Prolonged Impact of Bisphosphonates and Glucocorticoids on Bone Mechanical Properties
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Mechanical Bending Test
4.3. Tensile Strength Test
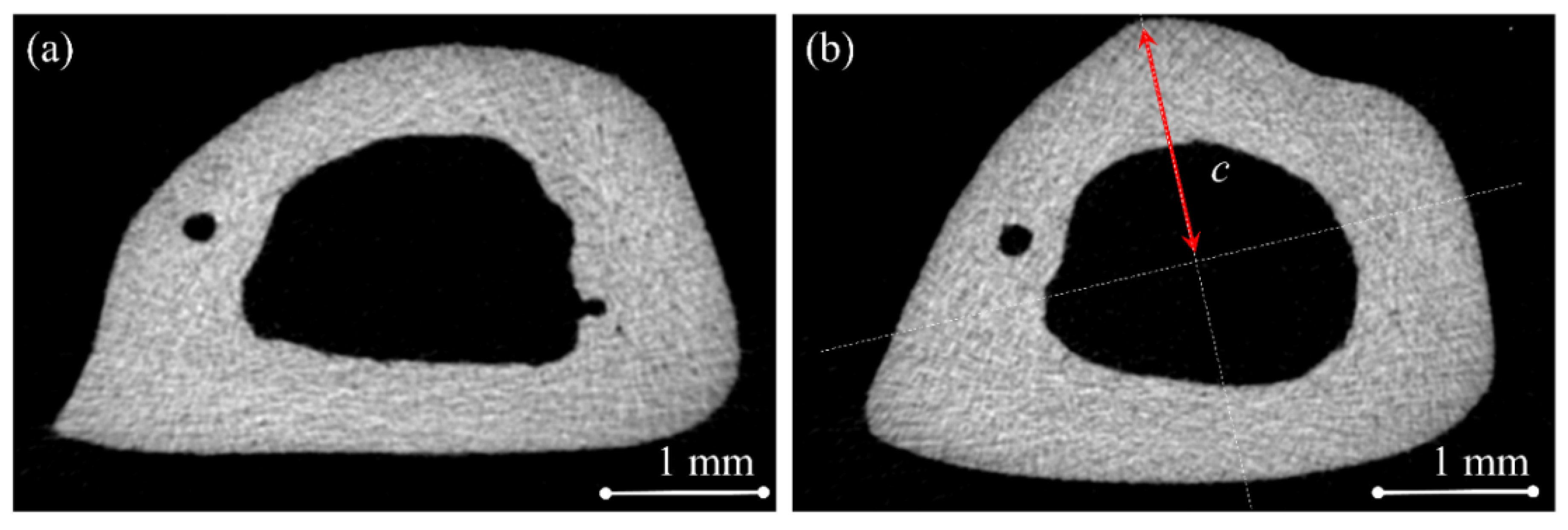
4.4. Micro-Computed Tomography (Micro-CT) Examination
4.5. Powder X-Ray Diffraction (PXRD)
4.6. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
4.7. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rizzoli, R.; Body, J.J.; Brandi, M.L.; Cannata-Andia, J.; Chappard, D.; El Maghraoui, A.; Glüer, C.C.; Kendler, D.; Napoli, N.; Papaioannou, A.; et al. Cancer-associated bone disease. Osteoporos. Int. 2013, 24, 2929–2953. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments. Curr. Osteoporos. Rep. 2019, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Green, J.R. Bisphosphonates in cancer therapy. Curr. Opin. Oncol. 2002, 14, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Geiger, E.J.; Eastell, R.; Vittinghoff, E.; Li, B.H.; Ryan, D.S.; Dell, R.M.; Adams, A.L. Atypical Femur Fracture Risk versus Fragility Fracture Prevention with Bisphosphonates. N. Engl. J. Med. 2020, 383, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Aldea, M.; Orillard, E.; Mansi, L.; Marabelle, A.; Scotte, F.; Lambotte, O.; Michot, J.M. How to manage patients with corticosteroids in oncology in the era of immunotherapy? Eur. J. Cancer 2020, 141, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, J.; Koeppen, V.; Aspenberg, P.; Michaelsson, K. Risk of atypical femoral fracture during and after bisphosphonate use. N. Engl. J. Med. 2014, 371, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Burr, D.B. Changes in bone matrix properties with aging. Bone 2019, 120, 85–93. [Google Scholar] [CrossRef]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2009, 21, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Gong, H.S.; Kim, T.H.; Park, S.Y.; Shin, J.H.; Cho, S.W.; Byun, D.W. Position Statement: Drug Holiday in Osteoporosis Treatment with Bisphosphonates in South Korea. J. Bone Metab. 2015, 22, 167–174. [Google Scholar] [CrossRef]
- McClung, M.R. Bisphosphonate therapy: How long is long enough? Osteoporos. Int. 2015, 26, 1455–1457. [Google Scholar] [CrossRef][Green Version]
- Strom, O.; Landfeldt, E.; Garellick, G. Residual effect after oral bisphosphonate treatment and healthy adherer effects—The Swedish Adherence Register Analysis (SARA). Osteoporos. Int. 2015, 26, 315–325. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Severcan, F.; Haris, P.I. Vibrational Spectroscopy in Diagnosis and Screening; IOS Press: Amsterdam, The Netherlands, 2012; Volume 6. [Google Scholar]
- Lebon, M.; Reiche, I.; Gallet, X.; Bellot-Gurlet, L.; Zazzo, A. Rapid quantification of bone collagen content by ATR-FTIR spectroscopy. Radiocarbon 2016, 58, 131–145. [Google Scholar] [CrossRef]
- Petibois, C.; Gouspillou, G.; Wehbe, K.; Delage, J.-P.; Déléris, G. Analysis of type I and IV collagens by FT-IR spectroscopy and imaging for a molecular investigation of skeletal muscle connective tissue. Anal. Bioanal. Chem. 2006, 386, 1961–1966. [Google Scholar] [CrossRef]
- Kourkoumelis, N.; Tzaphlidou, M. Spectroscopic assessment of normal cortical bone: Differences in relation to bone site and sex. Sci. World J. 2010, 10, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Miller, L.M.; Carlson, C.S.; Chance, M.R. In situ chemistry of osteoporosis revealed by synchrotron infrared microspectroscopy. Bone 2003, 33, 514–521. [Google Scholar] [CrossRef]
- Paschalis, E.; Verdelis, K.; Doty, S.; Boskey, A.; Mendelsohn, R.; Yamauchi, M. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef]
- Lozano, L.; Pena-Rico, M.; Heredia, A.; Ocotlan-Flores, J.; Gomez-Cortes, A.; Velazquez, R.; Belio, I.; Bucio, L. Thermal analysis study of human bone. J. Mater. Sci. 2003, 38, 4777–4782. [Google Scholar] [CrossRef]
- León-Mancilla, B.; Araiza-Téllez, M.; Flores-Flores, J.; Piña-Barba, M. Physico-chemical characterization of collagen scaffolds for tissue engineering. J. Appl. Res. Technol. 2016, 14, 77–85. [Google Scholar] [CrossRef]
- Shane, E.; Burr, D.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; Dempster, D.W.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2014, 29, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.J.; Montgomery, S.M.; Al Dabbagh, Z.; Jansson, K.A. National data of 6409 Swedish inpatients with femoral shaft fractures: Stable incidence between 1998 and 2004. Injury 2009, 40, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Lin, S.C.; Chang, F.L.; Hsieh, S.S.; Liu, S.H.; Yang, R.S. Effects of different exercise modes on mineralization, structure, and biomechanical properties of growing bone. J. Appl. Physiol. 2003, 95, 300–307. [Google Scholar] [CrossRef]
- Jonas, J.; Burns, J.; Abel, E.W.; Cresswell, M.J.; Strain, J.J.; Paterson, C.R. A technique for the tensile testing of demineralised bone. J. Biomech. 1993, 26, 271–273. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef]
- Munch, E.; Launey, M.E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Tough, bio-inspired hybrid materials. Science 2008, 322, 1516–1520. [Google Scholar] [CrossRef]
- Wegst, U.G.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Seton, M.P.; Saag, K.G. Should bisphosphonates be used for long-term treatment of glucocorticoid-induced osteoporosis? Arthritis Rheumatol. 2011, 63, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Ferrari, S.; Russell, R.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Essex, A.L.; Pin, F.; Huot, J.R.; Bonewald, L.F.; Plotkin, L.I.; Bonetto, A. Bisphosphonate Treatment Ameliorates Chemotherapy-Induced Bone and Muscle Abnormalities in Young Mice. Front. Endocrinol. 2019, 10, 809. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Bertoldo, F.; Valenti, M.T.; Zenari, S.; Zanatta, M.; Sella, S.; Giannini, S.; Cascio, V.L. Histomorphometric analysis of glucocorticoid-induced osteoporosis. Micron 2005, 36, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kaibuchi, N.; Iwata, T.; Onizuka, S.; Yano, K.; Tsumanuma, Y.; Yamato, M.; Okano, T.; Ando, T. Allogeneic multipotent mesenchymal stromal cell sheet transplantation promotes healthy healing of wounds caused by zoledronate and dexamethasone in canine mandibular bones. Regen. Ther. 2019, 10, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Watkins, B.A.; Lyng, G.D.; Lerman, M.A.; Anderson, K.C. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol. 2009, 45, 164–172. [Google Scholar] [CrossRef]
- Jabbour, Z.; El-Hakim, M.; Henderson, J.E.; de Albuquerque Junior, R.F. Bisphosphonates inhibit bone remodeling in the jaw bones of rats and delay healing following tooth extractions. Oral Oncol. 2014, 50, 485–490. [Google Scholar] [CrossRef]
- Tarantino, U.; Cerocchi, I.; Celi, M.; Scialdoni, A.; Saturnino, L.; Gasbarra, E. Pharmacological agents and bone healing. Clin. Cases Miner. Bone Metab. 2009, 6, 144–148. [Google Scholar]
- Turner, C.H.; Burr, D.B. Basic biomechanical measurements of bone: A tutorial. Bone 1993, 14, 595–608. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelieres, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Bowman, S.M.; Zeind, J.; Gibson, L.J.; Hayes, W.C.; McMahon, T.A. The tensile behavior of demineralized bovine cortical bone. J. Biomech. 1996, 29, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
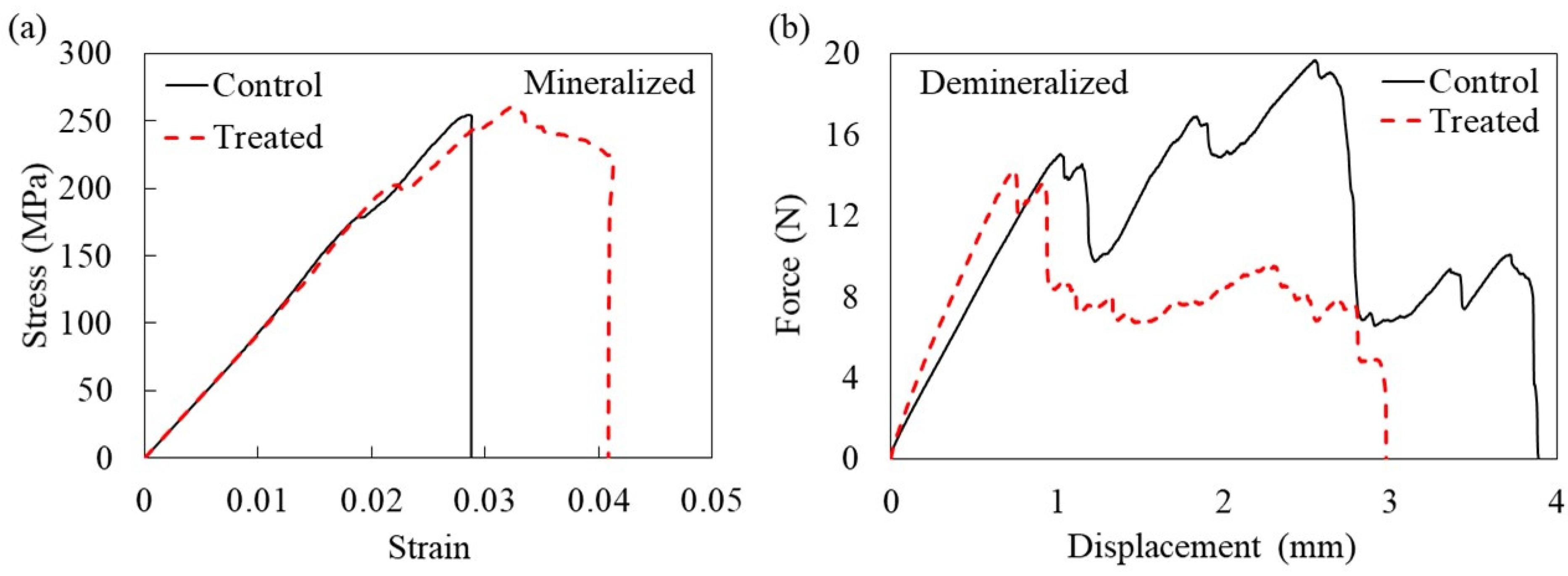
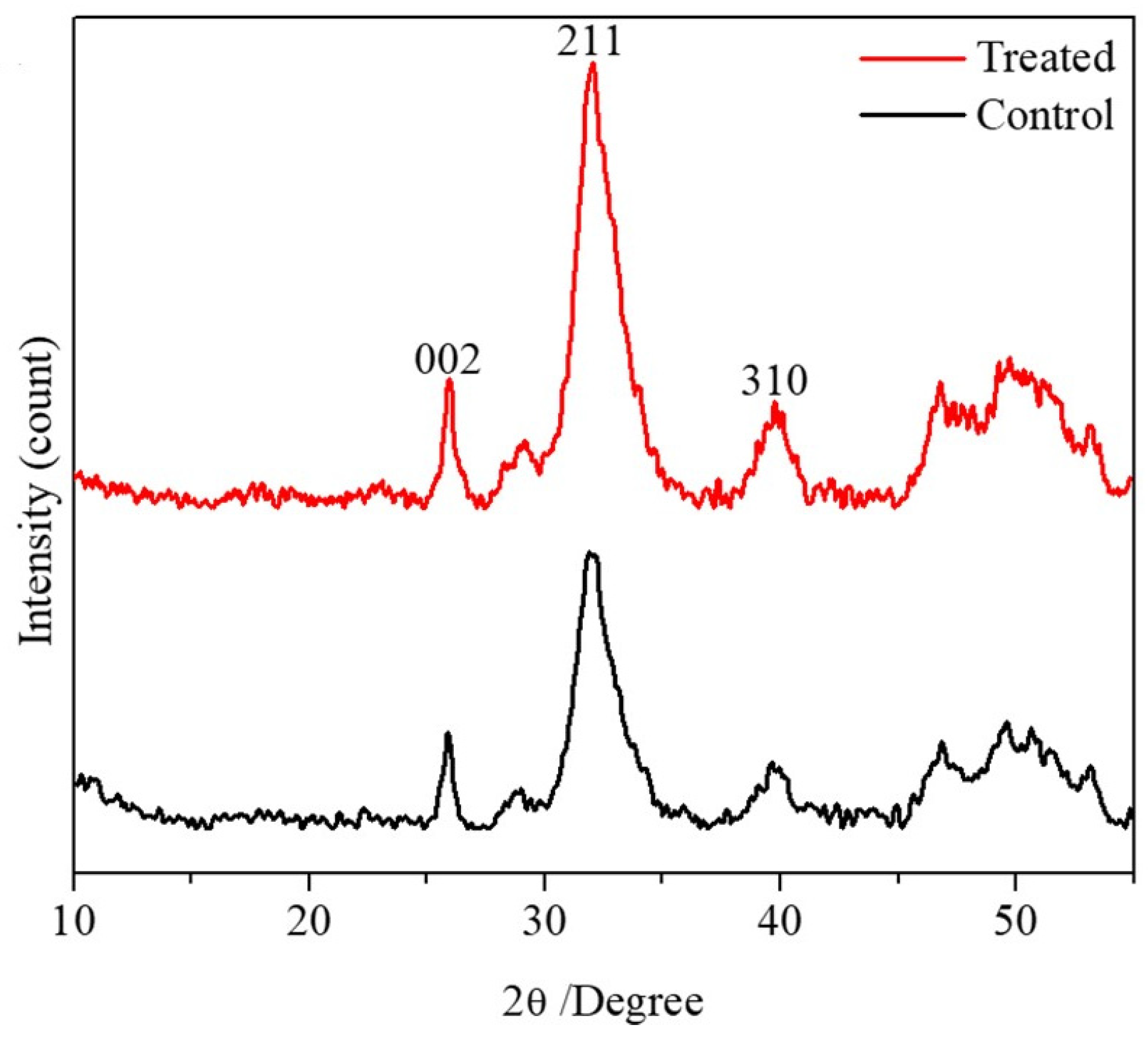
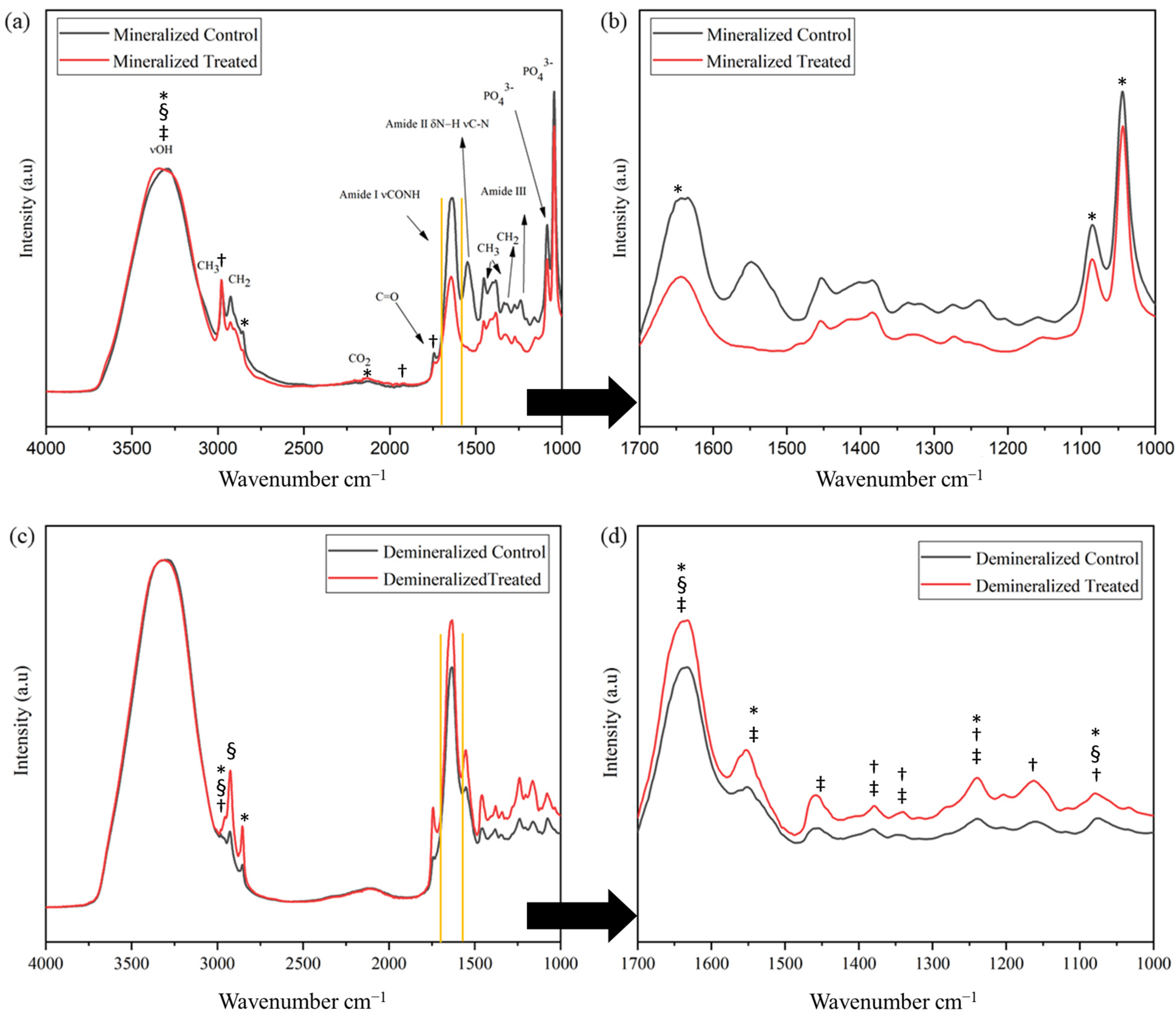
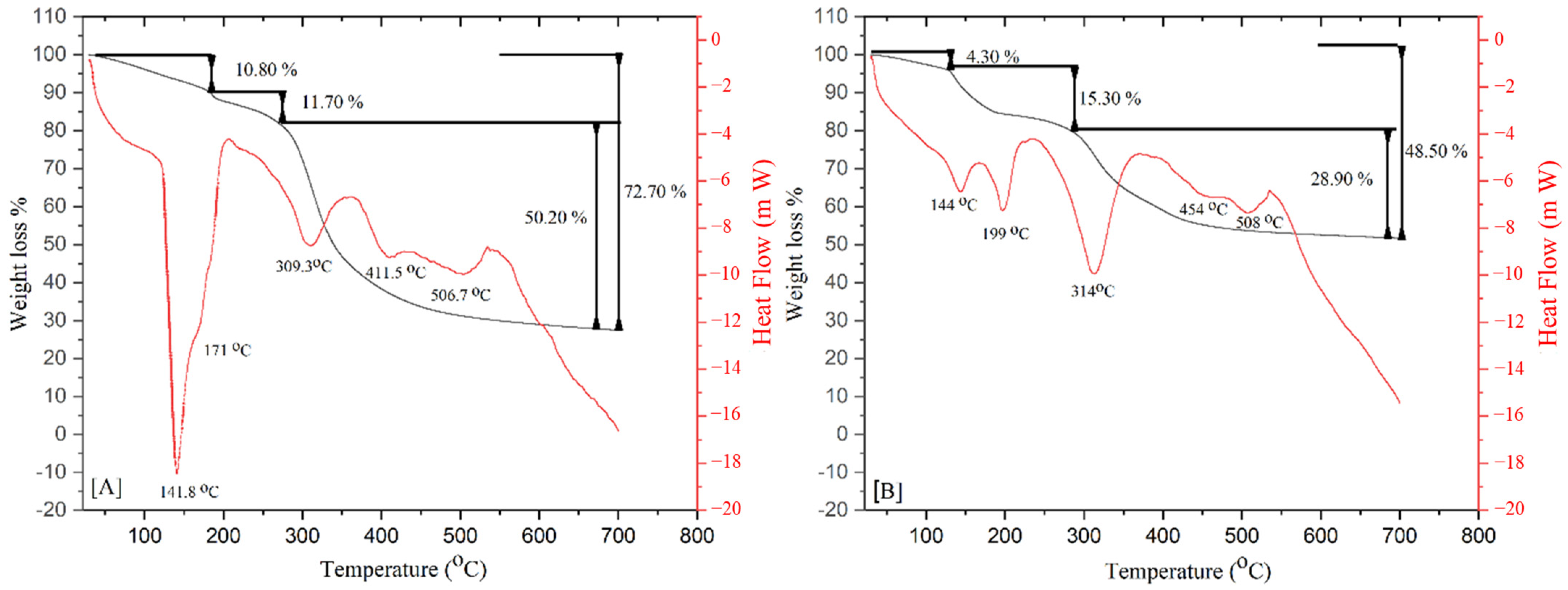
| Property Mineralized Samples | Control (n = 5) | Treatment (n = 8) | p-Value |
|---|---|---|---|
| Bone length (mm) | 49.73 ± 2.7 | 49.26 ± 1.76 | 0.284 |
| Max chord length from centroidal axis (mm) | 1.64 ± 0.1 | 1.63 ± 0.1 | 0.943 |
| Cross-sectional area of cortical bone (mm2) | 6.37 ± 0.4 | 6.94 ± 0.67 | 0.065 |
| Average cortical thickness (mm) | 0.78 ± 0.06 * | 0.88 ± 0.04 * | 0.006 |
| 2nd moment of area (mm4) | 4.51 ± 0.75 | 4.85 ± 1.01 | 0.512 |
| Max force (N) | 137 ± 11.6 * | 157 ± 11.5 * | 0.030 |
| Stiffness (N mm−1) | 278 ± 107 | 290 ± 20 | 0.354 |
| Flexural strength (MPa) | 250.8 ± 9 | 269 ± 25 | 0.152 |
| Elastic modulus (GPa) | 10.1 ± 2.5 | 10.3 ± 2.2 | 0.943 |
| Flexural strain | 0.032 ± 0.01 | 0.037 ± 0.01 | 0.354 |
| Energy to ultimate load (toughness) (MPa) | 4.7 ± 1.6 * | 8.1 ± 1.8 * | 0.003 |
| Property Demineralized Samples | Control (n = 9) | Treatment (n = 8) | p-Value |
| Max force (N) | 19.48 ± 2.96 | 17.17 ± 3.6 | 0.167 |
| Tensile strength (MPa) | 3.4 ± 0.28 * | 2.47 ± 0.44 * | 0.001 |
| Stiffness (N mm−1) | 16.3 ± 4.1 | 13.8 ± 5.4 | 0.339 |
| Strain at failure | 0.40 ± 0.14 | 0.27 ± 0.1 | 0.057 |
| Crystal Size of Mineralized Samples | Control (n = 10) | Treatment (n = 10) | p-Value |
|---|---|---|---|
| Crystal size 002 plane (Å) | 186.5 ± 30.6 | 195.1 ± 24 | 0.6 |
| Crystal size 211 plane (Å) | 50.9 ± 1.5 * | 53.6 ± 3.4 * | 0.037 |
| Crystal size 310 plane (Å) | 85.6 ± 16.5 | 102.3 ± 17.5 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, A.; Jabbour, Z.; Alsheghri, A.; Elhadad, A.; Berridi, K.R.; Moussa, H.; Ramirez-Garcialuna, J.L.; Tamimi, I.; Santos dos Santos, S.; Henderson, J.; et al. Prolonged Impact of Bisphosphonates and Glucocorticoids on Bone Mechanical Properties. Pharmaceuticals 2025, 18, 164. https://doi.org/10.3390/ph18020164
Mansour A, Jabbour Z, Alsheghri A, Elhadad A, Berridi KR, Moussa H, Ramirez-Garcialuna JL, Tamimi I, Santos dos Santos S, Henderson J, et al. Prolonged Impact of Bisphosphonates and Glucocorticoids on Bone Mechanical Properties. Pharmaceuticals. 2025; 18(2):164. https://doi.org/10.3390/ph18020164
Chicago/Turabian StyleMansour, Alaa, Zaher Jabbour, Ammar Alsheghri, Amir Elhadad, Karla R. Berridi, Hanan Moussa, Jose Luis Ramirez-Garcialuna, Iskandar Tamimi, Sailer Santos dos Santos, Janet Henderson, and et al. 2025. "Prolonged Impact of Bisphosphonates and Glucocorticoids on Bone Mechanical Properties" Pharmaceuticals 18, no. 2: 164. https://doi.org/10.3390/ph18020164
APA StyleMansour, A., Jabbour, Z., Alsheghri, A., Elhadad, A., Berridi, K. R., Moussa, H., Ramirez-Garcialuna, J. L., Tamimi, I., Santos dos Santos, S., Henderson, J., Song, J., & Tamimi, F. (2025). Prolonged Impact of Bisphosphonates and Glucocorticoids on Bone Mechanical Properties. Pharmaceuticals, 18(2), 164. https://doi.org/10.3390/ph18020164









