Abstract
Research is increasingly revealing that inflammation significantly contributes to various diseases, particularly inflammatory bowel disease (IBD). IBD is a major medical challenge due to its chronic nature, affecting at least one in a thousand individuals in many Western countries, with rising incidence in developing nations. Historically, indigenous people have used natural products to treat ailments, including IBD. Ethnobotanically guided studies have shown that plant-derived extracts and compounds effectively modulate immune responses and reduce inflammation. Similarly, helminths and their products offer unique mechanisms to modulate host immunity and alleviate inflammatory responses. This review explored the pharmaceutical potential of Aboriginal remedial plants and helminths for treating IBD, emphasizing recent advances in discovering anti-inflammatory small-molecule drug leads. The literature from Scopus, MEDLINE Ovid, PubMed, Google Scholar, and Web of Science was retrieved using keywords such as natural product, small molecule, cytokines, remedial plants, and helminths. This review identified 55 important Aboriginal medicinal plants and 9 helminth species that have been studied for their anti-inflammatory properties using animal models and in vitro cell assays. For example, curcumin, berberine, and triptolide, which have been isolated from plants; and the excretory-secretory products and their protein, which have been collected from helminths, have demonstrated anti-inflammatory activity with lower toxicity and fewer side effects. High-throughput screening, molecular docking, artificial intelligence, and machine learning have been engaged in compound identification, while clustered regularly interspaced short palindromic repeats (CRISPR) gene editing and RNA sequencing have been employed to understand molecular interactions and regulations. While there is potential for pharmaceutical application of Aboriginal medicinal plants and gastrointestinal parasites in treating IBD, there is an urgent need to qualify these plant and helminth therapies through reproducible clinical and mechanistic studies.
1. Introduction
Inflammation is a defense response to injuries, chemicals, or microorganisms, involving by tissue damage, as well as metabolic and microcirculation disorders. It involves the release of mediators such as nitric oxide, prostaglandins, and cytokines, which are essential for removing harmful stimuli, restoring normal physiology, and controlling inflammation [1]. Different cytokines, markedly, interleukin-1 (IL-1) and tumor necrosis factor (TNF), regulate inflammation. These chemical mediators, primarily triggered by bacterial lipopolysaccharide [2], are released by monocytes, macrophages, and other cells [3]. Anti-inflammatory drugs have been used to address severe inflammation and pain. Presently, the United States Food and Drug Administration (USFDA) has approved nonsteroidal anti-inflammatory drugs (NSAIDs), including indomethacin, ibuprofen, cyclooxygenase-2 enzyme (COX-2) inhibitors like celecoxib and naproxen, for managing inflammation. However, these NSAIDs are linked to side effects on various bodily systems [4]. For instance, the long-term use of NSAIDs increases the risk of ulcers and complications in the upper gastrointestinal tract by about four times, while in the lower gastrointestinal tract, they cause mucosal ulcers or erosions and alter the diversity of microbiota, thereby modulating their toxicity [5].
Among the various inflammatory disorders, the chronic-relapsing condition of IBD, which mainly affects the gastrointestinal tract, has become a global health burden [5]. The etiology and pathogenesis of IBD are multifaceted, and it is difficult to determine a single causative agent [6]. Overall, these disorders are marked by persistent inflammation, often resulting in complications such as a heightened likelihood of hospitalization, surgical intervention, colorectal cancer, and disability. Acknowledging the chronic and advancing characteristics of IBD, commencing effective treatment during the early phases of the disease is crucial to minimize relapses and prevent complications [7]. In this view, NSAIDs such as 5-aminosalicylic acids (5-ASA), corticosteroids, and immunomodulators such as 6-mercaptopurine and azathioprine are used for managing IBD [7]. These medications can induce and sustain remission [8]; unfortunately, they are linked to numerous side effects [9,10]. For instance, 5-ASA and corticosteroids, often prescribed for patients with mild-to-moderate ulcerative colitis, have exhibited only moderate efficacy. Long-term medication use is associated with side effects such as stunted growth, depression, hypertension, and osteoporosis [11].
Similarly, immunomodulators such as 6-mercaptopurine and azathioprine are also associated with pancreatic inflammation, decreased hematopoiesis, and liver toxicity [12]. Among the biological agents or biologics, infliximab is commonly utilized for IBD treatment. However, biologics are expensive and associated with adverse infusion lupus-like syndrome and infections, including sepsis [11,13]. Historically, natural products (NPs) have been a vital source of structurally diverse and pharmacologically important anti-inflammatory small molecules (SMs, <10 kDa molecular weight) [14,15,16,17,18]. For instance, in the period spanning from 1981 to 2019, the USFDA approved 33.5% (1394 SMs) of the small-molecule (SM) drugs derived from natural products, underscoring the significant role of NPs in shaping the global healthcare landscape [18]. A total of 1881 new drugs were approved, which included 71 drugs sourced from unaltered natural products and 14 from botanicals. Among the 1602 new chemical entities, 53 were identified to exhibit anti-inflammatory properties [18].
Overall, the treatment of inflammatory disorders such as IBD has seen a transition from focusing on symptomatic control to emphasizing more objective endpoints [19]. This has resulted in the development of large-molecule biologics and synthetic small-molecule drugs (SMDs). However, these drugs have efficacy, safety, cost, and management time frame limitations. Considering this, more cost-effective oral drugs that offer improved efficacy and tolerability are in demand. Consequently, there is rising interest in developing new, targeted anti-inflammatory SMDs for IBD and other immune-mediated inflammatory conditions. Due to their low molecular weight, SMDs can quickly diffuse through cell membranes. The variance in size notably influences aspects such as the route of administration, target site, pharmacokinetics, antigenicity, and drug interactions. When administered orally, these drugs resist gastric degradation and quickly enter into the systemic circulation. The short half-life of SMDs can prove advantageous, especially in settings where instant drug elimination occurs, such as in infections, during surgical procedures, or during pregnancy. Hence, herbal and natural product compounds have been screened for therapeutic potential in anti-inflammatory-related diseases due to their chemical diversity, lower toxicity, and cost-effectiveness. However, biocompatibility and toxicity concerns have impeded clinical trial progress [20]. To address issues like poor bioavailability, solubility, and targeted delivery, advanced drug delivery systems such as nanoparticles, bioadhesive microspheres, chitosan-based hydrogels, liposomes, and phytosomes are being developed [21,22,23,24,25,26]. Nanoparticles can encapsulate or attach therapeutic drugs, ensuring precise targeting and controlled release, enhancing drug delivery and efficacy. FDA-approved nanoparticle-based therapies, such as liposomes and micelles, improve drug delivery by preventing gastrointestinal degradation and enhancing the delivery of poorly soluble drugs [27,28,29]. Similarly, the phytophospholipid complex technique converts water-soluble herbal extracts into lipid-compatible complexes, improving absorption and protecting compounds from degradation, thus enhancing therapeutic efficacy [30]. Examples of nanoformulated phytochemicals include silymarin, hypericin, and curcumin [31].
While general anti-inflammatory drugs have been reviewed elsewhere in more detail, we examined emerging the SMDs with anti-inflammatory properties derived from natural sources for treating IBD. Specifically, we explored anti-inflammatory SMs in medicinal plants and gastrointestinal parasites, offering insights into their potential future roles and impact. Databases such as Scopus, MEDLINE Ovid, PubMed, Google Scholar and Web of Science were queried using keywords like ‘Aboriginal remedial plants’, ‘helminths therapy’, and ‘anti-inflammatory small molecules’ to uncover the literature discussing the biological activities, phytochemistry, and ethnomedical applications of both Aboriginal plants and helminths against inflammatory-related disorders.
2. Plant-Derived Anti-Inflammatory Compounds
Plants have been a healing source since time immemorial [32]. Since their chemical analysis in the 19th century, bioactive compounds, referred to as phytochemicals in plants, animals, and living organisms, which influence physiological processes and have therapeutic potential and offer health benefits [33,34], have been crucial in advancing drug development [35]. Nearly 150,000 plant species, including medicinal plants, have been studied, with numerous harboring valuable therapeutic compounds, particularly anti-inflammatory compounds [36,37]. For example, the Eucalyptus and Melaleuca species are herbal remedies used by Australian Aboriginal people to treat inflammatory ailments such as muscular aches, sores, internal pains, and painful joints [38]. Likewise, Bhutanese traditional medicine involves the use of Aconitum laciniatum Stapf and the entire plant of Aconitum orochryseum Stapf to treat chronic parasitic and microbial infections, inflammatory diseases, and bilious fever [39]. Considering these traditional practices, researchers isolated the compound 14-O-acetylneoline, which exhibited anticolitis activity in TNBS (2,4,6-trinitrobenzene sulfonic acid)-induced colitis [40,41]. This practice emphasizes the long-standing recognition of the anti-inflammatory properties of NPs. Patients with IBD often turn to botanicals due to their perceived safety and effectiveness. Popular herbal remedies include Tripterygium wilfordii Hook. f., Plantago ovata Phil., Artemisia absinthium L., Aloe vera L., Curcuma longa L., Boswellia serrata Roxb., and Cannabis sativa L. [42]. A review of 27 studies and 1874 patients with IBD found seven herbal remedies were beneficial for ulcerative colitis (UC) and four induced remissions in cystic fibrosis diseases [42]. The oral administration of Aloe vera gel and Plantago ovata Phil. seeds showed promising anti-inflammatory results in UC [42]. Overall, ethnopharmacology insight has been a guiding force in the biodiscovery of anti-inflammatory SMs from NPs.
Inflammatory mediators play a crucial role in modulating IBD through various mechanisms. For example, nitric oxide (NO) is a signaling molecule produced by inducible nitric oxide synthase (iNOS) during inflammation and plays a significant role in vasodilation, modulation of blood flow, and immune defense. However, excess NO production can contribute to inflammation and tissue damage [43]. Both crude extracts and compounds isolated from natural sources exhibit anti-inflammatory effects by regulating key cytokines [44,45]. Pro-inflammatory cytokines like IL-6 promote the immune response by stimulating acute-phase protein production and influencing B-cell differentiation, while TNF and IL-1β, produced mainly by macrophages, mediate inflammation by promoting leukocyte recruitment, fever, and apoptosis in infected cells. Conversely, anti-inflammatory cytokines such as IL-10, IL-4, and IL-13, released by T helper 2 (Th2) cells, inhibit pro-inflammatory responses, modulating immune functions and inflammation [46]. However, excess cytokines are associated with chronic inflammation and autoimmune diseases.
Both crude extracts and compounds isolated from medicinal plant species have demonstrated wide-spectrum anti-inflammatory activity in both in vitro and in vivo screening (Table 1). For example, a methanol extract of Blainvillea acmella (L.) Philipson inhibited interleukin (IL)-1β and IL-6 expression levels in LPS-stimulated RAW264.7 macrophage cells [47]. This bioactivity guided the isolation of the bioactive compound spilanthol from B. acmella, inhibiting iNOS expression NO production and reducing TNF levels in LPS- and IFNγ-induced RAW264.7 cells [48]. In another study, a methanol extract of Corymbia terminalis (F.Muell.) K.D.Hill and L.A.S.Johnson inhibited IL-6, IL-8, and COX-1 expression levels in LPS-stimulated mammalian keratinocyte (HaCaT) cells [49,50]. Small molecules such as axifolin, aromadendrin, cianidanol, and farrerol isolated from C. terminalis showed anti-inflammatory properties by suppressing IL-6, IL-8, and cyclooxygenase-1 (COX-1) expression in LPS-stimulated HaCaT cells [49] (Table 1). Likewise, an ethanol root extract of Euphorbia tirucalli L. inhibited the TNF and interferon-gamma (IFN-γ) production in carrageenan-induced acute inflammation in the albino rat hind paw edema model [51]. Phytochemically, Eucalyptus species were reported to contain 1,8-cineole and α-pinene and aromadenderene as the main compounds in their leaves and essential oils [52]. The compound 1,8-cineole demonstrated the downregulation of inflammatory responses in dextran sulfate sodium (DSS)-induced colitis in mice and decreased proinflammatory chemokine production in TNF-stimulated HT-29 cells, proving to be a potent compound in treating human IBD [53]. Monoterpene acid and gallic acid glucose esters and phenolic compounds have also been reported in Eucalyptus species, which have shown a substantial reduction in the production of pro-inflammatory cytokines, including TNF, IL-1β, and IL-6 in LPS-stimulated peripheral blood mononuclear cells (PBMCs) and LPS-stimulated murine macrophage (RAW264.7) cells, indicating significant anti-inflammatory effects [54,55]. Studies have also reported the presence of phenolic compounds (including flavonoids, phenylpropanoids, and polyphenols) [56,57,58] and triterpenoids (including lupine, ursane, and oleanane derivatives) in Melaleuca species [59,60,61,62]. Many of these compounds showed pharmacological activities, including anti-inflammatory properties [63]. For instance, galloyl-lawsoniaside A and (4S)-α-terpineol 8-O-β-D-(6-O-galloyl) glucopyranoside, isolated from Uromyrtus metrosideros (F.M.Bailey) A.J.Scott, demonstrated remarkable in vitro inhibition of proinflammatory cytokines, which are linked to the development of IBD. Specifically, the releases of IFN-γ, IL-17A, and IL-8 from phorbol myristate acetate/ionomycin (P/I) and anti-CD3/anti-CD28-activated T cells were significantly suppressed [64]. On a similar note, the isolated compound hispidulin from Clerodendrum inerme R.Br. exhibited anti-inflammatory properties characterized by the inhibition of prostaglandin E2 (PGE2) production. It suppressed the expression of inducible nitric oxide synthase (iNOS) and COX-2 by blocking nuclear factor-kappa B (NF-κB) DNA-binding activity in LPS-stimulated RAW264.7 macrophages [2].
Many researchers have conducted studies to assess the effectiveness of plant-derived extracts and compounds in chronic IBD models. These have included curcumin (1E,6E)-1,7-bis (4-hy- droxy-3-methoxyphenyl) hepta-1,6-diene-3,5-dione, isolated from Curcuma longa L. (Zingiberaceae) [65]; colchicine, a major alkaloid from Colchicum autumnale L. [66]; resveratrol from Veratrum grandiflorum O.Loes. [67]; capsaicin from Capsicum species [68]; and epigallocatechin-3-gallate (EGCG) from Camellia sinensis (L.) Kuntze [69]. Additional small bioactive molecules, such as quercetin and berberine, have been shown to suppress IFN-γ- and IL-17A; while berberrubine, which inhibits myeloperoxidase (MPO), has been identified and isolated from Berberis vulgaris [70,71,72]. In clinical studies, curcumin inhibits important proinflammatory signaling cascades, such as NF-κB, mitogen-activated protein kinases (MAPK), COX and lipoxygenase (LOX) pathways in LPS-stimulated RAW264.7 cells and HT-29 human colon cancer cells. Additional proinflammatory cytokines like TNF, IL-1β, and IL-6 were downregulated [73,74,75]. According to numerous studies, curcumins exhibit a good safety profile (well tolerated and nontoxic).
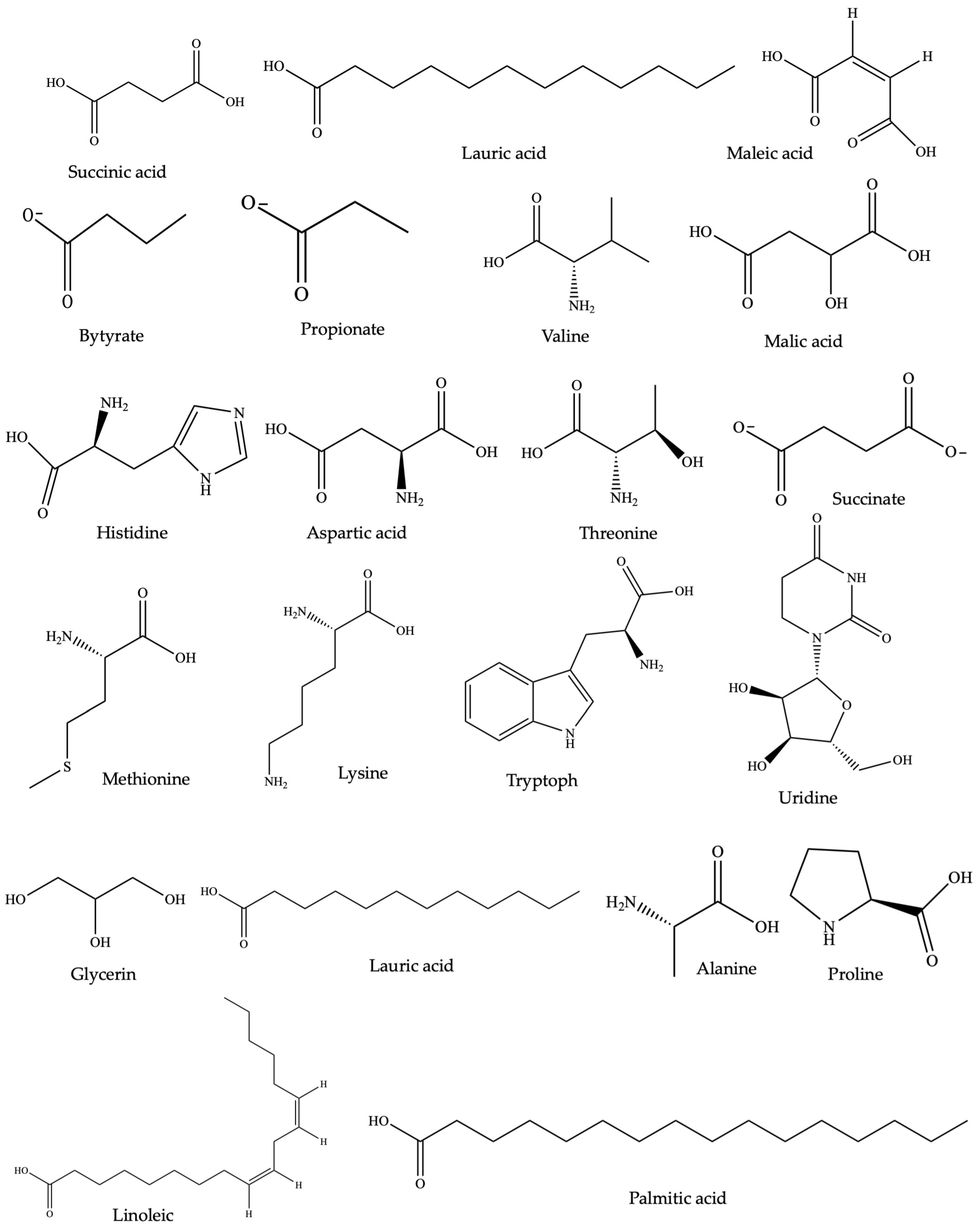
Other anti-inflammatory lead compounds such as brevilin A, centiplide A and H (Centipeda minima) [76,77], geraniin, corilagin (Acalypha wilkesiana Mull.Arg.) [78], 5′-methoxy nobiletin, 1,2-benzopyrone, (Ageratum conyzoides L.) [79], costatamins A–C (Angophora costata Britten), quercetin 3-O-(2″-acetyl)-glucoside, oumarinolignoid, cleomiscosins A–C (Arivela viscosa (L.) Raf.) [80,81], and quercetin 7-O-β-D-glucopyranoside (Brasenia schreberi J.F. Gmel.) have been isolated following ethnopharmacology and bioassay-guided approaches. The quercetin and quercitrin isolated from Merremia tridentata (L.) Hallier f. exhibited inhibitory effects on NO production and proinflammatory cytokines such as IL-6, TNF, and IL-1β in LPS-stimulated RAW264.7 cells [82]. Apigenin demonstrated similar bioactivity in LPS-stimulated BV2 microglia [83]. Additionally, calophyllolide and 27-[(E)-p-coumaroyl] canophyllic derived from Calophyllum inophyllum L. downregulated IL-6, TNF, IL-1β, and NO production while upregulating IL-10 in LPS-stimulated RAW 264.7 cells [4]. Compounds including antidesoside, podocarpusflavone A, and amentoflavone from Antidesma bunius Wall. also reduced NO levels in LPS-stimulated BV2 cells and RAW 264.7 cells [4]. The crude extracts and a few isolated compounds from NPs showing anti-inflammatory activity in diverse colitis animal models are summarized in Table 1. Representative chemical structures are shown in Figure 1.

Table 1.
Anti-inflammatory activity of crude extract and bioassay-guided isolated SMs from Aboriginal medicinal plants.
Table 1.
Anti-inflammatory activity of crude extract and bioassay-guided isolated SMs from Aboriginal medicinal plants.
| Botanical Name and Family | Plant Parts and Traditional Uses | Anti-Inflammatory Compounds/Extracts. | Model/Cell Used for Testing | Main Effect on Inflammation | Ref |
|---|---|---|---|---|---|
| Abrus precatorius L. (Fabaceae) | Seeds; abortion | Olean-12-ene-3β, 16β,23,28-tetrahydroxy-3-O-{[β-D-glucopyranosyl-(→ 4)-β-D-glucopyranosyl-( 1→ 3)]-[β-D-glucopyranosyl-(→ 2)]-β-D-fucopyranose} | Croton oil ear model | ↓ Inflammation on the ear tissue of rats | [4] |
| (S)-8-Hydroxy 6,7, 5′-trimethoxyisoflavan-1′,4′-quino Abruquinone A and B | Polymorphonuclear cells (PMNs) | ↓ TNF and ROS | [84] | ||
| Acacia melanoxylon R.Br. (Fabaceae) | Bark: headache, cold and fever | Kolavic acid 15-methyl ester | LPS- LPS-stimulated J774 cell | ↓IL-6 | [85] |
| Acalypha wilkesiana Müll.Arg. (Euphorbiaceae) | Shoot: sores/skin lesions/wounds/cuts | Polyphenol-enriched fraction | LPS-stimulated RAW264.7 macrophage | ↓ TNF, IL- 1β, and IL-6 | [78,86] |
| Ageratum conyzoides L. (Asteraceae) | Whole plant: sores/skin lesions/wounds/cuts | 5′-Methoxy nobiletin 1, 2-benzopyrone | Carrageenan-induced pleurisy | ↓ p-p65 NF-κB and p-p38 MAPK ↑ IL-17A, IL-6, TNF and IFN-γ levels | [87,88] |
| Leaves extract and aerial extract | Cotton pellet-induced granuloma and formaldehyde-induced arthritis models | ↓ Paw edema | |||
| Alphitonia petriei Braid and C.T. White (Rhamnaceae) | Bark, leaves, stem; Body pain | Embolic acid | LPS + IFN-γ activated RAW 264.7 cells | ↓ NO and TNF production | [89] |
| Alphitonia excelsa Reissek ex Endl (Rhamnaceae). | Whole plants: headache, colds, fever, stomach upset, skin lesions, wounds, cuts | Betulinic acid | λ-carrageenan-induced paw edema mice | ↓ COX-2, NO, TNF, and IL1-β | [90] |
| Angophora costata Britten (Myrtaceae) | Costatamins A-C | LPS-stimulated RAW264.7 cell | ↓ NO production and TNF | [91] | |
| Alstonia scholaris (L.) R.Br. (Apocynaceae) | Juice, sap, bark; toothache, fever, sores, skin lesions, wounds, and cuts | 12-ursene-2,3,18,19-tetrol-28 Acetate | Carrageenan-induced. paw edema (Wistar rats) | ↓ Paw edema | [92] |
| Picrinine, vallesamine, and scholaricine | Mice air pouch model (In vivo) | ↑ SOD activity ↓ NO production, PGE2, and MD | [93] | ||
| Antidesma bunius Wall. (Phyllanthaceae) | Fruit: colds, fever, and headache | Antidesoside, podocarpusflavone A, byzantionoside B, (6S,9R)-roseoside | LPS-stimulated BV2 cells and RAW264.7 macrophages | ↓ NO production | [94] |
| Arivela viscosa (L.) Raf. (Cleomaceae) | Whole plants; Colds and fever | Quercetin 3-O-(2″-acetyl)-glucoside | Carrageenan-induced rat paw edema | ↓ Carrageenan-induced rat paw edema | [80] |
| Coumarinolignoid cleomiscosins A, B and C | Female Swiss albino mice | ↓ IL-6, TNF NO production ↑ IL-4 in a dose-dependent manner | [81] | ||
| ↓ Carrageenan-induced rat paw edema ↓ IL-4, TNF, and NO production ↓ COX-1 | |||||
| Cembrenoid diterpene Malabaric acid | Cyclooxygenase enzyme (COX-1 and -2) inhibitory assays | ↓ COX-1 and COX-2 enzyme | [95] | ||
| Stigmast-4-ene-3,6-dione, cleomaldeic acid, stigmast-4-en-3-one, and lupeol | ↓ COX-1 and COX-2 enzyme | ||||
| Barringtonia racemose (L.) Spreng. (Lecythidaceae) | Bark, fruits; tonic, pain and inflammatory | Ethyl acetate fraction | Carrageenan-induced rat paw edema model | Demonstrated dose-dependent anti-inflammatory activity | [96] |
| Barringosides I | LPS-stimulated RAW264.7 cell | ↓ Production of NO | [97] | ||
| Blainvillea acmella (L.) Philipson (Asteraceae) | Bark, fruits, and roots; muscle sprain, bone aches, dislocation, broken bones | Spilanthol | LPS and IFNγ induced RAW264.7 cells | ↓ iNOS expression, NO production, and TFN | [48] |
| Methanol extracts | LPS-stimulated RAW264.7 macrophages | ↓ IL-1β and IL-6 | [47] | ||
| Boerhavia diffusa L. (Nyctaginaceae) | Whole plants; asthma | Boeravinone B and N | Carrageenan-induced paw edema | ↓ COX-1 and COX-2 Exhibited anti-inflammatory activity | [98] |
| Rotenoid-rich fraction | Sprague–Dawley rats | Exhibited ↑ anti-inflammatory potential and ↑ plasma level | [99] | ||
| Brasenia schreberi J.F.Gmel. (Cabombaceae) | Leaves: stomach cancer, boil, dysentery, and tuberculosis | Quercetin 7-0-β-d-glucopyranoside | LPS-stimulated RAW 264.7 cells | ↓ Expression of iNOS and NO Overexpression of COX-2 ↓ Granulocyte macrophage-colony-stimulating factor | [100] |
| Brucea javanica (L.) Merr. (Simaroubaceae) | Leaves and roots; pain | Ethyl acetate fraction of seeds | LPS-stimulated RAW264.7 macrophages | ↓ NO, PGE2, TNF, IL-1β, and IL-6 ↑ IL-10 | [101] |
| Carrageenan-induced paw edema | Inhibited carrageenan-induced paw edema | ||||
| Oil emulsion | DSS-induced colitis mice | ↓ TNF, IL-1β, IL-6, IL-8, IL-17, and IFN-γ ↓ mRNA expression of MPO, iNOS, and COX-2 | [102] | ||
| Cleomiscosin A and E | LPS-stimulated RAW264.7 macrophages | ↓ NO production | [103] | ||
| Brujavanoid E | LPS-induced RAW264.7 cells | ↓ Caspase1, CD206, IL-1β, IL-18, MCP-1, TNF, NIK, NLRP3, and p65 in a dose-dependent manner | [104] | ||
| Brusatol | LPS-induced RAW 264.7 cells TNBS-induced colitis mice | ↓ TNF, IL-1β, PGE2, and NO levels ↓ NF-κB signaling pathway. ↓ IL-1β and IL-18 levels ↓ Catalase, glutathione, and superoxide dismutase enzymes in the colon tissue | [105,106,107] | ||
| Calophyllum inophyllum L. (Clusiaceae) | Fruits; body pain and purgative | Acetone extract | LPS-stimulated RAW 264.7 cells | ↓ NO production, iNOS, COX-2, and NF-κB | [108] |
| Calophyllolide | Albino male mice | ↓ IL-1β, IL-6, TNF ↑IL-10 | [109] | ||
| E-coumaroyl triterpenoid (27-[(E)-p-coumaroyloxy] canophyllic acid) | LPS-induced RAW 264.7 cells | ↓ NO production, IL-1β, and TNF, iNOS ↓ NF-κB signaling pathway | [110] | ||
| Capparis mitchellii Lindl. (Capparaceae) | Bark: cuts, wounds, and skin lesions | Luteolin, kaempferol, apigenin and catechin | LPS-induced RAW 264.7 cells | Inhibition with IC50 values of 26.24 ± 1.78, 20.38 ± 1.36, 22.8 ± 1.57, and 42.5 ± 2.24 μM, respectively ↓ NO production | [111] |
| Salvia plebeian R.Br. (Labiatae) | Colds and tumors | Ethanol extract | BALB/c mice | ↓ IL-4, IL-17, MMP-1, and MMP-3 ↓ Akt and MAPKs pathways, ↓ Akt and ERK p38 expression | [112] |
| Carpobrotus rossii Schwantes (Aizoaceae) | Leaves: (extract juice) respiratory tract and throat infections, gastrointestinal discomfort, insect bites, wounds burns, eczema, bluebottle, and jellyfish stings | Aqueous extract | PBMC | ↓ IL-10, TNF, and MCP-1 | [113] |
| Clematis pickeringii A. Gray (Ranunculaceae) | Headache, pain, rheumatism, infections, common colds. | Ethanolic extract | HepG2 cells | ↑ PPARa and PPARy at the dose of 60 µg/mL | [114] |
| Centipeda minima (L.) A.Br. (Apiaceae) | Nasal allergy, diarrhea, asthma | Whole-plant extract | LPS-induced RAW 264.7 cells and λ-carrageenan-induced paw edema | ↓ NO production | [115] |
| DSS-induced colitis mouse model | ↓ TNF-α and IL-1β | [116,117] | |||
| Brevilin A, centiplide A, centiplide H, and Helenalin isova lerate | LPS-induced RAW 264.7 cells | Attenuated NF-κB pathway activation and oxidative stress ↓ NO production | [76,77] | ||
| 6-O-angeloylplenolin | LPS-induced macrophage cells (BV2 cells) | ↓ TNF and IL-1β ↓ Phosphorylation of NF-κB ↓ IκB-α, NO, and PGE2 | [76,118] | ||
| Centella asiatica (L.) Urb. (Apiaceae) | Whole plants; lupus, varicose ulcers, eczema, and psoriasis | Triterpenoid saponin-rich fraction | LPS-stimulated RAW 264.7 cells | ↓ IL-13, NF-κB pathway | [119] |
| Asiatic acid, isomadecassoside, asiaticoside B and G, rosmarinic acid | LPS-stimulated RAW 264.7 cells | ↓ NO production | [120,121] | ||
| Clematis microphylla DC. (Ranunculaceae) | Whole plants; headache, colds, sores, gastric disorder and fever | Ethanolic plant extracts | HepG2 cells | ↓ COX-1, 5-LOX | [114] |
| Clerodendrum inerme R.Br. (Lamiaceae) | Leaves and roots; sores, skin lesions, wounds, cuts, and sprains | Ethanol leaf extract | LPS-induced RAW 264.7 cells | ↓ NO production ↓ mRNA and protein expressions of iNOS | [122] |
| Hispidulin | LPS-stimulated RAW 264.7 cells | ↓ NO production, NF-κB DNA-binding activity, and JNK signaling pathway ↓ iNOS and COX-2 expression | |||
| Methanolic extract | Formalin-induced hind-paw edema | ↑ Anti-inflammatory activity at dose 200 mg/kg | [123] | ||
| Ethyl acetate fraction | LPS-stimulated RAW264.7 macrophage cells | ↓ NO production ↓ iNOS | [122,124] | ||
| Cleome viscosa L. (Capparidaceae) | Whole plants; diarrhea, fever, cuts, and ulcers | Quercetin 3-O-(2″-acetyl)-glucoside | Carrageenan-induced paw edema | ↓ Carrageenan-induced rat paw edema | [80,124] |
| Cleomiscosins A-C | LPS-stimulated Peritoneal Macrophages (Swiss albino mice) | ↓ IL-4, TNF, and NO production | [81] | ||
| Malabaric acid | ↓ COX-1 and two activities | [95] | |||
| Hispidulin | ↓ PGE2 production, and iNOS and COX-2 expressions ↓ NF-κB DNA-binding activity and JNK pathway | [122] | |||
| Crinum pedunculatum R.Br. (Amaryllidoideae) | Whole plants; stings from marine life | Methanol, ethanol, and ethyl acetate extracts | Carrageenin-induced Wistar albino paw edema | ↓ Carrageenin-induced rat paw edema | [124,125] |
| Morinda citrifolia (Rubiaceae) | Fruits: cough and cold, sore throat | LPS-stimulated human monocyte | ↓ Matrix metalloproteinase-9 (MMP-9) production | [126] | |
| Fruit juice | DSS-induced colitis model | ↓ TNF and IFN-γ, NO and IL-17 | [127] | ||
| Ethyl acetate extract | Caco-2 cells | ↓ COX-2, IL-8, and prostaglandin E2 production and neutrophil chemotaxis | [128] | ||
| Fruit extract | LPS-stimulated RAW 264.7 cells | ↓ NO synthase and TNF | [129] | ||
| Rutin, nonioside A, (2E,4E,7Z)-deca-2,4,7-trienoate-2-O-β-d-glucopyranosyl-β-d-glucopyranoside, and tricetin | LPS-stimulated RAW 264.7 cells | ↓ NO production, expression of IKKα/β, I-κBα, and NF-κB p65 | [126,130] | ||
| Eucalyptus camaldulensis Dehnh. (Myrtaceae) | Leaves and gum from barks; diarrhea | Ethanol extract | Carrageenan-induced paw edema | ↓ Carrageenan-induced paw edema | [124,131] |
| 12-O -tetradecanoyl-phorbol-13-acetate | ↓ Edema and leukocyte infiltration | ||||
| Acalypha wilkesiana Mull.Arg. (Euphorbiaceae) | Shoots: skin lesions, sores, cuts, wounds | Leaf extract | LPS-stimulated RAW 264.7 cells | ↓ NO and PGE2 ↓ iNOS productions and COX-2 expression ↓ TNF, IL-1β) and IL-6 | [2] |
| Corymbia terminalis (F.Muell.) K.D.Hill and L.A.S.Johnson (Myrtaceae) | Bark; dysentery | Methanol leaves extracts | LPS- stimulated mammalian keratinocyte (HaCaT) cell | ↓ IL-6, IL-8, and COX-1 and 2 enzyme activities | [49,50] |
| Axifolin, aromadendrin, cianidanol, and farrerol | ↓ IL-6, IL-8, and COX-1 and 2 enzyme activities | [49] | |||
| Dodonaea polyandra Merr. and L.M.Perry (Sapindaceae) | Roots: sores, skin lesions, wounds, cuts, and toothache | Nonpolar hexane and methylene chloride/methanol | TPA-induced mouse ear edema | ↓ Inflammation in TPA-induced mouse ear edema by 72.12 and 79.81%, respectively, at 0.4 mg/ear; 12 and 79.81%, respectively, at 0.4 mg/ear | [132] |
| Polyandric acid A | Mouse Ear Tissue (male BALB/c mice) | ↓ IL-1β | [133] | ||
| 5,16-Epoxy-8α-(benzyloxy) methyl-2α-hydroxycleroda-3,13(16),14-tried-18-oic acid and 15,16-Epoxy-2α-benzoyloxycleroda-3,13(16),14-tried-18-oic acid | TPA-induced mouse ear edema | Exhibited optimum inhibition of inflammation (70–76%) at doses of 0.22 and 0.9 μmol/ear, respectively | [134] | ||
| Dodonaea viscosa Jacq. (Sapindaceae) | Leaves, rheumatism, skin infections, and diarrhea | Hydroalcoholic extract | Carrageenin-induced paw edema | ↓ Edema induced in rats by carrageenin | [135] |
| Dichloromethane extract | TPA-induced edema model in CD-1 mice | ↓ 97.8% of the edema | [136] | ||
| Hautriwaic acid | 12-O-tetradecanoylphorbol 13-acetate (TPA) mice ear edema models | 97% of edema inhibition with an ED50 = 0.158 mg/ear | |||
| Excoecaria agallocha L. (Euphorbiaceae) | Latex; stings (marine) | Agallochanin K | RAW264.7 cells | ↓ NF-κB | [137] |
| Agallolides I and J | NF-κB (p65) RAW264.7 cells | ↓ NF-κB with inhibition rates of 23.4% and 19.4%, respectively, at the concentration of 100.0 µM | [138] | ||
| Eleocharis dulcis Hensch. (Cyperaceae) | Whole plants; antiseptic, wounds | Ethyl acetate extract | LPS-induced RAW 264.7 macrophages | ↓ TNF, iNOS, and COX-2 | [124,139] |
| Ipomoea pes-caprae (L.) R.Br. (Convolvulaceae) | Whole plants; diseases, boils, and swelling | Ethanol extract from leaves and stems | Trypsin-, histamine-, and bradykinin-induced paw edema in mice | ↓ Trypsin-, histamine-, and bradykinin-induced paw edema in mice | [140] |
| Heliotropium ovalifolium Forssk. (Boraginaceae) | Body wash and fever | 4,7,8-Trimethoxy-naphthalene-2-carboxylic acid and 6-hydroxy-5,7-dimethoxy-naphthalene-2-carbaldehyde | LPS-stimulated THP-1 cell | ↓ IL-6 and TNF | [141] |
| Euphorbia hirta L. (Euphorbiaceae) | Leaves; hypertension, respiratory ailments, tumors, and wounds | Ethanol extract | LPS-induced inflamed rat knees | ↓ TNF and NO production | [142] |
| Butanol extract | LPS-stimulated RAW 264.7 cells | ↓ NO production and iNO protein expressions | [143] | ||
| Melaleuca leucadendra L. (Myrtaceae) | Leaves and bark; cough and cold; wounds, cuts, and sores | Butanol extract | LPS-stimulated RAW 264.7 cells | ↓ NO and PGE2 production ↓ Expression of COX-2 and iNOS protein ↓ NF-κB transcriptional activity in a dose-dependent manner | [144] |
| Casuarinin | Ethanol-induced gastric ulceration in rats | ↓ NF-κB, COX-2, iNOS | [145] | ||
| Euphorbia tirucalli L. (Euphorbiaceae) | Latex; skin cancer | Ethanol extract from roots | Carrageenan-induced acute-inflammation in albino rat model | ↓ TNF and IFN-γ production | [51] |
| Manihot esculenta Crantz (Euphorbiaceae) | Roots: diarrhea and stomachache | Carrageenan-induced paw edema in rats and xylene-induced ear edema in mice | ↓ Carrageenan-induced rat paw edema and xylene-induced ear swelling in mice | [146] | |
| Flueggea virosa (Roxb. Ex willd.) Royle (Phyllanthaceae) | Root; toothache | Flueggrenes A | N-formylmethionyl-leucyl-phenylalanine (FMLP)/cytochalasin B (CB) activated-human neutrophils | ↓ Superoxide anion generation and elastase release | [147] |
| Litsea glutinosa (Lour.) C.B.Rob. (Auraceae) | Leaves and bark; fever, scabies, gastritis pain, cuts, and wounds | Hydroalcoholic extracts of leaves | Carrageenan-induced edema | Carrageenan-induced rat paw edema | [148] |
| Merremia tridentata (L.) Hallier f. (Convolaceae) | Whole plants; sores, antiseptic | Apigenin | LPS-stimulated BV2 microglia | ↓ TNF, IL-1β, and IL-6 production ↓ NF-κB activation | [83,124] |
| Quercetin, quercitrin | LPS-induced RAW264.7 Cells | ↓ NO production, TNF, IL-1β, and IL-6 | [82] | ||
| Ocimum tenuiflorum L. (Lamiaceae) | Leaves and stems; pain and stomachache | Leaf extract | LPS-induced RAW264.7 Cells | ↓ Lipopolysaccharide-induced inflammation in RAW 264.7 cells | [149] |
| Nauclea orientalis (L.) L. (Rubiaceae) | Bark; colds, stomachache, and snake bite | Ethyl acetate (EA) and ethyl alcohol (ET) lotus petal extracts | LPS-stimulated human monocyte-derived macrophages | ↓ TNF, NF-κB | [150] |
| Nelumbo nucifera Gaertn. (Nelumbonaceae) | Ethanol extract from fruits | Carrageenan-induced paw edema (Wistar male rats) | ↓ Carrageenan-induced rat paw edema | [151] | |
| Leaf extract | 293T cells | ↑ IL-10 and IL-12 ↓ IL-6, IL-1β, TNF-α, and IFN-γ | [152] | ||
| Neferine | DSS-induce colitis mice model (C57BL/6J male mice) | TNF, IL-1β, and IL-6 ↑ IL-10 | [153,154] | ||
| Murine macrophage RAW264.7 cells | IL-6 and TNF production ↑ Peroxisome proliferator-activated receptor (PPARα and PPARγ) | [155] | |||
| LPS-stimulated RAW 264.7 cells | ↓ NO release | [156] | |||
| Ochrosia elliptica Labill. (Apocynaceae) | Bark; malaria | Quercetin-3-O-β-d-glucuronide | LPS-stimulated RAW 264.7 cells and human peripheral blood monocytes | ↓ NO production and TNF | [157,158] |
| Phyllanthus urinaria L. (Phyllanthaceae) | Leaves; colds | Corilagin | IB3-1 cells | ↓ IL-8 gene expression in TNF-treated IB3-1 cells TNF-alpha-induced secretion of MCP-1 | [159] |
| Scoparia dulcis L. (Plantaginaceae) | Whole plants; sores, stomachache, skin lesions, wounds, and cuts | Ethanol extract | Carrageenan-induced paw edema | ↓ COX-2, NO, TNF and IL1-β | [160] |
| Betulinic acid | ↓ TNF, IL-1β, COX-2, and NO production | ||||
| Terminalia catappa L. (Combretaceae) | Bark, young green fruit; sore throat and thrush | Ethanol extract of leaves | 12-O-TPA-induced edema (Male ICR mice) | ↓ Myeloperoxidase (MPO) activity | [161] |
| Ursolic acid and 2α,3β,23-trihydroxyurs-12-en-28-oic acid | ↓ TPA-induced ear edema and inhibited MPO activity | ||||
| Terminalia muelleri Benth. (Combretaceae) | Leaves: Scabies, sores, skin lesions, wounds, and cuts | Polyphenol-rich fraction | Carrageenan-induced paw edema model | ↓ PGE2 and IL-6, IL-1β and TNF, IL-1β | [162] |
| Uromyrtus metrosideros (F.M.Bailey) A.J.Scott (Myrtaceae) | Leaves and branches | (4S)-α-terpineol 8-O-β-D-(6-O-galloyl) glucopyranoside | Human PBMCs stimulated by P/I or anti-CD3/anti-CD28 | ↓ IFN-γ, IL-17A and IL-8 | [64] |
| Vitex trifolia L. (Labiatae) | Leaves; inflammatory ailments | Aqueous extract | LPS-induced RAW 264.7 cells | ↓ COX-2, NF-κB, L-1 β and caspase-3 | [163] |
| Galloyl-lawsoniaside | ↓IFN-γ and IL-17A | ||||
| Lophostemon suaveolens (Soland. ex Gaertn.) Peter G.Wilson and J.T.Waterh. (Myrtaceae) | N-hexane and dichloromethane extracts from leaves | LPS-stimulated RAW264.7 cells | ↓ NO production ↓ PGE2 in 3T3 murine fibroblasts | [164] | |
| Betulinic acid | LPS-induced lung inflammation in Sprague-Dawley rats | Inhibit cell recruitment, ↓ TNF, NO, and TGF-β1 expression ↓ MDA production | [165] | ||
| Tasmannia lanceolate (Poir.) A.C.Sm. (Winteraceae) | Berries, bark, and leaves | Polyphenolic-rich extracts (gallic acid, 2-hydroxybenzoic acid and chlorogenic acid were identified through their MS/MS spectra) | LPS-stimulated RAW264.7 cells | ↓ iNOS and COX-2 ↓ NO and prostaglandin E2 (PGE2) levels | [166] |
↓, Downregulated; ↑, upregulated: TNF, tumor necrosis factor; ROS, reactive oxygen species; IL, interleukin; TNF, tumor necrosis factor; IFN-γ, interferon-gamma; NF-kB, nuclear factor kappa beta; MAPk, mitogen-activated protein kinase; NO, nitric oxide; PGE2, prostaglandin 2; COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; IL, interleukin; LPS, lipopolysaccharide; NO, nitric oxide; DSS, dextran sulfate sodium; RAW, Ralph and William’s cell line; MPO, myeloperoxidase; DMSO, dimethyl sulfoxide; MMP, matrix metalloproteinase; MDA, malondialdehyde.
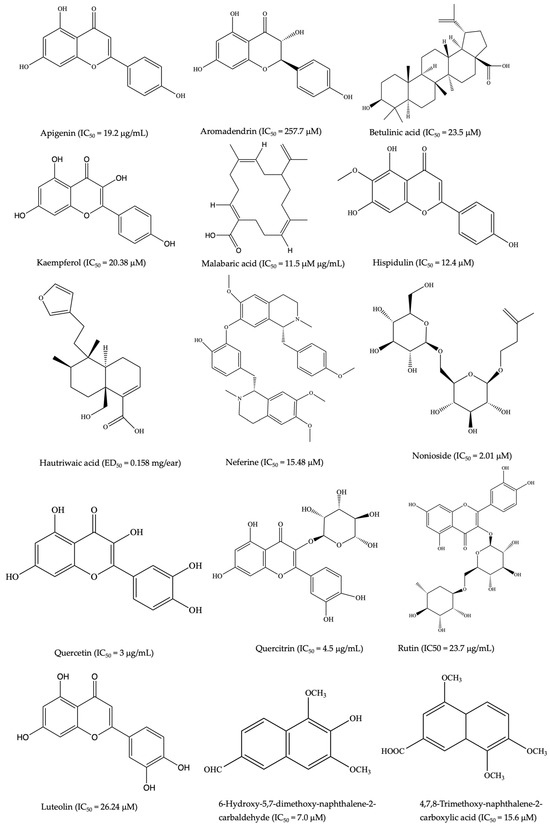
Figure 1.
Selected common chemical structures of plant-derived anti-inflammatory SMs (the half-maximal inhibitory concentration (IC50) measures a small molecule’s efficacy by indicating the amount needed to inhibit inflammation by 50%, thus reflecting its potency in treating inflammatory-related disorders) [167].
Figure 1.
Selected common chemical structures of plant-derived anti-inflammatory SMs (the half-maximal inhibitory concentration (IC50) measures a small molecule’s efficacy by indicating the amount needed to inhibit inflammation by 50%, thus reflecting its potency in treating inflammatory-related disorders) [167].
3. Helminth-Derived Anti-Inflammatory Compounds
Several studies have highlighted the therapeutic significance of helminths and helminth-derived excretory/secretory products (ESP) [168] in modulating host immune responses and protecting against inflammatory conditions (Table 2). Helminth-induced immune modulation is multifactorial and often involves the potent stimulation of Th2 responses in conjunction with IL10- and transforming growth factor-β (TGFβ)-dependent immune regulation [169,170,171] and decreased type 1 and 17 T helper (Th1/Th17) inflammation [172,173,174,175]. Helminths are also potent drivers of regulatory cell responses, including Foxp3+ regulatory T cells (Tregs) [171,175,176], IL10-producing type 1 regulatory T (Tr1) cells [177], type 2 innate lymphoid cells [178], alternatively activated macrophages [179] and regulatory dendritic cells [180,181]. Other studies have reported helminths like Hymenolepis diminuta, Trichinella spiralis, and Trichuris suis offering protection against colitis in 2,4-dinitrobenzene sulfonic acid (DNBS) and TNBS-induced mouse models of colitis [182,183] and randomized human trials [184]. For instance, TNBS-treated mice infected with S. mansoni eggs showed decreased Th1-type inflammation and alleviated colon pathology, possibly through IL-4 signaling and increased IL-4 levels [185]. The soluble molecules from S. mansoni eggs and hookworm (Ancylostoma caninum) ESPs were found to counteract the detrimental effects of INF-γ and IL-12 induced by DSS, inducing the secretion of IL-10 [185]. In a piroxicam-induced IL-10−/− mouse model, infection with Heligmosomoides polygyrus inhibited IFN-γ and IL-12 production while promoting the production of Th2 cytokine IL-13 [185]. The succinic acid secreted by Nippostrongylus brasiliensis ESPs was found to be involved in initiating an early Th2-type immune response [186], which involves the activation of Th2 cells and the production of cytokines like IL-4, IL-5, IL-10, and IL-13. These cytokines promote B-cell differentiation, antibody production, and the activation of eosinophils, contributing to allergic inflammation and the defense against extracellular pathogens [187].
Similar work on A. caninum by Wangchuk et al. [188] showed the protection of mice from colitis by low-molecular-weight metabolites of somatic extracts, ESPs, and the latter when used to treat mice resulted in a significant reduction in inflammatory cytokines such as IL-23, TNF, and IL-1β. Similarly, different concentrations of the hexane-dichloromethane-acetonitrile somatic fraction of A. caninum exhibited notable reductions in TNF, IL-1β, IL-6, and monocyte chemoattractant protein-1 (MCP-1) production [188]. Hence, helminth therapy is gaining attention in IBD treatment. For example, preliminary investigations into the positive impacts of helminths on IBD revealed that the oral administration of Trichuris muris whipworm eggs notably decreased TNBS-induced colitis in IL-10−/− mice [189]. Similarly, Trichuris suis ova showed promise in human trials, inducing remission in 21 out of 29 patients with active Crohn’s disease (CD) [184]. Hence, identifying and characterizing helminth molecules offers a unique opportunity to create nature-inspired, effective, safe, and minimally immunogenic drugs.
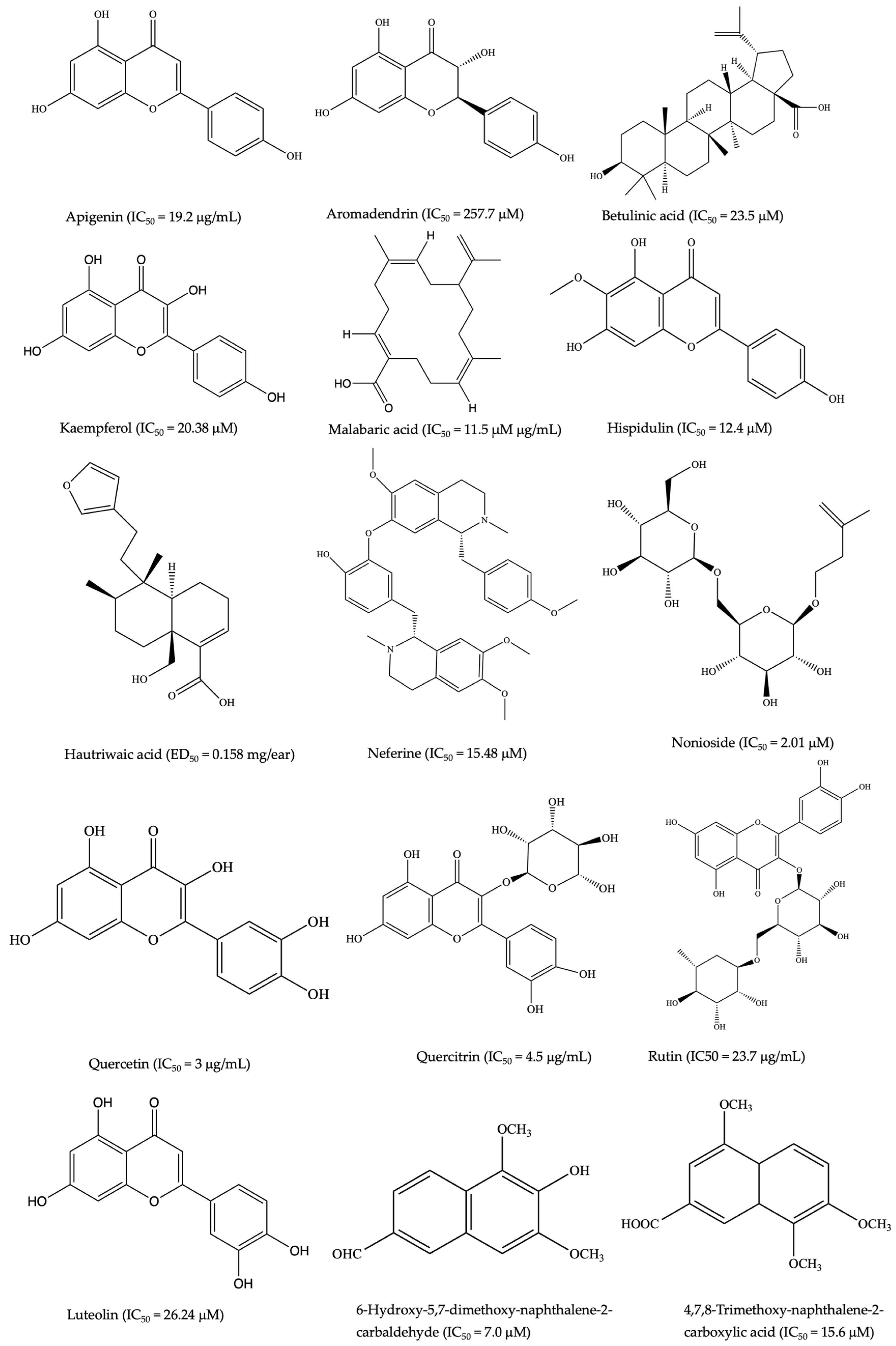
Wangchuk et al. [190] identified 54 SMs in the ESP of Trichuris muris and N. brasiliensis, of which 17 SMs had exhibited pharmacological activities in other studies. Similarly, in the case of Dipylidium caninum, 49 SMs were characterized by gas chromatography/mass spectrometry (GC-MS), with succinic acid as the chief constituent of its ESP [191]. The study also highlighted that among the 35 polar metabolites, lactic acid, malic acid, methionine, glycerol, and fructose were previously reported to exhibit anti-inflammatory and proinflammatory activities [191]. Furthermore, Wangchuk et al. [192] carried out the initial metabolomic and lipidomic examination of the infective third-stage filariform larvae of the human hookworm Necator americanus, utilizing liquid chromatography–mass spectrometry. The study unveiled 645 SMs, with 495 metabolites exclusive to the somatic tissue extract and 34 found solely in the ESP component. Of these, 45 SMs were identified as polar metabolites; 26 SMs had previously been documented for their anti-inflammatory properties [192]. For example, in a study conducted to investigate the impact of L-arginine on the inflammatory response and casein expression, the findings indicated that arginine mitigated the LPS-induced production of inflammatory markers such as IL-1β, IL-6, TNF, and iNOS [193]. Among the SMs identified, lactic acid, malic acid, methionine, glycerol, and fructose, sourced from various NPs, have demonstrated anti-inflammatory properties [194,195,196,197,198]. However, the immunomodulatory properties of nonprotein SMs secreted or excreted by helminths remain relatively underexplored for medicinal applications [199]. Therefore, based on these promising preliminary results, the somatic tissues and ESPs of helminths could be sources of anti-inflammatory SMs that can be used for treating IBD and other inflammatory conditions. The chemical structure of common anti-inflammatory SMs identified through metabolomic studies from Trichuris muris and Ancylostoma caninum are shown in Figure 2.
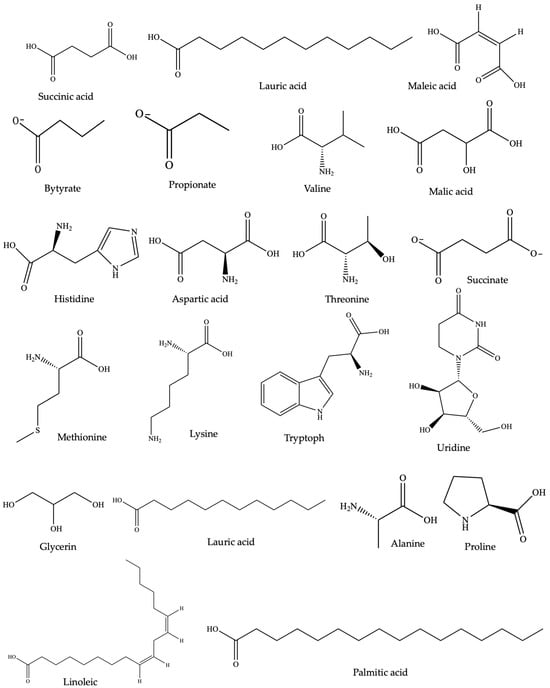
Figure 2.
Chemical structure of anti-inflammatory SMs identified through metabolomic studies of Trichuris muris and Ancylostoma caninum (common to both).

Table 2.
Anti-inflammatory activities of different stages of helminths and their components in various colitis animal models and cell lines.
Table 2.
Anti-inflammatory activities of different stages of helminths and their components in various colitis animal models and cell lines.
| Helminth Species | SMs/ESP/Somatic Tissue/Larva/Egg/EVs/Protein | Animal Model or Cell Line | Mechanism of Action | Reference |
|---|---|---|---|---|
| Ancylostoma caninum | Low-molecular-weight somatic tissue extract metabolites | TNBS-induced colitis model | ↓ TNF, IL-1β, and IL-13 | [188] |
| Low-molecular-weight excretory-secretory product metabolites | LPS-stimulated PBMC | ↓ TNF, IL-1β, and IL-13 | ||
| Dichloromethane-acetonitrile somatic extract | ↓ TNF, IL-1β, IL-6 and the chemokine MCP-1 | |||
| DSS-induced colitis model mice | ↓ Proinflammatory mediators iNOS, IL-6, and IL-17A ↑ IL-4 and IL-10 levels | [200] | ||
| Ancylostoma ceylanicum | Crude extracts and excretory-secretory products | DSS-induced colitis model | ↓ Th1 and Th17 cytokines ↓ Colonic microscopic ↓ EPO and MPO activity | [201] |
| Trichinella spiralis | 53 kDa ES protein | TNBS-induced colitis model | ↑ IL-4, IL-13, IL-10, TGF-β, AAM | [202] |
| ↓ IFN-γ, TNF, IL-6 ↓ inflammatory score | ||||
| Extracellular vesicles | TNBS-induced colitis model | ↓ IL 1β, TNF IFN-γ, and IL-17A ↑ IL-10 and TGF-β, IL-4, and IL-13 | [203] | |
| Nippostrongylus brasiliensis | Extracellular vesicles | Murine small intestinal organoids | ↓ IL-6, IL-1β, IFN γ, and IL-17a ↑ IL-10 | [204] |
| Spirometra erinaceieuropaei | Extracellular vesicles | LPS-stimulated RAW 264.7 cell | ↓ NO production ↓ TNF, IL-1β, and IL-6) | [205] |
| Schistosoma mansoni | Egg | TNBS-induced colitis model | ↓ IFN-γ ↑ IL-10 mRNA expression | [172] |
| Egg | DSS-induced colitis model. | ↑ FoxP3+ T regulatory cells and Th2 cytokines. | [206] | |
| Trichinella spiralis | Infective larvae | TNBS-induced colitis model | ↓ DAI score and weight | [207] |
| ↓ IFN-γ ↑ IL-4 | ||||
| T. spiralis | Infective larvae | TNBS-induced colitis model | ↑ IL-4, IL-13- induction of a Th2 response. | [182] |
| ↓ IL-12, IFN-γ, MPO activity | ||||
| Heligmosomoides polygyrus | Infective larvae | Piroxicam- induced IL-10–/– mice | ↓ IL-12 and IFN-γ production ↑ IL-13, | [170] |
| Infective larvae | TNBS-induced colitis model | ↑ IL-4, IL-5, IL-10, IL-13 | [208] | |
| ↓ IL-12p40, IFN-γ ↑ IL-4, IL-5, IL-13, and IL-10 secretion | ||||
| Infective larvae | IL-10−/− mice | ↓ IL-17 ↓ IL-4 and IL-10 | [209] | |
| Infective third-stage larvae | TNBS-induced colitis model | ↑ IL-4, IL-13, mucosal mast cells, and resistance | [210] | |
| ↓ IFN-γ, TNF | ||||
| Trichuris muris | Embryonated eggs | Mdr1a−/− mice | ↑ IFNγ, TNF ↑ IL-13 and IL-5 | [211] |
| Infective larvae | IL-10−/− mice | ↑ IFN-γ, IL-17 | [212] | |
| ↓ IL-13 ↑ IL-13Rα2 | ||||
| Necator americanus | Netrin-domain-containing proteins (prophylactic Na-AIP-1) | TNBS-induced colitis model | ↓ TNF | [213] |
4. Anti-Inflammatory Agents in Clinical Trials
Several plant species and their allied small molecules are being examined for their potential in treating IBD, specifically UC and CD. The small molecule epigallocatechin-3-gallate derived from Camellia sinensis is being investigated for its safety in patients with mild to moderately active UC during clinical remission and maintenance therapy, with the study currently in phase II. Another anti-inflammatory SM, curcumin, is also being investigated at various clinical phases. In pediatric patients with IBD, a phase I study is aiming to assess the tolerability of curcumin. In patients with UC, a phase I trial is investigated the effectiveness of a combined therapy involving curcumin and 5-ASA compared to that of 5-ASA alone (Table 3).

Table 3.
Nature-derived anti-inflammatory compounds and helminth products in clinical trials for treating inflammatory bowel diseases.
In contrast, a phase III trial is exploring the impact of combining curcumin with thiopurines to prevent postoperative recurrence. The bioactive compound berberine, isolated from Coptis chinensis, is the focus of a phase I study evaluating its safety in patients with UC in clinical remission and undergoing maintenance therapy. Finally, triptolide, reported as a bioactive compound in Tripterygium wilfordii, is being studied to assess its impact and safety in inducing remission in CD, comparing its efficacy with that of mesalamine, with ongoing trials in both phase II and III. Overall, studies underscore the potential of natural compounds in treating BD, offering insights into safety, efficacy, and therapeutic strategies at the various stages of clinical development (Table 3).
Following the positive tolerance and absence of side effects observed in the open trial involving patients with active UC and CD, researchers conducted clinical trials primarily using Trichuris suis, the pig whipworm, and Necator americanus, a hematophagous hookworm [169]. In an open-label trial (phase 1) at the University of Iowa, seven patients with IBD received 2500 TSO, resulting in remission for six patients per the IBD Quality of Life Index [169,214]. Subsequently, a randomized, double-blind, placebo-controlled trial (NCT01433471) involved 54 active UC patients treated with 2500 TSO or placebo every two weeks for 12 weeks. The study showed significant improvement in 43.3% of patients treated with TSO compared to 16.7% of placebo recipients [169,214]. Similarly, a trial with 29 patients with active CD demonstrated a 79.3% decrease in the CD activity index (CDAI) and a 72.4% remission rate after 24 weeks of TSO treatment (Table 3). N. americanus was also tested in patients with CD, showing clinical improvement and remission in eight of nine patients after 20 weeks despite mild side effects. Additionally, helminth-derived products, such as P28 S-glutathione transferase (P28SGT), demonstrated promise, reducing disease activity and inflammatory markers in patients with CD in clinical trials (NCT02281916) [169] (Table 3). Considering the reported immunomodulatory properties, helminths show significant potential for treating inflammatory bowel disease (IBD). However, further research is needed to fully understand their mechanisms and ensure their safe and effective application in clinical settings.
5. Advances and Recent Approaches in SM Drug Lead Discovery
In drug discovery, including SMs, drug leads demand the physical screening of large chemical libraries for biological targets, which is common but time-consuming and expensive. As such, there have been substantial advancements in techniques for SM drug discovery, including high-throughput screening, structure-based drug design, virtual screening, and the refinement of lead compounds. High-throughput screening (HTS) analyzes over a million compounds biochemically, requiring substantial time and investment [215]. To address this, virtual high-throughput screening was developed as a cost-effective computational method that is extensively applied in early drug discovery. It aims to identify novel, active small molecules by searching vast compound libraries, supporting the goals of HTS while reducing costs by evaluating only selected compounds for pharmacological activity [216,217].
Furthermore, molecular docking has proved to be a crucial approach in drug discovery in predicting the interaction patterns between proteins and small molecules and indicating the presence of bioactive compounds in natural products. For instance, GC-MS-identified bioactive ingredients and molecular docking against key targets revealed 3,5-dehydro-6-methoxy, ethyl iso-allocholate cholest-22-ene-21-ol, alpha-cadinol, and pivalate of Phyllanthus nivosus as promising compounds for UC drug development [218]. Computational methods, particularly in silico discovery, enhance traditional drug development, ensuring sustainable and cost-effective drug discovery with increased efficacy [219]. Modern techniques like pharmacophore modeling are also crucial for virtual screening, utilizing advanced compound databases and computing power to find small molecules of lead compounds [17].
To expedite the discovery of bioactive NPs in extracts, metabolomics data have been subjected to chemometric methods like multivariate data analysis, which correlate measured activity with nuclear magnetic resonance (NMR) and MS spectra signals, facilitating the tracking of active compounds in complex mixtures without additional bioassays [168,220]. Recent advancements in analytical technologies, particularly higher-field NMR instruments and probe technology, have allowed for precise structure determination of NPs even from limited quantities (<10 µg) [221]. Microcrystal electron diffraction, a cryo-electron-microscopy-based technique, is being increasingly applied for unambiguous structure determination of SMs in NP research [222]. Bioactivity-guided fractionation techniques with NMR-based methods have recently been utilized for screening, identifying, and isolating anti-inflammatory bioactive compounds from natural products. For instance, a methanolic extract of Uraria crinite (L.) roots was screened to isolate the immunomodulatory isoflavone genistein. This compound exhibited immunomodulatory activity against producing proinflammatory cytokines (IL-6 and TNF) [223].
6. Challenges and Future Directions
Exploring the small anti-inflammatory molecules derived from remedial plants and helminths represents a promising frontier in pharmacopoeia research. These naturally sourced compounds often exhibit unique mechanisms of action, lower toxicity, and fewer side effects. However, challenges exist in identifying and isolating bioactive compounds from diverse natural products. The use of HTS, computational approaches like molecular docking and virtual screening, and integrating artificial intelligence (AI) and machine learning (ML) algorithms can enhance the accuracy and efficiency of identifying promising candidates from natural compound libraries. It is crucial to understand the molecular mechanisms through which these natural compounds exert their anti-inflammatory effects. Research should focus on investigating how these compounds interact with key inflammatory mediators. Advanced techniques such as clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) gene editing and RNA sequencing can provide better insights into these interactions. Additionally, addressing challenges related to bioavailability and unfavorable pharmacokinetic profiles is essential. Techniques like nanoencapsulation, liposomal delivery, and phytosome technology should be incorporated to improve small anti-inflammatory molecules’ absorption, stability, and targeted delivery. Research has shown that helminths are therapeutic in treating inflammatory disorders due to their mechanisms evolved for modulating host immune responses. Further research should explore isolating and characterizing the small molecules from helminths to assess their therapeutic potential in inflammatory diseases. Techniques like proteomics and metabolomics can be instrumental in identifying bioactive helminth-derived compounds. Continued interdisciplinary research and collaboration will unlock the full therapeutic potential of these natural compounds.
7. Conclusions
In conclusion, our comprehensive analysis of the anti-inflammatory properties inherent in natural products, encompassing both crude extracts and isolated SMs, underscores their remarkable capacity to modulate a spectrum of inflammatory pathways. The anti-inflammatory efficacy of natural products is manifested by inhibiting key inflammatory mediators such as NO, Cox-2, and proinflammatory cytokines, alongside the stimulation of anti-inflammatory cytokine production. While certain reported extracts or SMs operate through singular or dual mechanisms, others exhibit a more diverse array of actions. Furthermore, emerging research highlights the therapeutic promise of helminths and their secretory products (ESPs) in coordinating host immune responses and alleviating inflammatory maladies. Helminth-induced immune modulation fosters a milieu conducive to Th2-, IL10-, and TGFβ-dependent immune regulation, effectively attenuating Th1/Th17 inflammatory responses. Several helminth species, including Schistosoma mansoni, Hymenolepis diminuta, Trichinella spiralis, and Trichuris suis, demonstrate significant protective effects against colitis in preclinical models and human trials, highlighting their potential as therapeutic agents.
In light of these findings, NPs remain a fertile ground for identifying and discovering diverse structures of anti-inflammatory SMs. These structures can be either directly developed or serve as initial frameworks or scaffolds for further optimization into innovative anti-inflammatory drugs. Despite the challenges associated with drug development, such as high attrition rates and obstacles related to accessibility, sustainable supply, and intellectual property constraints, we remain optimistic that ongoing scientific and technological advancements will establish a robust foundation for NP-based drug discovery and harness the vast potential of nature’s pharmacopeia.
Author Contributions
P.W. and T.J. conceptualized the project; T.J. took the lead in preparing the original draft; P.W. and A.L. contributed to reviewing and editing the manuscript. Funding was acquired by P.W. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this research was provided through various sources: T.J. was supported by the James Cook University Postgraduate Research Scholarship (JCUPRS), P.W. by the NHMRC Ideas Grant (APP1183323), and A.L. by an NHMRC program grant (APP1132975).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tung, B.T.; Linh, T.V.; Thao, T.P.; Thuan, N.D. Anti-inflammatory Agents from Medicinal Plants. In Phytochemical Drug Discovery for Central Nervous System Disorders: Biochemistry and Therapeutic Effects, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 219–250. [Google Scholar]
- Morgan, R.E.; Ahn, S.; Nzimiro, S.; Fotie, J.; Phelps, M.A.; Cotrill, J.; Yakovich, A.J.; Sackett, D.L.; Dalton, J.T.; Werbovetz, K.A. Inhibitors of tubulin assembly identified through screening a compound library. Chem. Biol. Drug. 2008, 72, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Vishal, V.; Ganesh, S.; Mukesh, G.; Ranjan, B. A review on some plants having anti-inflammatory activity. J. Phytopharmacol. 2014, 3, 214–221. [Google Scholar]
- Yeshi, K.; Turpin, G.; Jamtsho, T.; Wangchuk, P. Indigenous uses, phytochemical analysis, and anti-inflammatory properties of Australian tropical medicinal plants. Molecules 2022, 27, 3849. [Google Scholar] [CrossRef] [PubMed]
- Hijos-Mallada, G.; Sostres, C.; Gomollón, F. NSAIDs, gastrointestinal toxicity and inflammatory bowel disease. Gastroenterol. Hepatol. 2022, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, Y.; Farag, M.A.; Li, Z.; Shao, P. Dendrobium as a new natural source of bioactive for the prevention and treatment of digestive tract diseases: A comprehensive review with future perspectives. Phytomedicine 2023, 114, 154784. [Google Scholar] [CrossRef] [PubMed]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the pipeline of novel therapies for inflammatory bowel disease; state of the art review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting inflammatory bowel Ddsease: Pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Braus, N.A.; Elliott, D.E. Advances in the pathogenesis and treatment of IBD. Clin. Immunol. 2009, 132, 1–9. [Google Scholar] [CrossRef]
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Talley, N.J.; Abreu, M.T.; Achkar, J.P.; Bernstein, C.N.; Dubinsky, M.C.; Hanauer, S.B.; Kane, S.V.; Sandborn, W.J.; Ullman, T.A.; Moayyedi, P. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am. J. Gastroenterol. 2011, 106 (Suppl. S1), S2–S25; quiz S26. [Google Scholar] [CrossRef]
- Coskun, M.; Steenholdt, C.; de Boer, N.K.; Nielsen, O.H. Pharmacology and optimization of thiopurines and methotrexate in inflammatory bowel disease. Clin. Pharmacokinet. 2016, 55, 257–274. [Google Scholar] [CrossRef]
- Zhao, L.; Xiao, H.T.; Mu, H.X.; Huang, T.; Lin, Z.S.; Zhong, L.L.D.; Zeng, G.Z.; Fan, B.M.; Lin, C.Y.; Bian, Z.X. Magnolol, a natural polyphenol, attenuates dextran sulfate sodium-induced colitis in mice. Molecules 2017, 22, 1218. [Google Scholar] [CrossRef] [PubMed]
- Madasu, C.; Xu, Y.-M.; Wijeratne, E.K.; Liu, M.X.; Molnár, I.; Gunatilaka, A.L. Semi-synthesis and cytotoxicity evaluation of pyrimidine, thiazole, and indole analogues of argentatins A–C from guayule (Parthenium argentatum) resin. Med. Chem. Res. 2022, 31, 1088–1098. [Google Scholar] [CrossRef]
- Shyni, G.; Renjitha, J.; B Somappa, S.; Raghu, K. Zerumin A attenuates the inflammatory responses in LPS-stimulated H9c2 cardiomyoblasts. J. Biochem. Mol. Toxicol. 2021, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jalaja, R.; Leela, S.G.; Mohan, S.; Nair, M.S.; Gopalan, R.K.; Somappa, S.B. Anti-hyperlipidemic potential of natural product based labdane-pyrroles via inhibition of cholesterol and triglycerides synthesis. Bioorg. Chem. 2021, 108, 104664. [Google Scholar] [CrossRef] [PubMed]
- El-Senduny, F.F.; Altouhamy, M.; Zayed, G.; Harsha, C.; Jalaja, R.; Somappa, S.B.; Nair, M.S.; Kunnumakkara, A.B.; Alsharif, F.M.; Badria, F.A. Azadiradione-loaded liposomes with improved bioavailability and anticancer efficacy against triple negative breast cancer. J. Drug Deliv. Sci. Technol. 2021, 65, 102665. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Danese, S.; Peyrin-Biroulet, L. Next generation of small molecules in inflammatory bowel disease. Gut 2017, 66, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef]
- Razzacki, S.Z.; Thwar, P.K.; Yang, M.; Ugaz, V.M.; Burns, M.A. Integrated microsystems for controlled drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 185–198. [Google Scholar] [CrossRef]
- Vasir, J.K.; Tambwekar, K.; Garg, S. Bioadhesive microspheres as a controlled drug delivery system. Int. J. Pharm. 2003, 255, 13–32. [Google Scholar] [CrossRef]
- Pellá, M.C.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Anabousi, S.; Ehrhardt, C.; Ravi Kumar, M. Liposomes as targeted drug delivery systems in the treatment of breast cancer. J. Drug Target. 2006, 14, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Haba, Y.; Kojima, C.; Harada, A.; Ura, T.; Horinaka, H.; Kono, K. Preparation of poly (ethylene glycol)-modified poly (amido amine) dendrimers encapsulating gold nanoparticles and their heat-generating ability. Langmuir 2007, 23, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Sun, K.; Baker, J.R., Jr. Spontaneous formation of functionalized dendrimer-stabilized gold nanoparticles. J. Phys. Chem. C 2008, 112, 8251–8258. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, M.M.; Aramwit, P.; Kwon, G.S. Nanotechnology in Drug Delivery; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Dent, M.; Matoba, N. Cancer biologics made in plants. Curr. Opin. Biotechnol. 2020, 61, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Muema, F.W.; Nanjala, C.; Oulo, M.A.; Wangchuk, P. Phytochemical content and antidiabetic properties of most commonly used antidiabetic medicinal plants of Kenya. Molecules 2023, 28, 7202. [Google Scholar] [CrossRef]
- Cláudia da Costa Rocha, A.; José de Andrade, C.; de Oliveira, D. Perspective on integrated biorefinery for valorization of biomass from the edible insect Tenebrio molitor. Trends Food Sci. Technol. 2021, 116, 480–491. [Google Scholar] [CrossRef]
- Inanoglu, S.; Goksen, G.; Nayik, G.A.; Mozaffari Nejad, A.S. Chapter 11—Essential oils from Lamiaceae family (rosemary, thyme, mint, basil). In Essential Oils; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 309–324. [Google Scholar]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef]
- Cao, Z.; Deng, Z. De novo assembly, annotation, and characterization of root transcriptomes of three caladium cultivars with a focus on necrotrophic pathogen resistance/defense-related genes. Int. J. Mol. Sci. 2017, 18, 712. [Google Scholar] [CrossRef]
- Shazhni, J.A.; Renu, A.; Vijayaraghavan, P. Insights of antidiabetic, anti-inflammatory and hepatoprotective properties of antimicrobial secondary metabolites of corm extract from Caladium x hortulanum. Saudi J. Biol. Sci. 2018, 25, 1755–1761. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Chalmers, A.C.; Jyoti Bhuyan, D.; Bowyer, M.C.; Scarlett, C.J. Botanical, phytochemical, and anticancer properties of the Eucalyptus Species. Chem. Biodivers. 2015, 12, 907–924. [Google Scholar] [CrossRef]
- Jamtsho, T.; Yeshi, K.; Samten; Wangchuk, P. Comparative analysis of two Himalayan Aconitum species for their phytopharmaceutical properties. J. Herb. Med. 2022, 32, 100497. [Google Scholar] [CrossRef]
- Wangchuk, P. Phytochemical Analysis, Bioassays and the Identification of Drug Lead Compounds from Seven Bhutanese Medicinal Plants. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2014. [Google Scholar]
- Wangchuk, P.; Navarro, S.; Shepherd, C.; Keller, P.A.; Pyne, S.G.; Loukas, A. Diterpenoid alkaloids of Aconitum laciniatum and mitigation of inflammation by 14-O-acetylneoline in a murine model of ulcerative colitis. Sci. Rep. 2015, 5, 12845. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. 2015, 28, 210. [Google Scholar]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef]
- Maroyi, A. Albizia Adianthifolia: Botany, Medicinal Uses, Phytochemistry, and Pharmacological Properties. Sci. World J. 2018, 2018, 7463584. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.C.; Kim, B.R.; Vuong, H.L.; Cho, S. Spilanthes acmella inhibits inflammatory responses via inhibition of NF-κB and MAPK signaling pathways in RAW 264.7 macrophages. Mol. Med. Rep. 2017, 16, 339–346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bakondi, E.; Singh, S.B.; Hajnády, Z.; Nagy-Pénzes, M.; Regdon, Z.; Kovács, K.; Hegedűs, C.; Madácsy, T.; Maléth, J.; Hegyi, P.; et al. Spilanthol Inhibits Inflammatory Transcription Factors and iNOS Expression in Macrophages and Exerts Anti-inflammatory Effects in Dermatitis and Pancreatitis. Int. J. Mol. Sci. 2019, 20, 4308. [Google Scholar] [CrossRef] [PubMed]
- Negahban, M. The Medicinal Effects of Two Australian Native Plants. Ph.D. Thesis, Queensland University of Technology, Queensland, Australia, 2020. [Google Scholar]
- Turpin, G.; Ritmejerytė, E.; Jamie, J.; Crayn, D.; Wangchuk, P. Aboriginal medicinal plants of Queensland: Ethnopharmacological uses, species diversity, and biodiscovery pathways. J. Ethnobiol. Ethnomed. 2022, 18, 54. [Google Scholar] [CrossRef] [PubMed]
- Palit, P.; Mukherjee, D.; Mahanta, P.; Shadab, M.; Ali, N.; Roychoudhury, S.; Asad, M.; Mandal, S.C. Attenuation of nociceptive pain and inflammatory disorders by total steroid and terpenoid fraction of Euphorbia tirucalli Linn root in experimental in vitro and in vivo model. Inflammopharmacology 2018, 26, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, M.; Rahimi-Nasrabadi, M.; Ahmadi, F.; Gandomi, H.; Abbaszadeh, S.; Batooli, H. The chemical composition and in vitro antifungal activities of essential oils of five Eucalyptus species. J. Essent. Oil-Bear. Plants 2015, 18, 666–677. [Google Scholar] [CrossRef]
- Venkataraman, B.; Almarzooqi, S.; Raj, V.; Bhongade, B.A.; Patil, R.B.; Subramanian, V.S.; Attoub, S.; Rizvi, T.A.; Adrian, T.E.; Subramanya, S.B. Molecular docking Identifies 1,8-Cineole (Eucalyptol) as A novel PPARγ agonist that alleviates colon inflammation. Int. J. Mol. Sci. 2023, 24, 6160. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M.; Wang, G.; Ma, H.; Mu, Y.; Zheng, D.; Huang, X.; Li, L. New monoterpene acid and gallic acid glucose esters with anti-Inflammatory activity from Blue gum (Eucalyptus globulus) Leaves. J. Agric. Food Chem. 2022, 70, 4981–4994. [Google Scholar] [CrossRef]
- Qabaha, K.; Ras, S.A.; Abbadi, J.; Al-Rimawi, F. Anti-inflammatory activity of Eucalyptus spp. and Pistascia lentiscus leaf extracts. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ito, H.; Miyashita, K.; Hatano, T.; Taniguchi, S.; Amakura, Y.; Yoshida, T. Flavonol glucuronides and C-glucosidic ellagitannins from Melaleuca squarrosa. Phytochemistry 2008, 69, 3062–3069. [Google Scholar] [CrossRef]
- Hussein, S.A.; Hashim, A.N.; El-Sharawy, R.T.; Seliem, M.A.; Linscheid, M.; Lindequist, U.; Nawwar, M.A. Ericifolin: An eugenol 5-O-galloylglucoside and other phenolics from Melaleuca ericifolia. Phytochemistry 2007, 68, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, E.; Wehde, R.; Dörr, M.; Lang, G.; Stevens, J.F. C-methyl-flavonoids from the leaf waxes of some Myrtaceae. Phytochemistry 2000, 55, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Abdel Bar, F.M.; Zaghloul, A.M.; Bachawal, S.V.; Sylvester, P.W.; Ahmad, K.F.; El Sayed, K.A. Antiproliferative triterpenes from Melaleuca ericifolia. J. Nat. Prod. 2008, 71, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Chang, M.H. Four new triterpenes from the heartwood of Melaleuca leucadendron. J. Nat. Prod. 1999, 62, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K. A New Norlupene from the Leaves of Melaleuca leucadendron. J. Nat. Prod. 1998, 61, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, T.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Melaleuca leucadendron: Inhibitors of induced histamine release from rat mast cells. Chem. Pharm. Bull. 1991, 39, 3276–3278. [Google Scholar] [CrossRef]
- Bar, F.M.A. Genus Melaleuca—A review on the phytochemistry and pharmacological activities of the non-volatile components. Rec. Nat. Prod. 2021, 15, 219–242. [Google Scholar] [CrossRef]
- Ritmejerytė, E.; Ryan, R.Y.; Byatt, B.J.; Peck, Y.; Yeshi, K.; Daly, N.L.; Zhao, G.; Crayn, D.; Loukas, A.; Pyne, S.G. Anti-inflammatory properties of novel galloyl glucosides isolated from the Australian tropical plant Uromyrtus metrosideros. Chem. Biol. Interact. 2022, 368, 110124. [Google Scholar] [CrossRef]
- Vogel, A.; Pelletier, J. Examen chimique de la racine de Curcuma. J. Pharm. 1815, 1, 289–300. [Google Scholar]
- Karamanou, M.; Tsoucalas, G.; Pantos, K.; Androutsos, G. Isolating colchicine in 19th century: An old drug revisited. Curr. Pharm. Des. 2018, 24, 654–658. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty years of growth, development and controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Escogido Mde, L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Bernini, R.; Santi, L.; Romani, A.; Lacetera, N.; Bernabucci, U. (-)-Epigallocatechin-3-gallate and hydroxytyrosol improved antioxidative and anti-inflammatory responses in bovine mammary epithelial cells. Animal 2019, 13, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Takaki, A.; Hiraoka, S.; Adachi, T.; Shimomura, Y.; Matsushita, H.; Nguyen, T.T.T.; Koike, K.; Ikeda, A.; Takashima, S. Berberine improved experimental chronic colitis by regulating interferon-γ-and IL-17A-producing lamina propria CD4+ T cells through AMPK activation. Sci. Rep. 2019, 9, 11934. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hu, Z.; Song, X.; Cui, Q.; Fu, Q.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Analgesic and anti-inflammatory activities of resveratrol through classic models in mice and rats. Evid. Based Complement. Altern. Med. 2017, 2017, 5197567. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-T.; Xu, Y.-F.; Huang, Y.-F.; Qu, C.; Xu, L.-Q.; Su, Z.-R.; Zeng, H.-F.; Zheng, L.; Yi, T.-G.; Li, H.-L. Berberrubine attenuates mucosal lesions and inflammation in dextran sodium sulfate-induced colitis in mice. PLoS ONE 2018, 13, e0194069. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.-H.; Chen, X.; Sang, S.; Lee, M.-J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: Effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-Y.; Kim, K.-H.; Lee, S.-H.; Yoon, M.-S.; Lee, H.-J.; Moon, D.-O.; Lee, C.-M.; Ahn, S.-C.; Park, Y.C.; Park, Y.-M. Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-κB as Potential Targets1. J. Immunol. 2005, 174, 8116–8124. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.O.; Ferguson, J.E.; Hunsaker, L.A.; Deck, L.M.; Vander Jagt, D.L. Natural products inhibit LPS-induced activation of pro-inflammatory cytokines in peripheral blood mononuclear cells. Nat. Prod. Res. 2010, 24, 1177–1188. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Yan, Y.-M.; Li, S.-Y.; He, D.-H.; Xiong, S.; Wei, S.-F.; Liu, W.; Hu, L.; Wang, Q.; Pan, H.-F. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol. Sin. 2020, 41, 10–21. [Google Scholar] [CrossRef]
- Xue, P.-H.; Zhang, N.; Liu, D.; Zhang, Q.-R.; Duan, J.-S.; Yu, Y.-Q.; Li, J.-Y.; Cao, S.-J.; Zhao, F.; Kang, N. Cytotoxic and anti-inflammatory sesquiterpenes from the whole plants of Centipeda minima. J. Nat. Prod. 2021, 84, 247–258. [Google Scholar] [CrossRef]
- Wu, H.; Pang, H.; Chen, Y.; Huang, L.; Liu, H.; Zheng, Y.; Sun, C.; Zhang, G.; Wang, G. Anti-inflammatory effect of a polyphenol-enriched fraction from Acalypha wilkesiana on lipopolysaccharide-stimulated RAW 264.7 macrophages and acetaminophen-induced liver injury in mice. Oxid. Med. Cell. Longev. 2018, 2018, 7858094. [Google Scholar] [CrossRef]
- Vigil de Mello, S.V.G.; da Rosa, J.S.; Facchin, B.M.; Luz, A.B.G.; Vicente, G.; Faqueti, L.G.; Rosa, D.W.; Biavatti, M.W.; Fröde, T.S. Beneficial effect of Ageratum conyzoides Linn (Asteraceae) upon inflammatory response induced by carrageenan into the mice pleural cavity. J. Ethnopharmacol. 2016, 194, 337–347. [Google Scholar] [CrossRef]
- Senthamilselvi, M.M.; Kesavan, D.; Sulochana, N. An anti-inflammatory and anti-microbial flavone glycoside from flowers of Cleome viscosa. Org. Med. Chem. Lett. 2012, 2, 19. [Google Scholar] [CrossRef]
- Bawankule, D.; Chattopadhyay, S.; Pal, A.; Saxena, K.; Yadav, S.; Faridi, U.; Darokar, M.; Gupta, A.; Khanuja, S.P.S. Modulation of inflammatory mediators by coumarinolignoids from Cleome viscosa in female swiss albino mice. Inflammopharmacology 2008, 16, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-induced RAW264. 7 cells: In vitro assessment and a theoretical model. BioMed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Okoro, E.E.; Maharjan, R.; Jabeen, A.; Ahmad, M.S.; Azhar, M.; Shehla, N.; Zaman, W.; Shams, S.; Osoniyi, O.R.; Onajobi, F.D.; et al. Isoflavanquinones from Abrus precatorius roots with their antiproliferative and anti-inflammatory effects. Phytochemistry 2021, 187, 112743. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.; Duarte, S.; Arsénio, P.; Gonçalves, J.; Rodrigues, C.; Lourenço, A.; Máximo, P. Exploring the phytochemicals of Acacia melanoxylon R. Br. Plants. 2021, 10, 2698. [Google Scholar] [CrossRef]
- Kim, J.E.; Min, S.K.; Hong, J.-M.; Kim, K.H.; Han, S.J.; Yim, J.H.; Park, H.; Kim, I.-C. Anti-inflammatory effects of methanol extracts from the Antarctic lichen, Amandinea sp. in LPS-stimulated raw 264.7 macrophages and zebrafish. Fish Shellfish. Immunol. 2020, 107, 301–308. [Google Scholar] [CrossRef]
- Vyas, A.V.; Mulchandani, N.B. Polyoxygenated flavones from Ageratum conyzoides. Phytochemistry 1986, 25, 2625–2627. [Google Scholar] [CrossRef]
- Moreira, M.D.; Picanço, M.C.; Barbosa, L.C.; Guedes, R.N.; Barros, E.C.; Campos, M.R. Compounds from Ageratum conyzoides: Isolation, structural elucidation and insecticidal activity. Pest Manag. Sci. 2007, 63, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Münch, G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.Y.; Kiendrebeogo, M.; Kehoe, P.G.; Sombie, P.A.; Lamien, C.E.; Millogo, J.F.; Nacoulma, O.G. Antioxidant and anti-inflammatory effects of Scoparia dulcis L. J. Med. Food 2011, 14, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Singh, A.; Bodkin, F.; Münch, G. Costatamins A–C, new 4-phenylcoumarins with anti-inflammatory activity from the Australian woodland tree Angophora costata (Myrtaceae). Fitoterapia 2019, 133, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Qazi, M.S.; Kamal, M. New anti-inflammatory triterpene esters and glycosides from Alstonia scholaris. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.-H.; Cai, X.-H.; Feng, T.; Zhao, Y.-L.; Wang, J.-K.; Zhang, L.-Y.; Yan, M.; Luo, X.-D. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J. Ethnopharmacol. 2010, 129, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Huyen, L.T.; Nhiem, N.X.; Quang, T.H.; Hang, D.T.T.; Yen, P.H.; Tai, B.H.; Anh, H.L.T.; Binh, N.Q.; Van Minh, C. Tirucallane glycoside from the leaves of Antidesma bunius and inhibitory NO production in BV2 cells and RAW264.7 macrophages. Nat. Prod. Commun. 2016, 11, 1934578X1601100717. [Google Scholar] [CrossRef]
- Dissanayake, A.A.; Georges, K.; Nair, M.G. Cyclooxygenase enzyme and lipid peroxidation inhibitory terpenoids and steroidal compounds as major constituents in Cleome viscosa leaves. Planta Med. 2022, 88, 1287–1292. [Google Scholar] [CrossRef]
- Patil, K.R.; Patil, C.R. Anti-inflammatory activity of bartogenic acid containing fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. J. Tradit. Complement. Med. 2017, 7, 86–93. [Google Scholar] [CrossRef]
- Van, Q.T.T.; Vien, L.T.; Hanh, T.T.H.; Huong, P.T.T.; Cuong, N.T.; Thao, N.P.; Thuan, N.H.; Dang, N.H.; Thanh, N.V.; Cuong, N.X. Acylated flavonoid glycosides from Barringtonia racemosa. Nat. Prod. Res. 2020, 34, 1276–1281. [Google Scholar] [CrossRef]
- Bairwa, K.; Singh, I.N.; Roy, S.K.; Grover, J.; Srivastava, A.; Jachak, S.M. Rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J. Nat. Prod. 2013, 76, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, K.; Jachak, S.M. Anti-inflammatory potential of a lipid-based formulation of a rotenoid-rich fraction prepared from Boerhavia diffusa. Pharm. Biol. 2015, 53, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; Xie, C.; Ye, H.; Tang, H.; Chen, L.; Peng, A. Anti-inflammatory activity of ethyl acetate fraction of the seeds of Brucea javanica. J. Ethnopharmacol. 2013, 147, 442–446. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Zhou, J.-T.; Qu, C.; Dou, Y.-X.; Huang, Q.-H.; Lin, Z.-X.; Xian, Y.-F.; Xie, J.-H.; Xie, Y.-L.; Lai, X.-P.; et al. Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-κB activation on dextran sulfate sodium-induced ulcerative colitis in mice. J. Ethnopharmacol. 2017, 198, 389–398. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Li, S.; Ye, H.; Tang, H.; Chen, L.; Peng, A. Coumarinolignans isolated from the seeds of Brucea javanica. Helv. Chim. Acta 2014, 97, 278–282. [Google Scholar] [CrossRef]
- Hu, Z.-F.; Su, J.-C.; Sun, X.; Xia, R.-F.; Wu, J.-L.; Fu, X.-N.; Zhang, B.-Z.; Chen, J.-C.; Wan, L.-S. Brujavanoids A–U, structurally diverse apotirucallane-type triterpenoids from Brucea javanica and their anti-inflammatory effects. Bioorg. Chem. 2022, 127, 106012. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, T.; Dou, Y.; Huang, Y.; Qu, C.; Gao, J.; Huang, Z.; Xie, Y.; Huang, P.; Lin, Z.; et al. Brusatol ameliorates 2, 4, 6-trinitrobenzenesulfonic acid-induced experimental colitis in rats: Involvement of NF-κB pathway and NLRP3 inflammasome. Int. Immunopharmacol. 2018, 64, 264–274. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Yang, Y.; Zhang, S.; Jiang, H.; Tian, K.; Arenbaoligao; Chen, D. The treatment of inflammatory bowel disease with monoclonal antibodies in Asia. Biomed. Pharmacother. 2023, 157, 114081. [Google Scholar] [CrossRef]
- Dalavaye, N.; Erridge, S.; Nicholas, M.; Pillai, M.; Bapir, L.; Holvey, C.; Coomber, R.; Rucker, J.J.; Hoare, J.; Sodergren, M.H. The effect of medical cannabis in inflammatory bowel disease: Analysis from the UK Medical Cannabis Registry. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-C.; Liang, Y.-H.; Chiang, J.-H.; Liu, F.-C.; Lin, W.-H.; Chang, S.-J.; Lin, W.-Y.; Wu, C.-H.; Weng, J.-R. Anti-inflammatory effects of Calophyllum inophyllum L. in RAW264.7 cells. Oncol. Rep. 2012, 28, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-L.; Truong, C.-T.; Nguyen, B.C.Q.; Vo, T.-N.V.; Dao, T.-T.; Nguyen, V.-D.; Trinh, D.-T.T.; Huynh, H.K.; Bui, C.-B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef]
- Van Thanh, N.; Jang, H.-J.; Vinh, L.B.; Linh, K.T.P.; Huong, P.T.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Kim, Y.H.; Yang, S.Y. Chemical constituents from Vietnamese mangrove Calophyllum inophyllum and their anti-inflammatory effects. Bioorg. Chem. 2019, 88, 102921. [Google Scholar] [CrossRef]
- Wu, F.-S.; Huang, C.-M.; Chen, L.-C.; Chang, T.-H.; Shu, C.-W.; Sung, P.-J.; Chu, Y.-C.; Cheng, M.-J.; Hsiao, J.-W.; Chen, J.-J. A new cinnamic acid derivative and anti-inflammatory constituents from Capparis lanceolaris. Chem. Nat. Compd. 2023, 59, 493–496. [Google Scholar] [CrossRef]
- Choi, J.K.; Oh, H.-M.; Park, J.H.; Choi, J.H.; Sa, K.H.; Kang, Y.M.; Park, P.-H.; Shin, T.-Y.; Rho, M.-C.; Kim, S.-H. Salvia plebeia extract inhibits the inflammatory response in human rheumatoid synovial fibroblasts and a murine model of arthritis. Phytomedicine 2015, 22, 415–422. [Google Scholar] [CrossRef]
- Geraghty, D.P.; Ahuja, K.D.; Pittaway, J.; Shing, C.; Jacobson, G.A.; Jager, N.; Jurković, S.; Narkowicz, C.; Saunders, C.I.; Ball, M. In vitro antioxidant, antiplatelet and anti-inflammatory activity of Carpobrotus rossii (pigface) extract. J. Ethnopharmacol. 2011, 134, 97–103. [Google Scholar] [CrossRef]
- Li, R.W.; Lin, G.D.; Leach, D.N.; Waterman, P.G.; Myers, S.P. Inhibition of COXs and 5-LOX and activation of PPARs by Australian Clematis species (Ranunculaceae). J. Ethnopharmacol. 2006, 104, 138–143. [Google Scholar] [CrossRef]
- Huang, S.-S.; Chiu, C.-S.; Lin, T.-H.; Lee, M.-M.; Lee, C.-Y.; Chang, S.-J.; Hou, W.-C.; Huang, G.-J.; Deng, J.-S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef]
- Li, S.-Y.; Zhou, Y.-L.; He, D.-H.; Liu, W.; Fan, X.-Z.; Wang, Q.; Pan, H.-F.; Cheng, Y.-X.; Liu, Y.-Q. Centipeda minima extract exerts antineuroinflammatory effects via the inhibition of NF-κB signaling pathway. Phytomedicine 2020, 67, 153164. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.-Y.; Lee, M.M.-L.; Leung, T.-W.; Shum, T.-Y.; Cho, W.C.-S.; Chen, S.; Tai, W.C.-S. Centipeda minima extract attenuates dextran sodium sulfate-induced acute colitis in mice by inhibiting macrophage activation and monocyte chemotaxis. Front. Pharmacol. 2021, 12, 738139. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, Anti-Inflammatory and Attenuating Intracellular Reactive Oxygen Species Activities of Nicotiana tabacum var. Virginia Leaf Extract Phytosomes and Shape Memory Gel Formulation. Gels 2023, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Tiwari, A.; Şahin, K.; Küçük, Ö.; Ali, S. Triterpenoid saponin-rich fraction of Centella asiatica decreases IL-1ß and NF-κB, and augments tissue regeneration and excision wound repair. Turk. J. Biol. 2016, 40, 399–409. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Tai, B.H.; Quang, T.H.; Van Kiem, P.; Van Minh, C.; Nam, N.H.; Kim, J.-H.; Im, L.-R.; Lee, Y.-M.; Kim, Y.H. A new ursane-type triterpenoid glycoside from Centella asiatica leaves modulates the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2011, 21, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Masi, F.; Cicia, D.; Ciceri, D.; Arpini, S.; Falzoni, M.; Pagano, E.; Taglialatela-Scafati, O. Isomadecassoside, a new ursane-type triterpene glycoside from Centella asiatica leaves, reduces nitrite levels in LPS-stimulated macrophages. Biomolecules 2021, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Srisook, K.; Srisook, E.; Nachaiyo, W.; Chan-In, M.; Thongbai, J.; Wongyoo, K.; Chawsuanthong, S.; Wannasri, K.; Intasuwan, S.; Watcharanawee, K. Bioassay-guided isolation and mechanistic action of anti-inflammatory agents from Clerodendrum inerme leaves. J. Ethnopharmacol. 2015, 165, 94–102. [Google Scholar] [CrossRef]
- Al Haidari, R.; Alshali, K.; Elkhayat, E.; Fouad, M.; Ibrahim, S.; Mohamed, G. Chemical constituents and biological investigations of the aerial parts of Egyptian Clerodendrum inerme. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 165–170. [Google Scholar] [CrossRef]
- Cock, I.E. Medicinal and aromatic plants–Australia. In Ethnopharmacology, Encyclopedia of Life Support Systems (EOLSS); Developed under the Auspices of the UNESCO; EOLSS Publishers: Oxford, UK, 2011. [Google Scholar]
- Doe, P.; Danquah, C.A.; Ohemeng, K.A.; Opare, A.E.; Sharif, A.; Akua-Abora, D.; Akuoko, A.K.; Kpabitey, A.; Quarshie, E.; Asante, O.O. Analgesic, anti-inflammatory, and anti-pyretic activities of Crinum pedunculatum R. Br. Bulb extracts. Pharmacogn. Res. 2022, 14, 24–29. [Google Scholar] [CrossRef]
- Deng, S.; Palu, A.K.; West, B.J.; Su, C.X.; Zhou, B.-N.; Jensen, J.C. Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. J. Nat. Prod. 2007, 70, 859–862. [Google Scholar] [CrossRef]
- Coutinho de Sousa, B.; Reis Machado, J.; da Silva, M.V.; da Costa, T.A.; Lazo-Chica, J.E.; Degasperi, T.d.P.; Rodrigues Junior, V.; Sales-Campos, H.; Uber Bucek, E.; Freire Oliveira, C.J. Morinda citrifolia (noni) fruit juice reduces inflammatory cytokines expression and contributes to the maintenance of intestinal mucosal integrity in DSS experimental colitis. Mediat. Inflamm. 2017, 2017, 6567432. [Google Scholar] [CrossRef]
- Huang, H.-L.; Liu, C.-T.; Chou, M.-C.; Ko, C.-H.; Wang, C.-K. Noni (Morinda citrifolia L.) fruit extracts improve colon microflora and exert anti-inflammatory activities in Caco-2 cells. J. Med. Food 2015, 18, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Kitamura, M.; Hayashi, Y.; Tomida, N.; Uwaya, A.; Isami, F.; Inoue, Y. Anti-inflammatory effects of Morinda citrifolia extract against lipopolysaccharide-induced inflammation in RAW264 cells. Medicines 2021, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, J.S.; Huang, P.; Qader, M.; Manavalan, A.; Wu, X.; Kim, J.-C.; Pang, C.; Cao, S.; Kang, K.S. Identification of anti-inflammatory compounds from Hawaiian noni (Morinda citrifolia L.) fruit juice. Molecules 2020, 25, 4968. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Quispe, C.; Sarkar, C.; Bepari, T.C.; Alam, M.J.; Saha, S.; Ray, P.; Rahim, M.A.; Islam, M.T.; Setzer, W.N. Analgesic and anti-inflammatory potential of essential oil of Eucalyptus camaldulensis leaf: In vivo and in silico studies. Nat. Prod. Commun. 2021, 16, 1934578X211007634. [Google Scholar] [CrossRef]
- Simpson, B.; Claudie, D.; Smith, N.; Wang, J.; McKinnon, R.; Semple, S. Evaluation of the anti-inflammatory properties of Dodonaea polyandra, a Kaanju traditional medicine. J. Ethnopharmacol. 2010, 132, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.S.; Luo, X.; Costabile, M.; Caughey, G.E.; Wang, J.; Claudie, D.J.; McKinnon, R.A.; Semple, S.J. Polyandric acid A, a clerodane diterpenoid from the Australian medicinal plant Dodonaea polyandra, attenuates pro-inflammatory cytokine secretion in vitro and in vivo. J. Nat. Prod. 2014, 77, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.S.; Claudie, D.J.; Gerber, J.P.; Pyke, S.M.; Wang, J.; McKinnon, R.A.; Semple, S.J. In vivo activity of benzoyl ester clerodane diterpenoid derivatives from Dodonaea polyandra. J. Nat. Prod. 2011, 74, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Sperotto, J.; Manfron, M. Antiinflammatory activity and acute toxicity of Dodonaea viscosa. Fitoterapia 2006, 77, 478–480. [Google Scholar] [CrossRef]
- Salinas-Sánchez, D.O.; Herrera-Ruiz, M.; Pérez, S.; Jiménez-Ferrer, E.; Zamilpa, A. Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. Molecules 2012, 17, 4292–4299. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Zou, B.-H.; Li, X.-J.; Liu, J.-J.; Shen, L.; Wu, J. Ent-kauranes from the Chinese Excoecaria agallocha L. and NF-κB inhibitory activity. Fitoterapia 2019, 133, 159–170. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Yu, Y.; Shen, L. Agallolides AM, including two rearranged ent-atisanes featuring a bicyclo [3.2. 1] octane motif, from the Chinese Excoecaria agallocha. Bioorg. Chem. 2020, 104, 104206. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, X.; Shuai, L.; Kwon, Y.S.; Kim, M.J. Chemical characterization, antioxidant properties and anti-inflammatory activity of Chinese water chestnut extracts. Sci. Asia 2020, 46, 151–156. [Google Scholar] [CrossRef]
- da Silva Barth, C.; de Souza, H.G.T.; Rocha, L.W.; da Silva, G.F.; Dos Anjos, M.F.; Pastor, V.D.A.; Bresolin, T.M.B.; Couto, A.G.; Santin, J.R.; Quintão, N.L.M. Ipomoea pes-caprae (L.) R. Br (Convolvulaceae) relieved nociception and inflammation in mice—A topical herbal medicine against effects due to cnidarian venom-skin contact. J. Ethnopharmacol. 2017, 200, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni-Almeida, A.; Suthar, A.; Goswami, H.; Vishwakarma, R.; Chauhan, V.S.; Balakrishnan, A.; Sharma, S. Novel leads from Heliotropium ovalifolium, 4, 7, 8-trimethoxy-naphthalene-2-carboxylic acid and 6-hydroxy-5, 7-dimethoxy-naphthalene-2-carbaldehyde show specific IL-6 inhibitory activity in THP-1 cells and primary human monocytes. Phytomedicine 2008, 15, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Bani, S.; Sultan, P.; Ali, S.A.; Bakheet, S.A.; Attia, S.M.; Abd-Allah, A.R. TNF-α inhibitory effect of Euphorbia hirta in rats. Pharm. Biol. 2013, 51, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-F.; Cheng, Y.-D.; Shen, C.-R.; Cherng, J.-Y. A molecular pharmacology study into the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J. Nat. Med. 2010, 64, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Yun, J.-M. Antioxidant and anti-inflammatory activities of butanol extract of Melaleuca leucadendron L. Prev. Nutr. Food Sci. 2012, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Michel, H.E.; Khattab, M.A.; El-Shazly, M.; Singab, A.N. Protective role of casuarinin from Melaleuca leucadendra against ethanol-induced gastric ulcer in rats. Planta Med. 2020, 86, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, O.O.; Yemitan, O.K.; Afolabi, L. Inhibition of chemically induced inflammation and pain by orally and topically administered leaf extract of Manihot esculenta Crantz in rodents. J. Ethnopharmacol. 2008, 119, 6–11. [Google Scholar] [CrossRef]
- Chao, C.-H.; Cheng, J.-C.; Hwang, T.-L.; Shen, D.-Y.; Wu, T.-S. Trinorditerpenes from the roots of Flueggea virosa. Bioorg. Med. Chem. Lett. 2014, 24, 447–449. [Google Scholar] [CrossRef]
- Bhowmick, R.; Sarwar, M.S.; RahmanDewan, S.M.; Das, A.; Das, B.; NasirUddin, M.M.; Islam, M.S.; Islam, M.S. In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res. 2014, 47, 56. [Google Scholar] [CrossRef] [PubMed]
- Narasimhulu, C.A.; Vardhan, S. Therapeutic potential of Ocimum tenuiflorum as MPO inhibitor with implications for atherosclerosis prevention. J. Med. Food 2015, 18, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Sranujit, R.P.; Noysang, C.; Tippayawat, P.; Kooltheat, N.; Luetragoon, T.; Usuwanthim, K. Phytochemical’s and immunomodulatory effect of Nelumbo nucifera flower extracts on human macrophages. Plants 2021, 10, 2007. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.A.; Zehra, T.; Fizzah, A.; Kumar, G. Evaluation of antiinflammatory activity of ethanol extract of Nelumbo nucifera fruit. Turk. J. Pharm. Sci. 2021, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Y.; Yang, Y.; Gou, Y.; Li, S.; Wang, R.; Zeng, S.; Zhao, X. Antioxidant and inflammatory effects of Nelumbo nucifera Gaertn. leaves. Oxid. Med. Cell. Longev. 2021, 2021, 8375961. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, Y.; Min, X.; Pei, L.; Chen, X. Neferine, a bisbenzylisoquinoline alkaloid, ameliorates dextran sulfate sodium-induced ulcerative colitis. Am. J. Chin. Med. 2018, 46, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Guo, Y.; Zhou, Y.; Chen, X. Protection against dextran sulfate sodium-induced ulcerative colitis in mice by neferine, a natural product from Nelumbo nucifera Gaertn. Cell J. 2021, 22, 523. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Yu, Y.; Yang, H.; Wang, N. Nuciferine inhibits proinflammatory cytokines via the PPARs in LPS-induced RAW264.7 cells. Molecules 2018, 23, 2723. [Google Scholar] [CrossRef]
- Li, F.; Sun, X.-Y.; Li, X.-W.; Yang, T.; Qi, L.-W. Enrichment and separation of quercetin-3-O-β-d-glucuronide from lotus leaves (Nelumbo nucifera gaertn.) and evaluation of its anti-inflammatory effect. J. Chromatogr. B Biomed. Appl. 2017, 1040, 186–191. [Google Scholar] [CrossRef]
- Fua, Y.-H. Structurally diverse indole alkaloids from Ochrosia elliptica. Heterocycles 2017, 94, 743–749. [Google Scholar] [CrossRef]
- Tian, L.-X.; Li, X.-Y.; Tang, X.; Zhou, X.-Y.; Luo, L.; Ma, X.-Y.; Tang, W.-Q.; Yu, J.; Ma, W.; Yang, X. Ellipticine conveys protective effects to lipopolysaccharide-activated macrophages by targeting the JNK/AP-1 signaling pathway. Inflammation 2020, 43, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Gambari, R.; Borgatti, M.; Lampronti, I.; Fabbri, E.; Brognara, E.; Bianchi, N.; Piccagli, L.; Yuen, M.C.-W.; Kan, C.-W.; Hau, D.K.-P. Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012, 13, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-C.; Peng, W.-H.; Chiu, T.-H.; Lai, S.-C.; Lee, C.-Y. Anti-inflammatory effects of Scoparia dulcis L. and betulinic acid. Am. J. Chin. Med. 2011, 39, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, L.; Gao, J.; Wang, Y.; Tang, X.; Zhao, X.; Zhang, Z. Phytochemical and antiinflammatory studies on Terminalia catappa. Fitoterapia 2004, 75, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, N.M.; Al-Sayed, E.; Abdel-Daim, M.M.; Singab, A.N. Anti-inflammatory and analgesic activities of Terminalia muelleri benth.(combretaceae). Drug Dev. Res. 2017, 78, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Adib-Conquy, M.; Coste, A.; Kumar-Roiné, S.; Pipy, B.; Laurent, D.; Pauillac, S. Aqueous extract of Vitex trifolia L. (Labiatae) inhibits LPS-dependent regulation of inflammatory mediators in RAW 264.7 macrophages through inhibition of Nuclear Factor kappa B translocation and expression. J. Ethnopharmacol. 2012, 143, 24–32. [Google Scholar] [CrossRef]
- Naz, T.; Packer, J.; Yin, P.; Brophy, J.J.; Wohlmuth, H.; Renshaw, D.E.; Smith, J.; Elders, Y.C.; Vemulpad, S.R.; Jamie, J.F. Bioactivity and chemical characterisation of Lophostemon suaveolens—An endemic Australian Aboriginal traditional medicinal plant. Nat. Prod. Res. 2016, 30, 693–696. [Google Scholar] [CrossRef]
- Oliveira-Costa, J.F.; Meira, C.S.; Neves, M.; Dos Reis, B.; Soares, M.B.P. Anti-Inflammatory Activities of Betulinic Acid: A Review. Front. Pharmacol. 2022, 13, 883857. [Google Scholar] [CrossRef]
- Guo, Y.; Sakulnarmrat, K.; Konczak, I. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicol. Rep. 2014, 1, 385–390. [Google Scholar] [CrossRef]
- Aykul, S.; Martinez-Hackert, E. Determination of half-maximal inhibitory concentration using biosensor-based protein interaction analysis. Anal. Biochem. 2016, 508, 97–103. [Google Scholar] [CrossRef]
- Graziani, V.; Scognamiglio, M.; Belli, V.; Esposito, A.; D’Abrosca, B.; Chambery, A.; Russo, R.; Panella, M.; Russo, A.; Ciardiello, F. Metabolomic approach for a rapid identification of natural products with cytotoxic activity against human colorectal cancer cells. Sci. Rep. 2018, 8, 5309. [Google Scholar] [CrossRef] [PubMed]
- Atagozli, T.; Elliott, D.E.; Ince, M.N. Helminth lessons in inflammatory bowel diseases (IBD). Biomedicines 2023, 11, 1200. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Setiawan, T.; Metwali, A.; Blum, A.; Urban, J.F., Jr.; Weinstock, J.V. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004, 34, 2690–2698. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.-L.; Bannick, N.; Henry, M.; Holm, A.N.; Metwali, A.; Urban, J.F.; Rothman, P.B.; Weiner, G.J.; Blazar, B.R. Intestinal helminths regulate lethal acute graft-versus-host disease and preserve the graft-versus-tumor effect in mice. J. Immunol. 2015, 194, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Li, J.; Blum, A.; Metwali, A.; Qadir, K.; Urban, J.F., Jr.; Weinstock, J.V. Exposure to Schistosome eggs protects mice from TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver. Physiol. 2003, 284, G385–G391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Guan, X.; Truscott, J.; Creemers, J.W.; Chen, H.-L.; Pesu, M.; El Abiad, R.G.; Karacay, B.; Urban, J.F. STAT6 and Furin Are Successive Triggers for the Production of TGF-β by T Cells. J. Immunol. 2018, 201, 2612–2623. [Google Scholar] [CrossRef] [PubMed]
- Metwali, A.; Winckler, S.; Urban, J.F., Jr.; Kaplan, M.H.; Ince, M.N.; Elliott, D.E. Helminth-induced regulation of T-cell transfer colitis requires intact and regulated T cell Stat6 signaling in mice. Eur. J. Immunol. 2021, 51, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhu, F.; Zheng, W.; Jacques, M.L.; Huang, J.; Guan, F.; Lei, J. Protective effect of Schistosoma japonicum eggs on TNBS-induced colitis is associated with regulating Treg/Th17 balance and reprogramming glycolipid metabolism in mice. Front. Cell. Infect. Microbiol. 2022, 12, 1028899. [Google Scholar] [CrossRef]
- Kitagaki, K.; Businga, T.R.; Racila, D.; Elliott, D.E.; Weinstock, J.V.; Kline, J.N. Intestinal helminths protect in a murine model of asthma. J. Immunol. 2006, 177, 1628–1635. [Google Scholar] [CrossRef]
- Metenou, S.; Dembele, B.; Konate, S.; Dolo, H.; Coulibaly, S.Y.; Coulibaly, Y.I.; Diallo, A.A.; Soumaoro, L.; Coulibaly, M.E.; Sanogo, D. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 2010, 184, 5375–5382. [Google Scholar] [CrossRef]
- Smith, K.A.; Löser, S.; Varyani, F.; Harcus, Y.; McSorley, H.J.; McKenzie, A.N.; Maizels, R.M. Concerted IL-25R and IL-4Rα signaling drive innate type 2 effector immunity for optimal helminth expulsion. eLife 2018, 7, e38269. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Bohnacker, S.; Esser-von Bieren, J. Macrophage regulation & function in helminth infection. Semin. Immunol. 2021, 53, 101526. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Setiawan, T.; Blum, A.M.; Urban, J.; Stoyanoff, K.; Arihiro, S.; Reinecker, H.-C.; Weinstock, J.V. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. J. Immunol. 2010, 185, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.M.; Hang, L.; Setiawan, T.; Urban, J.P.; Stoyanoff, K.M.; Leung, J.; Weinstock, J.V. Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J. Immunol. 2012, 189, 2512–2520. [Google Scholar] [CrossRef]
- Khan, W.; Blennerhasset, P.; Varghese, A.; Chowdhury, S.; Omsted, P.; Deng, Y.; Collins, S. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun. 2002, 70, 5931–5937. [Google Scholar] [CrossRef] [PubMed]
- Melon, A.; Wang, A.; Phan, V.; McKay, D.M. Infection with Hymenolepis diminuta is more effective than daily corticosteroids in blocking chemically induced colitis in mice. J. Biomed. Biotechnol. 2009, 2010, 384523. [Google Scholar] [CrossRef]
- Summers, R.W.; Elliott, D.; Urban, J.; Thompson, R.; Weinstock, J. Trichuris suis therapy in Crohn’s disease. Gut 2005, 54, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xu, N.; Wang, X.; Vallée, I.; Liu, M.; Liu, X. Helminth therapy for immune-mediated inflammatory diseases: Current and future perspectives. J. Inflamm. Res. 2022, 15, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, A.J.; Levy, C.W.; Jowitt, T.A.; Hayes, K.S.; Thompson, S.; McKenzie, E.A.; Ball, M.D.; Dubaissi, E.; France, A.P.; Bellina, B.; et al. The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nat. Commun. 2019, 10, 2344. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Wangchuk, P.; Shepherd, C.; Constantinoiu, C.; Ryan, R.Y.M.; Kouremenos, K.A.; Becker, L.; Jones, L.; Buitrago, G.; Giacomin, P.; Wilson, D.; et al. Hookworm-derived metabolites suppress pathology in a mouse model of colitis and inhibit secretion of key inflammatory cytokines in primary human leukocytes. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Urban, J.F., Jr.; Argo, C.K.; Weinstock, J.V. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? FASEB J. 2000, 14, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Kouremenos, K.; Eichenberger, R.M.; Pearson, M.; Susianto, A.; Wishart, D.S.; McConville, M.J.; Loukas, A. Metabolomic profiling of the excretory–secretory products of hookworm and whipworm. Metabolomics 2019, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Constantinoiu, C.; Eichenberger, R.M.; Field, M.; Loukas, A. Characterization of tapeworm metabolites and their reported biological activities. Molecules 2019, 24, 1480. [Google Scholar] [CrossRef]
- Wangchuk, P.; Anderson, D.; Yeshi, K.; Loukas, A. Identification of small molecules of the infective stage of human hookworm using LCMS-based metabolomics and lipidomics protocols. ACS Infect. Dis. 2021, 7, 3264–3276. [Google Scholar] [CrossRef]
- Wu, T.; Wang, C.; Ding, L.; Shen, Y.; Cui, H.; Wang, M.; Wang, H. Arginine relieves the inflammatory response and enhances the casein Expression in bovine mammary epithelial cells induced by lipopolysaccharide. Mediat. Inflamm. 2016, 2016, 9618795. [Google Scholar] [CrossRef]
- Unnikrishnan, M.; Rao, M. Antiinflammatory activity of methionine, methionine sulfoxide and methionine sulfone. Agents Actions 1990, 31, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, O.; Kosugi, T.; Roncal, C.; Mu, W.; Heinig, M.; Cirillo, P.; Sanchez-Lozada, L.G.; Johnson, R.J.; Nakagawa, T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J. Am. Soc. Nephrol. 2008, 19, 1712. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The cardioprotective effects of citric acid and L-malic acid on myocardial ischemia/reperfusion injury. Evid. Based Complement. Altern. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef]
- Ahmad, M.; Baba, W.N.; Gani, A.; Wani, T.A.; Gani, A.; Masoodi, F. Effect of extraction time on antioxidants and bioactive volatile components of green tea (Camellia sinensis), using GC/MS. Cogent Food Agric. 2015, 1, 1106387. [Google Scholar] [CrossRef]
- Hearps, A.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.; Aldunate, M.; Cone, R.; Gugasyan, R.; Anderson, D.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- Shepherd, C.; Giacomin, P.; Navarro, S.; Miller, C.; Loukas, A.; Wangchuk, P. A medicinal plant compound, capnoidine, prevents the onset of inflammation in a mouse model of colitis. J. Ethnopharmacol. 2018, 211, 17–28. [Google Scholar] [CrossRef]
- Ferreira, I.; Smyth, D.; Gaze, S.; Aziz, A.; Giacomin, P.; Ruyssers, N.; Artis, D.; Laha, T.; Navarro, S.; Loukas, A.; et al. Hookworm Excretory/Secretory Products Induce Interleukin-4 (IL-4)+ IL-10+ CD4+ T Cell Responses and Suppress Pathology in a Mouse Model of Colitis. Infect. Immun. 2013, 81, 2104–2111. [Google Scholar] [CrossRef]
- Cançado, G.G.L.; Fiuza, J.A.; de Paiva, N.C.N.; de Carvalho Dhom Lemos, L.; Ricci, N.D.; Gazzinelli-Guimarães, P.H.; Martins, V.G.; Bartholomeu, D.C.; Negrão-Corrêa, D.A.; Carneiro, C.M.; et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. J. Leukoc. Biol. 2011, 17, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Tang, H.; Ma, Z.; Xu, J.; Gao, W.; Chen, J.; Gan, W.; Zhang, Z.; Yu, X.; Zhou, X. The protective effect of the recombinant 53-kDa protein of Trichinella spiralis on experimental colitis in mice. Dig. Dis. Sci. 2011, 56, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Liu, X.; Zhang, Y.; Shi, H.; Jia, W.; Zhu, H.; Jia, H.; Liu, M.; Bai, X. Extracellular vesicles derived from Trichinella spiralis muscle larvae ameliorate TNBS-induced colitis in mice. Front. pharmacol. 2020, 11, 1174. [Google Scholar] [CrossRef]
- Eichenberger, R.M.; Ryan, S.; Jones, L.; Buitrago, G.; Polster, R.; Montes de Oca, M.; Zuvelek, J.; Giacomin, P.R.; Dent, L.A.; Engwerda, C.R. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front. pharmacol. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Ito, D.; Taniguchi, R.; Tademoto, S.; Horie, T.; Otsuki, H. Extracellular vesicles derived from Spirometra erinaceieuropaei plerocercoids inhibit activation of murine macrophage RAW264.7 cells. Parasitol. Int. 2023, 95, 102742. [Google Scholar] [CrossRef]
- Hasby, E.A.; Hasby Saad, M.A.; Shohieb, Z.; El Noby, K. FoxP3+ T regulatory cells and immunomodulation after Schistosoma mansoni egg antigen immunization in experimental model of inflammatory bowel disease. Cell. Immunol. 2015, 295, 67–76. [Google Scholar] [CrossRef]
- Xu, J.; Yu, P.; Wu, L.; Liu, M.; Lu, Y. Effect of Trichinella spiralis intervention on TNBS-induced experimental colitis in mice. Immunobiol. 2019, 224, 147–153. [Google Scholar] [CrossRef]
- Setiawan, T.; Metwali, A.; Blum, A.M.; Ince, M.N.; Urban, J.F., Jr.; Elliott, D.E.; Weinstock, J.V. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 2007, 75, 4655–4663. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.E.; Metwali, A.; Leung, J.; Setiawan, T.; Blum, A.M.; Ince, M.N.; Bazzone, L.E.; Stadecker, M.J.; Urban, J.F.; Weinstock, J.V. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J. Immunol. 2008, 181, 2414–2419. [Google Scholar] [CrossRef]
- Sutton, T.L.; Zhao, A.; Madden, K.B.; Elfrey, J.E.; Tuft, B.A.; Sullivan, C.A.; Urban, J.F., Jr.; Shea-Donohue, T. Anti-Inflammatory mechanisms of enteric Heligmosomoides polygyrus infection against trinitrobenzene sulfonic acid-induced colitis in a murine model. Infect. Immun. 2008, 76, 4772–4782. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, E.K.; Else, K.J.; Rogan, M.T.; Warhurst, G. Increased susceptibility to Trichuris muris infection and exacerbation of colitis in Mdr1a-/- mice. World J. Gastroenterol. 2014, 20, 1797–1806. [Google Scholar] [CrossRef]
- Wilson, M.S.; Ramalingam, T.R.; Rivollier, A.; Shenderov, K.; Mentink–Kane, M.M.; Madala, S.K.; Cheever, A.W.; Artis, D.; Kelsall, B.L.; Wynn, T.A. Colitis and intestinal inflammation in IL10−/− mice results from IL-13Rα2–mediated attenuation of IL-13 activity. Gastroenterology 2011, 140, 254–264. [Google Scholar] [CrossRef]
- Buitrago, G.; Pickering, D.; Ruscher, R.; Cobos Caceres, C.; Jones, L.; Cooper, M.; Van Waardenberg, A.; Ryan, S.; Miles, K.; Field, M.; et al. A netrin domain-containing protein secreted by the human hookworm Necator americanus protects against CD4 T cell transfer colitis. Transl. Res. 2021, 232, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Eichenberger, R.M.; Ruscher, R.; Giacomin, P.R.; Loukas, A. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLoS Pathog. 2020, 16, e1008508. [Google Scholar] [CrossRef] [PubMed]
- Jamtsho, T.; Yeshi, K.; Perry, M.J.; Loukas, A.; Wangchuk, P. Approaches, Strategies and Procedures for Identifying Anti-Inflammatory Drug Lead Molecules from Natural Products. Pharmaceuticals 2024, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Ojeda-Montes, M.J.; Tomás-Hernández, S.; Cereto-Massagué, A.; Beltrán-Debón, R.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. The light and dark sides of virtual screening: What is there to know? Int. J. Mol. Sci. 2019, 20, 1375. [Google Scholar] [CrossRef]
- Lin, X.; Li, X.; Lin, X. A review on applications of computational methods in drug screening and design. Molecules 2020, 25, 1375. [Google Scholar] [CrossRef]
- Johnson, T.O.; Odoh, K.D.; Nwonuma, C.O.; Akinsanmi, A.O.; Adegboyega, A.E. Biochemical evaluation and molecular docking assessment of the anti-inflammatory potential of Phyllanthus nivosus leaf against ulcerative colitis. Heliyon 2020, 6, e03893. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnson, T.O.; Akinsanmi, A.O.; Ejembi, S.A.; Adeyemi, O.E.; Oche, J.-R.; Johnson, G.I.; Adegboyega, A.E. Modern drug discovery for inflammatory bowel disease: The role of computational methods. World J. Gastroenterol. 2023, 29, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Foster, P.A.; Zwirchmayr, J.; Tahir, A.; Rollinger, J.M.; Mikros, E. 1H NMR-MS-based heterocovariance as a drug discovery tool for fishing bioactive compounds out of a complex mixture of structural analogues. Sci. Rep. 2019, 9, 11113. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.; Monteiro, A.F.; Bertonha, A.F.; Bernardi, D.I.; Gubiani, J.R.; Slivinski, J.; Michaliski, L.F.; Tonon, L.A.; Venancio, V.A.; Freire, V.F. Approaches for the isolation and identification of hydrophilic, light-sensitive, volatile and minor natural products. Nat. Prod. Rep. 2019, 36, 981–1004. [Google Scholar] [CrossRef]
- Jones, C.G.; Martynowycz, M.W.; Hattne, J.; Fulton, T.J.; Stoltz, B.M.; Rodriguez, J.A.; Nelson, H.M.; Gonen, T. The CryoEM method MicroED as a powerful tool for small molecule structure determination. ACS Cent. Sci. 2018, 4, 1587–1592. [Google Scholar] [CrossRef]
- Tu, P.-C.; Chan, C.-J.; Liu, Y.-C.; Kuo, Y.-H.; Lin, M.-K.; Lee, M.-S. Bioactivity-guided fractionation and NMR-based identification of the immunomodulatory isoflavone from the roots of Uraria crinita (L.) Desv. ex DC. Foods 2019, 8, 543. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).