Advances in Ferroptosis Research: A Comprehensive Review of Mechanism Exploration, Drug Development, and Disease Treatment
Abstract
1. Introduction
2. Study on the Mechanism of Ferroptosis
2.1. Molecules and Pathways Related to Ferroptosis
2.2. Studies on Metabolic Pathways Related to Ferroptosis
2.3. Ferroptosis-Related Signaling Pathways
3. Ferroptosis Inducers: New Synthesis and Old Compound Activity
3.1. Design and Synthesis of Novel Ferroptosis Inducer
3.2. Ferroptosis-Induced Activity of Known Compounds
4. Research on Ferroptosis Inhibitors: Creation, Activity, and Modification
4.1. Design and Synthesis of Novel Ferroptosis Inhibitors
4.2. Ferroptosis Inhibitory Activity of Known Compounds
4.3. Optimization and Modification of Ferroptosis Inhibitors
5. Ferroptosis and Disease Treatment
5.1. Ferroptosis Application in Cancer Treatment
5.2. Application of Ferroptosis in the Treatment of Neurodegenerative Diseases
5.3. Application of Ferroptosis in the Treatment of Cardiovascular Diseases
5.4. Application of Ferroptosis in the Treatment of Other Diseases
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Du, Y.X.; Guo, Z. Recent progress in ferroptosis: Inducers and inhibitors. Cell Death Discov. 2022, 8, 501. [Google Scholar] [CrossRef]
- Xu, R.; Wu, X.; Zhao, Q.Q.; Yang, Q.L. Ferroptosis synergistically sensitizes wee1 inhibitors: A bibliometric study. Am. J. Transl. Res. 2022, 14, 8473. [Google Scholar] [PubMed]
- Kulkarni, N.; Gadde, R.; Betharia, S. Dithiolethiones D3T and ACDT Protect Against Iron Overload-Induced Cytotoxicity and Serve as Ferroptosis Inhibitors in U-87 MG Cells. Neurochem. Res. 2023, 48, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.K.; Mohandas, S.; Mohanram, R.K. Role of ferroptosis inhibitors in the management of diabetes. Biofactors 2023, 49, 270–296. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Fu, J.H.; Wang, S.M.; Wang, H.; Liu, X.Y. Emerging ferroptosis inhibitors as a novel therapeutic strategy for the treatment of neonatal hypoxic-ischemic encephalopathy. Eur. J. Med. Chem. 2024, 271, 116453. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.L.; Xiang, Y.; Bai, X.Y.; Qiang, R.R.; Zhang, X.; Yang, Y.L.; Liu, X.L. Ferroptosis inhibitors: Past, present and future. Front. Pharmacol. 2024, 15, 1407335. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zeng, M.; Lv, N.; Wang, L.M.; Yang, Q.Y.; Gan, J.L.; Li, H.H.; Yu, B.; Jiang, X.J.; Yang, L. Ferroptosis in neurodegenerative diseases: Inhibitors as promising candidate mitigators. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 46–65. [Google Scholar] [PubMed]
- Deng, J.J.; Zhou, M.; Liao, T.T.; Kuang, W.L.; Xia, H.; Yin, Z.R.; Tan, Q.; Li, Y.M.; Song, S.W.; Zhou, E.; et al. Targeting Cancer Cell Ferroptosis to Reverse Immune Checkpoint Inhibitor Therapy Resistance. Front. Cell Dev. Biol. 2022, 10, 818453. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.Y.; Wu, D.Y.; Lin, Y.T.; Ai, H.P.; Xu, J.; Zhou, H.H.; Gu, Q. Identifying eleven new ferroptosis inhibitors as neuroprotective agents from FDA-approved drugs. Bioorg. Chem. 2024, 146, 107261. [Google Scholar] [CrossRef] [PubMed]
- Yehia, A.; Abulseoud, O.A. Melatonin: A ferroptosis inhibitor with potential therapeutic efficacy for the post-COVID-19 trajectory of accelerated brain aging and neurodegeneration. Mol. Neurodegener. 2024, 19, 36. [Google Scholar] [CrossRef]
- Emmons, M.F.; Smalley, K.S.M. Ironing-Out the Details: New Strategies for Combining Ferroptosis Inhibitors with Immunotherapy in Melanoma. J. Investig. Dermatol. 2022, 142, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Leng, J.F.; Tan, J.; Zhao, Y.J.; Xie, S.S.; Zhao, S.F.; Yan, X.Y.; Zhu, L.Q.; Luo, J.; Kong, L.Y.; et al. Discovery of Novel Potent Covalent Glutathione Peroxidase 4 Inhibitors as Highly Selective Ferroptosis Inducers for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2023, 66, 10036–10059. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.M.; Wang, X.C.; Huang, S.Y.; Zhang, L.; Lan, B.; Li, X.Y.; Chen, H.; Liu, Z.F.; Su, Y.J.; Xi, L.S.; et al. A CRISPR-Cas9 library screening identifies CARM1 as a critical inhibitor of ferroptosis in hepatocellular carcinoma cells. Mol. Ther. Nucleic Acids 2023, 34, 102063. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.F.; Yuan, Z.H.; Wang, J. Autophagy inhibition and ferroptosis activation during atherosclerosis: Hypoxia-inducible factor 1α inhibitor PX-478 alleviates atherosclerosis by inducing autophagy and suppressing ferroptosis in macrophages. Biomed. Pharmacother. 2023, 161, 114333. [Google Scholar] [CrossRef]
- Huang, C.; Santofimia-Castaño, P.; Liu, X.; Xia, Y.; Peng, L.; Gotorbe, C.; Neira, J.L.; Tang, D.L.; Pouyssegur, J.; Iovanna, J. NUPR1 inhibitor ZZW-115 induces ferroptosis in a mitochondria-dependent manner. Cell Death Discov. 2021, 7, 269. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ru, Y.; Luo, Y.L.; Luo, X.Y.; Liu, D.D.; Ma, Y.C.; Zhou, X.R.; Linghu, M.; Xu, W.; Gao, F.; et al. Identification of a targeted ACSL4 inhibitor to treat ferroptosis-related diseases. Sci. Adv. 2024, 10, eadk1200. [Google Scholar] [CrossRef]
- Kato, I.; Kasukabe, T.; Kumakura, S. Menin-MLL inhibitors induce ferroptosis and enhance the anti-proliferative activity of auranofin in several types of cancer cells. Int. J. Oncol. 2020, 57, 1057–1071. [Google Scholar] [CrossRef] [PubMed]
- Kumada, H.; Itoh, M.; Tohda, S. Effect of Ferroptosis Inducers and Inhibitors on Cell Proliferation in Acute Leukemia. Anticancer Res. 2024, 44, 1003–1010. [Google Scholar] [CrossRef]
- Kumar, S.; Ziegler, Y.; Plotner, B.N.; Flatt, K.M.; Kim, S.H.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Resistance to FOXM1 inhibitors in breast cancer is accompanied by impeding ferroptosis and apoptotic cell death. Breast Cancer Res. Treat. 2024, 208, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Cao, Y.M.; Xiao, J.; Shang, J.W.; Tan, Q.; Ping, F.; Huang, W.F.; Wu, F.; Zhang, H.J.; Zhang, X.P. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020, 27, 2635–2650. [Google Scholar] [CrossRef]
- Shaghaghi, Z.; Motieian, S.; Alvandi, M.; Yazdi, A.; Asadzadeh, B.; Farzipour, S.; Abbasi, S. Ferroptosis Inhibitors as Potential New Therapeutic Targets for Cardiovascular Disease. Mini-Rev. Med. Chem. 2022, 22, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Su, X.D.; Xie, Y.; Zhang, J.W.; Li, M.X.; Zhang, Q.; Jin, G.S.; Liu, F.S. HIF-α activation by the prolyl hydroxylase inhibitor roxadustat suppresses chemoresistant glioblastoma growth by inducing ferroptosis. Cell Death Dis. 2022, 13, 861. [Google Scholar] [CrossRef]
- Sun, Y.R.; He, L.B.; Wang, W.; Xie, Z.S.; Zhang, X.W.; Wang, P.; Wang, L.; Yan, C.C.; Liu, Z.W.; Zhao, J.; et al. Activation of Atg7-dependent autophagy by a novel inhibitor of the Keap1-Nrf2 protein-protein interaction from Penthorum chinense Pursh. attenuates 6-hydroxydopamine-induced ferroptosis in zebrafish and dopaminergic neurons. Food Funct. 2022, 13, 7885–7900. [Google Scholar] [CrossRef] [PubMed]
- Taufani, I.P.; Situmorang, J.H.; Febriansah, R.; Tasminatun, S.; Sunarno, S.; Yang, L.Y.; Chiang, Y.T.; Huang, C.Y. Mitochondrial ROS induced by ML385, an Nrf2 inhibitor aggravates the ferroptosis induced by RSL3 in human lung epithelial BEAS-2B cells. Hum. Exp. Toxicol. 2023, 42, 09603271221149663. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.N.; Chen, Y.B.; Mi, Y.J.; Qiao, J.H.; Jin, H.; Li, J.T.; Lu, Z.D.; Wang, Q.M.; Zou, Z.Z. ATF2 inhibits ani-tumor effects of BET inhibitor in a negative feedback manner by attenuating ferroptosis. Biochem. Biophys. Res. Commun. 2021, 558, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.M.; Tian, L.L.; Xi, J.H.; Girimpuhwe, D.; Huang, C.F.; Ma, R.X.; Yao, X.J.; Shi, D.F.; Bai, Z.T.; Wu, Q.X.; et al. Alterperylenol as a Novel Thioredoxin Reductase Inhibitor Induces Liver Cancer Cell Apoptosis and Ferroptosis. J. Agric. Food Chem. 2022, 70, 15763–15775. [Google Scholar] [CrossRef]
- Xu, C.J.; Xiao, Z.H.; Wang, J.; Lai, H.L.; Zhang, T.; Guan, Z.L.; Xia, M.; Chen, M.X.; Ren, L.L.; He, Y.F.; et al. Discovery of a Potent Glutathione Peroxidase 4 Inhibitor as a Selective Ferroptosis Inducer. J. Med. Chem. 2021, 64, 13312–13326. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.; Wen, B.; Liu, Y.Z.; Zhang, W.; Hu, X.L.; Chen, L.; Hang, W.J.; Chen, J. GSK-J4, a Specific Histone Lysine Demethylase 6A Inhibitor, Ameliorates Lipotoxicity to Cardiomyocytes via Preserving H3K27 Methylation and Reducing Ferroptosis. Front. Cardiovasc. Med. 2022, 9, 907747. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, X.L.; Song, C.L.; Ji, S.; Yang, J.H.; Liu, Y.; You, J.; Zhang, J.; Huang, S.Z.; Cheng, W.; et al. Structure-activity relationship studies of phenothiazine derivatives as a new class of ferroptosis inhibitors together with the therapeutic effect in an ischemic stroke model. Eur. J. Med. Chem. 2021, 209, 112842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Hong, L.H.; Fan, S.Y.; Zhu, L.; Yu, Z.P.; Chen, C.; Kong, L.Y.; Luo, J.G. Discovery of piperine derivatives as inhibitors of human dihydroorotate dehydrogenase to induce ferroptosis in cancer cells. Bioorg. Chem. 2024, 150, 107594. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.J.; Li, T.; Wang, K.W.; Huang, X.B.; Shi, J.F.; Wang, Y. Mechanisms and inhibitors of ferroptosis in psoriasis. Front. Mol. Biosci. 2022, 9, 1019447. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.F.; Peng, F.; Li, J.F.; Cui, X.D.; Qiao, X.; Zhu, W.L. Ferroptosis-Specific Inhibitor Ferrostatin-1 Relieves H2O2-Induced Redox Imbalance in Primary Cardiomyocytes through the Nrf2/ARE Pathway. Dis. Markers 2022, 2022, 4539932. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Zhang, Z.Q.; Tang, H.; Chen, B.; Cai, Y.; Wei, Y.X.; Zhao, W.G.; Wu, Z.B.; Shang, H.B. Mitochondrial Inhibitor Rotenone Triggers and Enhances Neuronal Ferroptosis Following Intracerebral Hemorrhage. Acs Chem. Neurosci. 2023, 14, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.; Shimanovsky, N.; Fedotcheva, N. Specific Features of Mitochondrial Dysfunction under Conditions of Ferroptosis Induced by t-Butylhydroperoxide and Iron: Protective Role of the Inhibitors of Lipid Peroxidation and Mitochondrial Permeability Transition Pore Opening. Membranes 2023, 13, 372. [Google Scholar] [CrossRef]
- Ning, N.N.; Shang, Z.Q.; Liu, Z.P.; Xia, Z.Z.; Li, Y.; Ren, R.B.; Wang, H.M.; Zhang, Y. A novel microtubule inhibitor promotes tumor ferroptosis by attenuating SLC7A11/GPX4 signaling. Cell Death Discov. 2023, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Ohta, T.; Obata, M.; Takahashi, K.; Seino, M.; Nagase, S. xCT inhibitor sulfasalazine depletes paclitaxel-resistant tumor cells through ferroptosis in uterine serous carcinoma. Oncol. Lett. 2020, 20, 2689–2700. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, W.X.; Li, X.M.; Wei, W.Z. NF-κB Inhibitor Myrislignan Induces Ferroptosis of Glioblastoma Cells via Regulating Epithelial-Mesenchymal Transformation in a Slug-Dependent Manner. Oxid. Med. Cell. Longev. 2023, 2023, 7098313. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Castell, D.; Currais, A.; Maher, P. Defining a pharmacological inhibitor fingerprint for oxytosis/ferroptosis. Free Radic. Biol. Med. 2021, 171, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Okazaki, R.; Sato, M.; Oh-hashi, K.; Takemori, H.; Furuta, K. Effect of ferroptosis inhibitors oxindole-curcumin hybrid compound and N, N-dimethylaniline derivatives on rotenone-induced oxidative stress. Eur. J. Pharmacol. 2022, 928, 175119. [Google Scholar] [CrossRef] [PubMed]
- Delehouzé, C.; Comte, A.; Leon-Icaza, S.A.; Cougoule, C.; Hauteville, M.; Goekjian, P.; Bulinski, J.C.; Dimanche-Boitrel, M.T.; Meunier, E.; Rousselot, M.; et al. Nigratine as dual inhibitor of necroptosis and ferroptosis regulated cell death. Sci. Rep. 2022, 12, 5118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Wang, Q.S.; Zhu, J.; Cai, J.; Feng, X.N.; Song, Q.Q.; Jiang, H.; Ren, W.Q.; He, Y.; Wang, P.; et al. Identification of KW-2449 as a dual inhibitor of ferroptosis and necroptosis reveals that autophagy is a targetable pathway for necroptosis inhibitors to prevent ferroptosis. Cell Death Dis. 2024, 15, 764. [Google Scholar] [CrossRef]
- Liang, Z.B.; Candib, A.; Soriano-Castell, D.; Fischer, W.; Finley, K.; Maher, P. Fragment-based drug discovery and biological evaluation of novel cannabinol-based inhibitors of oxytosis/ferroptosis for neurological disorders. Redox Biol. 2024, 72, 103138. [Google Scholar] [CrossRef]
- Fan, C.Y.; Guo, X.H.; Zhang, J.; Zheng, W.; Shi, C.L.; Qin, Y.W.; Shen, H.L.; Lu, Y.; Fan, Y.H.; Li, Y.L.; et al. BRD4 inhibitors broadly promote erastin-induced ferroptosis in different cell lines by targeting ROS and FSP1. Discov. Oncol. 2024, 15, 98. [Google Scholar] [CrossRef]
- Linkermann, A. Telomerase inhibitor imetelstat kills AML cells via lipid ROS and ferroptosis. Nat. Cancer 2024, 5, 12–13. [Google Scholar] [CrossRef]
- Xi, J.M.; Zhang, Z.J.; Wang, Z.; Wu, Q.F.; He, Y.; Xu, Y.Y.; Ding, Z.J.; Zhao, H.H.; Da, H.H.; Zhang, F.; et al. Hinokitiol functions as a ferroptosis inhibitor to confer neuroprotection. Free Radic. Biol. Med. 2022, 190, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Kumfu, S.; Sripetchwandee, J.; Thonusin, C.; Sumneang, N.; Maneechote, C.; Arunsak, B.; Chunchai, T.; Oo, T.T.; Kongkaew, A.; Chattipakorn, S.C.; et al. Ferroptosis inhibitor improves cardiac function more effectively than inhibitors of apoptosis and necroptosis through cardiac mitochondrial protection in rats with iron-overloaded cardiomyopathy. Toxicol. Appl. Pharmacol. 2023, 479, 116727. [Google Scholar] [CrossRef]
- Cheng, Y.; Qu, W.H.; Li, J.; Jia, B.W.; Song, Y.T.; Wang, L.Y.; Rui, T.Y.; Li, Q.Q.; Luo, C.L. Ferristatin II, an Iron Uptake Inhibitor, Exerts Neuroprotection against Traumatic Brain Injury via Suppressing Ferroptosis. Acs Chem. Neurosci. 2022, 13, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Fayzullina, D.; Yakushov, S.; Kantserova, K.; Belyaeva, E.; Aniskin, D.; Tsibulnikov, S.; Fayzullina, N.; Kalinin, S.; Romantsova, O.; Timashev, P.S.; et al. Carbonic Anhydrase Inhibitors Induce Ferroptosis through Inhibition of AKT/FTH1 Signaling in Ewing Sarcoma Tumor Cells. Cancers 2023, 15, 5225. [Google Scholar] [CrossRef]
- Liu, D.; Cao, J.X.; Ding, X.; Xu, W.; Yao, X.J.; Dai, M.Y.; Tai, Q.D.; Shi, M.X.; Fei, K.; Xu, Y.P.; et al. Disulfiram/copper complex improves the effectiveness of the WEE1 inhibitor Adavosertib in p53 deficient non-small cell lung cancer via ferroptosis. Biochim. Biophys. Acta-Mol. Basis Dis. 2024, 1870, 167455. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Liu, R.; Chen, L. Ferroptosis inhibitor ferrostatin-1 alleviates homocysteine-induced ovarian granulosa cell injury by regulating TET activity and DNA methylation. Mol. Med. Rep. 2022, 25, 130. [Google Scholar] [CrossRef]
- Yin, J.X.; Chen, J.F.; Hong, J.H.; Huang, Y.L.; Xiao, R.; Liu, S.N.; Deng, P.; Sun, Y.C.; Chai, K.X.Y.; Zeng, X.; et al. 4EBP1-mediated SLC7A11 protein synthesis restrains ferroptosis triggered by MEK inhibitors in advanced ovarian cancer. JCI Insight 2024, 9, 177857. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, Z.G.; Shen, H.; Xiong, Y.; Shah, Y.M.; Liu, Y.; Fan, X.G.; Rui, L.Y. Hepatic NF-κB-Inducing Kinase and Inhibitor of NF-κB Kinase Subunit α Promote Liver Oxidative Stress, Ferroptosis, and Liver Injury. Hepatol. Commun. 2021, 5, 1704–1720. [Google Scholar] [CrossRef]
- Feng, L.M.; Wang, C.; Zhang, C.; Zhang, W.L.; Zhu, W.M.; He, Y.; Xia, Z.H.; Song, W.T. p38 MAPK inhibitor SB202190 suppresses ferroptosis in the glutamate-induced retinal excitotoxicity glaucoma model. Neural Regen. Res. 2024, 19, 2299–2309. [Google Scholar] [CrossRef]
- Shu, J.K.; Du, K.Y.; Peng, Y.; Liu, L.; Fang, J. HIF-PHD Inhibitor Roxadustat Promotes Erastin-Induced Ferroptosis in Lung Cancer Cells by Stabilizing HIF-2α and Targeting Hepcidin/FPN1 Axis. J. Biol. Regul. Homeost. Agents 2024, 38, 161–172. [Google Scholar] [CrossRef]
- Cheff, D.M.; Huang, C.Y.; Scholzen, K.C.; Gencheva, R.; Ronzetti, M.H.; Cheng, Q.; Hall, M.D.; Arnér, E.S.J. The ferroptosis inducing compounds RSL3 and ML162 are not direct inhibitors of GPX4 but of TXNRD1. Redox Biol. 2023, 62, 102703. [Google Scholar] [CrossRef] [PubMed]
- Chhillar, B.; Kadian, R.; Kumar, M.; Yadav, M.; Sodhi, N.; da Silva, T.N.X.; Angeli, J.P.F.; Singh, V.P. Aminic Organoselenium Compounds as Glutathione Peroxidase Mimics and Inhibitors of Ferroptosis. ChemBioChem 2024, 25, e202400074. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.R.; Fan, R.M.; Zhao, T.; Lin, R.Z.; Zhuang, J.Y.; Deng, A.H.; Meng, S.S.; Hou, Z.; Wei, G.F. A novel mitochondria-targeting DHODH inhibitor induces robust ferroptosis and alleviates immune suppression. Pharmacol. Res. 2024, 202, 107115. [Google Scholar] [CrossRef]
- Hendricks, J.M.; Doubravsky, C.E.; Wehri, E.; Li, Z.P.; Roberts, M.A.; Deol, K.K.; Lange, M.; Lasheras-Otero, I.; Momper, J.D.; Dixon, S.J.; et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem. Biol. 2023, 30, 1090. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, J.; Wang, J.; Fan, X.J.; Xu, X.M.; Wang, H.; Xiong, Y.; Li, X.Y.; Zhang, X.M.; Zhang, Q.E.; et al. How does ferrocene correlate with ferroptosis? Multiple approaches to explore ferrocene-appended GPX4 inhibitors as anticancer agents. Chem. Sci. 2024, 15, 10477–10490. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.L.; Zhang, S.; Liu, J.J.; Zhou, X.Q.; Ariffin, N.S.; Wei, J.H.; Shi, C.Y.; Ma, X.L.; Zhang, Y.; Huang, R.Z. Discovery of novel 1,8-naphthalimide piperazinamide based benzenesulfonamides derivatives as potent carbonic anhydrase IX inhibitors and ferroptosis inducers for the treatment of triple-negative breast cancer. Bioorg. Chem. 2024, 150, 107596. [Google Scholar] [CrossRef]
- Morcos, C.A.; Khattab, S.N.; Haiba, N.S.; Bassily, R.W.; Abu-Serie, M.M.; Teleb, M. Battling colorectal cancer via s-triazine-based MMP-10/13 inhibitors armed with electrophilic warheads for concomitant ferroptosis induction; the first-in-class dual-acting agents. Bioorg. Chem. 2023, 141, 106839. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Fan, Y.F.; Zhou, L.; Duan, Y.J.; Li, S.; Sun, Z.K.; Zhang, C.Q.; Yang, H.Y.; Yuan, W.X.; et al. Reactivation of MAPK-SOX2 pathway confers ferroptosis sensitivity in KRASG12C inhibitor resistant tumors. Redox Biol. 2024, 78, 103419. [Google Scholar] [CrossRef]
- Ying, D.Z.; Shen, X.; Wang, S.Q.; Chen, J.Y.; Wu, Z.Y.; Chen, W.T.; Wang, F.D.; Min, J.X.; Yu, Y.P. Discovery of 4-hydroxyl pyrazole derivatives as potent ferroptosis inhibitors. Eur. J. Med. Chem. 2024, 263, 115913. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Yang, W.; Ma, R.G.; Xia, A.J.; Zhang, G.; Fang, Z.; Guo, N.H.; Yang, S.Y.; Li, L.L. Discovery of 2-vinyl-10H-phenothiazine derivatives as a class of ferroptosis inhibitors with minimal human Ether-a-go-go related gene (hERG) activity for the treatment of DOX-induced cardiomyopathy. Bioorg. Med. Chem. Lett. 2022, 74, 128911. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Tian, C.Y.; Wang, Q.; Qiu, X.Y.; Wang, Y.F.; Jiang, H.L.; Hao, J.F.; He, Y.J. Synergistic Amplification of Ferroptosis with Liposomal Oxidation Catalyst and Gpx4 Inhibitor for Enhanced Cancer Therapy. Adv. Healthc. Mater. 2023, 12, 2301292. [Google Scholar] [CrossRef]
- Cheng, J.M.; Yu, Q.L.; Li, J.X.; Xu, Z.Y.; Li, J.H.; Guan, L.Y.; Xiao, J.S.; Duan, X.P. Intrinsic tumor-targeted murine Ferritin nanocage co-delivers GPX4 and FSP1 inhibitors for synergistic ferroptosis-immunotherapy. Nano Today 2024, 58, 102411. [Google Scholar] [CrossRef]
- Hao, J.F.; Chen, Q.G.; Feng, Y.M.; Jiang, Q.Y.; Sun, H.W.; Deng, B.T.; Huang, X.; Guan, J.B.; Chen, Q.P.; Liu, X.C.; et al. Combination treatment with FAAH inhibitors/URB597 and ferroptosis inducers significantly decreases the growth and metastasis of renal cell carcinoma cells via the PI3K-AKT signaling pathway. Cell Death Dis. 2023, 14, 247. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeong, K.J.; Poire, A.; Zhang, D.; Tsang, Y.H.; Blucher, A.S.; Mills, G.B. Irreversible HER2 inhibitors overcome resistance to the RSL3 ferroptosis inducer in non-HER2 amplified luminal breast cancer. Cell Death Dis. 2023, 14, 532. [Google Scholar] [CrossRef]
- Reed, A.; Ichu, T.A.; Milosevich, N.; Melillo, B.; Schafroth, M.A.; Otsuka, Y.; Scampavia, L.; Spicer, T.P.; Cravatt, B.F. LPCAT3 Inhibitors Remodel the Polyunsaturated Phospholipid Content of Human Cells and Protect from Ferroptosis. Acs Chem. Biol. 2022, 17, 1607–1618. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.S.; Li, X.Q.; Xiong, X.F.; Xue, R.Y.; Zang, L.L.; Wang, Z.Q.; Wang, L.J. Novel methyltransferase G9a inhibitor induces ferroptosis in multiple myeloma through Nrf2/HO-1 pathway. Ann. Hematol. 2024, 103, 2405–2417. [Google Scholar] [CrossRef]
- Han, J.X.; Luo, L.L.; Wang, Y.C.; Miyagishi, M.; Kasim, V.; Wu, S.R. SGLT2 inhibitor empagliflozin promotes revascularization in diabetic mouse hindlimb ischemia by inhibiting ferroptosis. Acta Pharmacol. Sin. 2023, 44, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Yan, J.R.; Sun, H.; Liang, Y.T.; Zhao, Q.Q.; Yu, S.; Zhang, Y. Ferroptosis inhibitor alleviates sorafenib-induced cardiotoxicity by attenuating KLF11-mediated FSP1-dependent ferroptosis. Int. J. Biol. Sci. 2024, 20, 2622–2639. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Kang, R.X.; Yang, N.; Pan, X.H.; Yang, J.; Yu, H.J.; Deng, W.L.; Jia, Z.G.; Zhang, J.; Shen, Q. Tetrahydrobiopterin inhibitor-based antioxidant metabolic strategy for enhanced cancer ferroptosis-immunotherapy. J. Colloid Interface Sci. 2024, 658, 100–113. [Google Scholar] [CrossRef]
- Bai, X.Y.; Yang, Y.L.; Luo, Y.L.; Zhang, D.; Zhai, T.Y.; Hu, Q.Q.; Zhang, N.; Dai, Q.F.; Liang, J.X.; Bian, H.Y.; et al. Design and synthesis of sulfonamide phenothiazine derivatives as novel ferroptosis inhibitors and their therapeutic effects in spinal cord injury. Bioorg. Chem. 2024, 148, 107458. [Google Scholar] [CrossRef] [PubMed]
- Balla, A.; Tran, B.; Valtari, A.; Steven, P.; Scarpellini, C.; Augustyns, K.; Urtti, A.; Vellonen, K.S.; Ruponen, M. A Novel Ferroptosis Inhibitor UAMC-3203, a Potential Treatment for Corneal Epithelial Wound. Pharmaceutics 2023, 15, 118. [Google Scholar] [CrossRef]
- Fan, Y.P.; Zhang, Y.H.; Shi, K.Y.; Cheng, S.; Pei, D.Q.; Shu, X.D. Identification of a group of bisbenzylisoquinoline (BBIQ) compounds as ferroptosis inhibitors. Cell Death Dis. 2022, 13, 1000. [Google Scholar] [CrossRef]
- Fang, Y.Y.; Tan, Q.Y.; Zhou, H.H.; Gu, Q.; Xu, J. Discovery of novel diphenylbutene derivative ferroptosis inhibitors as neuroprotective agents. Eur. J. Med. Chem. 2022, 231, 114151. [Google Scholar] [CrossRef] [PubMed]
- Günther, M.; Dabare, S.; Fuchs, J.; Gunesch, S.; Hofmann, J.; Decker, M.; Culmsee, C. Flavonoid-Phenolic Acid Hybrids Are Potent Inhibitors of Ferroptosis via Attenuation of Mitochondrial Impairment. Antioxidants 2024, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Hinder, L.; Pfaff, A.L.; Emmerich, R.E.; Michels, S.; Schlitzer, M.; Culmsee, C. Characterization of Novel Diphenylamine Compounds as Ferroptosis Inhibitors. J. Pharmacol. Exp. Ther. 2021, 378, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Hashimoto, T.; Ando, K.; Kamatari, Y.O.; Takemori, H.; Furuta, K. Structural features localizing the ferroptosis inhibitor GIF-2197-r to lysosomes. RSC Adv. 2023, 13, 32276–32281. [Google Scholar] [CrossRef]
- Ji, H.L.; Zhang, Y.F.; Zhang, N.Y.; Wang, K.M.; Meng, N.; Zhang, J.; Jiang, C.S. Design, synthesis, and evaluation of formylpiperazine analogs of Ferrostatin-1 as novel improved ferroptosis inhibitors. Bioorg. Med. Chem. 2024, 105, 117716. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.F.; Teng, X.F.; Shi, H.W.; Cao, L.D.; He, L.; Gu, Q. Discovery and optimization of olanzapine derivatives as new ferroptosis inhibitors. Bioorg. Chem. 2023, 133, 106393. [Google Scholar] [CrossRef] [PubMed]
- Kuganesan, N.; Dlamini, S.; McDaniel, J.; Tillekeratne, V.L.M.; Taylor, W.R. Identification and initial characterization of a potent inhibitor of ferroptosis. J. Cell. Biochem. 2021, 122, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Li, X.C.; Hua, Y.J.; Zhang, W.H.; Zhou, X.X.; He, J.F.; Chen, D.F. Tannic Acid as a Natural Ferroptosis Inhibitor: Mechanisms and Beneficial Role of 3′-O-Galloylation. ChemistrySelect 2021, 6, 1562–1569. [Google Scholar] [CrossRef]
- Lu, Y.; Shen, Z.X.; Xu, Y.P.; Lin, H.R.; Shen, L.T.; Jin, Y.Z.; Guo, Y.; Lu, J.L.; Li, L.J.; Zhuang, Y.X.; et al. Discovery of New Phenyltetrazolium Derivatives as Ferroptosis Inhibitors for Treating Ischemic Stroke: An Example Development from Free Radical Scavengers. J. Med. Chem. 2024, 67, 11712–11731. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.X.; Liu, K.D.; Deng, L.Y.; Wang, W.J.; Lai, T.L.; Li, X.L. Chicoric acid acts as an ALOX15 inhibitor to prevent ferroptosis in asthma. Int. Immunopharmacol. 2024, 142, 113187. [Google Scholar] [CrossRef]
- Peng, X.; Tan, Q.Y.; Zhou, H.H.; Xu, J.; Gu, Q. Discovery of phloroglucinols from Hypericum japonicum as ferroptosis inhibitors. Fitoterapia 2021, 153, 104984. [Google Scholar] [CrossRef] [PubMed]
- Poon, J.F.; Zilka, O.; Pratt, D.A. Potent Ferroptosis Inhibitors Can Catalyze the Cross-Dismutation of Phospholipid-Derived Peroxyl Radicals and Hydroperoxyl Radicals. J. Am. Chem. Soc. 2020, 142, 14331–14342. [Google Scholar] [CrossRef] [PubMed]
- Su, S.K.; Wu, X.Z.; Zhu, Y.X.; Yang, S.; Lu, K.Y.; Zhang, X.L.; Zhang, D.; Wang, X.Y. Screening of orthopedic medicines identifies raloxifene hydrochloride as a novel ferroptosis inhibitor for spinal cord injury therapy. Int. Immunopharmacol. 2024, 143, 113542. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.Y.; Fang, Y.Y.; Peng, X.; Zhou, H.H.; Xu, J.; Gu, Q. A new ferroptosis inhibitor, isolated from Ajuga nipponensis, protects neuronal cells via activating NRF2-antioxidant response elements (AREs) pathway. Bioorg. Chem. 2021, 115, 105177. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, Y.X.; Shi, Y.F.; Su, S.K.; Liang, H.B.; Li, S.L.; Wu, Z.W.; Miao, J.S.; Chen, Y.L.; Zhang, X.L.; et al. Screening of NSAIDs library identifies Tinoridine as a novel ferroptosis inhibitor for potential intervertebral disc degeneration therapy. Free Radic. Biol. Med. 2024, 221, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Lu, Y.; Zhang, J.Q.; Zang, A.J.; Ren, J.H.; Zheng, Z.Y.; Fan, M.L.; Xie, Y.Y. Novel 3-hydroxypyridin-4(1H)-One derivatives as ferroptosis inhibitors with iron-chelating and reactive oxygen species scavenging activities and therapeutic effect in cisplatin-induced cytotoxicity. Eur. J. Med. Chem. 2024, 263, 115945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Y.; Liu, J.Y.; Zheng, H.; Wang, K.M.; Zhang, J.; Meng, N.; Jiang, C.S. Design, Synthesis, and Biological Evaluation of New Improved Ferrostatin-1 Derived Ferroptosis Inhibitors. Chem. Biodivers. 2024, e202402141. [Google Scholar] [CrossRef]
- Geng, Y.Q.; Qiu, L.N.; Cheng, Y.Q.; Li, J.J.; Ma, Y.L.; Zhao, C.C.; Cai, Y.; Zhang, X.B.; Chen, J.L.; Pan, Y.C.; et al. Alleviating Recombinant Tissue Plasminogen Activator-induced Hemorrhagic Transformation in Ischemic Stroke via Targeted Delivery of a Ferroptosis Inhibitor. Adv. Sci. 2024, 11, e2309517. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.S.; Zhao, C.X.; Xu, Z.Y.; Liu, D.R.; Shen, W.Y.; Yuan, W.L.; Li, Y.; Ma, J.; Wang, Z.S.; Feng, S.Q. ROS-responsive nanoparticle delivery of ferroptosis inhibitor prodrug to facilitate mesenchymal stem cell-mediated spinal cord injury repair. Bioact. Mater. 2024, 38, 438–454. [Google Scholar] [CrossRef]
- Huang, K.K.; Deng, H.Y.; Wang, S.; Zhang, F.X.; Huang, G.; Wang, L.; Liu, J.Y.; Zhao, X.L.; Ren, H.; Yang, G.; et al. Melanin-Like Nanomedicine Functions as a Novel RPE Ferroptosis Inhibitor to Ameliorate Retinal Degeneration and Visual Impairment in Dry Age-Related Macular Degeneration. Adv. Healthc. Mater. 2024, 13, e2401613. [Google Scholar] [CrossRef]
- Ryan, F.; Blex, C.; Ngo, T.D.; Kopp, M.A.; Michalke, B.; Venkataramani, V.; Curran, L.; Schwab, J.M.; Ruprecht, K.; Otto, C.; et al. Ferroptosis inhibitor improves outcome after early and delayed treatment in mild spinal cord injury. Acta Neuropathol. 2024, 147, 106. [Google Scholar] [CrossRef]
- Xin, W.; Gong, S.Q.; Chen, Y.; Yao, M.Y.; Qin, S.Z.; Chen, J.; Zhang, A.H.; Yu, W.R.; Zhou, S.Y.; Zhang, B.; et al. Self-Assembling P38 Peptide Inhibitor Nanoparticles Ameliorate the Transition from Acute to Chronic Kidney Disease by Suppressing Ferroptosis. Adv. Healthc. Mater. 2024, 13, e2400441. [Google Scholar] [CrossRef]
- Adelusi, O.B.; Etemadi, Y.; Akakpo, J.Y.; Ramachandran, A.; Jaeschke, H. Effect of ferroptosis inhibitors in a murine model of acetaminophen-induced liver injury. J. Biochem. Mol. Toxicol. 2024, 38, e23791. [Google Scholar] [CrossRef]
- Aleem, A.M.; Kang, W.X.; Lin, S.Y.; Milad, M.; Kingsley, P.J.; Crews, B.C.; Uddin, M.J.; Rouzer, C.A.; Marnett, L.J. Ferroptosis Inhibitors Suppress Prostaglandin Synthesis in Lipopolysaccharide-Stimulated Macrophages. ACS Chem. Biol. 2023, 18, 404–418. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; He, C.; Yan, F.; Li, J.R.; Xu, H.Z.; Zhuang, J.F.; Zhou, H.; Peng, Y.C.; Fu, X.J.; et al. Selective Ferroptosis Inhibitor Liproxstatin-1 Attenuates Neurological Deficits and Neuroinflammation After Subarachnoid Hemorrhage. Neurosci. Bull. 2021, 37, 535–549. [Google Scholar] [CrossRef]
- Chung, C.H.; Lin, C.Y.; Chen, C.Y.; Hsueh, C.W.; Chang, Y.W.; Wang, C.C.; Chu, P.Y.; Tai, S.K.; Yang, M.H. Ferroptosis Signature Shapes the Immune Profiles to Enhance the Response to Immune Checkpoint Inhibitors in Head and Neck Cancer. Adv. Sci. 2023, 10, 2204514. [Google Scholar] [CrossRef] [PubMed]
- Dirik, H.; Taskiran, A.S.; Joha, Z. Ferroptosis inhibitor ferrostatin-1 attenuates morphine tolerance development in male rats by inhibiting dorsal root ganglion neuronal ferroptosis. Korean J. Pain 2024, 37, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.Y.; Pang, Y.L.; Li, W.X.; Zhao, C.X.; Zhang, Y.; Wang, X.; Ning, G.Z.; Kong, X.H.; Liu, C.; Yao, X.; et al. Liproxstatin-1 is an effective inhibitor of oligodendrocyte ferroptosis induced by inhibition of glutathione peroxidase 4. Neural Regen. Res. 2021, 16, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Q.; Peng, T.T.; Wu, C.B.; Pan, X.; Huang, Z.W. Engineering Ferroptosis Inhibitors as Inhalable Nanomedicines for the Highly Efficient Treatment of Idiopathic Pulmonary Fibrosis. Bioengineering 2023, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Uchiyama, A.; Kosaka, K.; Nishio, M.; Ogino, S.; Yokoyama, Y.; Torii, R.; Akai, R.; Iwawaki, T.; Torii, S.; et al. Exposure to volatile ferroptosis inhibitor, TEMPO, reduced cutaneous ischemia-reperfusion injury progression to pressure ulcer formation in a mouse model. J. Dermatol. Sci. 2024, 115, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Alluri, R.K.; Wu, K.L.; Kalayjian, R.C.; Bush, W.S.; Palella, F.J.; Koletar, S.L.; Hileman, C.O.; Erlandson, K.M.; Ellis, R.J.; et al. Sex-Biased Associations of Circulating Ferroptosis Inhibitors with Reduced Lipid Peroxidation and Better Neurocognitive Performance in People with HIV. Antioxidants 2024, 13, 1042. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Chen, X.; Xu, F.D.; Tao, F.F.; Liu, L.Y.; Cheng, R.; Li, N.; Pan, Y. Self-assembled miR-134-5p inhibitor nanoparticles ameliorate experimental bronchopulmonary dysplasia (BPD) via suppressing ferroptosis. Microchim. Acta 2023, 190, 491. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Cao, F.Y.; Chen, Y.; Zhang, L.L.; Li, H.; Cao, J.B.; Song, J.; Ma, Y.L.; Mi, W.D.; et al. The Ferroptosis Inhibitor Liproxstatin-1 Ameliorates LPS-Induced Cognitive Impairment in Mice. Nutrients 2022, 14, 4599. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Zhang, X.; Cai, B.L.; Qi, M.M.; Chi, Y.B.; Peng, B.; Zhang, D.H. Ferroptosis inhibitors reduce celastrol toxicity and preserve its insulin sensitizing effects in insulin resistant HepG2 cells. J. Integr. Med. 2024, 22, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.W.; Chen, L.; Sun, H.; Zhang, S.Q.; Dong, X.W.; Pan, J.J.; Xiao, W.M.; Lu, G.T.; Wang, Y.D.; Xu, H.W. Soat2 inhibitor avasimibe alleviates acute pancreatitis by suppressing acinar cell ferroptosis. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 397, 5989–5999. [Google Scholar] [CrossRef]
- Ma, P.W.; Wang, W.L.; Chen, J.W.; Yuan, H.; Lu, P.H.; Gao, W.; Ding, X.R.; Lun, Y.Q.; Liang, R.; He, Z.H.; et al. Treatment with the Ferroptosis Inhibitor Ferrostatin-1 Attenuates Noise-Induced Hearing Loss by Suppressing Ferroptosis and Apoptosis. Oxid. Med. Cell. Longev. 2022, 2022, 3373828. [Google Scholar] [CrossRef] [PubMed]
- Minnella, A.; McCusker, K.P.; Amagata, A.; Trias, B.; Weetall, M.; Latham, J.C.; O’Neill, S.; Wyse, R.K.; Klein, M.B.; Trimmer, J.K. Targeting ferroptosis with the lipoxygenase inhibitor PTC-041 as a therapeutic strategy for the treatment of Parkinson’s disease. PLoS ONE 2024, 19, e0309893. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, A.; Watanabe, C.; Yamada, N.; Jimbo, E.F.; Kobayashi, M.; Ohishi, N.; Nagayoshi, A.; Aoki, S.; Kishita, Y.; Ohtake, A.; et al. Apomorphine is a potent inhibitor of ferroptosis independent of dopaminergic receptors. Sci. Rep. 2024, 14, 4820. [Google Scholar] [CrossRef] [PubMed]
- Rashidipour, N.; Karami-Mohajeri, S.; Mandegary, A.; Mohammadinejad, R.; Wong, A.; Mohit, M.; Salehi, J.; Ashrafizadeh, M.; Najafi, A.; Abiri, A. Where ferroptosis inhibitors and paraquat detoxification mechanisms intersect, exploring possible treatment strategies. Toxicology 2020, 433, 152407. [Google Scholar] [CrossRef]
- Scarpellini, C.; Klejborowska, G.; Lanthier, C.; Hassannia, B.; Vanden Berghe, T.; Augustyns, K. Beyond ferrostatin-1: A comprehensive review of ferroptosis inhibitors. Trends Pharmacol. Sci. 2023, 44, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Lan, X.T.; Zhang, Z.; Liu, Y.; Sun, D.Y.; Wang, X.J.; Ou-Yang, S.X.; Zhuang, C.L.; Shen, F.M.; Wang, P.; et al. Ferroptosis inhibitor liproxstatin-1 alleviates metabolic dysfunction-associated fatty liver disease in mice: Potential involvement of PANoptosis. Acta Pharmacol. Sin. 2023, 44, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, B.; Zhou, Y.F.; Yang, Z.B.; Jiang, L.C.; Kou, Z.Y.; Li, J.X.; Ma, X.D.; Song, J.N. NLRP3 inflammasome inhibitor MCC950 reduces cerebral ischemia/reperfusion induced neuronal ferroptosis. Neurosci. Lett. 2023, 795, 137032. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Hou, S.H.; Huang, S.Q.; Li, H.W.; Li, Y.P. Ferroptosis Inhibitor Regulates the Disease Progression of Systematic Lupus Erythematosus Mice Model Through Th1/Th2 Ratio. Curr. Mol. Med. 2023, 23, 799–807. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhang, N.Y.; Zheng, H.; Wang, K.M.; Zhang, J.; Meng, N.; Jiang, C.S. Synthesis and biological evaluation of ferrostatin-based diamide derivatives as new ferroptosis inhibitors. Bioorg. Med. Chem. Lett. 2024, 113, 129974. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yi, X.; Huo, B.; He, Y.; Guo, X.; Zhang, Z.H.; Zhong, X.X.; Feng, X.; Fang, Z.M.; Zhu, X.H.; et al. BRD4770 functions as a novel ferroptosis inhibitor to protect against aortic dissection. Pharmacol. Res. 2022, 177, 106122. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, X.B.; Chen, S.Q.; Luo, H.S.; Huo, B.; Guo, X.; Li, R.; Chen, Y.; Yi, X.; Wei, X.; et al. SP2509 functions as a novel ferroptosis inhibitor by reducing intracellular iron level in vascular smooth muscle cells. Free Radic. Biol. Med. 2024, 219, 49–63. [Google Scholar] [CrossRef]
- Wu, J.J.; Liu, C.; Wang, T.; Liu, H.; Wei, B. Deubiquitinase inhibitor PR-619 potentiates colon cancer immunotherapy by inducing ferroptosis. Immunology 2023, 170, 439–451. [Google Scholar] [CrossRef]
- Zakaria, S.; Ibrahim, N.; Abdo, W.; El-Sisi, A.E. JNK inhibitor and ferroptosis modulator as possible therapeutic modalities in Alzheimer disease (AD). Sci. Rep. 2024, 14, 23293. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.Z.; Wan, T.H.; Sun, G.X.; Chen, X.W.; Zhang, S.Y. A ROS-responsive microsphere capsule encapsulated with NADPH oxidase 4 inhibitor ameliorates macrophage inflammation and ferroptosis. Heliyon 2024, 10, e23589. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, N.; Wang, Y.; Song, G.N.; Li, P.; Fang, Y.K.; Xu, L.; Wang, W.; Xie, M.J. Soluble epoxide hydrolase inhibitor (TPPU) alleviates ferroptosis by regulating CCL5 after intracerebral hemorrhage in mice. Biomed. Pharmacother. 2024, 172, 116301. [Google Scholar] [CrossRef]
- Rai, A.; Patwardhan, R.S.; Jayakumar, S.; Pachpatil, P.; Das, D.; Panigrahi, G.C.; Gota, V.; Patwardhan, S.; Sandur, S.K. Clobetasol propionate, a Nrf-2 inhibitor, sensitizes human lung cancer cells to radiation-induced killing via mitochondrial ROS-dependent ferroptosis. Acta Pharmacol. Sin. 2024, 45, 1506–1519. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, B.; Liang, S.S.; Zheng, L.N.; Fang, H.; Xu, S.; Zhang, T.F.; Wang, M.; He, X.; Feng, W.Y. Fullerenols as efficient ferroptosis inhibitor by targeting lipid peroxidation for preventing drug-induced acute kidney injury. J. Colloid Interface Sci. 2025, 680, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.Q.; Liu, F.; Zhang, J.G.; Fu, S.M.; Zhan, H.; Qian, K.J. miR-3587 Inhibitor Attenuates Ferroptosis Following Renal Ischemia-Reperfusion Through HO-1. Front. Mol. Biosci. 2022, 8, 789927. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Zhong, L.S.; Huang, C.; Guo, Y.Y.; Jin, F.J.; Hu, Y.Z.; Zhao, Z.B.; Ren, Z.; Wang, Y.F. β-Caryophyllene Acts as a Ferroptosis Inhibitor to Ameliorate Experimental Colitis. Int. J. Mol. Sci. 2022, 23, 16055. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.H.; Chen, Y.B.; Mi, Y.J.; Jin, H.; Huang, T.; Liu, L.F.; Gong, L.L.; Wang, L.N.; Wang, Q.M.; Zou, Z.Z. NR5A2 synergizes with NCOA3 to induce breast cancer resistance to BET inhibitor by upregulating NRF2 to attenuate ferroptosis. Biochem. Biophys. Res. Commun. 2020, 530, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.S.; Liu, P.; Bao, R.D.; Chen, J.; Zhou, M.H.; Mo, Z.X.; Ma, Y.R.; Liu, H.Q.; Zhou, Y.P.; Cai, X.; et al. A Dual PI3K/HDAC Inhibitor Induces Immunogenic Ferroptosis to Potentiate Cancer Immune Checkpoint Therapy. Cancer Res. 2021, 81, 6233–6245. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, K.; Gong, H.M.; Li, D.Z.; Chu, W.F.; Zhao, D.; Wang, X.F.; Xu, D.Y. Death by histone deacetylase inhibitor quisinostat in tongue squamous cell carcinoma via apoptosis, pyroptosis, and ferroptosis. Toxicol. Appl. Pharmacol. 2021, 410, 115363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.W.; Zhao, Y.M.; Chen, S.; Cui, G.H.; Fu, W.K.; Li, S.Y.; Lin, X.R.; Hu, H. Cisplatin Promotes the Efficacy of Immune Checkpoint Inhibitor Therapy by Inducing Ferroptosis and Activating Neutrophils. Front. Pharmacol. 2022, 13, 870178. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, L.; Li, T.E.; Lin, X.X.; Zheng, Y.; Xu, D.; Guo, Y.; Zhang, Z.; Fu, Y.; Wang, H.; et al. Disruption of MerTK increases the efficacy of checkpoint inhibitor by enhancing ferroptosis and immune response in hepatocellular carcinoma. Cell Rep. Med. 2024, 5, 101415. [Google Scholar] [CrossRef]
- Cui, Y.S.; Li, Y.; Xu, Y.Y.; Liu, X.X.; Kang, X.F.; Zhu, J.W.; Long, S.; Han, Y.C.; Xue, C.Y.; Sun, Z.J.; et al. SLC7A11 protects luminal A breast cancer cells against ferroptosis induced by CDK4/6 inhibitors. Redox Biol. 2024, 76, 103304. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.W.; Pan, Y.F.; Shang, T.Y.; Jiang, T.Y.; Lin, Y.K.; Yang, C.; Pang, S.J.; Cui, X.W.; Wang, Y.X.; Feng, X.F.; et al. URI alleviates tyrosine kinase inhibitors-induced ferroptosis by reprogramming lipid metabolism in p53 wild-type liver cancers. Nat. Commun. 2023, 14, 6269. [Google Scholar] [CrossRef]
- Xie, X.X.; Chen, C.C.; Wang, C.; Guo, Y.J.; Sun, B.H.; Tian, J.X.; Yan, J.; Li, D.K.; Chen, G. Targeting GPX4-mediated ferroptosis protection sensitizes BRCA1-deficient cancer cells to PARP inhibitors. Redox Biol. 2024, 76, 103350. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.G.; Lin, W.H.; Zhang, Q.; Ma, Y.R.; Wang, X.; Guo, A.; Zhu, G.L.; Zhou, Z.D.; Song, W.W.; Zhao, Z.Y.; et al. MGST1 facilitates novel KRASG12D inhibitor resistance in KRASG12D-mutated pancreatic ductal adenocarcinoma by inhibiting ferroptosis. Mol. Med. 2024, 30, 199. [Google Scholar] [CrossRef]
- Zheng, S.T.; Hu, H.L.; Hou, M.R.; Zhu, K.; Wu, Z.D.; Qi, L.; Xia, H.; Liu, G.Q.; Ren, Y.Y.; Xu, Y.K.; et al. Proton pump inhibitor-enhanced nanocatalytic ferroptosis induction for stimuli-responsive dual-modal molecular imaging guided cancer radiosensitization. Acta Biomater. 2023, 162, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.N.; Lou, S.Y.; Lu, J.; Zheng, F.L.; Tang, Y.M.; Zhang, E.J.; Cui, S.L.; Zhao, H.J. Selective PI3Kδ inhibitor TYM-3-98 suppresses AKT/mTOR/SREBP1-mediated lipogenesis and promotes ferroptosis in KRAS-mutant colorectal cancer. Cell Death Dis. 2024, 15, 474. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Liu, X.; Du, M.W.; Xie, S.S.; Dou, L.; Mi, X.; Zhou, D.H.; Su, Y.; Shen, T.Y.; Zhang, Y.Y.; et al. LATS1 inhibitor and zinc supplement synergistically ameliorates contrast-induced acute kidney injury: Induction of Metallothionein-1 and suppression of tubular ferroptosis. Free Radic. Biol. Med. 2024, 223, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.B.; Cui, J.; Chen, Y. Polydopamine Nanoparticles-Encapsulated Ferroptosis Inhibitor Ferstatin-1 Promotes GPX4 Expression by Down-Regulating NOX4 to Alleviate Myocardial Ischemia-Reperfusion Injury. Ann. Clin. Lab. Sci. 2024, 54, 190–200. [Google Scholar] [PubMed]
- Dai, Y.Q.; Li, Y.; Xu, J.J.; Zhang, J. A highly selective inhibitor of discoidin domain receptor-1 (DDR1-IN-1) protects corneal epithelial cells from YAP/ACSL4-mediated ferroptosis in dry eye. Br. J. Pharmacol. 2024, 181, 4245–4261. [Google Scholar] [CrossRef]
- Schneider, C.; Hilbert, J.; Genevaux, F.; Höfer, S.; Krauss, L.; Schicktanz, F.; Contreras, C.T.; Jansari, S.; Papargyriou, A.; Richter, T.; et al. A Novel AMPK Inhibitor Sensitizes Pancreatic Cancer Cells to Ferroptosis Induction. Adv. Sci. 2024, 11, e2307695. [Google Scholar] [CrossRef]
- Bao, R.; Wang, Q.S.; Yu, M.X.; Zeng, Y.L.; Wen, S.P.; Liu, T.Y.; Wang, M.; Li, Y.Y.; Chang, S.R.; Chi, H.Y.; et al. AAV9-HGF cooperating with TGF-beta/Smad inhibitor attenuates silicosis fibrosis via inhibiting ferroptosis. Biomed. Pharmacother. 2023, 161, 114537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Shi, J.Y.; Liu, R.Z.; Liu, Y.; Liu, R.F.; Wu, Z.M.; Xu, W.; Ma, H.B.; Luo, H.B.; Cheng, Z.B. Structure Revisions of Phenolic Bisabolane Sesquiterpenes and a Ferroptosis Inhibitor from the Marine-Derived Fungus Aspergillus versicolor YPH93. J. Nat. Prod. 2023, 86, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.M.; Wei, Y.D.; Hou, X.Z.; Guo, Q.; Liang, H.; Zeng, K.W.; Tu, P.F.; Zhang, Q.Y. Triterpenoids from Uncaria macrophylla as ferroptosis inhibitors. Phytochemistry 2023, 206, 113530. [Google Scholar] [CrossRef]
- Hong, Y.; Feng, J.; Dou, Z.J.; Sun, X.X.; Hu, Y.Y.; Chen, Z.X.; Liu, L.; Xu, H.H.; Du, M.H.; Tang, P.P.; et al. Berberine as a novel ACSL4 inhibitor to suppress endothelial ferroptosis and atherosclerosis. Biomed. Pharmacother. 2024, 177, 117081. [Google Scholar] [CrossRef] [PubMed]
- Jenke, R.; Oliinyk, D.; Zenz, T.; Korfer, J.; Schäker-Hübner, L.; Hansen, F.K.; Lordick, F.; Meier-Rosar, F.; Aigner, A.; Büch, T. HDAC inhibitors activate lipid peroxidation and ferroptosis in gastric cancer. Biochem. Pharmacol. 2024, 225, 116257. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.X.; Jiang, N.; Zhang, H.; Wang, S.; Pi, Q.; Chen, H.; He, X.; Luo, W.; Lu, Y.; Deng, Y.; et al. Inhibitors of APE1 redox and ATM synergistically sensitize osteosarcoma cells to ionizing radiation by inducing ferroptosis. Int. Immunopharmacol. 2024, 139, 112672. [Google Scholar] [CrossRef]
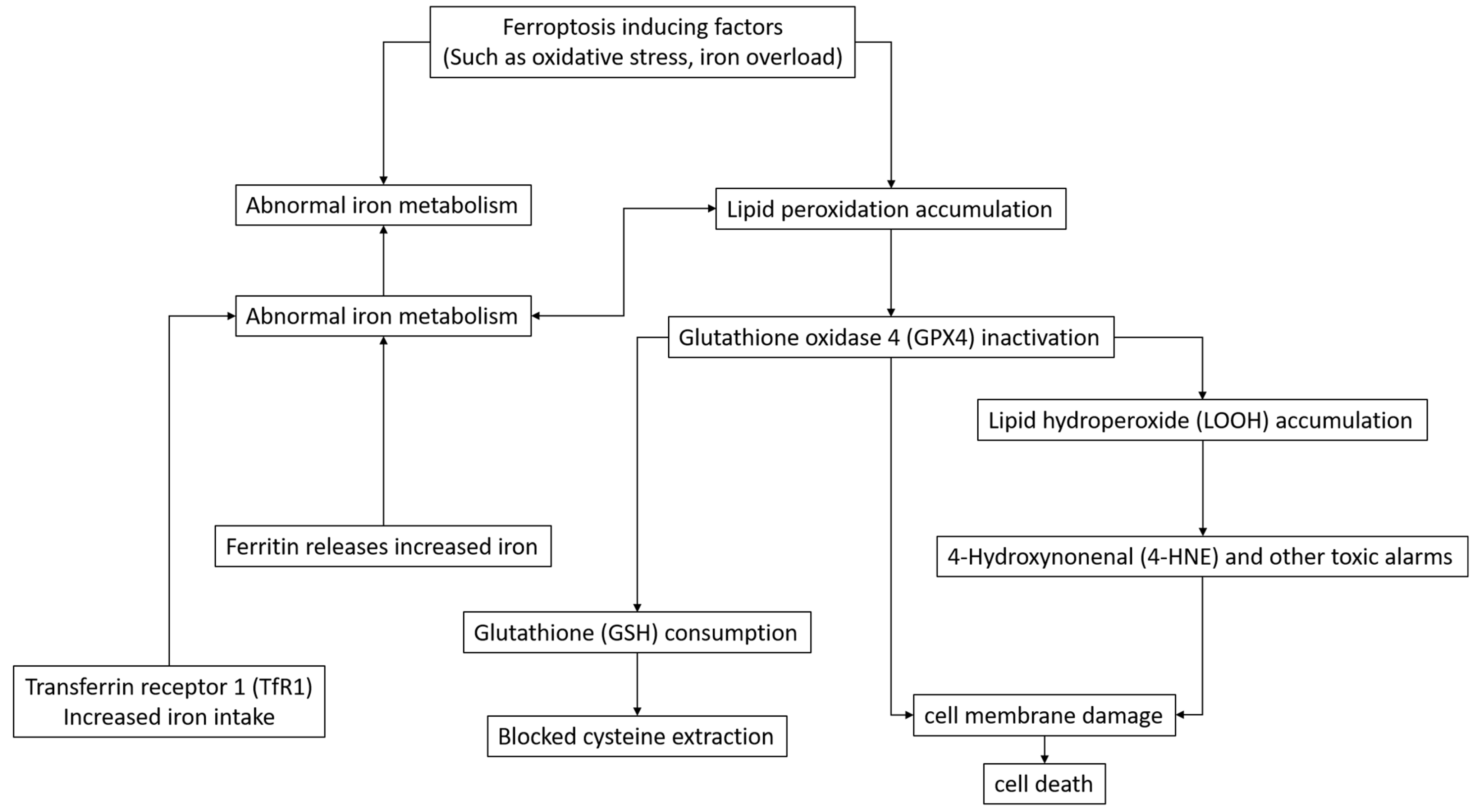
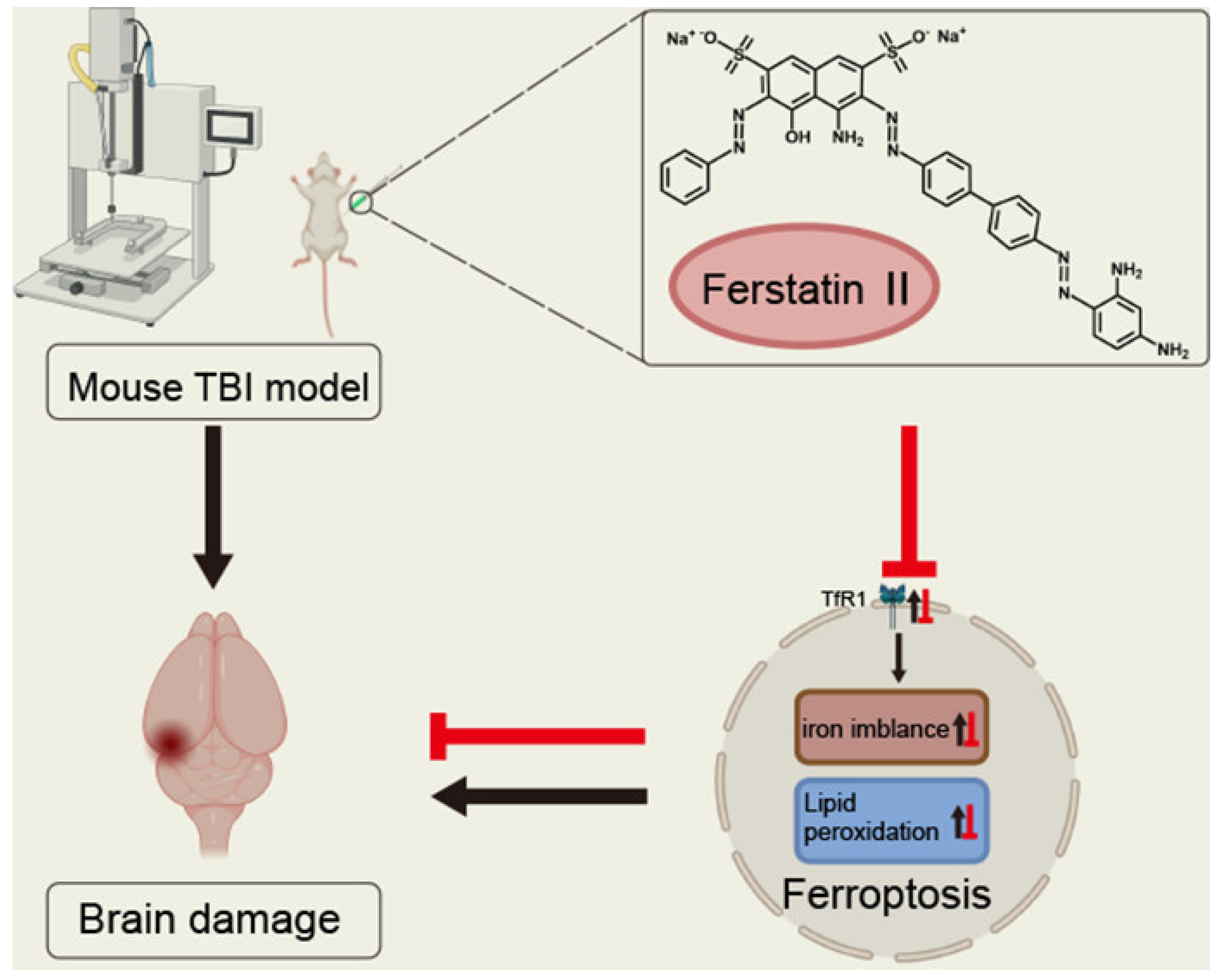

| Inducer Compound Name/Type | Mechanism of Action | Related Disease Research | Refs. |
|---|---|---|---|
| RSL3 and ML162 | Affect redox balance and promote lipid peroxidation and ferroptosis | Proliferation of leukemia cells | [18,55] |
| Compound 26a and other targeted GPX4 inhibitors | Targeted inhibition of GPX4 | Breast cancer research | [27] |
| Erastin | Interrupts cystine uptake and depletes intracellular glutathione | Study of leukemia cells | [18] |
| Synergistic inducers such as dihydroartemisinin and JKE1674 | Synergistic effect with ferroptosis inducer to trigger ferroptosis | Breast cancer research | [19] |
| Sorafenib and other signaling pathway-related inducers | The expression of related transcription factors changes, promoting ferroptosis | Hepatocellular carcinoma and cardiotoxicity studies | [72] |
| Drug-resistant tumor inducers such as lapatinib | Induced ferroptosis | KRASG12C mutant cancer cells | [62] |
| MTX-LDH@MnO and other nano platform inducers | Disrupt the metabolic activity of antioxidants and promote ferroptosis | Cancer immunotherapy | [73] |
| DOX and other chemotherapy drug inducers | Induced by affecting oxidative stress-related pathways | Cardiomyopathy research | [64] |
| 1-(4-(4-Methylpiperazin-1-yl) phenyl) ethyl-10H-thiazide (51) and other new inducers | The mechanism of action is not mentioned in detail | Ischemic stroke model studies | [29] |
| 9D and other novel hybrid triazine inducers | Induce lipid peroxidation and initiate ferroptosis | Colorectal cancer research | [61] |
| Type of Inhibitor | Representative Compounds/Inhibitors | Overview of Mechanism of Action | Refs. |
|---|---|---|---|
| Free radical capture antioxidants | Ferrostatin-1 and its analogs | Capture free radicals | [31,81,93,104,112] |
| Mitochondrial protectant | Liproxstatin-1 | Protects mitochondrial function; | [101,104,117] |
| Iron-chelating agent | Deferriamine | chelates iron ions | [46,105] |
| Active oxygen scavenger | N-Acetylcysteine | Removes reactive oxygen species | [110,115] |
| Enzyme inhibitor | PD146176 | Inhibits specific enzyme activity | [86] |
| Analogs/metabolites/combination effects/synergistic enhancement/synergistic weakening inhibitors | Idebenone, oleic acid, kinofen, SCD1 inhibitor, zinc protoporphyrin-9 | Regulate metabolic influence pathway | [17] |
| Antioxidant capacity inhibitors | Olanzapine and its derivatives | Antioxidant capacity | [82] |
| Multipurpose inhibitor | UAMC-3203 | Effects on corneal injury, liver injury, etc. | [75,99] |
| Multifunctional inhibitor | Necrostatin-1 | Inhibits a variety of cell death-related proteins | [100] |
| Histone methyltransferase inhibitors | BRD4770 | Regulates histone methylation | [121] |
| Lysine-specific demethylase 1 inhibitors | SP2509 | Reduces intracellular iron levels | [122] |
| Deubiquitin enzyme inhibitors | PR-619 | Degrades related proteins | [123] |
| Inflammatory body inhibitors | MCC950 | Inhibits the inflammatory body | [118] |
| Nrf2-related inhibitors | Tinoridine | Binds to Nrf2 and promotes its expression and activity | [91] |
| Multifunctional regulator | Melatonin | Anti-inflammatory, iron chelator, and antioxidant | [10] |
| Iron-chelating neuroprotectant | CPX-O | Iron chelation and regulation of related pathways | [124] |
| Autophagy pathway-targeting inhibitors | KW-2449 | Targets the autophagy pathway | [41] |
| NOX4 inhibitors | GLX351322 | Inhibits NOX4 activity and inhibits iron sagging | [125] |
| Multifunctional inhibitor | Formylpiperazine-derived compounds | Multifaceted regulation of cell state | [81] |
| Antioxidant activator | Dithiothiones | Activate transcription factors; upregulates GSH levels | [3] |
| Enzyme inhibitor | TPPU | Inhibits specific enzymes | [126] |
| Multimechanism inhibitor | Hinokitiol | Chelates iron and activates transcription factors; upregulates antioxidant genes | [45] |
| Covalent-binding inhibitor | GPX4 inhibitors | Covalently bind to GPX4-specific locations | [12] |
| Structure optimization inhibitors | NecroX-7 and eriodictyol-7-O-glucoside | Modify structure-related parameters | [85] |
| Lipoxygenase inhibitors | PTC-041 | Inhibits specific lipoxygenase | [113] |
| Dopamine-receptor-independent inhibitors | Apomorphine | Inhibits lipid peroxidation | [114] |
| Inhibitors of signaling pathway regulation | Valproic acid | Inhibits specific signaling pathways and induces ferroptosis | [127] |
| Free radical-scavenging inhibitors | Fullerenol nanoparticles | Use free radical scavenging | [128] |
| Gene regulatory inhibitors | miR-3587 inhibitors | Regulate gene expression and inhibit ferroptosis | [129] |
| 4-Hydroxypyrazole derivatives (e.g., HW-3, Compound 25) | Antioxidant through free radical capture; inhibits ferroptosis | Ferroptosis inhibition and related diseases | [63] |
| Disease-related inhibitors | Soat2 inhibitors | Inhibit ferroptosis and improve disease Symptoms | [111] |
| Receptor activation inhibitor | Beta-Caryophyllene | Activates receptors | [130] |
| Disease Type | The Role of Ferroptosis in Disease | Specific Diseases | Refs. |
|---|---|---|---|
| Cancer | Inhibits tumor growth and has great potential for combined application with chemotherapy, radiotherapy, and immunotherapy | Breast cancer, lung cancer, liver cancer, and other cancers | [19,22,30,43,48,51,60,62,66,70,72,113,123,131,132,133,135,136,137,138,139,141,145] |
| Neurodegenerative diseases | Associated with nerve cell death and neurological impairment; inhibition of ferroptosis provides neuroprotection | Parkinson’s disease, glaucoma, spinal cord injury, and neonatal hypoxic-ischemic encephalopathy | [5,7,9,23,42,45,53,90,95,97,101,104,109,112,113,114] |
| Cardiovascular disease | Inhibiting ferroptosis is of great significance for the treatment of cardiovascular diseases | Myocardial infarction, heart failure, aortic dissection, and contrast-induced acute kidney injury | [28,46,71,120,121,122,142,143] |
| Other diseases | Intervention against ferroptosis brings new targets and ideas for the treatment of these diseases | Diabetes, pulmonary fibrosis, ulcerative colitis, acute pancreatitis, asthma, dry eye, low back pain, acne, acute kidney injury, and contrast-induced acute kidney injury | [4,10,11,50,52,85,91,92,105,110,111,115,117,124,125,126,127,128,130,144,146,147,148,149,150,151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xie, Y. Advances in Ferroptosis Research: A Comprehensive Review of Mechanism Exploration, Drug Development, and Disease Treatment. Pharmaceuticals 2025, 18, 334. https://doi.org/10.3390/ph18030334
Wang H, Xie Y. Advances in Ferroptosis Research: A Comprehensive Review of Mechanism Exploration, Drug Development, and Disease Treatment. Pharmaceuticals. 2025; 18(3):334. https://doi.org/10.3390/ph18030334
Chicago/Turabian StyleWang, Haojie, and Yuanyuan Xie. 2025. "Advances in Ferroptosis Research: A Comprehensive Review of Mechanism Exploration, Drug Development, and Disease Treatment" Pharmaceuticals 18, no. 3: 334. https://doi.org/10.3390/ph18030334
APA StyleWang, H., & Xie, Y. (2025). Advances in Ferroptosis Research: A Comprehensive Review of Mechanism Exploration, Drug Development, and Disease Treatment. Pharmaceuticals, 18(3), 334. https://doi.org/10.3390/ph18030334





