Extraction Yields of Psilocybin and Psilocin: A Short Review of Current Methods and Their Implications
Abstract
:1. Introduction
2. Methodology
2.1. Elegibility Criteria
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection
2.4. Quality Analysis in Individual Studies
3. Results and Discussion
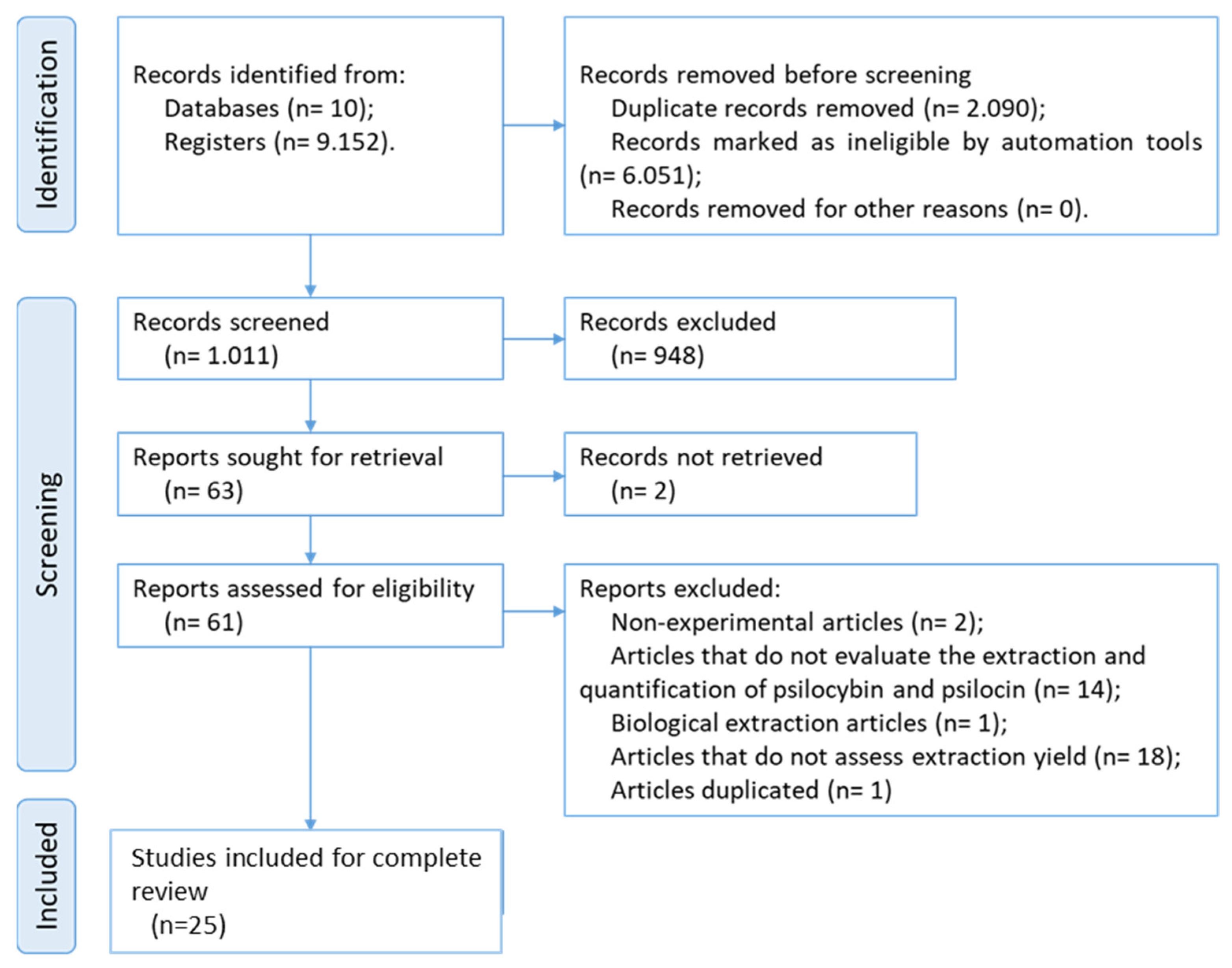
3.1. Study Selection
3.2. Characteristics of the Included Studies
3.3. Quality of Individual Studies
3.4. Extraction Techniques of Psilocybin and Psilocin in Hallucinogenic Mushrooms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Construction of the Systematic Review
| Database | String | Results | Search Date |
|---|---|---|---|
| Scopus | (ALL ((psilocybin OR psilocibina OR psilocin OR psilocina)) AND ALL ((extraction OR extração OR extracción OR identification OR identificação OR identificación OR characterization OR caracterização OR descripción OR quantification OR quantificação OR cuantificación))) | 3882 | 11 September 2024 |
| Science Direct | (Psilocybin OR Psilocin) AND (Extraction OR Identification OR Identificação OR Characterization OR Quantification) | 3065 | 11 September 2024 |
| Sage Journal | (Psilocybin OR Psilocibina OR Psilocin OR Psilocina) AND (Extraction OR Extração OR Extracción OR Identification OR Identificação OR Identificación OR Characterization OR Caracterização OR Descripción OR Quantification OR Quantificação OR Cuantificación) | 818 | 11 September 2024 |
| Web of Science | ALL = ((Psilocybin OR Psilocibina OR Psilocin OR Psilocina) AND (Extraction OR Extração OR Extracción OR Identification OR Identificação OR Identificación OR Characterization OR Caracterização OR Descripción OR Quantification OR Quantificação OR Cuantificación)) | 195 | 11 September 2024 |
| BVS | ((Psilocybin OR Psilocibina OR Psilocin OR Psilocina)) AND ((Extraction OR Extração OR Extracción OR Identification OR Identificação OR Identificación OR Characterization OR Caracterização OR Descripción OR Quantification OR Quantificação OR Cuantificación)) | 253 | 11 September 2024 |
| LILACS | ((Psilocybin OR Psilocibina OR Psilocin OR Psilocina)) AND ((Extraction OR Extração OR Extracción OR Identification OR Identificação OR Identificación OR Characterization OR Caracterização OR Descripción OR Quantification OR Quantificação OR Cuantificación)) | 253 | 11 September 2024 |
| PubMed | (Psilocybin OR Psilocibina OR Psilocin OR Psilocina) AND (Extraction OR Extração OR Extracción OR Identification OR Identificação OR Identificación OR Characterization OR Caracterização OR Descripción OR Quantification OR Quantificação OR Cuantificación) | 332 | 11 September 2024 |
| Embase | (‘psilocybin’/exp OR psilocybin OR psilocibina OR ‘psilocin’/exp OR psilocin OR psilocina) AND (‘extraction’/exp OR extraction OR extração OR extracción OR ‘identification’/exp OR identification OR identificação OR identificación OR ‘characterization’/exp OR characterization OR caracterização OR descripción OR ‘quantification’/exp OR quantification OR quantificação OR cuantificación) | 293 | 11 September 2024 |
| Cochrane | (Psilocybin OR Psilocibina OR Psilocin OR Psilocina) AND (Extraction OR Extração OR Extracción OR Identification OR Identificação OR Identificación OR Characterization OR Caracterização OR Descripción OR Quantification OR Quantificação OR Cuantificación) | 39 | 11 September 2024 |
| Engineering Village | (Psilocybin OR Psilocin) AND (Extraction OR Identification OR Identificação OR Characterization OR Quantification) | 22 | 11 September 2024 |
| Inclusion Criteria (I) | Exclusion Criteria (E) |
|---|---|
| We accept articles with only psilocybin or psilocin. | We reject articles with a score less than or equal to 5. |
| We accept articles that contain the words psilocybin or psilocin in the title, abstract, and/or keywords. | We reject articles that are not experimental. |
| We reject articles that do not deal with the extraction, identification, quantification, or chromatography of psilocybin and psilocin. | |
| We reject articles containing biological/in vivo/clinical tissues/fluids analysis. |
Appendix B. Quality Analysis of Selected Studies
| 1 | Was the aim of the study clearly defined and relevant to psilocybin extraction? |
| 2 | Were the extraction methodologies (e.g., solvents, time, temperature) described in sufficient detail? |
| 3 | Was the sample preparation process adequately explained (e.g., drying, grinding, storage conditions)? |
| 4 | Were the solvents and reagents properly justified and aligned with the goals of the extraction process? |
| 5 | Was the extraction efficiency (yield of psilocybin/psilocin) clearly reported? |
| 6 | Were the analytical methods (e.g., HPLC, TLC, GC-MS) for psilocybin quantification validated and appropriate? |
| 7 | Was the reproducibility and consistency in the replication of the experiments? |
| 8 | Were the data and results presented in a clear, organized, and statistically valid manner? |
| 9 | Were the limitations or challenges of the extraction or quantification process discussed? |
| 10 | Were the conclusions supported by the data and consistent with the study’s objectives? |
| 11 | Does the study discuss potential sources of bias and how they were addressed during the experimental process? |
| 12 | Was the study’s contribution to the field clearly articulated and placed within the context of existing literature? |
References
- Hirschfeld, T.; Schmidt, T. Dose–response relationships of psilocybin-induced subjective experiences in humans. J. Psychopharmacol. 2021, 35, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, J.; Wilson, C.; Hannan, A.; Renoir, T. Psilocybin as a lead candidate molecule in preclinical therapeutic studies of psychiatric disorders: A systematic review. J. Neurochem. 2023, 168, 1687–1720. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, P.B.; Fuentes, J.J.; Kaptein, A.A.; Schoones, J.W.; De Waal, M.M.; Goudriaan, A.E.; Kramers, K.; Schellekens, A.; Somers, M.; Bossong, M.G. Therapeutic effect of psilocybin in addiction: A systematic review. Front. Psychiatry 2023, 14, 1134454. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Pavlova, B. Long-term effects of depression treatment. Lancet Psychiatry 2016, 3, 95–96. [Google Scholar] [CrossRef]
- Johnson, M.W.; Griffiths, R.R. Potential therapeutic effects of psilocybin. Neurotherapeutics 2017, 14, 734–740. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The therapeutic potential of psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef]
- Whinkin, E.; Opalka, M.; Watters, C.; Jaffe, A.; Aggarwal, S. Psilocybin in palliative care: An update. Curr. Geriatr. Rep. 2023, 12, 50–59. [Google Scholar] [CrossRef]
- Muttoni, S.; Ardissino, M.; John, C. Classical psychedelics for the treatment of depression and anxiety: A systematic review. J. Affect. Disord. 2019, 258, 11–24. [Google Scholar] [CrossRef]
- Bird, C.I.; Modlin, N.L.; Rucker, J.J.H. Psilocybin and MDMA for the treatment of trauma-related psychopathology. Int. Rev. Psychiatry 2021, 33, 229–249. [Google Scholar] [CrossRef]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef]
- Ehrmann, K.; Allen, J.J.; Moreno, F.A. Psilocybin for the treatment of obsessive-compulsive disorders. In Disruptive Psychopharmacology; Springer: Cham, Switzerland, 2021; Volume 56, pp. 247–259. [Google Scholar] [CrossRef]
- Tullis, P. How ecstasy and psilocybin are shaking up psychiatry. Nature 2021, 589, 506–510. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Psychedelics. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=psychedelics&timeline=expanded (accessed on 13 January 2025).
- World Health Organization. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 18 June 2024).
- Luppi, A.I.; Hansen, J.Y.; Adapa, R.; Carhart-Harris, R.L.; Roseman, L.; Timmermann, C.; Golkowski, D.; Ranft, A.; Ilg, R.; Jordan, D. In vivo mapping of pharmacologically induced functional reorganization onto the human brain’s neurotransmitter landscape. Sci. Adv. 2023, 9, eadf8332. [Google Scholar] [CrossRef] [PubMed]
- Musikaphongsakul, P.; Ya, K.; Subsoontorn, P.; Lohitnavy, M. Development of a physiologically based pharmacokinetic (PBPK) model of psilocybin and psilocin from magic mushroom in rats and humans. F1000Research 2021, 10, 209. [Google Scholar] [CrossRef]
- Rickli, A.; Moning, O.; Hoener, M.; Liechti, M. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016, 26, 1327–1337. [Google Scholar] [CrossRef]
- Smausz, R.; Neill, J.; Gigg, J. Neural mechanisms underlying psilocybin’s therapeutic potential–the need for preclinical in vivo electrophysiology. J. Psychopharmacol. 2022, 36, 781–793. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet 2016, 3, 619–627. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Roseman, L.; Bolstridge, M.; Demetriou, L.; Pannekoek, J.N.; Wall, M.B.; Tanner, M.; Kaelen, M.; McGonigle, J.; Murphy, K. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci. Rep. 2017, 7, 13187. [Google Scholar] [CrossRef]
- Mertens, L.J.; Wall, M.B.; Roseman, L.; Demetriou, L.; Nutt, D.J.; Carhart-Harris, R.L. Therapeutic mechanisms of psilocybin: Changes in amygdala and prefrontal functional connectivity during emotional processing after psilocybin for treatment-resistant depression. J. Psychopharmacol. 2020, 34, 167–180. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Leech, R.; Hellyer, P.J.; Shanahan, M.; Feilding, A.; Tagliazucchi, E.; Chialvo, D.R.; Nutt, D. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front. Hum. Neurosci. 2014, 8, 20. [Google Scholar] [CrossRef]
- Petri, G.; Expert, P.; Turkheimer, F.; Carhart-Harris, R.; Nutt, D.; Hellyer, P.J.; Vaccarino, F. Homological scaffolds of brain functional networks. J. R. Soc. Interface 2014, 11, 20140873. [Google Scholar] [CrossRef]
- Raithatha, S.A.; Hagel, J.M.; Matinkhoo, K.; Yu, L.; Press, D.; Cook, S.G.; Sharma, G.; Dhananjaya, D.; Jensen, G.; Lee, J.B. Novel psilocin prodrugs with altered pharmacological properties as candidate therapies for treatment-resistant anxiety disorders. J. Med. Chem. 2023, 67, 1024–1043. [Google Scholar] [CrossRef] [PubMed]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and serotonin 5-HT 2A receptor-mediated signaling pathways. Behav. Neurobiol. Psychedelic Drugs 2018, 36, 45–73. [Google Scholar] [CrossRef]
- Nkadimeng, S.M.; Steinmann, C.M.L.; Eloff, J.N. Anti-inflammatory effects of four psilocybin-containing magic mushroom water extracts in vitro on 15-lipoxygenase activity and on lipopolysaccharide-induced cyclooxygenase-2 and inflammatory cytokines in human U937 macrophage cells. J. Inflamm. Res. 2021, 14, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Tylš, F.; Pálenícek, T.; Kaderábek, L.; Lipski, M.; Kubešová, A.; Horácek, J. Sex differences and serotonergic mechanisms in the behavioural effects of psilocin. Behav. Pharmacol. 2016, 27, 309–320. [Google Scholar] [CrossRef]
- Londesbrough, D.J.; Brown, C.; Northen, J.S.; Moore, G.; Patil, H.; Nichols, D. Preparation of Psilocybin, Different Polymorphic Forms, Intermediates, Formulations and Their Use. Patent WO2019073379, 18 April 2019. [Google Scholar]
- Matzopoulos, R.; Morlock, R.; Morlock, A.; Lerer, B.; Lerer, L. Psychedelic mushrooms in the USA: Knowledge, patterns of use, and association with health outcomes. Front. Psychiatry 2022, 12, 780696. [Google Scholar] [CrossRef]
- Siegel, J.S.; Daily, J.E.; Perry, D.A.; Nicol, G.E. Psychedelic drug legislative reform and legalization in the US. JAMA Psychiatry 2023, 80, 77–83. [Google Scholar] [CrossRef]
- Lash, N.; Tadros, G.; Tadros, E. Therapeutic Implications of Psilocybin in the Wake of Decriminalization. Integr. Complement. Ther. 2024, 30, 3. [Google Scholar] [CrossRef]
- Nutt, D.; Crome, I.; Young, A. Is it now time to prepare psychiatry for a psychedelic future? Br. J. Psychiatry J. Ment. Sci. 2024, 225, 308–310. [Google Scholar] [CrossRef]
- Švecová, M.; Novák, V.; Bartůněk, V.; Člupek, M. Lanthanum trilactate: Vibrational spectroscopic study—Infrared/Raman spectroscopy. Vib. Spectrosc. 2016, 87, 123–128. [Google Scholar] [CrossRef]
- Marks, M.; Cohen, I.G. Patents on psychedelics: The next legal battlefront of drug development. Harv. Law Rev. Forum 2021, 135, 24. [Google Scholar] [CrossRef]
- Rafati, H.; Riahi, H.; Mohammadi, A. Enhancement of indole alkaloids produced by Psilocybe cubensis (earle) singer (agaricomycetideae) in controlled harvesting light conditions. Int. J. Med. Mushrooms 2009, 11, 419–426. [Google Scholar] [CrossRef]
- Kolaczynska, K.E.; Liechti, M.E.; Duthaler, U. Development and validation of an LC-MS/MS method for the bioanalysis of psilocybin’s main metabolites, psilocin and 4-hydroxyindole-3-acetic acid, in human plasma. J. Chromatogr. B 2021, 1164, 122486. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, A.; Janusson, E.; Shah, S.; Roggen, M. Rapid quantification of Psilocybin with reversed-phase HPLC and single-wavelength detection. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, R.; Yuan, S.; Qiang, H.; Shen, M.; Shen, B.; Drummer, O.H.; Yu, Z.; Zhao, Y.; Xiang, P. UHPLC-MS/MS method for simultaneously detecting 16 tryptamines and their metabolites in human hair and applications to real forensics cases. J. Chromatogr. B 2020, 1159, 122392. [Google Scholar] [CrossRef] [PubMed]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University Technical Report No. TR/SE-0401; NICTA Technical Report No. 0400011T. 1; Keele University: Keele, UK, 2004. [Google Scholar]
- Goff, R.; Smith, M.; Islam, S.; Sisley, S.; Ferguson, J.; Kuzdzal, S.; Badal, S.; Kumar, A.B.; Sreenivasan, U.; Schug, K.A. Determination of psilocybin and psilocin content in multiple Psilocybe cubensis mushroom strains using liquid chromatography—Tandem mass spectrometry. Anal. Chim. Acta 2024, 1288, 342161. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Évid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 372, n71. [Google Scholar] [CrossRef]
- Koçak, A.; De Cotiis, L.; Hoffman, D. Comparative study of ATR and transflection IR spectroscopic techniques for the analysis of hallucinogenic mushrooms. Forensic Sci. Int. 2010, 195, 36–41. [Google Scholar] [CrossRef]
- Van Court, R.C.; Wiseman, M.S.; Meyer, K.W.; Ballhorn, D.J.; Amses, K.R.; Slot, J.C.; Dentinger, B.T.M.; Garibay-Orijel, R.; Uehling, J.K. Diversity, biology, and history of psilocybin-containing fungi: Suggestions for research and technological development. Fungal Biol. 2022, 126, 308–319. [Google Scholar] [CrossRef]
- Bigwood, J.; Beug, M.W. Variation of psilocybin and psilocin levels with repeated flushes (harvests) of mature sporocarps of Psilocybe cubensis (Earle) Singer. J. Ethnopharmacol. 1982, 5, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Garrigues, S.; de la Guardia, M. Green extraction techniques in green analytical chemistry: A 2019–2023 up-date. TrAC Trends Anal. Chem. 2023, 170, 117464. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, K.S. Supercritical Fluid Extraction of Natural Products: A Review. Int. J. Eng. Dev. Res. 2014, 2, 14–17. [Google Scholar]
- Stríbrný, J.; Borovicka, J.; Sokol, M. Levels of psilocybin and psilocin in various types of mushrooms. Soud. Lek. 2003, 48, 45–49. [Google Scholar] [PubMed]
- Musshoff, F.; Madea, B.; Beike, J. Hallucinogenic mushrooms on the German market—Simple instructions for examination and identification. Forensic Sci. Int. 2000, 113, 389–395. [Google Scholar] [CrossRef]
- Qi, L.I.; Haijiao, L.I.; Yizhe, Z.; Yajuan, Z.; Shu, Z.H.U.; Fei, X.U.; Xiaoke, X.; Gang, D. A preliminary study on chemical components of the hallucinogenic poisonous mushroom Psilocybe ovoideocystidiata. Mycosystema 2022, 41, 1704–1715. [Google Scholar] [CrossRef]
- Repke, D.B.; Leslie, D.T. Baeocystin in Psilocybe semilanceata. J. Pharm. Sci. 1977, 66, 113–114. [Google Scholar] [CrossRef]
- Alexander, J.B.; Talia, B.; Ramírez-Cruz, V.; Dale, L.F.; Jaclyn, M.W.; Guzmán-Dávalos, L.; Furci, G.; Stamets, P.; Dentinger, B. DNA Authentication and Chemical Analysis of Psilocybe Mushrooms Reveal Widespread Misdeterminations in Fungaria and Inconsistencies in Metabolites. Appl. Environ. Microbiol. 2022, 88, e0149822. [Google Scholar] [CrossRef]
- Hannah, T.R.; Vijayakumar, V.; Emile, G.-T.; Korotkin, H.B.; Matheny, P.B.; Slot, J. Horizontal gene cluster transfer increased hallucinogenic mushroom diversity. Evol. Lett. 2017, 2, 88–101. [Google Scholar] [CrossRef]
- Sebastian, D.; Kai, R.; Janis, F.; Schäfer, T.; Jacob, M.W.; Markus, G.; Duyen, N.K.P.; David, R.M.; Andrew, R.C.; Hoffmeister, D. Genetic Survey of Psilocybe Natural Products. Chembiochem 2022, 23, e202200249. [Google Scholar] [CrossRef]
- Tsujikawa, K.; Kanamori, T.; Iwata, Y.; Ohmae, Y.; Sugita, R.; Inoue, H.; Kishi, T. Morphological and chemical analysis of magic mushrooms in Japan. Forensic Sci. Int. 2003, 138, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Rotolo, M.C.; Marchei, E.; Pacifici, R.; Saggio, F.; Pichini, S. Magic truffles or Philosopher’s stones: A legal way to sell psilocybin? Drug Test. Anal. 2013, 5, 182–185. [Google Scholar] [CrossRef]
- Gambaro, V.; Roda, G.; Visconti, G.L.; Arnoldi, S.; Casagni, E.; Dell’Acqua, L.; Farè, F.; Paladino, E.; Rusconi, C.; Arioli, S.; et al. DNA-based taxonomic identification of basidiospores in hallucinogenic mushrooms cultivated in “grow-kits” seized by the police: LC-UV quali-quantitative determination of psilocybin and psilocin. J. Pharm. Biomed. Anal. 2016, 125, 427–432. [Google Scholar] [CrossRef]
- Gotvaldová, K.; Borovička, J.; Hájková, K.; Cihlářová, P.; Rockefeller, A.; Kuchař, M. Extensive Collection of Psychotropic Mushrooms with Determination of Their Tryptamine Alkaloids. Int. J. Mol. Sci. 2022, 23, 14068. [Google Scholar] [CrossRef]
- Christiansen, A.L.; Rasmussen, K.E.; Høiland, K. Detection of psilocybin and psilocin in norwegian species of pluteus and conocybe. Planta Med. 1984, 50, 341–343. [Google Scholar] [CrossRef]
- Anastos, N.; Barnett, N.W.; Lewis, S.W.; Gathergood, N.; Scammells, P.J.; Sims, D.N. Determination of psilocin and psilocybin using flow injection analysis with acidic potassium permanganate and tris (2, 2′-bipyridyl) ruthenium (II) chemiluminescence detection respectively. Talanta 2005, 67, 354–359. [Google Scholar] [CrossRef]
- Anastos, N.; Lewis, S.W.; Barnett, N.W.; Sims, D.N. The Determination of Psilocin and Psilocybin in Hallucinogenic Mushrooms by HPLC Utilizing a Dual Reagent Acidic Potassium Permanganate and Tris (2, 2′-bipyridyl) ruthenium (II) Chemiluminescence Detection System. J. Forensic Sci. 2006, 51, 45–51. [Google Scholar] [CrossRef]
- Gurevich, L.S. Indole derivatives in certain Panaeolus species from East Europe and Siberia. Mycol. Res. 1993, 97, 251–254. [Google Scholar] [CrossRef]
- Saito, K.; Toyo’oka, T.; Kato, M.; Fukushima, T.; Shirota, O.; Goda, Y. Determination of psilocybin in hallucinogenic mushrooms by reversed-phase liquid chromatography with fluorescence detection. Talanta 2005, 66, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Y.-Z.; Liang, J.-Q.; Liu, F.; Li, Z.-F.; Li, H.-J.; Xu, F. Mushroom poisoning of Panaeolus subbalteatus from Ningxia, northwest China, with species identification and tryptamine detection. Toxicon 2024, 247, 107849. [Google Scholar] [CrossRef] [PubMed]
- Halama, M.; Poliwoda, A.; Jasicka-Misiak, I.; Wieczorek, P.P.; Rutkowski, R. Pholiotina cyanopus, a rare fungus producing psychoactive tryptamines. Open Life Sci. 2014, 10, 40–51. [Google Scholar] [CrossRef]
- Zhuk, O.; Jasicka-Misiak, I.; Poliwoda, A.; Kazakova, A.; Godovan, V.V.; Halama, M.; Wieczorek, P.P. Research on acute toxicity and the behavioral effects of methanolic extract from psilocybin mushrooms and psilocin in mice. Toxins 2015, 7, 1018–1029. [Google Scholar] [CrossRef]
- Beug, M.W.; Bigwood, J. Quantitative analysis of psilocybin and psilocin and Psilocybe baecystis (Singer and Smith) by high-performance liquid chromatography and by thin-layer chromatography. J. Chromatogr. A 1981, 207, 379–385. [Google Scholar] [CrossRef]
- Wurst, M.; Semerdžieva, M.; Vokoun, J. Analysis of psychotropic compounds in fungi of the genus psilocybe by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1984, 286, 229–235. [Google Scholar] [CrossRef]
- Laussmann, T.; Meier-Giebing, S. Forensic analysis of hallucinogenic mushrooms and khat (Catha edulisForsk) using cation-exchange liquid chromatography. Forensic Sci. Int. 2010, 195, 160–164. [Google Scholar] [CrossRef]
- Repke, D.B.; Leslie, D.T.; Mandell, D.M.; Kish, N.G. GLC-mass spectral analysis of psilocin and psilocybin. J. Pharm. Sci. 1977, 66, 743–744. [Google Scholar] [CrossRef]
- Keller, T.; Schneider, A.; Regenscheit, P.; Dirnhofer, R.; Rücker, T.; Jaspers, J.; Kisser, W. Analysis of psilocybin and psilocin in Psilocybe subcubensis Guzmán by ion mobility spectrometry and gas chromatography-mass spectrometry. Forensic Sci. Int. 1999, 99, 93–105. [Google Scholar] [CrossRef]
- Miller, D.R.; Jacobs, J.T.; Rockefeller, A.; Singer, H.; Bollinger, I.M.; Conway, J.; Slot, J.C.; Cliffel, D.E. Cultivation, chemistry, and genome of Psilocybe zapotecorum. Biorxiv Prepr. Serv. Biol. 2023, 8, 63–81. [Google Scholar] [CrossRef]
- Waldbillig, A.; Baranova, M.; Neumann, S.; Andrade, J.; Sidhu, S. Exploring Psilocybe spp. mycelium and fruiting body chemistry for potential therapeutic compounds. Front. Fungal Biol. 2023, 4, 1295223. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.S.; Cunha, K.F.d.; Izabelly Geraldes, S.; Fiorentin, T.R.; Campos, E.D.d.; José Luiz, C. Development and Validation of a Sensitive LC-MS/MS Method to Quantify Psilocin in Authentic Oral Fluid Samples. J. Anal. Toxicol. 2023, 47, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G. Psilocybin Mushrooms of the World: An identification guide. Paul Stamets. Berkeley, California: Ten Speed Press. 1996. 245pp. ISBN 0 89815 839 7. $24.95 (paperback). Edinb. J. Bot. 1999, 56, 466–467. [Google Scholar] [CrossRef]
- Beug, M.W.; Bigwood, J. Psilocybin and psilocin levels in twenty species from seven genera of wild mushrooms in the Pacific Northwest, USA. J. Ethnopharmacol. 1982, 5, 271–285. [Google Scholar] [CrossRef]
- Strauss, D.; Ghosh, S.; Murray, Z.; Gryzenhout, M. An Overview on the Taxonomy, Phylogenetics and Ecology of the Psychedelic Genera Psilocybe, Panaeolus, Pluteus and Gymnopilus. Front. For. Glob. Chang. 2022, 5, 813998. [Google Scholar] [CrossRef]


| Whole Mushroom | Cap | Stem | ||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Species | Methodology | %PSCB | %PSC | %PSCB | %PSC | %PSCB | %PSC |
| [48,56] | - | Shaking and maceration | 0.100–0.760 | 0.040–0.840 | NA | NA | NA | NA |
| - | Unrest | 0.060–0.160 | NI | NI | NI | NI | NI | |
| [49] | Inocybe aeruginascens | Vortex agitation | 0.012–0.013 | 0.0005 | NI | NI | NI | NI |
| [49] | Inocybe calamistrata | Vortex agitation | ND | ND | NI | NI | NI | NI |
| [49] | Inocybe corydalina | Vortex agitation | 0.008–0.028 | 0–0.0006 | NI | NI | NI | NI |
| [49] | Agaricus bisporus | Vortex agitation | ND | ND | NI | NI | NI | NI |
| [40] | Blue Meanie | Vortex agitation | 1140–1220 | 0.021–0.051 | NI | NI | NI | NI |
| [40] | B-Plus | Vortex agitation | 0.860–1.360 | 0.013–0.031 | NI | NI | NI | NI |
| [47] | Conocybe cyanopus | - | 0.330–0.55 | 0.004–0.007 | NA | NA | NA | NA |
| [47] | Conocybe tenera | - | ND | ND | NA | NA | NA | NA |
| [62] | Copelandia | Ultrasonic bath | 0.080–0.220 | 0.430–0.760 | 0.020–0.220 | 0.360–0.740 | 0.010–0.390 | 0.310–0.780 |
| [47] | Coprinus plicatilis | - | ND | ND | NI | NI | NI | NI |
| [40] | Creeper | Vortex agitation | 1000–1600 | 0.017–0.031 | NI | NI | NI | NI |
| [49] | Gymnopilus dilepis | Vortex agitation | 0.003–0.013 | 0.0024–0.0063 | NI | NI | NI | NI |
| [47] | Gymnopilus spectabilis | - | ND | ND | NI | NI | NI | NA |
| [44,45] | Orange Hypholoma | Maceration | 0.930 | 0.021 | NA | NA | NA | NA |
| 0.970–0.990 | ND | NA | NA | NA | NA | |||
| [61] | Bloodless | Shaker | ND | ND | NA | NA | NA | NA |
| [47] | Marasmius oreades | - | ND | ND | NA | NA | NA | NA |
| [61] | Panaeolus acuminatus | Shaker | ND | ND | NA | NA | NA | NA |
| [50] | Black panther | Maceration | ND | ND | NA | NA | NA | NA |
| [49] | Belted panaeolus | Vortex agitation | 0.011–0.158 | 0.0007–0.0257 | NA | NA | NA | NA |
| [55,60] | Panaeolus cyanescens | Ultrasonic bath | 0.020–1.150 | 0.140–0.90 | NI | NI | NI | NI |
| ND | NI | NI | NI | NI | NI | |||
| [45,47,49] | Panaeolus foenisecii | Vortex agitation | ND | ND | NI | NI | NI | NI |
| NI | ND | ND | NI | NI | NI | NI | ||
| Maceration | 0.680–0.730 | ND | NI | NI | NI | NI | ||
| [49] | Panaeolus olivaceus | Vortex agitation | ND | ND | NI | NI | NI | NI |
| [49,50] | Panaeolus papilionaceus | Vortex agitation | ND | ND | NI | NI | NI | NI |
| Maceration | ND | ND | NI | NI | NI | NI | ||
| [47,49] | Panaeolus rickenii | - | ND | ND | NA | NA | NA | NA |
| Maceration | ND | ND | NA | NA | NA | NA | ||
| [50] | Panaeolus sphinctrinus | Maceration | ND | ND | NA | NA | NA | NA |
| [50,65] | Panaeolus subbalteatus | Maceration | 1.100–1.300 | NA | 2.900–4.130 | NA | 1.100–1.900 | NI |
| Ultrasonic bath | 0.153–0.176 | 0.012–0.014 | NI | NI | NI | NI | ||
| [49,51,66] | Pholiotina cyanopus | Vortex agitation | 0.000–0.086 | 0.000–0.062 | NI | NI | NI | NI |
| Ultrasonic bath | 0.820–0.980 | 0.160–0.180 | NI | NI | NI | NI | ||
| Ultrasonic bath | 0.820–0.980 | 0.160–0.180 | NI | NI | NI | NI | ||
| [49] | American Pluteus | Vortex agitation | 0.117–0.243 | 0.012–0.035 | NI | NI | NI | NI |
| [49] | Pluteus glaucotinctus | Vortex agitation | 0.194 | 0.0013 | NI | NI | NI | NI |
| [47,49,61] | Pluteus salicinus | - | 0.031–0.135 | 0.037–0.070 | NI | NI | NI | NI |
| Vortex agitation | 0.350 | 0.011 | NO | NO | NO | NO | ||
| Shaker | ND | ND | NO | NO | NO | NO | ||
| [46] | Psathyrella foenisecii | Agitation | 0.150–0.850 | 0.00–0.590 | NO | NO | NO | NO |
| [47] | Psathyrella multipedata | - | ND | ND | YES | YES | YES | YES |
| [47] | Psathyrella velutina | - | ND | ND | NI | NI | NI | NI |
| [49] | Psilocybe | Vortex agitation | 0.051–0.189 | 0.149–0.205 | NI | NI | NI | NI |
| [47] | Psilocybe atrobrunnea | - | ND | ND | NI | NI | NI | NI |
| [61,64] | Psilocybe bohemica | Shaker | 0.306–0.622 | 0.318–0.884 | NA | NA | NA | NA |
| 0.250–1.150 | 0.000–0.020 | NA | NA | NA | NA | |||
| [49] | Psilocybe aerulescens | Vortex agitation | 0.022–0.031 | 0.034–0.041 | NA | NA | NA | NA |
| [49] | Psilocybe aureulipes | Vortex agitation | 0.223–0.567 | 0.050–0.028 | NA | NA | NA | NA |
| [35,49,53,55,59,60,62] | Psilocybe cubensis | Vortex agitation | 0.065–0.351 | 0.021–0.534 | NI | NI | NI | NI |
| Ultrasonic bath | 0.000–1.070 | 0.010–0.230 | NI | NI | NI | NI | ||
| 0.370–1.300 | 0.140–0.420 | 0.440–1.350 | 0.170–0.780 | 0.050–1.270 | 0.090–0.900 | |||
| 0.00048 | NI | NI | NI | NI | NI | |||
| Agitation | NI | NI | 0.42 | 0.168 | NI | NI | ||
| Ultrasonic bath and mixer | 0.923–1.379 | 0.060–0.192 | NI | NI | NI | NI | ||
| - | 0.010–1.000 | NI | NI | NI | NI | NI | ||
| [49,53,61] | Psilocybe cyanescens | Vortex agitation | 0.234–1.380 | 0.041–1.002 | NI | NI | NI | NI |
| Shaker | 0.455–1.565 | 0.450–1.588 | NI | NI | NI | NI | ||
| Ultrasonic bath and mixer | 1606–3422 | 0.621–1.767 | NI | NI | NI | NI | ||
| [49] | Psilocybe fimetaria | Vortex agitation | ND | ND | NI | NI | NI | NI |
| [49] | Psilocybe fuscofulva | Vortex agitation | ND | ND | NI | NI | NI | NI |
| [49] | Psilocybe medullosa | Vortex agitation | 0.014–0.100 | 0.000–0.005 | NI | NI | NI | NI |
| [49] | Psilocybe mexicana | Vortex agitation | 0.328–0.393 | 0.194–0.197 | NI | NI | NI | NI |
| [49,57] | Psilocybe ovoideocystidiata | Ultrasonic bath | NI | NI | 1020–1790 | 0.140–0.460 | 0.170–0.190 | 0.040 |
| Vortex agitation | 0.091–0.717 | 0.003–0.546 | NI | NI | NI | NI | ||
| [58] | Psilocybe pelliculosa | Ultrasonic bath | NI | NI | 1020–1790 | 0.140–0.460 | 0.170–0.190 | 0.040 |
| [49,55,58,61,64,66] | Psilocybe semilanceata | Vortex agitation | 0.128–1.142 | 0.003–0.062 | NI | NI | NI | NI |
| Shaker | 0.300–0.322 | 0.146–0.158 | NI | NI | NI | NI | ||
| Ultrasonic bath | 0.010–0.910 | 0.010–0.900 | NI | NI | NI | NI | ||
| Ultrasonic bath | 1340–1580 | 0.228–0.252 | NI | NI | NI | NI | ||
| Shaker | 0.250–1.150 | 0.000–0.020 | NI | NI | NI | NI | ||
| Agitation | 0.120–0.360 | NI | NI | NI | NI | NI | ||
| [49,61] | Psilocybe serbica | Vortex agitation | 0.156–0.396 | 0.021–0.381 | NI | NI | NI | NI |
| Shaker | 0.094–0.820 | 0.310–0.370 | NI | NI | NI | NI | ||
| [49] | Psilocybe serbica var. arcana | Vortex agitation | 0.0002–0.878 | 0.041–0.792 | NI | NI | NI | NI |
| [49] | Psilocybe serbica var. bohemica | Vortex agitation | 0.155–1.554 | 0.003–0.248 | NA | NA | NA | NA |
| [49] | Psilocybe serbica var. moravica | Vortex shaking | 0.565–1.416 | 0.006–0.038 | NA | NA | NA | NA |
| [44,45,49] | Psilocybe subaeruginosa | Vortex shaking | 0.010–0.019 | 0.008–0.033 | NA | NA | NA | NA |
| Maceration | 1410 | 0.038 | NA | NA | NA | NA | ||
| 1.070–1.120 | 0.011–0.019 | NA | NA | NA | NA | |||
| [47] | Psilocybe subcoprophila | - | ND | ND | NA | NA | NA | NA |
| [52] | Psilocybe subcubensis | Ultrasonic bath | NA | NA | 0.86 | 0.02 | 0.8 | 0.03 |
| [53,55] | Psilocybe tampanensis | Ultrasonic bath | 0.000–0.190 | 0.010–0.030 | NA | NI | NI | NI |
| Ultrasonic bath and mixer | 0.057–0.181 | 0.015–0.101 | NI | NI | NI | NI | ||
| [49,54] | Psilocybe zapotecorum | Vortex agitation | 0.902–0.965 | 0.029–0.037 | NI | NI | NI | NI |
| Ultrasonic bath | 0.110–0.260 | 0.038–0.650 | 0.190–0.310 | 0.110–0.510 | 0.080–0.240 | 0.030–0.220 | ||
| [63] | Psilocybe | Vortex agitation | 0.950–1.030 | NI | NI | NI | NI | NI |
| [49] | Stropharia aeruginosa | Vortex agitation | ND | ND | NI | NI | NI | NI |
| [47] | Stropharia semiglobara | - | ND | ND | NI | NI | NI | NI |
| [60] | Alleged Psilocybe cubensis | Ultrasonic bath | 0.0008 | NI | NI | NI | NI | NI |
| [40] | Texas Yellow | Vortex agitation | 1000–1160 | 0.019–0.028 | NI | NI | NI | NI |
| [40] | Thai Cubensis | Vortex agitation | 0.740–0.880 | 0.050–0.090 | NI | NI | NI | NI |
| Reference | Methodology | Mushroom Form | Spraying Technique | Solvent Type | Proportion (m/v) | Time (h) | Number of Extractions | Temperature (°C) | Performance | |
|---|---|---|---|---|---|---|---|---|---|---|
| PSCB (%) | PSC(%) | |||||||||
| [56] | Agitation | Dust | Mill | Methanol | (1:50) | one night | 1 | NI | 0.10–0.76 | 0.04–0.84 |
| [48] | Agitation and maceration | NI | NI | Methanol | (1:10) | 0.75 and one night | 1 | NI | 0.06–0.16 | NI |
| [49] | Vortex agitation | Dust | Mortar | Methanol/acetic acid + Methanol | (1:100) | 1 | NI | 20 | 0.0002–1.5543 | 0–1.0018 |
| [40] | Vortex Agitation | Dust | Mill | Methanol/acetic acid | (1:100) | 0.5 | 2 | NI | 0.74–1.6 | 0.013–0.051 |
| [47] | - | NI | NI | Methanol/ammonium nitrate | (9:1) | NI | 2 | NI | ND–0.55 | ND–0.011 |
| [62] | Ultrasonic bath | Dust | Mortar | Methanol | (1:100) | 0.5 | 1 | NI | 0.08–0.22 | 0.43–0.76 |
| [44] | Maceration | NI | NI | Methanol/sodium polyphosphate | (1:100) | one night | 1 | NI | 0.93–1.41 | 0.021–0.038 |
| [45] | Maceration | Dust | Mill | Methanol | (1:1) | 24 | 1 | NI | 0.68–1.12 | 0.011–0.019 |
| [61] | Shaker | Dust | Mortar | Methanol | (1:50) | 0.5 | 1 | NI | 0.094–1.565 | 0.146–1.588 |
| [50] | Maceration | Dust | Mortar | Water/ethanol | (1:100) | One night | 2 | 20–25 | 1.1–1.9 | ND |
| [55] | Ultrasonic bath | Dust | NI | Methanol | (1:90) | 2 | 1 | 50 | 0–1.15 | 0.01–0.9 |
| [60] | Ultrasonic bath | Dust | Mill | Methanol/disodium 3-indoxyl phosphate | (1:1000) | 0.5 | 2 | <50 | 0.001–1.30 | 0.014–0.42 |
| [65] | Ultrasonic bath | Dust | - | Methanol/water | (1:20) | 0.25 | 1 | NI | 0.153–0.176 | 0.012–0.014 |
| [51] | Ultrasonic bath | Dust | Mortar | Methanol | (1:100) | 3 | 1 | NI | 0.82–0.98 | 0.16–0.18 |
| [66] | Ultrasonic bath | NI | NI | Methanol | (1:100) | 3 | 1 | NI | 0.82–1.58 | 0.16–0.252 |
| [46] | Agitation | Dust | Mill | Methanol | (1:28) | 12 | 1 | 20–25 | 0.15–0.85 | 0–0.59 |
| [64] | Agitation | NI | NI | Methanol | (1:100 and 1:10) | 16 | 1 | 25 | 0.25–1.15 | 0–0.02 |
| [53] | Ultrasonic bath and mixer | Powder and whole | Mill | Methanol/hydrochloric acid | (1:1000) and NI | 1 | 1 | 20–25 | 0.00048–3.422 | 0.015–1.767 |
| [35] | Ultrasonic bath | Dust | Mortar | Chloroform | (1:15) | 1 | 1 | NI | NI | NI |
| [59] | Agitation | Dust | Mortar | Methanol | (1:100) | 24 | 1 | 20–25 | 0.923–1.379 | 0.06–0.192 |
| [57] | Ultrasonic bath + vortex | Dust | Mill | Methanol/water | (1:10) | 0.25 + 0.5 | 1 | NI | NI | NI |
| [58] | Agitation | Dust | Mill | Methanol | NI | 20 | 1 | 20–25 | 0.08–0.36 | NI |
| [52] | Ultrasonic bath | Dust | Mortar | Chloroform | (1:20) | 1 | 1 | NI | NI | NI |
| [54] | Ultrasonic bath | Dust | Mortar | Methanol and Methanol/water + formic acid | (1:33) | 0.17 | 3 | 20–25 | 0.11–0.26 | 0.038–0.65 |
| [63] | Vortex agitation + ultrasonic bath | Dust | Mill | Methanol | (1:200) | NI + 0.083 | 2 | NI | 0.95–1.03 | NI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galdino, T.P.; Oliveira, L.C.; Luz, M.A.; Jesus, R.A.; Lima, E.P.N.; Torres, M.C.M.; Sivieri, K.; Afonso, V.I.; Delgado, J.M.P.Q.; Lima, A.G.B.; et al. Extraction Yields of Psilocybin and Psilocin: A Short Review of Current Methods and Their Implications. Pharmaceuticals 2025, 18, 380. https://doi.org/10.3390/ph18030380
Galdino TP, Oliveira LC, Luz MA, Jesus RA, Lima EPN, Torres MCM, Sivieri K, Afonso VI, Delgado JMPQ, Lima AGB, et al. Extraction Yields of Psilocybin and Psilocin: A Short Review of Current Methods and Their Implications. Pharmaceuticals. 2025; 18(3):380. https://doi.org/10.3390/ph18030380
Chicago/Turabian StyleGaldino, Taynah P., Lucas C. Oliveira, Mateus A. Luz, Raquel A. Jesus, Eunice P. N. Lima, Maria C. M. Torres, Katia Sivieri, Victor I. Afonso, João M. P. Q. Delgado, Antonio G. B. Lima, and et al. 2025. "Extraction Yields of Psilocybin and Psilocin: A Short Review of Current Methods and Their Implications" Pharmaceuticals 18, no. 3: 380. https://doi.org/10.3390/ph18030380
APA StyleGaldino, T. P., Oliveira, L. C., Luz, M. A., Jesus, R. A., Lima, E. P. N., Torres, M. C. M., Sivieri, K., Afonso, V. I., Delgado, J. M. P. Q., Lima, A. G. B., Silva, S. M. L., & Fook, M. V. L. (2025). Extraction Yields of Psilocybin and Psilocin: A Short Review of Current Methods and Their Implications. Pharmaceuticals, 18(3), 380. https://doi.org/10.3390/ph18030380









