Nanofibrous Scaffolds’ Ability to Induce Mesenchymal Stem Cell Differentiation for Soft Tissue Regenerative Applications
Abstract
1. Introduction
2. Nanofibrous Scaffolds Inducing MSC Differentiation
2.1. Cardiogenic Differentiation
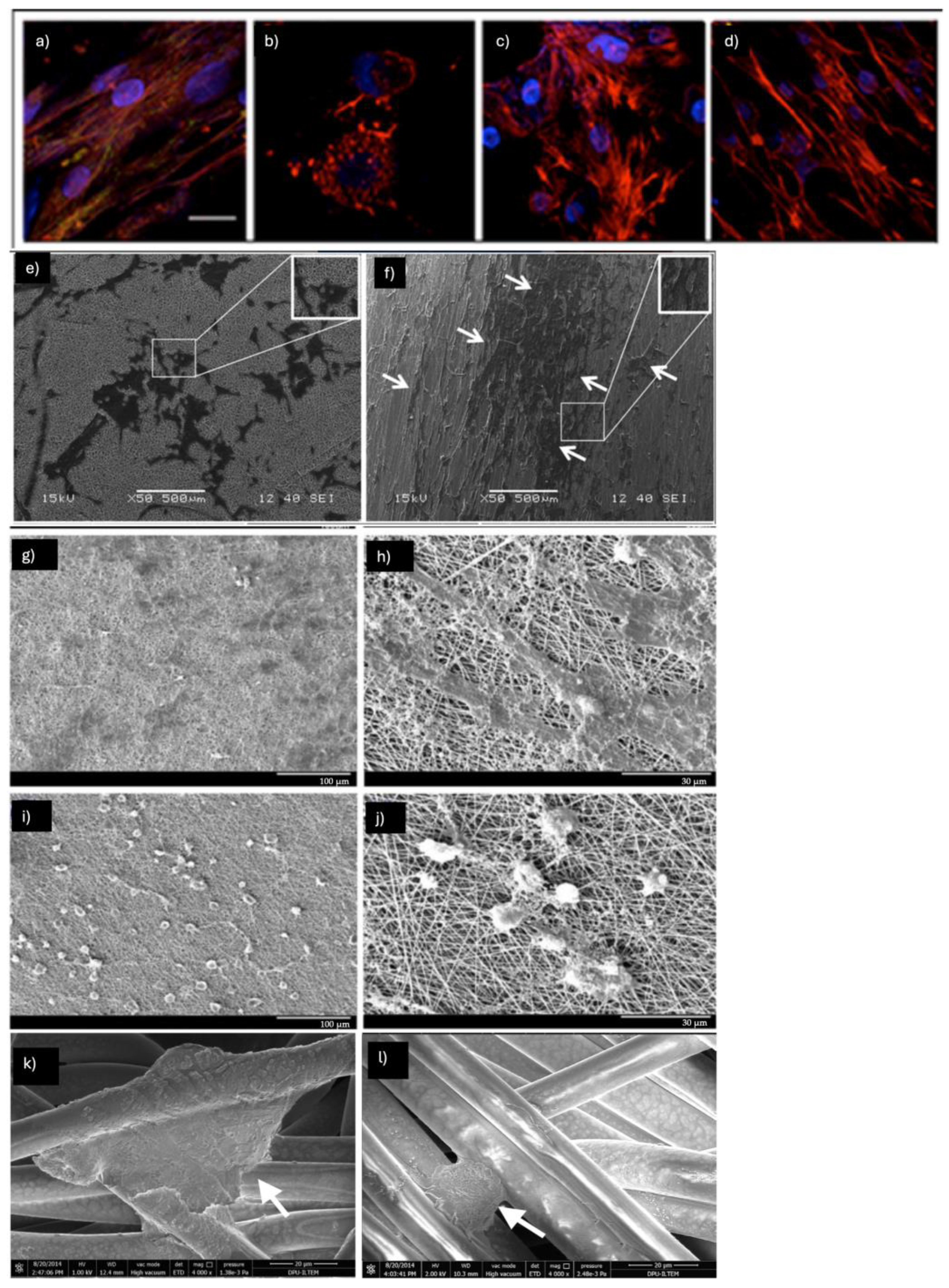
2.2. Epithelial Differentiation
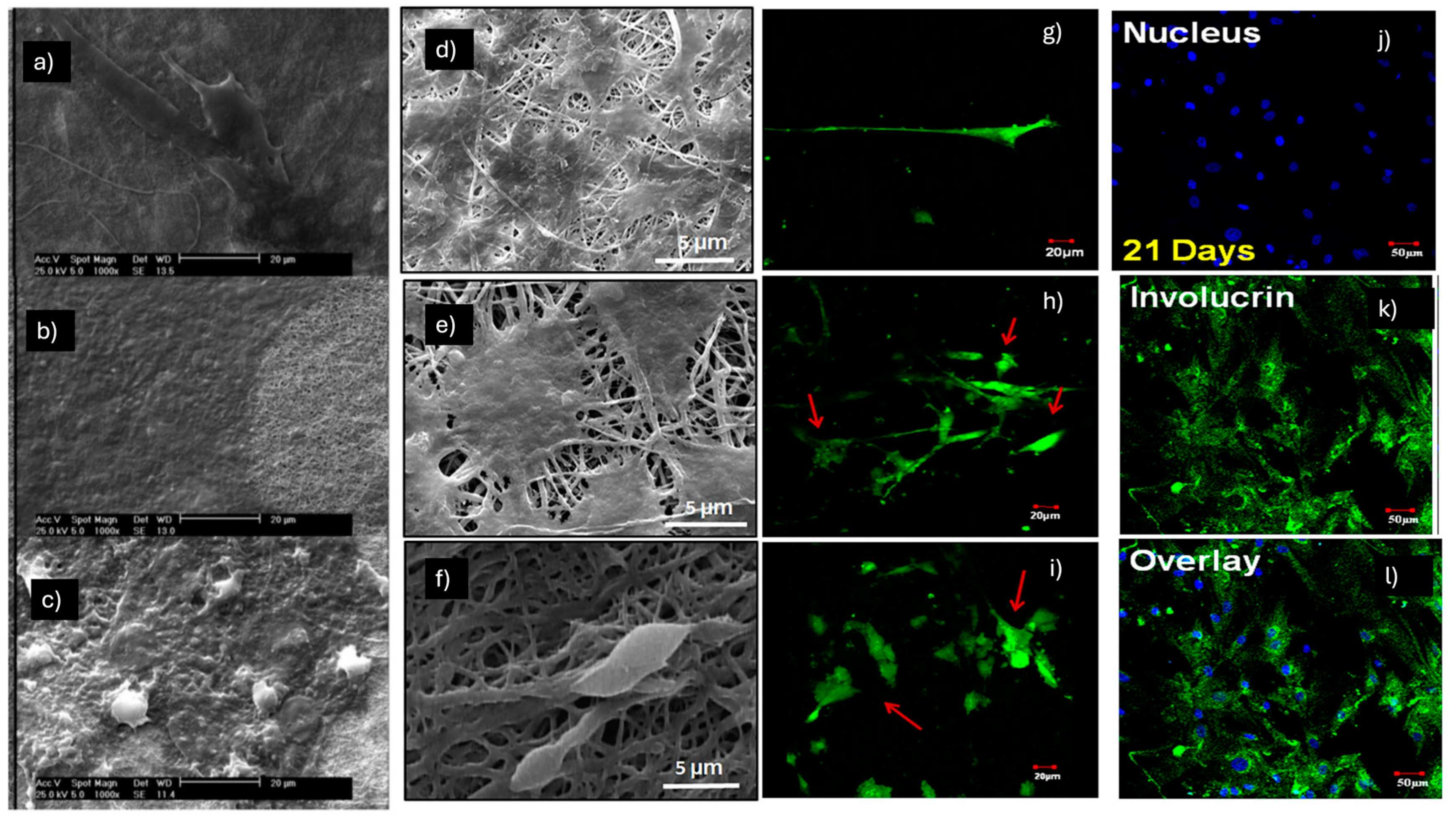
2.3. Myogenic Differentiation
2.4. Tendon Differentiation
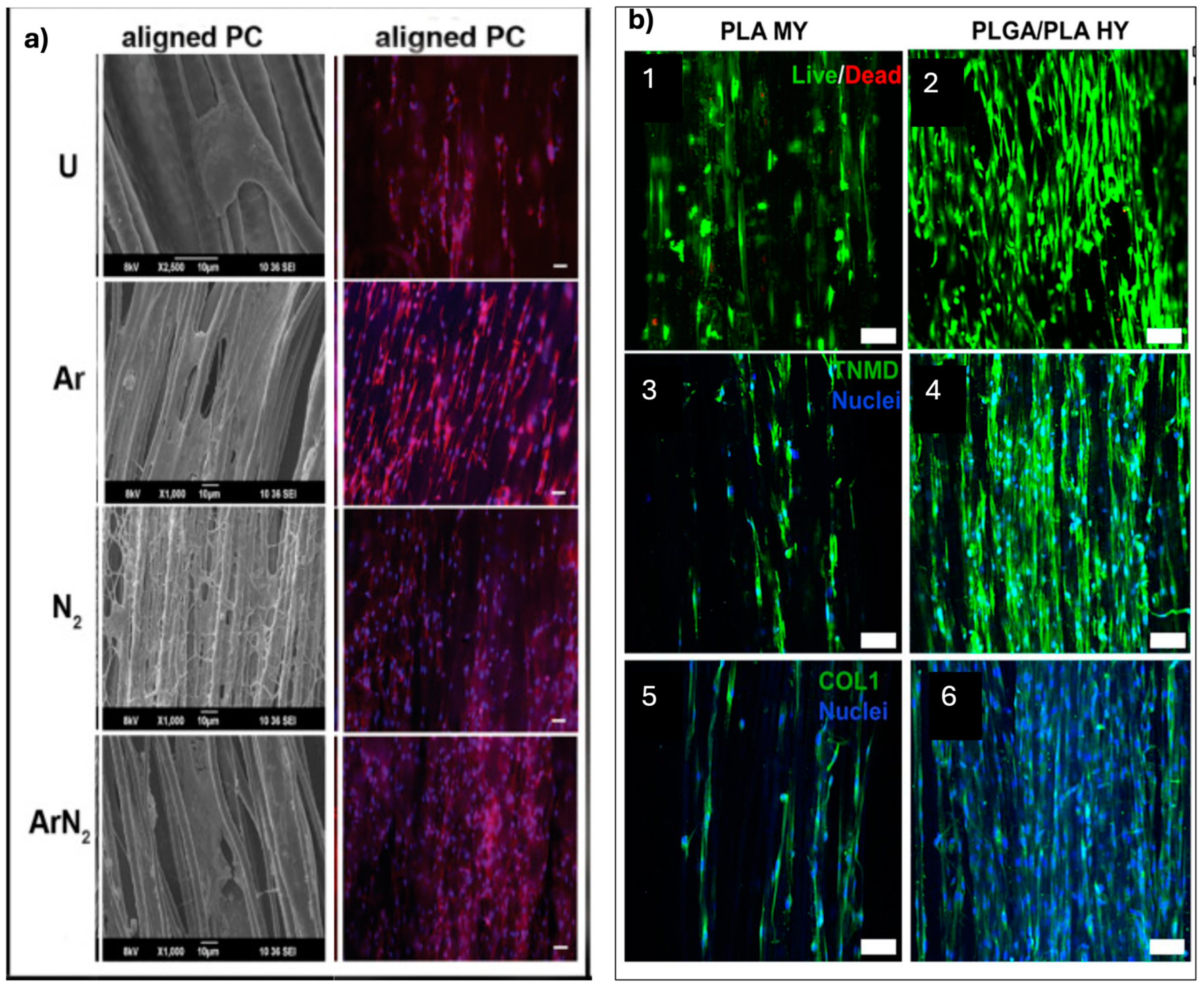
2.5. Vascular Differentiation
3. Future Research Directions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 10T1/2 | Mouse embryonic multipotent mesenchymal progenitor cell line |
| 5-Aza | 5-Azacytidine |
| A549 | Human lung epithelial cells |
| AA | Ascorbic acid |
| ACL | Anterior cruciate ligament |
| ACTA1 | Skeletal alpha actin 1 |
| Ad | Adipose tissue-derived |
| ADH | Alcohol dehydrogenase |
| ADM | Acellular dermal matrix |
| Ad-MSCs | Adipose-derived mesenchymal stem cells |
| AM | Amniotic membrane |
| Ang-1 | Angiopoietina-1 |
| ASA | Acetylsalicylic acid |
| ASAP | L-ascorbic acid 2-phosphate |
| ASMA | Alpha-smooth muscle actin |
| ATE | Adipose tissue extract |
| AuNPs | Gold nanoparticles |
| AV | Aloe vera |
| B. vulgaris | Beta vulgaris |
| B@P | BMP-2-loaded CS/HA multilayer-modified PCL scaffold |
| bFGF | Basic fibroblast growth factor |
| BM | Bone marrow-derived |
| BMP-2 | Bone morphogenetic protein-2 |
| BM-MSCs | Bone marrow mesenchymal stem cells |
| BNFSs | Braided nanofibrous scaffolds |
| BSA | Bovine serum albumin |
| CC | Calcium chloride |
| CK-19 | Cytokeratin-19 |
| CK-MB | Creatine kinase-MB |
| CMs | Cardiomyocytes |
| CNF | Chitin nanofiber |
| CNT | Carbon nanotube |
| Ch | Chitosan |
| CS | Chondroitin sulfate |
| CTGF | Connective tissue growth factor |
| cTnI | Troponin I |
| cTnT | Troponin T |
| Cx43 | Connexin 43 |
| DCM | Dichloromethane |
| DHS | Donor horse serum |
| DM | Differentiation medium |
| DMF | Dimethylformamide |
| DXM | Dexamethasone |
| E | Young’s modulus |
| EBs | Embryonic bodies |
| ECG | Electrocardiogram |
| ECM | Extracellular matrix |
| ECs | Endotelial cells |
| EGF | Epidermal growth factor |
| EO | Emu oil |
| Fb | Fibrin |
| Fbg | Fibrinogen |
| FBS-HI | Fetal bovine serum—heat-inactivated |
| FE | Field emission |
| FGF-2 | Fibroblast growth factor-2 |
| FN | Fibronectin |
| GA | Glutaraldehyde |
| GEL | Gelatin |
| GELMa | Gelatin methacryloyl |
| GDF-5 | Growth and differentiation factor-5 |
| GO | Graphene oxide |
| H9c2 | Rat cardio myoblast |
| Hb | Hemoglobin |
| HA | Native hyaluronan |
| HaCaT | Keratinocyte cell line from adult human skin |
| HC | Hydrocortisone |
| HFIP | 1,1,1,3,3,3-Hexafluoroisopropanol |
| HG | HydroGel |
| HGF | Hepatocyte growth factor |
| hiPSCs | Induced pluripotent stem cells |
| hMSCs | Human mesenchymal stem cells |
| HPCL | Heparin polycaprolactone |
| hTCs | Human tenocytes |
| hUVECs | Human umbilical vein endotelial cells |
| hWJ | Human Wharton’s jelly-derived |
| HY | Hybrid yarns |
| ICC | Immunocytochemistry |
| IGF-1 | Insulin-like growth factor 1 |
| IMDM | Iscove’s modified Dulbecco’s medium |
| ITS | Insulin–transferrin–selenium |
| IVL | Involucrin |
| KGF | Keratinocyte growth factor |
| L-AA | L-ascorbic acid |
| LbL | Layer-by-layer |
| LDH | Lactate dehydrogenase |
| MAAB | Magnesium l-ascorbic acid 2 phosphate, salts |
| Mb | Myoblasts |
| MHC | Myosin heavy chain |
| MI | Myocardial infarction |
| MnO2–NPs | Manganese nanoparticles |
| MSC-FBS | MSC-fetal bovine serum |
| MSCs | Mesenchimal stem cells |
| MTS assay | 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| MY | Microfiber yarns |
| MYH2 | Myosin heavy chain 2 |
| MYOG | Myogenin |
| N NFS | Non-composite nylon66 nanofibrous scaffold |
| nanoHA | Nanophased hydroxyapatite |
| N-B.v NFS | Composite nylon66 nanofibrous scaffold |
| NGF | Nerve growth factor |
| NP(TGF) | TGF-β1-loaded chitosan nanoparticles |
| P/S | Penicillin/streptomycin |
| PAN | Polyacrylonitrile |
| PαAPz-A | Poly(organophosphazene)—L-alanine |
| PEO | Polyethylene oxide |
| PCL | Polycaprolactone |
| PDGF | Platelet-derived growth factor |
| PEG | Polyethylene glycol |
| PenStrep | Penicillin-streptomycin |
| PG | Propylene glycol |
| PGS | Poly (glycerol sebacate) |
| PHBV | Poly 3-hydroxybutyrate-co-3-hydroxyvalerate |
| PLA BNFSs | Poly(lactic acid) braided nanofibrous scaffolds |
| PLCL | Poly(L-lactide-co-ε-caprolactone) |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLLA | Poly-L-lactic acid |
| PDLLA/Lam—a PDLLA/NaOH | Hydrolyzed poly-L-lactic acid with laminin |
| PEA | Poly(ester amide) |
| poly-P | Poly-phosphate |
| PPDO | Poly(p-dioxanone) |
| PS | Polystyrene |
| PVA | Polyvinyl alcohol |
| PU | Polyurethane |
| PTMC-MA | Poly(trimethylene carbonate)-methacrylated |
| RT-PCR | Real-time PCR |
| S + B@P | SDF-1α- and BMP-2-loaded CS/HA multilayer-modified PCL scaffold |
| Sal B | Salvianolic acid B |
| SCs | Schwann cells |
| SDVGs | Small-diameter vascular grafts |
| SEM | Scanning electron microscopy |
| SF | Silk fibroin |
| SF/Fe | Silk fibroin/Fe3O4 nanoparticles |
| sGAG | Sulfated glycosaminoglycans |
| sHA | Sulfated hyaluronan |
| SJES | Stable jet electrospinning |
| SM-22a | Smooth muscle 22 alpha |
| SMC | Smooth muscle cells |
| SP | Substance P |
| SS | Silk sericin |
| Tβ4 | Thymosin β4 |
| T3 | 3,3′,5-Triiodo-L-thyronine |
| TCPS | Tissue culture polystyrene |
| TE | Tissue engineering |
| TGF-β | Transforming growth factor-β |
| THF | Tetrahydrofuran |
| T/L | Tendon and ligament |
| UC | Umbilical cord |
| UCB | Umbilical cord blood |
| USCs | Urine-derived stem cells |
| VB12 | Vitamin B12 |
| VD3 | Vitamin D3 |
| VEGF | Vascular endothelial growth factor |
| VGPs | VEGF-encapsulated gelatin particles |
| Vim | Vimentin |
| vSMCs | Vascular smooth muscle cells |
| YM | Young’s modulus |
| WNSs | Wavy nanofibrous scaffolds |
References
- Gomes, M.E.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L. Tissue Engineering and Regenerative Medicine: New Trends and Directions—A Year in Review. Tissue Eng. Part B Rev. 2017, 23, 211–224. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadban, S.; Artiles, M.; Bunnell, B.A. Adipose Stem Cells in Regenerative Medicine: Looking Forward. Front. Bioeng. Biotechnol. 2022, 9, 837464. [Google Scholar] [CrossRef] [PubMed]
- McClarren, B.; Olabisi, R. Strain and Vibration in Mesenchymal Stem Cells. Int. J. Biomater. 2018, 2018, 8686794. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-B.; Kim, J.-K.; Lee, G.; Kim, D.-H. Mechanical Properties of Materials for Stem Cell Differentiation. Adv. Biosyst. 2020, 4, 2000247. [Google Scholar] [CrossRef]
- Budhwani, K.; Wood, A.; Gangrade, A.; Sethu, P.; Thomas, V. Nanofiber and Stem Cell Enabled Biomimetic Systems and Regenerative Medicine. J. Nanosci. Nanotechnol. 2016, 16, 8923–8934. [Google Scholar] [CrossRef]
- Do, A.-V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Wise, S.G.; Li, Z.; Maitz, P.K.; Young, C.J.; Wang, Y.; Weiss, A.S. Tailoring the porosity and pore size of electrospun synthetic human elastin scaffolds for dermal tissue engineering. Biomaterials 2011, 32, 6729–6736. [Google Scholar] [CrossRef]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bünger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef]
- He, Y.; Lu, F. Development of Synthetic and Natural Materials for Tissue Engineering Applications Using Adipose Stem Cells. Stem Cells Int. 2016, 2016, 5786257. [Google Scholar] [CrossRef] [PubMed]
- Raut, H.; Das, R.; Liu, Z.; Liu, X.; Ramakrishna, S. Biocompatibility of Biomaterials for Tissue Regeneration or Replacement. Biotechnol. J. 2020, 15, 2000160. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Haj, J.; Haj Khalil, T.; Falah, M.; Zussman, E.; Srouji, S. An ECM-Mimicking, Mesenchymal Stem Cell-Embedded Hybrid Scaffold for Bone Regeneration. BioMed Res. Int. 2017, 2017, 8591073. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, C.S.; Rath, S.N.; Ramakrishna, S. Electrospun Fibers for Recruitment and Differentiation of Stem Cells in Regenerative Medicine. Biotechnol. J. 2017, 12, 1700263. [Google Scholar] [CrossRef]
- Rajasekaran, R.; Seesala, V.S.; Sunka, K.C.; Ray, P.G.; Saha, B.; Banerjee, M.; Dhara, S. Role of nanofibers on MSCs fate: Influence of fiber morphologies, compositions and external stimuli. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110218. [Google Scholar] [CrossRef]
- Bowers, D.T.; Brown, J.L. Nanofibers as Bioinstructive Scaffolds Capable of Modulating Differentiation through Mechanosensitive Pathways for Regenerative Engineering. Regen. Eng. Transl. Med. 2019, 5, 22–29. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- Ghosh, L.D.; Jain, A.; Sundaresan, N.R.; Chatterjee, K. Elucidating molecular events underlying topography mediated cardiomyogenesis of stem cells on 3D nanofibrous scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 88, 104–114. [Google Scholar] [CrossRef]
- Pushp, P.; Sahoo, B.; Ferreira, F.C.; Sampaio Cabral, J.M.; Fernandes-Platzgummer, A.; Gupta, M.K. Functional comparison of beating cardiomyocytes differentiated from umbilical cord-derived mesenchymal/stromal stem cells and human foreskin-derived induced pluripotent stem cells. J. Biomed. Mater. Res. A 2020, 108, 496–514. [Google Scholar] [CrossRef]
- Tambrchi, P.; Mahdavi, A.H.; DaliriJoupari, M.; Soltani, L. Polycaprolactone-co-polylactic acid nanofiber scaffold in combination with 5-azacytidine and transforming growth factor-β to induce cardiomyocyte differentiation of adipose-derived mesenchymal stem cells. Cell Biochem. Funct. 2022, 40, 668–682. [Google Scholar] [CrossRef] [PubMed]
- Tambrchi, P.; DaliriJoupari, M.; Mahdavi, A.H.; Soltani, L. Adipose-Derived Mesenchymal Stem Cells Differentiation Toward Cardiomyocyte-Like Cells on the PCL/PANI Nanofibrous Scaffold: An Experimental Study. Iran. J. Biotechnol. 2022, 20, e3205. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Venugopal, J.R.; Sridhar, R.; Ramakrishna, S. Cardiogenic differentiation of mesenchymal stem cells with gold nanoparticle loaded functionalized nanofibers. Colloids Surf. B Biointerfaces 2015, 134, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sridhar, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Ramakrishna, S. Gold nanoparticle loaded hybrid nanofibers for cardiogenic differentiation of stem cells for infarcted myocardium regeneration. Macromol. Biosci. 2014, 14, 515–525. [Google Scholar] [CrossRef]
- Joshi, J.; Brennan, D.; Beachley, V.; Kothapalli, C.R. Cardiomyogenic differentiation of human bone marrow-derived mesenchymal stem cell spheroids within electrospun collagen nanofiber mats. J. Biomed. Mater. Res. A 2018, 106, 3303–3312. [Google Scholar] [CrossRef]
- Ghosh, L.D.; Ravi, V.; Jain, A.; Panicker, A.G.; Sundaresan, N.R.; Chatterjee, K. Sirtuin 6 mediated stem cell cardiomyogenesis on protein coated nanofibrous scaffolds. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 145–155. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Tian, L.; Ramakrishna, S. Potential of VEGF-encapsulated electrospun nanofibers for in vitro cardiomyogenic differentiation of human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2017, 11, 1002–1010. [Google Scholar] [CrossRef]
- Ravichandran, R.; Venugopal, J.R.; Mukherjee, S.; Sundarrajan, S.; Ramakrishna, S. Elastomeric core/shell nanofibrous cardiac patch as a biomimetic support for infarcted porcine myocardium. Tissue Eng. Part A 2015, 21, 1288–1298. [Google Scholar] [CrossRef]
- Kishta, M.S.; Ahmed, H.H.; Ali, M.A.M.; Aglan, H.A.; Mohamed, M.R. Mesenchymal stem cells seeded onto nanofiber scaffold for myocardial regeneration. Biotech. Histochem. 2022, 97, 322–333. [Google Scholar] [CrossRef]
- Kang, B.-J.; Kim, H.; Lee, S.K.; Kim, J.; Shen, Y.M.; Jung, S.; Kang, K.-S.; Im, S.; Lee, S.; Choi, M.; et al. Umbilical cord blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized PCL nanofiber improve the cardiac function. Acta Biomater. 2014, 10, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.Y.; Huang, A.L.; Lee, P.C.; Chung, T.W.; Wang, S.S. Morphological transformation of hBMSC from 2D monolayer to 3D microtissue on low-crystallinity SF-PCL patch with promotion of cardiomyogenesis. J. Tissue Eng. Regen. Med. 2018, 12, e1852–e1864. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, B.; Qin, X.; Butcher, J.T. Nanofiber-structured hydrogel yarns with pH-response capacity and cardiomyocyte-drivability for bio-microactuator application. Acta Biomater. 2017, 60, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Celebi Saltik, B.; Oteyaka, M. Cardiac patch design: Compatibility of nanofiber materials prepared by electrospinning method with stem cells. Turk. J. Biol. 2016, 40, 510–518. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. Strategic design of cardiac mimetic core-shell nanofibrous scaffold impregnated with Salvianolic acid B and Magnesium l-ascorbic acid 2 phosphate for myoblast differentiation. Mater. Sci. Eng. C 2018, 90, 131–147. [Google Scholar] [CrossRef]
- Sierra-Sánchez, Á.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. npj Regen. Med. 2021, 6, 35. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Soleimani, M.; Vossoughi, M.; Ranjbarvan, P.; Hamedi, S.; Zamanlui, S.; Mahmoudifard, M. Study of epithelial differentiation and protein expression of keratinocyte-mesenchyme stem cell co-cultivation on electrospun nylon/B. vulgaris extract composite scaffold. Mater. Sci. Eng. C 2017, 75, 653–662. [Google Scholar] [CrossRef]
- Fathi, A.; Khanmohammadi, M.; Goodarzi, A.; Foroutani, L.; Mobarakeh, Z.T.; Saremi, J.; Arabpour, Z.; Ai, J. Fabrication of chitosan-polyvinyl alcohol and silk electrospun fiber seeded with differentiated keratinocyte for skin tissue regeneration in animal wound model. J. Biol. Eng. 2020, 14, 27. [Google Scholar] [CrossRef]
- Shou, K.; Huang, Y.; Qi, B.; Hu, X.; Ma, Z.; Lu, A.; Jian, C.; Zhang, L.; Yu, A. Induction of mesenchymal stem cell differentiation in the absence of soluble inducer for cutaneous wound regeneration by a chitin nanofiber-based hydrogel. J. Tissue Eng. Regen. Med. 2018, 12, e867–e880. [Google Scholar] [CrossRef]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Epidermal differentiation of stem cells on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) nanofibers. Ann. Biomed. Eng. 2014, 42, 2589–2599. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Nouri, M.; Martino, M.M.; Fattahi, A.; Alizadeh, E.; Darabi, M.; Rahmati-Yamchi, M.; Zarghami, N. Cytoprotection, proliferation and epidermal differentiation of adipose tissue-derived stem cells on emu oil based electrospun nanofibrous mat. Exp. Cell Res. 2017, 357, 192–201. [Google Scholar] [CrossRef]
- Mirzaei-Parsa, M.J.; Ghanbari, H.; Alipoor, B.; Tavakoli, A.; Najafabadi, M.R.H.; Faridi-Majidi, R. Nanofiber-acellular dermal matrix as a bilayer scaffold containing mesenchymal stem cell for healing of full-thickness skin wounds. Cell Tissue Res. 2019, 375, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Scharnweber, D.; Koul, V. Co-cultivation of keratinocyte-human mesenchymal stem cell (hMSC) on sericin loaded electrospun nanofibrous composite scaffold (cationic gelatin/hyaluronan/chondroitin sulfate) stimulates epithelial differentiation in hMSCs: In vitro study. Biomaterials 2016, 88, 83–96. [Google Scholar] [CrossRef]
- Bhowmick, S.; Rother, S.; Zimmermann, H.; Lee, P.S.; Moeller, S.; Schnabelrauch, M.; Koul, V.; Scharnweber, D. Reciprocal influence of hMSCs/HaCaT cultivated on electrospun scaffolds. J. Mater. Sci. Mater. Med. 2017, 28, 128. [Google Scholar] [CrossRef]
- Shahmohammadi, A.; Samadian, H.; Heidari Keshel, S.; Rashidi, K.; Kiani, A.; Soleimani, M.; Goudarzi, F. Burn wound healing using adipose-derived mesenchymal stem cells and manganese nanoparticles in polycaprolactone/gelatin electrospun nanofibers in rats. Bioimpacts 2024, 14, 30193. [Google Scholar] [CrossRef] [PubMed]
- Majidansari, S.; Vahedi, N.; Rekabgardan, M.; Ganjoury, C.; Najmoddin, N.; Tabatabaei, M.; Sigaroodi, F.; Naraghi-Bagherpour, P.; Taheri, S.A.A.; Khani, M.-M. Enhancing endothelial differentiation of human mesenchymal stem cells by culture on a nanofibrous polycaprolactone/(poly-glycerol sebacate)/gelatin scaffold. Polym. Adv. Technol. 2023, 34, 740–747. [Google Scholar] [CrossRef]
- Ribeiro, N.; Sousa, A.; Cunha-Reis, C.; Oliveira, A.L.; Granja, P.L.; Monteiro, F.J.; Sousa, S.R. New prospects in skin regeneration and repair using nanophased hydroxyapatite embedded in collagen nanofibers. Nanomedicine 2021, 33, 102353. [Google Scholar] [CrossRef] [PubMed]
- Steffens, D.; Mathor, M.B.; Santi, B.T.; Luco, D.P.; Pranke, P. Development of a biomaterial associated with mesenchymal stem cells and keratinocytes for use as a skin substitute. Regen. Med. 2015, 10, 975–987. [Google Scholar] [CrossRef]
- Yan, S.; Li, X.; Dai, J.; Wang, Y.; Wang, B.; Lu, Y.; Shi, J.; Huang, P.; Gong, J.; Yao, Y. Electrospinning of PVA/sericin nanofiber and the effect on epithelial-mesenchymal transition of A549 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 436–444. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Shi, X.; Sadeghian, R.B.; Salehi, S.; Fujie, T.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Stem Cell Differentiation Toward the Myogenic Lineage for Muscle Tissue Regeneration: A Focus on Muscular Dystrophy. Stem Cell Rev. Rep. 2015, 11, 866–884. [Google Scholar] [CrossRef]
- Rezaei, H.; Rezaie, Z.; Seifati, S.M.; Ardeshirylajimi, A.; Basiri, A.; Taheri, M.; Omrani, M.D. Poly-phosphate increases SMC differentiation of mesenchymal stem cells on PLGA-polyurethane nanofibrous scaffold. Cell Tissue Bank. 2020, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Fakhrieh, M.; Darvish, M.; Ardeshirylajimi, A.; Taheri, M.; Omrani, M.D. Improved bladder smooth muscle cell differentiation of the mesenchymal stem cells when grown on electrospun polyacrylonitrile/polyethylene oxide nanofibrous scaffold. J. Cell. Biochem. 2019, 120, 15814–15822. [Google Scholar] [CrossRef] [PubMed]
- Mohamadali, M.; Irani, S.; Soleimani, M.; Hosseinzadeh, S. PANi/PAN copolymer as scaffolds for the muscle cell-like differentiation of mesenchymal stem cells. Polym. Adv. Technol. 2017, 28, 1078–1087. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Ghaderian, S.M.-H.; Omrani, M.D.; Moradi, S.L. Biomimetic scaffold containing PVDF nanofibers with sustained TGF-β release in combination with AT-MSCs for bladder tissue engineering. Gene 2018, 676, 195–201. [Google Scholar] [CrossRef]
- Mirzaei, A.; Saburi, E.; Islami, M.; Ardeshirylajimi, A.; Omrani, M.D.; Taheri, M.; Moghadam, A.S.; Ghafouri-Fard, S. Bladder smooth muscle cell differentiation of the human induced pluripotent stem cells on electrospun Poly(lactide-co-glycolide) nanofibrous structure. Gene 2019, 694, 26–32. [Google Scholar] [CrossRef]
- Boroujeni, S.; Mashayekhan, S.; Vakilian, S.; Ardeshirylajimi, A.; Soleimani, M. The synergistic effect of surface topography and sustained release of TGF-1 on myogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Research. Part A 2016, 104, 1610–1621. [Google Scholar] [CrossRef]
- Wang, J.; Feng, C.; Zhu, Y.; Wang, Z.; Ren, X.; Li, X.; Ying, Y.; Tian, Y.; Yu, K.; Liu, S.; et al. A multilayered nanofibrous patch functionalized with adipose tissue extract for the treatment of bladder regeneration. Mater. Des. 2022, 220, 110821. [Google Scholar] [CrossRef]
- Awadalla, A.; Elkhooly, T.A.; El-Assmy, A.; Hamam, E.T.; Ali, M.; Sena, A.M.; Shokeir, D.; Shokeir, A.A.; Gabal, R.A.; Khirallah, S.M. Electrospun nanostructured heparin conjugated-poly-ε-caprolactone based scaffold promote differentiation of smooth muscle cells from adipose mesenchymal stem cells. Process Biochem. 2024, 143, 148–162. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.M.; Cai, A.; Dippold, D.; Boccaccini, A.R.; Schubert, D.W.; Hardt, M.; Lange, C.; Arkudas, A.; et al. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef]
- Cai, A.; Hardt, M.; Schneider, P.; Schmid, R.; Lange, C.; Dippold, D.; Schubert, D.W.; Boos, A.M.; Weigand, A.; Arkudas, A.; et al. Myogenic differentiation of primary myoblasts and mesenchymal stromal cells under serum-free conditions on PCL-collagen I-nanoscaffolds. BMC Biotechnol. 2018, 18, 75. [Google Scholar] [CrossRef]
- Cai, A.; Zheng, Z.M.; Himmler, M.; Schubert, D.W.; Fuchsluger, T.A.; Weisbach, V.; Horch, R.E.; Arkudas, A. Schwann Cells Promote Myogenic Differentiation of Myoblasts and Adipogenic Mesenchymal Stromal Cells on Poly-ɛ-Caprolactone-Collagen I-Nanofibers. Cells 2022, 11, 1436. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cheng, Q.; Zhou, G.; Wei, T.; Zhong, S.; Lu, L.; Yan, C.; Wang, Y.; Fang, M.; Yang, M.; et al. Electrospinning Aligned SF/Magnetic Nanoparticles-Blend Nanofiber Scaffolds for Inducing Skeletal Myoblast Alignment and Differentiation. ACS Appl. Bio Mater. 2024, 7, 7710–7718. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, S.; Mequanint, K. Electrospun Biodegradable α-Amino Acid-Substituted Poly(organophosphazene) Fiber Mats for Stem Cell Differentiation towards Vascular Smooth Muscle Cells. Polymers 2022, 14, 1555. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, X.; Chen, J.L.; Shen, W.L.; Hieu Nguyen, T.M.; Gao, L.; Ouyang, H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials 2010, 31, 2163–2175. [Google Scholar] [CrossRef]
- Zhou, K.; Feng, B.; Wang, W.; Jiang, Y.; Zhang, W.; Zhou, G.; Jiang, T.; Cao, Y.; Liu, W. Nanoscaled and microscaled parallel topography promotes tenogenic differentiation of ASC and neotendon formation in vitro. Int. J. Nanomed. 2018, 13, 3867–3881. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Xie, S.; Wang, N.; Wu, S.; Duan, Y.; Zhang, M.; Shui, L. Fabrication of Photo-Crosslinkable Poly(Trimethylene Carbonate)/Polycaprolactone Nanofibrous Scaffolds for Tendon Regeneration. Int. J. Nanomed. 2020, 15, 6373–6383. [Google Scholar] [CrossRef]
- Sankar, D.; Mony, U.; Rangasamy, J. Combinatorial effect of plasma treatment, fiber alignment and fiber scale of poly (ε-caprolactone)/collagen multiscale fibers in inducing tenogenesis in non-tenogenic media. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112206. [Google Scholar] [CrossRef]
- Rothrauff, B.B.; Lauro, B.B.; Yang, G.; Debski, R.E.; Musahl, V.; Tuan, R.S. Braided and Stacked Electrospun Nanofibrous Scaffolds for Tendon and Ligament Tissue Engineering. Tissue Eng. Part A 2017, 23, 378–389. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Streubel, P.N.; Duan, B. Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation. Acta Biomater. 2017, 62, 102–115. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, R.; Zhou, F.; Streubel, P.N.; Chen, S.; Duan, B. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/microfiber hybrid yarns for tendon tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110268. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.; Qi, Y.; Cai, J.; Zhao, J.; Duan, B.; Chen, S. Tendon-bioinspired wavy nanofibrous scaffolds provide tunable anisotropy and promote tenogenesis for tendon tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112181. [Google Scholar] [CrossRef] [PubMed]
- Sarıkaya, B.; Gümüşderelioğlu, M. Aligned silk fibroin/poly-3-hydroxybutyrate nanofibrous scaffolds seeded with adipose-derived stem cells for tendon tissue engineering. Int. J. Biol. Macromol. 2021, 193, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Rathbone, S.R.; Bradley, R.S.; Cartmell, S.H. Dynamic loading of electrospun yarns guides mesenchymal stem cells towards a tendon lineage. J. Mech. Behav. Biomed. Mater. 2014, 39, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Olvera, D.; Schipani, R.; Sathy, B.N.; Kelly, D.J. Electrospinning of highly porous yet mechanically functional microfibrillar scaffolds at the human scale for ligament and tendon tissue engineering. Biomed. Mater. 2019, 14, 035016. [Google Scholar] [CrossRef]
- Su, W.; Wang, Z.; Jiang, J.; Liu, X.; Zhao, J.; Zhang, Z. Promoting tendon to bone integration using graphene oxide-doped electrospun poly(lactic-co-glycolic acid) nanofibrous membrane. Int. J. Nanomed. 2019, 14, 1835–1847. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, H.; Liu, H.; Chen, X.; Lu, P.; Zhu, T.; Yang, L.; Yin, Z.; Heng, B.C.; Zhang, Y.; et al. Well-aligned chitosan-based ultrafine fibers committed teno-lineage differentiation of human induced pluripotent stem cells for Achilles tendon regeneration. Biomaterials 2015, 53, 716–730. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, X.; Song, H.X.; Hu, J.J.; Tang, Q.M.; Zhu, T.; Shen, W.L.; Chen, J.L.; Liu, H.; Heng, B.C.; et al. Electrospun scaffolds for multiple tissues regeneration in vivo through topography dependent induction of lineage specific differentiation. Biomaterials 2015, 44, 173–185. [Google Scholar] [CrossRef]
- Han, F.; Zhang, P.; Chen, T.; Lin, C.; Wen, X.; Zhao, P. A LbL-Assembled Bioactive Coating Modified Nanofibrous Membrane for Rapid Tendon-Bone Healing in ACL Reconstruction. Int. J. Nanomed. 2019, 14, 9159–9172. [Google Scholar] [CrossRef]
- Popielarczyk, T.L.; Nain, A.S.; Barrett, J.G. Aligned Nanofiber Topography Directs the Tenogenic Differentiation of Mesenchymal Stem Cells. Appl. Sci. 2017, 7, 59. [Google Scholar] [CrossRef]
- Xue, Y.; Kim, H.J.; Lee, J.; Liu, Y.; Hoffman, T.; Chen, Y.; Zhou, X.; Sun, W.; Zhang, S.; Cho, H.J.; et al. Co-Electrospun Silk Fibroin and Gelatin Methacryloyl Sheet Seeded with Mesenchymal Stem Cells for Tendon Regeneration. Small 2022, 18, e2107714. [Google Scholar] [CrossRef]
- Jia, L.; Prabhakaran, M.P.; Qin, X.; Ramakrishna, S. Stem cell differentiation on electrospun nanofibrous substrates for vascular tissue engineering. Mater. Sci. Eng. C 2013, 33, 4640–4650. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xue, F.; Zhang, H.; Sanyour, H.J.; Rickel, A.P.; Uttecht, A.; Fanta, B.; Hu, J.; Hong, Z. Fabrication and Characterization of Pectin Hydrogel Nanofiber Scaffolds for Differentiation of Mesenchymal Stem Cells into Vascular Cells. ACS Biomater. Sci. Eng. 2019, 5, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Pennings, I.; van Haaften, E.E.; Jungst, T.; Bulsink, J.A.; Rosenberg, A.; Groll, J.; Bouten, C.V.C.; Kurniawan, N.A.; Smits, A.; Gawlitta, D. Layer-specific cell differentiation in bi-layered vascular grafts under flow perfusion. Biofabrication 2019, 12, 015009. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Xu, Z.; Ikegami, Y.; Yamane, S.; Tsurashima, M.; Ijima, H. Co-culture of mesenchymal stem cells and human umbilical vein endothelial cells on heparinized polycaprolactone/gelatin co-spun nanofibers for improved endothelium remodeling. Int. J. Biol. Macromol. 2020, 151, 186–192. [Google Scholar] [CrossRef]
- Shafiq, M.; Jung, Y.; Kim, S.H. Covalent immobilization of stem cell inducing/recruiting factor and heparin on cell-free small-diameter vascular graft for accelerated in situ tissue regeneration. J. Biomed. Mater. Res. A 2016, 104, 1352–1371. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Wang, X.F.; Xu, Y.Y.; Qiao, Y.H.; Guo, X.; Wang, D.F.; Li, Q.; Turng, L.S. Polycaprolactone Nanofibers Containing Vascular Endothelial Growth Factor-Encapsulated Gelatin Particles Enhance Mesenchymal Stem Cell Differentiation and Angiogenesis of Endothelial Cells. Biomacromolecules 2018, 19, 3747–3753. [Google Scholar] [CrossRef]
- Ezhilarasu, H.; Sadiq, A.; Ratheesh, G.; Sridhar, S.; Ramakrishna, S.; Ab Rahim, M.H.; Yusoff, M.M.; Jose, R.; Reddy, V.J. Functionalized core/shell nanofibers for the differentiation of mesenchymal stem cells for vascular tissue engineering. Nanomedicine 2019, 14, 201–214. [Google Scholar] [CrossRef]
- Fu, Y.; Guan, J.; Guo, S.; Guo, F.; Niu, X.; Liu, Q.; Zhang, C.; Nie, H.; Wang, Y. Human urine-derived stem cells in combination with polycaprolactone/gelatin nanofibrous membranes enhance wound healing by promoting angiogenesis. J. Transl. Med. 2014, 12, 274. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, Z.; Tahtinen, M.; Qi, S.; Zhao, F. Prevascularization of natural nanofibrous extracellular matrix for engineering completely biological three-dimensional prevascularized tissues for diverse applications. J. Tissue Eng. Regen. Med. 2018, 12, e1325–e1336. [Google Scholar] [CrossRef]
- Aslani, S.; Kabiri, M.; Kehtari, M.; Hanaee-Ahvaz, H. Vascular tissue engineering: Fabrication and characterization of acetylsalicylic acid-loaded electrospun scaffolds coated with amniotic membrane lysate. J. Cell Physiol. 2019, 234, 16080–16096. [Google Scholar] [CrossRef]
- Kiros, S.; Lin, S.; Xing, M.; Mequanint, K. Embryonic Mesenchymal Multipotent Cell Differentiation on Electrospun Biodegradable Poly(ester amide) Scaffolds for Model Vascular Tissue Fabrication. Ann. Biomed. Eng. 2020, 48, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Szczesny, S.E.; Kim, D.H.; Saleh, K.S.; Mauck, R.L. Expansion of mesenchymal stem cells on electrospun scaffolds maintains stemness, mechano-responsivity, and differentiation potential. J. Orthop. Res. 2018, 36, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.H.; Bidone, T. 3D Model of Cell Migration and Proliferation in a Tissue Scaffold. Biophys. J. 2021, 120, 265a. [Google Scholar] [CrossRef]
- Leach, J.K.; Whitehead, J. Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 1115–1127. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dodda, J.M.; Liu, X.; Knitter, M.; Oosterbeek, R.N.; Salinas, P.; Pozo, E.; Ferreira, A.M.; Sadiku, E.R. Engineering of Bioresorbable Polymers for Tissue Engineering and Drug Delivery Applications. Adv. Healthc. Mater. 2024, 13, 2401674. [Google Scholar] [CrossRef]
- Holmes, T.C. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002, 20, 16–21. [Google Scholar] [CrossRef]
- Wang, L.; Hu, D.; Xu, J.; Hu, J.; Wang, Y. Complex in vitro Model: A Transformative Model in Drug Development and Precision Medicine. Clin. Transl. Sci. 2023, 17, e13695. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Tayebi, L. Vascularization strategies in tissue engineering approaches for soft tissue repair. J. Tissue Eng. Regen. Med. 2021, 15, 747–762. [Google Scholar] [CrossRef]
- Pérez-Gutiérrez, L.; Ferrara, N. Biology and therapeutic targeting of vascular endothelial growth factor A. Nat. Rev. Mol. Cell Biol. 2023, 24, 816–834. [Google Scholar] [CrossRef]
- Chung, L.; Maestas, D.R.; Housseau, F.; Elisseeff, J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017, 114, 184–192. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; He, M.; Zhang, L.; Yang, L.; Wei, L. Recent advances in the role of mesenchymal stem cells as modulators in autoinflammatory diseases. Front. Immunol. 2024, 15, 1525380. [Google Scholar] [CrossRef] [PubMed]
- Norrie, J. The importance of long-term follow-up in clinical trials. Lancet Glob. Health 2023, 11, e995–e996. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hou, W.; Zhao, B.; Fan, P.; Wang, S.; Wang, L.; Gao, J. Mesenchymal stem cells lineage and their role in disease development. Mol. Med. 2024, 30, 207. [Google Scholar] [CrossRef] [PubMed]
- Arinzeh, T.L.; Weber, N.; Jaffe, M. Electrospun Electroactive Polymers for Regenerative Medicine Applications. U.S. Patent US10052412B2, 21 August 2018. [Google Scholar]
- Brunelle, J.N. Hydrogel Scaffold for Three Dimensional Cell Culture. U.S. Patent Application 16/050,510, 31 January 2019. [Google Scholar]
- Kuo, C.K.; Zamarripa, N.; Thomas, A.H. Scaffolds for Tissue Engineering and Regenerative Medicine. U.S. Patent US20110293685A1, 1 December 2011. [Google Scholar]
- Bice, C.; Ida, G.; Rossella, D.; Marco, B.; Silvia, P.; Antonietta, A.M. Metodo per la Produzione di un Tessuto a Memoria di Forma e Usi. Relativi. Patent 202100019256, 20 January 2021. [Google Scholar]
- Liu, Z.; Wei, P.; Cui, Q.; Mu, Y.; Zhao, Y.; Deng, J.; Zhi, M.; Wu, Y.; Jing, W.; Liu, X.; et al. Guided bone regeneration with extracellular matrix scaffold of small intestinal submucosa membrane. J. Biomater. Appl. 2022, 37, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.M.; La Francesca, S.; Olson, S.D.; Triolo, F.; Bouchard, J.; Mondano, L.; Sundaram, S.; Roffidal, C.; Cox, C.S., Jr.; Wong Kee Song, L.M.; et al. First-in-Human Segmental Esophageal Reconstruction Using a Bioengineered Mesenchymal Stromal Cell-Seeded Implant. JTO Clin. Res. Rep. 2021, 2, 100216. [Google Scholar] [CrossRef] [PubMed]
- Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol 2023, 3, 524–553. [Google Scholar] [CrossRef]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef]
- Pisani, S.; Mauri, V.; Negrello, E.; Mauramati, S.; Alaimo, G.; Auricchio, F.; Benazzo, M.; Dorati, R.; Genta, I.; Conti, B.; et al. Assessment of different manufacturing techniques for the production of bioartificial scaffolds as soft organ transplant substitutes. Front. Bioeng. Biotechnol. 2023, 11, 1186351. [Google Scholar] [CrossRef]
- Asadi Sarabi, P.; Shabanpouremam, M.; Eghtedari, A.R.; Barat, M.; Moshiri, B.; Zarrabi, A.; Vosough, M. AI-Based solutions for current challenges in regenerative medicine. Eur. J. Pharmacol. 2024, 984, 177067. [Google Scholar] [CrossRef]
- Kim, S.; Colicchia, C.; Menachof, D. Ethical Sourcing: An Analysis of the Literature and Implications for Future Research. J. Bus. Ethics 2018, 152, 1033–1052. [Google Scholar] [CrossRef]
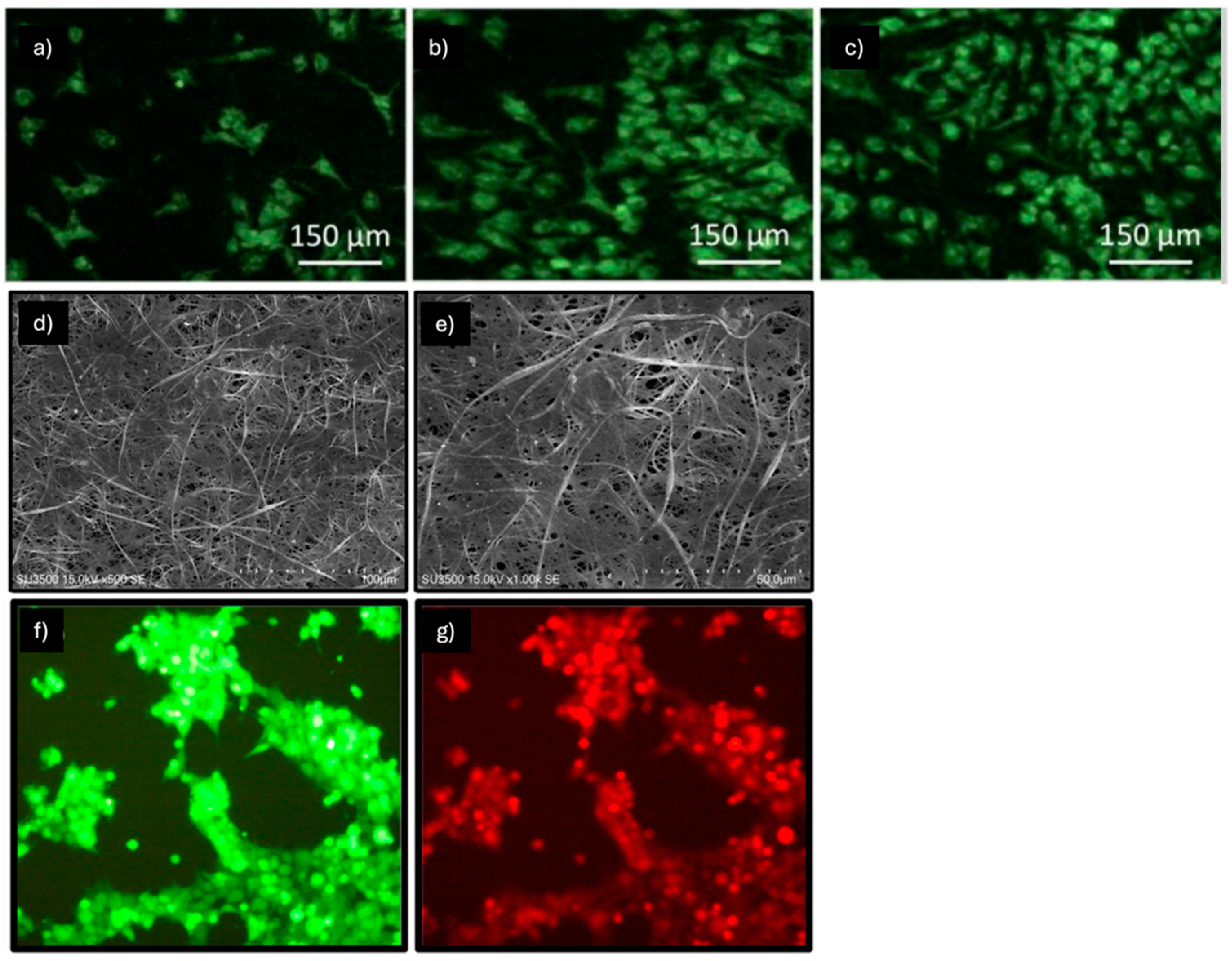
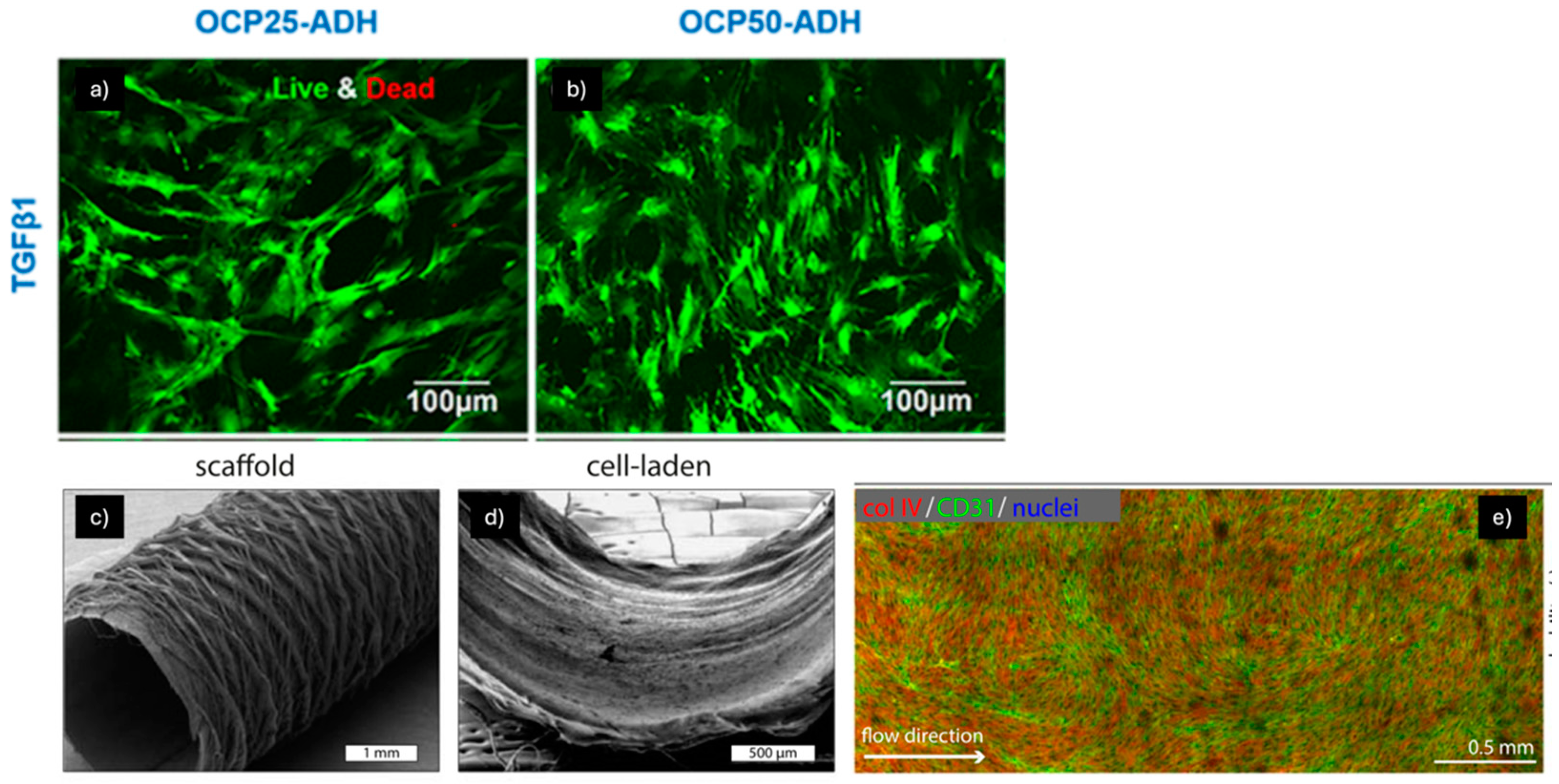
| Nanofibers | Mechanical Properties | MSCs Source | Supplemented Factors | Ref. |
|---|---|---|---|---|
| PCL-GEL | 2 ± 1 MPa (random) 1 ± 0.7 MPa (aligned) | hMSCs | 5-aza | [19] |
| PCL | - | UCB hiPSC | 5-aza GSK3 inhibitor (6 μM) | [20] |
| PLA-PCL | 6.41 ± 0.48 MPa | Ad-MSCs | 5-aza and TGF-β | [21] |
| PCL/PANI | - | Ad-MSCs | 5-aza and TGF-β | [22] |
| PVA-Ch-CNT | PVA-Ch = 30 ± 3.01 MPa PVA-Ch-CNT1 = 130 ± 3.605 MPa PVA-Ch-CNT3 = 121 ± 2.516 MPa PVA-Ch-CNT5 = 101 ± 1.527 MPa | Rat | 20 μL 5-aza, 1.5 μL AA 1 ng·ml−1 TGF-β | [23] |
| PCL/AV/VB12/SF/AuNPs | PCL = 4.5 MPa PCL/Collagen = 1.56 MPa PCL/SF/AV = 3.27 MPa PCL/SF/AV/VB12/AuNPs = 2.56 MPa | hMSCs (PT-2501; Lonza) | - | [24] |
| BSA/PVA/AuNPs | 4.77 MPa | hMSCs (PT-2501; Lonza) | 5-aza | [25] |
| Collagen I | - | BM-MSCs | 10 μM 5-aza | [26] |
| PCL/Collagen (Aligned) | 3 ± 1 MPa | hMSCs (PT-2501; Lonza) | 3 μM 5-aza | [27] |
| PCL/G-VEGF | E = 8.48 ± 0.53 MPa Tensile Strength = 3.82 ± 0.91 MPa Elongation at Break = 35.8 ± 4.8% | hMSCs (PT-2501; Lonza) | 10 μM 5-aza | [28] |
| PGS/Fbg | 3.28 ± 0.31 MPa | BM-MSCs | VEGF | [29] |
| Polyamide | - | Ad-MSCs | - | [30] |
| FN-PCL | Elongation at break = 7.9% | UCB | FN Cyclosporine | [31] |
| SF-PCL | 120 MPa | BM-MSCs | 5-aza | [32] |
| PAN HG yarns | 100 MPa | Ad-MSCs Chicken CMs | NaOH TGF-β1 | [33] |
| PU | - | BM-MSCs | 10 µM 5-aza | [34] |
| Sal B/MAAP-PCL/GEL | UTS = 40 ± 2.2 MPa | H9c2 | 2.5 μg mL−1 amphotericin B | [35] |
| Nanofibers | Mechanical Properties | MSCs Source | Supplemented Factors | Ref. |
|---|---|---|---|---|
| NFM | Stress Value = 13.8 MPa (NFM) 8.8 MPa (NFM + B. vulgaris) | Ad-MSCs | B. vulgaris | [37] |
| Ch-PVA + Silk | UTS = ~2.4 MPa E = ~2 MPa Strain Break = ~240% | Rat BM-MSCs | 2 mM CC, 5 μg/mL Insulin, 10 ng/mL EGF, 10 ng/mL KGF | [38] |
| CNF hydrogel | - | Rat BM-MSCs | - | [39] |
| PHBV | - | BM-MSCs | 400 ng/mL HC, 500 ng/mL Insulin, 1 nM T3, 10 ng/mL EGF, 1 μM vD3, 50 μg/mL L-AA | [40] |
| EO-PCL/PEG | UTS = 5.8 MPa | Ad-MSCs | 0.4 µg/mL HC, 5 µg/mL Insulin, 1 nM T3, 10 ng/mL EGF, 1 µM VD3, 50 µg/mL L-AA | [41] |
| PCL/Fbg | - | Ad-MSCs | 1× ITS, 0.5 μg/mL HC, 10 ng/mL EGF, 10 ng/mL KGF, 50 μg/mL L-AA | [42] |
| Cationic GEL/HA/CS + Sericin | - | BM-MSCs | 2 mM L-Glutamin | [43] |
| GEL/sHA HA/CS | - | BM-MSCs | GAGs | [44] |
| PCL/GEL—MnO2–NPs | UTS = 0.327 MPa (PCL/GEL) 2.525 MPa (PCL/GEL −5% MnO2–NPs | Ad-MSCs | - | [45] |
| PCL/PGS/GEL | E = 1.32 ± 0.27 MPa UTS = 1.23 ± 0.18 MPa | Ad-MSCs | 50 ng/mL VEGF | [46] |
| Collagen/nanoHA mats | E = 8.46 ± 1.05 kPa UTS = 428 ± 54 kPa | BM-MSCs | - | [47] |
| PDLLA/Lam—a PDLLA/NaOH | - | MSCs | - | [48] |
| PVA/SS | - | A549 | - | [49] |
| Nanofibers | Mechanical Properties | MSC Source | Supplemented Factors | Ref. |
|---|---|---|---|---|
| PLGA/PU PLGA/PU/polyP | UTS = 15.12 ± 2.16 MPa (PLGA/PU) 13.23 ± 1.25 MPa (PLGA/PU/polyP) | Ad-MSCs | 30 mM AA, 5 ng/mL TGF-β1 | [51] |
| PAN-PEO | E = 109.42 ± 4.55 MPa Elongation at break = 28.42 ± 2.12% | Ad-MSCs | 30 mM AA, 5 ng/mL TGF-β1 | [52] |
| PANi-PAN | - | hBM-MSCs | HS, hydrocortisone, and dexamethasone. | [53] |
| PVDF-TGFβ | E = 9.15 MPa Strain% = 61.7% | Ad-MSCs | 2.5 ng/mL TGFβ1 30 mM Ascorbic Acid | [54] |
| PLGA | UTS = 11.32 ± 2.02 MPa Elongation at break = 32.43 ± 1.97% | hiPSCs | 30 mM AA, 5 ng/mL TGF-β1 | [55] |
| PCL/NP(TGF)-PLLA | - | hWJ-derived UC-MSCs | 30 mM AA, 2.5 ng/mL TGF-β1 | [56] |
| PLA-PCL/ATE/HA | E = 14.93 ± 0.13 MPa | Ad-MSCs | - | [57] |
| HPCL | - | Ad-MSCs | 100 U/mL heparin | [58] |
| PCL/Collagen I | - | Lewis 1WR2 Rat (BM-MSC) + Mb | 0.4 μg/mL DXM, 1 ng/mL bFGF, HGF (10, 30, 60, 100 ng/mL), IGF-1 (5, 10, 30, 60 ng/mL), 10 ng/mL HGF + 10 ng/mL IGF1 | [59] |
| PCL/Collagen I | - | Lewis Rat (BM-MSC, Ad-MSC) + Mb | AIM V, AIM V + 0,1% Ultroser® G, DMEM/Ham’s F12 + 0,2% Ultroser® G | [60] |
| PCL/Collagen I | - | Ad-MSC + Mb + SCs | 2% DHS, 1% L-GlutaMAX, 0.4 μg/mL DXM, 1 ng/mL bFGF | [61] |
| SF/Fe | E = 233.62 ± 5.27 MPa Elongation at break = 10% | MSCs | - | [62] |
| PαAPz-A | - | iPSCs BM-MSCs | 82.5 μg/mL L-AA 2 ng/mL TGF-β1 | [63] |
| Nanofibers | Mechanical Properties | MSCs Source | Supplemented Factors | Ref. |
|---|---|---|---|---|
| GEL/PCL PGA | - | Ad-MSCs | - | [65] |
| PCL/PTMC-MA (1:3) | E = 31.13 ± 1.30 MPa Maximum Stress at Break = 23.80 ± 3.44 MPa Yield Strain = 170 ± 22% | Mouse C57BL/6 | - | [66] |
| Aligned PCL/Collagen multiscale fibers | - | hMSCs (Lonza) | - | [67] |
| PCL Braided | Ultimate elongation (mm) = 24.47 ± 5.61 Stiffness (N/mm) = 11.25 ± 5.04 | BM-MSCs | 50 μg/mL ascorbic acid 2-phosphate + 10 ng/mL TGF-β3 | [68] |
| PLLA Braided | Ultimate elongation (mm) = 17.01 ± 3.86 Stiffness (N/mm) = 5.94 ± 2.68 | |||
| PCL Stacked | Ultimate elongation (mm) = 9.47 ± 1.15 Stiffness (N/mm) = 36.51 ± 7.33 | |||
| PLLA Stacked | Ultimate elongation (mm) = 6.43 ± 3.06 Stiffness (N/mm) = 24.31 ± 7.61 | |||
| PCL/PLA | - | Ad-MSCs | 20 ng/mL TGF-β3 hTC/hUVEC | [69] |
| PLGA/PLA HY | - | Ad-MSCs | 20 ng/mL TGF-β3, TGF-β4 | [70] |
| PPDO/SF | UTS = 31 ± 1 MPa PPDO WNS 25 ± 1 MPa PPDO WNS 4/1 17 ± 1 MPa PPDO WNS 2/1 | Ad-MSCs | 20 ng/mL TGF-β3 | [71] |
| SF/P3HB | UTS = 197.0 ± 7.7 MPa | Ad-MSCs | GDF-5 | [72] |
| PCL dissolved in HFIP | UTS = ~55 MPa E = ~100 MPa | BM-MSCs | 1% P/S | [73] |
| PCL | Young’s modulus = 121.5 ± 3.8 MPa Yield stress = 6.3 ± 0.09 MPa Yield Strain = ~10% | BM-MSCs | 50 μg/mL L-AA 2-phosphate, 0.25 μg/mL amphotericin B, 100 ng/mL CTGF | [74] |
| GO-PLGA | UTS = 2.37 ± 0.31 MPa (PLGA) 2.05 ± 0.29 MPa (GO-PLGA) | BM-MSCs | - | [75] |
| Ch-PLLA-GEL-PEO | UTS = 14.23 ± 1.08 MPa (aligned), 2.43 ± 0.28 MPa (randomly) E = 325.01 ± 25.05 MPa (aligned), 74.46 ± 12.99 MPa (randomly) | hiPSC | 2 mM L-glutamine, 10 ng/mL bFGF, 1% AA, | [76] |
| Aligned and Randomly oriented PLLA | E = 24.42 ± 2.20 MPa (aligned) 20.86 ± 3.56 MPa (randomly) | C3H10T1/2 Rat MSCs | 10 mM β-glycerophosphate, 0.1 mM DXM, 50 mg/mL AA | [77] |
| PCL B@P S + B@P | - | SD Rats BM-MSCs | SDF-1α, BMP-2 | [78] |
| PS | - | BM-MSCs | - | [79] |
| SF/GelMa | UTS = 500–1000 kPa (%GElMa dependent) | BM-MSCs | - | [80] |
| Nanofibers | Mechanical Properties | MSC Source | Supplemented Factors | Ref. |
|---|---|---|---|---|
| Pectin HG | - | BM-MSCs | 10 ng/mL TGFβ1, 50 ng/mL VEGF | [82] |
| PCL (SES diameter 1,4 ± 0,2 μm MEW diameter 15,2 ± 4,8 μm) | - | BM-MSCs | 30 μM ASAP, 5 ng/mL TGF-β1 | [83] |
| PCL/GEL co-spun functionalized with Hep-PG | Strain = 50% | Ad of Sprague–Dawley (SD) Rats | VEGF | [84] |
| PLCL + S-PLCL + H-PLCL | UTS = 11.38 ± 0.34 MPa E = 0.99 ± 0.10 MPa Elongation = 776.4 ± 44.9% | hMSCs | - | [85] |
| PCL | - | - | VEGF | [86] |
| Core/shell (PCL + GEL 3:6 150 ng SF + 250 ng VEGF) | E = 3.0 MPa (PCL/GEL) 3.3 MPa (PCL/GEL + SF) 2.8 MPa for 150 ng, 2.6 MPa for 250 ng (PCL/GEL + SF + VEGF) | hMSCs from Lonza, Singapore | VEGF | [87] |
| PCL/GEL | E = 0.86 MPa | USCs | - | [88] |
| ECM derived from decellularized hDF cell sheets | - | hMSCs | - | [89] |
| PLLA | - | hWJ from the UC | ASA as an antithrombogenic agent | [90] |
| PEA | - | 10T1/2 | TGF-b1 | [91] |
| N° | Title | Field of the Invention | Ref. |
|---|---|---|---|
| US10052412B2 | Electrospun electroactive polymers for regenerative medicine applications | Synthetic electroactive, or piezoelectric, biomaterial useful as an electroactive scaffold for repairing tissues. | [104] |
| US20190030211A1 | Hydrogel scaffold for three-dimensional cell culture | A scaffolding system that includes a thermosensitive hydrogel and a biodegradable polymer blended into a composite, electrospun microfibrous structure. | [105] |
| US20110293685A1 | Scaffolds for tissue engineering and regenerative medicine | The present invention relates to the field of tissue regeneration and replacement. | [106] |
| EP4374006A1 | Method for the production of a shape-memory tissue and relative uses | The present invention involves a method for producing a shape-memory tissue using polymeric matrices that can temporarily alter their shape in response to an external stimulus. These matrices are also designed to support the delivery of cells and/or drugs. | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisani, S.; Evangelista, A.; Chesi, L.; Croce, S.; Avanzini, M.A.; Dorati, R.; Genta, I.; Benazzo, M.; Comoli, P.; Conti, B. Nanofibrous Scaffolds’ Ability to Induce Mesenchymal Stem Cell Differentiation for Soft Tissue Regenerative Applications. Pharmaceuticals 2025, 18, 239. https://doi.org/10.3390/ph18020239
Pisani S, Evangelista A, Chesi L, Croce S, Avanzini MA, Dorati R, Genta I, Benazzo M, Comoli P, Conti B. Nanofibrous Scaffolds’ Ability to Induce Mesenchymal Stem Cell Differentiation for Soft Tissue Regenerative Applications. Pharmaceuticals. 2025; 18(2):239. https://doi.org/10.3390/ph18020239
Chicago/Turabian StylePisani, Silvia, Aleksandra Evangelista, Luca Chesi, Stefania Croce, Maria Antonietta Avanzini, Rossella Dorati, Ida Genta, Marco Benazzo, Patrizia Comoli, and Bice Conti. 2025. "Nanofibrous Scaffolds’ Ability to Induce Mesenchymal Stem Cell Differentiation for Soft Tissue Regenerative Applications" Pharmaceuticals 18, no. 2: 239. https://doi.org/10.3390/ph18020239
APA StylePisani, S., Evangelista, A., Chesi, L., Croce, S., Avanzini, M. A., Dorati, R., Genta, I., Benazzo, M., Comoli, P., & Conti, B. (2025). Nanofibrous Scaffolds’ Ability to Induce Mesenchymal Stem Cell Differentiation for Soft Tissue Regenerative Applications. Pharmaceuticals, 18(2), 239. https://doi.org/10.3390/ph18020239












