; Qiao, C.; Chen, Z.F. Optically pure chiral copper(II) complexes of rosin derivative as attractive anticancer agents with potential anti-metastatic and anti-angiogenic activities. Eur. J. Med. Chem. 2019, 176, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Man, X.; Tongfu, Y.; Li, W.; Li, S.; Xu, G.; Zhang, Z.; Liang, H.; Yang, F. Developing a Hetero-Trinuclear Erbium(III)-Copper(II) Complex Based on Apoferritin: Targeted Photoacoustic Imaging and Multimodality Therapy of Tumor. J. Med. Chem. 2023, 66, 15424–15436. [Google Scholar] [CrossRef] [PubMed]
- Icsel, C.; Yilmaz, V.T.; Aygun, M.; Erkisa, M.; Ulukaya, E. Water-soluble copper(II) 5-fluorouracil complexes bearing polypyridyl co-ligands: Synthesis, structures and anticancer activity. Dalton Trans. 2023, 52, 7048–7058. [Google Scholar] [CrossRef] [PubMed]
- Amiri Rudbari, H.; Saadati, A.; Aryaeifar, M.; Blacque, O.; Cuevas-Vicario, J.V.; Cabral, R.; Raposo, L.R.; Fernandes, A.R. Platinum(II) and Copper(II) complexes of asymmetric halogen-substituted [NN’O] ligands: Synthesis, characterization, structural investigations and antiproliferative activity. Bioorg. Chem. 2022, 119, 105556. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Salem, N.M.H.; Fischer, R.C.; Torvisco, A.; Mautner, F.A.; Vančo, J.; Belza, J.; Dvořák, Z.; Trávníček, Z. Dinuclear doubly bridged phenoxido copper(II) complexes as efficient anticancer agents. Eur. J. Med. Chem. 2023, 246, 114992. [Google Scholar] [CrossRef] [PubMed]
- Trávníček, Z.; Malina, T.; Vančo, J.; Šebela, M.; Dvořák, Z. Heteroleptic Copper(II) Complexes Containing 2’-Hydroxy-4-(Dimethylamino)Chalcone Show Strong Antiproliferative Activity. Pharmaceutics 2023, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Polloni, L.; Seni Silva, A.C.; Teixeira, S.C.; Azevedo, F.; Zóia, M.A.P.; da Silva, M.S.; Lima, P.; Correia, L.I.V.; do Couto Almeida, J.; da Silva, C.V.; et al. Action of copper(II) complex with β-diketone and 1,10-phenanthroline (CBP-01) on sarcoma cells and biological effects under cell death. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 112, 108586. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Chen, Z.; Chu, B.; Liu, D.; Liang, Y.; Liang, F. Structure and anticancer activities of four Cu(ii) complexes bearing tropolone. Met. Integr. Biometal Sci. 2019, 11, 1952–1964. [Google Scholar] [CrossRef]
- Lee, Z.Y.; Leong, C.H.; Lim, K.U.L.; Wong, C.C.S.; Pongtheerawan, P.; Arikrishnan, S.A.; Tan, K.L.; Loh, J.S.; Low, M.L.; How, C.W.; et al. Induction of Apoptosis and Autophagy by Ternary Copper Complex Towards Breast Cancer Cells. Anti-Cancer Agents Med. Chem. 2022, 22, 1159–1170. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Fu, Y.; Liu, Y.; Zhang, Y.; Zhang, Y.; Zhou, P.; Yuan, Y.; Zhou, S.; Li, S.; et al. Redox cycling of a copper complex with benzaldehyde nitrogen mustard-2-pyridine carboxylic acid hydrazone contributes to its enhanced antitumor activity, but no change in the mechanism of action occurs after chelation. Oncol. Rep. 2016, 35, 1636–1644. [Google Scholar] [CrossRef]
- Zhong, W.; Zhu, H.; Sheng, F.; Tian, Y.; Zhou, J.; Chen, Y.; Li, S.; Lin, J. Activation of the MAPK11/12/13/14 (p38 MAPK) pathway regulates the transcription of autophagy genes in response to oxidative stress induced by a novel copper complex in HeLa cells. Autophagy 2014, 10, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Machura, B.; Erfurt, K.; Casimiro, A.R.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Copper(II) Complexes with 2,2’:6’,2″-Terpyridine Derivatives Displaying Dimeric Dichloro-μ-Bridged Crystal Structure: Biological Activities from 2D and 3D Tumor Spheroids to In Vivo Models. J. Med. Chem. 2024, 67, 5813–5836. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.H.A.; Paixão, D.A.; Lino, R.C.; de Souza, T.R.; de Souza Bontempo, N.J.; Sousa, L.M.; Van Petten de Vasconcelos Azevedo, F.; Orsolin, P.C.; Lima, P.; Martins, I.C.; et al. A selective Cu(II) complex with 4-fluorophenoxyacetic acid hydrazide and phenanthroline displays DNA-cleaving and pro-apoptotic properties in cancer cells. Sci. Rep. 2021, 11, 24450. [Google Scholar] [CrossRef]
- Trejo-Solís, C.; Jimenez-Farfan, D.; Rodriguez-Enriquez, S.; Fernandez-Valverde, F.; Cruz-Salgado, A.; Ruiz-Azuara, L.; Sotelo, J. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer 2012, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Bisceglie, F.; Alinovi, R.; Pinelli, S.; Galetti, M.; Pioli, M.; Tarasconi, P.; Mutti, A.; Goldoni, M.; Pelosi, G. Autophagy and apoptosis: Studies on the effects of bisthiosemicarbazone copper(ii) complexes on p53 and p53-null tumour cell lines. Metallomics 2016, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Zhou, Y.; Karges, J.; Du, K.; Shen, J.; Lin, M.; Wei, F.; Kou, J.; Chen, Y.; Ji, L.; et al. Autophagy-Dependent Apoptosis Induced by Apoferritin-Cu(II) Nanoparticles in Multidrug-Resistant Colon Cancer Cells. ACS Appl. Mater. Interfaces 2021, 13, 38959–38968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, D.; Yang, L.; Ma, P.; Si, Y.; Kortz, U.; Niu, J.; Wang, J. Nona-copper(II)-containing 18-tungsto-8-arsenate(III) exhibits antitumor activity. Chem. Commun. 2013, 49, 5189–5191. [Google Scholar] [CrossRef]
- Boya, P.; Reggiori, F.; Codogno, P. Emerging regulation and functions of autophagy. Nat. Cell Biol. 2013, 15, 713–720. [Google Scholar] [CrossRef]
- Schütz, I.; Lopez-Hernandez, T.; Gao, Q.; Puchkov, D.; Jabs, S.; Nordmeyer, D.; Schmudde, M.; Rühl, E.; Graf, C.M.; Haucke, V. Lysosomal Dysfunction Caused by Cellular Accumulation of Silica Nanoparticles. J. Biol. Chem. 2016, 291, 14170–14184. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, Y.; Jin, S.; Tian, Y.; Zhang, X.; Zhao, Y.; Yu, L.; Liang, X.J. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 2011, 5, 8629–8639. [Google Scholar] [CrossRef]
- Hu, X.; Wang, M.; Shi, S.; Keerthi Raja, M.; Gupta, G.; Chen, H.; Xu, P. Polymer/copper nanocomplex-induced lysosomal cell death promotes tumor lymphocyte infiltration and synergizes anti-PD-L1 immunotherapy for triple-negative breast cancer. Biomater. Sci. 2023, 11, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Tian, H.; Jiang, J.; Zhou, L.; Li, L.; Luo, M.; Ding, N.; Nice, E.C.; Huang, C.; Zhang, H. Brain-Targeted HFn-Cu-REGO Nanoplatform for Site-Specific Delivery and Manipulation of Autophagy and Cuproptosis in Glioblastoma. Small 2023, 19, e2205354. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liu, A.; Ren, Q.; Xue, Y.; Yu, X.; Ying, Y.; Gao, H.; Tan, H.; Zhang, Z.; Li, W.; et al. Cuprous oxide nanoparticles trigger reactive oxygen species-induced apoptosis through activation of erk-dependent autophagy in bladder cancer. Cell Death Dis. 2020, 11, 366. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, R.; Liu, X.; Li, X.; Qiao, G.; Li, C.; Gedanken, A.; Lin, X. Zn-doped CuO nanocomposites inhibit tumor growth by NF-κB pathway cross-linked autophagy and apoptosis. Nanomedicine 2019, 14, 131–149. [Google Scholar] [CrossRef]
- Xiong, K.; Lin, X.; Kou, J.; Wei, F.; Shen, J.; Chen, Y.; Ji, L.; Chao, H. Apoferritin-Cu(II) Nanoparticles Induce Oncosis in Multidrug-Resistant Colon Cancer Cells. Adv. Healthc. Mater. 2024, 13, e2302564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Y. Near-infrared optically active Cu2−xS nanocrystals: Sacrificial template-ligand exchange integration fabrication and chirality dependent autophagy effects. J. Mater. Chem. 2020, 8, 7921–7930. [Google Scholar] [CrossRef]
- Song, H.; Xu, Q.; Zhu, Y.; Zhu, S.; Tang, H.; Wang, Y.; Ren, H.; Zhao, P.; Qi, Z.; Zhao, S. Serum adsorption, cellular internalization and consequent impact of cuprous oxide nanoparticles on uveal melanoma cells: Implications for cancer therapy. Nanomedicine 2015, 10, 3547–3562. [Google Scholar] [CrossRef]
- Kang, M.; Luo, J.; Zhao, L.; Shi, F.; Ye, G.; He, X.; Hao, S.; Yang, D.; Chen, H.; Guo, H.; et al. Autophagy was activated against the damages of placentas caused by nano-copper oral exposure. Ecotoxicol. Environ. Saf. 2021, 220, 112364. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Li, C.; Qiao, G.; Farooqi, A.A.; Gedanken, A.; Liu, X.; Lin, X. Zinc-Doped Copper Oxide Nanocomposites Inhibit the Growth of Pancreatic Cancer by Inducing Autophagy Through AMPK/mTOR Pathway. Front. Pharmacol. 2019, 10, 319. [Google Scholar] [CrossRef]
- Xu, P.; Cao, M.; Dong, X.; Yu, Z.; Liu, J.; Tan, J.; Wang, Y.; Li, T.; Zhao, S. Nanosized copper particles induced mesangial cell toxicity via the autophagy pathway. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas Biol. 2022, 55, e12252. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Luo, J.; Kang, M.; Hou, J.; Tang, R.; Zhao, L.; Shi, F.; Ye, G.; He, X.; et al. Autophagy and apoptosis mediated nano-copper-induced testicular damage. Ecotoxicol. Environ. Saf. 2022, 229, 113039. [Google Scholar] [CrossRef]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef]
- Kwan, T.Y.; Chowdhury, E.H. Clinical Outcomes of Chemotherapeutic Molecules as Single and Multiple Agents in Advanced Non-Small-Cell Lung Carcinoma (NSCLC) Patients. Medicina 2021, 57, 1252. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.M.; Liu, Y.; Park, G.Y.; Murai, J.; Koch, C.E.; Eisen, T.J.; Pritchard, J.R.; Pommier, Y.; Lippard, S.J.; Hemann, M.T. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat. Med. 2017, 23, 461–471. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Peng, X. NDC80 Enhances Cisplatin-resistance in Triple-negative Breast Cancer. Arch. Med. Res. 2022, 53, 378–387. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Wang, P.; Chen, H.; Xu, Y.; Ge, J.; Tian, Z.; Yan, Z. Potential of Copper and Copper Compounds for Anticancer Applications. Pharmaceuticals 2023, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Y.; Fu, Y.; Huang, T.; Yang, Y.; Li, S.; Li, C. Antiproliferative activity of di-2-pyridylhydrazone dithiocarbamate acetate partly involved in p53 mediated apoptosis and autophagy. Int. J. Oncol. 2017, 51, 1909–1919. [Google Scholar] [CrossRef]
- Liu, R.X.; Luo, R.Y.; Tang, M.T.; Liu, Y.C.; Chen, Z.F.; Liang, H. The first copper(I) complex of anthrahydrazone with potential ROS scavenging activity showed significant in vitro anticancer activity by inducing apoptosis and autophagy. J. Inorg. Biochem. 2021, 218, 111390. [Google Scholar] [CrossRef]
- Kanellis, D.C.; Zisi, A.; Skrott, Z.; Lemmens, B.; Espinoza, J.A.; Kosar, M.; Björkman, A.; Li, X.; Arampatzis, S.; Bartkova, J.; et al. Actionable cancer vulnerability due to translational arrest, p53 aggregation and ribosome biogenesis stress evoked by the disulfiram metabolite CuET. Cell Death Differ. 2023, 30, 1666–1678. [Google Scholar] [CrossRef]
- Canonico, B.; Carloni, R.; Sanz Del Olmo, N.; Papa, S.; Nasoni, M.G.; Fattori, A.; Cangiotti, M.; de la Mata, F.J.; Ottaviani, M.F.; García-Gallego, S. Fine-Tuning the Interaction and Therapeutic Effect of Cu(II) Carbosilane Metallodendrimers in Cancer Cells: An In Vitro Electron Paramagnetic Resonance Study. Mol. Pharm. 2020, 17, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Pramanik, A.; Maity, J.; Mukherjee, A.; Pramanik, P.; Laskar, A.; Karmakar, P. Interplay between autophagy and apoptosis mediated by copper oxide nanoparticles in human breast cancer cells MCF7. Biochim. Biophys. Acta 2014, 1840, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.J.; Zhang, Y.; Chen, B.Q.; Zhao, Y.; Wang, J.Y.; Li, C.Y.; Zhang, D.G.; Kankala, R.K.; Wang, S.B.; Liu, G.; et al. NIR-II light triggered burst-release cascade nanoreactor for precise cancer chemotherapy. Bioact. Mater. 2024, 33, 311–323. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, H.; Yan, B.; Zhang, S.; Xu, B.; Lin, M.; Shuai, X.; Huang, J.; Pang, J. Tumor acidity-activatable macromolecule autophagy inhibitor and immune checkpoint blockade for robust treatment of prostate cancer. Acta Biomater. 2023, 168, 593–605. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, W.; Dong, B.; Xin, Z.; Ji, Y.; Su, R.; Shen, K.; Pan, J.; Wang, Q.; Xue, W. Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. Theranostics 2022, 12, 4965–4979. [Google Scholar] [CrossRef]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef]
- Wang, T.; Fu, Y.; Huang, T.; Liu, Y.; Wu, M.; Yuan, Y.; Li, S.; Li, C. Copper Ion Attenuated the Antiproliferative Activity of Di-2-pyridylhydrazone Dithiocarbamate Derivative; However, There Was a Lack of Correlation between ROS Generation and Antiproliferative Activity. Molecules 2016, 21, 1088. [Google Scholar] [CrossRef] [PubMed]
- Nurmamat, M.; Yan, H.; Wang, R.; Zhao, H.; Li, Y.; Wang, X.; Nurmaimaiti, K.; Kurmanjiang, T.; Luo, D.; Baodi, J.; et al. Novel Copper(II) Complex with a 4-Acylpyrazolone Derivative and Coligand Induce Apoptosis in Liver Cancer Cells. ACS Med. Chem. Lett. 2021, 12, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.J.; Otake, A.H.; Bustos, S.O.; Fazzi, R.B.; Chammas, R.; Da Costa Ferreira, A.M. Unlike reactivity of mono- and binuclear imine-copper(II) complexes toward melanoma cells via a tyrosinase-dependent mechanism. Chem. Biol. Interact. 2019, 311, 108789. [Google Scholar] [CrossRef]
- Wang, M.M.; Li, H.M.; Deng, D.P.; Su, Y.; Su, Z. Performance of Ir(III)-Based Anticancer Agents in the Treatment of Cisplatin-Resistant Cancer Cells. ChemMedChem 2022, 17, e202200273. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Zhang, L.; Fang, D.; Zhu, Z.; He, W.; Hu, L.; Di, L.; Guo, Z.; Wang, X. Surmounting tumor resistance to metallodrugs by co-loading a metal complex and siRNA in nanoparticles. Chem. Sci. 2021, 12, 4547–4556. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Yu, L.; Hou, Z.; Liu, H.; Kong, L.; Xu, Y.; He, J.; Lan, J.; Ou, Q.; et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy 2024, 20, 114–130. [Google Scholar] [CrossRef]
- Jia, C.; Guo, Y.; Wu, F.G. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2022, 18, e2103868. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Fan, Z.; Chen, L.; Liu, J.; Wan, Z.; Xiao, Z.; Chen, W.; Wu, L.; Chen, D.; Zhu, X. Copper-based theranostic nanocatalysts for synergetic photothermal-chemodynamic therapy. Acta Biomater. 2022, 147, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Munk, D.E.; Vendelbo, M.H.; Kirk, F.T.; Rewitz, K.S.; Bender, D.A.; Vase, K.H.; Munk, O.L.; Vilstrup, H.; Ott, P.; Sandahl, T.D. Distribution of non-ceruloplasmin-bound copper after i.v. (64)Cu injection studied with PET/CT in patients with Wilson disease. JHEP Rep. Innov. Hepatol. 2023, 5, 100916. [Google Scholar] [CrossRef]
- Ahuja, A.; Dev, K.; Tanwar, R.S.; Selwal, K.K.; Tyagi, P.K. Copper mediated neurological disorder: Visions into amyotrophic lateral sclerosis, Alzheimer and Menkes disease. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2015, 29, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet. Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Muchenditsi, A.; Talbot, C.C.; Gottlieb, A.; Yang, H.; Kang, B.; Boronina, T.; Cole, R.; Wang, L.; Dev, S.; Hamilton, J.P.; et al. Systemic deletion of Atp7b modifies the hepatocytes’ response to copper overload in the mouse models of Wilson disease. Sci. Rep. 2021, 11, 5659. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, E.V.; Garbuz, M.M.; Ovchinnikova, A.A.; Kumeiko, V.V. Epidemiology of Wilson’s Disease and Pathogenic Variants of the ATP7B Gene Leading to Diversified Protein Disfunctions. Int. J. Mol. Sci. 2024, 25, 2402. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, H.; Zhang, H.; Wang, T.; Zheng, Q.; Li, Z. Controlled Activation of TRPV1 Channels on Microglia to Boost Their Autophagy for Clearance of Alpha-Synuclein and Enhance Therapy of Parkinson’s Disease. Adv. Mater. 2022, 34, e2108435. [Google Scholar] [CrossRef]
- Quintanova, C.; Keri, R.S.; Chaves, S.; Santos, M.A. Copper(II) complexation of tacrine hybrids with potential anti-neurodegenerative roles. J. Inorg. Biochem. 2015, 151, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Mercer, J.F.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Binesh, A.; Venkatachalam, K. Copper in Human Health and Disease: A Comprehensive Review. J. Biochem. Mol. Toxicol. 2024, 38, e70052. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, X.; Xu, C.; Gao, M.; Wang, S.; Zhang, J.; Tong, H.; Wang, L.; Han, Y.; Cheng, N.; et al. Inhibiting NLRP3 inflammasome activation prevents copper-induced neuropathology in a murine model of Wilson’s disease. Cell Death Dis. 2021, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Langner, C.; Fuchsbichler, A.; Heinz-Erian, P.; Ellemunter, H.; Schlenck, B.; Bavdekar, A.R.; Pradhan, A.M.; Pandit, A.; Müller-Höcker, J.; et al. Immunohistochemical analysis of Mallory bodies in Wilsonian and non-Wilsonian hepatic copper toxicosis. Hepatology 2004, 39, 963–969. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Wang, S.; Yang, D.; Zhang, Y.; Xu, L.; Ma, L.; Zheng, J.; Petersen, R.B.; Zheng, L.; et al. Copper and iron ions accelerate the prion-like propagation of α-synuclein: A vicious cycle in Parkinson’s disease. Int. J. Biol. Macromol. 2020, 163, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, X.; Zheng, C.; Zhang, C.; Li, P.; He, K.; Liu, G.; Huang, X.; Liu, J.; Xie, Y.; et al. Low-dose Cu exposure enhanced α-synuclein accumulation associates with mitochondrial impairments in mice model of Parkinson’s disease. Toxicol. Lett. 2023, 387, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Paris, I.; Perez-Pastene, C.; Couve, E.; Caviedes, P.; LeDoux, S.; Segura-Aguilar, J. Copper dopamine complex induces mitochondrial autophagy preceding caspase-independent apoptotic cell death. J. Biol. Chem. 2009, 284, 13306–13315. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Y.; Kang, Y.J. Copper reverses cardiomyocyte hypertrophy through vascular endothelial growth factor-mediated reduction in the cell size. J. Mol. Cell. Cardiol. 2008, 45, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Matute, J.; de Bruijn, J.; van Kuijk, K.; Riascos-Bernal, D.F.; Diaz, A.; Tasset, I.; Martín-Segura, A.; Gijbels, M.J.J.; Sander, B.; Kaushik, S.; et al. Protective role of chaperone-mediated autophagy against atherosclerosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2121133119. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R. Copper’s dual role: Unravelling the link between copper homeostasis, cuproptosis, and cardiovascular diseases. Hypertens. Res. 2024, 47, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Mavil-Guerrero, E.; Vazquez-Duhalt, R.; Juarez-Moreno, K. Exploring the cytotoxicity mechanisms of copper ions and copper oxide nanoparticles in cells from the excretory system. Chemosphere 2024, 347, 140713. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ouyang, Y.; Wang, J.; Cui, H.; Deng, H.; Zhong, X.; Jian, Z.; Liu, H.; Fang, J.; Zuo, Z.; et al. Cu-induced spermatogenesis disease is related to oxidative stress-mediated germ cell apoptosis and DNA damage. J. Hazard. Mater. 2021, 416, 125903. [Google Scholar] [CrossRef]
- Zhong, C.C.; Zhao, T.; Hogstrand, C.; Chen, F.; Song, C.C.; Luo, Z. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J. Nutr. Biochem. 2022, 100, 108883. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Ghosh, S.; Chatterji, A.; Chakraborty, K. Neuron-glia: Understanding cellular copper homeostasis, its cross-talk and their contribution towards neurodegenerative diseases. Met. Integr. Biometal Sci. 2020, 12, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.M.; Wagener, A.; Fietzek, U.M.; Klopstock, T.; Mosharov, E.V.; Zucca, F.A.; Sulzer, D.; Zecca, L.; Burbulla, L.F. Interactions of dopamine, iron, and alpha-synuclein linked to dopaminergic neuron vulnerability in Parkinson’s disease and Neurodegeneration with Brain Iron Accumulation disorders. Neurobiol. Dis. 2022, 175, 105920. [Google Scholar] [CrossRef]
- Bademosi, A.T.; Decet, M.; Kuenen, S.; Calatayud, C.; Swerts, J.; Gallego, S.F.; Schoovaerts, N.; Karamanou, S.; Louros, N.; Martin, E.; et al. EndophilinA-dependent coupling between activity-induced calcium influx and synaptic autophagy is disrupted by a Parkinson-risk mutation. Neuron 2023, 111, 1402–1422.e13. [Google Scholar] [CrossRef] [PubMed]
- Sachan, N.; Tiwari, N.; Patel, D.K.; Katiyar, D.; Srikrishna, S.; Singh, M.P. Dyshomeostasis of Iron and Its Transporter Proteins in Cypermethrin-Induced Parkinson’s Disease. Mol. Neurobiol. 2023, 60, 5838–5852. [Google Scholar] [CrossRef]
- Pradhan, S.H.; Liu, J.Y.; Sayes, C.M. Evaluating Manganese, Zinc, and Copper Metal Toxicity on SH-SY5Y Cells in Establishing an Idiopathic Parkinson’s Disease Model. Int. J. Mol. Sci. 2023, 24, 16129. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.; Nevitt, S.J.; Tuohy, O.; Cook, P. Biomarkers for diagnosis of Wilson’s disease. Cochrane Database Syst. Rev. 2019, 2019, CD012267. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Tumor Types | Molecular Target/Mechanisms | Effect on Autophagy | Pathophysiological Effect | Refs. |
|---|---|---|---|---|---|
| Elesclomol-CuCl2 | Prostate cancer | DLAT/mTOR pathway | Inhibiting | Enhancing the chemosensitivity of docetaxel | [17] |
| Cu2+-LFH | Human gastric cancer AGS cells | Enhanced apoptosis induction and autophagy inhibition | Inhibiting | Enhanced anticancer effects in AGS cells via PI3K-Akt-mTOR pathway activation | [39] |
| DSF/Cu | Human pancreatic cancer cells | Transcription factor p8 and PI3K/mTOR/p70S6K signaling pathways | Inducing | Inhibiting cell proliferation and induced apoptosis | [15] |
| Colorectal cancer | ULK1 | Inducing | Inducing autophagic cell death | [46] | |
| Human HCC cell lines HepG2, Hep3B, SNU387, and SNU423 | Inducing autophagy and apoptosis | Inducing | Improving the therapeutic efficacy of sorafenib | [47] | |
| Copper (I) nicotinate complex (CNC) | Human breast cancer cell line HCC1806 | Inhibiting autophagy and inducing cell cycle arrest | Inhibiting | Enhancing the efficacy of DOX and reducing the use of DOX in HCC1806 cells | [18] |
| HSA-IND-C4 complex | BxPC-3 and HK-2 cells | Inducing apoptosis and autophagy | Inducing | Inhibiting tumor growth by remodeling the tumor microenvironment | [43] |
| Copper (II) complex C2 | T24 cancer cells | Oxidizing GSH and affecting autophagy | Inhibiting | Enhancing chemodynamic therapy (CDT) | [50] |
| Cu(L4)2 and Cu(L10)2 | T24 cancer cells | Mitochondrial dysfunctions, ER stress, and autophagy flux inhibition | Inhibiting | Enhancing the effects of CDT and exhibiting strong tumor suppression in the T24 xenograft model | [53] |
| [Cu(4′-(2-quin)-terpy) Cl] (PF6) | Cancer cell lines A2780 and HCT116 | Intercalating DNA and inducing intracellular ROS | Inducing | Exhibiting cytotoxicity against cancer cells | [54] |
| CuL1Cl2/CuL2Cl2 | A549 cells | Mitochondria-mediated apoptosis and autophagy | Inducing | Inducing bimodal death through apoptosis and autophagy | [56] |
| Schiff base Cu (II) complexes | Human breast cancer cells (MCF-7) | Autophagy | Inducing | Exhibiting antiproliferative activity against cancer cells but not against healthy cells | [60] |
| Cu (Cl2-L1) Cl | Ovarian cancer A2780 cell line | - | Inducing | Exhibiting antiproliferative activities | [61] |
| SBCCC | Gastric cancer cell lines SGC-7901 and BGC-823 | NF-κB, ROS production, and autophagy | Inducing | Inducing cancer cell death | [63] |
| Cu2(µ2-O)(L)4(DMF)2 | MCF-7 | Increasing ROS, GSSG/GSH ratio, and Ca2+ production, etc. | Inducing | Killing MCF-7 cells and displaying anti-metastatic activities, together with anti-angiogenesis properties | [69] |
| [Cu2(μ-Cl)2L2]-CH2Cl2 | MCF-7 | Cell cycle arrest, apoptosis, etc. | Inducing | Exhibiting cytotoxic activity | [70] |
| [CuL4Cl]Cl·2CH2Cl2·H2O | MCF-7 | ROS production, cell-cycle arrest, etc. | Inducing | Promoting MCF-7 cell death through activation of autophagy and possessing anti-metastatic and anti-angiogenic effects | [71] |
| CBP-01 | Sarcoma cells | ROS augmentation | Inducing | Showing in vitro antitumor activity and cytotoxic selectivity toward the sarcoma 180 cells | [77] |
| [Cu (phen) (Ltyr) Cl] 3H2O | MCF-7 and MDA-MB-231 | Inducing apoptosis and cell cycle, inducing autophagy | Inducing | Promoting cell survival | [79] |
| BNMPH-Cu complex | HepG2 and HCT-116 | Inducing ROS generation | Inducing | Suppressing the growth of cancer cells | [80] |
| Copper (II) complexes 2, 3, 5, and 7 | HCT116DoxR cells | ROS/affecting cell cycle | Inducing | Inducing cell death through both autophagy and apoptosis | [82] |
| Cas III-ia | Rat malignant glioma C6 cells | ROS and JNK | Inducing | Inducing cell death by autophagy and apoptosis | [84] |
| DpdtaA-Cu complex | HepG2 cells | Through p53 meditated apoptosis/ROS generation | Inducing | Exhibiting antiproliferative activities, but copper ion attenuated the antiproliferative activity of DpdtaA alone | [109] |
| [CuI(9-AQH)2] · NO3 | MGC-803 cells | Mitochondria-mediated cell apoptosis and autophagy | Inducing | Showing significant in vitro anticancer activity | [110] |
| CuET | U2OS osteosarcoma, A549 lung epithelial carcinoma, and MDA-MB-231 breast carcinoma | Translational arrest, p53 aggregation, and ribosome biogenesis stress | Inducing | Protecting tumor cells from death and thus reducing the clinical efficacy of CuET | [111] |
| Apoferritin–Cu (II) NPs | Multidrug-resistant colon tumor | Autophagy-dependent apoptosis | Inducing | Inducing autophagy-dependent apoptosis in multidrug-resistant colon cancer cells | [86] |
| Nona-copper(ii)-containing 18-tungsto-8-arsenate(iii) | K562 leukemia cells and HepG2 cells | Inducing cell apoptosis and autophagy | Inducing | Exhibiting antitumor activity | [87] |
| Poly/Cu nanocomplexes | 4T1 cells | Facilitating copper ion uptake and lysosomal escaping | Inhibiting | Exhibiting synergetic effect with PD-L1 antibody through ICD-boosted T-cell infiltration | [91] |
| CONPs | Bladder cancer cell lines (T24, J82, 5637, and UMUC3) | Triggering ROS-induced apoptosis through activation of ERK-dependent autophagy | Inducing | Suppressing the growth of bladder cancer | [93] |
| Cu2O-NPs | Uveal melanoma cells | Elevating ROS level and over-stimulating apoptosis and autophagy | Inducing | Inhibiting cancer cell growth and impairing the ability of uveal melanoma cell migration, invasion, and the cytoskeleton assembly | [97] |
| Copper (II) carbosilane metallodendrimers | U937 tumor cells | Mitochondria–lysosome axis as well as autophagic vesicle formation | Inducing | Inducing death processes of U937 tumor cells | [112] |
| CuO NPs | MCF7 | Autophagy | Inducing | CuO NP-induced autophagy is a survival strategy of MCF7 cells and inhibition of autophagy renders the cellular fate to apoptosis | [113] |
| DSF/Cu2−xSe@PCM | 4T1 cells | Autophagy | Inducing | Inducing tumor cell death | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Zeng, S.; Wang, Z.; Huang, H.; Zhao, X.; Li, M. Mechanisms of Copper-Induced Autophagy and Links with Human Diseases. Pharmaceuticals 2025, 18, 99. https://doi.org/10.3390/ph18010099
Fu Y, Zeng S, Wang Z, Huang H, Zhao X, Li M. Mechanisms of Copper-Induced Autophagy and Links with Human Diseases. Pharmaceuticals. 2025; 18(1):99. https://doi.org/10.3390/ph18010099
Chicago/Turabian StyleFu, Yuanyuan, Shuyan Zeng, Zhenlin Wang, Huiting Huang, Xin Zhao, and Min Li. 2025. "Mechanisms of Copper-Induced Autophagy and Links with Human Diseases" Pharmaceuticals 18, no. 1: 99. https://doi.org/10.3390/ph18010099
APA StyleFu, Y., Zeng, S., Wang, Z., Huang, H., Zhao, X., & Li, M. (2025). Mechanisms of Copper-Induced Autophagy and Links with Human Diseases. Pharmaceuticals, 18(1), 99. https://doi.org/10.3390/ph18010099










