Development and Blood–Brain Barrier Penetration of Nanovesicles Loaded with Cannabidiol
Abstract
1. Introduction
2. Results and Discussion
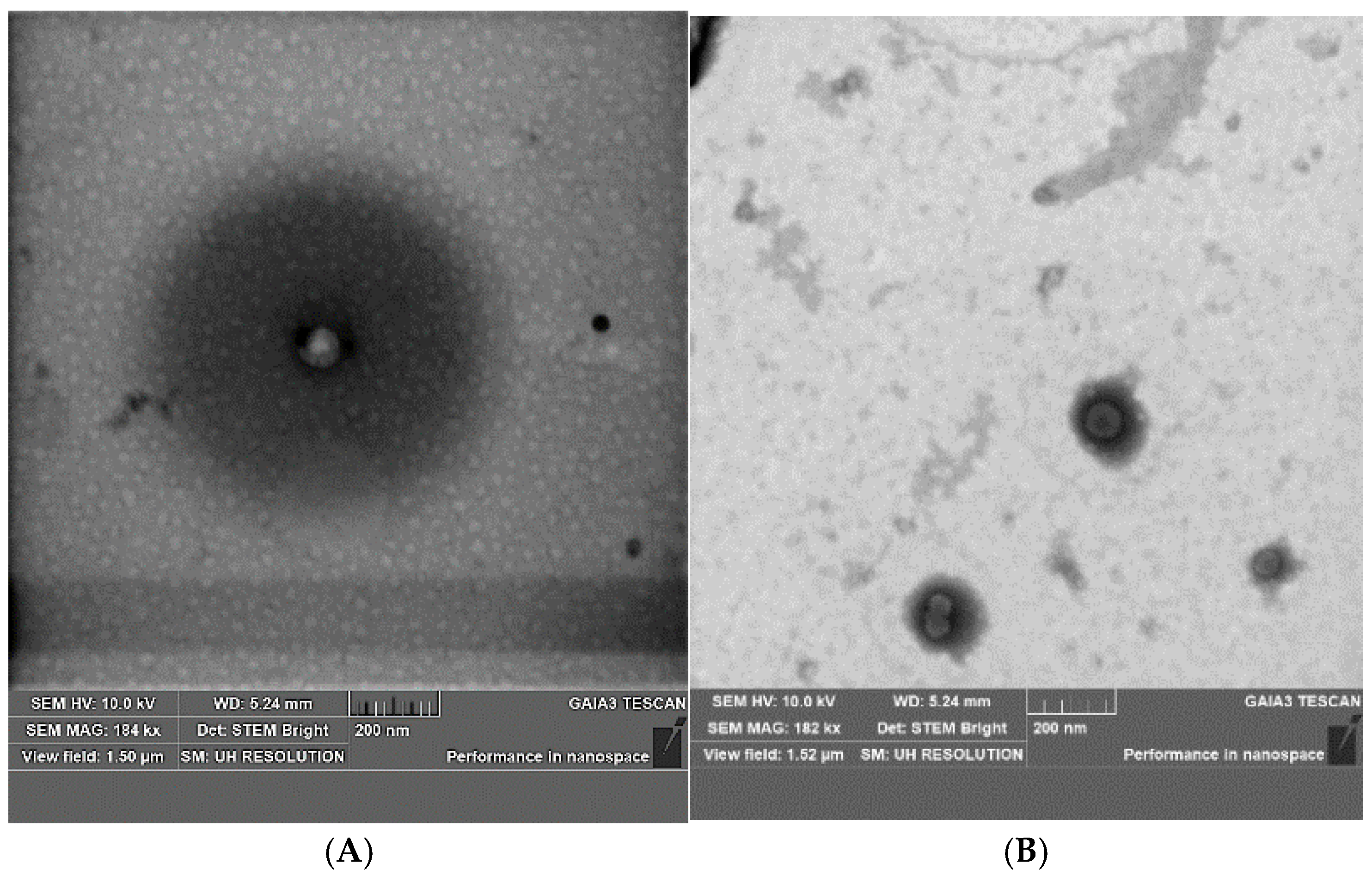
2.1. Development and Optimization of CBD Nanovesicles (DLS, ELS, HPLC, and TEM Analysis)
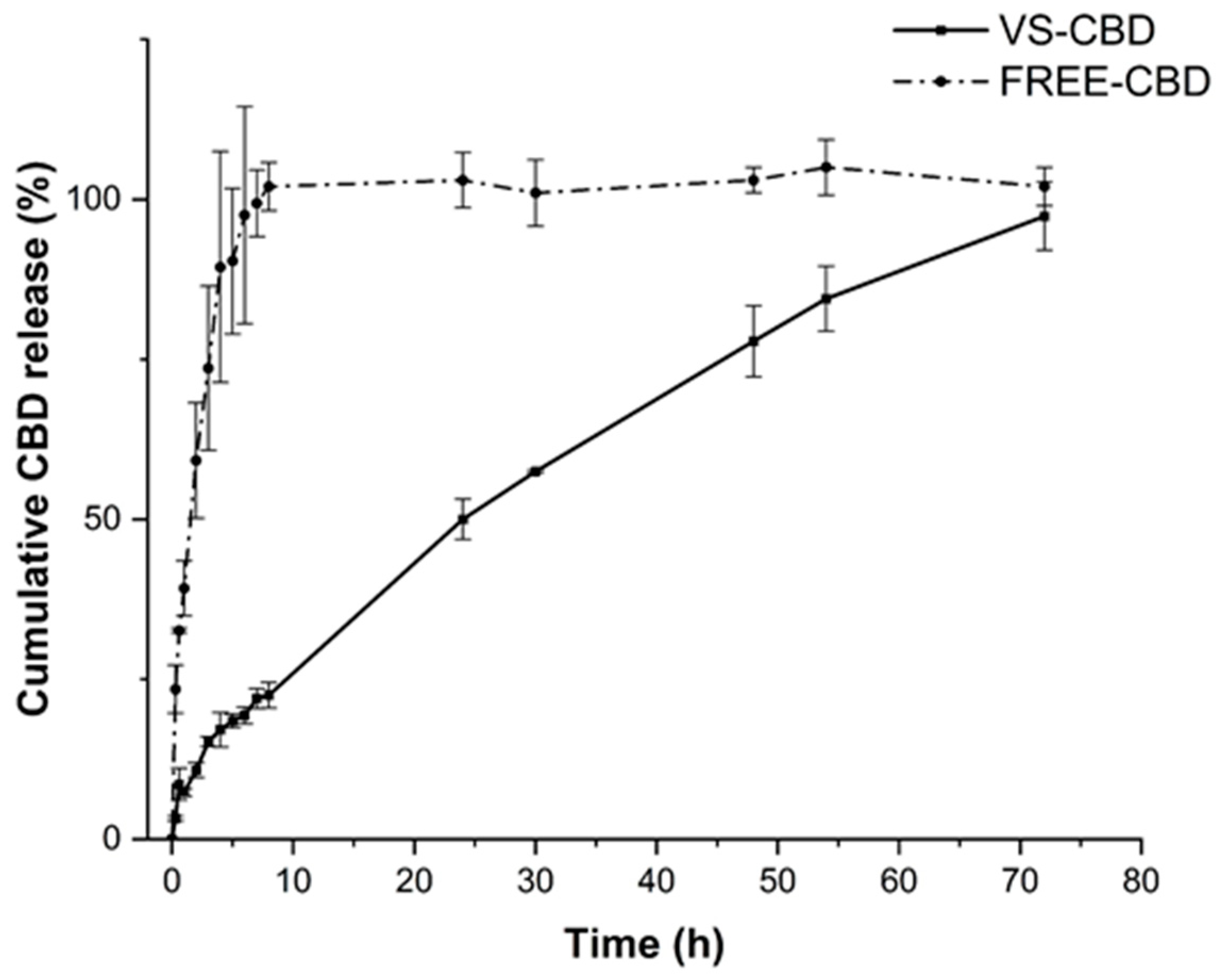
2.2. In Vitro Release Studies and Stability Studies
2.3. Diffusion Behaviour Through Artificial Membrane (PAMPA-BBB)
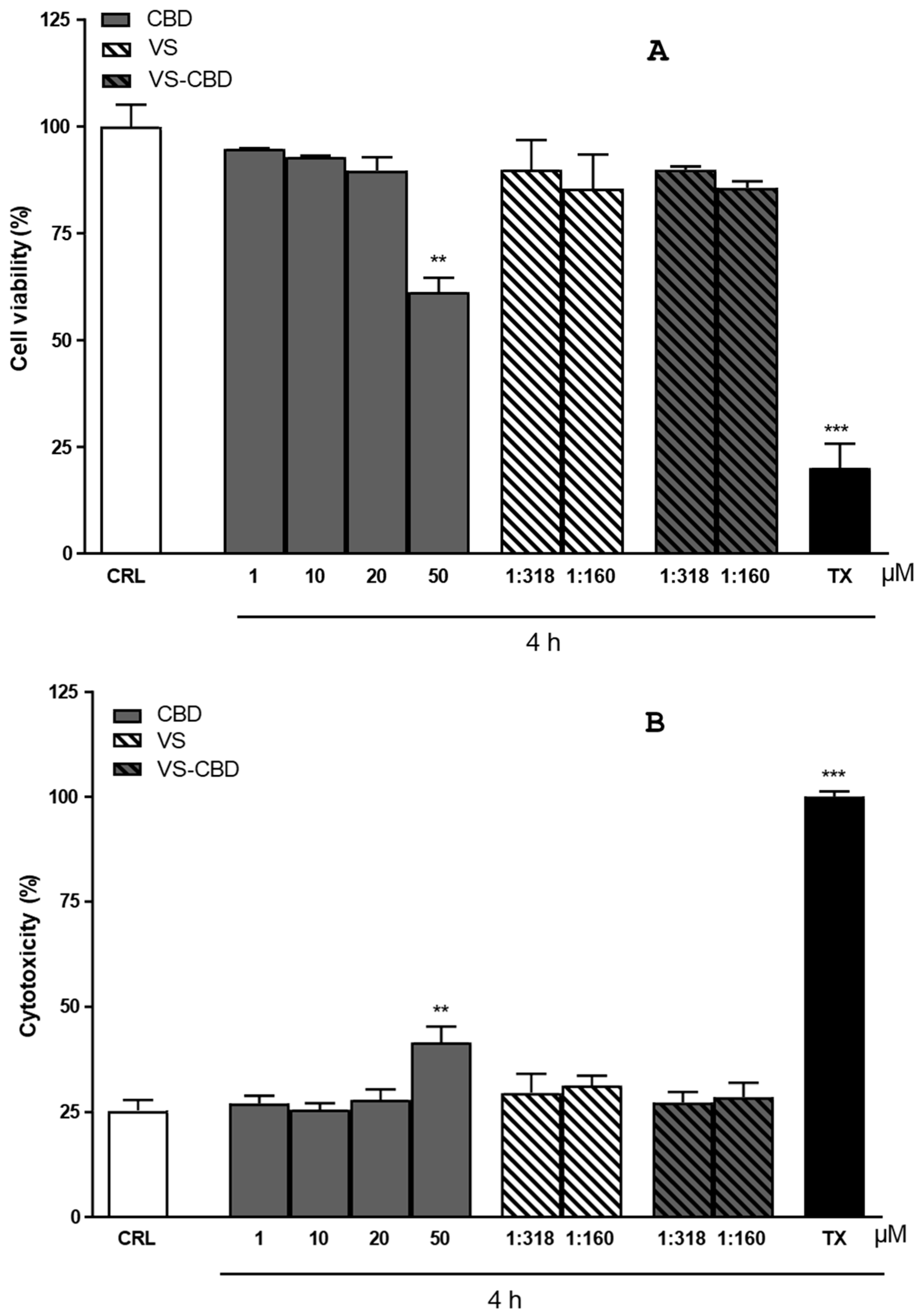
2.4. Cytotoxicity and Cell Viability on HCMEC/D3 Cell Line
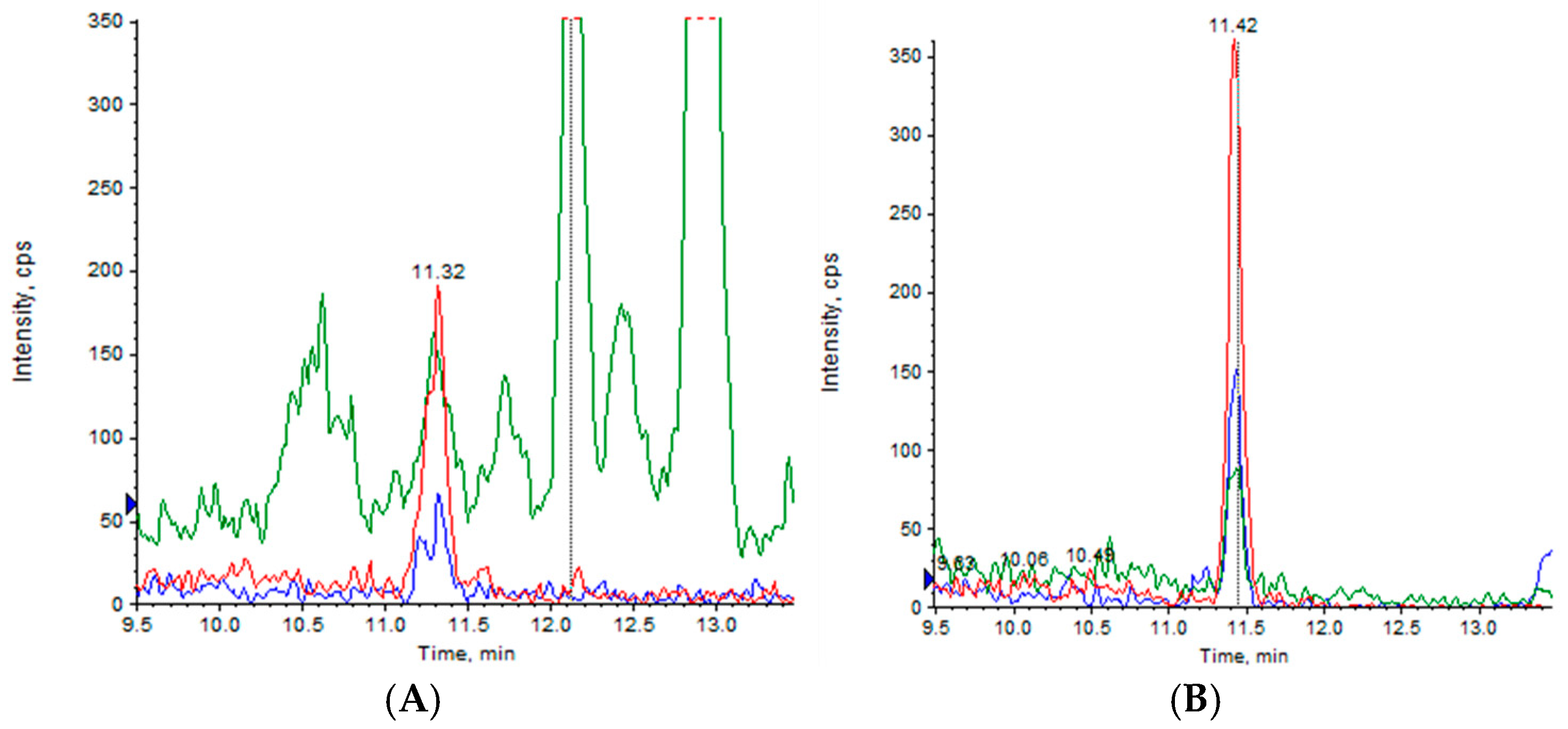
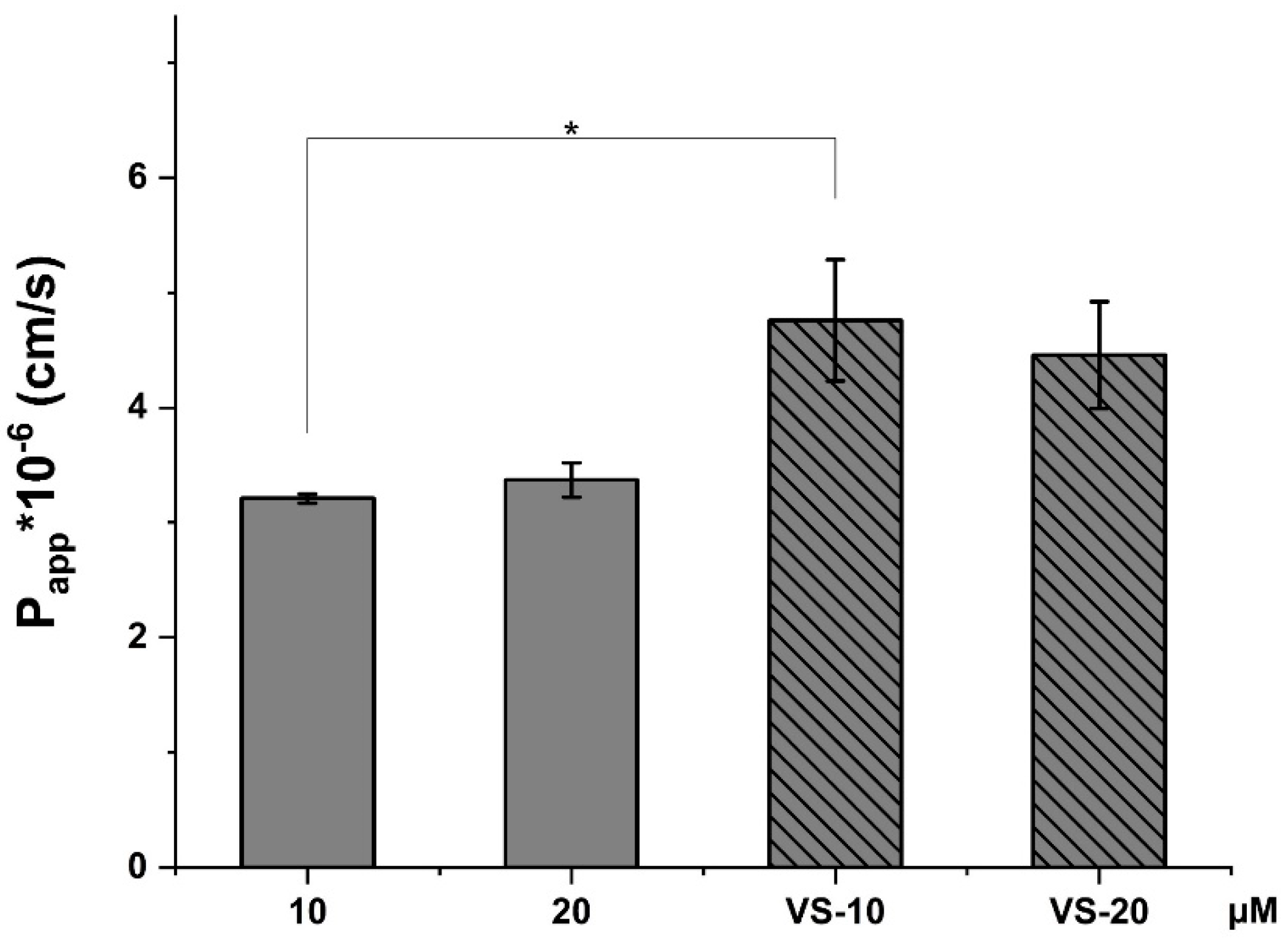
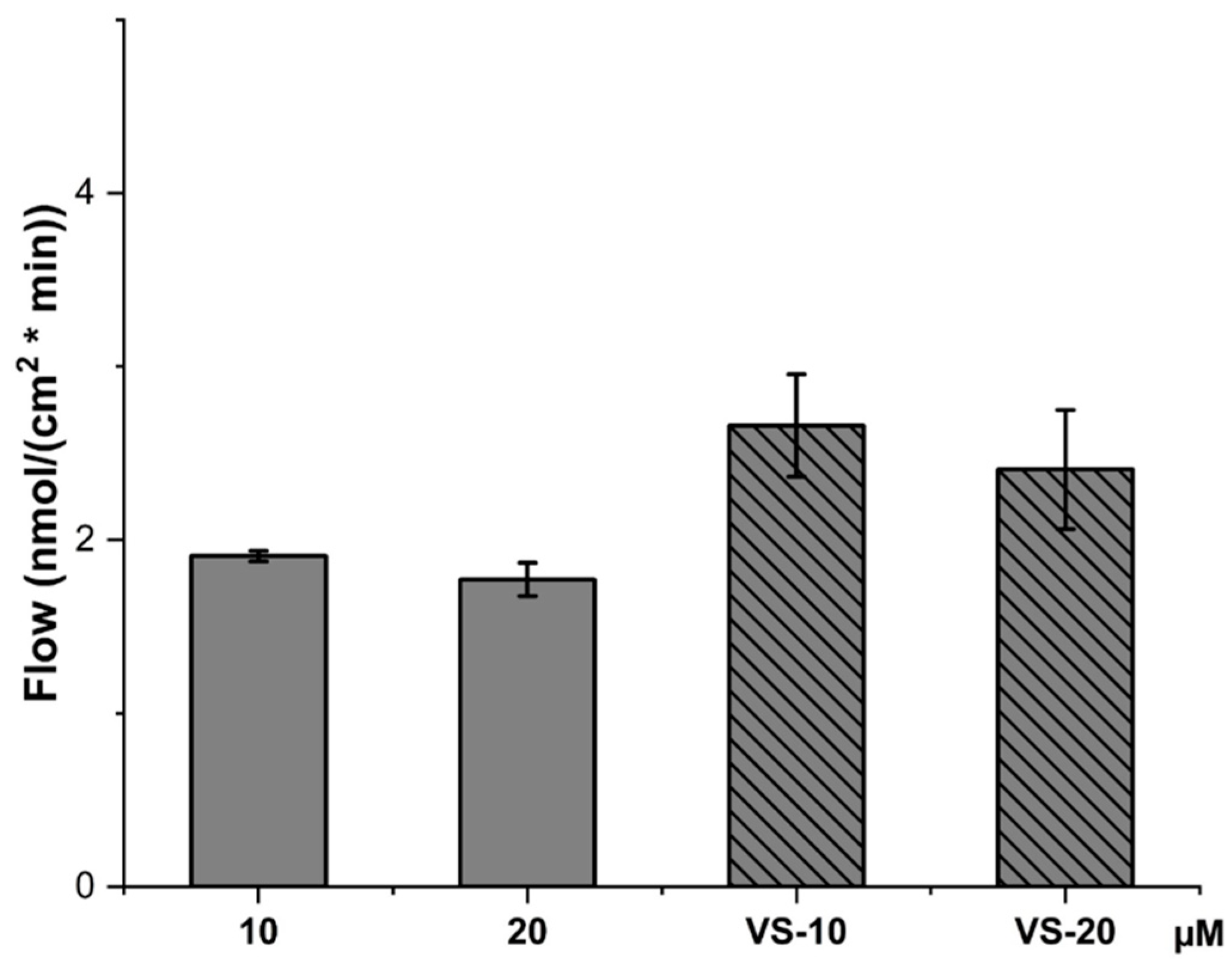
2.5. CBD Permeability Evaluation Across HCMEC/D3 Cell Line
3. Materials and Methods
3.1. Materials
3.2. Liposome and Nanovesicle Preparation
3.3. Dynamic and Electrophoretic Light Scattering
3.4. Scanning Transmission Electron Microscopy (STEM)
3.5. Quantification of CBD
3.5.1. HPLC-DAD and HPLC-FLD
3.5.2. HPLC-MS/MS
3.6. Recovery and Encapsulation Efficiency
3.7. CBD Release from VS-CBD
3.8. PAMPA-BBB Assay
3.9. Stability Studies
3.10. MTT and LDH Assays
3.11. Permeability Through hCMEC/D3 Cell Line
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Mazzantini, C.; Lana, D.; Davolio, P.L.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective effects of cannabidiol but not Δ9-tetrahydrocannabinol in rat hippocampal slices exposed to oxygen-glucose deprivation: Studies with cannabis extracts and selected cannabinoids. Int. J. Mol. Sci. 2021, 22, 9773. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.M.W.; Kohara, N.A.; Milani, H. Cannabidiol in experimental cerebral ischemia. Int. Rev. Neurobiol. 2024, 177, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Mazzantini, C.; Lana, D.; Calvani, M.; Magni, G.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Cannabidiol inhibits microglia activation and mitigates neuronal damage induced by kainate in an in-vitro seizure model. Neurobiol. Dis. 2022, 174, 105895. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Russo, E.; Elia, M. Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol. Res. 2016, 107, 85–92. [Google Scholar] [CrossRef]
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic efficacy of cannabidiol (CBD): A review of the evidence from clinical trials and human laboratory studies. Curr. Addict. Rep. 2020, 7, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sawwa, R.; Scutt, B.; Park, Y. Emerging use of Epidiolex (cannabidiol) in epilepsy. J. Pediatr. Pharmacol. Ther. 2020, 25, 485–499. [Google Scholar] [CrossRef]
- Epidyolex (Cannabidiol) An Overview of Epidyolex and Why It Is Authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/epidyolex-epar-medicine-overview_en.pdf (accessed on 12 October 2024).
- World Health Organization. Cannabidiol (CBD) pre-review report. In Proceedings of the Expert Committee on Drug Dependence, Thirty-Ninth Meeting, Geneva, Switzerland, 6–10 November 2017; pp. 6–10. [Google Scholar]
- Stella, B.; Baratta, F.; Della Pepa, C.; Arpicco, S.; Gastaldi, D.; Dosio, F. Cannabinoid formulations and delivery systems: Current and future options to treat pain. Drugs 2021, 81, 1513–1557. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A systematic review on the pharmacokinetics of cannabidiol in humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef]
- Lust, C.A.C.; Lin, X.; Rock, E.M.; Limebeer, C.L.; Parker, L.A.; Ma, D.W.L. Tissue distribution of major cannabinoids following intraperitoneal injection in male rats. PLoS ONE 2022, 17, e0262633. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Jensen, S.S.; Kolli, A.R.; Nikolajsen, G.N.; Bruun, H.Z.; Hoeng, J. Strategies to improve cannabidiol bioavailability and drug delivery. Pharmaceuticals 2024, 17, 244. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Donato, R.; Sacco, C.; Bilia, A.R. Promising nanocarriers to enhance solubility and bioavailability of cannabidiol for a plethora of therapeutic opportunities. Molecules 2022, 27, 6070. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards better delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Pande, S. Liposomes for drug delivery: Review of vesicular composition, factors affecting drug release and drug loading in liposomes. Art. Cells Nanomed. Biotechnol. 2023, 51, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Cipolla, D.; Wu, H.; Gonda, I.; Eastman, S.; Redelmeier, T.; Chan, H.-K. Tween-20 induces the structural remodeling of single lipid vesicles modifying the release properties of liposomes toward personalized medicine. J. Pharm. Sci. 2014, 103, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.K.; Zhang, C.X.; Zhang, W.T.; Zhao, C.H.; Guan, S.X. Characteristics and pharmacokinetics of docetaxel liposome containing Tween 80 or PEG-DSPE. J. Drug Deliv. Sci. Technol. 2008, 18, 253–257. [Google Scholar] [CrossRef]
- Tasi, L.-M.; Liu, D.-Z.; Chen, W.-Y. Microcalorimetric investigation of the interaction of polysorbate surfactants with unilamellar phosphatidylcholines liposomes, Colloids. Surf. A 2003, 213, 7–14. [Google Scholar] [CrossRef]
- Bnyan, R.; Khan, I.; Ehtezazi, T.; Saleem, I.; Gordon, S.; O’Neill, F.; Roberts, M. Surfactant effects on lipid-based vesicles properties. J. Pharm. Sci. 2018, 107, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Kronberg, B.; Dahlman, A.; Carlfors, J.; Karlsson, J.; Artursson, P. Preparation and evaluation of sterically stabilized liposomes: Colloidal stability, serum stability, macrophage uptake, and toxicity. J. Pharm. Sci. 1990, 79, 667–671. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Yu, B.; Browne, C.; Garnier, G. Reversible pH responsive bovine serum albumin hydrogel sponge nanolayer. Front. Bioeng. Biotechnol. 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. Physical basis of self-organization and function of membranes: Physics of vesicles. In Handbook of Biological Physics; Lipowsky, R., Sackmann, E., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 1, pp. 213–303. [Google Scholar]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids. 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Glavas-Dodov, M.; Fredro-Kumbaradzi, E.; Goracinova, K.; Calis, S.; Simonoska, M.; Hincal, A.A. 5-Fluorouracil in topical liposome gels for anticancer treatment--formulation and evaluation. Acta Pharm. 2003, 53, 241–250. [Google Scholar] [PubMed]
- Glavas-Dodov, M.; Fredro-Kumbaradzi, E.; Goracinova, K.; Simonoska, M.; Calis, S.; Trajkovic-Jolevska, S.; Hincal, A.A. The effects of lyophilization on the stability of liposomes containing 5-FU. Int. J. Pharm. 2005, 291, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kato, R.; Zeng, W.; Siramshetty, V.B.; Williams, J.; Kabir, M.; Hagen, N.; Padilha, E.C.; Wang, A.Q.; Mathé, E.A.; Xu, X.; et al. Development and validation of PAMPA-BBB QSAR model to predict brain penetration potential of novel drug candidates. Front. Pharmacol. 2023, 14, 1291246. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.; Romero, I.A.; Couraud, P.O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Lin, H.; Hu, B.; Wei, Y. A review on in vitro model of the blood-brain barrier (BBB) based on hCMEC/D3 cells. J. Control. Release 2023, 358, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Vree, T.B. Mass spectrometry of cannabinoids. J. Pharm. Sci. 1977, 66, 1444–1450. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Panseri, S.; Vezzalini, F.; Forni, F.; Vandelli, M.A.; Cannazza, G. Cannabinoid profiling of hemp seed oil by liquid chromatography coupled to high-resolution mass spectrometry. Front. Plant Sci. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.; Capizzi, M.; Di Cesare Mannelli, L.; Lucarini, E.; Bergonzi, M.C.; Ghelardini, C.; Bilia, A.R. Escinosomes: Safe and successful nanovesicles to deliver andrographolide by a subcutaneous route in a mice model of oxaliplatin-induced neuropathy. Pharmaceutics 2022, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, L.; Sacco, C.; Donato, R.; Tziakas, S.; Tomou, E.M.; Skaltsa, H.; Vanti, G.; Bergonzi, M.C.; Bilia, A.R. Environmentally friendly microemulsions of essential oils of Artemisia annua and Salvia fruticosa to protect crops against Fusarium verticillioides. Nanomaterials 2024, 14, 1715. [Google Scholar] [CrossRef]
- Askarizadeh, M.; Esfandiari, N.; Honarvar, B.; Sajadian, S.A.; Azdarpour, A. Kinetic modeling to explain the release of medicine from drug delivery systems. ChemBioEng Rev. 2023, 10, 1006–1049. [Google Scholar] [CrossRef]
- Radan, M.; Djikic, T.; Obradovic, D.; Nikolic, K. Application of in vitro PAMPA technique and in silico computational methods for blood-brain barrier permeability prediction of novel CNS drug candidates. Eur. J. Pharm. Sci. 2022, 168, 106056. [Google Scholar] [CrossRef] [PubMed]
- Gualbert, J.; Shahgaldian, P.; Coleman, A.W. Interactions of amphiphilic calix[4]arene-based Solid Lipid Nanoparticles with bovine serum albumin. Int. J. Pharm. 2003, 257, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Landucci, E.; Urru, M.; Chiarugi, A.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Bergonzi, M.C. Enhanced dissolution, permeation and oral bioavailability of aripiprazole mixed micelles: In vitro and In vivo evaluation. Int. J. Pharm. 2020, 583, 119361. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef] [PubMed]


| Tween 20 (mg/mL) | Time Sonication (min) | Time: 0 | Time: 2 Weeks | |||||
|---|---|---|---|---|---|---|---|---|
| Size (nm) | PdI | Z-Potential (mV) | Size (nm) | PdI | Z-Potential (mV) | |||
| L | 0 | 4 | 90.7 ± 0.2 | 0.295 ± 0.003 | −43.35 ± 2.25 | 96.28 ± 0.3 | 0.291 ± 0.003 | −28.35 ± 2.25 |
| VS | 10 | 4 | 67.7 ± 0.8 | 0.288 ± 0.008 | −32.13 ± 0.800 | 65.7 ± 1.0 | 0.263 ± 0.002 | −33.15 ± 0.700 |
| Tween 20 (mg/mL) | CBD (mg/mL) | Size (nm) | PdI | Z-Potential (mV) | R % | EE % | |
|---|---|---|---|---|---|---|---|
| L-CBD | - | 1 | 70.1 ± 2.3 | 0.181 ± 0.011 | −56.20 ± 7.561 | 96.89 ± 1.52 | 91.80 ± 0.76 |
| VS-CBD | 10 | 1 | 65.27 ± 1.27 | 0.230± 0.005 | −30.31 ± 0.54 | 99.89 ± 0.52 | 96.80 ± 0.96 |
| Kinetic Model | Function | R2 |
|---|---|---|
| After 8 h | ||
| Zero-order | y = 2.56x + 4.56 | 0.9052 |
| First-order | y = −0.01x + 1.98 | 0.9232 |
| Higuchi | y = 8.14x + −0.10 | 0.9815 |
| Korsmeyer–Peppas | y = −0.07x + 1.96 | 0.8465 |
| After 70 h | ||
| Zero-order | y = 1.37x + 9.80 | 0.9642 |
| First-order | y = −0.02x + 2.02 | 0.9331 |
| Higuchi | y = 11.92x + −5.41 | 0.9901 |
| Korsmeyer–Peppas | y = −0.44x + 2.05 | 0.8516 |
| Pe × 10−6 (cm/s) | R% | MR% | |
|---|---|---|---|
| Progesterone | 2.29 ±0.51 A | 87.47 ± 0.06 | 12.53 ± 0.06 |
| FREE-CBD | 1.90 ± 0.03 A,B | 72.82 ± 0.01 | 27.16 ± 0.01 |
| VS-CBD | 1.11 ± 0.01 B | 100.95 ± 0.02 | / |
| NaF | / | 100.31 ± 2.30 | / |
| Ratio (AMeOH/Abuffer) | Buffer in Solution (% v/v) | CBD (ng/mL) |
|---|---|---|
| 1.56 | 80 | 725 |
| 1.65 | 40 | 362.5 |
| 1.24 | 20 | 181.2 |
| 0.98 | 10 | 90.6 |
| Sample Analyzed | % MeOH | Correlation | Slope | Intercept |
|---|---|---|---|---|
| Time 0 | 100 | 0.9998 | 0.000131323 | 1.21 |
| Donor | 80 | 0.9998 | 0.000130587 | −3.12 |
| Lysate | 33 | 0.9997 | 0.000131504 | 0.93 |
| Acceptor | 0 | 0.9998 | 0.005018337 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grifoni, L.; Landucci, E.; Pieraccini, G.; Mazzantini, C.; Bergonzi, M.C.; Pellegrini-Giampietro, D.E.; Bilia, A.R. Development and Blood–Brain Barrier Penetration of Nanovesicles Loaded with Cannabidiol. Pharmaceuticals 2025, 18, 160. https://doi.org/10.3390/ph18020160
Grifoni L, Landucci E, Pieraccini G, Mazzantini C, Bergonzi MC, Pellegrini-Giampietro DE, Bilia AR. Development and Blood–Brain Barrier Penetration of Nanovesicles Loaded with Cannabidiol. Pharmaceuticals. 2025; 18(2):160. https://doi.org/10.3390/ph18020160
Chicago/Turabian StyleGrifoni, Lucia, Elisa Landucci, Giuseppe Pieraccini, Costanza Mazzantini, Maria Camilla Bergonzi, Domenico E. Pellegrini-Giampietro, and Anna Rita Bilia. 2025. "Development and Blood–Brain Barrier Penetration of Nanovesicles Loaded with Cannabidiol" Pharmaceuticals 18, no. 2: 160. https://doi.org/10.3390/ph18020160
APA StyleGrifoni, L., Landucci, E., Pieraccini, G., Mazzantini, C., Bergonzi, M. C., Pellegrini-Giampietro, D. E., & Bilia, A. R. (2025). Development and Blood–Brain Barrier Penetration of Nanovesicles Loaded with Cannabidiol. Pharmaceuticals, 18(2), 160. https://doi.org/10.3390/ph18020160









