Huperzine A Production and Acetylcholinesterase Inhibition by Phlegmariurus taxifolius Cell Suspension Culture: A Comparative Study in Flasks and an Airlift Bioreactor †
Abstract
:1. Introduction
2. Results
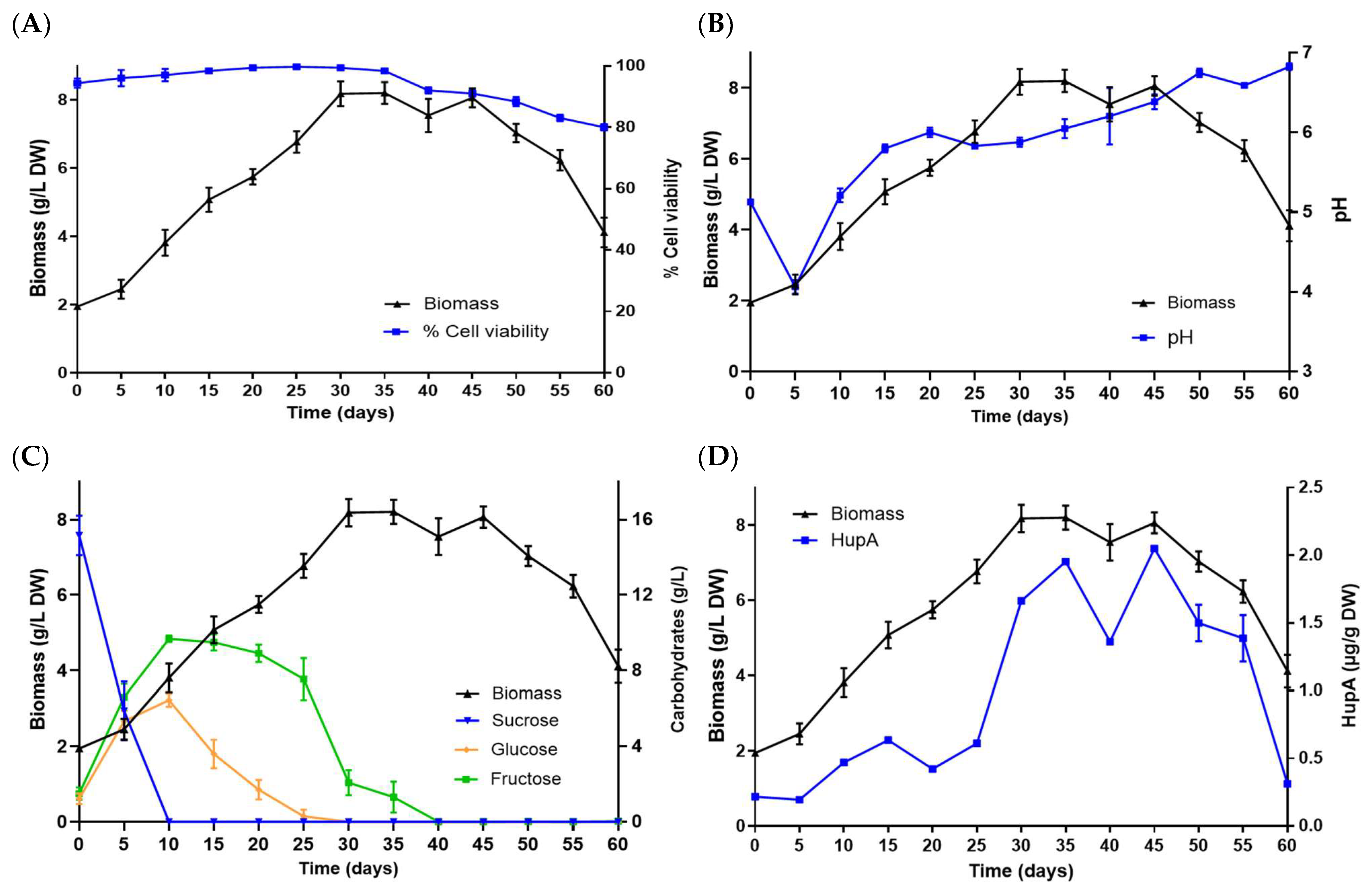
2.1. Cell Suspension Cultures of Line HupS21
2.2. Kinetic Study of the Suspension Cells of the HupS21 Line in Flask
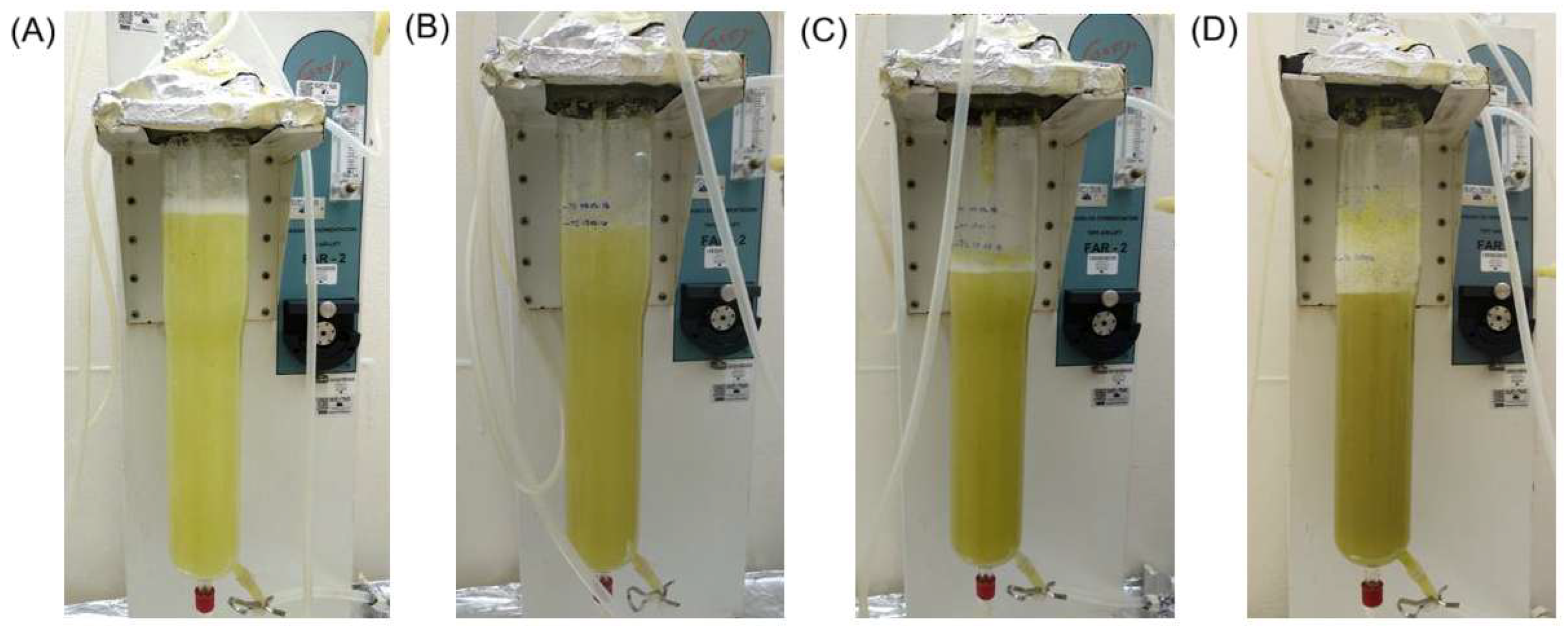
2.3. Kinetic Study and HupA Production of Airlift Bioreactor-Grown Cell Suspension Culture of P. taxifolius
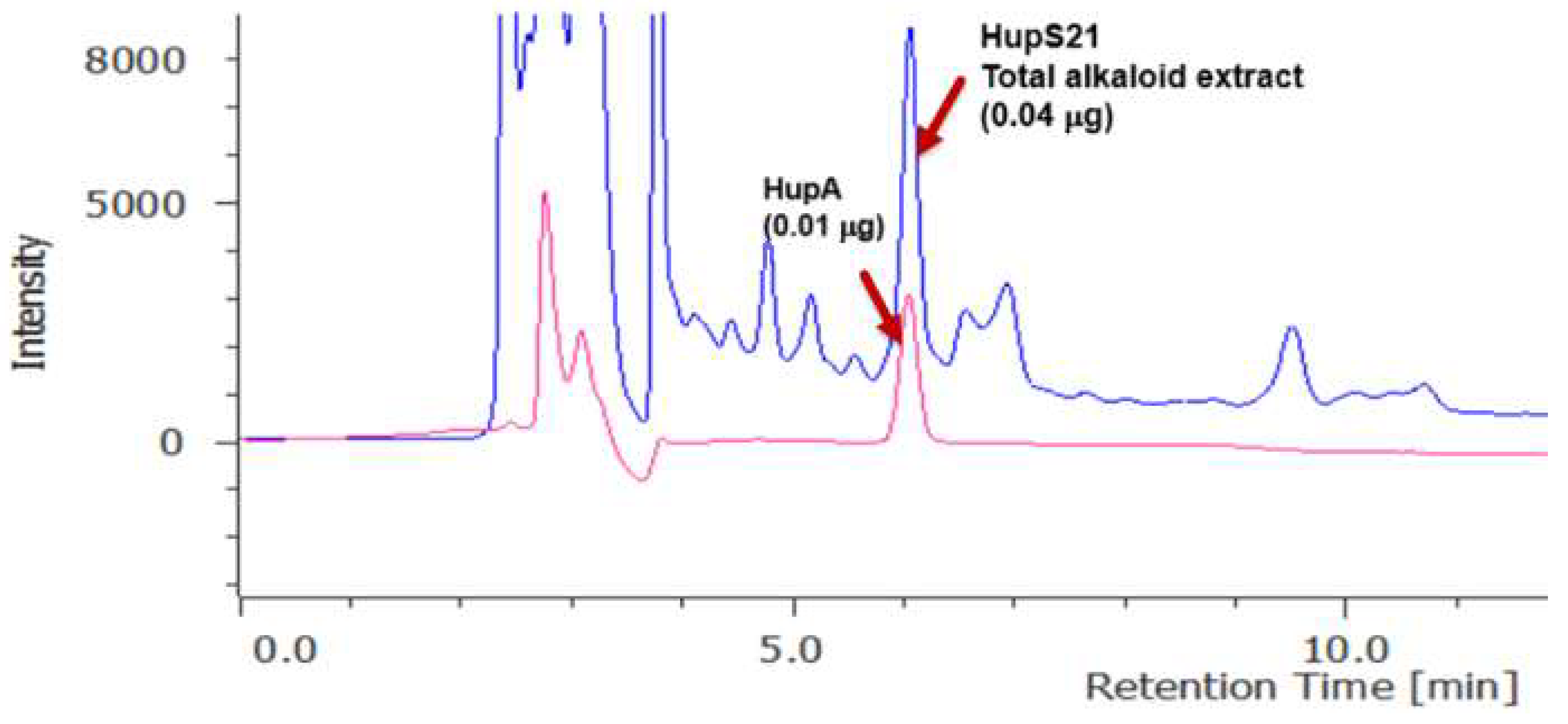
2.4. In Vitro Acetylcholinesterase Inhibitory Activity
3. Discussion
4. Materials and Methods
4.1. Callus Growth in Semisolid Medium
4.2. Establishment of Cell Suspension Cultures in Shake Flasks
4.3. Kinetic Studies of Cell Suspension Cultures in Shake Flasks
4.4. Cell Suspension Cultures of Cell Line HupS21 in Airlift Bioreactor
4.5. Growth Kinetics
4.6. Identification and Quantification of HupA
4.7. Inhibitory Activity of the AChE
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deutsch, L.H.; Rovner, B.W. Agitation and other noncognitive abnormalities in Alzheimer’s disease. Psychiatr. Clini. N. Am. 1991, 14, 341–351. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499922/ (accessed on 21 February 2025).
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539735/ (accessed on 21 February 2025).
- Liu, J.-S.; Zhu, Y.-L.; Yu, C.-M.; Zhou, Y.-Z.; Han, Y.-Y.; Wu, F.-W.; Qi, B.-F. The Structures of Huperzine A and B, Two New Alkaloids Exhibiting Marked Anticholinesterase Activity. Can. J. Chem. 1986, 64, 837–839. [Google Scholar] [CrossRef]
- Friedli, M.J.; Inestrosa, N.C. Huperzine A and Its Neuroprotective Molecular Signaling in Alzheimer’s Disease. Molecules 2021, 26, 6531. [Google Scholar] [CrossRef]
- Ma, X.; Tan, C.; Zhu, D.; Gang, D.R. A survey of potential huperzine A natural resources in China: The Huperziaceae. J. Ethnopharmacol. 2006, 104, 54–67. [Google Scholar] [CrossRef]
- Armenta-Montero, S.; Carvajal-Hernández, C.I.; Ellis, E.A.; Krömer, T. Distribution and conservation status of Phlegmariurus (Lycopodiaceae) in the state of Veracruz, Mexico. Trop. Conserv. Sci. 2015, 8, 114–137. [Google Scholar] [CrossRef]
- Rodríguez Salgado, T.; Villarreal Ortega, M.L.; Cardoso Taketa, A.T. Método de Inducción a Desdiferenciación Celular de Phlegmariurus taxifolius. MX/a/2019/014063. 22 October 2024. Available online: https://patentscope.wipo.int/search/es/detail.jsf?docId=MX394089143&_cid=P22-M4W3NX-41705-1 (accessed on 21 February 2025).
- García, M.V.; Poser, G.L.V.; Apel, M.; Tlatilpa, R.C.; Mendoza-Ruiz, A.; Villarreal, M.L.; Henriques, A.T.; Taketa, A.C. Anticholinesterase activity and identification of Huperzine A in three Mexican lycopods: Huperzia cuernavacensis, Huperzia dichotoma and Huperzia linifolia (Lycopodiaceae). Pak. J. Pharm. Sci. 2017, 30 (Suppl. 1), 235–239. [Google Scholar] [PubMed]
- Baskaran, P.; Kumari, A.; Ncube, B.; Van Staden, J. Acetylcholinesterase Inhibition and Antibacterial Activity of Mondia whitei Adventitious Roots and Ex Vitro-Grown Somatic Embryogenic Biomass. Front. Pharmacol. 2016, 7, 335. [Google Scholar] [CrossRef]
- Motolinía-Alcántara, E.A.; Castillo-Araiza, C.O.; Rodríguez-Monroy, M.; Román-Guerrero, A.; Cruz-Sosa, F. Engineering considerations to produce bioactive compounds from plant cell suspension culture in bioreactors. Plants 2021, 10, 2762. [Google Scholar] [CrossRef]
- Fett-Neto, A.G.; Zhang, W.Y.; Dicosmo, F. Kinetics of taxol production, growth, and nutrient uptake in cell suspensions of Taxus cuspidata. Biotechnol. Bioengineer. 1994, 44, 205–210. [Google Scholar] [CrossRef]
- Osuna, L.; Pereda-Miranda, R.; Villarreal, M.L. In vitro production of sedative galphimine B by cell suspension cultures of Galphimia glauca. Biotechnol. Lett. 2002, 24, 257–261. [Google Scholar] [CrossRef]
- Mavituna, F. Applications of plant biotechnology in industry and agriculture. In Recent Advances in Biotechnology; Vardar-Sukan, F., Sukan, S.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 209–226. [Google Scholar]
- Sánchez-Ramos, M.; Marquina-Bahena, S.; Alvarez, L.; Bernabé-Antonio, A.; Cabañas-García, E.; Román-Guerrero, A.; Cruz-Sosa, F. Obtaining 2,3-dihydrobenzofuran and 3-epilupeol from Ageratina pichinchensis (Kunth) R.King & Ho.Rob. cell cultures grown in shake flasks under photoperiod and darkness, and its scale-up to an airlift bioreactor for enhanced production. Molecules 2023, 28, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.; Tian, P.; Tan, T. Delineating biosynthesis of huperzine A, a plant-derived medicine for the treatment of Alzheimer’s disease. Biotechnol. Adv. 2022, 60, 108026. [Google Scholar] [CrossRef] [PubMed]
- Tomilova, S.V.; Globa, E.B.; Demidova, E.V.; Nosov, A.M. Secondary metabolism in Taxus spp. plant cell culture in vitro. Russ. J. Plant Physiol. 2023, 70, 227–240. [Google Scholar] [CrossRef]
- Cruz-Miranda, O.L.; Folch-Mallol, J.; Martínez-Morales, F.; Gesto-Borroto, R.; Villarreal, M.L.; Cardoso-Taketa, A. Identification of a Huperzine A-producing endophytic fungus from Phlegmariurus taxifolius. Mol. Biol. Rep. 2019, 47, 489–495. [Google Scholar] [CrossRef]
- Sagita, R.; Quax, W.J.; Haslinger, K. Current state and future directions of genetics and genomics of endophytic fungi for bioprospecting efforts. Front. Bioeng. Biotechnol. 2021, 9, 649906. [Google Scholar] [CrossRef]
- Ma, X.; Gang, D.R. In vitro production of huperzine A, a promising drug candidate for Alzheimer’s disease. Phytochemistry 2008, 69, 2022–2028. [Google Scholar] [CrossRef]
- Gamborg, O.L. The effects of amino acids and ammonium on the growth of plant cells in suspension culture. Plant Physiol. 1970, 45, 372–375. [Google Scholar] [CrossRef]
- Widholm, J.M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972, 4, 189–194. [Google Scholar] [CrossRef]
- Caspeta, L.; Quintero, R.; Villarreal, M.L. Novel Airlift Reactor Fitting for Hairy Root Cultures: Developmental and Performance Studies. Biotechnol. Progr. 2008, 21, 735–740. [Google Scholar] [CrossRef]
- Arias, M.; Aguirre, A.; Angarita, M.; Montoya, C.; Restrepo, J. Aspectos ingenieriles del cultivo in vitro de células vegetales para la producción de metabolitos secundarios. Dyna 2009, 76, 109–121. [Google Scholar]
- Carreño-Campos, C.; Arevalo-Villalobos, J.I.; Villarreal, M.L.; Ortiz-Caltempa, A.; Rosales-Mendoza, S. Establishment of the carrot-made LTB-syn antigen cell line in shake flask and airlift bioreactor cultures. Planta Med. 2022, 88, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Emerich, D.F. Cholinergic markers in Alzheimer disease. JAMA 1999, 282, 2208–2209. [Google Scholar] [CrossRef] [PubMed]


| Kinetic Parameters | 250 mL Flasks Culture | 2 L Bioreactor Culture |
|---|---|---|
| Day of maximum growth | 30 | 15 |
| Maximum biomass production | 8.17 ± 0.03 g/L DW | 16.70 ± 0.08 g/L DW |
| Growth rate (µ) | 0.045 day−1 | 0.062 day−1 |
| Duplication time (dt) | 15.40 days | 11.20 days |
| Day of maximum HupA production | 45 | 15 |
| Maximum HupA production | 2.03 μg/g DW | 2.48 μg/g DW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez Aguilar, R.d.C.; Rodríguez Salgado, T.; Cruz-Miranda, O.L.; Soto Díaz, A.U.; Zenil Rodríguez, A.; Bensaddek, L.; Carreño-Campos, C.; Villarreal, M.L.; Ortiz-Caltempa, A.; Cardoso-Taketa, A.T. Huperzine A Production and Acetylcholinesterase Inhibition by Phlegmariurus taxifolius Cell Suspension Culture: A Comparative Study in Flasks and an Airlift Bioreactor. Pharmaceuticals 2025, 18, 383. https://doi.org/10.3390/ph18030383
Pérez Aguilar RdC, Rodríguez Salgado T, Cruz-Miranda OL, Soto Díaz AU, Zenil Rodríguez A, Bensaddek L, Carreño-Campos C, Villarreal ML, Ortiz-Caltempa A, Cardoso-Taketa AT. Huperzine A Production and Acetylcholinesterase Inhibition by Phlegmariurus taxifolius Cell Suspension Culture: A Comparative Study in Flasks and an Airlift Bioreactor. Pharmaceuticals. 2025; 18(3):383. https://doi.org/10.3390/ph18030383
Chicago/Turabian StylePérez Aguilar, Rocío del Carmen, Talia Rodríguez Salgado, Olga Lidia Cruz-Miranda, Alexis Uriel Soto Díaz, Ariadna Zenil Rodríguez, Lamine Bensaddek, Christian Carreño-Campos, María Luisa Villarreal, Anabel Ortiz-Caltempa, and Alexandre Toshirrico Cardoso-Taketa. 2025. "Huperzine A Production and Acetylcholinesterase Inhibition by Phlegmariurus taxifolius Cell Suspension Culture: A Comparative Study in Flasks and an Airlift Bioreactor" Pharmaceuticals 18, no. 3: 383. https://doi.org/10.3390/ph18030383
APA StylePérez Aguilar, R. d. C., Rodríguez Salgado, T., Cruz-Miranda, O. L., Soto Díaz, A. U., Zenil Rodríguez, A., Bensaddek, L., Carreño-Campos, C., Villarreal, M. L., Ortiz-Caltempa, A., & Cardoso-Taketa, A. T. (2025). Huperzine A Production and Acetylcholinesterase Inhibition by Phlegmariurus taxifolius Cell Suspension Culture: A Comparative Study in Flasks and an Airlift Bioreactor. Pharmaceuticals, 18(3), 383. https://doi.org/10.3390/ph18030383






