Is Copper-61 the New Gallium-68? Automation and Preclinical Proof-of-Concept of 61Cu-Based Radiopharmaceuticals for Prostate Cancer Imaging
Abstract
:1. Introduction
2. Results
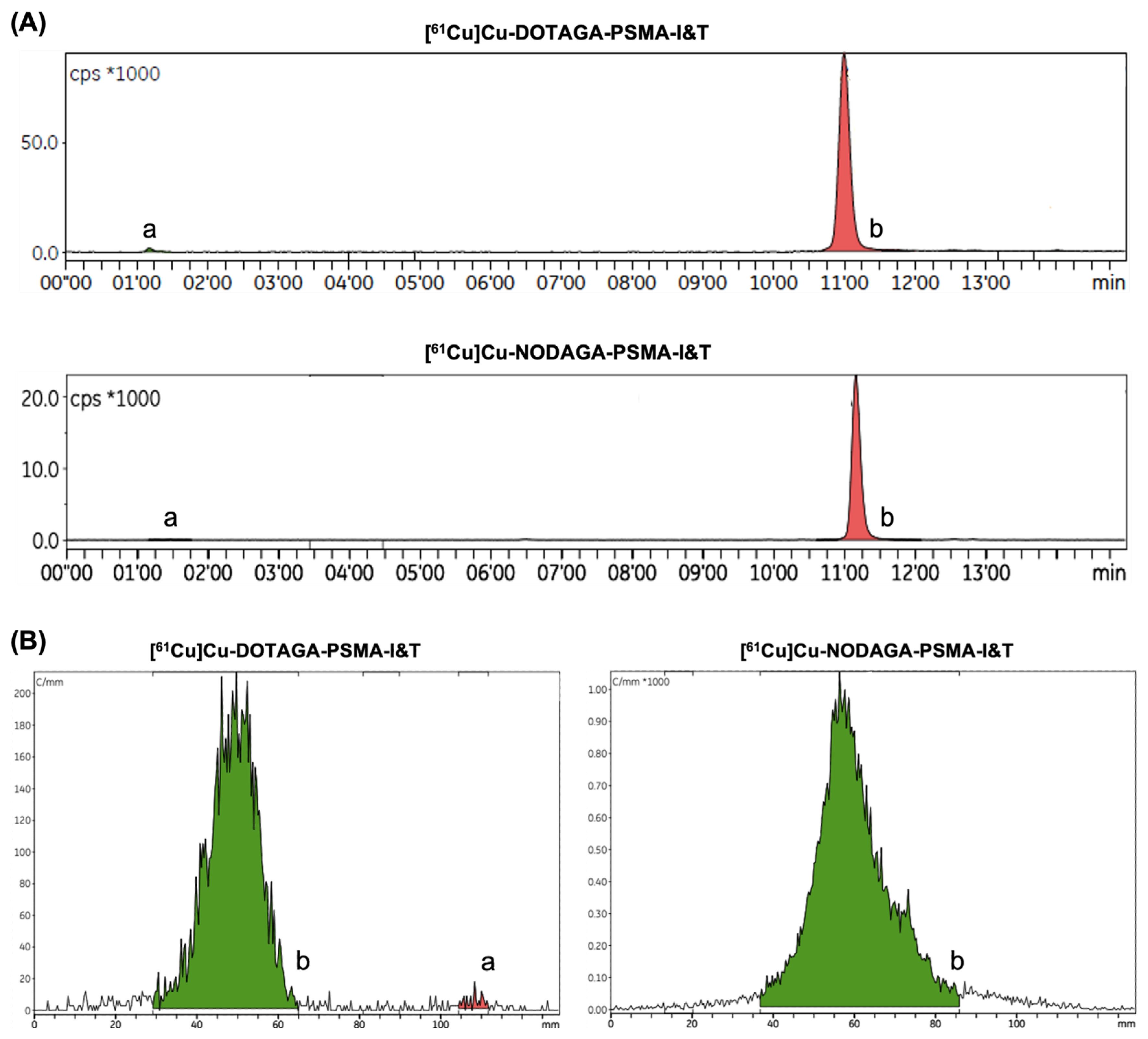
2.1. Automated Radiopharmaceutical Synthesis and Radiochemical Purity
2.2. In Vitro Stability and Uptake Studies
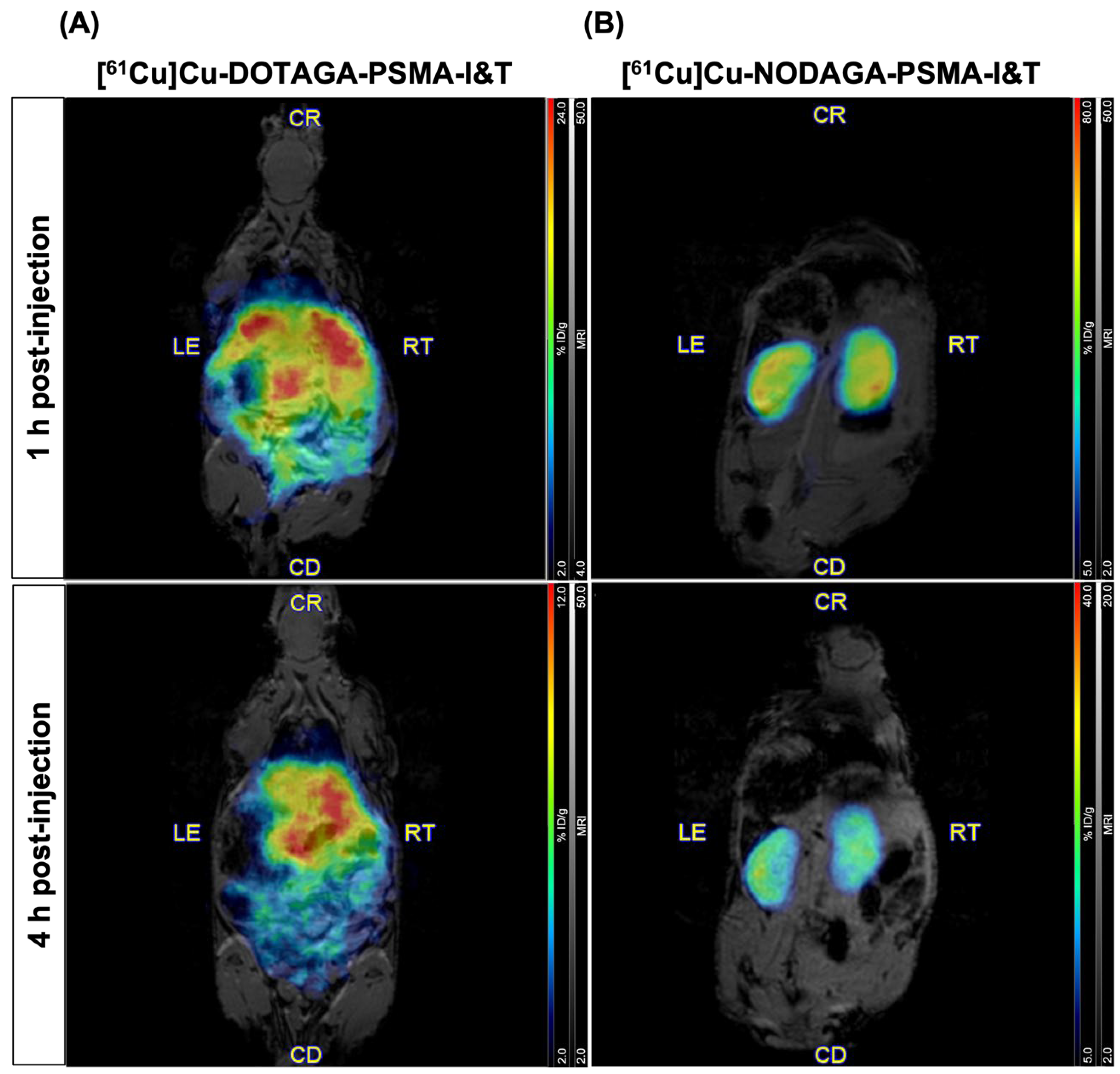
2.3. PET/MR Imaging in Healthy Mice
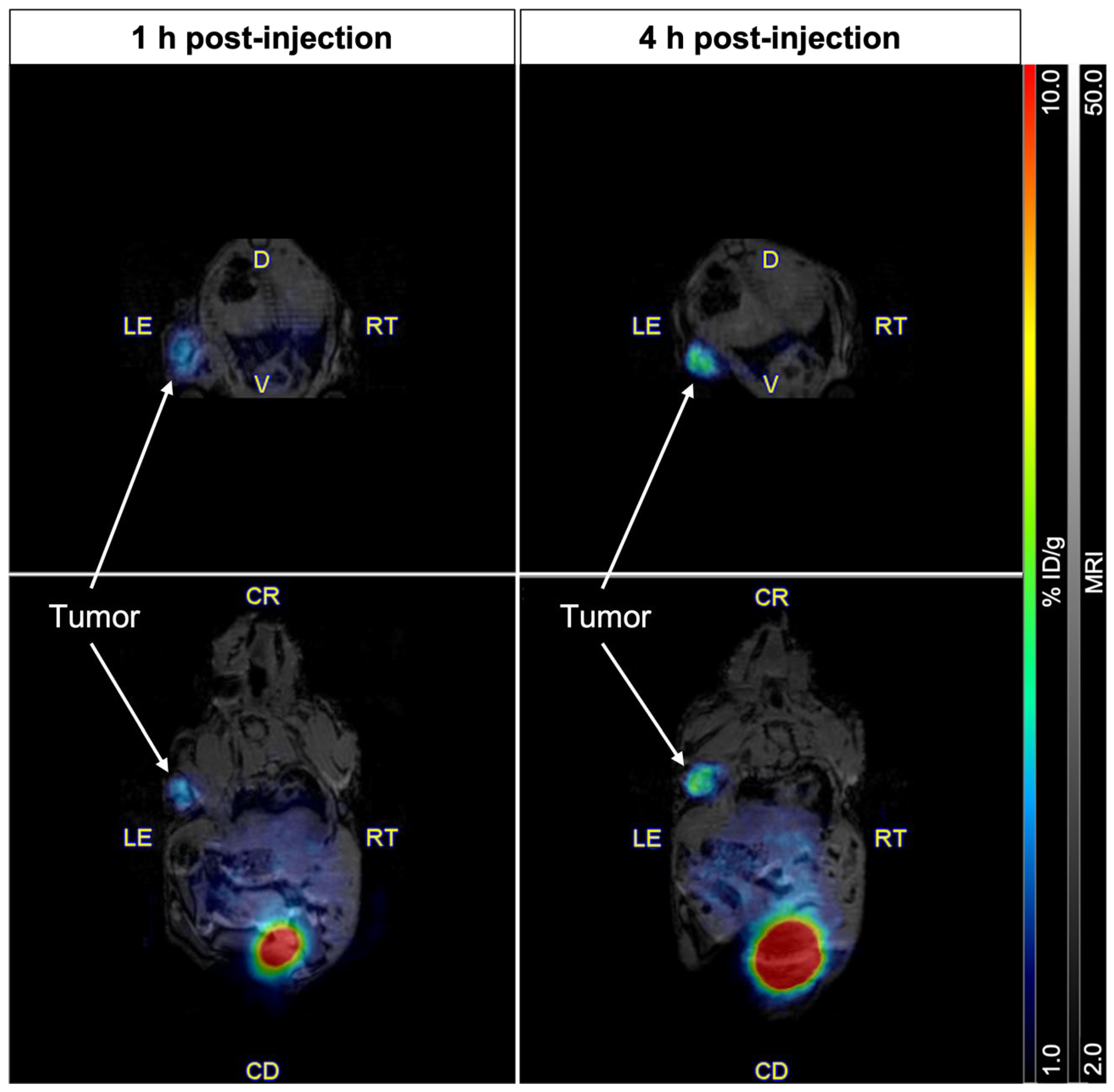
2.4. Imaging of PSMA Receptors with [61Cu]Cu-NODAGA-PSMA-I&T
3. Discussion
4. Materials and Methods
4.1. [61Cu]CuCl2 Production and Purification
- Prior to use, the SAX cartridge is preconditioned with water (10 mL) followed by HCl 8 M (8 mL);
- Water (3mL) is added to the radioactivity-receiver vial to adjust pH;
- The CU cartridge is preconditioned with water (10 mL);
- After the irradiated solution is transferred into the hot-cell, the CU cartridge is loaded using a peristaltic pump to avoid cross-contamination of the tubing system with zinc;
- The CU cartridge is rinsed with HNO3 1 mM (10 mL) and dried for 2 min;
- The CU cartridge is eluted with HCl 8M (2 mL) to Vial A containing 3.3 mL of water;
- The solution is then passed through a smaller SAX cartridge (SAX 2.0—1 mL cartridge; 0.40–0.45 g of resin) in order to remove residual zinc and transferred to Vial B containing > 5.5 mL of HCl 30%;
- Finally, the second SAX cartridge (2 mL cartridge; full) is loaded, dried for 2 min, and eluted with 3 mL of water into the final product vial (FPV).
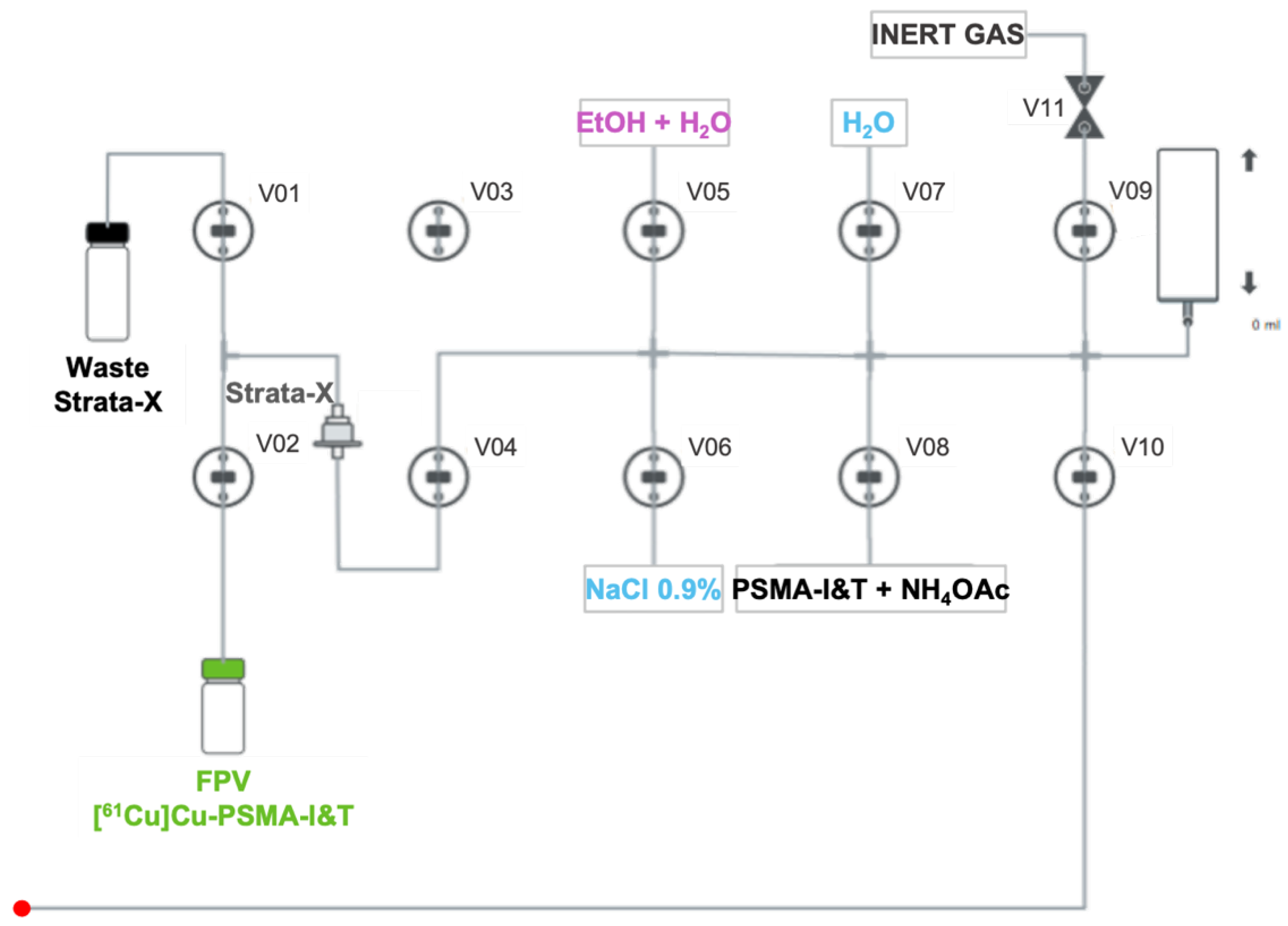
4.2. Automated Synthesis of PSMA-Based Radiopharmaceuticals
- A mass of 30 µg of DOTAGA/NODAGA-PSMA-I&T diluted in 5 mL of NH4OAc 2 M is transferred to the reaction vial (Figure 6) and mixed with [61Cu]CuCl2;
- The radiolabeling reaction occurs for 10 min at 100 °C with pH fixed between 3.5- 4.5;
- After that time, the reaction mixture is diluted with water (5 mL) and passed through a Strata-X cartridge into the waste container to remove free copper-61;
- The Strata-X cartridge is rinsed with water (5 mL) and eluted with 2 mL of a water/EtOH solution (1:1) into the FPV;
- Finally, 8 mL of NaCl 0.9% is added to the FPV in order to get the radiopharmaceutical in its injectable final formulation.
4.3. Radiochemical Purity
4.4. Stability
4.5. Cell Culture
4.6. In Vitro Uptake Assay
4.7. Experimental Mouse Model of PCa
4.8. In Vivo PET/MR Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDTA | Ethylenediaminetetraacetic acid |
| EOB | End of bombardment |
| EOS | End of synthesis |
| EtOH | Ethanol |
| FDA | Food and Drug Agency |
| FOV | Field of view |
| FPV | Final product vial |
| HCl | Hydrochloric acid |
| HLB | Hydrophilic/lipophilic balanced |
| HNO3 | Nitric acid |
| HPLC | High-performance liquid chromatography |
| iTLC | Instant thin layer chromatography |
| mAb | Monoclonal antibody |
| MRI | Magnetic resonance imaging |
| NaOAc | Sodium acetate |
| NH4OAc | Ammonium acetate |
| PBS | Phosphate-buffered saline |
| PCa | Prostate cancer |
| PET | Positron emission tomography |
| PSMA | Prostate specific membrane antigen |
| RCY | Radiochemical yield |
| SAX | Strong anion exchange |
| SPECT | Single-photon emission computed tomography |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Prostate SEER Relative Survival Rates by Time Since Diagnosis, 2000–2020. Available online: https://seer.cancer.gov/statistics-network/explorer/application.html?site=66&data_type=4&graph_type=6&compareBy=stage&chk_stage_104=104&chk_stage_105=105&chk_stage_106=106&chk_stage_107=107&hdn_sex=2&race=1&age_range=1&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0 (accessed on 22 October 2024).
- Mouraviev, V.; Madden, J.F.; Broadwater, G.; Mayes, J.M.; Burchette, J.L.; Schneider, F.; Smith, J.; Tsivian, M.; Wong, T.; Polascik, T.J. Use of 111In-Capromab Pendetide Immunoscintigraphy to Image Localized Prostate Cancer Foci Within the Prostate Gland. J. Urol. 2009, 182, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Sodee, D.B.; Ellis, R.J.; Samuels, M.A.; Spirnak, J.P.; Poole, W.F.; Riester, C.; Martanovic, D.M.; Stonecipher, R.; Bellon, E.M. Prostate cancer and prostate bed SPECT imaging with ProstaScint®: Semiquantitative correlation with prostatic biopsy results. Prostate 1998, 37, 140–148. [Google Scholar] [CrossRef]
- Ellis, R.J.; Kaminsky, D.A.; Zhou, E.H.; Fu, P.; Chen, W.-D.; Brelin, A.; Faulhaber, P.F.; Bodner, D. Ten-Year Outcomes: The Clinical Utility of Single Photon Emission Computed Tomography/Computed Tomography Capromab Pendetide (Prostascint) in a Cohort Diagnosed with Localized Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 29–34. [Google Scholar] [CrossRef]
- Elgamal, A.A.A.; Troychak, M.J.; Murphy, G.P. ProstaScint Scan May Enhance Identification of Prostate Cancer Recurrences After Prostatectomy, Radiation, or Hormone Therapy: Analysis of 136 Scans of 100 Patients. Prostate 1998, 37, 261–269. [Google Scholar] [CrossRef]
- Lamb, H.M.; Faulds, D. Capromab Pendetide A Review of its Use as an Imaging Agent in Prostate Cancer. Drugs Aging 1998, 12, 293–304. [Google Scholar] [CrossRef]
- Manyak, M.J. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev. Anticancer. Ther. 2008, 8, 175–181. [Google Scholar] [CrossRef]
- Dammes, N.; Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 2020, 10, 938–955. [Google Scholar] [CrossRef]
- Rahbar, K.; Afshar-Oromieh, A.; Seifert, R.; Wagner, S.; Schäfers, M.; Bögemann, M.; Weckesser, M. Diagnostic performance of 18F-PSMA-1007 PET/CT in patients with biochemical recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2055–2061. [Google Scholar] [CrossRef]
- Metser, U.; Zukotynski, K.; Mak, V.; Langer, D.; MacCrostie, P.; Finelli, A.; Kapoor, A.; Chin, J.; Lavallee, L.; Klotz, L.H.; et al. Effect of 18F-DCFPyL PET/CT on the Man-agement of Patients with Recurrent Prostate Cancer: Results of a Prospective Multicenter Registry Trial. Radiology 2022, 303, 414–422. [Google Scholar] [CrossRef]
- Barbaud, M.; Frindel, M.; Ferrer, L.; Le Thiec, M.; Rusu, D.; Rauscher, A.; Maucherat, B.; Baumgartner, P.; Fleury, V.; Colombie, M.; et al. 68Ga-PSMA-11 PET-CT study in prostate cancer patients with biochemical recurrence and non-contributive 18F-Choline PET-CT: Impact on therapeutic decision-making and biomarker changes. Prostate 2019, 79, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Ferraro, D.A.; Muehlematter, U.J.; Schüler, H.I.G.; Kedzia, S.; Eberli, D.; Guckenberger, M.; Kroeze, S.G.C.; Sulser, T.; Schmid, D.M.; et al. Clinical impact of 68Ga-PSMA-11 PET on patient management and outcome, including all patients referred for an increase in PSA level during the first year after its clinical introduction. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, T.B.; Mansi, R.; Del Pozzo, L.; Zanger, S.; Gaonkar, R.H.; McDougall, L.; De Rose, F.; Jaafar-Thiel, L.; Herz, M.; Eiber, M.; et al. 61Cu-PSMA–Targeted PET for Prostate Cancer: From Radiotracer Development to First-in-Human Imaging. J. Nucl. Med. 2024, 65, 1427–1434. [Google Scholar] [CrossRef]
- Cantiello, F.; Crocerossa, F.; Russo, G.I.; Gangemi, V.; Ferro, M.; Vartolomei, M.D.; Lucarelli, G.; Mirabelli, M.; Scafuro, C.; Ucciero, G.; et al. Comparison Between 64Cu-PSMA-617 PET/CT and 18F-Choline PET/CT Imaging in Early Diagnosis of Prostate Cancer Biochemical Recurrence. Clin. Genitourin. Cancer 2018, 16, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Grubmüller, B.; Baum, R.P.; Capasso, E.; Singh, A.; Ahmadi, Y.; Knoll, P.; Floth, A.; Righi, S.; Zandieh, S.; Meleddu, C.; et al. 64Cu-PSMA-617 PET/CT Imaging of Prostate Adenocarcinoma: First In-Human Studies. Cancer Biother. Radiopharm. 2016, 31, 277–286. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration: FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 27 June 2024).
- U.S. Food & Drug Administration: FDA Approves Second PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. 2021. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-second-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 27 June 2024).
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Fani, M.; Nicolas, G.P. 61Cu-labeled radiotracers: Alternative or choice? J. Nucl. Med. 2023, 64, 1855–1857. [Google Scholar] [CrossRef]
- Meisenheimer, M.; Saenko, Y.; Eppard, E. Gallium-68: Radiolabeling of Radiopharmaceuticals for PET Imaging—A Lot to Consider. In Medical Isotopes; Naqvi, S.A.R., Imrani, M.B., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Carrasquillo, J.A.; Fine, B.M.; Pandit-Taskar, N.; Larson, S.M.; Fleming, S.E.; Fox, J.J.; Cheal, S.M.; O’donoghue, J.A.; Ruan, S.; Ragupathi, G.; et al. Imaging Patients with Metastatic Castration-Resistant Prostate Cancer Using89Zr-DFO-MSTP2109A Anti-STEAP1 Antibody. J. Nucl. Med. 2019, 60, 1517–1523. [Google Scholar] [CrossRef]
- Joraku, A.; Hatano, K.; Kawai, K.; Kandori, S.; Kojima, T.; Fukumitsu, N.; Isobe, T.; Mori, Y.; Sakata, M.; Hara, T.; et al. Phase I/IIa PET imaging study with 89zirconium labeled anti-PSMA minibody for urological malignancies. Ann. Nucl. Med. 2019, 33, 119–127. [Google Scholar] [CrossRef]
- Pretze, M.; van der Meulen, N.P.; Wängler, C.; Schibli, R.; Wängler, B. Targeted 64Cu-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Compd. Radiopharm. 2019, 62, 471–482. [Google Scholar] [CrossRef]
- Almeida, S.F.; Fonseca, A.; Sereno, J.; Ferreira, H.R.S.; Lapo-Pais, M.; Martins-Marques, T.; Rodrigues, T.; Oliveira, R.C.; Miranda, C.; Almeida, L.P.; et al. Osteosarcoma-Derived Exosomes as Potential PET Imaging Nanocarriers for Lung Metastasis. Small 2022, 18, e2203999. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bai, Z.; Zhong, W.; Li, C.; Wang, J.; Xiang, J.; Du, J.; Jia, B.; Zhu, Z. Synthesis, preclinical evaluation and pilot clinical translation of [68Ga]Ga-PMD22, a novel nanobody PET probe targeting CLDN18.2 of gastrointestinal cancer. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Schollhammer, R.; Robert, G.; Asselineau, J.; Yacoub, M.; Vimont, D.; Balamoutoff, N.; Bladou, F.; Benard, A.; Hindie, E.; de Clermont Gallerande, H.; et al. Comparison of 68Ga-PSMA-617 PET/CT and 68Ga-RM2 PET/CT in Patients with Localized Prostate Cancer Who Are Candidates for Radical Prostatectomy: A Prospective, Single-Arm, Single-Center, Phase II Study. J. Nucl. Med. 2023, 64, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.I.; Alves, V.H.; Carmo, S.J.C.D.; Silva, M.; Hrynchak, I.; Alves, F.; Falcão, A.; Abrunhosa, A.J. Production of GMP-Compliant Clinical Amounts of Copper-61 Radiopharmaceuticals from Liquid Targets. Pharmaceuticals 2022, 15, 723. [Google Scholar] [CrossRef]
- Rösch, F. Past, present and future of 68Ge/68Ga generators. Appl. Radiat. Isot. 2013, 76, 24–30. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F.; Spreckelmeyer, S. Good Practices for 68Ga Radiopharmaceutical Production. In EJNMMI Radiopharmacy and Chemistry; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2022; Volume 7. [Google Scholar]
- do Carmo, S.J.C.; Scott, P.J.H.; Alves, F. Production of Radiometals in Liquid Targets. In EJNMMI Radiopharmacy and Chemistry; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2020; Volume 5. [Google Scholar]
- Ghiani, S.; Hawala, I.; Szikra, D.; Trencsényi, G.; Baranyai, Z.; Nagy, G.; Vágner, A.; Stefania, R.; Pandey, S.; Maiocchi, A. Synthesis, radiolabeling, and pre-clinical evaluation of [44Sc]Sc-AAZTA conjugate PSMA inhibitor, a new tracer for high-efficiency imaging of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2351–2362. [Google Scholar] [CrossRef]
- Müller, C.; Singh, A.; Umbricht, C.A.; Kulkarni, H.R.; Johnston, K.; Benešová, M.; Senftleben, S.; Müller, D.; Vermeulen, C.; Schibli, R.; et al. Preclinical investigations and first-in-human application of 152Tb-PSMA-617 for PET/CT imaging of prostate cancer. EJNMMI Res. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Saini, S.; Mullen, G.E.D.; Blower, P.J.; Lapi, S.E. Radiochemistry and In Vivo Imaging of [45Ti]Ti-THP-PSMA. Mol. Pharm. 2024, 21, 822–830. [Google Scholar] [CrossRef]
- Alves, F.; Alves, V.H.P.; Do Carmo, S.J.C.; Neves, A.C.B.; Silva, M.; Abrunhosa, A.J. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod. Phys. Lett. A 2017, 32, 1740013. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Wilson, J.; Richter, S.; Duke, M.J.M.; Wuest, M.; Wuest, F. Taking cyclotron 68Ga production to the next level: Expeditious solid target production of 68Ga for preparation of radiotracers. Nucl. Med. Biol. 2020, 80–81, 24–31. [Google Scholar] [CrossRef]
- Carmo, S.J.C.D.; Alves, V.H.P.; Alves, F.; Abrunhosa, A.J. Fast and cost-effective cyclotron production of61Cu using a natZn liquid target: An opportunity for radiopharmaceutical production and R&D. Dalton Trans. 2017, 46, 14556–14560. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Schaedler, A.W.; Ho, K.-V.; Golzy, M.; Mathur, A.; Pun, M.; Gallazzi, F.; Watkinson, L.D.; Carmack, T.L.; Sikligar, K.; et al. Evaluation of a bimodal, matched pair theranostic agent targeting prostate-specific membrane antigen. Nucl. Med. Biol. 2024, 136–137, 108938. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, U.; Thomas, K.E.; Søborg Pedersen, K.; Kranz, M.; Sundset, R.; Moldes-Anaya, A.; Jensen, M. Production of 67Cu at a biomedical cyclotron via 70Zn(p,α)67Cu reaction and its evaluation in a preclinical study using small animal SPECT/CT. Appl. Radiat. Isot. 2025, 215, 111551. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Sabet, M.; Rowshanfarzad, P.; Kamali-Dehghan, M.; Akhlaghi, M.; Mirzaii, M. Optimization of the production of [61Cu]Diacetyl-bis(N4-methylthiosemicarbazone) for PET studies. J. Radioanal. Nucl. Chem. 2006, 269, 147–154. [Google Scholar] [CrossRef]
- Brühlmann, S.A.; Walther, M.; Kopka, K.; Kreller, M. Production of the PET radionuclide 61Cu via the 62Ni(p,2n)61Cu nuclear reaction. EJNMMI Radiopharm. Chem. 2024, 9, 1–16. [Google Scholar] [CrossRef]
- Alves, V.H.; Carmo, S.J.C.D.; Alves, F.; Abrunhosa, A.J. Automated Purification of Radiometals Produced by Liquid Targets. Instruments 2018, 2, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Engle, J.W.; Bean, J.; Yang, Y.; Leigh, B.R.; Barnhart, T.E.; Cai, W. Positron Emission Tomography Imaging of CD105 Expression with a 64Cu-Labeled Monoclonal Antibody: NOTA Is Superior to DOTA. PLoS ONE 2011, 6(12), e28005. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Pullambhatla, M.; Foss, C.A.; Nimmagadda, S.; Ferdani, R.; Anderson, C.J.; Mease, R.C.; Pomper, M.G. 64Cu-Labeled Inhibitors of Prostate-Specific Membrane Antigen for PET Imaging of Prostate Cancer. J. Med. Chem. 2014, 57, 2657–2669. [Google Scholar] [CrossRef]
- Lee, I.; Kim, M.H.; Lee, K.; Oh, K.; Lim, H.; Ahn, J.H.; Lee, Y.J.; Cheon, G.J.; Chi, D.Y.; Lim, S.M. Comparison of the Effects of DOTA and NOTA Chelators on 64Cu-Cudotadipep and 64Cu-Cunotadipep for Prostate Cancer. Diagnostics 2023, 13, 2649. [Google Scholar] [CrossRef]
- Tschan, V.J.; Busslinger, S.D.; Bernhardt, P.; Grundler, P.V.; Zeevaart, J.R.; Kӧster, U.; van der Meulen, N.P.; Schibli, R.; Muller, C. Albumin-Binding and Conventional PSMA Ligands in Combination with 161Tb: Biodistribution, Dosimetry, and Preclinical Therapy. J. Nucl. Med. 2023, 64, 1625–1631. [Google Scholar] [CrossRef]
- Vaccarin, C.; Mapanao, A.K.; Deberle, L.M.; Becker, A.E.; Borgna, F.; Marzaro, G.; Schibli, R.; Müller, C. Design and Preclinical Evaluation of a Novel Prostate-Specific Membrane Antigen Radioligand Modified with a Transthyretin Binder. Cancers 2024, 16, 1262. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Alves, V.; Carmo, S.D.; Alves, F.; Abrunhosa, A. Copper-61 from liquid targets: Optimized purification for GMP labelling. Nucl. Med. Biol. 2021, 96–97 (Suppl. S1), S98–S99. [Google Scholar] [CrossRef]
- Martins, P.; Blanco, A.; Crespo, P.; Marques, M.F.F.; Marques, R.F.; Gordo, P.M.; Kajetanowicz, M.; Korcyl, G.; Lopes, L.; Michel, J.; et al. Towards very high resolution RPC-PET for small animals. J. Instrum. 2014, 9, C10012. [Google Scholar] [CrossRef]



| HPLC Equipment Specifications | |||
|---|---|---|---|
| Model | Agilent 1260 Infinity II | ||
| Detector | VWD 1260 Infinity II G7114A | ||
| Column | Avantor® ACE® C18, 150 × 3 mm, 3 μm | ||
| Acquisition software | Gina X | ||
| Operating conditions of the system | |||
| Wavelength | 264 nm | ||
| Volume injected | 20 μL | ||
| Mobile phase A | Water/TFA 0.1% (v/v) | ||
| Mobile phase B | Acetonitrile/TFA 0.1% (v/v) | ||
| Method characterization | Time (min) | Mobile phase A (%) | Mobile phase B (%) |
| 0 | 80 | 20 | |
| 15 | 70 | 30 | |
| Running time | 15 min | ||
| Flow | 0.6 mL/min | ||
| iTLC equipment specifications | |||
| Model | miniGita | ||
| Detector | Scintillation | ||
| Acquisition software | TLC Control Software, version 2.30 | ||
| Operating conditions of the system | |||
| Chromatographic strips | iTLC-SG | ||
| Volume applied | 5 μL | ||
| Mobile phase | EDTA 0.1 M | ||
| Elution length | 9 cm (origin: 1.5 cm from the bottom end; elution front: 1 cm from the top end) | ||
| Running time | 5 min | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, D.; Fonseca, A.I.; do Carmo, S.; Sereno, J.; Hrynchak, I.; Moreira, J.N.; Gomes, C.; Abrunhosa, A. Is Copper-61 the New Gallium-68? Automation and Preclinical Proof-of-Concept of 61Cu-Based Radiopharmaceuticals for Prostate Cancer Imaging. Pharmaceuticals 2025, 18, 469. https://doi.org/10.3390/ph18040469
Rodrigues D, Fonseca AI, do Carmo S, Sereno J, Hrynchak I, Moreira JN, Gomes C, Abrunhosa A. Is Copper-61 the New Gallium-68? Automation and Preclinical Proof-of-Concept of 61Cu-Based Radiopharmaceuticals for Prostate Cancer Imaging. Pharmaceuticals. 2025; 18(4):469. https://doi.org/10.3390/ph18040469
Chicago/Turabian StyleRodrigues, Diana, Alexandra I. Fonseca, Sérgio do Carmo, José Sereno, Ivanna Hrynchak, João N. Moreira, Célia Gomes, and Antero Abrunhosa. 2025. "Is Copper-61 the New Gallium-68? Automation and Preclinical Proof-of-Concept of 61Cu-Based Radiopharmaceuticals for Prostate Cancer Imaging" Pharmaceuticals 18, no. 4: 469. https://doi.org/10.3390/ph18040469
APA StyleRodrigues, D., Fonseca, A. I., do Carmo, S., Sereno, J., Hrynchak, I., Moreira, J. N., Gomes, C., & Abrunhosa, A. (2025). Is Copper-61 the New Gallium-68? Automation and Preclinical Proof-of-Concept of 61Cu-Based Radiopharmaceuticals for Prostate Cancer Imaging. Pharmaceuticals, 18(4), 469. https://doi.org/10.3390/ph18040469











