LPS-Induced Liver Inflammation Is Inhibited by Psilocybin and Eugenol in Mice
Abstract
:1. Introduction
2. Results
2.1. Recapitulating an Inflammatory Mouse Model to Test Novel Anti-Inflammatory Compounds
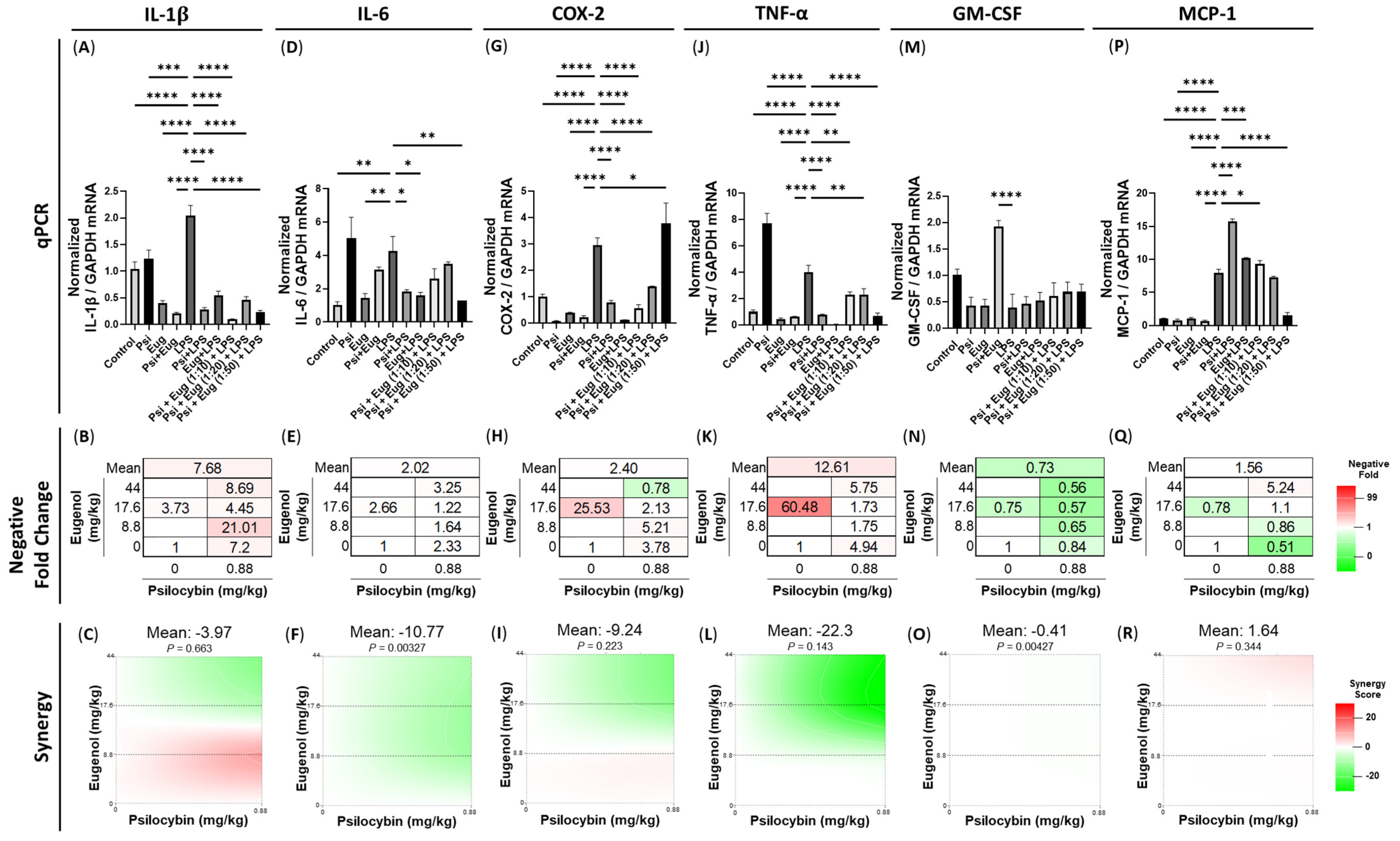
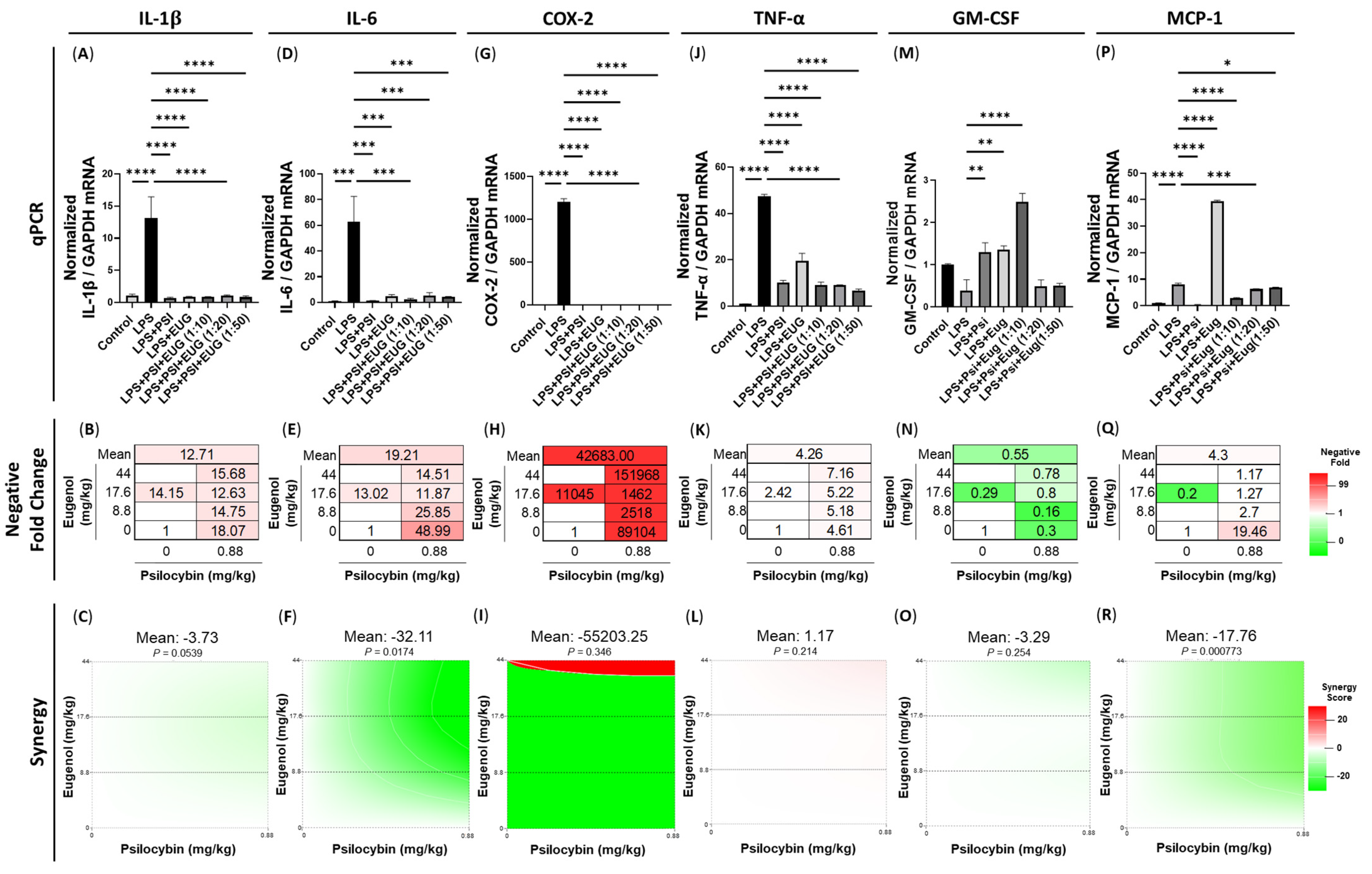
2.2. Pre-Treatment and Post-Treatment with Psilocybin and/or Eugenol Decreases Cytokine mRNA Levels
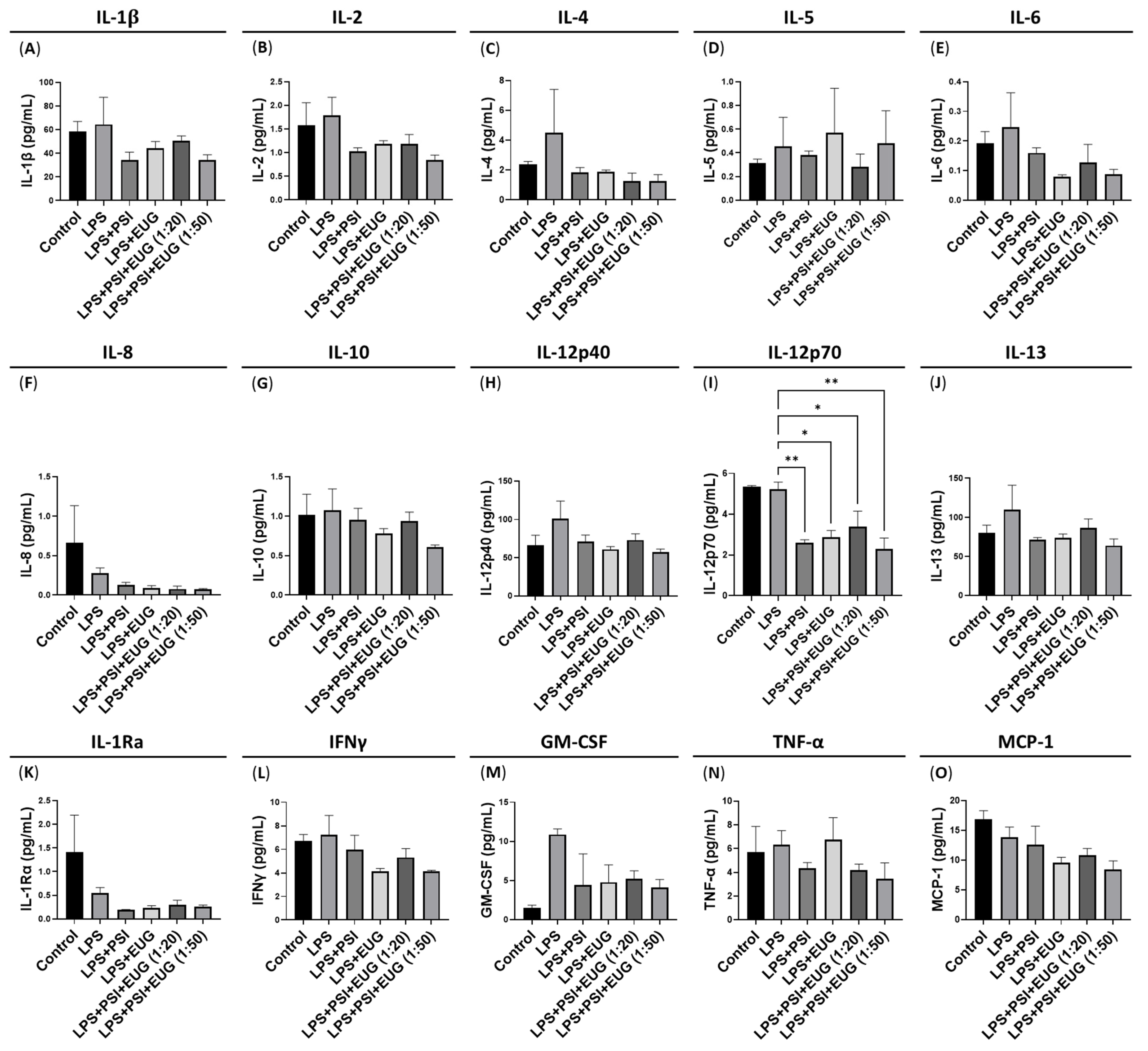
2.3. Post-Treatment with Psilocybin and/or Eugenol Decreases IL-12p70 Protein Levels
2.4. Pre-Treatment and Post-Treatment with Psilocybin and/or Eugenol Ameliorates LPS-Induced Histological Changes
3. Discussion
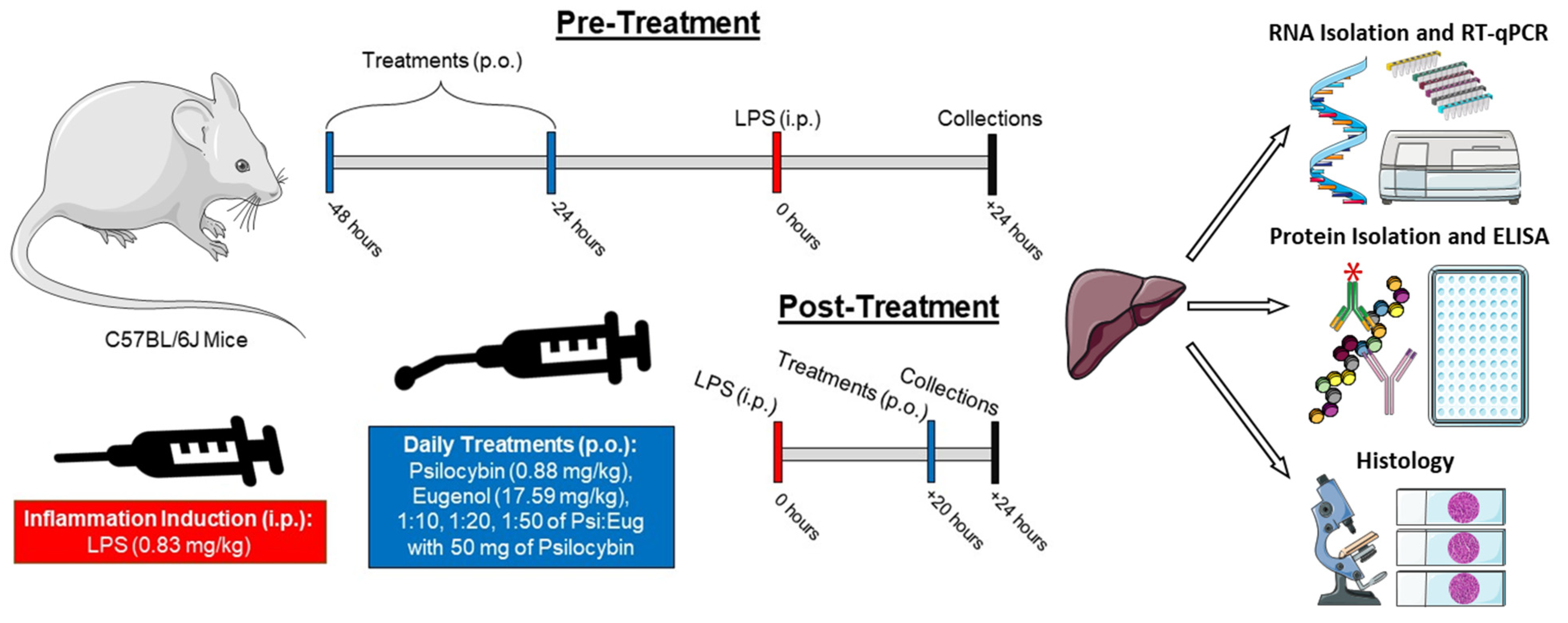
4. Materials and Methods
4.1. Animal Handling, Exposure, and Harvesting
4.2. Protein Extraction and Quantification
4.3. Multiplex Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
4.5. Synergy Testing
4.6. Histology and Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Pan, X.; Li, L. Crosstalk between liver macrophages and gut microbiota: An important component of inflammation-associated liver diseases. Front. Cell Dev. Biol. 2022, 10, 1070208. [Google Scholar] [CrossRef]
- Schaefer, T.J.; John, S. Acute Hepatitis. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK551570/ (accessed on 18 December 2022).
- Li, S.; Hong, M.; Tan, H.-Y.; Wang, N.; Feng, Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhang, Y.; Liu, Y.; Zhou, T.; Zhou, B.; Zhang, Y.; Chen, R.; Xing, J.; He, L.; et al. Current Perspectives of Neuroendocrine Regulation in Liver Fibrosis. Cells 2022, 11, 3783. [Google Scholar] [CrossRef]
- Su, G.L. Lipopolysaccharides in liver injury: Molecular mechanisms of Kupffer cell activation. Am. J. Physiol. Liver Physiol. 2002, 283, G256–G265. [Google Scholar] [CrossRef]
- Soares, J.-B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Soares, J.B., Jr.; Roncon-Albuquerque, R.; Dinis-Ribeiro, M.; Leite-Moreira, A.F. Toll-like receptors as therapeutic targets in gastrointestinal diseases. Expert Opin. Ther. Targets 2010, 14, 347–368. [Google Scholar] [CrossRef] [PubMed]
- Hamesch, K.; Borkham-Kamphorst, E.; Strnad, P.; Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 2015, 49 (Suppl. S1), 37–46. [Google Scholar] [CrossRef]
- Niazi, A.A.; Kourkinejad Gharaei, F.; Saebinasab, Z.; Maleki, M.; Maghool, F.; Fereidooni, F.; Safari, T. Eugenol Administration Improves Liver Damage Induced by a Fructose-Rich Diet. Adv. Biomed. Res. 2021, 10, 42. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Yang, H.; Xuefeng, Y.; Shandong, W.; Jianhua, X. COX-2 in liver fibrosis. Clin. Chim. Acta 2020, 506, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Brenner, D.A. Mechanisms of Liver Injury. I. TNF-α-induced liver injury: Role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G583–G589. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Guo, C.-J.; Li, Y.; Douglas, S.D.; Qi, X.-X.; Song, L.; Ho, W.-Z. Interleukin-1β induces macrophage inflammatory protein-1β expression in human hepatocytes. Cell. Immunol. 2003, 226, 45–53. [Google Scholar] [CrossRef]
- Negash, A.A.; Ramos, H.J.; Crochet, N.; Lau, D.T.Y.; Doehle, B.; Papic, N.; Delker, D.A.; Jo, J.; Bertoletti, A.; Hagedorn, C.H.; et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013, 9, e1003330. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Kumar, A.; Siddiqi, N.J.; Alrashood, S.T.; Khan, H.A.; Dubey, A.; Sharma, B. Protective effect of eugenol on hepatic inflammation and oxidative stress induced by cadmium in male rats. Biomed. Pharmacother. 2021, 139, 111588. [Google Scholar] [CrossRef]
- Yousef, H.N.; Ibraheim, S.S.; Ramadan, R.A.; Aboelwafa, H.R. The Ameliorative Role of Eugenol against Silver Nanoparticles-Induced Hepatotoxicity in Male Wistar Rats. Oxidative Med. Cell. Longev. 2022, 2022, 3820848. [Google Scholar] [CrossRef]
- Yogalakshmi, B.; Viswanathan, P.; Anuradha, C.V. Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 2010, 268, 204–212. [Google Scholar] [CrossRef]
- Yu, B.; Becnel, J.; Zerfaoui, M.; Rohatgi, R.; Boulares, A.H.; Nichols, C.D. Serotonin 5-Hydroxytryptamine2A Receptor Activation Suppresses Tumor Necrosis Factor-α-Induced Inflammation with Extraordinary Potency. J. Pharmacol. Exp. Ther. 2008, 327, 316–323. [Google Scholar] [CrossRef]
- Nau, F.; Yu, B.; Martin, D.; Nichols, C.D. Serotonin 5-HT2A Receptor Activation Blocks TNF-α Mediated Inflammation In Vivo. PLoS ONE 2013, 8, e75426. [Google Scholar] [CrossRef] [PubMed]
- Nkadimeng, S.M.; Steinmann, C.M.; Eloff, J.N. Anti-Inflammatory Effects of Four Psilocybin-Containing Magic Mushroom Water Extracts in vitro on 15-Lipoxygenase Activity and on Lipopolysaccharide-Induced Cyclooxygenase-2 and Inflammatory Cytokines in Human U937 Macrophage Cells. J. Inflamm. Res. 2021, 14, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, T.W.; Nichols, C.D. Psychedelics as anti-inflammatory agents. Int. Rev. Psychiatry 2018, 30, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.I.; Li, D.; Wang, B.; Zahoruiko, Y.; Gerasymchuk, M.; Hudson, D.; Kovalchuk, O.; Kovalchuk, I. Anti-Inflammatory Effects of Serotonin Receptor and Transient Receptor Potential Channel Ligands in Human Small Intestinal Epithelial Cells. Curr. Issues Mol. Biol. 2023, 45, 6743–6774. [Google Scholar] [CrossRef]
- Robinson, G.I.; Li, D.; Wang, B.; Rahman, T.; Gerasymchuk, M.; Hudson, D.; Kovalchuk, O.; Kovalchuk, I. Psilocybin and Eugenol Reduce Inflammation in Human 3D EpiIntestinal Tissue. Life 2023, 13, 2345. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Trauner, M. Gut-liver axis: Pathophysiological concepts and clinical implications. Cell Metab. 2022, 34, 1700–1718. [Google Scholar] [CrossRef]
- Zanikov, T.; Gerasymchuk, M.; Gojani, E.G.; Robinson, G.I.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cameron, M.; et al. The Effect of Combined Treatment of Psilocybin and Eugenol on Lipopolysaccharide-Induced Brain Inflammation in Mice. Molecules 2023, 28, 2624. [Google Scholar] [CrossRef]
- Zanikov, T.; Gerasymchuk, M.; Robinson, G.I.; Gojani, E.G.; Asghari, S.; Groves, A.; Cameron, M.; Rodriguez-Juarez, R.; Snelling, A.; Hudson, D.; et al. Psilocybin and eugenol prevent DSS-induced neuroinflammation in mice. Biocatal. Agric. Biotechnol. 2024, 56, 103033. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Lymphocyte recruitment to the liver: Molecular insights into the pathogenesis of liver injury and hepatitis. Toxicology 2008, 254, 136–146. [Google Scholar] [CrossRef]
- She, S.; Ren, L.; Chen, P.; Wang, M.; Chen, D.; Wang, Y.; Chen, H. Functional Roles of Chemokine Receptor CCR2 and Its Ligands in Liver Disease. Front. Immunol. 2022, 13, 812431. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.D.; Hall, B.M.; Plain, K.M.; Robinson, C.M.; Boyd, R.; Tran, G.T.; Wang, C.; Bishop, G.A.; Hodgkinson, S.J. Interleukin-12 (IL-12p70) Promotes Induction of Highly Potent Th1-Like CD4+CD25+ T Regulatory Cells That Inhibit Allograft Rejection in Unmodified Recipients. Front. Immunol. 2014, 5, 190. [Google Scholar] [CrossRef]
- Said, M.M. The protective effect of eugenol against gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Fundam. Clin. Pharmacol. 2010, 25, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Y. Eugenol attenuates concanavalin A-induced hepatitis through modulation of cytokine levels and inhibition of mitochondrial oxidative stress. Arch. Biol. Sci. 2019, 71, 339–346. [Google Scholar] [CrossRef]
- Satoh, T.; Otsuka, A.; Contassot, E.; French, L. E The inflammasome and IL-1β: Implications for the treatment of inflammatory diseases. Immunotherapy 2015, 7, 243–254. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Han, J.; Zhou, P.; Yu, X.; Shen, Z.; Mao, R.; Lu, M.; Huang, Y.; Zhang, J. MicroRNA-124 expression in Kupffer cells modulates liver injury by targeting IL-6/STAT3 signaling. Antivir. Res. 2022, 211, 105510. [Google Scholar] [CrossRef] [PubMed]
- Gough, P.; Myles, I.A. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Zhao, S.; Jiang, J.; Jing, Y.; Liu, W.; Yang, X.; Hou, X.; Gao, L.; Wei, L. The concentration of tumor necrosis factor-α determines its protective or damaging effect on liver injury by regulating Yap activity. Cell Death Dis. 2020, 11, 70. [Google Scholar] [CrossRef]
- Tiegs, G.; Horst, A.K. TNF in the liver: Targeting a central player in inflammation. Semin. Immunopathol. 2022, 44, 445–459. [Google Scholar] [CrossRef]
- Yang, Y.M.; Seki, E. TNFα in Liver Fibrosis. Curr. Pathobiol. Rep. 2015, 3, 253–261. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.H.; Roberts, A.I.; Das, J.; Xu, G.; Ren, G.; Zhang, Y.; Zhang, L.; Yuan, Z.R.; Tan, H.S.; et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: What we do and don’t know. Cell Res. 2006, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Tan-Garcia, A.; Lai, F.; Yeong, J.P.S.; Irac, S.E.; Ng, P.Y.; Msallam, R.; Lim, J.C.T.; Wai, L.-E.; Tham, C.Y.; Choo, S.P.; et al. Liver fibrosis and CD206+ macrophage accumulation are suppressed by anti-GM-CSF therapy. JHEP Rep. 2020, 2, 100062. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, M.B.; Hada, B.; Park, S.R.; Kim, K.H.; Choi, B.H. Granulocyte-macrophage colony-stimulating factor (GM-CSF) shows therapeutic effect on dimethylnitrosamine (DMN)-induced liver fibrosis in rats. PLoS ONE 2022, 17, e0274126. [Google Scholar] [CrossRef]
- Chouaib, S.; Chehimi, J.; Bani, L.; Genetet, N.; Tursz, T.; Gay, F.; Trinchieri, G.; Mami-Chouaib, F. Interleukin 12 induces the differentiation of major histocompatibility complex class I-primed cytotoxic T-lymphocyte precursors into allospecific cytotoxic effectors. Proc. Natl. Acad. Sci. USA 1994, 91, 12659–12663. [Google Scholar] [CrossRef] [PubMed]
- Pinkoski, M.J.; A Heibein, J.; Barry, M.; Bleackley, R.C. Nuclear translocation of granzyme B in target cell apoptosis. Cell Death Differ. 2000, 7, 17–24. [Google Scholar] [CrossRef]
- Velotti, F.; Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Granzyme B in Inflammatory Diseases: Apoptosis, Inflammation, Extracellular Matrix Remodeling, Epithelial-to-Mesenchymal Transition and Fibrosis. Front. Immunol. 2020, 11, 587581. [Google Scholar] [CrossRef]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef]
- El Motteleb, D.M.A.; Selim, S.A.; Mohamed, A.M. Differential effects of eugenol against hepatic inflammation and overall damage induced by ischemia/re-perfusion injury. J. Immunotoxicol. 2014, 11, 238–245. [Google Scholar] [CrossRef]
- Carvalho, R.P.R.; Ribeiro, F.C.D.; Lima, T.I.; Ervilha, L.O.G.; de Oliveira, E.L.; Faustino, A.d.O.; Lima, G.D.d.A.; Machado-Neves, M. High doses of eugenol cause structural and functional damage to the rat liver. Life Sci. 2022, 304, 120696. [Google Scholar] [CrossRef]
- Thomann, J.; Kolaczynska, K.E.; Stoeckmann, O.V.; Rudin, D.; Vizeli, P.; Hoener, M.C.; Pryce, C.R.; Vollenweider, F.X.; Liechti, M.E.; Duthaler, U. In vitro and in vivo metabolism of psilocybin’s active metabolite psilocin. Front. Pharmacol. 2024, 15, 1391689. [Google Scholar] [CrossRef]
- Mckenna, D.; Repke, D.; Lo, L.; Peroutka, S. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 1990, 29, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016, 26, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Viohl, N.; Zanjani, A.A.H.; Khandelia, H. Molecular insights into the modulation of the 5HT2A receptor by serotonin, psilocin, and the G protein subunit Gqα. FEBS Lett. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, W.; Aldahdooh, J.; Malyutina, A.; Shadbahr, T.; Tanoli, Z.; Pessia, A.; Tang, J. SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets. Genom. Proteom. Bioinform. 2022, 20, 587–596. [Google Scholar] [CrossRef]



| Pre-Treatments | Psilocybin (mg/kg) | Eugenol (mg/kg) |
|---|---|---|
| Control-Pre | 0 | 0 |
| Psi | 0.88 | 0 |
| Eug | 0 | 17.59 |
| Psi + Eug | 0.88 | 8.8 |
| LPS | N/A | N/A |
| Psi + LPS | 0.88 | 0 |
| Eug + LPS | 0 | 17.59 |
| Psi + Eug (1:10) + LPS | 0.88 | 8.8 |
| Psi + Eug (1:20) + LPS | 0.88 | 17.6 |
| Psi + Eug (1:50) + LPS | 0.88 | 44.0 |
| Control-Post | 0 | 0 |
| LPS + Psi | 0.88 | 0 |
| LPS + Eug | 0 | 17.59 |
| LPS + Psi + Eug (1:10) | 0.88 | 8.8 |
| LPS + Psi + Eug (1:20) | 0.88 | 17.59 |
| LPS + Psi + Eug (1:50) | 0.88 | 44.0 |
| Target Gene | Forward Sequence (5′ → 3′) | Reverse Sequence (5′ → 3′) |
|---|---|---|
| Il1b Acc #: NM_008361.4 | CAGGCAGGCAGTATCACTCATT | AAGAAGGTGCTCATGTCCTCATC |
| Il6 Acc #: NM_001314054.1 | GACTTCCATCCAGTTGCCTTCT | TATCCTCTGTGAAGTCTCCTCTCC |
| Ptgs2 Acc #: NM_011198.4 | CCTTCTCCAACCTCTCCTACTACA | AGCTCCTTATTTCCCTTCACACC |
| Tnf Acc #: NM_001278601.1 | GCCTCTTCTCATTCCTGCTTGT | TGGGAACTTCTCATCCCTTTGG |
| Csf2 Acc #: NM_009969(1) | AGCTCTGAATCCAGCTTCTC | CCACATCTCTTGGTCCCTTTA |
| Ccl2 Acc #: NM_011333(1) | CTCGGACTGTGATGCCTTAAT | TGGATCCACACCTTGCATTTA |
| Gapdh Acc #: XM_036165840.1 | CATCACTGCCACCCAGAAGA | AGTGGATGCAGGGATGATGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, G.I.; Gerasymchuk, M.; Zanikov, T.; Gojani, E.G.; Asghari, S.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Cruz, C.; et al. LPS-Induced Liver Inflammation Is Inhibited by Psilocybin and Eugenol in Mice. Pharmaceuticals 2025, 18, 451. https://doi.org/10.3390/ph18040451
Robinson GI, Gerasymchuk M, Zanikov T, Gojani EG, Asghari S, Groves A, Haselhorst L, Nandakumar S, Stahl C, Cruz C, et al. LPS-Induced Liver Inflammation Is Inhibited by Psilocybin and Eugenol in Mice. Pharmaceuticals. 2025; 18(4):451. https://doi.org/10.3390/ph18040451
Chicago/Turabian StyleRobinson, Gregory Ian, Marta Gerasymchuk, Timur Zanikov, Esmaeel Ghasemi Gojani, Shima Asghari, Alyssa Groves, Lucie Haselhorst, Sanjana Nandakumar, Cora Stahl, Ceejay Cruz, and et al. 2025. "LPS-Induced Liver Inflammation Is Inhibited by Psilocybin and Eugenol in Mice" Pharmaceuticals 18, no. 4: 451. https://doi.org/10.3390/ph18040451
APA StyleRobinson, G. I., Gerasymchuk, M., Zanikov, T., Gojani, E. G., Asghari, S., Groves, A., Haselhorst, L., Nandakumar, S., Stahl, C., Cruz, C., Cameron, M., Zahoruiko, Y., Li, D., Rodriguez-Juarez, R., Snelling, A., Hudson, D., Fiselier, A., Kovalchuk, O., & Kovalchuk, I. (2025). LPS-Induced Liver Inflammation Is Inhibited by Psilocybin and Eugenol in Mice. Pharmaceuticals, 18(4), 451. https://doi.org/10.3390/ph18040451








