Mechanistic Insights into the Anticancer Potential of Asparagus racemosus Willd. Against Triple-Negative Breast Cancer: A Network Pharmacology and Experimental Validation Study
Abstract
:1. Introduction
2. Results
2.1. Screening of Phytochemical Compounds of A. racemosus
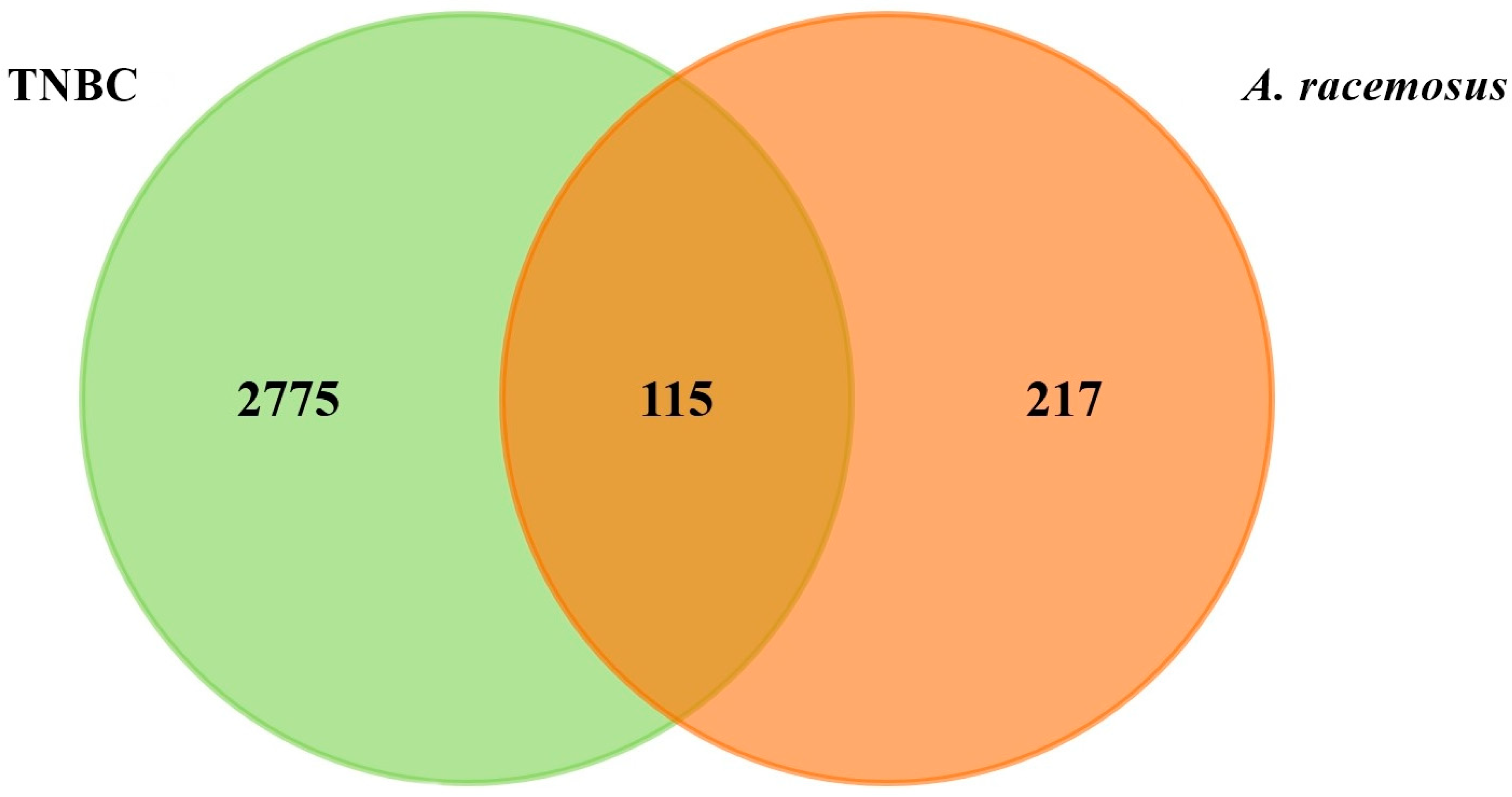
2.2. Prediction of Targets and Screening for Potential Targets
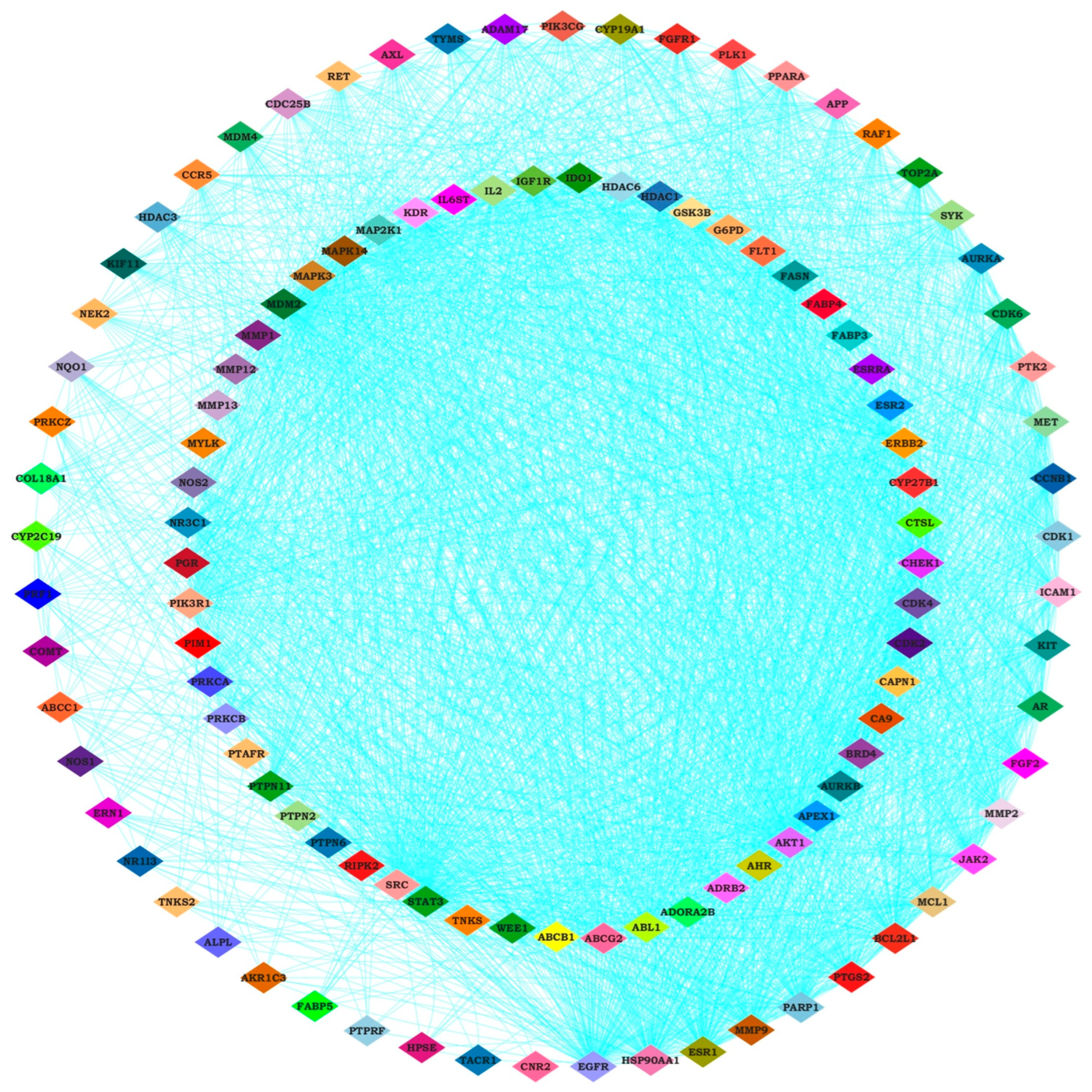
2.3. Identifying Hub Genes and Construction of Compounds-Disease Common Target Network
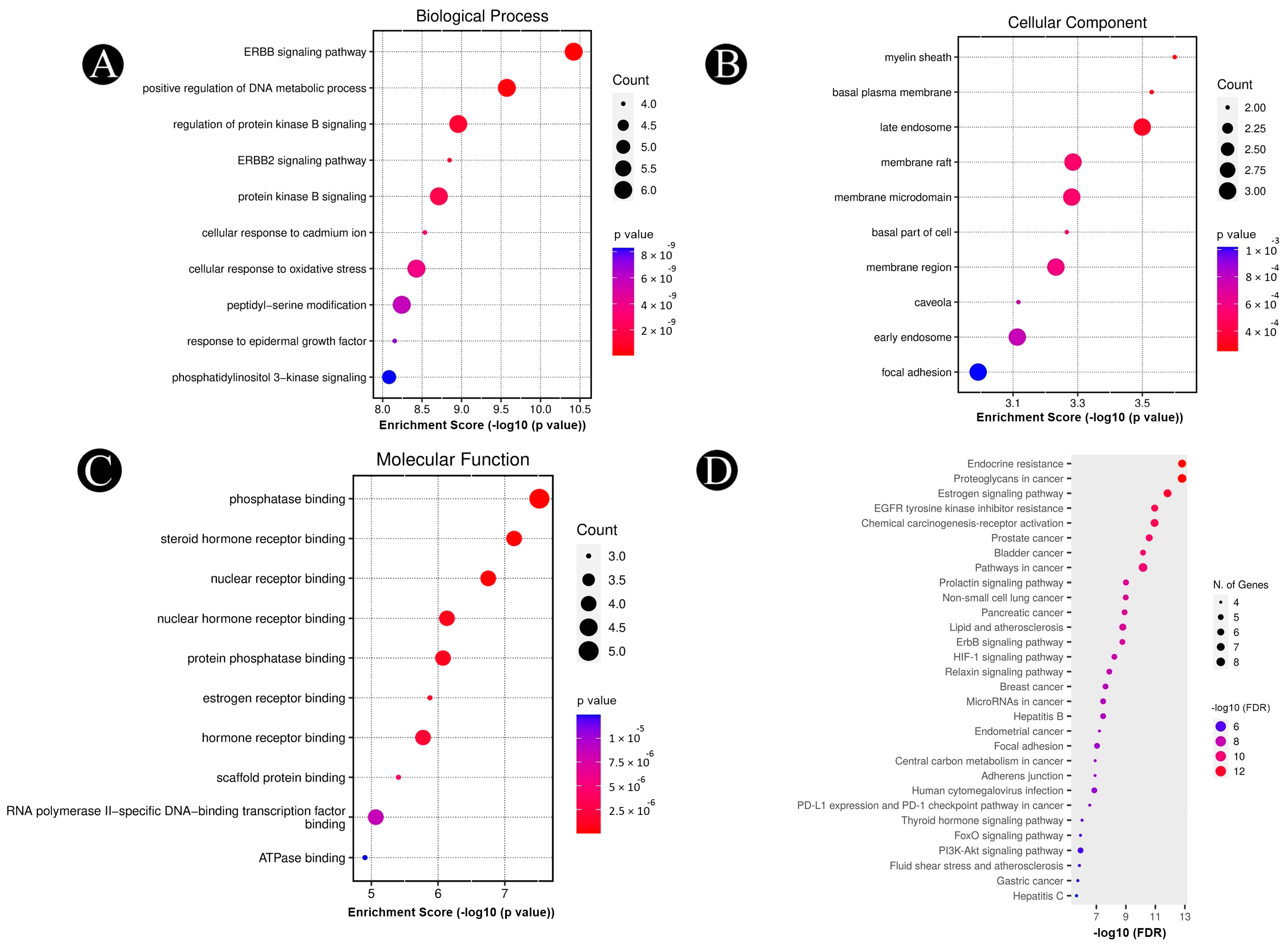
2.4. Analysis of Pathways and Functional Enrichment
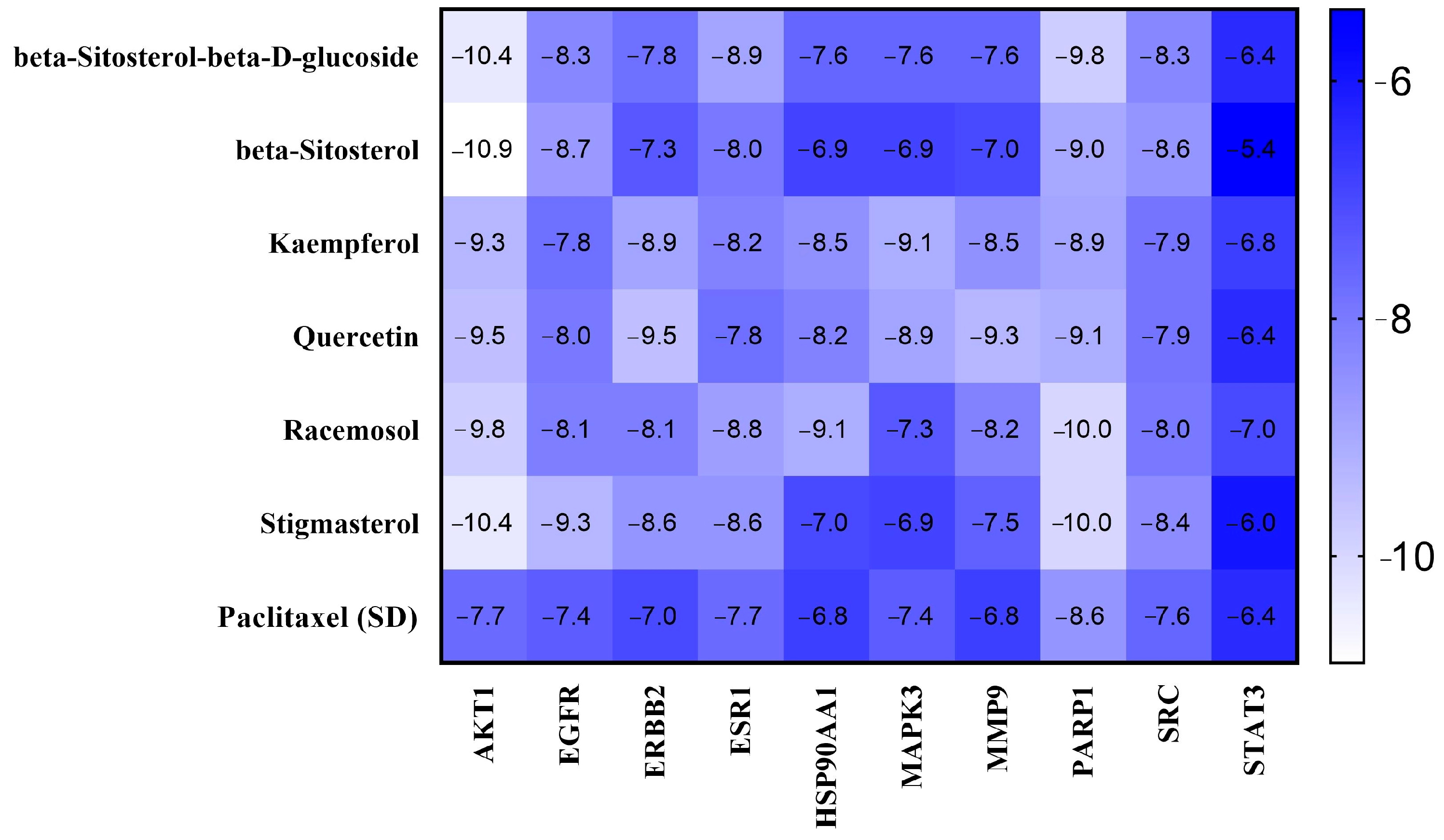
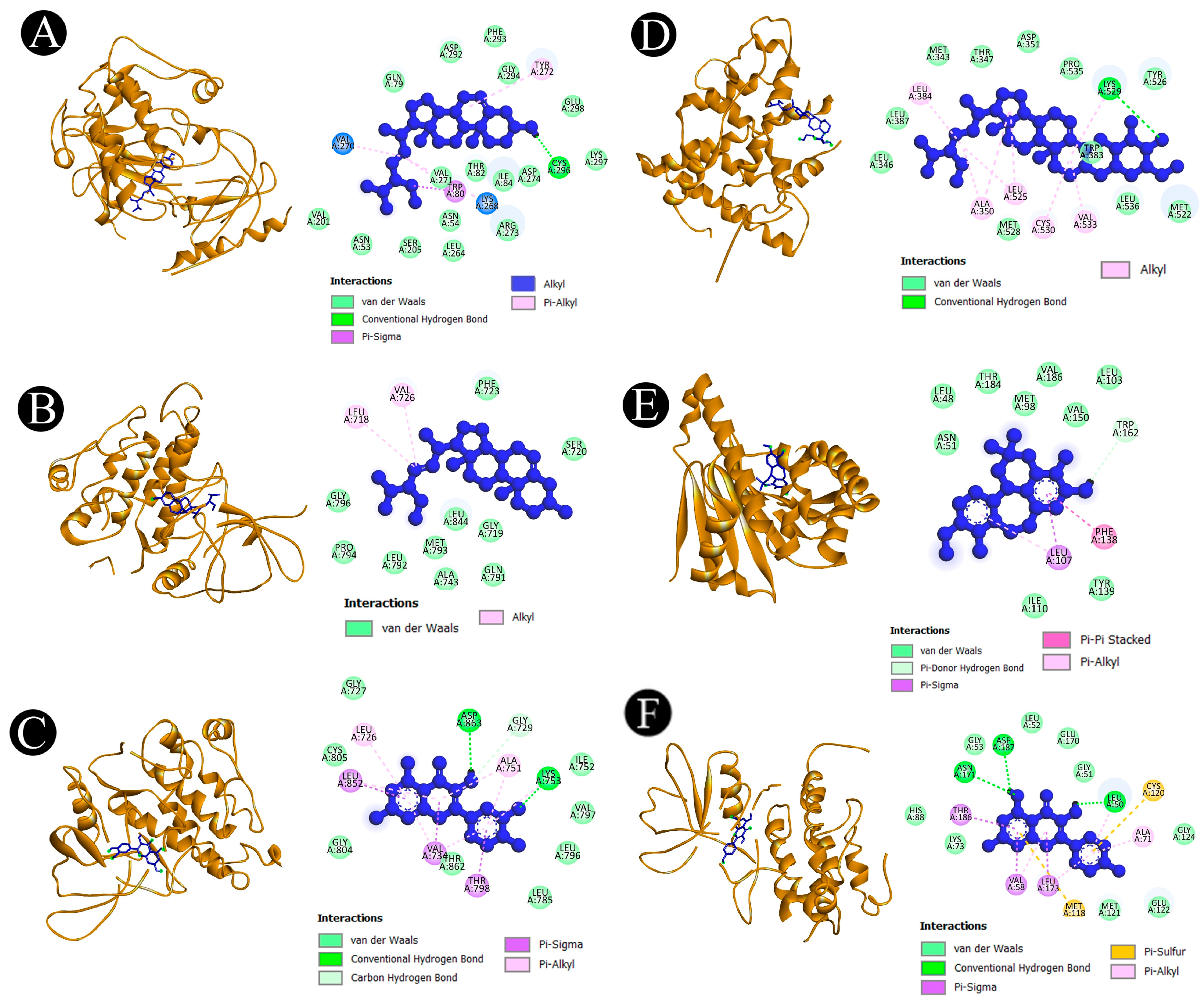
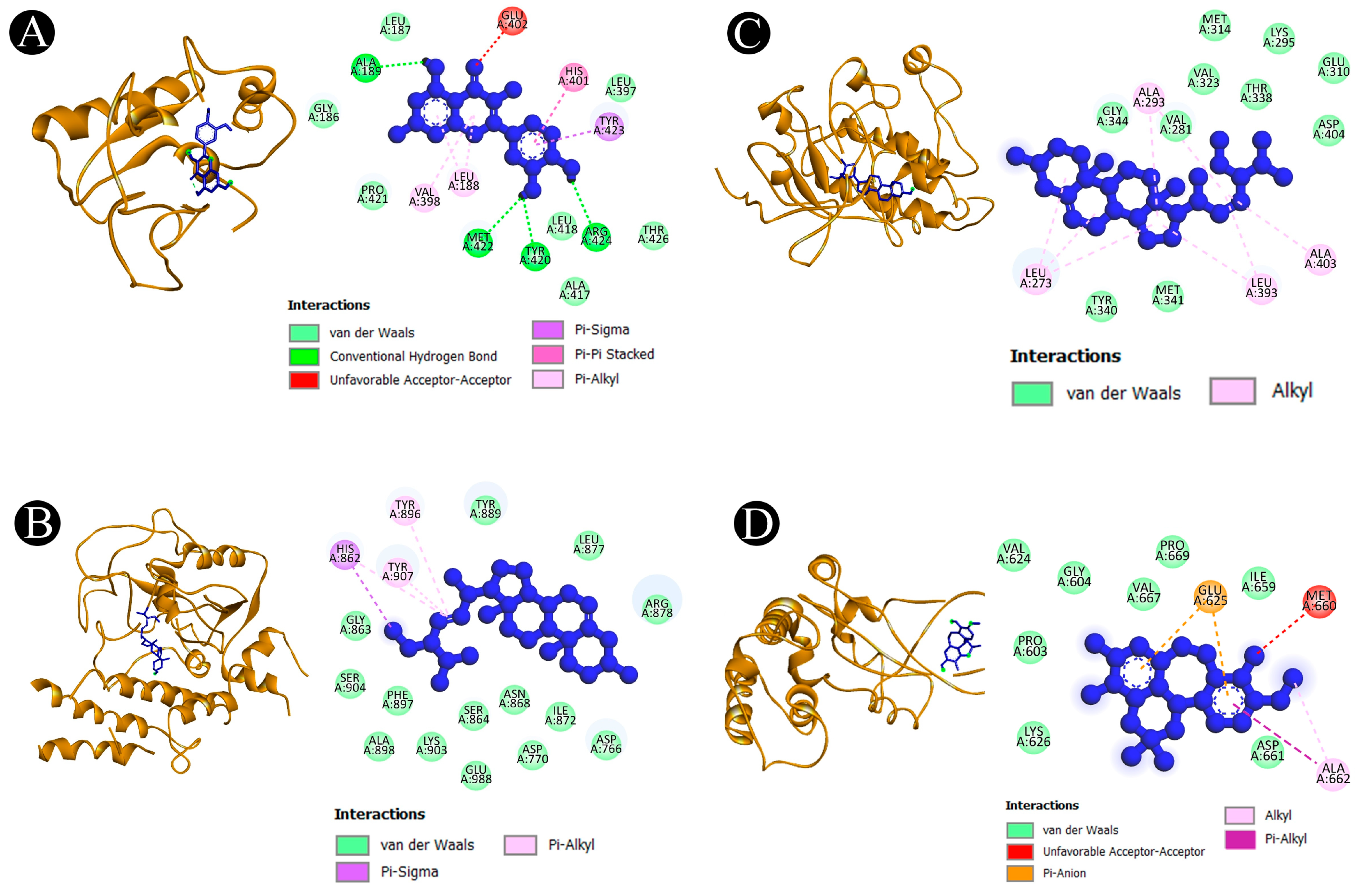
2.5. Binding Affinity and Molecular Interaction Analysis
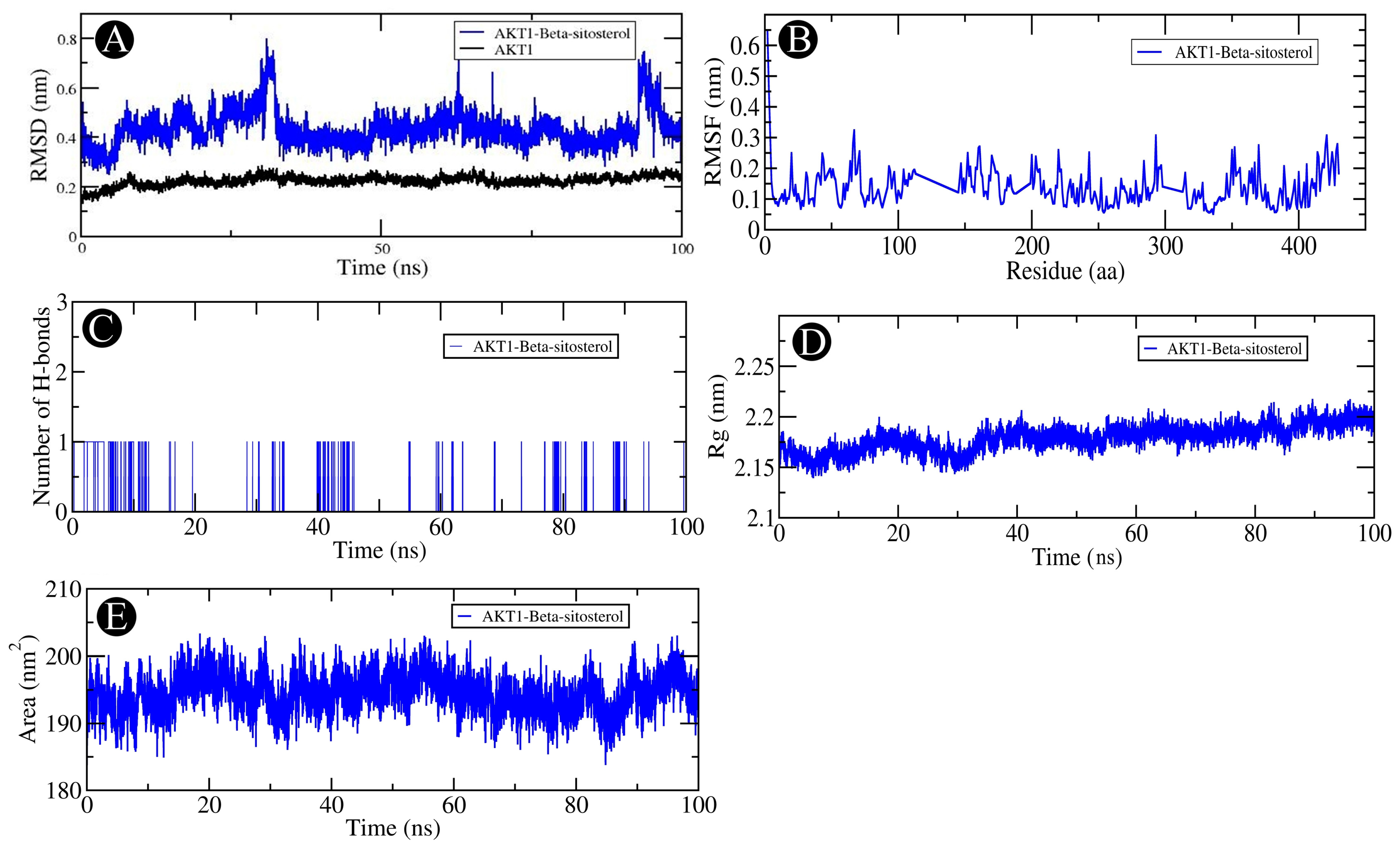
2.6. MD Simulation Analysis
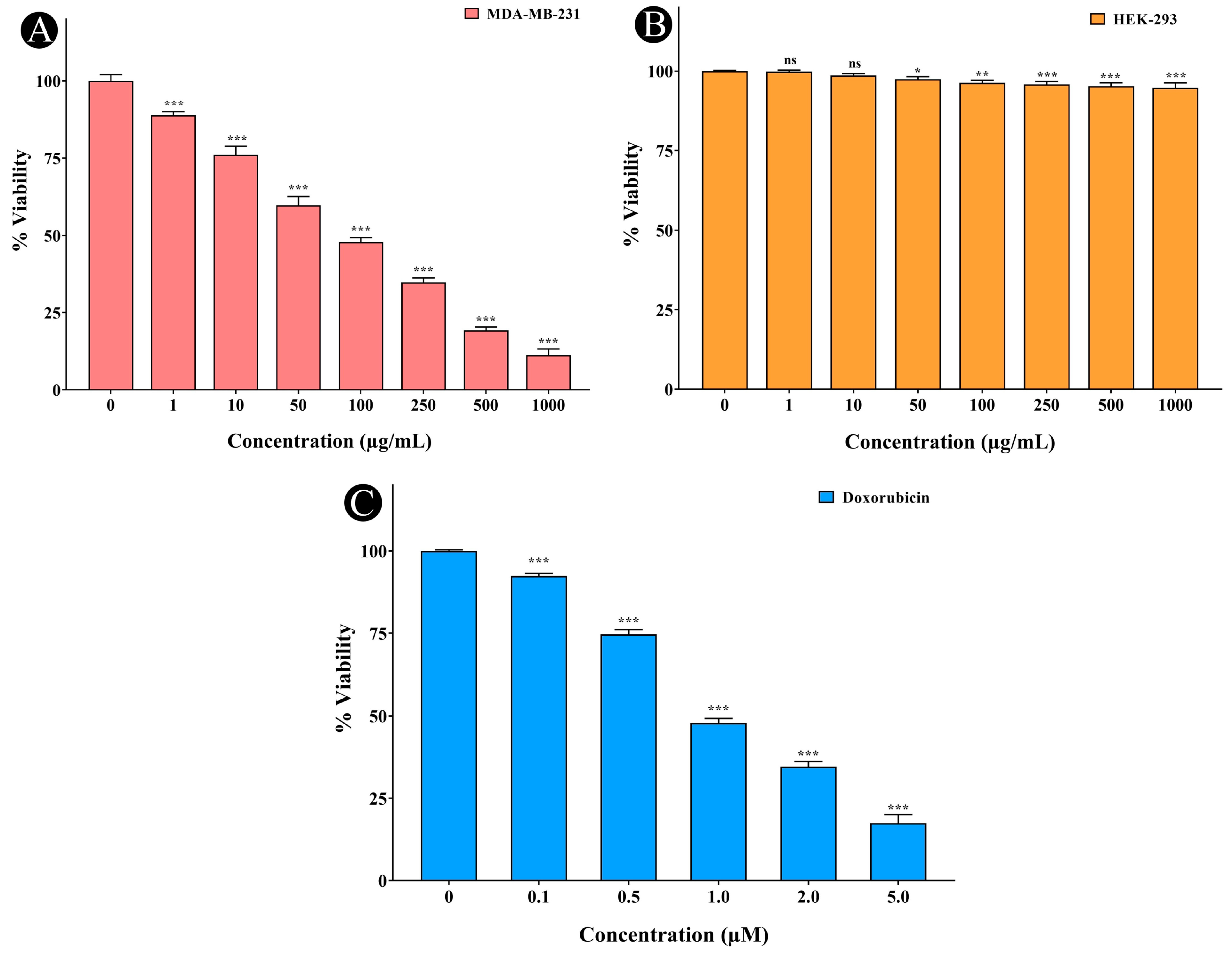
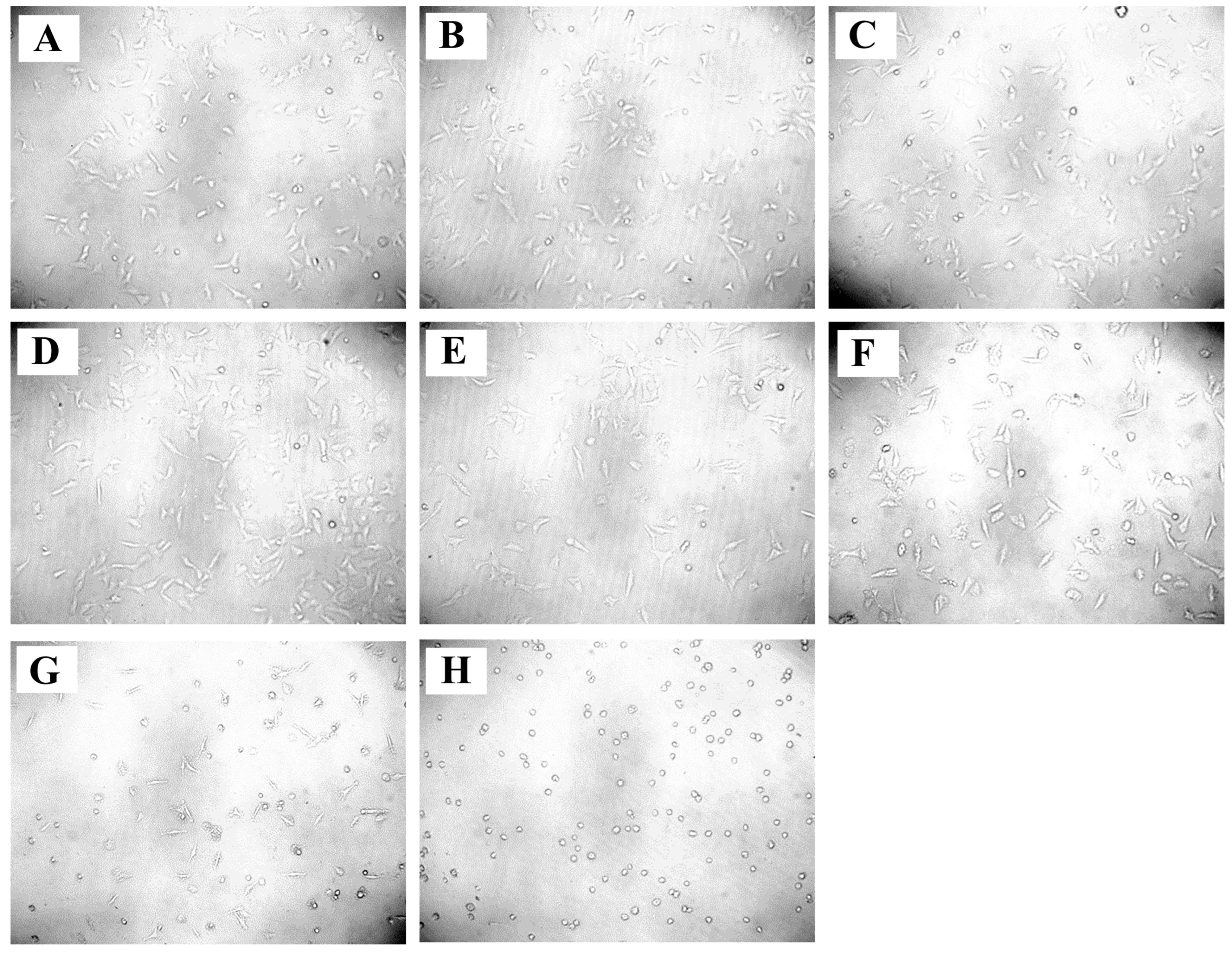
2.7. Anticancer Activity of A. racemosus Crude Extract
2.8. Impact on Cell Cycle Regulation
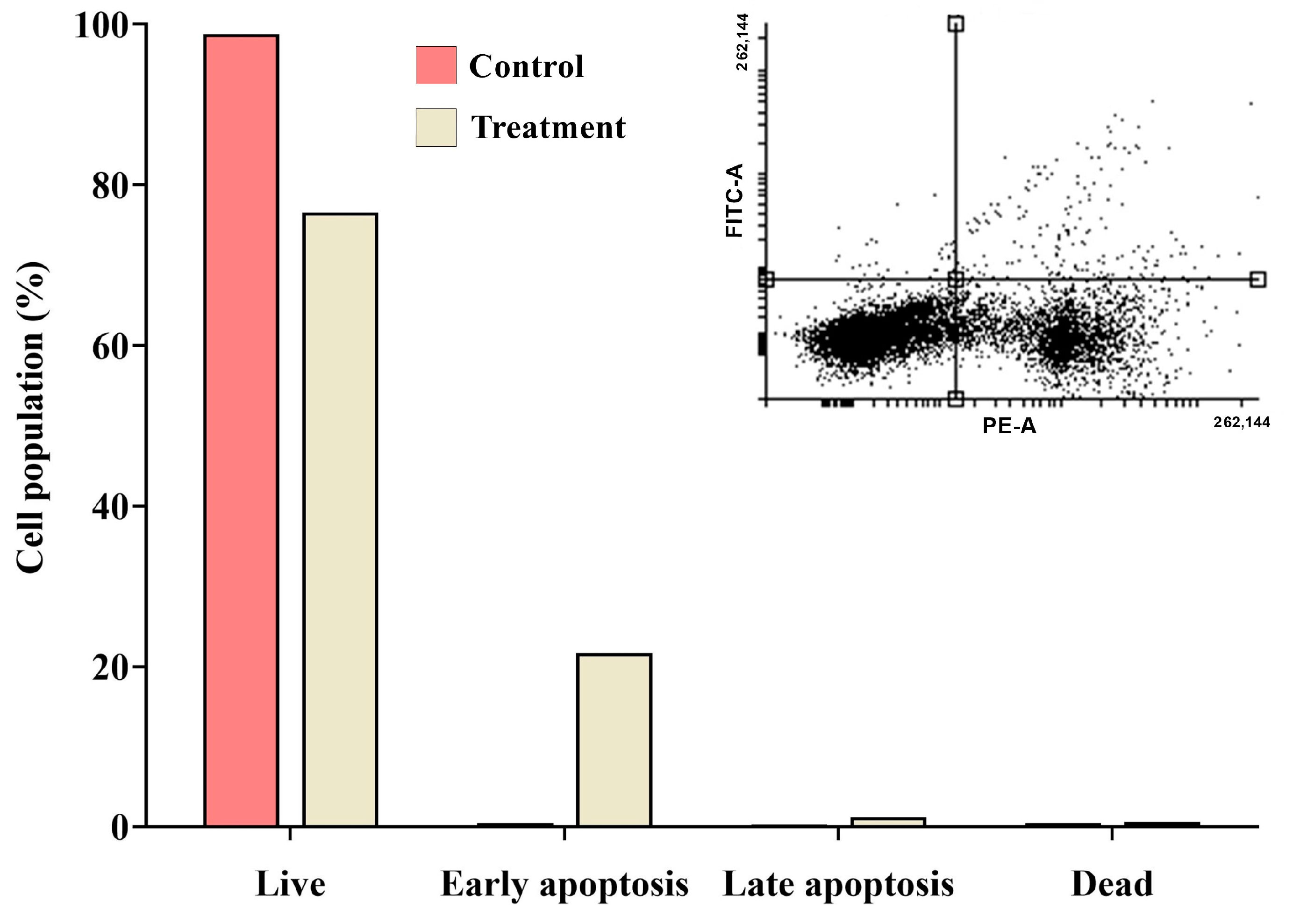
2.9. Detection of Apoptosis Using Propidium Iodide (PI) Staining with Annexin V
3. Discussion
4. Materials and Methods
4.1. In Silico Screening of Bioactive Molecules
4.2. Exploring Targets in Compound-Disease Relationships
4.3. Identifying and Obtaining Potential Targets
4.4. Hub Gene Identification and Protein Interaction Network Analysis
4.5. GO and KEGG Pathway Enrichment Analysis
4.6. Molecular Docking Studies
4.7. Molecular Dynamics (MD) Simulation
4.8. Plant Material Collection and Preparation of Extract
4.9. Culturing of Cells
4.10. Cell Viability Study
4.11. Cell Cycle Analysis
4.12. Annexin-V Apoptosis Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spradlin, J.N.; Hu, X.; Ward, C.C.; Brittain, S.M.; Jones, M.D.; Ou, L.; To, M.; Proudfoot, A.; Ornelas, E.; Woldegiorgis, M. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat. Chem. Biol. 2019, 15, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Prakash, R.; Dixit, A.; Srivastava, A. Chemical Analysis and their Therapeutic activity of Ethanolic extract of Asparagus racemesus (Shatawari) Root by GC-MS analysis. J. Ultra Chem. (Chem. Chem. Sci. Chem. Eng.) 2018, 14, 163. [Google Scholar] [CrossRef]
- Chawla, A.; Chawla, P.; Mangalesh, R.R.; Roy, R. Asparagus racemosus (Willd): Biological activities & its active principles. Indo-Glob. J. Pharm. Sci. 2011, 2, 113–120. [Google Scholar]
- Kaushik, P.; Sansanwal, R.; Sharma, R. A comparative analysis of the in-vitro effects of various sterilization techniques and different disinfectants on the micropropagation of explants of Asparagus racemosus. Int. J. Health Sci. 2022, 6, 11288–11294. [Google Scholar] [CrossRef]
- Patil, D.; Gautam, M.; Gairola, S.; Jadhav, S.; Patwardhan, B. HPLC/Tandem mass spectrometric studies on steroidal saponins: An example of quantitative determination of shatavarin IV from dietary supplements containing Asparagus racemosus. J. AOAC Int. 2014, 97, 1497–1502. [Google Scholar] [CrossRef]
- Chaudhary, B.; Jyothi, Y.; Rabbani, S. Potential effect of Asparagus racemosus root extract on experimental anemic and thrombocytopenic conditions in rats. J. Pharm. Res. 2016, 15, 15–19. [Google Scholar] [CrossRef]
- Sharma, V.; Verma, R.B.; Sharma, S. Atherosclerotic Regulation of Asparagus racemosus Root Extract on Lipid Profile in Male Swiss Albino Mice, Subjected to Inorganic Lead Compound. Biomed. Pharmacol. J. 2015, 4, 107–114. [Google Scholar] [CrossRef]
- Vishwakarma, P.; Chatterjee, M.; Sharma, M.; Saini, M.; Goel, R.; Saxena, K. Evaluation of Cardioprotective activity of Asparagus racemosus against Doxorubicin induced cardiotoxicity in albino rats: An experimental study. Int. J. Basic Clin. Pharmacol. 2019, 8, 1693. [Google Scholar] [CrossRef]
- Behera, S.K. Phytochemical screening and antioxidant properties of methanolic extract of root of Asparagus racemosus Linn. Int. J. Food Prop. 2018, 21, 2681–2688. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Kollu, P.; Khan, S.; Pammi, S. Antibacterial activity assessment and characterization of green synthesized CuO nano rods using Asparagus racemosus roots extract. SN Appl. Sci. 2019, 1, 421. [Google Scholar] [CrossRef]
- El-Senosiy, Y.A.; Ahmad, S.A.; Farid, A.S. Hepatoprotective effect of Asparagus racemosus in paracetamol induced hepatotoxicity in rats. Benha Vet. Med. J. 2015, 28, 133–137. [Google Scholar] [CrossRef]
- Kumar, S.; Akhtar, N.; Jassal, P.S. Enhanced antioxidant and phytochemical properties of in vitro grown Asparagus racemosus. Plant Arch. 2020, 20, 2665–2669. [Google Scholar]
- Jagdish, P. Neuroprotective Activity of Phytoestrogens Against Scopolamine Induced Oxidative Stress in Mice. Int. J. Zool. Investig. 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Bishoyi, S.K.; Tripathy, U.P. Asparagus racemosus: Many problems, one solution on its phytochemical and pharmacological potential. J. Med. Plants Stud. 2023, 11, 3–7. [Google Scholar] [CrossRef]
- Thakur, S.; Kaurav, H.; Chaudhary, G. Shatavari (Asparagus racemosus)-the best female reproductive tonic. Int. J. Res. Rev. 2021, 8, 73–84. [Google Scholar] [CrossRef]
- Kongkaneramit, L.; Witoonsaridsilp, W.; PeungvichA, P.; Ingkaninan, K.; Waranuch, N.; Sarisuta, N. Antioxidant activity and antiapoptotic effect of Asparagus racemosus root extracts in human lung epithelial H460 cells. ExpErimEntal Ther. Med. 2011, 2, 143–148. [Google Scholar] [CrossRef]
- Tripathi, Y.; Tiwari, S.; Anjum, N.; Tewari, D. Phytochemical, antioxidant and antimicrobial screening of roots of Asparagus racemosus Willd. World J. Pharm. Res. 2015, 4, 709–722. [Google Scholar]
- Karass, M.; Bareja, R.; Shelkey, E.; Vlachostergios, P.J.; Robinson, B.D.; Khani, F.; Mosquera, J.M.; Scherr, D.S.; Sboner, A.; Tagawa, S.T. Oncogenic addiction to ERBB2 signaling predicts response to trastuzumab in urothelial cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 194–200. [Google Scholar] [CrossRef]
- Lee, H.J.; So, J.-Y.; DeCastro, A.; Smolarek, A.; Paul, S.; Maehr, H.; Uskokovic, M.; Suh, N. Gemini vitamin D analog suppresses ErbB2-positive mammary tumor growth via inhibition of ErbB2/AKT/ERK signaling. J. Steroid Biochem. Mol. Biol. 2010, 121, 408–412. [Google Scholar] [CrossRef]
- Zhang, E.Y.; Cristofanilli, M.; Robertson, F.; Reuben, J.M.; Mu, Z.; Beavis, R.C.; Im, H.; Snyder, M.; Hofree, M.; Ideker, T. Genome wide proteomics of ERBB2 and EGFR and other oncogenic pathways in inflammatory breast cancer. J. Proteome Res. 2013, 12, 2805–2817. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, L.; Liu, R.; Shi, Q.; Li, X.; Wang, M. MiR-125 inhibited cervical cancer progression by regulating VEGF and PI3K/AKT signaling pathway. World J. Surg. Oncol. 2020, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, L.; Wang, M.; Jia, Y.; Wang, R.; Hao, S.; Feng, C.; Li, G.; Su, S.; Du, L. Sodium Selenite Inhibits the Proliferate of Cervical Cancer Cells Through PI3K/AKT Pathway; Research Square: Durham, NC, USA, 2023. [Google Scholar]
- Jiang, E.; Sun, X.; Kang, H.; Sun, L.; An, W.; Yao, Y.; Hu, X. Dehydrocostus lactone inhibits proliferation, antiapoptosis, and invasion of cervical cancer cells through PI3K/Akt signaling pathway. Int. J. Gynecol. Cancer 2015, 25, 1179–1186. [Google Scholar] [CrossRef]
- Ursini-Siegel, J.; Hardy, W.; Zheng, Y.; Ling, C.; Zuo, D.; Zhang, C.; Podmore, L.; Pawson, T.; Muller, W. The ShcA SH2 domain engages a 14-3-3/PI3′ K signaling complex and promotes breast cancer cell survival. Oncogene 2012, 31, 5038–5044. [Google Scholar] [CrossRef]
- Tamaskovic, R.; Schwill, M.; Nagy-Davidescu, G.; Jost, C.; Schaefer, D.C.; Verdurmen, W.P.; Schaefer, J.V.; Honegger, A.; Plückthun, A. Intermolecular biparatopic trapping of ErbB2 prevents compensatory activation of PI3K/AKT via RAS–p110 crosstalk. Nat. Commun. 2016, 7, 11672. [Google Scholar] [CrossRef]
- So, J.Y.; Wahler, J.E.; Yoon, T.; Smolarek, A.K.; Lin, Y.; Shih, W.J.; Maehr, H.; Uskokovic, M.; Liby, K.T.; Sporn, M.B. Oral administration of a gemini vitamin D analog, a synthetic triterpenoid and the combination prevents mammary tumorigenesis driven by ErbB2 overexpression. Cancer Prev. Res. 2013, 6, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Tome-Garcia, J.; Li, D.; Ghazaryan, S.; Shu, L.; Wu, L. ERBB2 increases metastatic potentials specifically in androgen-insensitive prostate cancer cells. PLoS ONE 2014, 9, e99525. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Al-Yousef, H.M.; Siddiqui, N.A.; Alhowiriny, T.A.; Alqasoumi, S.I.; Amina, M.; Hassan, W.H.B.; Abdelaziz, S.; Abdalla, R.H. Anticancer activity and concurrent analysis of ursolic acid, β-sitosterol and lupeol in three different Hibiscus species (aerial parts) by validated HPTLC method. Saudi Pharm. J. 2018, 26, 1060–1067. [Google Scholar] [CrossRef]
- Vundru, S.S.; Kale, R.K.; Singh, R.P. β-sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement. Altern. Med. 2013, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Harfita, N.L.; Santoni, A.; Suryati, S. Beta-sitosterol glycoside from Paraboea leuserensis and Cytotoxicity Test against MCF-7 Human Breast Cancer Cells. Ris. Inf. Kesehat. 2023, 12, 272–276. [Google Scholar] [CrossRef]
- Sundarraj, S.; Thangam, R.; Sreevani, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef]
- Fan, T.; Huang, Y.; Liu, Z.; Huang, J.; Ke, B.; Rong, Y.; Qiu, H.; Zhang, B. Unveiling the Mechanism of the ChaiShao Shugan Formula Against Triple-Negative Breast Cancer. Drug Des. Dev. Ther. 2024, 18, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Song, G.; Lim, W. Stigmasterol causes ovarian cancer cell apoptosis by inducing endoplasmic reticulum and mitochondrial dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef]
- Ferrer, A.M.; David, J.R.; Taquiqui, A.; Bautista, A.; Deocaris, C.C.; Alinsug, M.V. Pharmacological mechanisms on the anti-breast cancer property of Coix lacryma-jobi: A network-based analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.-Y.; Sun, Y.-S.; Ma, R.-H.; Thakur, K.; Zhang, J.-G.; Wei, Z.-J. Asparanin A from Asparagus officinalis L. induces G0/G1 cell cycle arrest and apoptosis in human endometrial carcinoma ishikawa cells via mitochondrial and PI3K/AKT signaling pathways. J. Agric. Food Chem. 2019, 68, 213–224. [Google Scholar] [CrossRef]
- Mohanraj, K.; Karthikeyan, B.S.; Vivek-Ananth, R.; Chand, R.B.; Aparna, S.; Mangalapandi, P.; Samal, A. IMPPAT: A curated database of I ndian M edicinal P lants, P hytochemistry A nd T herapeutics. Sci. Rep. 2018, 8, 4329. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Bardakci, F.; Alreshidi, M.; Badraoui, R.; Noumi, E.; Tepe, B.; Sachidanandan, M.; Patel, M. Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation to Elucidate the Molecular Targets and Potential Mechanism of Phoenix dactylifera (Ajwa Dates) against Candidiasis. Pathogens 2023, 12, 1369. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Keerthikumar, S.; Ang, C.S.; Gangoda, L.; Quek, C.Y.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Rao, V.S.; Srinivas, K.; Sujini, G.; Kumar, G.S. Protein-protein interaction detection: Methods and analysis. Int. J. Proteom. 2014, 2014, 147648. [Google Scholar] [CrossRef]
- Dennis, R.; Le T, M.M.; Brown, J. Cloud chamber. Wabash J. Phys. 2015, 4, 1–11. [Google Scholar]
- Huey, R.; Morris, G.M.; Forli, S. Using AutoDock 4 and AutoDock vina with AutoDockTools: A tutorial. Scripps Res. Inst. Mol. Graph. Lab. 2012, 10550, 1000. [Google Scholar]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Schrodinger, L. The PyMOL molecular graphics system. Version 2015, 1, 8. [Google Scholar]
- Dassault Systèmes. BIOVIA Discovery Studio Visualizer, Discovery Studio, Accelrys 2.1; Dassault Systèmes: San Diego, CA, USA, 2008. [Google Scholar]
- Alotaibi, N.M.; Alotaibi, M.O.; Alshammari, N.; Adnan, M.; Patel, M. Network Pharmacology Combined with Molecular Docking, Molecular Dynamics, and In Vitro Experimental Validation Reveals the Therapeutic Potential of Thymus vulgaris L. Essential Oil (Thyme Oil) against Human Breast Cancer. ACS Omega 2023, 8, 48344–48359. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum philippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: An in vitro-in silico approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef]
- Ahirwar, B.; Ahirwar, D. In vivo and in vitro investigation of cytotoxic and antitumor activities of polyphenolic leaf extract of Hibiscus sabdariffa against breast cancer cell lines. Res. J. Pharm. Technol. 2020, 13, 615–620. [Google Scholar] [CrossRef]
- Elasbali, A.M.; Al-Soud, W.A.; Al-Oanzi, Z.H.; Qanash, H.; Alharbi, B.; Binsaleh, N.K.; Alreshidi, M.; Patel, M.; Adnan, M. Cytotoxic Activity, Cell Cycle Inhibition, and Apoptosis-Inducing Potential of Athyrium hohenackerianum (Lady Fern) with Its Phytochemical Profiling. Evid.-Based Complement. Altern. Med. 2022, 2022, 2055773. [Google Scholar] [CrossRef]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of cell cycle by flow cytometry. In Checkpoint Controls and Cancer: Volume 2: Activation and Regulation Protocols; Humana Press: Totowa, NJ, USA, 2004; pp. 301–311. [Google Scholar]
- Surti, M.; Patel, M.; Redhwan, A.; Al-Keridis, L.A.; Adnan, M.; Alshammari, N.; Reddy, M.N. Ilimaquinone (marine sponge metabolite) induces apoptosis in HCT-116 human colorectal carcinoma cells via mitochondrial-mediated apoptosis pathway. Mar. Drugs 2022, 20, 582. [Google Scholar] [CrossRef] [PubMed]



| Compound Name | Pubchem ID | Molecular Formula | Molecular Weight (g/mol) | Drug Likeliness | Bioavailability Score | BBB Permeant | Structure |
|---|---|---|---|---|---|---|---|
| Stigmasterol | 5280794 | C29H48O | 412.7 | 0.62 | 0.55 | No | 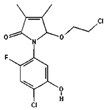 |
| Quercetin | 5280343 | C15H10O7 | 302.23 | 0.52 | 0.55 | No | 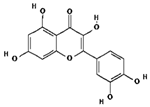 |
| Kaempferol | 5280863 | C15H10O6 | 286.24 | 0.50 | 0.55 | No | 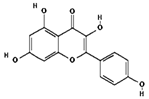 |
| beta-Sitosterol- beta-D-glucoside | 12309055 | C35H60O6 | 576.8 | 0.5 | 0.55 | No | 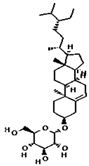 |
| beta-Sitosterol | 222284 | C29H50O | 414.7 | 0.78 | 0.55 | No | 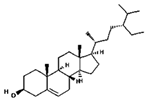 |
| Racemosol | 624971 | C21H24O4 | 340.4 | 0.36 | 0.55 | Yes | 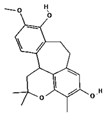 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, A.J.; Elkahoui, S.; Alshammari, A.M.; Patel, M.; Ghoniem, A.E.M.; Abdalla, R.A.H.; Dwivedi-Agnihotri, H.; Badraoui, R.; Adnan, M. Mechanistic Insights into the Anticancer Potential of Asparagus racemosus Willd. Against Triple-Negative Breast Cancer: A Network Pharmacology and Experimental Validation Study. Pharmaceuticals 2025, 18, 433. https://doi.org/10.3390/ph18030433
Siddiqui AJ, Elkahoui S, Alshammari AM, Patel M, Ghoniem AEM, Abdalla RAH, Dwivedi-Agnihotri H, Badraoui R, Adnan M. Mechanistic Insights into the Anticancer Potential of Asparagus racemosus Willd. Against Triple-Negative Breast Cancer: A Network Pharmacology and Experimental Validation Study. Pharmaceuticals. 2025; 18(3):433. https://doi.org/10.3390/ph18030433
Chicago/Turabian StyleSiddiqui, Arif Jamal, Salem Elkahoui, Ahmed Mohajja Alshammari, Mitesh Patel, Ahmed Eisa Mahmoud Ghoniem, Randa Abdeen Husien Abdalla, Hemlata Dwivedi-Agnihotri, Riadh Badraoui, and Mohd Adnan. 2025. "Mechanistic Insights into the Anticancer Potential of Asparagus racemosus Willd. Against Triple-Negative Breast Cancer: A Network Pharmacology and Experimental Validation Study" Pharmaceuticals 18, no. 3: 433. https://doi.org/10.3390/ph18030433
APA StyleSiddiqui, A. J., Elkahoui, S., Alshammari, A. M., Patel, M., Ghoniem, A. E. M., Abdalla, R. A. H., Dwivedi-Agnihotri, H., Badraoui, R., & Adnan, M. (2025). Mechanistic Insights into the Anticancer Potential of Asparagus racemosus Willd. Against Triple-Negative Breast Cancer: A Network Pharmacology and Experimental Validation Study. Pharmaceuticals, 18(3), 433. https://doi.org/10.3390/ph18030433












