Puerarin Targets HIF-1α to Modulate Hypoxia-Related Sphingolipid Metabolism in Diabetic Hepatopathy via the SPTLC2/Ceramide Pathway
Abstract
1. Introduction
2. Results
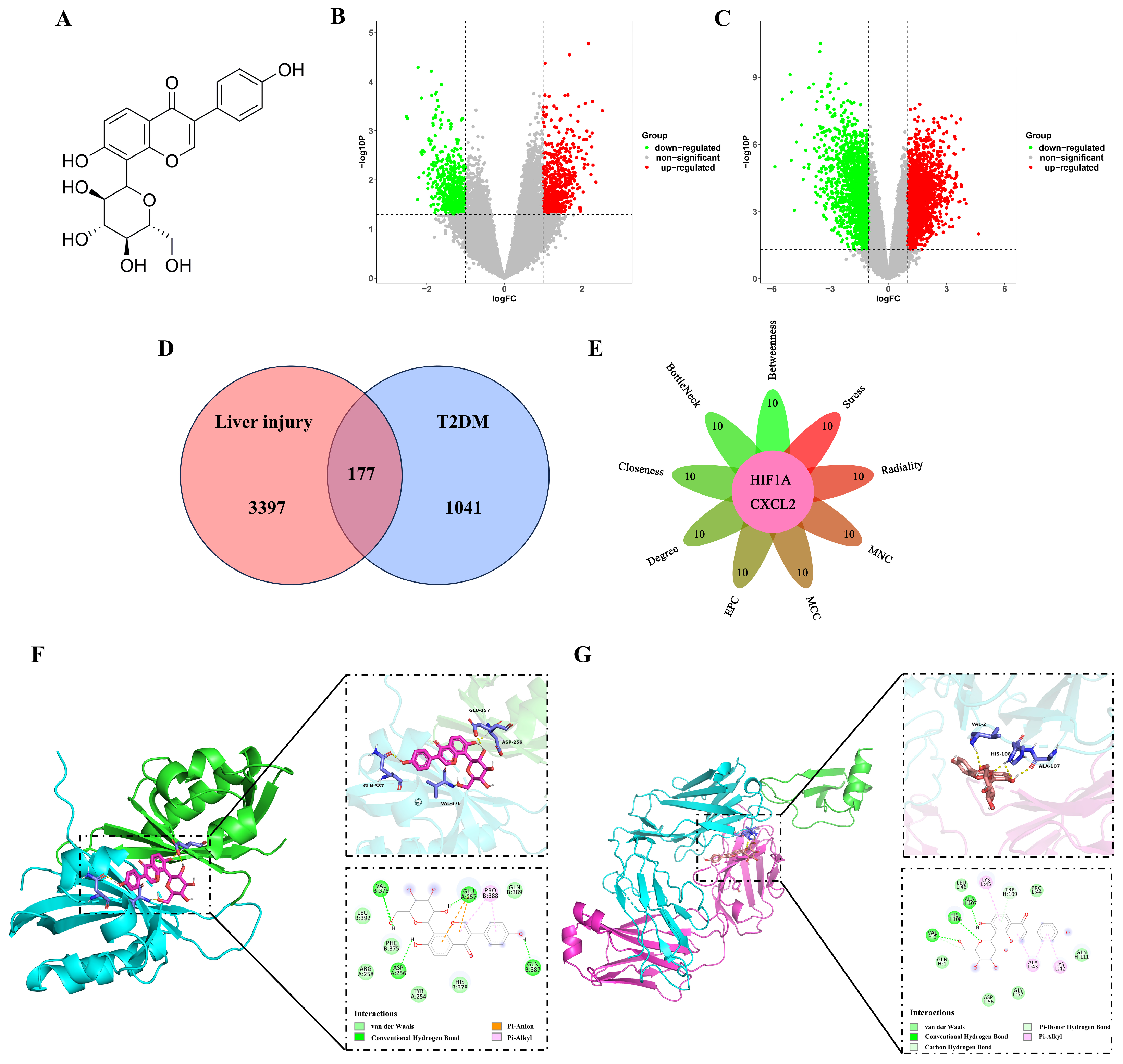
2.1. Analysis of the Hub Genes Between T2DM and Liver Injury Using Bioinformatics
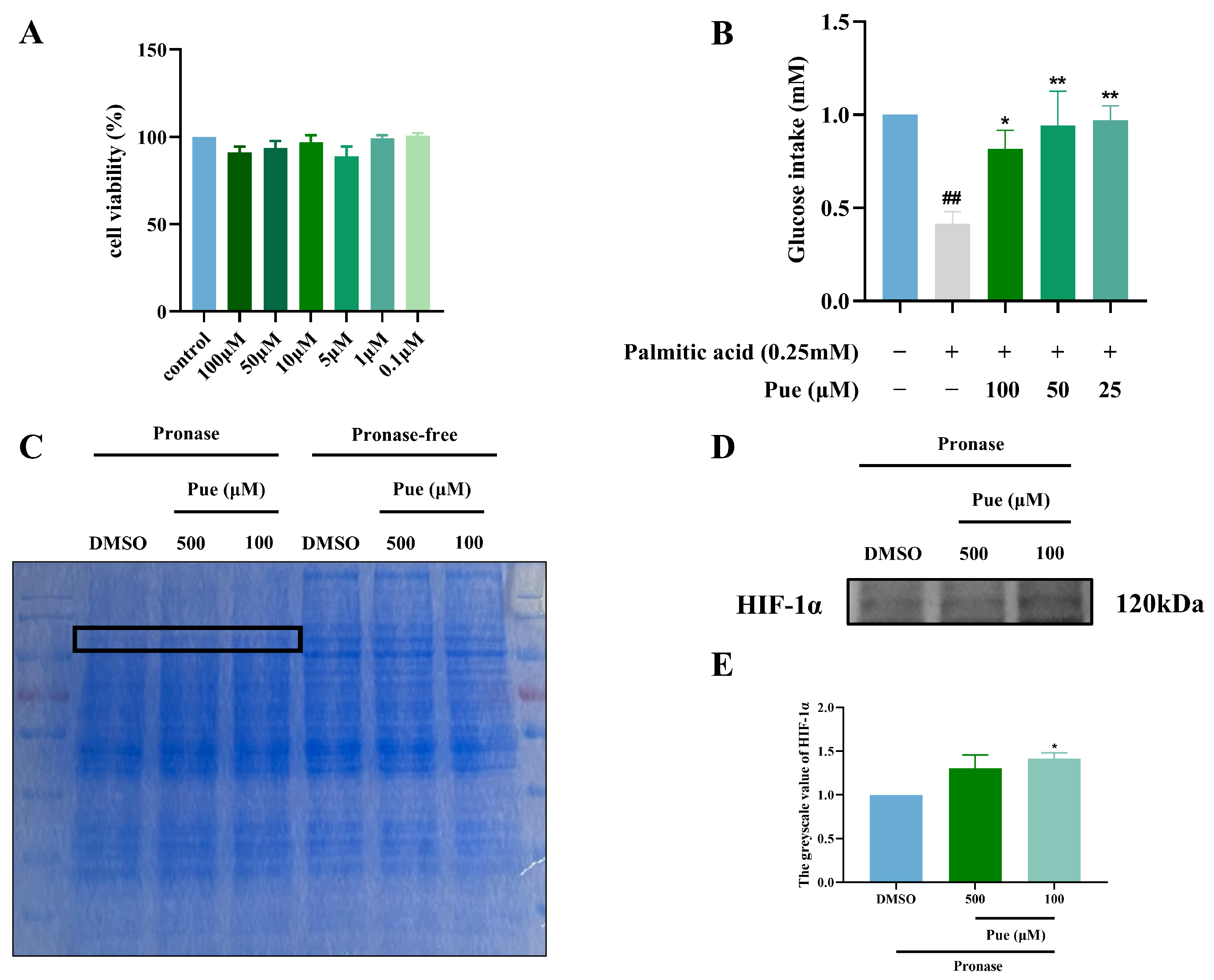
2.2. Puerarin Increases Glucose Intake in Insulin-Resistant Hepatocytes HepG2
2.3. Puerarin Exhibits a Binding Affinity for HIF-1α
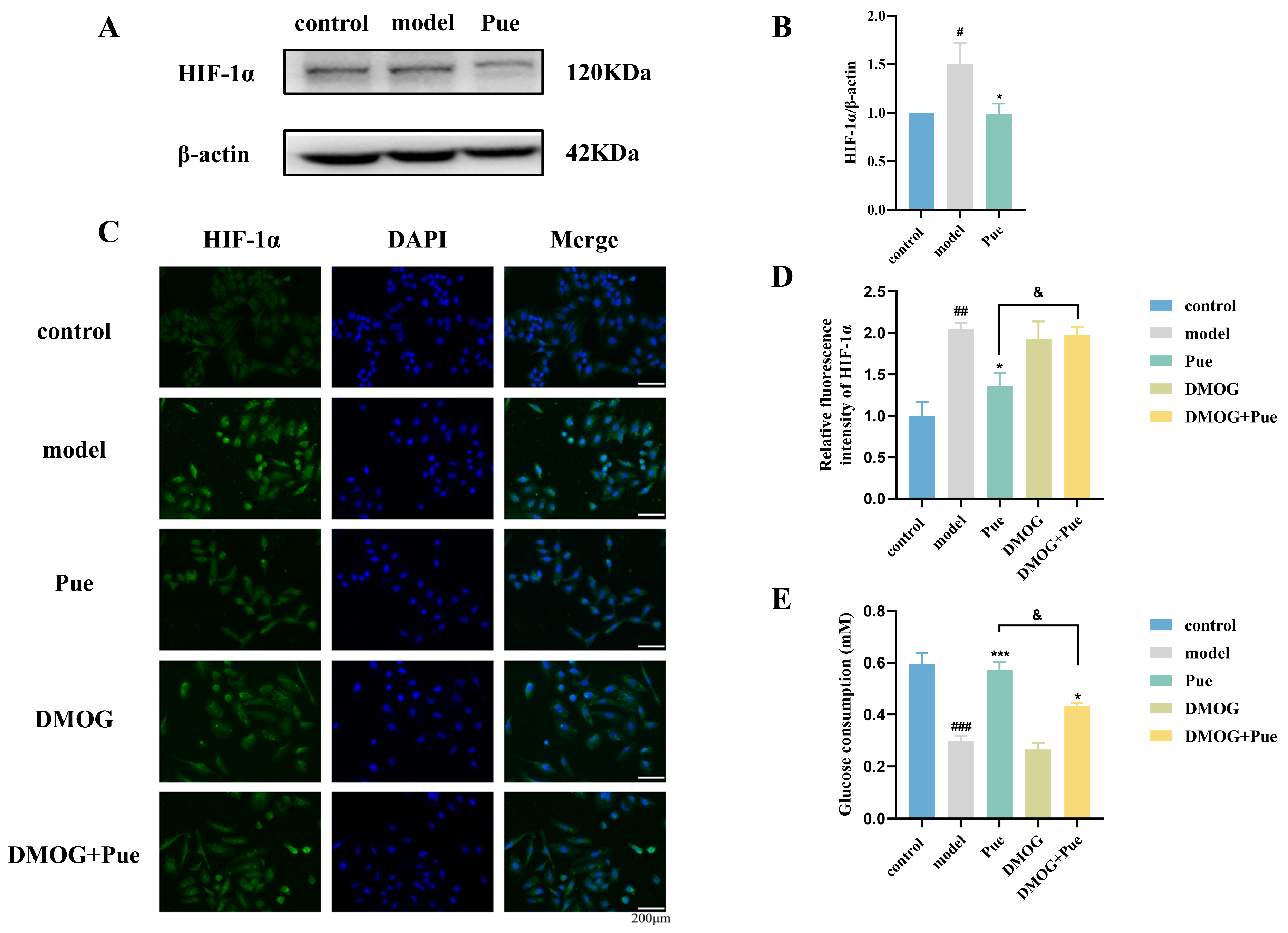
2.4. Inhibition of HIF-1α by Puerarin Ameliorates Insulin Resistance in HepG2 Cells
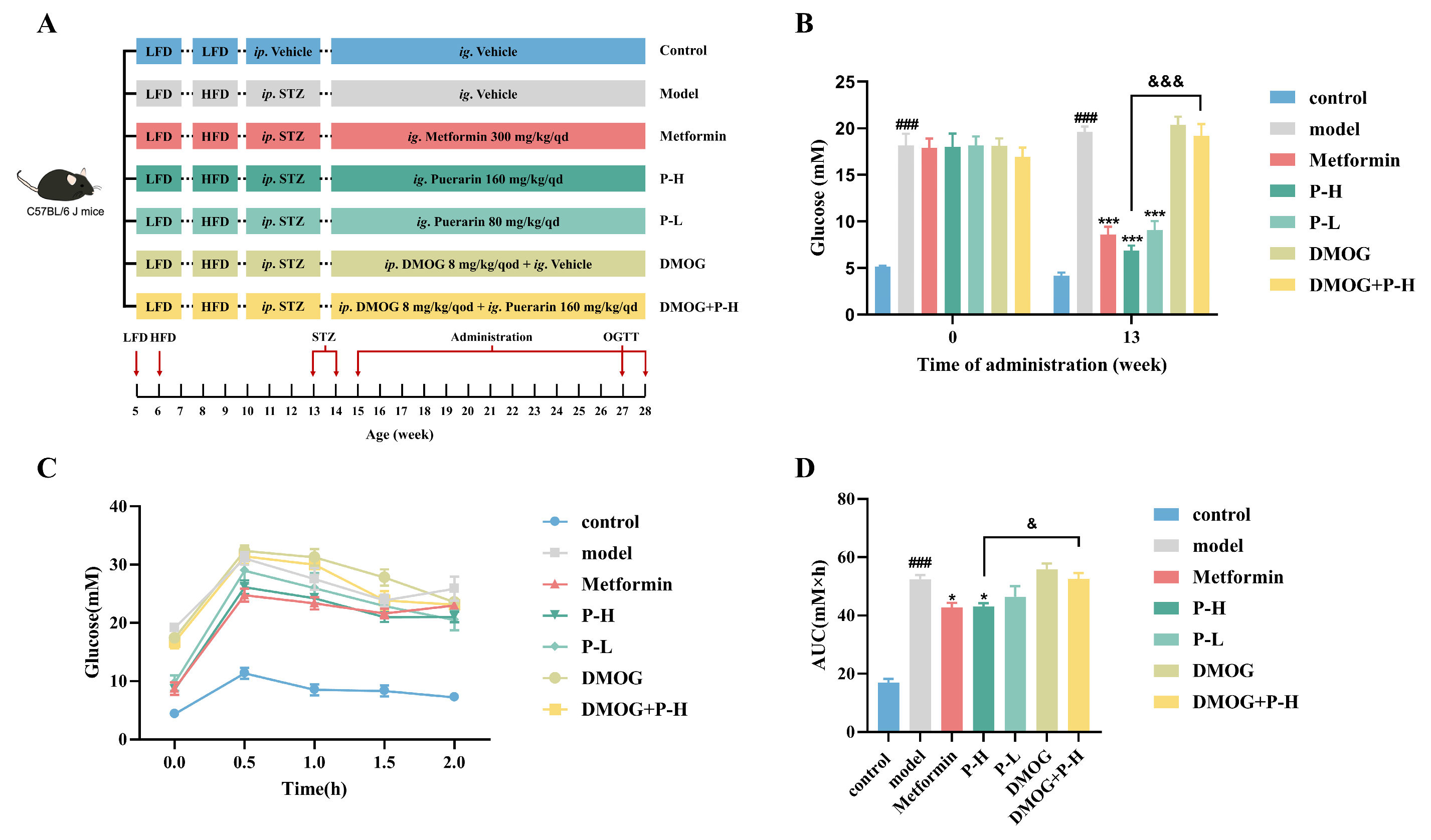
2.5. Effect of Puerarin on Fasting Blood Glucose and Oral Glucose Tolerance in Diabetic Mice, Reversed by HIF-1α Stabilizer DMOG
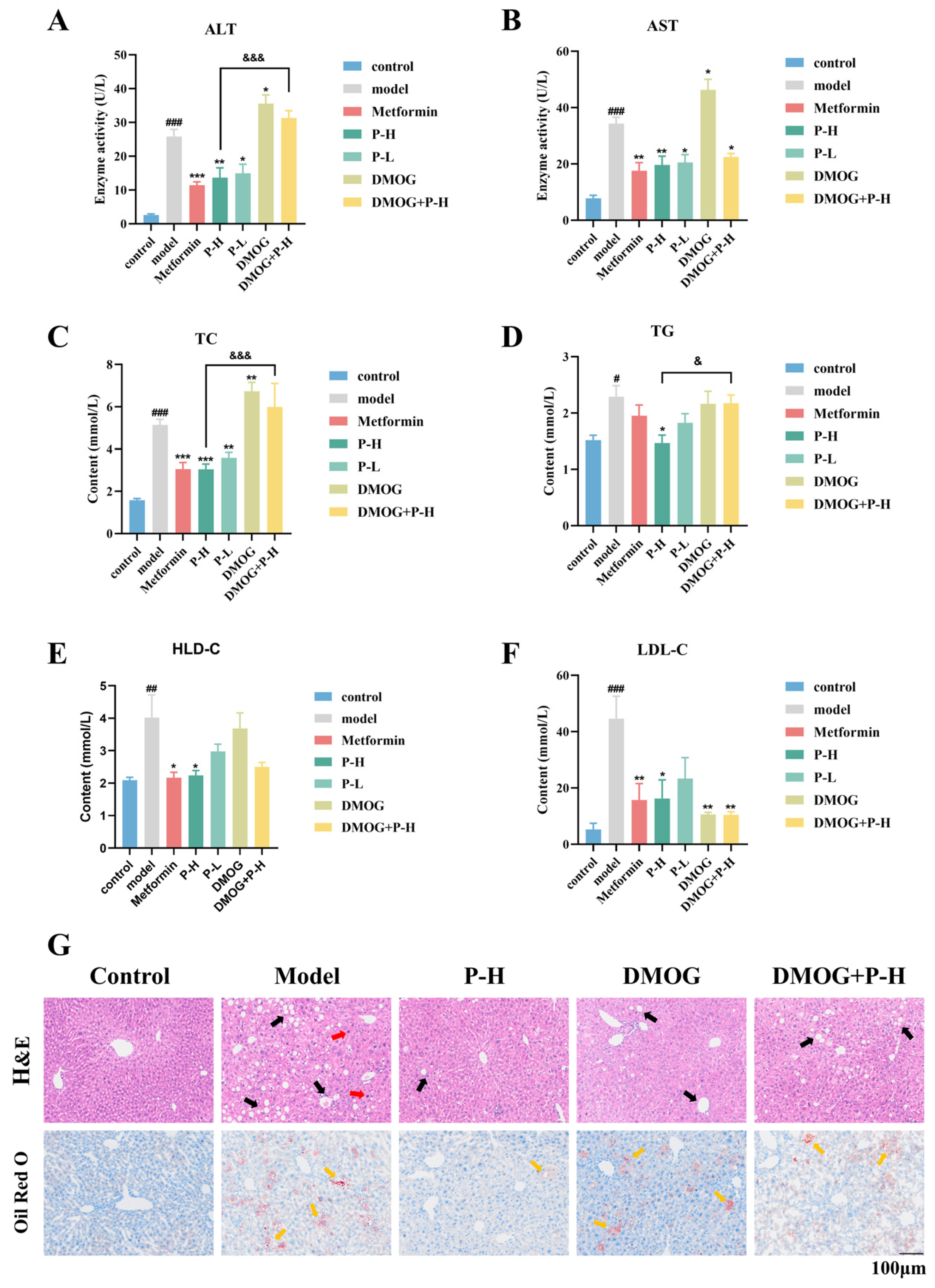
2.6. The Hepatoprotective Effects of Puerarin in Diabetic Mice Are Reversed by the HIF-1α Stabilizer DMOG
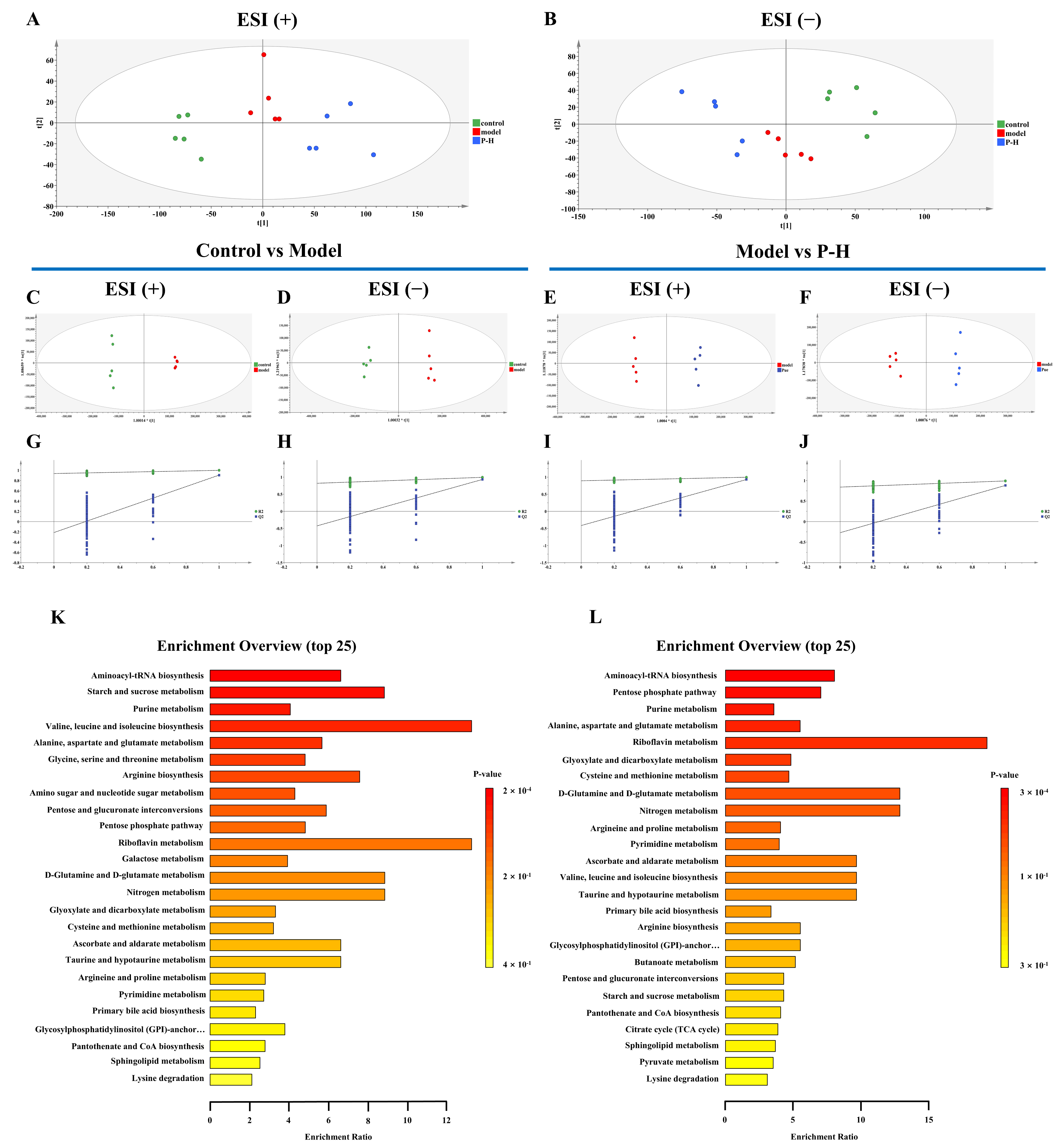
2.7. Metabolic Profiling Reveals Key Metabolic Pathways in the Treatment of Diabetic Mice with Puerarin
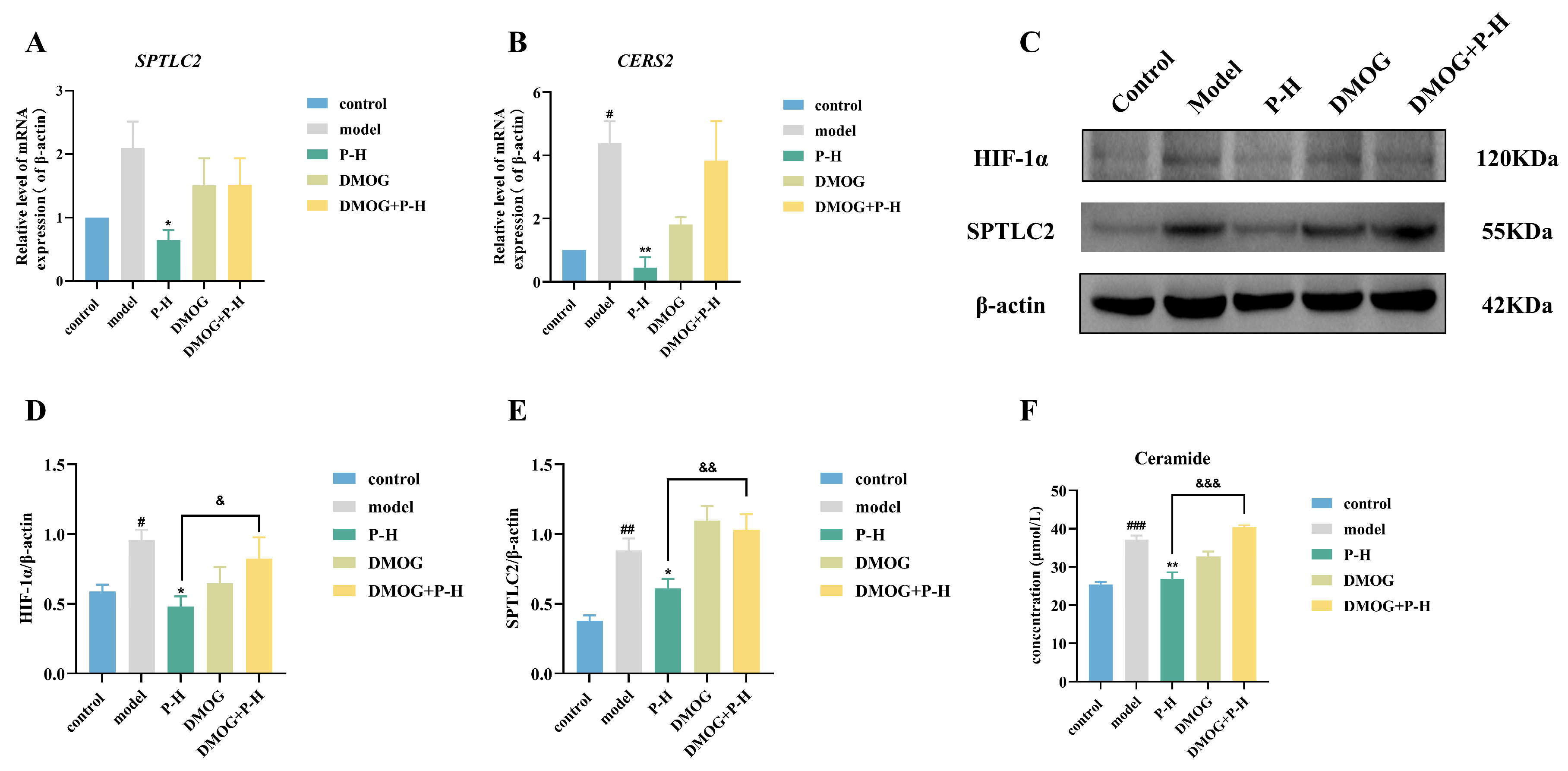
2.8. Puerarin Inhibits Ceramide Production Through the HIF-1α/SPTLC2 Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Data Preparation for Network Pharmacology
4.3. Molecular Docking
4.4. Cell Culture and Viability Analysis
4.5. Preparation of Palmitic Acid (PA) and Establishment of Insulin-Resistant HepG2 Cell Model
4.6. Detection of Glucose Intake
4.7. Drug Affinity Responsive Target Stability
4.8. Immunofluorescence
4.9. Experimental Grouping and Drug Administration
4.10. Fasting Blood Glucose and Oral Glucose Tolerance Test
4.11. Biochemical Analyses
4.12. Histopathologic Examinations
4.13. Metabolomics Analysis
4.14. Real-Time Quantitative PCR (RT-qPCR)
4.15. Western Blot Analysis
4.16. Statistical Analysis
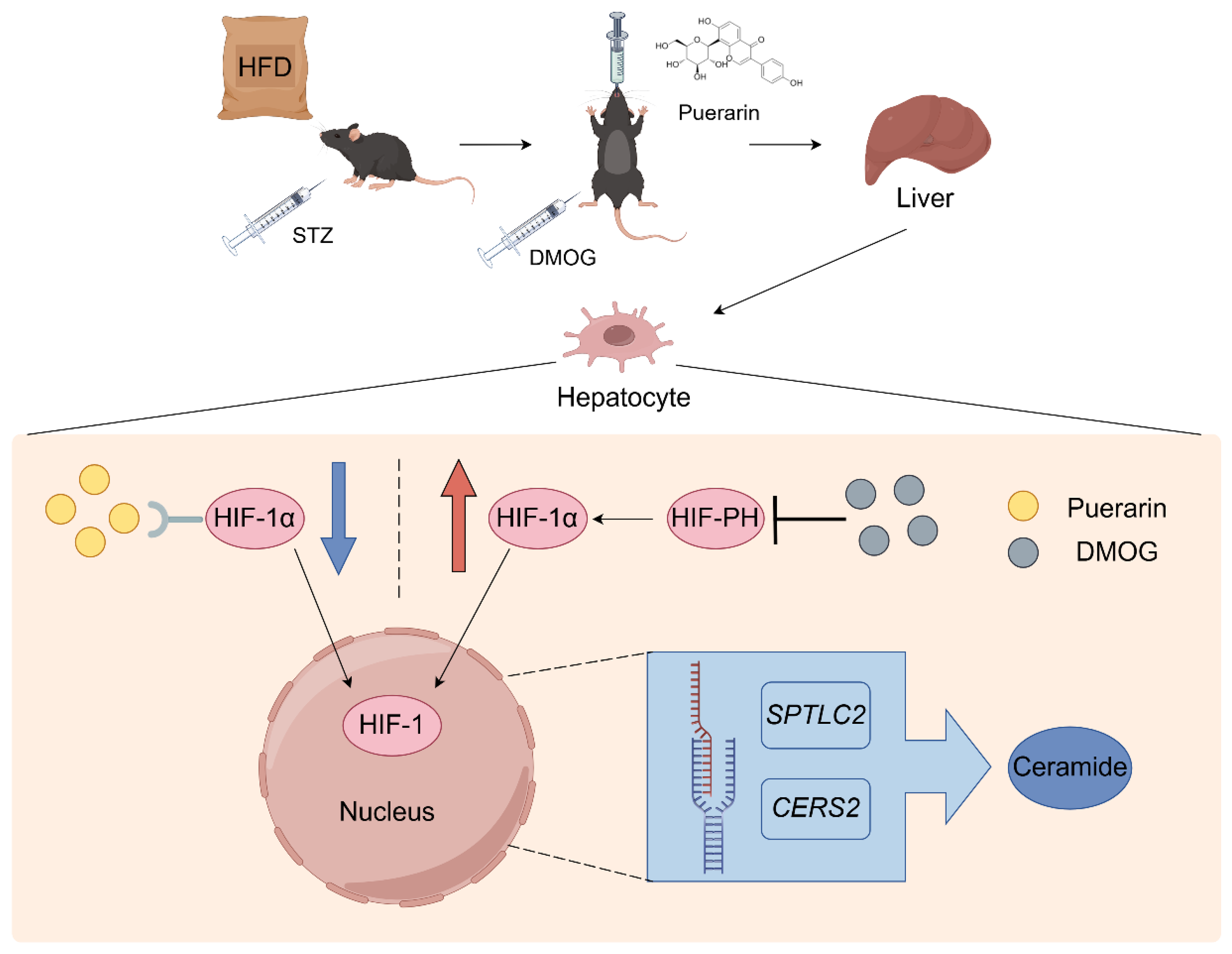
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| AUC | Area under curve |
| BSA | Bovine serum albumin |
| CERS2 | Ceramide synthase 2 |
| DARTS | Drug affinity responsive target stability |
| DEGs | Differentially Expressed Genes |
| DMOG | Dimethyloxallyl glycine |
| FBG | Fasting blood glucose |
| GO | Gene Ontology |
| H&E | Histopathologic examinations |
| HDL-C | High-density lipoprotein cholesterol |
| HFD | High-fat diet |
| HIF-1α | Hypoxia inducible factor-1α |
| IR | Insulin resistance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDL-C | Low-density lipoprotein cholesterol |
| OGTT | Oral glucose tolerance test |
| PA | Palmitic acid |
| PPI | Protein-Protein Interaction Networks |
| Pue | Puerarin |
| PVDF | Polyvinylidene fluoride |
| RT | Room temperature |
| ROS | reactive oxygen species |
| SPTLC2 | Serine palmitoyltransferase 2 |
| STZ | Streptozotocin |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglyceride |
| UPLC | Ultra-high performance liquid chromatography |
References
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, Z.H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the incidence of diabetes mellitus: Results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef]
- Sumida, Y.; Shima, T.; Mitsumoto, Y.; Katayama, T.; Umemura, A.; Yamaguchi, K.; Itoh, Y.; Yoneda, M.; Okanoue, T. Epidemiology: Pathogenesis, and Diagnostic Strategy of Diabetic Liver Disease in Japan. Int. J. Mol. Sci. 2020, 21, 4337. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Cheng, X.F.; Liu, Y.; Lv, Q.Z.; Liu, G.L.; Zhang, J.G.; Li, X.Y. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front. Pharmacol. 2018, 9, 1566. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.; Nazratun Nafizah, A.H.; Zariyantey, A.H.; Budin, S.B. Mechanisms of Diabetes-Induced Liver Damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132–e141. [Google Scholar] [CrossRef]
- Honma, M.; Sawada, S.; Ueno, Y.; Murakami, K.; Yamada, T.; Gao, J.; Kodama, S.; Izumi, T.; Takahashi, K.; Tsukita, S.; et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int. J. Obes. 2018, 42, 1544–1555. [Google Scholar] [CrossRef]
- Anavi, S.; Harmelin, N.B.; Madar, Z.; Tirosh, O. Oxidative stress impairs HIF1α activation: A novel mechanism for increased vulnerability of steatotic hepatocytes to hypoxic stress. Free Radic. Biol. Med. 2012, 52, 1531–1542. [Google Scholar] [CrossRef]
- Xie, L.; Wang, Q.; Ma, J.; Zeng, Y.E. Hypoxia-induced reactive oxygen species in organ and tissue fibrosis. Biocell 2022, 47, 261–267. [Google Scholar] [CrossRef]
- Gabryelska, A.; Karuga, F.F.; Szmyd, B.; Białasiewicz, P. HIF-1α as a Mediator of Insulin Resistance, T2DM, and Its Complications: Potential Links With Obstructive Sleep Apnea. Front. Physiol. 2020, 11, 1035. [Google Scholar] [CrossRef]
- Lee, Y.S.; Riopel, M.; Cabrales, P.; Bandyopadhyay, G.K. Hepatocyte-specific HIF-1α ablation improves obesity-induced glucose intolerance by reducing first-pass GLP-1 degradation. Sci. Adv. 2019, 5, eaaw4176. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.J.; Xie, C.; Jiang, C. The role of hypoxia-inducible factors in metabolic diseases. Nat. Rev. Endocrinol. 2018, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, T.; Sang, H. The role of hypoxia-inducible factor 1α in hepatic lipid metabolism. J. Mol. Med. 2023, 101, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhao, X.; Li, S.; Zhou, H.; Chen, W.; Ren, L.; Zhou, X.; Zhang, H.; Shi, R. Hypoxia induces dysregulation of lipid metabolism in HepG2 cells via activation of HIF-2α. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2014, 34, 1427–1441. [Google Scholar] [CrossRef]
- Sun, R.; Deng, X.; Zhang, D.; Xie, F.; Wang, D.; Wang, J.; Tavallaie, M.S.; Jiang, F.; Fu, L. Anti-diabetic potential of Pueraria lobata root extract through promoting insulin signaling by PTP1B inhibition. Bioorganic Chem. 2019, 87, 12–15. [Google Scholar] [CrossRef]
- Kang, A.W.; Sun, C.; Li, H.T.; Zhong, K.; Zeng, X.H.; Gu, Z.F.; Li, B.Q.; Zhang, X.N.; Gao, J.L.; Chen, T.X. Puerarin extends the lifespan of Drosophila melanogaster by activating autophagy. Food Funct. 2023, 14, 2149–2161. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Wang, S.; Gao, P.; Dai, L. A comprehensive review on Pueraria: Insights on its chemistry and medicinal value. Biomed. Pharmacother. 2020, 131, 110734. [Google Scholar] [CrossRef]
- Li, Z.; Shangguan, Z.; Liu, Y.; Wang, J.; Li, X.; Yang, S.; Liu, S. Puerarin protects pancreatic β-cell survival via PI3K/Akt signaling pathway. J. Mol. Endocrinol. 2014, 53, 71–79. [Google Scholar] [CrossRef]
- Xiong, F.L.; Sun, X.H.; Gan, L.; Yang, X.L.; Xu, H.B. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur. J. Pharmacol. 2006, 529, 1–7. [Google Scholar] [CrossRef]
- Yang, L.; Yao, D.; Yang, H.; Wei, Y.; Peng, Y.; Ding, Y.; Shu, L. Puerarin Protects Pancreatic β-Cells in Obese Diabetic Mice via Activation of GLP-1R Signaling. Mol. Endocrinol. 2016, 30, 361–371. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Wang, Q.; Huang, H.; Zhao, Y.; Du, F.; Chen, Y.; Shen, J.; Luo, H.; Zhao, Q.; et al. Pueraria lobata starch regulates gut microbiota and alleviates high-fat high-cholesterol diet induced non-alcoholic fatty liver disease in mice. Food Res. Int. 2022, 157, 111401. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, J.; Hou, J.; Hui, M.; Qi, H.; Lei, T.; Zhang, X.; Zhao, L.; Du, H. Induction of autophagy via the PI3K/Akt/mTOR signaling pathway by Pueraria flavonoids improves non-alcoholic fatty liver disease in obese mice. Biomed. Pharmacother. 2023, 157, 114005. [Google Scholar] [CrossRef] [PubMed]
- Engel, H.; Xiong, L.; Reichenberger, M.A.; Germann, G.; Roth, C.; Hirche, C. Rodent models of diet-induced type 2 diabetes mellitus: A literature review and selection guide. Diabetes Metab. Syndr. 2019, 13, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Xiao, L.; Lai, B.; Yao, Q.; Liu, J.; Yang, H.; Wang, N. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway. Br. J. Pharmacol. 2018, 175, 4218–4228. [Google Scholar] [CrossRef]
- Kumar, G.V.N.; Hoshitsuki, K.; Rathod, S.; Ramsey, M.J.; Kokai, L.; Kershaw, E.E.; Xie, W.; Fernandez, C.A. Mechanistic studies of PEG-asparaginase-induced liver injury and hepatic steatosis in mice. Acta Pharm. Sin. B 2021, 11, 3779–3790. [Google Scholar] [CrossRef]
- Hoofnagle, A.N.; Vaisar, T.; Mitra, P.; Chait, A. HDL lipids and insulin resistance. Curr. Diabetes Rep. 2010, 10, 78–86. [Google Scholar] [CrossRef]
- Berk, P.D.; Verna, E.C. Nonalcoholic Fatty Liver Disease: Lipids and Insulin Resistance. Clin. Liver Dis. 2016, 20, 245–262. [Google Scholar] [CrossRef]
- Ng, M.L.; Wadham, C.; Sukocheva, O.A. The role of sphingolipid signalling in diabetes-associated pathologies (Review). Int. J. Mol. Med. 2017, 39, 243–252. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, S.; Funcke, J.B.; Straub, L.G.; Lin, Q.; Zhao, S.; Joung, C.; Zhang, Z.; Kim, D.S.; Li, N.; et al. PAQR4 regulates adipocyte function and systemic metabolic health by mediating ceramide levels. Nat. Metab. 2024, 6, 1347–1366. [Google Scholar] [CrossRef]
- Lallement, J.; Raho, I.; Merlen, G.; Rainteau, D.; Croyal, M.; Schiffano, M.; Kassis, N.; Doignon, I.; Soty, M.; Lachkar, F.; et al. Hepatic deletion of serine palmitoyl transferase 2 impairs ceramide/sphingomyelin balance, bile acids homeostasis and leads to liver damage in mice. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2023, 1868, 159333. [Google Scholar] [CrossRef]
- Ren, Y.S.; Li, H.L.; Piao, X.H.; Yang, Z.Y.; Wang, S.M.; Ge, Y.W. Drug affinity responsive target stability (DARTS) accelerated small molecules target discovery: Principles and application. Biochem. Pharmacol. 2021, 194, 114798. [Google Scholar] [CrossRef] [PubMed]
- Kucejova, B.; Sunny, N.E.; Nguyen, A.D.; Hallac, R.; Fu, X.; Peña-Llopis, S.; Mason, R.P.; Deberardinis, R.J.; Xie, X.J.; Debose-Boyd, R.; et al. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene 2011, 30, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Xiang, D.; Wu, D.; Liu, C.; Fang, Y.; Chen, P.; Hu, Y.P. Hepatic PHD2/HIF-1α axis is involved in postexercise systemic energy homeostasis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 4670–4680. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.B.; Zheng, X. Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 2021, 64, 709–716. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Scherer, P.E. Lowering ceramides to overcome diabetes. Science 2019, 365, 319–320. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, G.; Yan, Y.; Zhang, S.Y.; Dong, Y.; Zhang, Y.; Zhang, X.; Liu, H.; Zhang, Z.; Jiang, C.; et al. Disruption of adipocyte HIF-1α improves atherosclerosis through the inhibition of ceramide generation. Acta Pharm. Sin. B 2022, 12, 1899–1912. [Google Scholar] [CrossRef]
- Kim, G.T.; Devi, S.; Sharma, A.; Cho, K.H.; Kim, S.J.; Kim, B.R.; Kwon, S.H.; Park, T.S. Upregulation of the serine palmitoyltransferase subunit SPTLC2 by endoplasmic reticulum stress inhibits the hepatic insulin response. Exp. Mol. Med. 2022, 54, 573–584. [Google Scholar] [CrossRef]
- Teng, W.; Li, Y.; Du, M.; Lei, X.; Xie, S.; Ren, F. Sulforaphane Prevents Hepatic Insulin Resistance by Blocking Serine Palmitoyltransferase 3-Mediated Ceramide Biosynthesis. Nutrients 2019, 11, 1185. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, C.; Jin, H.; Wu, Z.; Lu, J.; Zhang, Z.; Sun, C.; Shan, Q.; Wu, D.; et al. Hepatocyte-specific deletion of LASS2 protects against diet-induced hepatic steatosis and insulin resistance. Free Radic. Biol. Med. 2018, 120, 330–341. [Google Scholar] [CrossRef]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A liver-derived secretory protein, selenoprotein P, causes insulin resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, L.; Wink, S.; Herpers, B.; Benedetti, G.; Hadi, M.; de Bont, H.; Groothuis, G.; Luijten, M.; Danen, E.; de Graauw, M.; et al. Drug-induced endoplasmic reticulum and oxidative stress responses independently sensitize toward TNFα-mediated hepatotoxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 140, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Fouda, K.; Mohamed, R.S. Molecular docking and in vivo protective effects of okra (Abelmoschus esculentus) against metabolic dysfunction in high-fat, high-sodium diet-fed rats. Food Funct. 2024, 15, 3566–3582. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Li, M.; Liu, B.; Xian, M.; Wang, S.; Liu, P. Bioinformatics combined with network pharmacology and experimental validation to identify key biomarkers of hepatocellular carcinoma and corresponding compounds in Radix Astragali and Pueraria Mirifica. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024. [Google Scholar] [CrossRef]
- Xiao, H.; Sun, X.; Lin, Z.; Yang, Y.; Zhang, M.; Xu, Z.; Liu, P.; Liu, Z.; Huang, H. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm. Sin. B 2022, 12, 2887–2904. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, H.; Wang, W.; Guan, S.; Yi, J.; Chu, L. Paeoniflorin suppresses lipid accumulation and alleviates insulin resistance by regulating the Rho kinase/IRS-1 pathway in palmitate-induced HepG2Cells. Biomed. Pharmacother. 2017, 90, 361–367. [Google Scholar] [CrossRef]
- Dubowski, K.M. An o-toluidine method for body-fluid glucose determination. Clin. Chem. 2008, 54, 1919–1920. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Fan, D. Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 2019, 10, 2538–2551. [Google Scholar] [CrossRef]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Models Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef]
- Pan, L.; Li, Z.; Wang, Y.; Zhang, B.; Liu, G.; Liu, J. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian Decoction in rats with type 2 diabetes mellitus. J. Ethnopharmacol. 2020, 258, 112842. [Google Scholar] [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience 2013, 2, 13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Lai, W.; Chen, H.; Cao, D.; Zhang, B.; Wang, F.; Xian, M.; Wang, S. Puerarin Targets HIF-1α to Modulate Hypoxia-Related Sphingolipid Metabolism in Diabetic Hepatopathy via the SPTLC2/Ceramide Pathway. Pharmaceuticals 2025, 18, 398. https://doi.org/10.3390/ph18030398
Cai M, Lai W, Chen H, Cao D, Zhang B, Wang F, Xian M, Wang S. Puerarin Targets HIF-1α to Modulate Hypoxia-Related Sphingolipid Metabolism in Diabetic Hepatopathy via the SPTLC2/Ceramide Pathway. Pharmaceuticals. 2025; 18(3):398. https://doi.org/10.3390/ph18030398
Chicago/Turabian StyleCai, Mangui, Wenxi Lai, Huien Chen, Dongmin Cao, Boyan Zhang, Feng Wang, Minghua Xian, and Shumei Wang. 2025. "Puerarin Targets HIF-1α to Modulate Hypoxia-Related Sphingolipid Metabolism in Diabetic Hepatopathy via the SPTLC2/Ceramide Pathway" Pharmaceuticals 18, no. 3: 398. https://doi.org/10.3390/ph18030398
APA StyleCai, M., Lai, W., Chen, H., Cao, D., Zhang, B., Wang, F., Xian, M., & Wang, S. (2025). Puerarin Targets HIF-1α to Modulate Hypoxia-Related Sphingolipid Metabolism in Diabetic Hepatopathy via the SPTLC2/Ceramide Pathway. Pharmaceuticals, 18(3), 398. https://doi.org/10.3390/ph18030398




