Mandragora autumnalis Distribution, Phytochemical Characteristics, and Pharmacological Bioactivities
Abstract
1. Introduction
2. Methods
3. Taxonomical Classification of Mandragora autumnalis
4. Geographical and Botanical Characteristics of Mandragora L.
4.1. Geographical Characteristics
4.2. Botanical Characteristics of Mandragora autumnalis
5. Phytochemical Constituents of Mandragora autumnalis
6. Pharmacological Bioactivities of Mandragora autumnalis
6.1. Antioxidant Activities
6.2. Antimicrobial Activities
6.3. Anticancer Activities
6.4. Antidiabetic, Anti-Enzymatic, and Anti-Obesity Activities
6.5. Anticholinesterase and Anti-Tyrosinase Activities
6.6. Anti-Inflammatory Activities
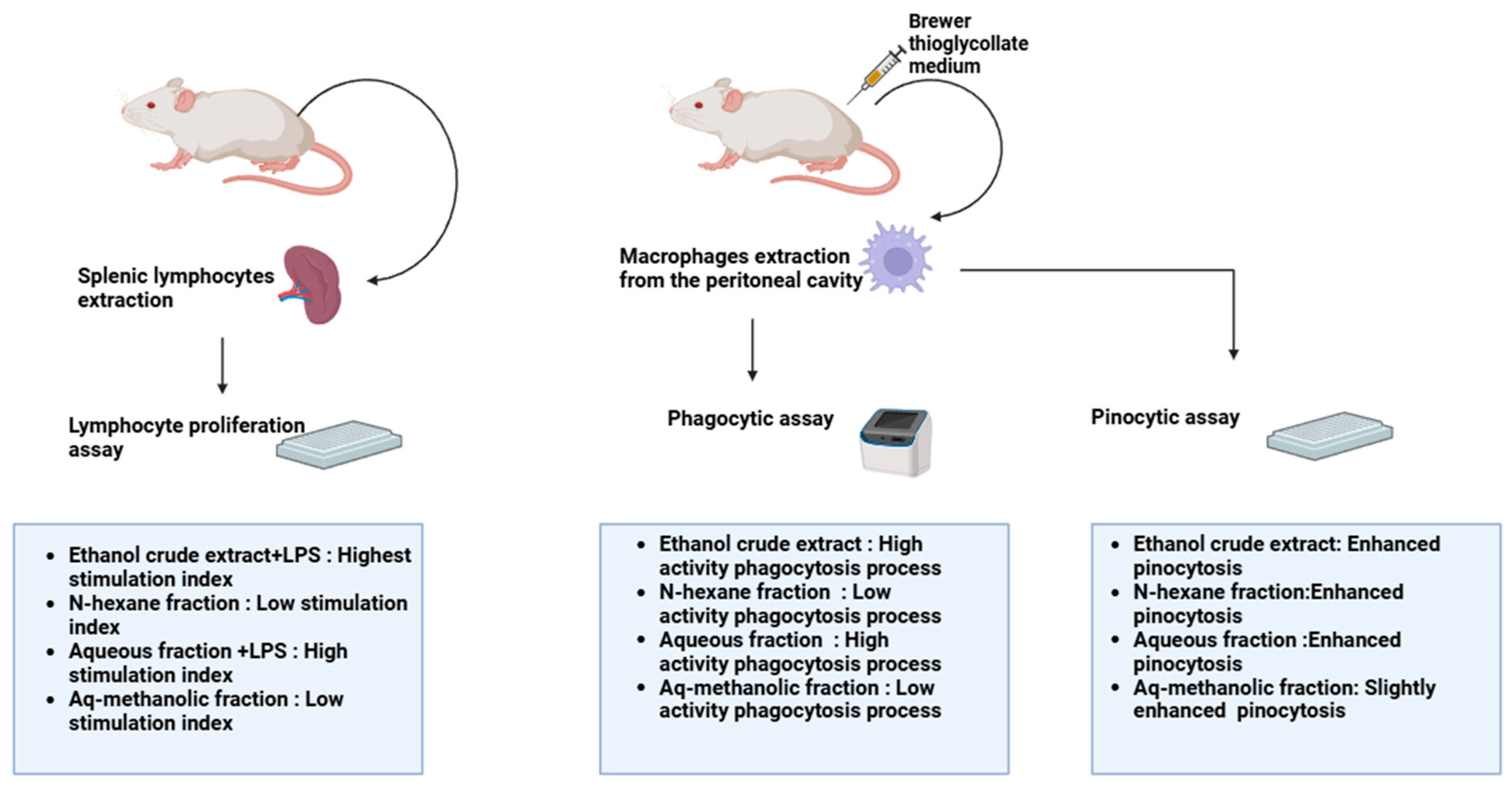
| Extract | Dose | Experimental Model | Observation | References |
|---|---|---|---|---|
| Ethanolic crude extract | 4 mg/mL | -Lymphocyte proliferation assay -Phagocytic assay -Pinocytosis assay | -Highest activity, with an LPS index value of 3.6 -Highest phagocytotic index of 325 -Highest pinocytotic index of 208 | [13] |
| n-hexane fraction | 4 mg/mL | -Lymphocyte proliferation assay -Phagocytic assay -Pinocytosis assay | -Lowest activity with Con A -Least phagocytotic index of 143 -Highest pinocytotic index of 208 | [13] |
| Aqueous fraction | 4 mg/mL | -Lymphocyte proliferation assay -Phagocytic assay -Pinocytosis assay | -Highest activity with LPS, with an index value of 2.5 -High phagocytotic index of 298 -High pinocytotic index of 200 | [13] |
| Aqueous-methanolic fraction | 4 mg/mL | -Lymphocyte proliferation assay -Phagocytic assay -Pinocytosis assay | -Lowest activity with Con A -Low phagocytotic index of 165 -High pinocytotic index of 200 | [13] |
6.7. Green Nanotechnology
6.8. Toxicological Studies
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alamgir, A.N.M. Therapeutic Use of Medicinal Plants and Their Extracts; Progress in drug research; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-63861-4. [Google Scholar]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Alum, E.U. The Role of Indigenous Knowledge in Advancing the Therapeutic Use of Medicinal Plants: Challenges and Opportunities. Plant Signal. Behav. 2024, 19, 2439255. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Ernest, T.B.; Elder, D.P.; Martini, L.G.; Roberts, M.; Ford, J.L. Developing Paediatric Medicines: Identifying the Needs and Recognizing the Challenges. J. Pharm. Pharmacol. 2007, 59, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug Discovery from Natural Sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [PubMed]
- Chihomvu, P.; Ganesan, A.; Gibbons, S.; Woollard, K.; Hayes, M.A. Phytochemicals in Drug Discovery-A Confluence of Tradition and Innovation. Int. J. Mol. Sci. 2024, 25, 8792. [Google Scholar] [CrossRef]
- Alzweiri, M.; Sarhan, A.A.; Mansi, K.; Hudaib, M.; Aburjai, T. Ethnopharmacological Survey of Medicinal Herbs in Jordan, the Northern Badia Region. J. Ethnopharmacol. 2011, 137, 27–35. [Google Scholar] [CrossRef]
- Old Names—New Growth: Proceedings of the 2nd ASPNS Conference, University of Graz, 6–10 June 2007, and Related Essays; Anglo-Saxon Plant-Name Survey; Bierbaumer, P., Klug, H.W., Eds.; Peter Lang: Frankfurt, Germany; New York, NY, USA, 2009; ISBN 978-3-631-58316-6. [Google Scholar]
- Piccillo, G.A.; Mondati, E.G.M.; Moro, P.A. Six Clinical Cases of Mandragora autumnalis Poisoning: Diagnosis and Treatment. Eur. J. Emerg. Med. 2002, 9, 342–347. [Google Scholar] [CrossRef]
- Piccillo, G.A.; Miele, L.; Mondati, E.; Moro, P.A.; Musco, A.; Forgione, A.; Gasbarrini, G.; Grieco, A. Anticholinergic Syndrome Due to ‘Devil’s Herb’: When Risks Come from the Ancient Time: DEVIL’S HERB. Int. J. Clin. Pract. 2006, 60, 492–494. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Řezanka, T.; Spížek, J.; Dembitsky, V.M. Substances Isolated from Mandragora Species. Phytochemistry 2005, 66, 2408–2417. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Bassalat, N.; Hawash, M.; Zaid, H. Isolation, Identification and Pharmacological Effects of Mandragora autumnalis Fruit Flavonoids Fraction. Molecules 2022, 27, 1046. [Google Scholar] [CrossRef] [PubMed]
- Mahmod, A.I.; Talib, W.H. Anticancer Activity of Mandragora autumnalis: An in Vitro and in Vivo Study. Pharmacia 2021, 68, 827–835. [Google Scholar] [CrossRef]
- Monadi, T.; Azadbakht, M.; Ahmadi, A.; Chabra, A. A Comprehensive Review on the Ethnopharmacology, Phytochemistry, Pharmacology, and Toxicology of the Mandragora Genus; from Folk Medicine to Modern Medicine. Curr. Pharm. Des. 2021, 27, 3609–3637. [Google Scholar] [CrossRef] [PubMed]
- Volis, S.; Fogel, K.; Tu, T.; Sun, H.; Zaretsky, M. Evolutionary History and Biogeography of Mandragora L. (Solanaceae). Mol. Phylogenet. Evol. 2018, 129, 85–95. [Google Scholar] [CrossRef]
- Dafni, A.; Blanché, C.; Khatib, S.A.; Petanidou, T.; Aytaç, B.; Pacini, E.; Kozuharova, E.; Geva-Kleinberger, A.; Shahvar, S.; Dajic, Z.; et al. In Search of Traces of the Mandrake Myth: The Historical, and Ethnobotanical Roots of Its Vernacular Names. J. Ethnobiol. Ethnomed. 2021, 17, 68. [Google Scholar] [CrossRef]
- Mandragora Autumnalis—Mandrake. Available online: https://www.magicgardenseeds.com/Autumn-Mandrake-Mandragora-autumnalis-seeds?srsltid=AfmBOorgLYodi6rr0PK5t_ohDKN9kgd3-A3yWT4uwe0riFzb0sOjWx_R (accessed on 10 February 2025).
- Azab, A. Solanaceae Plants of Israel and Palestine. Rich Source of Medicinally Active Natural Products. Eur. Chem. Bull. 2020, 9, 199. [Google Scholar] [CrossRef]
- Fleisher, A.; Fleisher, Z. The Fragrance of Biblical Mandrake. Econ. Bot. 1994, 48, 243–251. [Google Scholar] [CrossRef]
- King, H. Hippocrates’ Woman: Reading the Female Body in Ancient Greece, 1st ed.; Routledge: London, UK, 2002; ISBN 978-0-203-02599-4. [Google Scholar]
- Théophraste; Hort, A. Enquiry into Plants and Minor Works on Odours and Weather Signs; Loeb classical library; Harvard University Press: Cambridge, MA, USA; London, UK, 1990; ISBN 978-0-674-99077-7. [Google Scholar]
- Byl, S. Suzanne AMIGUES, Théophraste. Recherches Sur Les Plantes. Tome V. Livre IX. L’Antiquité Class. 2007, 76, 276–278. [Google Scholar]
- Salazar, C.F. L. Y. Beck (Tr.), Pedanius Dioscorides of Anazarbus, De Materia Medica (Altertumswissenschaftliche Texte Und Studien, Vol. 38), Hildesheim-Zurich-New York: Olms-Weidmann, 2005, 540 + Xxviii Pp., ISBN 3-487-12881-0. Ex. Class. 2007, 11, 397–400. [Google Scholar] [CrossRef]
- Safadi, R. The Liver in Greco-Arabic and Islamic Medicine. Clin. Liver Dis. 2024, 23, e0137. [Google Scholar] [CrossRef]
- Benítez, G.; Leonti, M.; Böck, B.; Vulfsons, S.; Dafni, A. The Rise and Fall of Mandrake in Medicine. J. Ethnopharmacol. 2023, 303, 115874. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2009; ISBN 978-0-470-74168-9. [Google Scholar]
- Jackson, B.P.; Berry, M.I. Hydroxytropane Tiglates in the Roots of Mandragora Species. Phytochemistry 1973, 12, 1165–1166. [Google Scholar] [CrossRef]
- Mahmod, A.I.; Talib, W.H. Chemical Composition, Antioxidant, Antimicrobial, and Immunomodulatory Activity of Mandragora autumnalis Grown in Jordan. Nat. Prod. J. 2023, 13, e020622205552. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kürkçüoglu, M.; Demirci, B.; Erdogmus, H. Composition of the Essential Oil and the Headspace Sample of Mandragora autumnalis Bertol. Fruits. J. Essent. Oil Res. 1998, 10, 632–634. [Google Scholar] [CrossRef]
- Kaya, A.; Demirci, B.; Başer, K.H.C. Study of the Essential Oils from the Flowers and Fruits of Scandix iberica Bieb. Growing in Turkey. J. Essent. Oil Res. 2007, 19, 155–156. [Google Scholar] [CrossRef]
- Suleiman, R.K.; Zarga, M.A.; Sabri, S.S. New Withanolides from Mandragora officinarum: First Report of Withanolides from the Genus Mandragora. Fitoterapia 2010, 81, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Comparative Study of Volatile Compounds in the Fresh Fruits of Mandragora autumnalis. Available online: https://www.researchgate.net/publication/237806241_Comparative_study_of_volatile_compounds_in_the_fresh_fruits_of_Mandragora_autumnalis (accessed on 10 February 2025).
- Cornara, L.; Smeriglio, A.; Frigerio, J.; Labra, M.; Di Gristina, E.; Denaro, M.; Mora, E.; Trombetta, D. The Problem of Misidentification between Edible and Poisonous Wild Plants: Reports from the Mediterranean Area. Food Chem. Toxicol. 2018, 119, 112–121. [Google Scholar] [CrossRef]
- Takemoto, Y.; Kuraoka, S.; Ohra, T.; Yonetoku, Y.; Iwata, C. Diastereoselective Total Synthesis of (−)-Solavetivone via a Copper-Catalyzed Conjugate Addition of Me3Al to a Cyclohexa-2,5-Dienone Intermediate. Tetrahedron 1997, 53, 603–616. [Google Scholar] [CrossRef]
- Fuchs, A.; Slobbe, W.; Mol, P.C.; Posthumus, M.A. GC/MS Analysis of Fungitoxic Terpenoids from Tobacco. Phytochemistry 1983, 22, 1197–1199. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic Phenolic Antioxidants: Metabolism, Hazards and Mechanism of Action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, L.; Kokot, S. Voltammetric Determination of Butylated Hydroxyanisole, Butylated Hydroxytoluene, Propyl Gallate and Tert-Butylhydroquinone by Use of Chemometric Approaches. Anal. Chim. Acta 2000, 412, 185–193. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Dogra, V.; Verma, D.; Fortunati, E. Biopolymers and Nanomaterials in Food Packaging and Applications. In Nanotechnology-Based Sustainable Alternatives for the Management of Plant Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 355–374. ISBN 978-0-12-823394-8. [Google Scholar]
- Uysal, S.; Zengin, G.; Aktümsek, A. Antioxidant Properties and Enzyme Inhibitory Effects of Extracts from Mandragora autumnalis and Its Fatty Acid Composition. Marmara Pharm. J. 2016, 20, 144. [Google Scholar] [CrossRef]
- Dağlioğlu, Y.; Öztürk, B. Green Synthesis of Silver Nanoparticles Using Mandragora autumnalis; Its Characterization, Antioxidant and Antimicrobial Activities. Erzincan Üniversitesi Fen Bilim. Enstitüsü Derg. 2021, 14, 1039–1054. [Google Scholar] [CrossRef]
- Jafri, L.; Saleem, S.; Ihsan-ul-Haq; Ullah, N.; Mirza, B. In Vitro Assessment of Antioxidant Potential and Determination of Polyphenolic Compounds of Hedera Nepalensis K. Koch. Arab. J. Chem. 2017, 10, S3699–S3706. [Google Scholar] [CrossRef]
- Cos, P.; Calomme, M.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure-Activity Relationship of Flavonoids as Antioxidant and Pro-Oxidant Compounds. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2000; Volume 22, pp. 307–341. ISBN 978-0-444-50588-0. [Google Scholar]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Ghagane, S.C.; Puranik, S.I.; Kumbar, V.M.; Nerli, R.B.; Jalalpure, S.S.; Hiremath, M.B.; Neelagund, S.; Aladakatti, R. In Vitro Antioxidant and Anticancer Activity of Leea Indica Leaf Extracts on Human Prostate Cancer Cell Lines. Integr. Med. Res. 2017, 6, 79–87. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Esin Çelik, S.; Baki, S.; Yıldız, L.; Karaman, Ş.; Apak, R. A Comprehensive Review of CUPRAC Methodology. Anal. Methods 2011, 3, 2439. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; De Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef]
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Kutikhin, A.G. Introduction. In Interleukins in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–16. ISBN 978-0-12-801121-8. [Google Scholar]
- Ma, X.; Yu, H. Global Burden of Cancer. Yale J. Biol. Med. 2006, 79, 85–94. Available online: https://pubmed.ncbi.nlm.nih.gov/17940618/ (accessed on 10 February 2025).
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Jiménez-González, V.; Benítez, G.; Pastor, J.E.; López-Lázaro, M.; Calderón-Montaño, J.M. Evaluation of Anticancer Activity of 76 Plant Species Collected in Andalusia (Spain) against Lung Cancer Cells. Plants 2023, 12, 3275. [Google Scholar] [CrossRef] [PubMed]
- Lieber, M.; Todaro, G.; Smith, B.; Szakal, A.; Nelson-Rees, W. A Continuous Tumor-cell Line from a Human Lung Carcinoma with Properties of Type II Alveolar Epithelial Cells. Int. J. Cancer 1976, 17, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J. Animal Models for Studying Prevention and Treatment of Breast Cancer. In Animal Models for the Study of Human Disease; Elsevier: Amsterdam, The Netherlands, 2013; pp. 997–1018. ISBN 978-0-12-415894-8. [Google Scholar]
- Westergard, L.; Christensen, H.M.; Harris, D.A. The Cellular Prion Protein (PrPC): Its Physiological Function and Role in Disease. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Al-Jaderi, Z.; Hachim, M.Y.; Maghazachi, A.A. HCT-116 Colorectal Cancer Cells Secrete Chemokines Which Induce Chemoattraction and Intracellular Calcium Mobilization in NK92 Cells. Cancer Immunol. Immunother. 2019, 68, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Twentyman, P.R.; Wright, K.A.; Fox, N.E. Characterisation of a Mouse Tumour Cell Line with in Vitro Derived Resistance to Verapamil. Br. J. Cancer 1990, 61, 279–284. [Google Scholar] [CrossRef]
- Ammerman, N.C.; Beier-Sexton, M.; Azad, A.F. Growth and Maintenance of Vero Cell Lines. Curr. Protoc. Microbiol. 2008, 11, A.4E.1–A.4E.7. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of Inhibition of α-Amylase and α-Glucosidase by Aqueous Extract of Morinda lucida Benth Leaf. BioMed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef]
- Matsui, T.; Ogunwande, I.; Abesundara, K.; Matsumoto, K. Anti-Hyperglycemic Potential of Natural Products. Mini-Reviews Med. Chem. 2006, 6, 349–356. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The Role of Medicinal Plants in the Treatment of Diabetes: A Systematic Review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P.A. Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Parhofer, K.G. Interaction between Glucose and Lipid Metabolism: More than Diabetic Dyslipidemia. Diabetes Metab. J. 2015, 39, 353. [Google Scholar] [CrossRef] [PubMed]
- Grimmelmann, I.; Momma, M.; Zimmer, L.; Hassel, J.C.; Heinzerling, L.; Pföhler, C.; Loquai, C.; Ruini, C.; Utikal, J.; Thoms, K.-M.; et al. Lipase Elevation and Type 1 Diabetes Mellitus Related to Immune Checkpoint Inhibitor Therapy—A Multicentre Study of 90 Patients from the German Dermatooncology Group. Eur. J. Cancer 2021, 149, 1–10. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Hu, X.; Huang, X.; Chen, G.-X. Current Understanding of Glucose Transporter 4 Expression and Functional Mechanisms. World J. Biol. Chem. 2020, 11, 76–98. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Minic, J.; Chatonnet, A.; Krejci, E.; Molgó, J. Butyrylcholinesterase and Acetylcholinesterase Activity and Quantal Transmitter Release at Normal and Acetylcholinesterase Knockout Mouse Neuromuscular Junctions. Br. J. Pharmacol. 2003, 138, 177–187. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lee, V.M.-Y.; Trojanowski, J.Q. Parkinson’s Disease Dementia: Convergence of α-Synuclein, Tau and Amyloid-β Pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef]
- Qi, S.; Guo, L.; Liang, J.; Wang, K.; Liao, Q.; He, S.; Lyu, W.; Cheng, Z.; Wang, J.; Luo, X.; et al. A New Strategy for the Treatment of Parkinson’s Disease: Discovery and Bio-Evaluation of the First Central-Targeting Tyrosinase Inhibitor. Bioorg. Chem. 2024, 150, 107612. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Shi, J.; Yu, C.; Zhao, M.; Xue, S.; Jiang, Y. Enhanced Antioxidant and Antityrosinase Activities of Longan Fruit Pericarp by Ultra-High-Pressure-Assisted Extraction. J. Pharm. Biomed. Anal. 2010, 51, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Okin, D.; Medzhitov, R. Evolution of Inflammatory Diseases. Curr. Biol. 2012, 22, R733–R740. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Festus-Ikhuoria, I.C.; Obiuto, N.C.; Adebayo, R.A.; Olajiga, O.K. Nanotechnology in Consumer Products: A Review of Applications and Safety Considerations. World J. Adv. Res. Rev. 2023, 21, 2050–2059. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abd-Elaziem, W.; Elsheikh, A.; Zayed, A.A. Advancements in Nanomaterials for Nanosensors: A Comprehensive Review. Nanoscale Adv. 2024, 6, 4015–4046. [Google Scholar] [CrossRef]
- Patel, B.; Darji, P.; Fnu, P.I.J.; Nalla, S.; Khatri, V.; Parikh, S. A Comprehensive Review and Insight into the Latest Advancements in Nanotechnology. Biosci. Biotech. Res. Asia 2024, 21, 985–1000. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Ponce, L.C.; Manavalan, K.; Awad, F.; Rajan, V.F. Nanoparticles as an Exotic Antibacterial, Antifungal, and Antiviral Agents. In Advances in Nanotechnology for Marine Antifouling; Elsevier: Amsterdam, The Netherlands, 2023; pp. 231–270. ISBN 978-0-323-91762-9. [Google Scholar]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of Silver Nanoparticles by Chemical and Biological Methods and Their Antimicrobial Properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of Silver Nanoparticles by Chemical Reduction Method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Alhamid, M.Z.; Hadi, B.S.; Khumaeni, A. Synthesis of Silver Nanoparticles Using Laser Ablation Method Utilizing Nd:YAG Laser. AIP Conf. Proc. 2019, 2202, 020013. [Google Scholar]
- Ali, I.; Qiang, T.; Ilahi, N.; Kakakhel, M.; Sajjad, W. Green Synthesis of Silver Nanoparticles by Using Bacterial Extract and Its Antimicrobial Activity against Pathogens. Int. J. Biosci. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Zhang, J.; Onakpoya, I.J.; Posadzki, P.; Eddouks, M. The Safety of Herbal Medicine: From Prejudice to Evidence. Evid.-Based Complement. Altern. Med. 2015, 2015, 316706. [Google Scholar] [CrossRef]
- Mssusa, A.K.; Holst, L.; Kagashe, G.; Maregesi, S. Safety Profile of Herbal Medicines Submitted for Marketing Authorization in Tanzania: A Cross-Sectional Retrospective Study. J. Pharm. Policy Pract. 2023, 16, 149. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing Herbal Medicine: Enhancing Product Quality and Safety through Robust Quality Control Practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- Van Wyk, A.S.; Prinsloo, G. Health, Safety and Quality Concerns of Plant-Based Traditional Medicines and Herbal Remedies. S. Afr. J. Bot. 2020, 133, 54–62. [Google Scholar] [CrossRef]
- An, I.; Ozturk, M. Phytodermatitis in East and Southeast of Turkey: A Prospective Study. Cutan. Ocul. Toxicol. 2019, 38, 176–181. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus Synthetic Anesthetic for Transport of Live Fish: A Review. Aquac. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Müller, J.; Wanke, K. Intoxikationspsychosen durch Atropin und Skopolamin. Fortschr. Neurol. Psychiatr. 1998, 66, 289–295. [Google Scholar] [CrossRef]
- Shim, K.H.; Kang, M.J.; Sharma, N.; An, S.S.A. Beauty of the Beast: Anticholinergic Tropane Alkaloids in Therapeutics. Nat. Prod. Bioprospect. 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Katırcı, Y.; Demirel, B.; Coşkun, F. Contact Dermatitis Associated with Mandragora Plant. Acta Med. Anatol. 2014, 2, 27. [Google Scholar] [CrossRef]
- Baysak, S.; Gönül, M.; Atacan, D.; Ergin, C. A Case Report of Allergic Contact Dermatitis Due to Mandragora Radix. Case Rep. Immunol. 2015, 2015, 591438. [Google Scholar] [CrossRef]

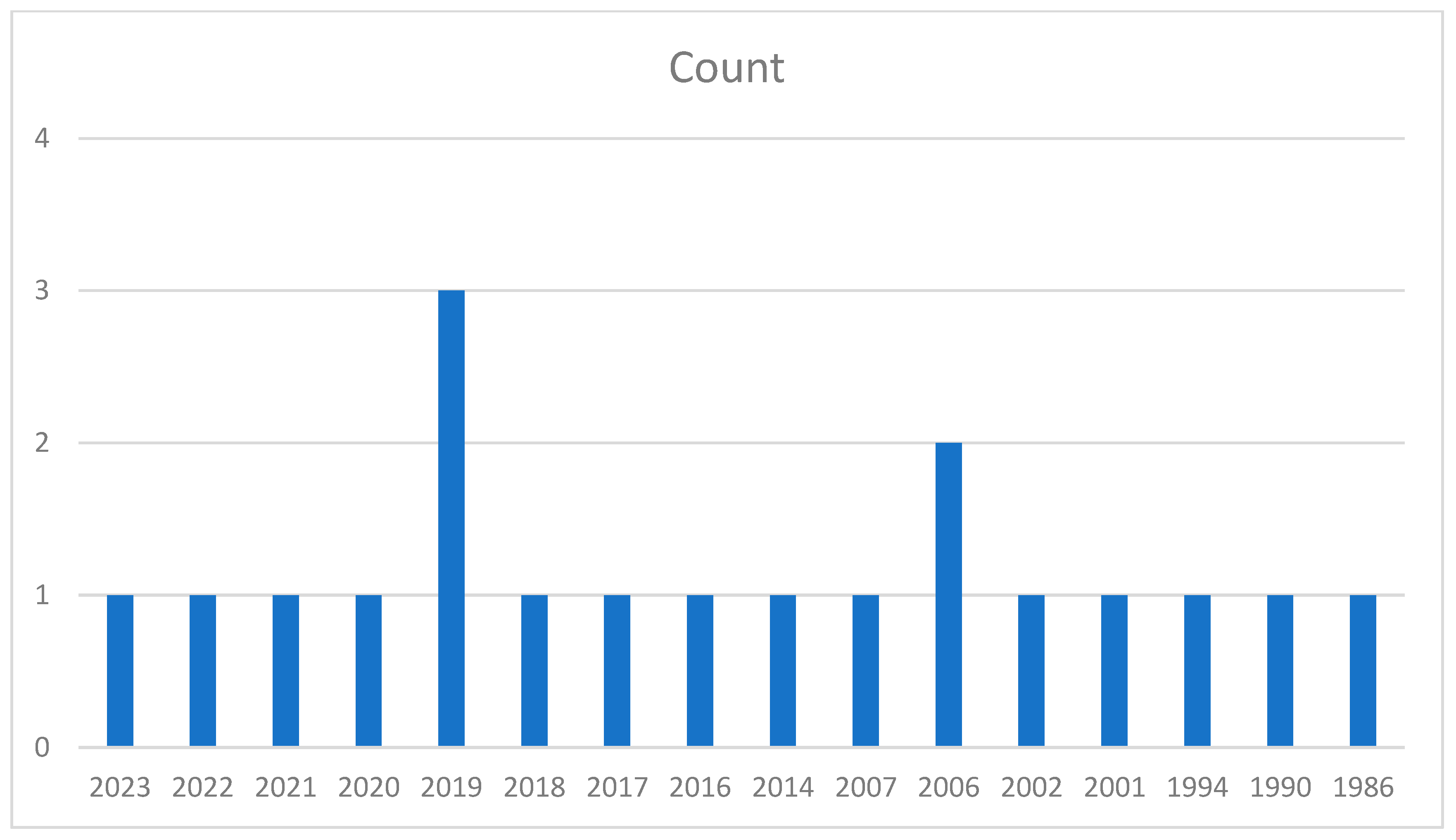
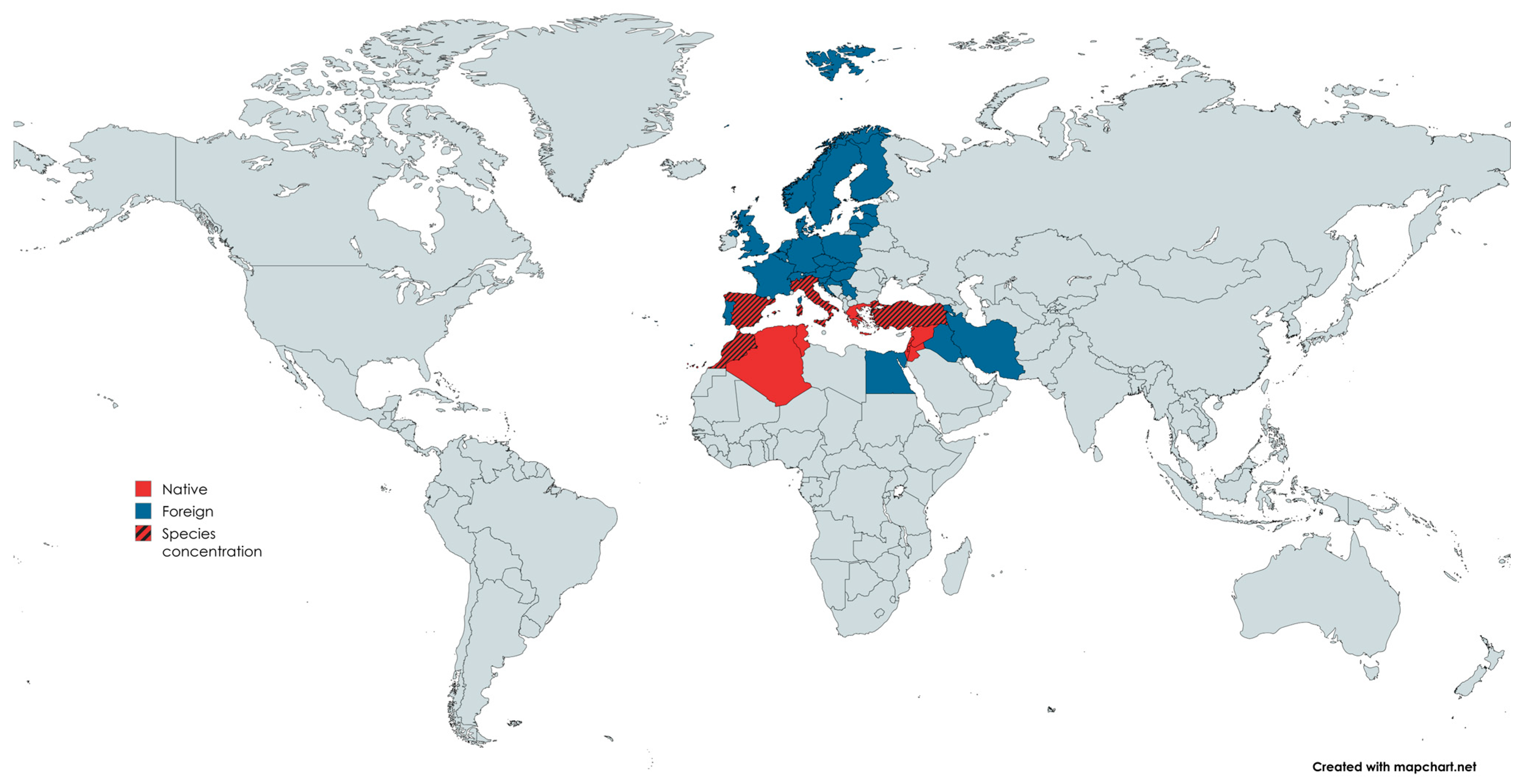
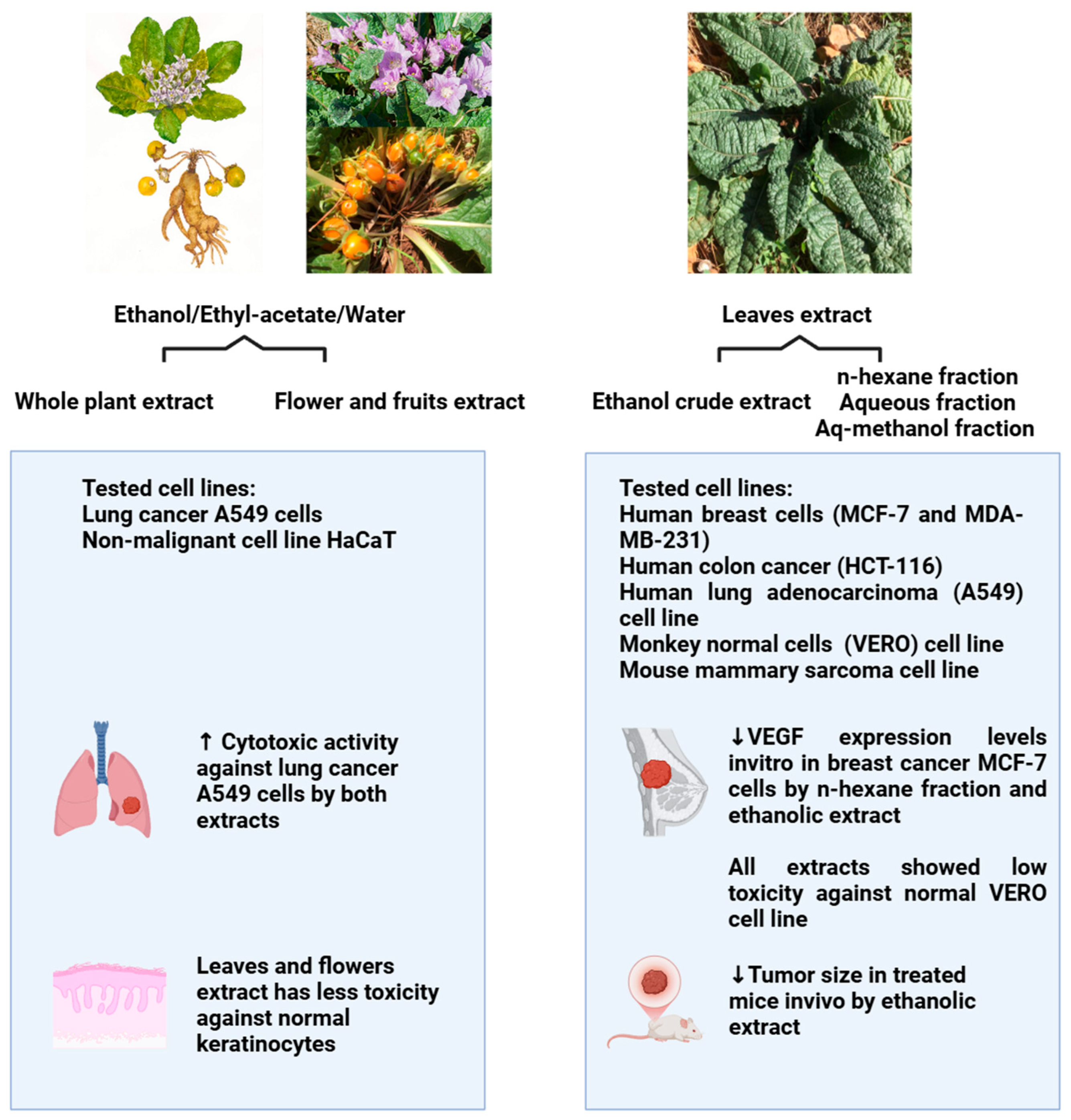

| Kingdom | Plantae |
|---|---|
| Phylum | Tracheophyta |
| Class | Magnoliopsida |
| Order | Solanales |
| Family | Solanaceae |
| Genus | Mandragora L. |
| Species | Mandragora autumnalis Bertol. |
| Plant Part | Mode of Usage | Traditional Use | Country | Reference |
|---|---|---|---|---|
| Roots | Warm pad | Treat tendons | Persia | [21] |
| Leaves | Poultice | Treat wounds | Cyprus | [22] |
| Roots | Soaked in vinegar | Gout | Persia | [23] |
| Seeds | Clyster | Uterus cleaning | Turkey North Africa | [24] |
| Leaves | External application | Abscesses Gland’s swellings small tumors | Egypt | [24] |
| Roots | Oral | Headache Snake bites Anesthesia Sedation Inflammation | Mediterranean area Europe | [25] |
| Leaves | Rubbed to skin | Freckles removal | Not identified | [26] |
| Leaves | Oral | Coughs Asthma Bronchitis | Jorden Morocco Northern Africa | [26] |
| Plant Part Extract | Extraction Solvent | Compounds | Major Metabolites | Reference |
|---|---|---|---|---|
| Fruits | Ethyl acetate | Flavonoids |  Kaempferol | [12] |
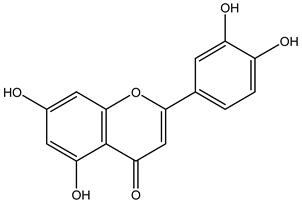 Luteolin | ||||
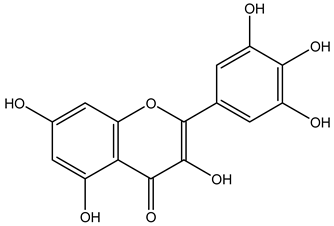 Myricetin | ||||
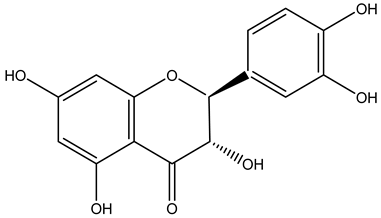 Taxifolin | ||||
| Fruits | Water distillation | Esters | 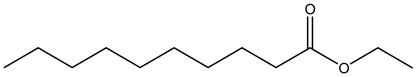 Ethyl caprate | [30,31] |
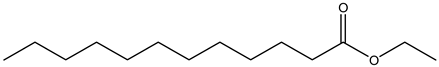 Ethyl laurate | ||||
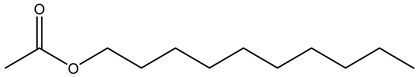 Decyl acetate | ||||
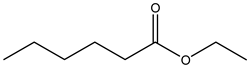 Ethyl caproate | ||||
| Leaves | Ethanolic extract | Phenolic acids | 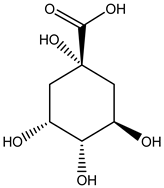 Quinic acid | [29] |
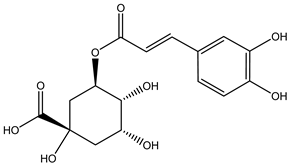 Chlorogenic acid | ||||
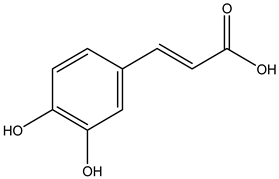 Caffeic acid | ||||
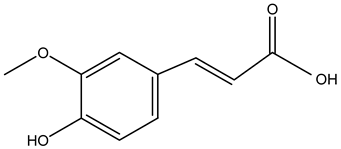 Ferulic acid | ||||
 Spiraeoside | ||||
| Leaves | Ethanolic extract | Fatty acids | 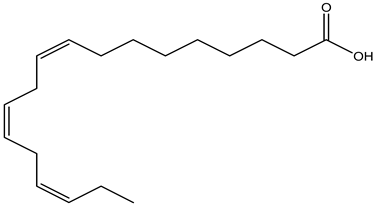 α-Linolenic acid | [14] |
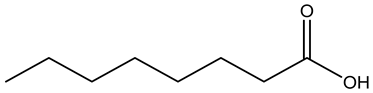 Caprylic acid | ||||
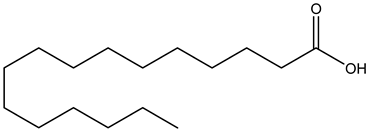 Palmitic acid | ||||
| Esters |  Ethyl butanoate | |||
| Alkanes | 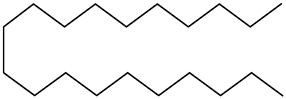 Eicosane | |||
| Monoterpenoids | 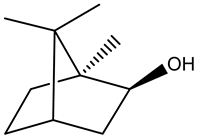 Borneol | |||
| Triterpenoids | 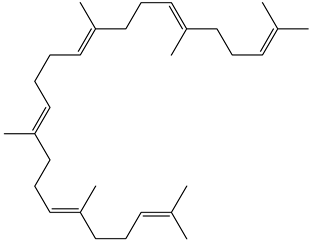 Squalene | |||
| Roots | Methanolic extract | Alkaloids | 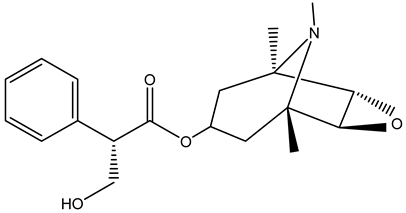 Scopolamine | [26,28] |
 Apoatropine | ||||
 3 α-tigloyloxytropane | ||||
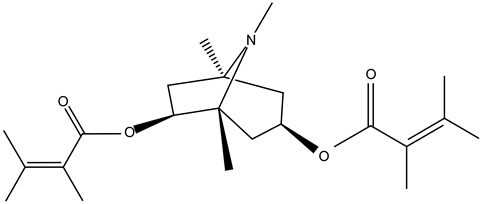 3,6-ditigloyloxytropane | ||||
 Beta-belladonnine | ||||
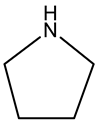 Pyrrolidine | ||||
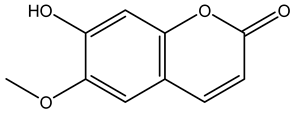 Scopoletin | ||||
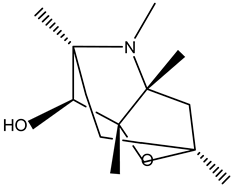 Scopoline |
| Extract | Plant Part | Dose | Methods | Observations | References |
|---|---|---|---|---|---|
| Flavonoid fraction | Ripe fruit | 20–120 µg/mL | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -Strong antioxidant activity -IC50 5.37 ± 0.41 µg/mL | [13] |
| -Methanolic extract -Acetone extract | Flowers | N/A | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -73.09 mg TE/g extract (strong antioxidant activity) -39.30 ± 0.10 mg TE/g extract (mild antioxidant activity) | [45] |
| -Methanolic extract -Acetone extract | Flowers | N/A | Phosphomolybdenum method | -mild antioxidant activity for both extracts -1.20 mmolTE/g extract -1.14 mmolTE/g extract | [45] |
| -Methanolic extract -Acetone extract | Flowers | N/A | Cupric ion reducing (CUPRAC) method | -113.24 mgTE/g extract (high antioxidant activity) -77.47 mgTE/g extract (mild antioxidant activity) | [45] |
| -Methanolic extract -Acetone extract | Flowers | N/A | Ferric reducing antioxidant power (FRAP) method | -90.88 mgTE/g extract (high antioxidant activity) -50.41 mgTE/g extract (mild antioxidant activity) | [45] |
| -Methanolic extract -Acetone extract | Flowers | N/A | Metal-chelating activity on ferrous ions | -high antioxidant activity for both extracts -15.94 mgEDTA/g extract -15.61 mgEDTA/g extract | [45] |
| -Methanolic extract -Acetone extract | Leaves | N/A | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -mild antioxidant activity for both extracts -51.44 ± 0.2 9 mg TE/g -33.19 ± 0.13 mg TE/g | [45] |
| -Methanolic extract -Acetone extract | Leaves | N/A | Phosphomolybdenum method | -1.03 mmolTE/g extract (mild antioxidant activity) -1.98 mmolTE/g extract (strong antioxidant activity) | [45] |
| -Methanolic extract -Acetone extract | Leaves | N/A | Cupric ion reducing (CUPRAC) method | -Mild antioxidant activities for both extracts -77.98 mgTE/g extract -86.55 mgTE/g extract | [45] |
| -Methanolic extract -Acetone extract | Leaves | N/A | Ferric reducing antioxidant power (FRAP) method | -Mild antioxidant activities for both extracts -53.29 mgTE/g extract -53.53 mgTE/g extract | [45] |
| -Methanolic extract -Acetone Extract | Leaves | N/A | Metal-chelating activity on ferrous ions | -11.64 mgEDTA/g extract (mild antioxidant activity) -6.15 mgEDTA/g extract (low antioxidant activity) | [45] |
| Ethanolic extract | Leaves | 1.56–200 µg/mL | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -High antioxidant activity -IC50 54.14 µg/mL | [29] |
| Aqueous fraction | Leaves | 1.56–200 µg/mL | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -High antioxidant activity -IC50 23.67 µg/mL | [29] |
| n-hexane fraction | Leaves | 1.56–200 µg/mL | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -Mild antioxidant activity -IC50 208.5 ± 3 µg/mL | [29] |
| Aqueous-Methanol fraction | Leaves | 1.56–200 µg/mL | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -Mild antioxidant activity -IC50 165.9 ± 13 µg/mL | [29] |
| Aqueous extract | Roots | 12.5–100 µg/mL | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -Strong antioxidant activity -IC50 47.16 ± 0.41 µg/mL | [46] |
| M.autumnalis synthesized silver nanoparticles | Roots | 12.5–100 µg/mL | 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay | -Strong antioxidant activity -IC50 51.81 ± 0.10 µg/mL | [46] |
| Extract | Dose | Experimental Model | Main Results | References |
|---|---|---|---|---|
| Antibacterial | ||||
| Fruit flavonoid fraction | Dose range: 0.5 to 500 µg/mL | -Method: Microdilution technique -Microorganisms: Gram-positive bacteria: Staphylococcus aureus, Enterococcus faecium and Methicillin-Resistant Staphylococcus aureus Gram negative bacteria: Shigella sonnie, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli | -Good activity against all of the tested bacterial strains -More effective against the K. pneumoniae strain | [13] |
| Extract of the leaves (ethanol extract, n-hexane fraction, aqueous -methanol fraction, aqueous fraction) | Dose range: 0.03 to 50 mg/mL | -Method: Microdilution technique -Microorganisms: Gram positive bacteria: Bacillus subtilis Gram negative bacteria: Pseudomonas aeruginosa and Escherichia coli | -Good activity of the ethanol extract and n-hexane fraction against B. subtilis and P. auriginosa, with MIC values of 25 mg/mL -No activity of aq-methanol and aqueous fractions | [29] |
| Aqueous extract of the roots | 100 µg/mL | -Method: Disk diffusion method -Microorganisms: gram positive bacteria: Staphylococcus aureus, Bacillus subtilis Gram negative bacteria: Pseudomonas aeruginosa, and Escherichia coli | -Good activity against all of the tested bacterial strains -More effective against the Bacillus subtilis strain | [46] |
| Aqueous extract of the synthesized silver nanoparticles of M. autumnalis roots | 100 µg/mL | -Method: Disk diffusion method -Microorganisms: Gram positive bacteria: Staphylococcus aureus, Bacillus subtilis Gram negative bacteria: Pseudomonas aeruginosa, and Escherichia coli | -Good activity against all of the tested bacterial strains -More effective against the Pseudomonas aeruginosa strain | [46] |
| Antifungal | ||||
| Fruit flavonoid fraction | Dose range: 0.5 to 500 µg/mL | -Method: Mircodilution technique -Microorganisms: Epidermatophyton floccosum and Candida albicans | -Potent antifungal activity against C. albicans, with MIC values of 6.25 ± 0.48 µg/mL. -Weak activity against E. floccosum with a MIC value of 12.5 ± 0.88 µg/mL | [13] |
| Extract of the leaves (ethanol extract, n-hexane fraction, aqueous-methanol fraction, aqueous fraction) | Dose range: 0.03 to 50 mg/mL | -Method: Microdilution technique -Microorganisms: Candida albicans | -n-hexane fraction exhibited antifungal activity towards C. albicans, with an MIC value of 12.5 mg/mL. Aqueous/methanol and aqueous fractions showed no activity at 50 mg/mL | [29] |
| The Solvent Used for Extraction | Plant Part | Dose | Experimental Model | Observation | References |
|---|---|---|---|---|---|
| Ethanol/ethyl acetate/water extract | Flowers and fruits | 0.1–1000 µg/mL | Cell availability assay Cell lines: A549, HaCat cells | -Selective toxicity on A549 cells, with IC50 369.5 ± 42.1 µg/mL -Less toxicity against HaCat cells, with IC50 > 1000 µg/mL | [61] |
| Ethanol/ethyl acetate/water extract | Whole plant | 0.1–1000 µg/mL | Cell availability assay Cell lines: A549, HaCat cells | -Selective toxicity on A549 cells, with IC50 201.9 ± 30.7 µg/mL -Less toxicity against HaCat cells, with IC50 645.7 ± 51.4 µg/mL | [61] |
| Ethanol crude extract | Leaves | 0.06–4 mg/mL | -MTT assay -Cell lines: MCF-7, MDA-MB-231, HCT-116, A549, VERO, EMT6/P | -Most effective against MCF-7 cells, with IC50 0.10 ± 0.01 µg/mL -Low activity against VERO normal cell line, with IC50 > 4 µg/mL -Significant decrease in tumor size in vivo with (−35.99%) compared with the untreated control group (+107.02%) | [14] |
| n-hexane fraction | Leaves | 0.06–4 mg/mL | -MTT assay -Cell lines: MCF-7, MDA-MB-231, HCT-116, A549, VERO, EMT6/P | -Most effective against MCF-7 cells with IC50 0.48 ± 0.02 µg/mL -Low activity against VERO normal cell line, with IC50 > 4 µg/mL | [14] |
| Aqueous fraction | Leaves | 0.06–4 mg/mL | -MTT assay -Cell lines: MCF-7, MDA-MB-231, HCT-116, A549, VERO, EMT6/P | -Low cytotoxic effect against all cancerous cell lines -low activity against VERO normal cell line, with IC50 > 4 µg/mL | [14] |
| Aqueous-methanol fraction | Leaves | 0.06–4 mg/mL | -MTT assay -Cell lines: MCF-7, MDA-MB-231, HCT-116, A549, VERO, EMT6/P | -Low cytotoxic effect against all cancerous cell lines -low activity against VERO normal cell line, with IC50 > 4 µg/mL | [14] |
| Extract | Dose | Experimental Model | Observation | Reference |
|---|---|---|---|---|
| Antidiabetic | ||||
| Ethyl acetate fraction of the methanolic extract of M. autumnalis fruits | 0–600 mg/mL | In vitro inhibition of α-glucosidase enzyme assay | -Dose-dependent increase in α-glucosidase inhibition -IC50 against this enzyme was 39.81 ± 0.74 µg/mL | [13] |
| Ethyl acetate fraction of the methanolic extract of M. autumnalis fruits | 0–600 mg/mL | In vitro inhibition of α-amylase enzyme assay | -Dose-dependent increase in α-amylase inhibition -IC50 against this enzyme was 72.44 ± 0.89 µg/mL | [13] |
| Ethyl acetate fraction of the methanolic extract of M. autumnalis fruits | 0–0.53 µg/mL | GLUT4 Translocation to the Plasma Membrane | -Dose-dependent increase in GLUT4 translocation was significantly noticed | [13] |
| Acetone extract from the leaves | N/A | -In vitro inhibition of α-amylase enzyme assay -In vitro inhibition of α-glucosidase enzyme assay | -Highest α-amylase inhibitory activity of 1.86 mmolACAE/g -No inhibitory activity of α-glucosidase | [45] |
| Acetone extract from the flowers | N/A | -In vitro inhibition of α-amylase enzyme assay -In vitro inhibition of α-glucosidase enzyme assay | -Mild α-amylase inhibitory activity of F-Ac of 1.27 mmolACAE/g -Mild inhibitory activity of α-glucosidase | [45] |
| The methanolic effect from the leaves | N/A | -In vitro inhibition of α-amylase enzyme assay -In vitro inhibition of α-glucosidase enzyme assay | -Mild α-amylase inhibitory activity of 0.51 mmolACAE/g -Mild inhibitory activity of α-glucosidase | [45] |
| Methanolic extract from the flowers | N/A | -In vitro inhibition of α-amylase enzyme assay -In vitro inhibition of α-glucosidase enzyme assay | -The least α-amylase inhibitory activity of 0.46 mmolACAE/g -Mild inhibitory activity of α-glucosidase | [45] |
| Anti-lipase | ||||
| Ethyl acetate fraction of the methanolic extract of M. autumnalis fruits | 0–500 mg/mL | In vitro inhibition of lipase enzyme assay | -Dose-dependent increase in lipase inhibition -IC50 against this enzyme was 39.81 ± 1.23 µg/mL | [13] |
| Anticholinesterase and anti-tyrosinase | ||||
| Acetone extract from the leaves | N/A | -Cholinesterase (ChE) inhibitory activity using Ellman’s method to assess the ability to inhibit AChE and BCh -Tyrosinase inhibitory activity was measured using the modified dopachrome method with L-DOPA as substrate | -Most potent inhibitory activity on BChE -Highest tyrosinase activity, with 29.68 mgKAE/g extract | [45] |
| Acetone extract from the flowers | N/A | -Cholinesterase (ChE) inhibitory activity using Ellman’s method to assess the ability to inhibit AChE and BCh -Tyrosinase inhibitory activity was measured using the modified dopachrome method with L-DOPA as substrate | -Least potent inhibitory activity on BChE -Mild tyrosinase activity | [45] |
| The methanolic effect from the leaves | N/A | -Cholinesterase (ChE) inhibitory activity using Ellman’s method to assess the ability to inhibit AChE and BCh -Tyrosinase inhibitory activity was measured using the modified dopachrome method with L-DOPA as substrate | -Potent inhibitory activity on AChE and BChE -Mild tyrosinase activity | [45] |
| The methanolic extract from the flowers | N/A | -Cholinesterase (ChE) inhibitory activity using Ellman’s method to assess the ability to inhibit AChE and BCh -Tyrosinase inhibitory activity was measured using the modified dopachrome method with L-DOPA as substrate | -Most potent activity for AChE -No inhibitory activity against tyrosinase | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albahri, G.; Badran, A.; Baki, Z.A.; Alame, M.; Hijazi, A.; Daou, A.; Mesmar, J.E.; Baydoun, E. Mandragora autumnalis Distribution, Phytochemical Characteristics, and Pharmacological Bioactivities. Pharmaceuticals 2025, 18, 328. https://doi.org/10.3390/ph18030328
Albahri G, Badran A, Baki ZA, Alame M, Hijazi A, Daou A, Mesmar JE, Baydoun E. Mandragora autumnalis Distribution, Phytochemical Characteristics, and Pharmacological Bioactivities. Pharmaceuticals. 2025; 18(3):328. https://doi.org/10.3390/ph18030328
Chicago/Turabian StyleAlbahri, Ghosoon, Adnan Badran, Zaher Abdel Baki, Mohamad Alame, Akram Hijazi, Anis Daou, Joelle Edward Mesmar, and Elias Baydoun. 2025. "Mandragora autumnalis Distribution, Phytochemical Characteristics, and Pharmacological Bioactivities" Pharmaceuticals 18, no. 3: 328. https://doi.org/10.3390/ph18030328
APA StyleAlbahri, G., Badran, A., Baki, Z. A., Alame, M., Hijazi, A., Daou, A., Mesmar, J. E., & Baydoun, E. (2025). Mandragora autumnalis Distribution, Phytochemical Characteristics, and Pharmacological Bioactivities. Pharmaceuticals, 18(3), 328. https://doi.org/10.3390/ph18030328








