The Anti-Inflammatory Potential of Tricyclic Antidepressants (TCAs): A Novel Therapeutic Approach to Atherosclerosis Pathophysiology
Abstract
1. Introduction
1.1. Strategy Search
- Studies that investigated the effects of tricyclic antidepressants (TCAs) on inflammatory pathways, particularly in the context of cardiovascular diseases, including atherosclerosis.
- Articles published in peer-reviewed journals.
- Studies involving human subjects or animal models.
- Research studies that provided primary data or detailed analyses of molecular mechanisms. Although some observational and small-scale human studies exist, robust RCTs that assess the therapeutic potential of TCAs in cardiovascular diseases remain scarce or unavailable.
- Exclusion criteria were as follows:
- Studies that focused on antidepressants other than TCAs.
- Articles not available in full text or lacking methodological details.
- Research not related to inflammation or cardiovascular disease.
1.2. Limitations of the Study
2. Mechanisms of Action
2.1. Reuptake Inhibition
2.2. Pharmacological Effects
2.3. Modulation of Pain Pathways
2.4. Impact on Neurotransmitter Systems
3. Inflammatory Pathways
- Patients with atherosclerosis and comorbid depression: Atherosclerosis, a condition marked by chronic inflammation, sees cytokines such as IL-6 and TNF-α playing critical roles in disease progression. TCAs, which influence these cytokines and reduce NF-κB activity, could be assessed as adjunctive treatments for patients suffering from both atherosclerosis and depression. Clinical trials in this setting could measure inflammatory biomarkers, endothelial function, and cardiovascular events, evaluating the combined effects of mood improvement and inflammation reduction.
- Chronic inflammatory diseases with psychiatric comorbidities: Diseases like rheumatoid arthritis, inflammatory bowel disease, and psoriasis are characterized by systemic inflammation, which contributes to disease severity. These conditions are frequently accompanied by higher rates of depression and anxiety, which in turn exacerbate inflammatory responses. TCAs could be explored in these populations to assess their ability to reduce inflammation while simultaneously improving mental health. Clinical endpoints could include cytokine profiles, disease activity scores, and quality of life measures.
- Post-acute coronary syndrome recovery: Inflammation following acute coronary syndromes, such as myocardial infarction, significantly contributes to adverse cardiovascular outcomes. TCAs may be examined as part of secondary prevention strategies for patients with elevated inflammatory markers, especially those with coexisting depressive symptoms. This clinical setting could focus on the impact of TCAs on inflammatory resolution, recurrence of cardiac events, and psychological well-being.
- Chronic pain syndromes with inflammatory components: Conditions such as fibromyalgia and neuropathic pain often involve low-grade inflammation and increased cytokine activity. Given the established role of TCAs in managing chronic pain, clinical trials could investigate whether their anti-inflammatory effects improve their efficacy in reducing pain severity and enhancing functionality.
- Cancer-related fatigue and inflammation: Cancer-related fatigue, a multifaceted condition, is often linked to systemic inflammation and cytokine dysregulation. In oncology, TCAs could be evaluated for their dual ability to alleviate depressive symptoms while mitigating inflammation. This approach could offer potential benefits for patients’ quality of life and treatment tolerance.
- Patients with metabolic syndrome or obesity: Metabolic syndrome is associated with chronic low-grade inflammation, which heightens the risk of cardiovascular disease. TCAs might be explored as adjunctive therapies in these patients, focusing on their potential to reduce inflammatory markers like IL-6 and improve endothelial function, alongside their effects on depressive symptoms [17,18,19,20].
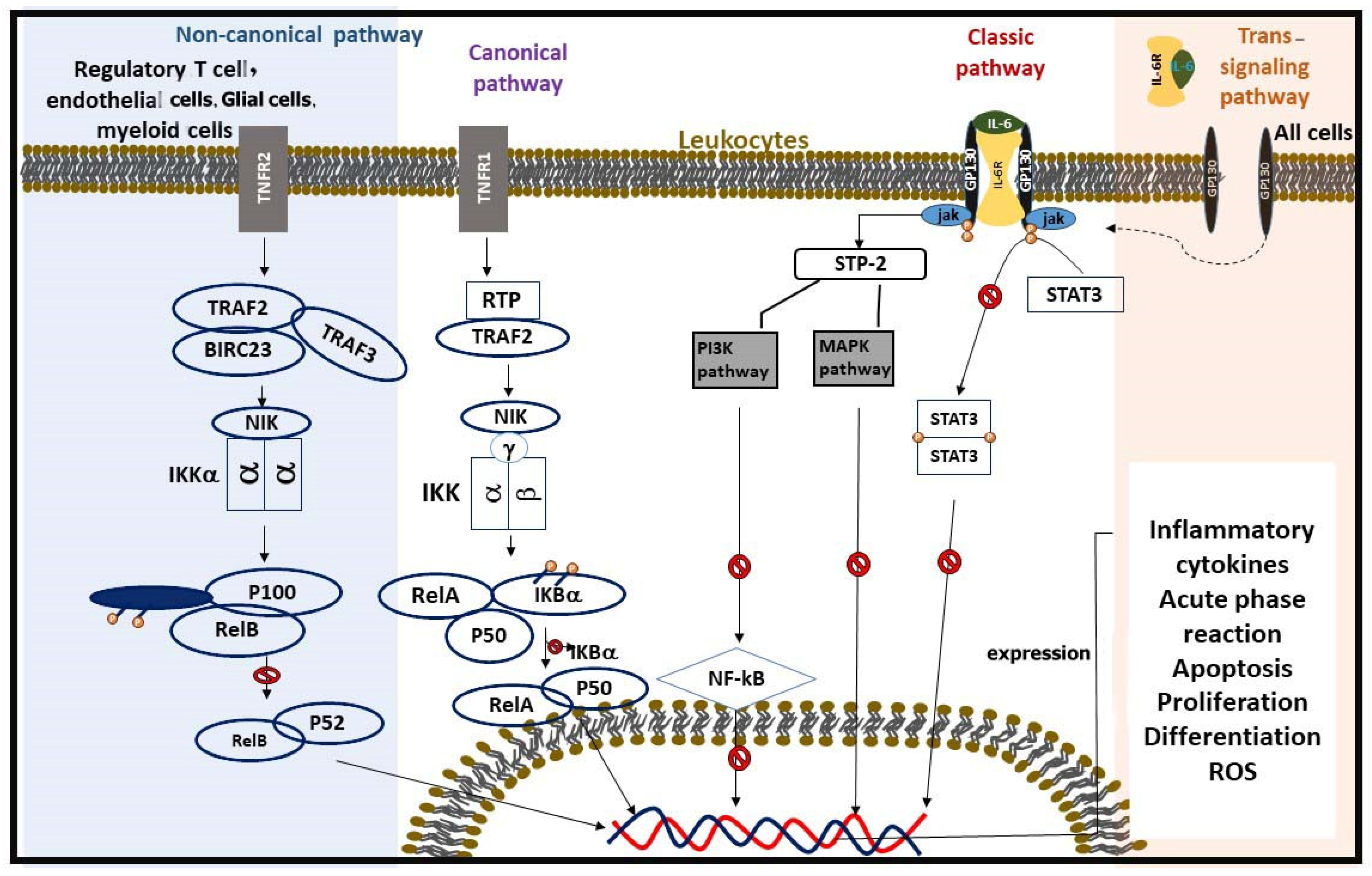
3.1. IL-6 Pathways, Effects, and the Role of TCAs
3.2. TNF-α in Atherosclerosis and the Modulatory Role of TCAs
3.3. NF-κB Pathway and the Effects of TCAs
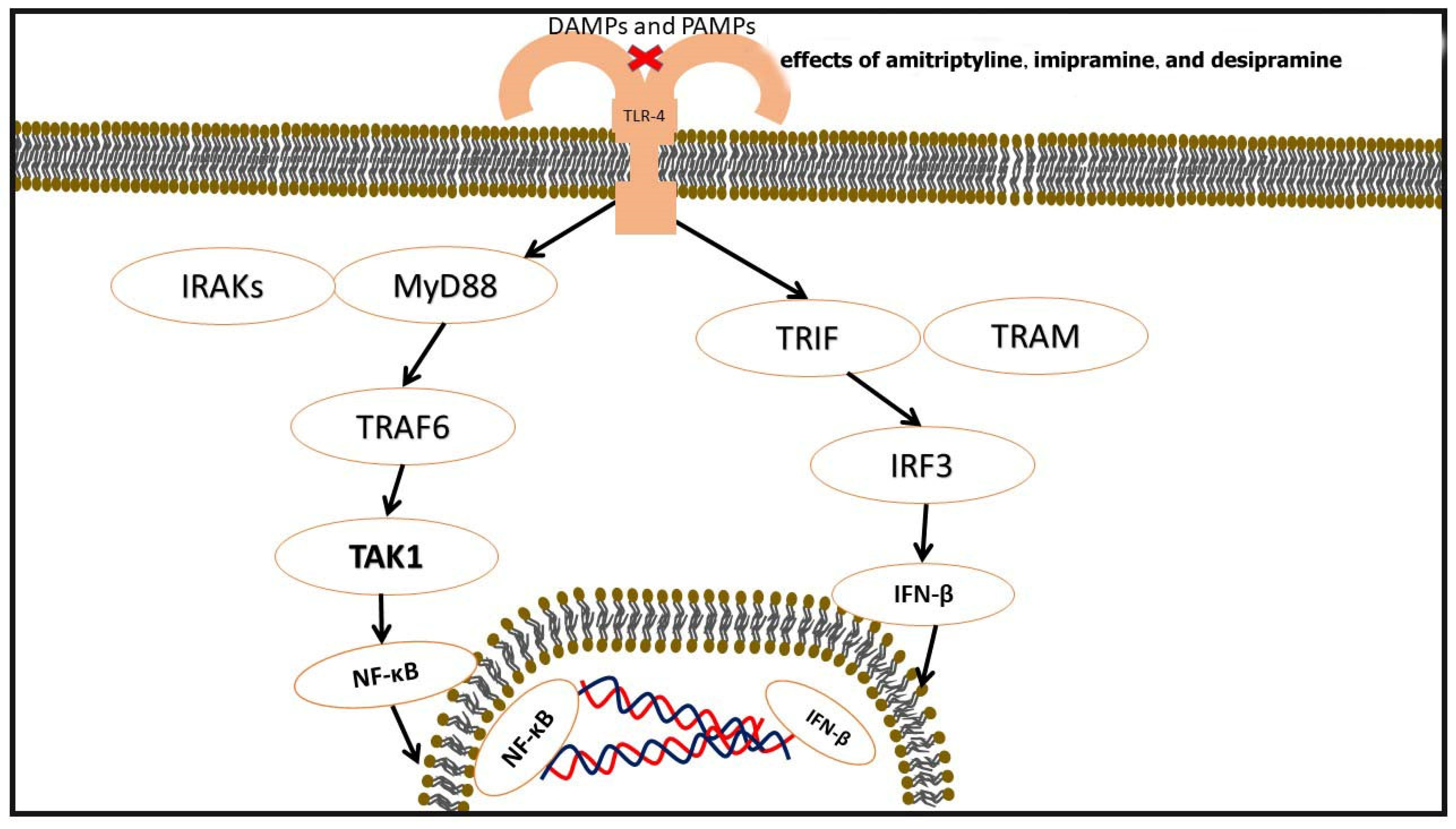
3.4. Toll-like Receptors (TLRs) in Atherosclerosis and Modulation by TCAs
Signaling Pathways Mediated by TLRs
| Category | Details | Ref. |
|---|---|---|
| TLR Pathways | MyD88-Dependent Pathway: Involves TLRs 1, 2, 4, 5, 6, 7, 8, 9. Activates IRAK4, IRAK1, and TRAF6. TRIF-Dependent Pathway: Involves TLRs 3 and 4. Activates TBK1 and IRF3 for IFN-β synthesis. Converts TRAF6 to NF-κB. | [65] |
| Impact on Atherosclerosis | TLR activation in endothelial cells increases NF-κB, ROS, IL-8, VCAM, and P-selectin. In platelets, ↑ CD40L, ↑ CCL5, ↑ ERK1/2, and ↑ NF-κB. In monocytes, macrophages, and foam cells, it upregulates inflammatory cytokines and adhesion molecules. | [61] |
| Effects of TCAs on TLRs | Imipramine decreases TLR-4 gene and protein expression in non-diabetic rats. Amitriptyline, imipramine, and desipramine inhibit TLR-2 and TLR-4 in a dose-dependent manner. Order of potency: amitriptyline > imipramine > desipramine. | [66] |
| Therapeutic Implications | TCAs modulate TLR activity, reducing inflammatory responses. Potential application in treating inflammation-related cardiovascular diseases, such as atherosclerosis. | [61] |
3.5. Adiponectin in Atherosclerosis and Effects of TCAs
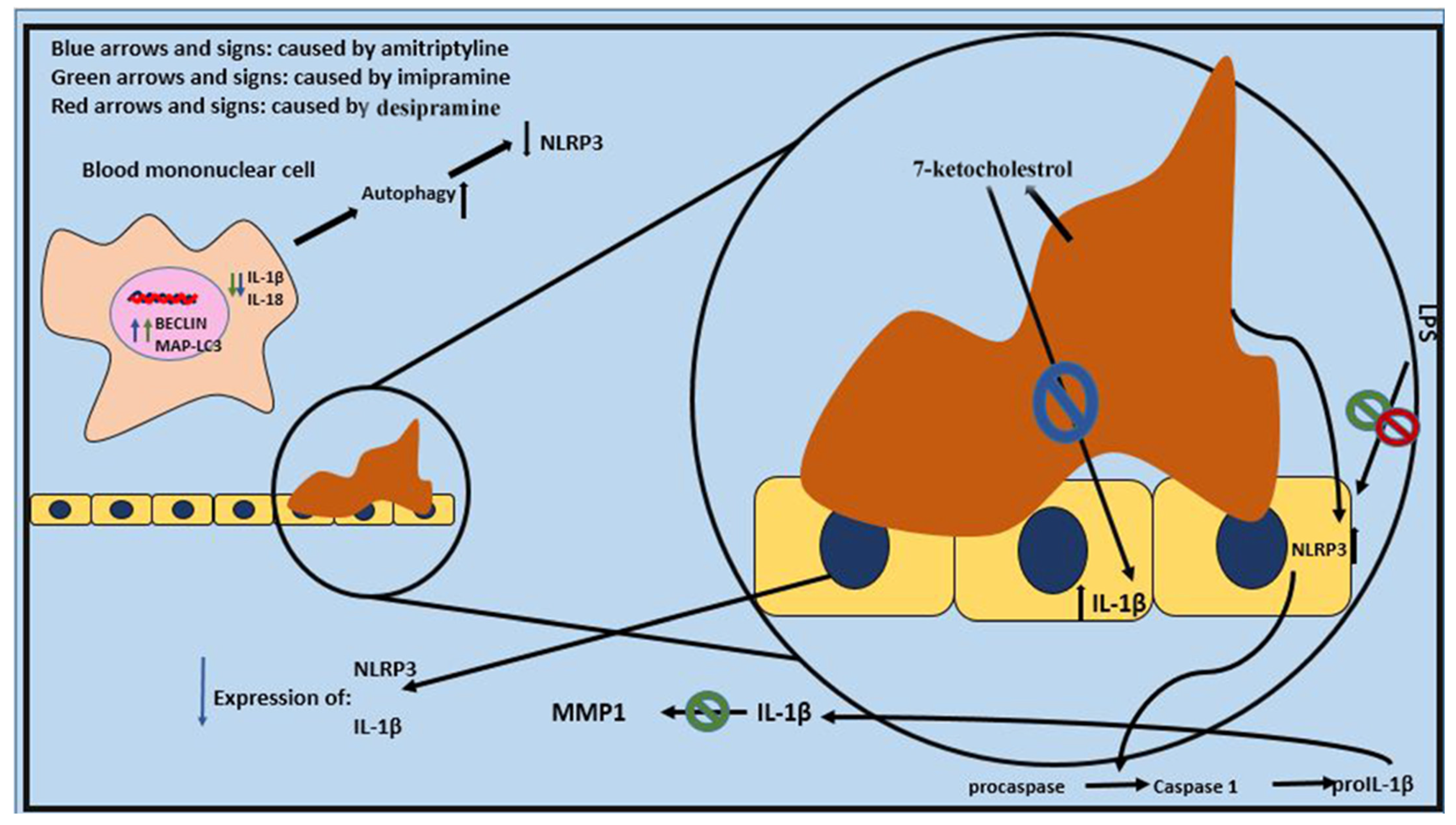
3.6. NLRP3 Inflammasome and the Effects of TCAs
4. Comprehensive Comparison of Contradictory Results in the Literature and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAMPs | damage-associated molecular patterns |
| GABA | γ-Aminobutyric acid |
| ICAM-1 | intercellular adhesion molecule-1 |
| IκBα | inhibitor of nuclear factor kappa B |
| JAK-STAT | Janus kinase–signal transducer and activator of transcription |
| MAPK | mitogen-activated protein kinase |
| MMPs | matrix metalloproteinases |
| NET | norepinephrine transporter |
| NIK | NF-κB-inducing kinase |
| NF-κB | nuclear factor kappa B |
| NLRP3 | NLR family pyrin domain-containing protein 3 |
| NMDA | N-methyl-D-aspartate |
| NOD | nucleotide-binding oligomerization domain |
| PAMPs | pathogen-associated molecular patterns |
| PI3K | phosphoinositide 3-kinase |
| SERT | serotonin transporter |
| STAT | signal transducer and activator of transcription |
| TBK1 | TANK-binding kinase-1 |
| TCA | tricyclic antidepressants |
| TLR | Toll-like receptors |
| TNFR1 | tumor necrosis factor receptor-1 |
| TRADD | receptor-associated death domain protein |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kolesova, E.P.; Maslyanskiy, A.L.; Rotar, O.P.; Konradi, A.O.; Mazurov, V.I. New approaches to pathogenetic anti-inflammatory therapy of cardiovascular diseases. HERALD North-West. State Med. Univ. Named After II Mechnikov 2023, 15, 19–32. [Google Scholar] [CrossRef]
- Rangarajan, S.; Orujyan, D.; Rangchaikul, P.; Radwan, M.M. Critical Role of Inflammation and Specialized Pro-Resolving Mediators in the Pathogenesis of Atherosclerosis. Biomedicines 2022, 10, 2829. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, A.J.; Clarke, J.-A.; Lu, S. Antidepressants in the elderly. Can. Fam. Physician 2019, 65, 340. [Google Scholar] [PubMed]
- Ferjan, I.; Lipnik-Štangelj, M. Chronic pain treatment: The influence of tricyclic antidepressants on serotonin release and uptake in mast cells. Mediat. Inflamm. 2013, 2013, 340473. [Google Scholar] [CrossRef]
- Xia, Z.; Depierre, J.W.; Nässberger, L. Tricyclic antidepressants inhibit IL-6, IL-1β and TNF-α release in human blood monocytes and IL-2 and interferon-γ in T cells. Immunopharmacology 1996, 34, 27–37. [Google Scholar] [CrossRef]
- Mu, W.; Xu, G.; Wang, Z.; Li, Q.; Sun, S.; Qin, Q.; Li, Z.; Shi, W.; Dai, W.; Zhan, X.; et al. Tricyclic antidepressants induce liver inflammation by targeting NLRP3 inflammasome activation. Cell Commun. Signal 2023, 21, 123. [Google Scholar] [CrossRef]
- Bao, M.-H.; Li, G.-Y.; Huang, X.-S.; Tang, L.; Dong, L.-P.; Li, J.-M. Long noncoding RNA LINC00657 acting as a miR-590-3p sponge to facilitate low concentration oxidized low-density lipoprotein–induced angiogenesis. Mol. Pharmacol. 2018, 93, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.M.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Chakal, D.A.; Breshenkov, D.G.; Charchyan, E.R. The role of cytokines in cholesterol accumulation in cells and atherosclerosis progression. Int. J. Mol. Sci. 2023, 24, 6426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B. Antidepressants: Mechanism of Action, Toxicity and Possible Amelioration. Medicine 2021, 3, 437–448. [Google Scholar] [CrossRef]
- Lawson, K. Tricyclic antidepressants and fibromyalgia: What is the mechanism of action? Expert. Opin. Investig. Drugs 2002, 11, 1437–1445. [Google Scholar] [CrossRef]
- Budziñski, M.L.; Sokn, C.; Gobbini, R.; Ugo, B.; Antunica-Noguerol, M.; Senin, S.; Bajaj, T.; Gassen, N.C.; Rein, T.; Schmidt, M.V.; et al. Tricyclic antidepressants target FKBP51 SUMOylation to restore glucocorticoid receptor activity. Mol. Psychiatry 2022, 27, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Xu, Z.; Yang, G.; Jia, Q.; Mo, F.; Jing, L.; Luo, J.; Jin, H.; Cai, X. Microelectrode Arrays for Detection of Neural Activity in Depressed Rats: Enhanced Theta Activity in the Basolateral Amygdala. Cyborg Bionic Syst. 2024, 5, 0125. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide attenuates inflammatory response in membranous glomerulo-nephritis rat via downregulation of NF-κB signaling pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903. [Google Scholar] [CrossRef] [PubMed]
- Martorana, F.; Guidotti, G.; Brambilla, L.; Rossi, D. Withaferin A Inhibits Nuclear Factor-κB-Dependent Pro-Inflammatory and Stress Response Pathways in the Astrocytes. Neural Plast. 2015, 2015, 381964. [Google Scholar] [CrossRef]
- Guo, J.; Garshick, E.; Si, F.; Tang, Z.; Lian, X.; Wang, Y.; Li, J.; Koutrakis, P. Environmental toxicant exposure and depressive symptoms. JAMA Netw. Open 2024, 7, e2420259. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.A.; Monemi, M.; Asli, S.; Mohammadi, S.; Foroozanmehr, B.; Haghmorad, D.; Oksenych, V.; Eslami, M. Using new technologies to analyze gut microbiota and predict cancer risk. Cells 2024, 13, 1987. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.; Ramji, D.P. Cytokines: Roles in atherosclerosis disease progression and potential therapeutic targets. Future Med. Chem. 2016, 8, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Silverio, A.; Cancro, F.P.; Esposito, L.; Bellino, M.; D’Elia, D.; Verdoia, M.; Vassallo, M.G.; Ciccarelli, M.; Vecchione, C.; Galasso, G.; et al. Secondary Cardiovascular Prevention after Acute Coronary Syndrome: Emerging Risk Factors and Novel Therapeutic Targets. J. Clin. Med. 2023, 12, 2161. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Oikonomou, E.; Economou, E.K.; Crea, F.; Kaski, J.C. Inflammatory cytokines in atherosclerosis: Current therapeutic approaches. Eur. Heart J. 2016, 37, 1723–1732. [Google Scholar] [CrossRef]
- Popa-Fotea, N.-M.; Ferdoschi, C.-E.; Micheu, M.-M. Molecular and cellular mechanisms of inflammation in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1200341. [Google Scholar] [CrossRef]
- Fontes, J.A.; Rose, N.R.; Čiháková, D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Liao, J.; Duan, Y.; Liu, Y.; Chen, H.; An, Z.; Chen, Y.; Su, Z.; Usman, A.M.; Xiao, G. Simvastatin alleviates glymphatic system damage via the VEGF-C/VEGFR3/PI3K-Akt pathway after experimental intracerebral hemorrhage. Brain Res. Bull. 2024, 216, 111045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, J.; Sun, X.; Yang, K.; Yang, L.; Kong, L.; Zhang, B.; Li, F.; Li, C.; Shi, B. Loss of m6A demethylase ALKBH5 promotes post-ischemic angiogenesis via post-transcriptional stabilization of WNT5A. Clin. Transl. Med. 2021, 11, e402. [Google Scholar] [CrossRef]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guan, J.; Xiong, J.; Wang, F. Effects of transcranial magnetic stimulation combined with sertraline on cognitive level, inflammatory response and neurological function in depressive disorder patients with non-suicidal self-injury behavior. Actas Esp. Psiquiatr. 2024, 52, 28. [Google Scholar] [PubMed]
- Roumestan, C.; Michel, A.; Bichon, F.; Portet, K.; Detoc, M.; Henriquet, C.; Jaffuel, D.; Mathieu, M. Anti-inflammatory properties of desipramine and fluoxetine. Respir. Res. 2007, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Gennarelli, M.; Uher, R.; Breen, G.; Farmer, A.; Aitchison, K.J.; Craig, I.W.; Anacker, C.; Zunsztain, P.A.; McGuffin, P.; et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: Differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology 2013, 38, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kubera, M.; Kenis, G.; Bosmans, E.; Kajta, M.; Basta-Kaim, A.; Scharpe, S.; Budziszewska, B.; Maes, M. Stimulatory effect of antidepressants on the production of IL-6. Int. Immunopharmacol. 2004, 4, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Martino, M.; Battaglia, F.; Colicchio, S.; Perugi, G. Increase in IL-6 levels among major depressive disorder patients after a 6-week treatment with duloxetine 60 mg/day: A preliminary observation. Neuropsychiatr. Dis. Treat. 2011, 7, 51–56. [Google Scholar] [CrossRef]
- Curzytek, K.; Maes, M.; Kubera, M. Immune-Regulatory and Molecular Effects of Antidepressants on the Inflamed Human Keratinocyte HaCaT Cell Line. Neurotox. Res. 2021, 39, 1211–1226. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Katkenov, N.; Mukhatayev, Z.; Kozhakhmetov, S.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. Systematic Review on the Role of IL-6 and IL-1β in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2024, 11, 206. [Google Scholar] [CrossRef]
- Al-Samhari, M.M.; Al-Rasheed, N.M.; Al-Rejaie, S.; Al-Rasheed, N.M.; Hasan, I.H.; Mahmoud, A.M.; Dzimiri, N. Possible involvement of the JAK/STAT signaling pathway in N-acetylcysteine-mediated antidepressant-like effects. Exp. Biol. Med. 2016, 241, 509–518. [Google Scholar] [CrossRef]
- Di Bartolo, B.; Chan, J.; Bennett, M.; Cartland, S.; Bao, S.; Tuch, B.; Kavurma, M. TNF-related apoptosis-inducing ligand (TRAIL) protects against diabetes and atherosclerosis in Apoe−/− mice. Diabetologia 2011, 54, 3157–3167. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.T.; Beckmann, N.; Winer, L.K.; Kim, Y.; Goetzman, H.S.; Veile, R.E.; Gulbins, E.; Goodman, M.D.; Nomellini, V.; Caldwell, C.C. Amitriptyline treatment mitigates sepsis-induced tumor necrosis factor expression and coagulopathy. Shock 2019, 51, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Pan, L.; Qian, M.; Sun, W.; Gu, C.; Chen, L.; Tang, X.; Hu, Y.; Xu, L.; Wei, Y.; et al. Tumor Necrosis Factor-α Variations in Patients With Major Depressive Disorder Before and After Antidepressant Treatment. Front. Psychiatry 2020, 11, 518837. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, J.; Pang, L.; Chen, C.; Ye, J.; Liu, H.; Chen, H.; Zhang, S.; Liu, S.; Liu, B. The acid sphingomyelinase inhibitor amitriptyline ameliorates TNF-α-induced endothelial dysfunction. Cardiovasc. Drugs Ther. 2024, 38, 43–56. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Plemmenos, G.; Evangeliou, E.; Polizogopoulos, N.; Chalazias, A.; Deligianni, M.; Piperi, C. Central regulatory role of cytokines in periodontitis and targeting options. Current medicinal chemistry 2021, 28, 3032–3058. [Google Scholar] [CrossRef]
- Verhelst, K.; Carpentier, I.; Beyaert, R. Regulation of TNF-induced NF-κB activation by different cytoplasmic ubiquitination events. Cytokine Growth Factor. Rev. 2011, 22, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shao, C.; Zhou, H.; Yu, L.; Bao, Y.; Mao, Q.; Yang, J.; Wan, H. Salvianolic acid B inhibits atherosclerosis and TNF-α-induced inflammation by regulating NF-κB/NLRP3 signaling pathway. Phytomedicine 2023, 119, 155002. [Google Scholar] [CrossRef]
- de Winther, M.P.J.; Kanters, E.; Kraal, G.; Hofker, M.H. Nuclear Factor κB Signaling in Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 904–914. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell Biol. 2005, 7, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Brand, K.; Page, S.; Walli, A.K.; Neumeier, D.; Baeuerle, P. Role of nuclear factor-B in atherogenesis. Exp. Physiol. 1997, 82, 297–304. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Wang, A.; Wu, B.; Cheng, Z.; Jiang, Y.; Gu, H.; Ding, L.; Mo, J.; Jiang, Y. Hemodynamic Impairment of Blood Pressure and Stroke Mechanisms in Symptomatic Intracranial Atherosclerotic Stenosis. Stroke 2024, 55, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Su, Y.H.; Hsu, W.H.; Wang, C.C.; Arbiser, J.L.; Yang, M.H. Imipramine blue halts head and neck cancer invasion through promoting F-box and leucine-rich repeat protein 14-mediated Twist1 degradation. Oncogene 2016, 35, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Zaafan, M.A.; Haridy, A.R.; Abdelhamid, A.M. Amitriptyline attenuates bleomycin-induced pulmonary fibrosis: Modulation of the expression of NF-κβ, iNOS, and Nrf2. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Nobile, B.; Durand, M.; Olié, E.; Guillaume, S.; Molès, J.-P.; Haffen, E.; Courtet, P. The anti-inflammatory effect of the tricyclic antidepressant clomipramine and its high penetration in the brain might be useful to prevent the psychiatric consequences of SARS-CoV-2 infection. Front. Pharmacol. 2021, 12, 615695. [Google Scholar] [CrossRef]
- Neumann, M.; Naumann, M. Beyond IκBs: Alternative regulation of NF-KB activity. FASEB J. 2007, 21, 2642–2654. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, Z.; Yang, L.; Fang, Y.; Lu, S.; Akakuru, O.U.; Huang, S.; Li, J.; Ma, S.; Wu, A. HPDA/Zn as a CREB inhibitor for ultrasound imaging and stabilization of atherosclerosis plaque. Chin. J. Chem. 2023, 41, 199–206. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, B.; Mukhopadhyay, R.; Naskar, K.; Sundar, S.; Dujardin, J.-C.; Roy, S. Imipramine exploits histone deacetylase 11 to increase the IL-12/IL-10 ratio in macrophages infected with antimony-resistant Leishmania donovani and clears organ parasites in experimental infection. J. Immunol. 2014, 193, 4083–4094. [Google Scholar] [CrossRef]
- Vollmer, S.; Strickson, S.; Zhang, T.; Gray, N.; Lee, K.L.; Rao, V.R.; Cohen, P. The mechanism of activation of IRAK1 and IRAK4 by interleukin-1 and Toll-like receptor agonists. Biochem. J. 2017, 474, 2027–2038. [Google Scholar] [CrossRef]
- Leulier, F.; Lemaitre, B. Toll-like receptors--taking an evolutionary approach. Nat. Rev. Genet. 2008, 9, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Loram, L.C.; Zhang, Y.; Shridhar, M.; Rezvani, N.; Berkelhammer, D.; Phipps, S.; Foster, P.S.; Landgraf, K.; Falke, J.J.; et al. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience 2010, 168, 551–563. [Google Scholar] [CrossRef]
- Roshan, M.H.; Tambo, A.; Pace, N.P. The Role of TLR2, TLR4, and TLR9 in the Pathogenesis of Atherosclerosis. Int. J. Inflamm. 2016, 2016, 1532832. [Google Scholar] [CrossRef]
- De Nardo, D.; Balka, K.R.; Cardona Gloria, Y.; Rao, V.R.; Latz, E.; Masters, S.L. Interleukin-1 receptor-associated kinase 4 (IRAK4) plays a dual role in myddosome formation and Toll-like receptor signaling. J. Biol. Chem. 2018, 293, 15195–15207. [Google Scholar] [CrossRef]
- Yan, B.; Yu, X.; Cai, X.; Huang, X.; Xie, B.; Lian, D.; Chen, J.; Li, W.; Lin, Y.; Ye, J.; et al. A Review: The Significance of Toll-Like Receptors 2 and 4, and NF-κB Signaling in Endothelial Cells during Atherosclerosis. Front. Biosci. (Landmark Ed.) 2024, 29, 161. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pérez, A.; Franco-Trepat, E.; Guillán-Fresco, M.; Jorge-Mora, A.; López, V.; Pino, J.; Gualillo, O.; Gómez, R. Role of toll-like receptor 4 on osteoblast metabolism and function. Front. Physiol. 2018, 9, 504. [Google Scholar] [CrossRef]
- Jin, M.; Fang, J.; Wang, J.-J.; Shao, X.; Xu, S.-W.; Liu, P.-Q.; Ye, W.-C.; Liu, Z.-P. Regulation of toll-like receptor (TLR) signaling pathways in atherosclerosis: From mechanisms to targeted therapeutics. Acta Pharmacol. Sin. 2023, 44, 2358–2375. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Shaker, S.; El-Gayar, N.; Aboul-Fotouh, S. The effects of antidepressants “fluoxetine and imipramine” on vascular abnormalities and Toll like receptor-4 expression in diabetic and non-diabetic rats exposed to chronic stress. PLoS ONE 2015, 10, e0120559. [Google Scholar] [CrossRef]
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Manthey, L.; Leeds, C.; Giltay, E.J.; van Veen, T.; Vreeburg, S.A.; Penninx, B.W.J.H.; Zitman, F.G. Antidepressant use and salivary cortisol in depressive and anxiety disorders. Eur. Neuropsychopharmacol. 2011, 21, 691–699. [Google Scholar] [CrossRef]
- Antoine, M.H.; Gall, D.; Schiffmann, S.N.; Lebrun, P. Tricyclic antidepressant imipramine reduces the insulin secretory rate in islet cells of Wistar albino rats through a calcium antagonistic action. Diabetologia 2004, 47, 909–916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lei, X.; Qiu, S.; Yang, G.; Wu, Q. Adiponectin and metabolic cardiovascular diseases: Therapeutic opportunities and challenges. Genes. Dis. 2023, 10, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Liu, L.; Shi, Z.; Ji, X.; Zhang, W.; Luan, J.; Zahr, T.; Qiang, L. Adipokines, adiposity, and atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 272. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-R.; Hou, P.-H.; Yang, W.-C.; Wang, C.-M.; Fan, P.-S.; Liao, H.-J.; Chen, T.-P. Doxepin exacerbates renal damage, glucose intolerance, nonalcoholic fatty liver disease, and urinary chromium loss in obese mice. Pharmaceuticals 2021, 14, 267. [Google Scholar] [CrossRef]
- Karasawa, T.; Takahashi, M. The crystal-induced activation of NLRP3 inflammasomes in atherosclerosis. Inflamm. Regen. 2017, 37, 18. [Google Scholar] [CrossRef]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Rajamäki, K.; Lappalainen, J.; Oörni, K.; Välimäki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS ONE 2010, 5, e11765. [Google Scholar] [CrossRef]
- Alcocer-Gómez, E.; Casas-Barquero, N.; Williams, M.R.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017, 121, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, X. NLRP3 inflammasome in atherosclerosis: Mechanisms and targeted therapies. Front. Pharmacol. 2024, 15, 1430236. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Ferrari, C.; Uher, R.; Bocchio-Chiavetto, L.; Riva, M.A.; Consortium, M.R.C.I.; Pariante, C.M. Absolute measurements of macrophage migration inhibitory factor and interleukin-1-β mRNA levels accurately predict treatment response in depressed patients. Int. J. Neuropsychopharmacol. 2016, 19, pyw045. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Huy, C.; Brenmoehl, J.; Obermeier, F.; Bock, J. Matrix metalloproteinase-1 expression induced by IL-1beta requires acid sphingomyelinase. FEBS Lett. 2009, 583, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, Y.; Cao, J.; Lu, Y.; Sun, G.; Yang, J. Role of ASM/Cer/TXNIP signaling module in the NLRP3 inflammasome activation. Lipids Health Dis. 2021, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Xia, M.; Chen, Y.; Bhat, O.M.; Yuan, X.; Boini, K.M.; Li, P.L. Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol. 2017, 13, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Mello, B.S.; Monte, A.S.; McIntyre, R.S.; Soczynska, J.K.; Custódio, C.S.; Cordeiro, R.C.; Chaves, J.H.; Vasconcelos, S.M.; Nobre, H.V., Jr.; Florenço de Sousa, F.C.; et al. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J. Psychiatr. Res. 2013, 47, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Obuchowicz, E.; Prymus, A.; Bielecka, A.M.; Drzyzga, Ł.; Paul-Samojedny, M.; Kot, M.; Daniel, W.A. Desipramine administered chronically inhibits lipopolysaccharide-stimulated production of IL-1β in the brain and plasma of rats. Cytokine 2016, 80, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.; Hajhashemi, V.; Minaiyan, M.; Movahedian, A.; Talebi, A. A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats. Eur. J. Pharmacol. 2011, 667, 396–401. [Google Scholar] [CrossRef]
- Sobieszczańska, A.; Lis, M.; Suszko-Pawłowska, A.; Szczypka, M. Clomipramine, a tricyclic antidepressant, and selegiline, a monoamine oxidase-B inhibitor, modulate the activity of phagocytic cells after oral administration in mice. J. Pharm. Pharmacol. 2020, 72, 836–842. [Google Scholar] [CrossRef]
- Yuan, X.; Bhat, O.M.; Zou, Y.; Li, X.; Zhang, Y.; Li, P.L. Endothelial Acid Sphingomyelinase Promotes NLRP3 Inflammasome and Neointima Formation During Hypercholesterolemia. J. Lipid Res. 2022, 63, 100298. [Google Scholar] [CrossRef]
- Witztum, J.L.; Lichtman, A.H. The influence of innate and adaptive immune responses on atherosclerosis. Annu. Rev. Pathol. 2014, 9, 73–102. [Google Scholar] [CrossRef]
- Hannestad, J.; DellaGioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef]
- Kalkman, H.O. Novel Treatment Targets Based on Insights in the Etiology of Depression: Role of IL-6 Trans-Signaling and Stress-Induced Elevation of Glutamate and ATP. Pharmaceuticals 2019, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Lotrich, F.E. Inflammatory cytokine-associated depression. Brain Res. 2015, 1617, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Boyce, P.; Judd, F. The place for the tricyclic antidepressants in the treatment of depression. Aust. N. Z. J. Psychiatry 1999, 33, 323–327. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry Off. J. World Psychiatr. Assoc. 2023, 22, 394–412. [Google Scholar] [CrossRef] [PubMed]


| Topic | Mechanisms/Details | Effects | Ref. |
|---|---|---|---|
| Role of IL-6 in Inflammation | Acts as both a pro-inflammatory and anti-inflammatory cytokine, depending on context. | Elevated levels linked to cardiovascular diseases and atherosclerosis. | [34] |
| Promotes adhesion molecule expression on endothelial cells. | Facilitates inflammatory cell recruitment to sites of injury. | [6] | |
| Stimulates production of acute-phase proteins in the liver. | Enhances systemic inflammatory responses. | ||
| IL-6 Signaling Pathways | Classical signaling: IL-6 binds to membrane-bound IL-6R, activates JAK-STAT pathway. | Targeted immune response in specific immune cells. | [35] |
| Trans-signaling: IL-6 binds to soluble IL-6R, interacts with gp130 on broader cell types. | Amplifies systemic inflammation and affects diverse cell types. | ||
| Anti-Inflammatory Effects of TCAs | Inhibits NF-κB activation, reducing inflammatory cytokine production. | Attenuates inflammation and improves endothelial function. | [21,23] |
| ↓ Oxidative stress through various molecular pathways. | Enhances vascular and systemic health. | ||
| TCAs and IL-6 Pathway | Nortriptyline and imipramine have been shown to ↓ IL-6 levels. | Suggests potential anti-inflammatory benefits. | [36] |
| Some studies indicate ↑ IL-6 levels with TCAs like duloxetine and amitriptyline. | Highlights variability in response across studies. | [31] | |
| Imipramine showed no significant impact on IL-6 levels in specific studies. | Demonstrates the complexity of TCA effects on IL-6 pathways. | [37] | |
| Proposed Mechanisms | TCAs inhibit NF-κB, JAK/STAT, MAPK, and PI3K pathways, ↓ IL-6 production. | Exerts anti-inflammatory effects through multiple molecular mechanisms. | [38] |
| Category | Details | Ref. |
|---|---|---|
| Production and Role of TNF-α | Produced by macrophages, lymphocytes, and endothelial cells. Regulates immune responses, cell proliferation, differentiation, and apoptosis. Contributes to atherosclerosis by upregulating VCAM-1, ICAM-1, and E-selectin, promoting leukocyte adhesion and transmigration. Induces IL-1β, IL-6, chemokine, and MMP production. | [21] |
| NF-κB Activation | TNF-α binds to TNFR1 and TNFR2, activating NF-κB via canonical (IκBα degradation) and non-canonical (NF-κB2/p100-to-p52 processing) pathways. Leads to inflammation, apoptosis, and ROS production. ROS causes endothelial damage and NO inactivation, worsening endothelial dysfunction. | [39,46] |
| Pathways of TNF-α-Induced Apoptosis | Extrinsic pathway: Mediated by death receptors. Intrinsic pathway: Mitochondrial-mediated apoptosis. Contributes to plaque instability. | |
| Effects of TCAs on TNF-α | ↓ TNF-α levels Inhibit NF-κB activation by stabilizing IκBα, reducing pro-inflammatory gene transcription. Reduce ROS via antioxidant actions (enhancing SOD, catalase). Restore NO bioavailability, improving endothelial function and vasodilation. | [39,41,42] |
| Clinical Implications | ↓ Inflammation and oxidative stress. Stabilization of atherosclerotic plaques. Potential as adjunctive therapy for patients with depression and atherosclerosis. | [21] |
| Category | Details | Ref. |
| NF-κB Family and Its Functions | Key transcription factor in inflammation and atherosclerosis. Regulates cytokines, chemokines, adhesion molecules, and apoptosis-related genes. Composed of proteins such as RELA (p65), NF-κB1 (p50; p105), and NF-κB2 (p52; p100). | [54] |
| Activation Pathways | Canonical Pathway: Triggered by stimuli like TNF-α, interleukins, and LPS. IKK complex phosphorylates IκB, leading to its degradation and NF-κB activation. Non-Canonical Pathway: Stimulated by factors like CD40 ligation and lymphotoxin-β. Relies on p100 processing to p52 via IKK1 and NIK. | [55] |
| Role in Atherosclerosis | NF-κB activation enhances the transcription of pro-inflammatory genes. Promotes endothelial dysfunction and adhesion molecule expression. Elevated activity was observed in unstable coronary plaques. | [40,41,42,56] |
| Effects of TCAs on NF-κB | ↓ NF-κB activation by inhibiting TNF-α release. Imipramine ↓ the nuclear translocation of p65 and the nuclear/total NF-κB ratio. Tianeptine and amitriptyline suppress the activation of p50, p65, and IκB. | [29,42,52] |
| Additional Mechanisms of TCAs | Imipramine remodels the IL-10 promoter, inhibiting p50/c-Rel binding. Imipramine blue (IB) reduces NADPH oxidase activity, lowering ROS, a key NF-κB activator. Reduced ROS prevents excessive inflammatory responses. | [57] |
| Therapeutic Implications | By suppressing NF-κB activation, TCAs mitigate inflammation, oxidative stress, and endothelial dysfunction. Potential benefits extend to conditions like atherosclerosis beyond their antidepressant effects. | [40,41,42] |
| Category | Details | Ref. |
|---|---|---|
| Function of Adiponectin | Adipokine is secreted by adipocytes with anti-atherogenic properties. Modulates AMPK and cAMP-PKA pathways to suppress apoptosis, ROS, and NF-κB pathways. Induces NO production, protecting against atherosclerosis. | [72,73] |
| Therapeutic Potential | Demonstrated by studies to have anti-atherogenic effects. Recognized as a potential therapeutic agent for cardiovascular protection. | [74] |
| Effects of TCAs on Adiponectin | Doxepin: Shown to suppress adiponectin expression in mice. Imipramine: No significant effect on adiponectin expression observed in vitro. | [74] |
| Research Gaps | Inconsistent findings regarding TCAs’ influence on adiponectin. Further studies required to clarify the effects of TCAs on adiponectin pathways and their therapeutic implications for atherosclerosis. | [74] |
| Factor | Effect | TCA Involvement | Ref. |
|---|---|---|---|
| NLRP3 Activation | Initiates inflammation. Promotes IL-1β and IL-18 release. Triggered by cholesterol crystals. | Amitriptyline and imipramine lower NLRP3 mRNA expression and inflammasome activation. Enhanced autophagy inhibits NLRP3 activation. | [78] |
| Caspase-1 Activation | Converts pro-IL-1β and pro-IL-18 into active cytokines. Drives inflammatory responses. | Imipramine inhibits caspase-1 activity. Amitriptyline alleviates caspase-1-mediated cytokine release. | [79] |
| IL-1β and IL-18 Release | Triggers endothelial dysfunction and oxidative stress. Contributes to atherosclerosis. | Imipramine and desipramine lower IL-1β levels. Amitriptyline significantly reduces IL-1β production. | [78] |
| MMP-1 Production Induction by IL-1β | Exacerbates atherosclerotic plaque instability. | Imipramine prevents IL-1β-induced MMP-1 expression, stabilizing plaques. | [78] |
| Predictive Role of IL-1β | High baseline IL-1β mRNA levels are associated with poor response to nortriptyline. | Suggested as a predictor of treatment response to TCAs like nortriptyline. | [80] |
| Conflicting Findings | ↑ IL-1 levels were observed in obese mice treated with doxepin. | Results highlight variability in TCAs’ effects. | [74] |
| Molecule/ Pathway | Role in Atherosclerosis | Effect of TCAs | Mechanisms of Action of TCAs | Ref. |
|---|---|---|---|---|
| IL-6 | Pro-inflammatory cytokine that ↑ atherosclerosis through various mechanisms, including the induction of adhesion molecule production, chemokine production, and T-cell proliferation | Some studies show that TCAs ↓ IL-6 levels, while others report an ↑ or no change | TCAs may ↓ IL-6 production by inhibiting NF-κB, JAK/STAT, and MAPK/PI3K pathways | [4] |
| TNF-α | Major pro-inflammatory cytokine that contributes to atherosclerosis by ↑ leukocyte adhesion, macrophage differentiation, and the production of other inflammatory mediators | Most studies indicate that TCAs ↓ TNF-α levels | TCAs inhibit NF-κB activation, scavenge ROS, ↑ antioxidant enzyme activity, and inhibit apoptosis, leading to ↓ inflammation and ↑ endothelial function | [2] |
| NF-κB | Key transcription factor that regulates the expression of numerous genes involved in inflammation and atherosclerosis, including cytokines, chemokines, and adhesion molecules | TCAs ↓ NF-κB activation | TCAs inhibit TNF-α release, ↓ total NF-κB levels, remodel the IL-10 promoter, inhibit NADPH oxidase activity, and ↓ ROS production | [52] |
| TLR | Pattern recognition receptors that play a role in inflammation and atherosclerosis by recognizing PAMPs and DAMPs and activating downstream signaling pathways | TCAs inhibit TLR expression, particularly TLR-4 and TLR-2 | The exact mechanisms by which TCAs inhibit TLRs are not fully elucidated | [52] |
| Adiponectin | Adipokine with anti-atherogenic properties due to its effects on AMPK and cAMP-PKA pathways and its ability to suppress apoptosis, ROS production, and NF-κB activation | Doxepin may suppress adiponectin expression, while imipramine may not have an effect | Further research is needed to clarify the impact of TCAs on adiponectin and its related pathways | [52,72] |
| NLRP3 and IL-1 | NLRP3 inflammasome activation leads to the processing and release of pro-inflammatory cytokines IL-1β and IL-18, contributing to endothelial dysfunction and atherosclerosis | TCAs ↓ NLRP3 inflammasome activation, IL-1β, and IL-18 levels | TCAs may inhibit NLRP3 inflammasome activation through autophagy induction, direct inhibition of NLRP3 and IL-1β expression, and prevention of IL-1β-induced MMP-1 production | [79,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eslami, M.; Monemi, M.; Nazari, M.A.; Azami, M.H.; Shariat Rad, P.; Oksenych, V.; Naderian, R. The Anti-Inflammatory Potential of Tricyclic Antidepressants (TCAs): A Novel Therapeutic Approach to Atherosclerosis Pathophysiology. Pharmaceuticals 2025, 18, 197. https://doi.org/10.3390/ph18020197
Eslami M, Monemi M, Nazari MA, Azami MH, Shariat Rad P, Oksenych V, Naderian R. The Anti-Inflammatory Potential of Tricyclic Antidepressants (TCAs): A Novel Therapeutic Approach to Atherosclerosis Pathophysiology. Pharmaceuticals. 2025; 18(2):197. https://doi.org/10.3390/ph18020197
Chicago/Turabian StyleEslami, Majid, Marzieh Monemi, Mohammad Ali Nazari, Mohammad Hossein Azami, Parand Shariat Rad, Valentyn Oksenych, and Ramtin Naderian. 2025. "The Anti-Inflammatory Potential of Tricyclic Antidepressants (TCAs): A Novel Therapeutic Approach to Atherosclerosis Pathophysiology" Pharmaceuticals 18, no. 2: 197. https://doi.org/10.3390/ph18020197
APA StyleEslami, M., Monemi, M., Nazari, M. A., Azami, M. H., Shariat Rad, P., Oksenych, V., & Naderian, R. (2025). The Anti-Inflammatory Potential of Tricyclic Antidepressants (TCAs): A Novel Therapeutic Approach to Atherosclerosis Pathophysiology. Pharmaceuticals, 18(2), 197. https://doi.org/10.3390/ph18020197







