1-Nitro-2-Phenylethane as a Multitarget Candidate for Cognitive and Psychiatric Disorders: Insights from In Silico and Behavioral Approaches
Abstract
1. Introduction
2. Results
2.1. ADME In Silico Analysis
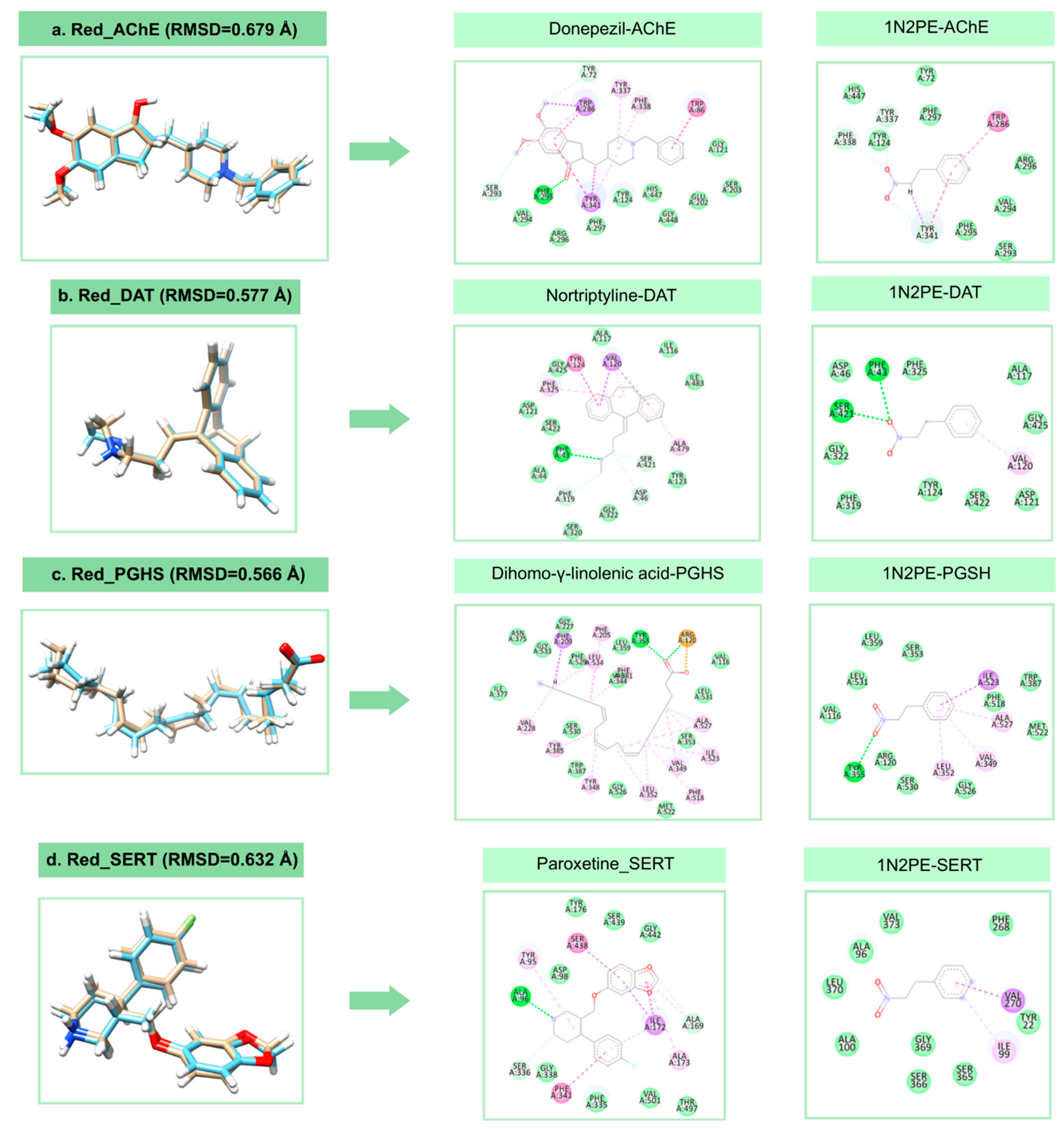
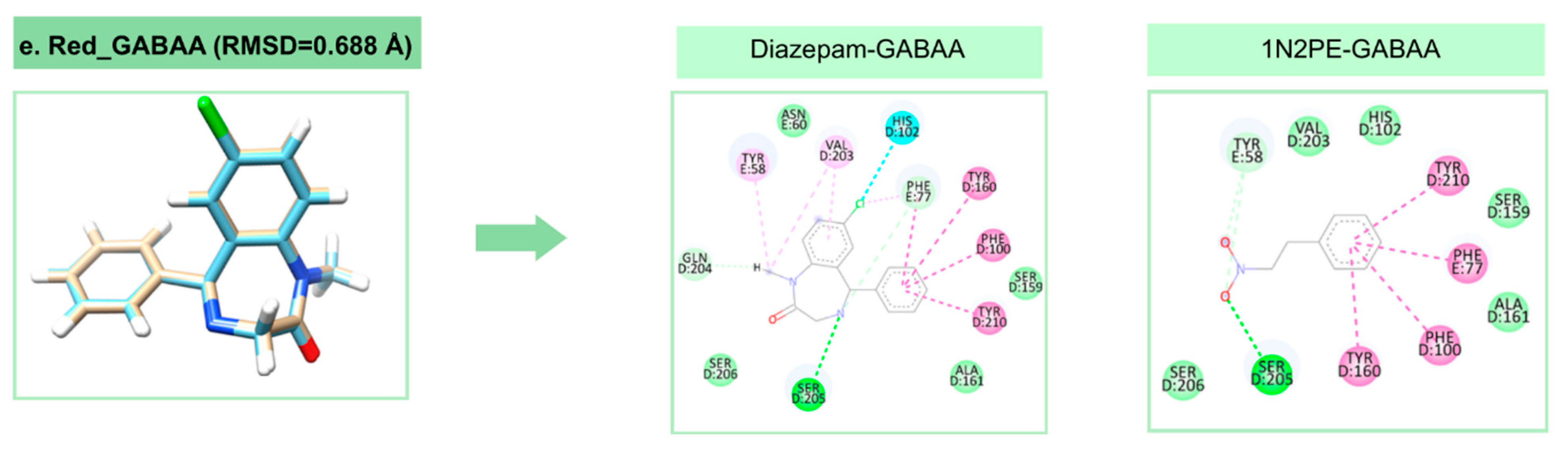
2.2. Molecular Docking Analysis
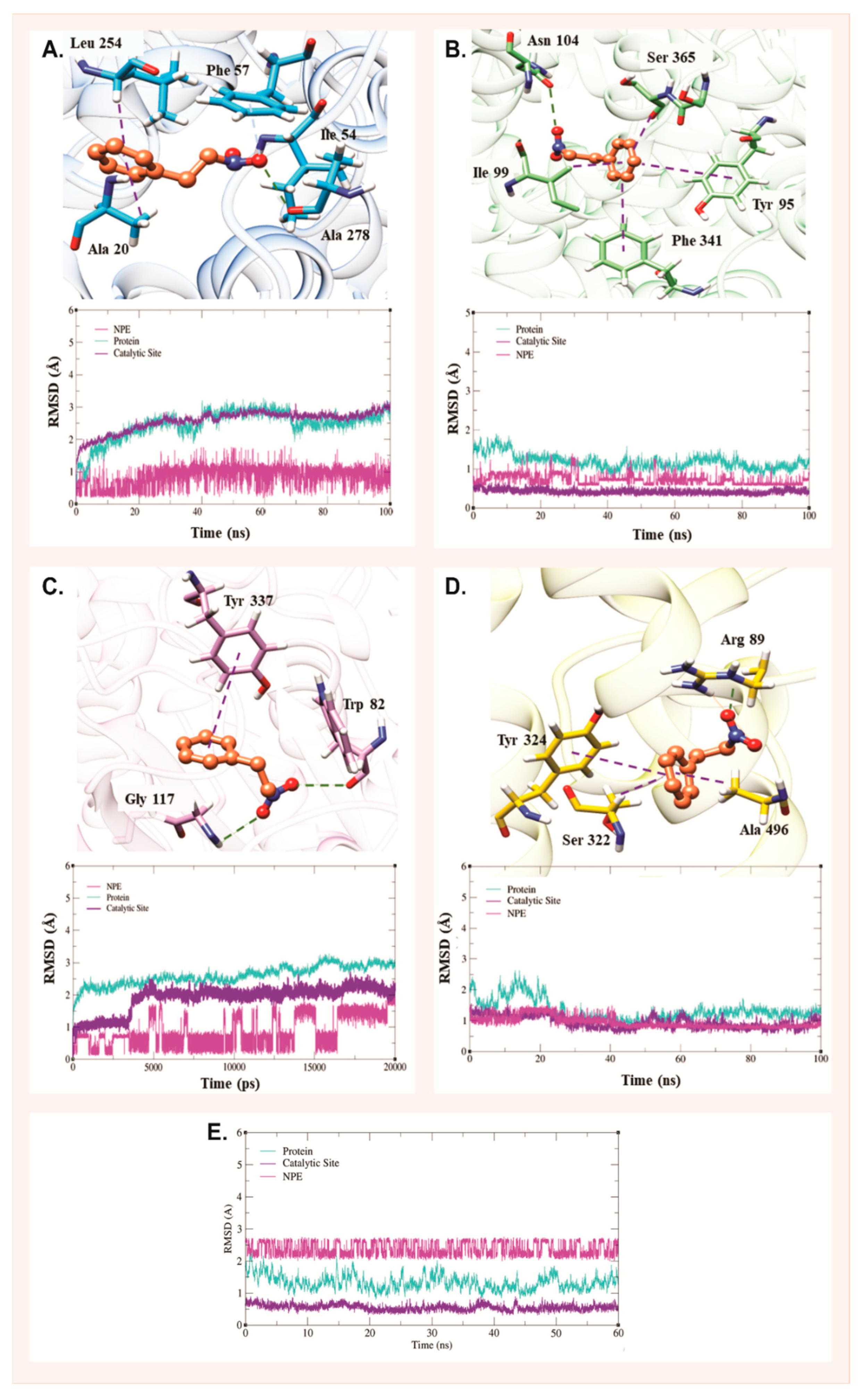
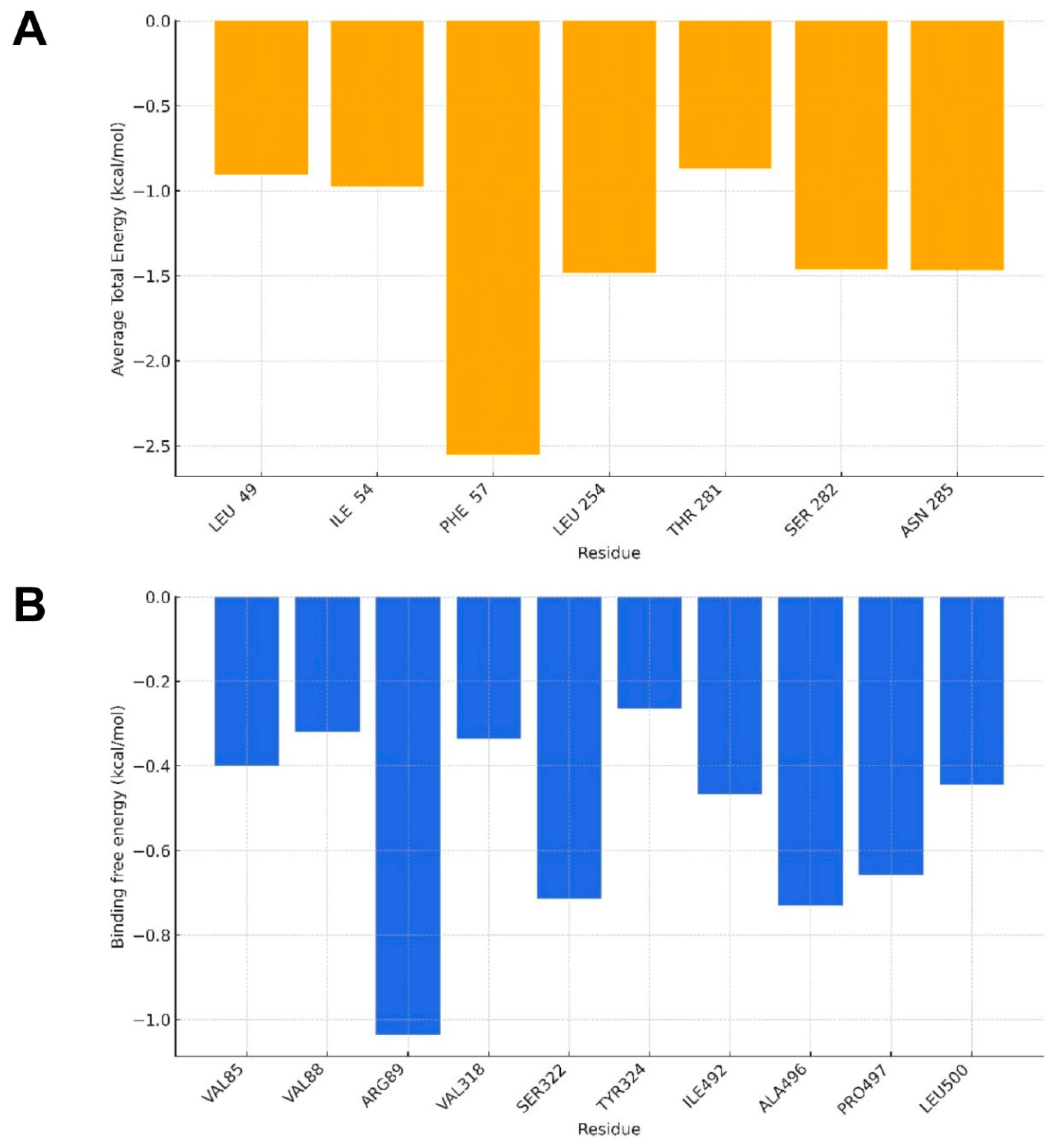
2.3. Molecular Dynamics Analysis
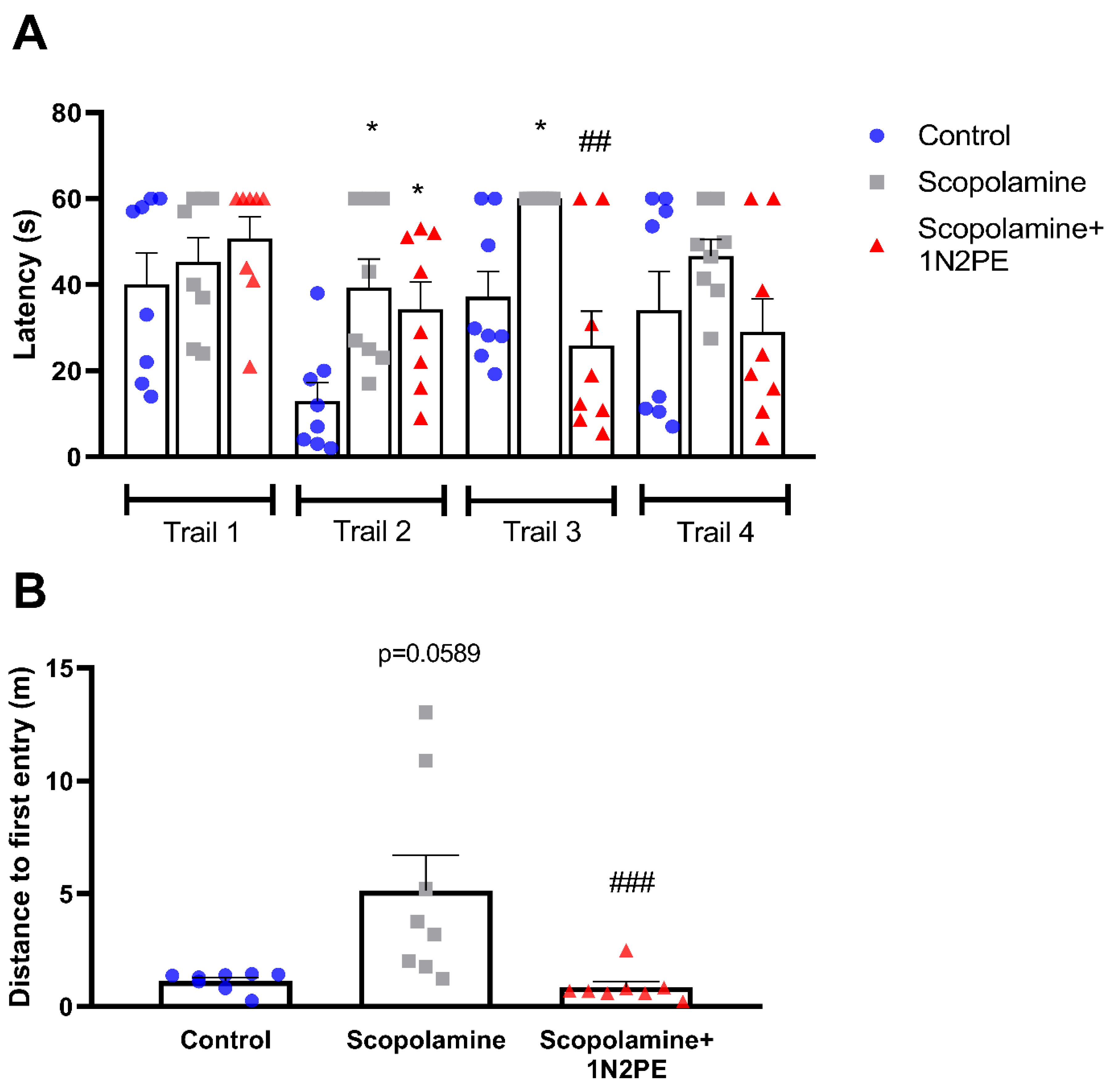
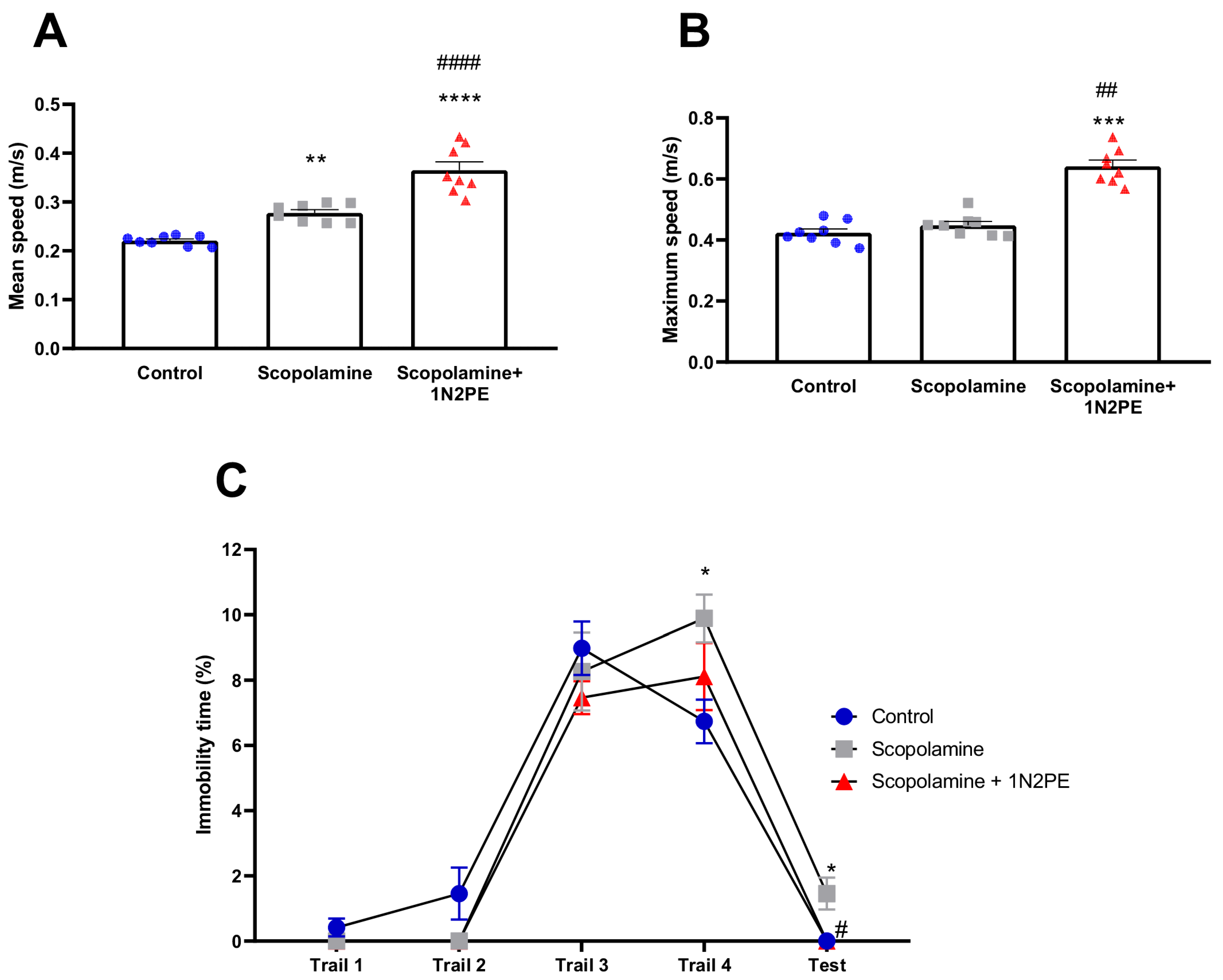
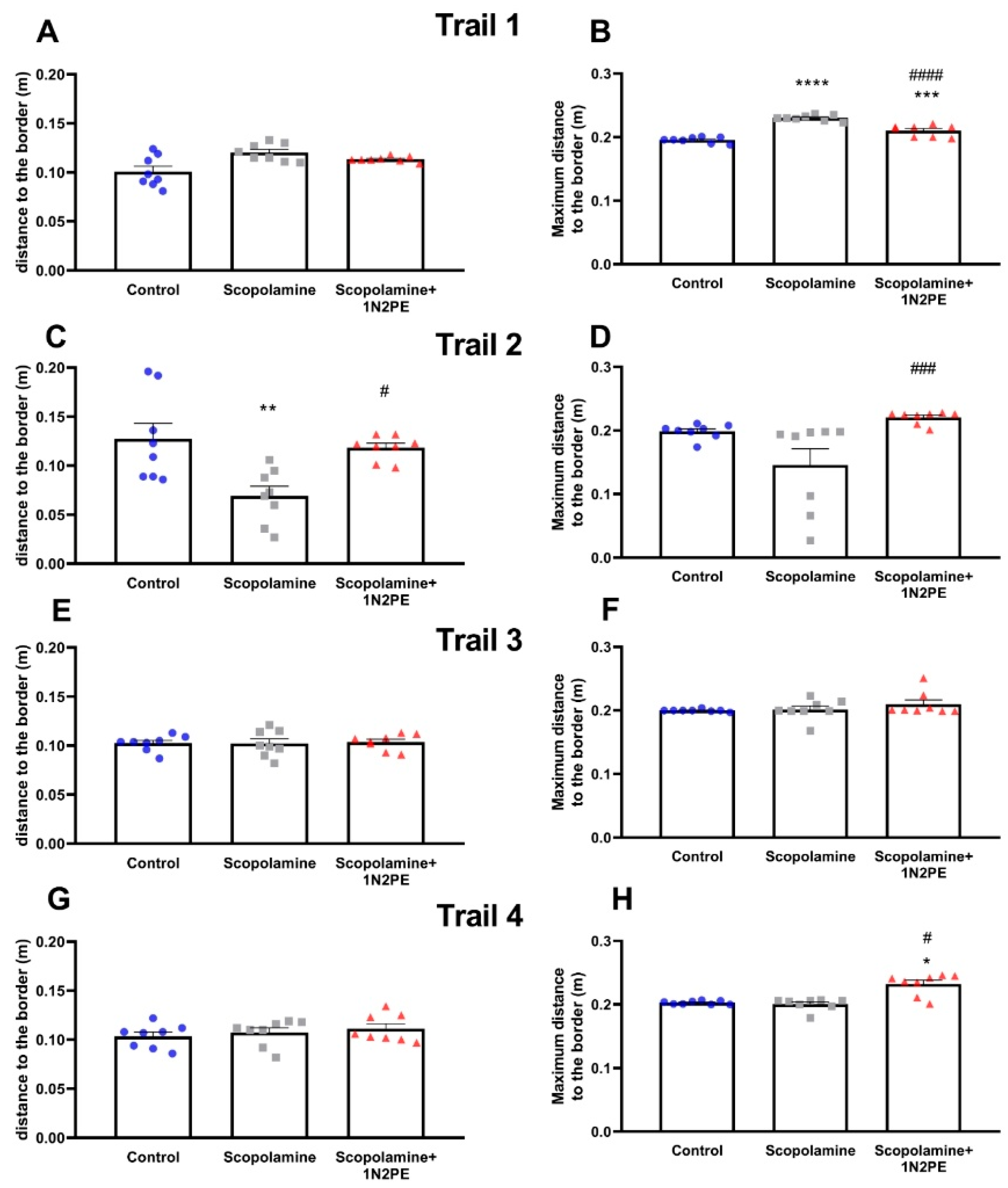
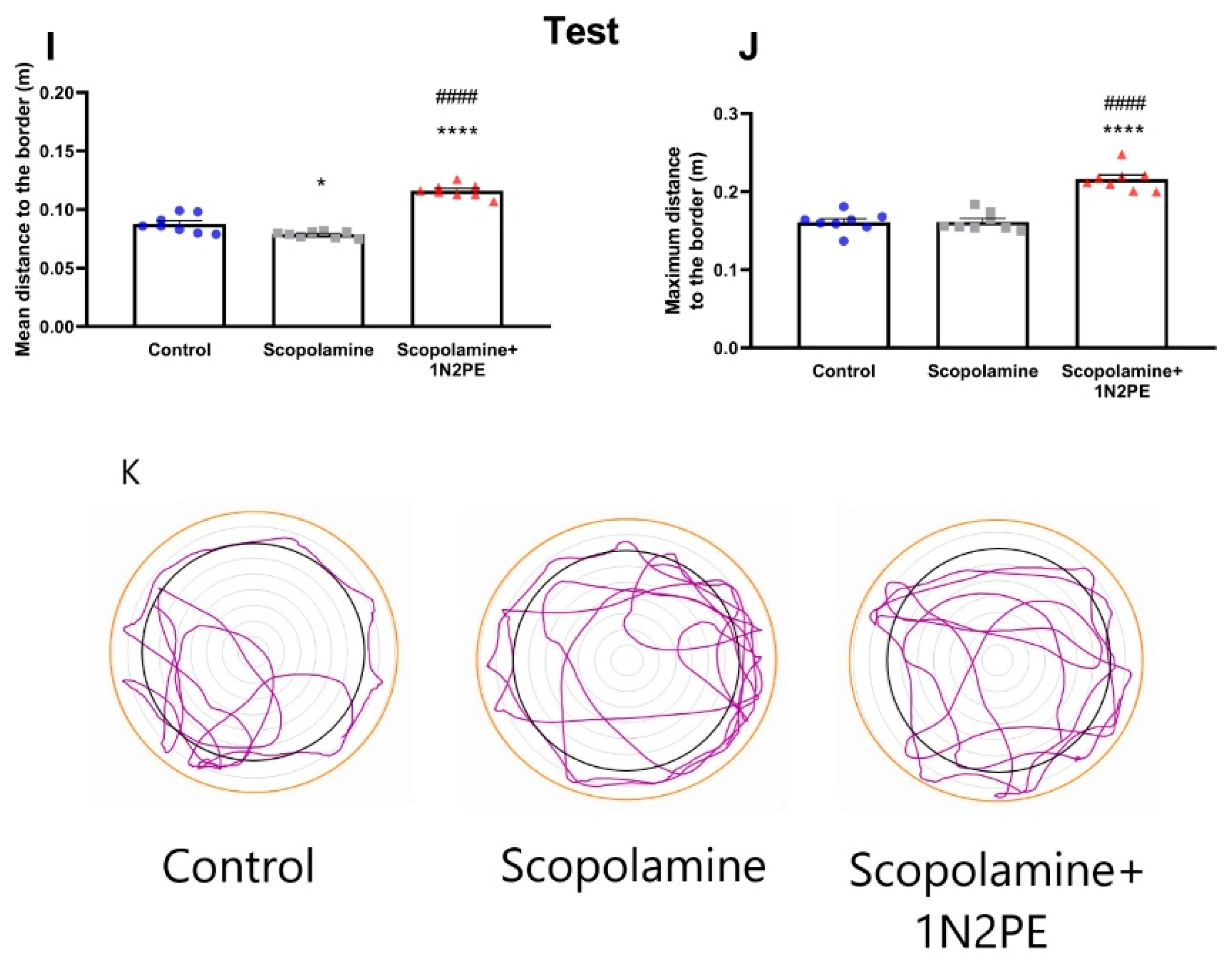
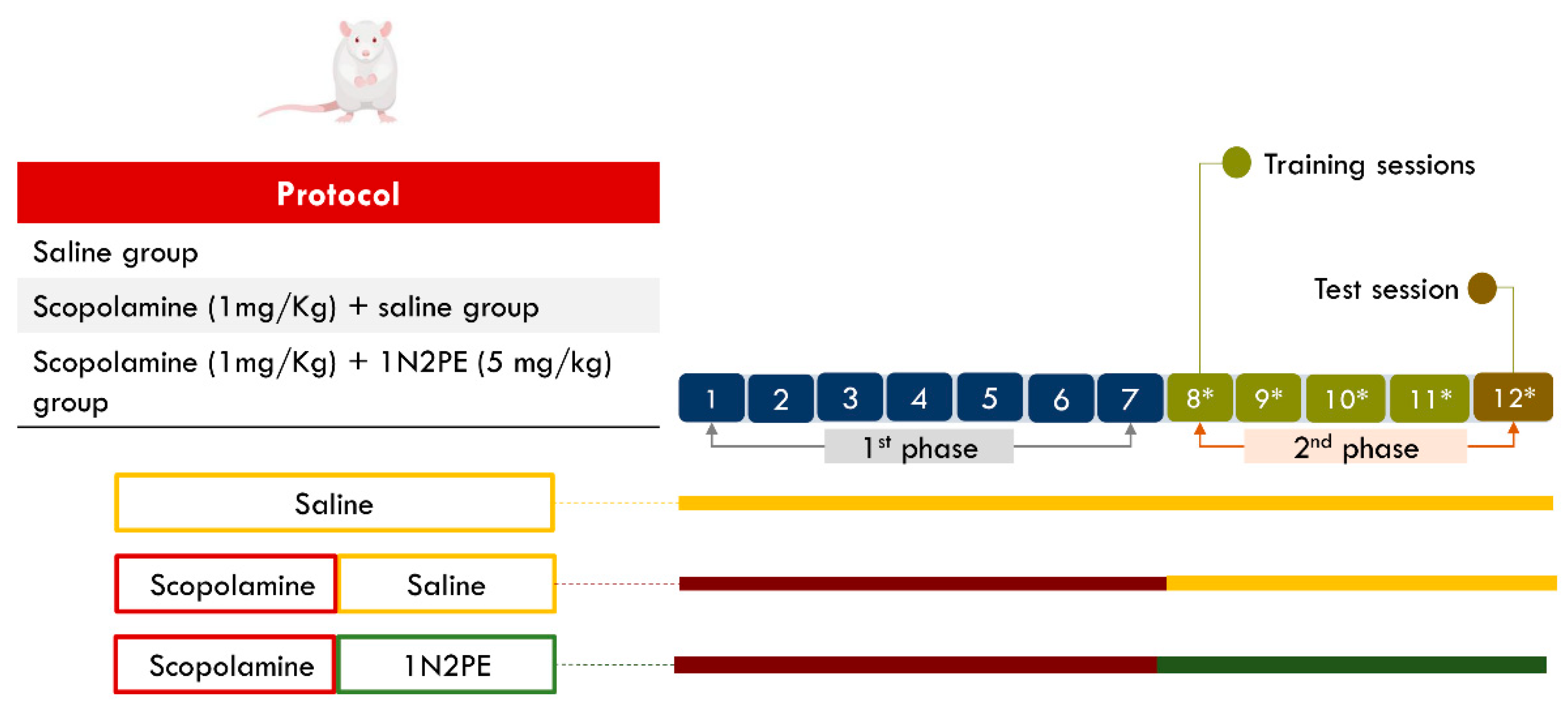
2.4. Behavioral Assays
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation of 1-Nitro-2-Phenylethane
4.2. In Silico Assessment of the Pharmacokinetics Profile
4.3. Preparation of the Molecular Docking In Silico
4.4. Preparation of Molecular Dynamics
4.5. Binding Free Energy Calculation
4.6. In Vivo Procedures
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChe | Acetilcolinesterase |
| AMBER | Assisted Model Building with Energy Refinement |
| BBB | Blood–brain barrier |
| COX-1 | Cyclo-oxygenase-1 |
| CL | Clearance |
| DAT | Dopamine transporter protein |
| DFT | Density Functional Theory |
| FU | Fraction unbound in plasma |
| GABA A | Gamma-aminobutyric acid A |
| HIA | Human Intestinal Absorption |
| MVD | Molegro Virtual Docker MMFF94 |
| MD | Molecular Dynamics |
| MMGBSA | Molecular Mechanics, General Born surface area |
| PGHS | Prostaglandin-H synthase |
| PDB | Protein Data Bank |
| PPB | Plasma Protein Binding |
| QSAR | Quantitative Structure–activity Relationship |
| RMDS | Root Mean squared deviation |
| SERT | serotonin transporter receptor |
| 1N2PE | 1-nitro-2-phenylethane |
| VD | Volume of Distribution |
References
- World Health Organization. International Classification of Diseases (ICD); World Health Organization: Geneva, Switzerland; Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 21 December 2024).
- Fan, Y.; Fan, A.; Yang, Z.; Fan, D. Global Burden of Mental Disorders in 204 Countries and Territories, 1990–2021: Results from the Global Burden of Disease Study 2021. BMC Psychiatry 2025, 25, 486. [Google Scholar] [CrossRef]
- Vervoort, I.; Delger, C.; Soubry, A. A Multifactorial Model for the Etiology of Neuropsychiatric Disorders: The Role of Advanced Paternal Age. Pediatr. Res. 2021, 91, 757–770. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Cogswell, I.E.; Dalos, J.; Tsakalos, G.; Lei, J.; Oros, A.; Rafferty, Q.; Santoni, S.; Steele, X.; Dieleman, J.L.; et al. Estimating Global Direct Health-Care Spending on Neurological and Mental Health between 2000 and 2019: A Modelling Study. Lancet Public Health 2025, 10, e401–e411. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, É.R.Q.; Maia, J.G.S.; Fontes-Júnior, E.A.; Maia, C.d.S.F. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Nasa, A.; Cullinane, D.; Raajakesary, K.; Gazzaz, A.; Sooknarine, V.; Haines, M.; Roman, E.; Kelly, L.; O’Neill, A.; et al. Childhood Trauma, the HPA Axis and Psychiatric Illnesses: A Targeted Literature Synthesis. Front. Psychiatry 2022, 13, 748372. [Google Scholar] [CrossRef] [PubMed]
- Saggu, S.; Pless, A.; Dew, E.; Ware, D.; Jiao, K.; Wang, Q. Monoamine Signaling and Neuroinflammation: Mechanistic Connections and Implications for Neuropsychiatric Disorders. Front. Immunol. 2025, 16, 1543730. [Google Scholar] [CrossRef]
- Thomson, F.; Craighead, M. Innovative Approaches for the Treatment of Depression: Targeting the HPA Axis. Neurochem. Res. 2008, 33, 691–707. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and Depression: A Review. Eur. J. Neurosci. 2020, 53, 151–171. [Google Scholar] [CrossRef]
- Wu, A.; Zhang, J. Neuroinflammation, Memory, and Depression: New Approaches to Hippocampal Neurogenesis. J. Neuroinflamm. 2023, 20. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation Mechanisms of Neuromodulation Therapies for Anxiety and Depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Santana de Oliveira, M. (Ed.) Essential Oils: Applications and Trends in Food Science and Technology; Springer: Cham, Germany, 2022. [Google Scholar]
- Pantoja, L.V.P.d.S.; Fonseca, E.C.M.; Souza-Júnior, J.C.C.; da Conceição, B.C.; Farias, S.V.; da Silva, J.K.R.; Maia, J.G.S.; Silva, P.I.C.; Freitas, J.J.S.; Prediger, R.D.; et al. 1-Nitro-2-Phenylethane: A Promising Phytoconstituent to Modulate Neuroinflammation and Oxidative Stress with Repercussions on Neurological and Psychiatric Disorders. Front. Pharmacol. 2025, 16, 1552295. [Google Scholar] [CrossRef]
- Cardoso, E.K.S.; Kubota, K.; Luz, D.A.; Mendes, P.F.S.; Figueiredo, P.L.B.; Lima, R.R.; Maia, C.S.F.; Fontes-Júnior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae) Essential Oil: Effects on Oxidative Stress and Vascular Permeability. Antioxidants 2022, 11, 1903. [Google Scholar] [CrossRef]
- Gottlieb, O.; Magalhaes, M. Communications Occurrence of 1-Nitro-2-Phenylethane in Octea Pretiosa and Aniba Canelilla. J. Org. Chem. 1959, 24, 2070–2071. [Google Scholar] [CrossRef]
- de Lima, A.B.; Santana, M.B.; Cardoso, A.S.; da Silva, J.K.R.; Maia, J.G.S.; Carvalho, J.C.T.; Sousa, P.J.C. Antinociceptive Activity of 1-Nitro-2-Phenylethane, the Main Component of Aniba canelilla Essential Oil. Phytomedicine 2009, 16, 555–559. [Google Scholar] [CrossRef] [PubMed]
- de Siqueira, R.J.B.; Macedo, F.I.B.; Interaminense, L.d.F.L.; Duarte, G.P.; Magalhães, P.J.C.; Brito, T.S.; da Silva, J.K.R.; Maia, J.G.S.; Sousa, P.J.d.C.; Leal-Cardoso, J.H.; et al. 1-Nitro-2-Phenylethane, the Main Constituent of the Essential Oil of Aniba Canelilla, Elicits a Vago-Vagal Bradycardiac and Depressor Reflex in Normotensive Rats. Eur. J. Pharmacol. 2010, 638, 90–98. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.K.R.; Sousa, P.J.C.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant Capacity and Cytotoxicity of Essential Oil and Methanol Extract of Aniba canelilla (H.B.K.) Mez. J. Agric. Food Chem. 2007, 55, 9422–9426. [Google Scholar] [CrossRef] [PubMed]
- de Campos, D.L.; Queiroz, L.Y.; Fontes-Junior, E.A.; Pinheiro, B.G.; da Silva, J.K.R.; Maia, C.S.F.; Maia, J.G.S. Aniba canelilla (Kunth) Mez Essential Oil and Its Primary Constituent, 1-Nitro-2-Phenylethane, Inhibits Acetylcholinesterase and Reverse Memory Impairment in Rodents. J. Ethnopharmacol. 2023, 303, 116036. [Google Scholar] [CrossRef]
- Oyemitan, I.A.; Elusiyan, C.A.; Akanmu, M.A.; Olugbade, T.A. Hypnotic, Anticonvulsant and Anxiolytic Effects of 1-Nitro-2-Phenylethane Isolated from the Essential Oil of Dennettia Tripetala in Mice. Phytomedicine 2013, 20, 1315–1322. [Google Scholar] [CrossRef]
- McNamara, R.K.; Skelton, R.W. The Neuropharmacological and Neurochemical Basis of Place Learning in the Morris Water Maze. Brain Res. Rev. 1993, 18, 33–49. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. Application of the SwissDrugDesign Online Resources in Virtual Screening. Int. J. Mol. Sci. 2019, 20, 4612. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Zhevlakova, A.; Selivanova, I. Insights into the Pharmacological Effects of Flavonoids: The Systematic Review of Computer Modeling. Int. J. Mol. Sci. 2022, 23, 6023. [Google Scholar] [CrossRef]
- Combs, S.; Kaufmann, K.; Field, J.R.; Blakely, R.D.; Meiler, J. Y95 and E444 Interaction Required for High-Affinity S-Citalopram Binding in the Human Serotonin Transporter. ACS Chem. Neurosci. 2010, 2, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.H.; Silman, I.; Sussman, J.L. Quaternary Ligand Binding to Aromatic Residues in the Active-Site Gorge of Acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar] [CrossRef] [PubMed]
- Thuresson, E.D.; Malkowski, M.G.; Lakkides, K.M.; Rieke, C.J.; Mulichak, A.M.; Ginell, S.L.; Garavito, R.M.; Smith, W.L. Mutational and X-Ray Crystallographic Analysis of the Interaction of Dihomo-γ-Linolenic Acid with Prostaglandin Endoperoxide H Synthases. J. Biol. Chem. 2000, 276, 10358–10365. [Google Scholar] [CrossRef]
- Murayama, M.A.; Arimitsu, N.; Shimizu, J.; Fujiwara, N.; Takai, K.; Okada, Y.; Hirotsu, C.; Takada, E.; Suzuki, T.; Suzuki, N. Dementia Model Mice Exhibited Improvements of Neuropsychiatric Symptoms as Well as Cognitive Dysfunction with Neural Cell Transplantation. Jikken Dobutsu 2021, 70, 387–397. [Google Scholar] [CrossRef]
- Murayama, M.A.; Arimitsu, N.; Shimizu, J.; Fujiwara, N.; Takai, K.; Ikeda, Y.; Okada, Y.; Hirotsu, C.; Takada, E.; Suzuki, T.; et al. Female Dominance of Both Spatial Cognitive Dysfunction and Neuropsychiatric Symptoms in a Mouse Model of Alzheimer’s Disease. Exp. Anim. 2021, 70, 398–405. [Google Scholar] [CrossRef]
- Souza-Junior, F.J.C.; Luz-Moraes, D.; Pereira, F.S.; Barros, M.A.; Fernandes, L.M.P.; Queiroz, L.Y.; Maia, C.F.; Maia, J.G.S.; Fontes-Junior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae): A Review of Ethnobotany, Phytochemical, Antioxidant, Anti-Inflammatory, Cardiovascular, and Neurological Properties. Front. Pharmacol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Pan, Y.; Huang, N.; Cho, S.; MacKerell, A.D. Consideration of Molecular Weight during Compound Selection in Virtual Target-Based Database Screening. J. Chem. Inf. Comput. Sci. 2002, 43, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Tuyet, T.; Tayara, H.; Chong, K.T. Artificial Intelligence in Drug Metabolism and Excretion Prediction: Recent Advances, Challenges, and Future Perspectives. Pharmaceutics 2023, 15, 1260. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The Role of Natural Products in Modern Drug Discovery. An. Acad. Bras. Ciências 2019, 91 (Suppl 3), e20190105. [Google Scholar] [CrossRef] [PubMed]
- Oashi, T.; Ringer, A.L.; Raman, E.P.; MacKerell, A.D. Automated Selection of Compounds with Physicochemical Properties to Maximize Bioavailability and Druglikeness. J. Chem. Inf. Model. 2011, 51, 148–158. [Google Scholar] [CrossRef]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of Physiological PH and Chemical PKa under the Umbrella of Physiologically Based Pharmacokinetic Modeling of Drug Absorption, Distribution, Metabolism, Excretion, and Toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef]
- Tsantili-Kakoulidou, A.; Demopoulos, V. Drug-like Properties and Fraction Lipophilicity Index as a Combined Metric. ADMET DMPK 2021, 9, 177–190. [Google Scholar] [CrossRef]
- Leeson, P.D.; Bento, A.P.; Gaulton, A.; Hersey, A.; Manners, E.J.; Radoux, C.J.; Leach, A.R. Target-Based Evaluation of “Drug-Like” Properties and Ligand Efficiencies. J. Med. Chem. 2021, 64, 7210–7230. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Volpe, D.A. Drug-Permeability and Transporter Assays in Caco-2 and MDCK Cell Lines. Future Med. Chem. 2011, 3, 2063–2077. [Google Scholar] [CrossRef]
- Li, Y.; Shin, Y.; Yu, C.; Kosmeder, J.; Hirschelman, W.; Pezzuto, J.; van Breemen, R. Increasing the Throughput and Productivity of Caco-2 Cell Permeability Assays Using Liquid Chromatography-Mass Spectrometry: Application to Resveratrol Absorption and Metabolism. Comb. Chem. High Throughput Screen. 2003, 6, 757–767. [Google Scholar] [CrossRef]
- Routledge, P. The Plasma Protein Binding of Basic Drugs. Br. J. Clin. Pharmacol. 1986, 22, 499–506. [Google Scholar] [CrossRef]
- Smith, D.A.; Di, L.; Kerns, E.H. The Effect of Plasma Protein Binding on in Vivo Efficacy: Misconceptions in Drug Discovery. Nat. Rev. Drug Discov. 2010, 9, 929–939. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-in Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Pariente, G.; Leibson, T.; Carls, A.; Adams-Webber, T.; Ito, S.; Koren, G. Pregnancy-Associated Changes in Pharmacokinetics: A Systematic Review. PLOS Med. 2016, 13, e1002160. [Google Scholar] [CrossRef]
- Deodhar, M.; Al Rihani, S.B.; Arwood, M.J.; Darakjian, L.; Dow, P.; Turgeon, J.; Michaud, V. Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions due to Mechanism-Based Inhibition in Clinical Practice. Pharmaceutics 2020, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chu, X.; Parrott, N.; Brouwer, K.L.; Hsu, V.; Nagar, S.; Matsson, P.; Sharma, P.; Snoeys, J.; Sugiyama, Y.; et al. Advancing Predictions of Tissue and Intracellular Drug Concentrations Using in Vitro, Imaging and Physiologically Based Pharmacokinetic Modeling Approaches. Clin. Pharmacol. Ther. 2018, 104, 865–889. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; dos Santos, R.; Oliva, G.; Andricopulo, A. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Sochacka, J. Docking of Thiopurine Derivatives to Human Serum Albumin and Binding Site Analysis with Molegro Virtual Docker. Acta Pol. Pharm.-Drug Res. 2014, 71, 343–349. [Google Scholar]
- Alnajjar, R.; Mostafa, A.; Kandeil, A.; Al-Karmalawy, A.A. Molecular Docking, Molecular Dynamics, and in Vitro Studies Reveal the Potential of Angiotensin II Receptor Blockers to Inhibit the COVID-19 Main Protease. Heliyon 2020, 6, e05641. [Google Scholar] [CrossRef]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-Ray Structure of Dopamine Transporter Elucidates Antidepressant Mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef]
- Bashir, M.A.; Khan, A.; Badshah, H.; Rodrigues-Filho, E.; Din, Z.U.; Khan, A. Synthesis, Characterization, Molecular Docking Evaluation, Antidepressant, and Anti—Alzheimer Effects of Dibenzylidene Ketone Derivatives. Drug Dev. Res. 2019, 80, 595–605. [Google Scholar] [CrossRef]
- Kufareva, I.; Abagyan, R. Methods of Protein Structure Comparison. Methods Mol. Biol. 2012, 857, 231–257. [Google Scholar] [CrossRef]
- Andersen, J.; Stuhr-Hansen, N.; Zachariassen, L.G.; Koldsø, H.; Schiøtt, B.; Strømgaard, K.; Kristensen, A.S. Molecular Basis for Selective Serotonin Reuptake Inhibition by the Antidepressant Agent Fluoxetine (Prozac). Mol. Pharmacol. 2014, 85, 703–714. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.N.S.; Silva, J.R.A.; Alves, C.N.; Andrade, E.H.A.; da Silva, J.K.R.; Maia, J.G.S. Acetylcholinesterase Inhibitory Activity and Molecular Docking Study of 1-Nitro-2-Phenylethane, the Main Constituent of Aniba canelilla Essential Oil. Chem. Biol. Drug Des. 2014, 84, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-J.; Oh, D.-K. Prostaglandin Synthases: Molecular Characterization and Involvement in Prostaglandin Biosynthesis. Prog. Lipid Res. 2017, 66, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Jafarian, S.; Ling, K.; Hassan, Z.; Perimal-Lewis, L.; Sulaiman, M.R.; Perimal, E.K. Effect of Zerumbone on Scopolamine—Induced Memory Impairment and Anxiety—Like Behaviours in Rats. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Bajo, R.; Pusil, S.; López, M.E.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine Effects on Functional Brain Connectivity: A Pharmacological Model of Alzheimer’s Disease. Sci. Rep. 2015, 5, 9748. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Blokland, A. The Validity of Scopolamine as a Pharmacological Model for Cognitive Impairment: A Review of Animal Behavioral Studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Al-Nusaif, M.; Li, S.; Tan, X.; Yang, H.; Cai, H.; Le, W. Progress on Early Diagnosing Alzheimer’s Disease. Front. Med. 2024, 18, 446–464. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Hritcu, L.; Dogan, G.; Hayta, S.; Bagci, E. The Effects of Inhaled Pimpinella Peregrina Essential Oil on Scopolamine-Induced Memory Impairment, Anxiety, and Depression in Laboratory Rats. Mol. Neurobiol. 2016, 53, 6557–6567. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, S.; Zia, M.A.; Uzair, M.; Alsubki, R.A.; Sajid, K.; Shoukat, S.; Attia, K.A.; Fiaz, S.; Ali, S.; Kimiko, I.; et al. Synergistic Neuroprotection by Phytocompounds of Bacopa Monnieri in Scopolamine-Induced Alzheimer’s Disease Mice Model. Mol. Biol. Rep. 2023, 50, 7967–7979. [Google Scholar] [CrossRef]
- Calabresi, P.; Centonze, D.; Gubellini, P.; Pisani, A.; Bernardi, G. Endogenous ACh Enhances Striatal NMDA-Responses via M1-like Muscarinic Receptors and PKC Activation. Eur. J. Neurosci. 1998, 10, 2887–2895. [Google Scholar] [CrossRef]
- Brown, M.A.; Sharp, P.E. Simulation of Spatial Learning in the Morris Water Maze by a Neural Network Model of the Hippocampal Formation and Nucleus Accumbens. Hippocampus 1995, 5, 171–188. [Google Scholar] [CrossRef]
- Luo, S.X.; Huang, E.J. Dopaminergic Neurons and Brain Reward Pathways. Am. J. Pathol. 2016, 186, 478–488. [Google Scholar] [CrossRef]
- Ozawa, T.; Yamada, K.; Ichitani, Y. D-Cycloserine Reverses Scopolamine-Induced Object and Place Memory Deficits in a Spontaneous Recognition Paradigm in Rats. Pharmacol. Biochem. Behav. 2019, 187, 172798. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ikegaya, Y.; Saito, H.; Abe, K. Roles of GABAA, NMDA and Muscarinic Receptors in Induction of Long-Term Potentiation in the Medial and Lateral Amygdala In Vitro. Neurosci. Res. 1995, 21, 317–322. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, D.; Huang, X.; Huang, L.; Tang, L.; Jiang, J.; Chen, L.; Li, S. Neuroprotective Effects of HTR1A Antagonist WAY-100635 on Scopolamine-Induced Delirium in Rats and Underlying Molecular Mechanisms. BMC Neurosci. 2016, 17, 66. [Google Scholar] [CrossRef]
- Bertrand, F.; Lehmann, O.; Galani, R.; Lazarus, C.; Jeltsch, H.; Cassel, J.-C. Effects of MDL 73005 on Water-Maze Performances and Locomotor Activity in Scopolamine-Treated Rats. Pharmacol. Biochem. Behav. 2001, 68, 647–660. [Google Scholar] [CrossRef]
- Morris, R. Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool Reverses Neuropathological and Behavioral Impairments in Old Triple Transgenic Alzheimer’s Mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Shin, M.; Park, Y.; Choi, B.; Jang, S.; Lim, C.; Yun, H.S.; Lee, I.-S.; Won, S.-Y.; Cho, K.S. Linalool Alleviates Aβ42-Induced Neurodegeneration via Suppressing ROS Production and Inflammation in Fly and Rat Models of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8887716. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, U.; Chaturbhuj, S.G.; Sankhe, R.; Das, M.; Surubhotla, R.; Krishnadas, N.; Gourishetti, K.; Nayak, P.G.; Kishore, A. Beta-Caryophyllene, a CB2R Selective Agonist, Protects against Cognitive Impairment Caused by Neuro-Inflammation and Not in Dementia due to Ageing Induced by Mitochondrial Dysfunction. CNS Neurol. Disord.-Drug Targets 2021, 20, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Bigdeli, Y.; Asle-Rousta, M.; Rahnema, M. Effects of Limonene on Chronic Restraint Stress-Induced Memory Impairment and Anxiety in Male Rats. Neurophysiology 2019, 51, 107–113. [Google Scholar] [CrossRef]
- Mehrjerdi, F.Z.; Niknazar, S.; Yadegari, M.; Akbari, F.A.; Pirmoradi, Z.; Khaksari, M. Carvacrol Reduces Hippocampal Cell Death and Improves Learning and Memory Deficits Following Lead-Induced Neurotoxicity via Antioxidant Activity. Naunyn-Schmiedeberg S Arch. Pharmacol. 2020, 393, 1229–1237. [Google Scholar] [CrossRef]
- Kostowski, W.; Giacalone, E.; Garattini, S.; Valzelli, L. Studies on Behavioral and Biochemical Changes in Rats after Lesion of Midbrain Raphé. Eur. J. Pharmacol. 1968, 4, 371–376. [Google Scholar] [CrossRef]
- Fibiger, H.C.; Campbell, B.A. The Effect of Para-Chlorophenylalanine on Spontaneous Locomotor Activity in the Rat. Neuropharmacology 1971, 10, 25–32. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A New Animal Model Sensitive to Antidepressant Treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Addy, N.A.; Nunes, E.J.; Wickham, R.J. Ventral Tegmental Area Cholinergic Mechanisms Mediate Behavioral Responses in the Forced Swim Test. Behav. Brain Res. 2015, 288, 54–62. [Google Scholar] [CrossRef]
- Karabeg, M.M.; Grauthoff, S.; Kollert, S.Y.; Weidner, M.; Heiming, R.S.; Jansen, F.; Popp, S.; Kaiser, S.; Lesch, K.-P.; Sachser, N.; et al. 5-HTT Deficiency Affects Neuroplasticity and Increases Stress Sensitivity Resulting in Altered Spatial Learning Performance in the Morris Water Maze but Not in the Barnes Maze. PLoS ONE 2013, 8, e78238. [Google Scholar] [CrossRef]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an Index of Anxiety in Mice. Influence of Dopaminergic Transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz-Jarosz, H.; Członkowska, A.I.; Siemiatkowski, M.; Maciejak, P.; Szyndler, J.; Planik, A. The Effects of Physostigmine and Cholinergic Receptor Ligands on Novelty-Induced Neophobia. J. Neural Transm. 2000, 107, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Goodman, J.; McIntyre, C.K. Impaired Spatial Memory and Enhanced Habit Memory in a Rat Model of Post-Traumatic Stress Disorder. Front. Pharmacol. 2017, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, W.; Zhang, Y. Bright Lighting Conditions during Testing Increase Thigmotaxis and Impair Water Maze Performance in BALB/c Mice. Behav. Brain Res. 2012, 226, 26–31. [Google Scholar] [CrossRef]
- Celebi, R.; Bear Don’t Walk, O.; Movva, R.; Alpsoy, S.; Dumontier, M. In-Silico Prediction of Synergistic Anti-Cancer Drug Combinations Using Multi-Omics Data. Sci. Rep. 2019, 9, 8949. [Google Scholar] [CrossRef]
- Ou-Yang, S.; Lu, J.; Kong, X.; Liang, Z.; Luo, C.; Jiang, H. Computational Drug Discovery. Acta Pharmacol. Sin. 2012, 33, 1131–1140. [Google Scholar] [CrossRef]
- de Azevedo, W.F., Jr. (Ed.) Docking Screens for Drug Discovery; Springer: New York, NY, USA, 2019. [Google Scholar]
- Martin, Y.C. A Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, J.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlegel, H.B.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Revision C.01; Science Open: Lexington, MA, USA, 2010. [Google Scholar]
- Elferich, J.; Williamson, D.M.; David, L.L.; Shinde, U. Determination of Histidine PKa Values in the Propeptides of Furin and Proprotein Convertase 1/3 Using Histidine Hydrogen–Deuterium Exchange Mass Spectrometry. Anal. Chem. 2015, 87, 7909–7917. [Google Scholar] [CrossRef]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Fonseca, E.C.M.; Costa, K.S.d.; Lameira, J.; Nahum Alves, C.; Lima, A.H. Investigation of the Target-Site Resistance of EPSP Synthase Mutants P106T and T102I/P106S against Glyphosate. RSC Adv. 2020, 10, 44352–44360. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA Methods to Estimate Ligand-Binding Affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Karplus, M.; Petsko, G.A. Molecular Dynamics Simulations in Biology. Nature 1990, 347, 631–639. [Google Scholar] [CrossRef]
- Alonso, H.; Bliznyuk, A.A.; Gready, J.E. Combining Docking and Molecular Dynamic Simulations in Drug Design. Med. Res. Rev. 2006, 26, 531–568. [Google Scholar] [CrossRef]
- Higaki, A.; Mogi, M.; Iwanami, J.; Min, L.-J.; Bai, H.-Y.; Shan, B.-S.; Kan-no, H.; Ikeda, S.; Higaki, J.; Horiuchi, M. Recognition of Early Stage Thigmotaxis in Morris Water Maze Test with Convolutional Neural Network. PLoS ONE 2018, 13, e0197003. [Google Scholar] [CrossRef] [PubMed]

| Physical-Chemical Parameters | Values | Pharmacokinetics | Result | Drug-Likeness | Result |
|---|---|---|---|---|---|
| Solubility (LogS) | −2.29 | GI absorption | High | Lipinski | 0 violation |
| Lipophilicity (LogP) | 2.03 | BBB permeant | Yes | Eagan | 0 violation |
| pKa | 9.21 | P-gp substrate | No | Veber | 0 violation |
| Molecular weight | 151.16 g/mol | Skin permeation | −5.75 cm/s | Bioavailability Score ** | 0.55 |
| ADME | Absorption | Value | Distribution | Value | Metabolism | Value | Excretion | Value |
|---|---|---|---|---|---|---|---|---|
| Parameters | Caco-2 permeability | −4.27 Log unit | PPB | 81.55% | CYP1A2 | I/S | CL | 8.77 mL/min/kg |
| MDCK permeability | 0.26 × 10−4 cm/s | FU | 16.12% | CYP2C19 CYP2C9 CYP2D6 CYP3A4 | I/S I/S I/S I/S | T1/2 | 45 min | |
| HIA | 0.6 × 10−2 cm/s | VD | 0.81 L/kg |
| Target | Binding Energy (NPE) (kcal/mol) | DSnorm (NPE) (kcal/mol) | Binding Energy (control) (kcal/mol) | DSnorm (control) (kcal/mol) |
|---|---|---|---|---|
| SERT | −68.00 | −93.61 | −108.28 | −115.30 |
| DAT | −62.53 | −83.08 | −107.09 | −123.31 |
| GABAA | −52.07 | −71.68 | −113.09 | −124.69 |
| AChE | −59.66 | −82.13 | −131.28 | −130.56 |
| PGHS-1 | −58.45 | −80.46 | −126.12 | −125.31 |
| Molecular Targets | GBSA |
|---|---|
| SERT | −18.20/1.59 |
| PGHS-1 | −20.27/1.86 |
| AChE | −16.58/2.38 |
| DAT | −26.26/1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, E.C.M.; Pantoja, L.V.P.d.S.; de Campos, D.L.; Souza-Junior, F.J.C.; Pinheiro, B.G.; da Conceição, B.C.; Maia, J.G.S.; de Lima, C.A.C.; Fontes-Júnior, E.A.; Carneiro, A.S.; et al. 1-Nitro-2-Phenylethane as a Multitarget Candidate for Cognitive and Psychiatric Disorders: Insights from In Silico and Behavioral Approaches. Pharmaceuticals 2025, 18, 1511. https://doi.org/10.3390/ph18101511
Fonseca ECM, Pantoja LVPdS, de Campos DL, Souza-Junior FJC, Pinheiro BG, da Conceição BC, Maia JGS, de Lima CAC, Fontes-Júnior EA, Carneiro AS, et al. 1-Nitro-2-Phenylethane as a Multitarget Candidate for Cognitive and Psychiatric Disorders: Insights from In Silico and Behavioral Approaches. Pharmaceuticals. 2025; 18(10):1511. https://doi.org/10.3390/ph18101511
Chicago/Turabian StyleFonseca, Emily Christie Maia, Lucas Villar Pedrosa da Silva Pantoja, Daniele Luz de Campos, Fábio José Coelho Souza-Junior, Bruno Gonçalves Pinheiro, Brenda Costa da Conceição, José Guilherme Soares Maia, Caroline Araujo Costa de Lima, Enéas Andrade Fontes-Júnior, Agnaldo Silva Carneiro, and et al. 2025. "1-Nitro-2-Phenylethane as a Multitarget Candidate for Cognitive and Psychiatric Disorders: Insights from In Silico and Behavioral Approaches" Pharmaceuticals 18, no. 10: 1511. https://doi.org/10.3390/ph18101511
APA StyleFonseca, E. C. M., Pantoja, L. V. P. d. S., de Campos, D. L., Souza-Junior, F. J. C., Pinheiro, B. G., da Conceição, B. C., Maia, J. G. S., de Lima, C. A. C., Fontes-Júnior, E. A., Carneiro, A. S., Alencar, N. A. N. d., Rocha, J. A. P. d., Freitas, J. J. S., Silva, J. K. d. R. d., Oliveira, M. S. d., & Maia, C. S. F. (2025). 1-Nitro-2-Phenylethane as a Multitarget Candidate for Cognitive and Psychiatric Disorders: Insights from In Silico and Behavioral Approaches. Pharmaceuticals, 18(10), 1511. https://doi.org/10.3390/ph18101511










