Enhanced Acute Muscle Activation in ALS Patients Following Liposomal Curcumin, Resveratrol, and Dutasteride Administration
Abstract
:1. Introduction
2. Results
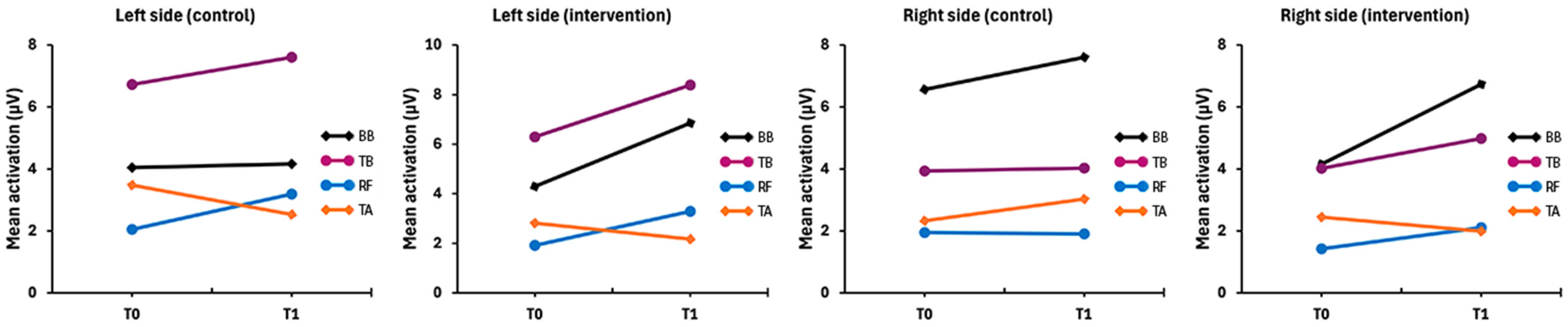
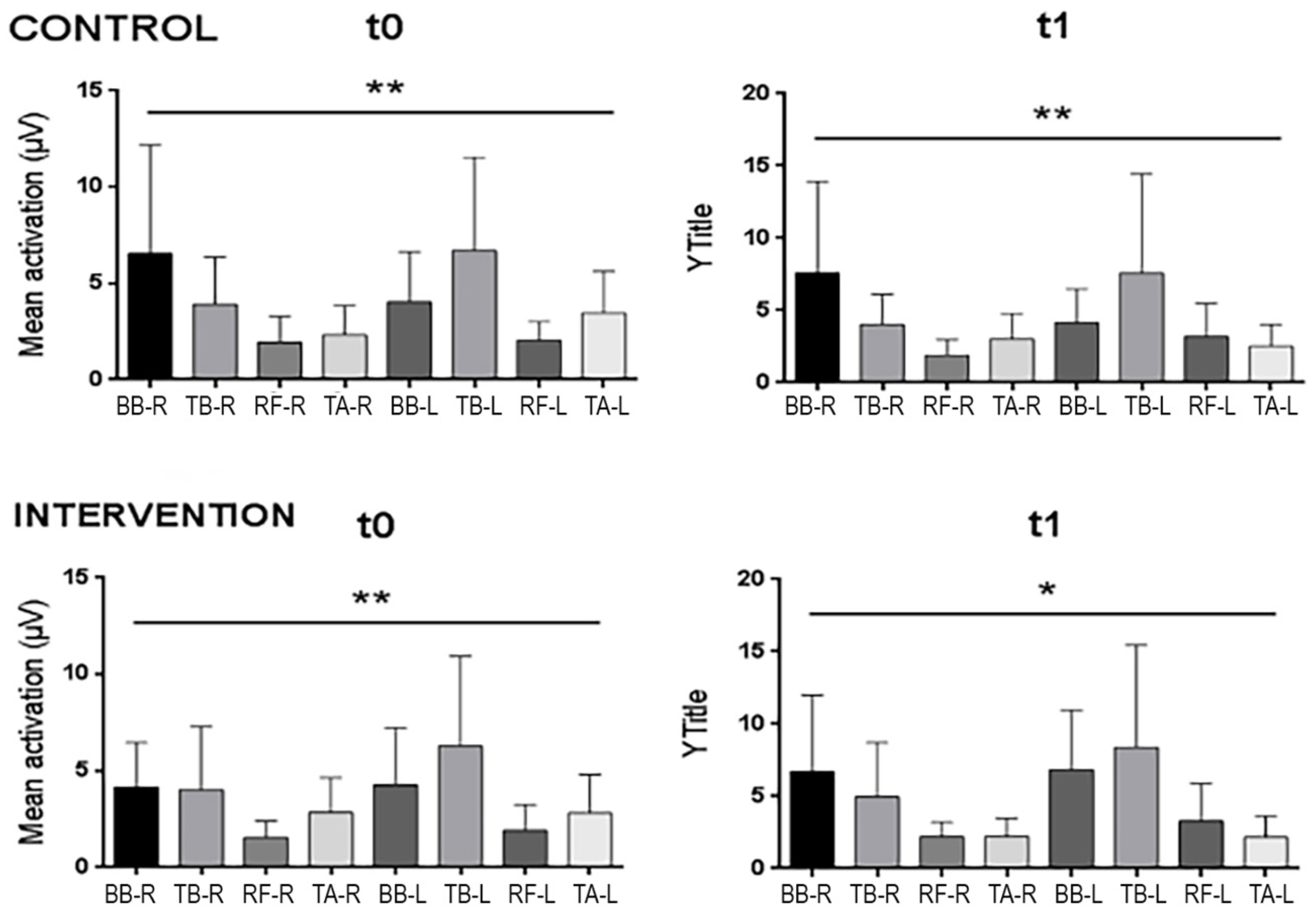
2.1. Baseline Records
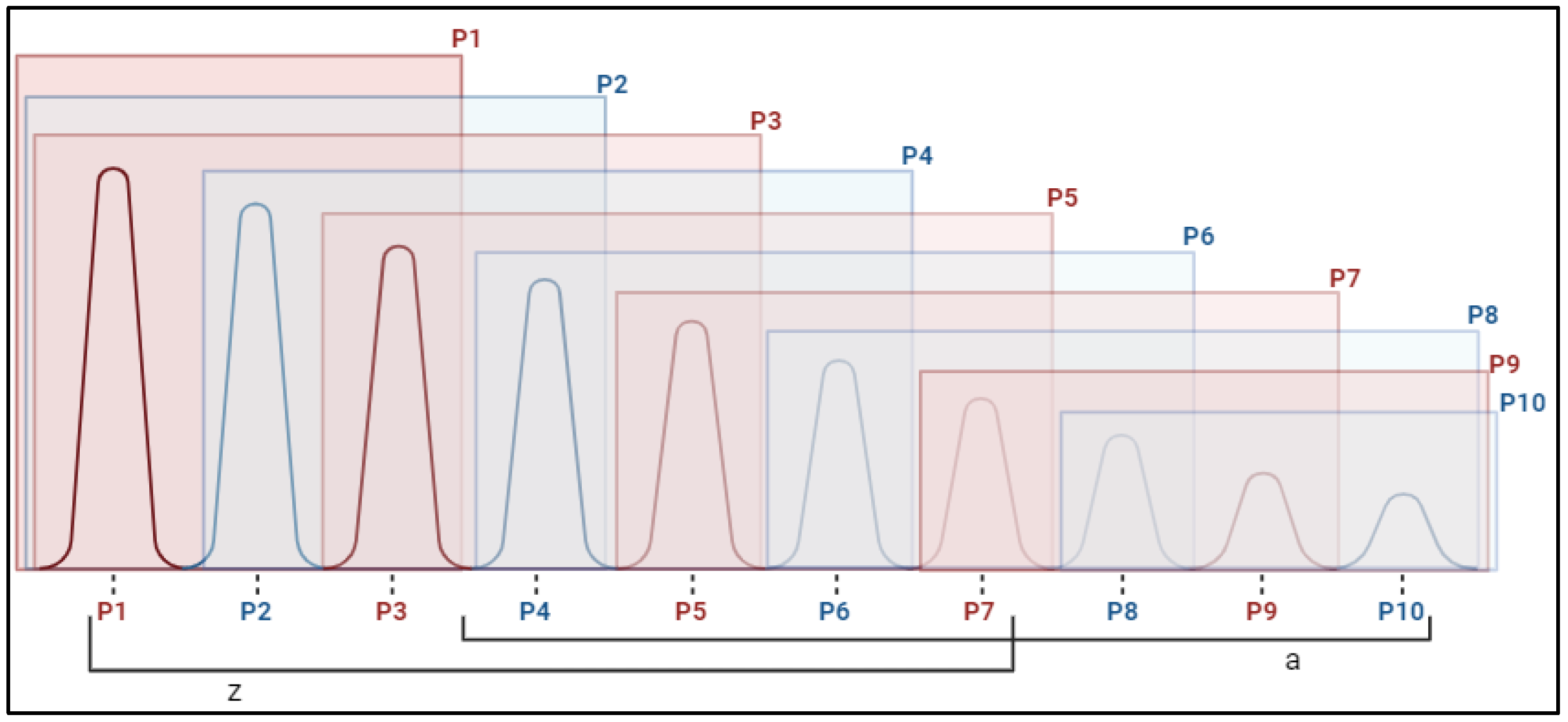
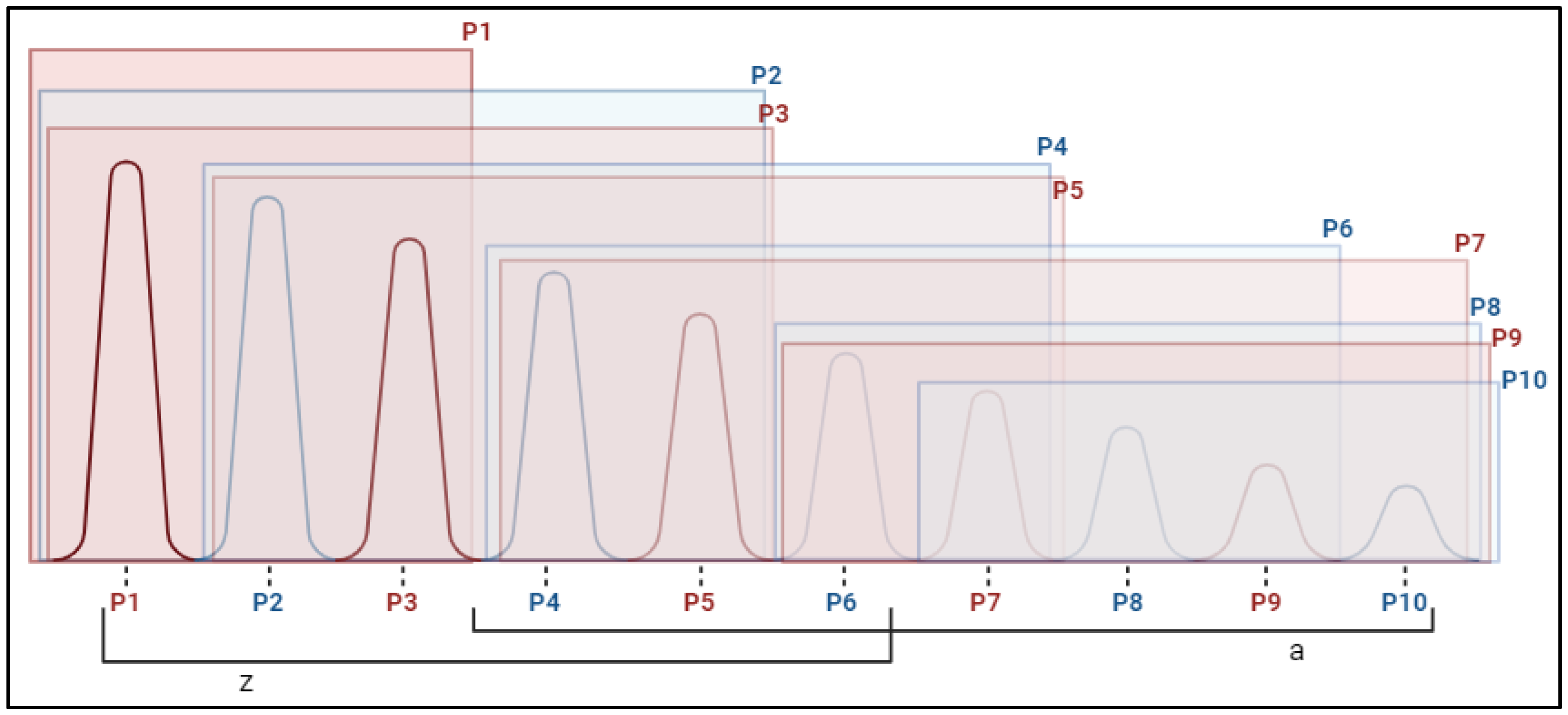
2.2. Fasciculations
3. Discussion
4. Materials and Methods
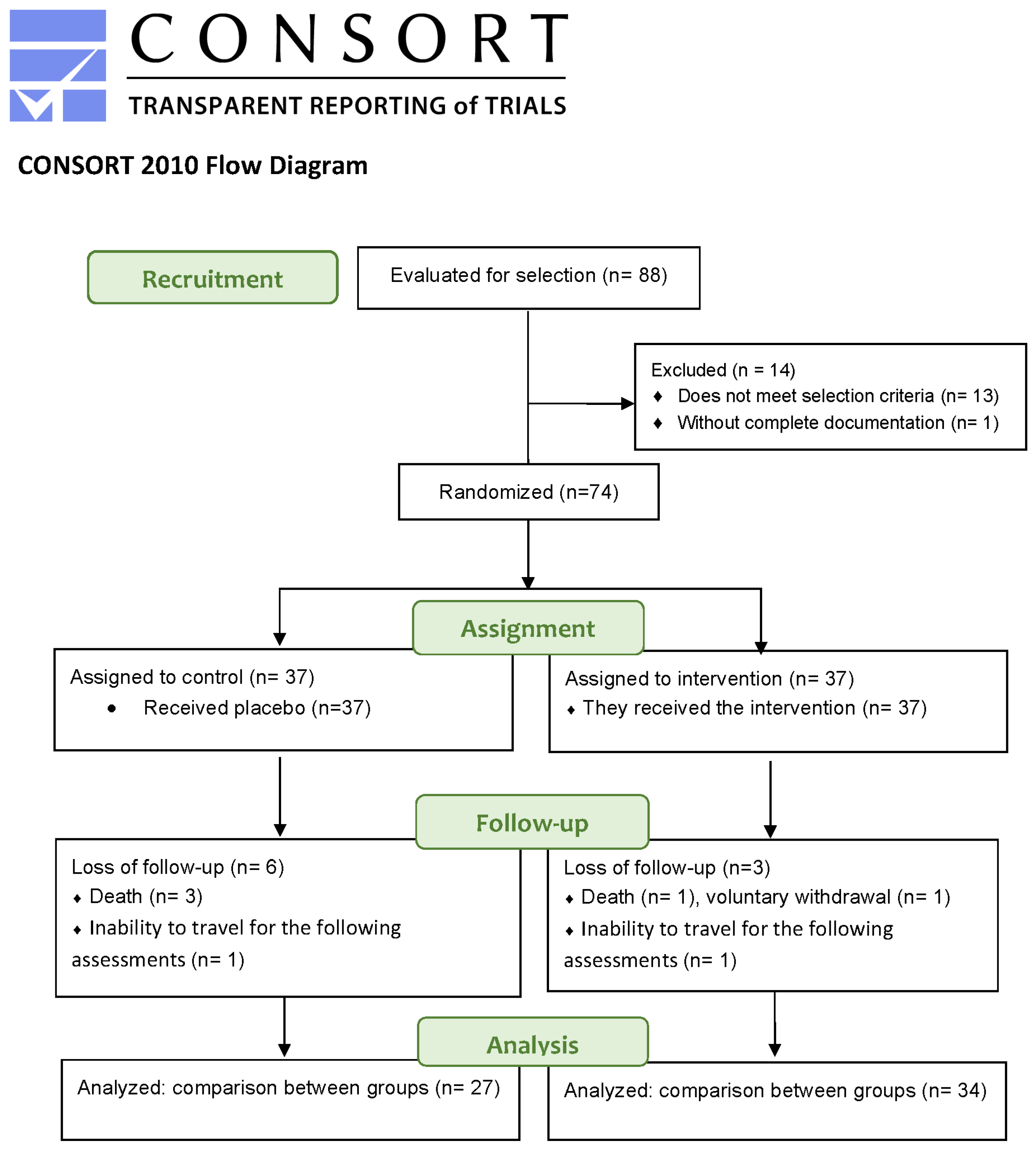
4.1. Study Design
4.2. Participants
4.3. Procedure
4.3.1. Pharmacological Administration
4.3.2. Determination of Functional Capacity
4.4. Instruments
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHP | Dihydroprogesterone |
| DHT | Dihydrotestosterone |
| ALS | Amyotrophic Lateral Sclerosis |
| ND | Neurodegenerative diseases |
| CG | Control group |
| IG | Intervention group |
| PROG | Progesterone |
| T | Testosterone |
| BB | Biceps brachii |
| TB | Triceps brachii |
| RF | Rectus femoris |
| TA | Tibialis anterior |
| sEMG | surface electromyography |
Appendix A
| CONTROL | INTERVENTION | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M ± SD | Me [RIC] | N | Min | Max | p-Value | M ± SD | Me [RIC] | N | Min | Max | p-Value | |
| Mean BB R T0 | 6.71 ± 5.6 | 5.65 [4.92] | 18 | 1.33 | 20.87 | <0.001 | 4.1 ± 3.64 | 2.84 [3.85] | 18 | 1.04 | 15.46 | 0.003 |
| Mean TB R T0 | 3.49 ± 2.57 | 3.06 [3.06] | 18 | 0.94 | 12 | 2.79 ± 1.63 | 2.28 [1.69] | 18 | 0.8 | 6.07 | ||
| Mean RF R T0 | 2.16 ± 1.24 | 1.72 [1.72] | 18 | 0.83 | 4.9 | 1.7 ± 1.06 | 1.43 [0.7] | 18 | 0.68 | 5.18 | ||
| Mean TA R T0 | 2.14 ± 1.5 | 1.54 [1.22] | 18 | 0.96 | 5.96 | 2.65 ± 2.17 | 1.89 [2.2] | 18 | 0.74 | 9.96 | ||
| Mean BB L T0 | 3.96 ± 2.71 | 2.9 [2.89] | 18 | 1.11 | 11.15 | 3.79 ± 3.33 | 2.81 [2.93] | 18 | 1.03 | 13.69 | ||
| Mean TB L T0 | 6.09 ± 4.01 | 5.1 [6.38] | 18 | 1.23 | 13.89 | 4.97 ± 4.43 | 3.5 [5.36] | 18 | 1.26 | 16.34 | ||
| Mean RF L T0 | 1.96 ± 1.15 | 1.44 [1.85] | 18 | 0.8 | 4.85 | 2.07 ± 1.44 | 1.38 [1.82] | 18 | 0.79 | 5.84 | ||
| Mean TA L T0 | 2.81 ± 1.85 | 1.99 [1.95] | 18 | 0.87 | 7.01 | 2.17 ± 1.21 | 1.83 [1.73] | 18 | 0.83 | 4.97 | ||
| Mean BB R T1 | 8.04 ± 6.58 | 5.36 [12.88] | 16 | 1.27 | 20.71 | <0.001 | 5.65 ± 5.48 | 3.44 [6.76] | 18 | 0.83 | 17.87 | 0.017 |
| Mean TB R T1 | 4.87 ± 3.93 | 4.62 [3.94] | 16 | 1.18 | 17.69 | 3.31 ± 2.15 | 2.67 [4.02] | 18 | 0.9 | 6.77 | ||
| Mean RF R T1 | 2.27 ± 1.61 | 1.8 [2.03] | 16 | 0.8 | 6.69 | 2.23 ± 1.28 | 2.18 [1.56] | 18 | 0.64 | 5.81 | ||
| Mean TA R T1 | 3.3 ± 2.36 | 2.25 [3.54] | 16 | 0.74 | 9.28 | 2.03 ± 1.24 | 1.83 [1.51] | 18 | 0.79 | 5.58 | ||
| Mean BB L T1 | 4.75 ± 2.92 | 4 [4.97] | 16 | 1.39 | 10.65 | 4.72 ± 3.7 | 3.89 [5.09] | 18 | 1.09 | 13.52 | ||
| Mean TB L T1 | 7.33 ± 6.22 | 3.7 [10.95] | 16 | 0.82 | 19.15 | 6.24 ± 6.84 | 3.72 [7.12] | 18 | 1.05 | 28.02 | ||
| Mean RF L T1 | 2.72 ± 1.13 | 2.81 [1.05] | 16 | 0.89 | 5.57 | 2.95 ± 2.26 | 2.46 [2.8] | 18 | 0.71 | 8.72 | ||
| Control | Intervention | |||
|---|---|---|---|---|
| Hedges’ g | IC 95% | Hedges’ g | IC 95% | |
| Basal activation | ||||
| Total basal | 0.012 | (−0.58; 0.6) | −0.647 | (−1.23; −0.05) |
| Total RH | −0.179 | (−0.67; 0.32) | −0.448 | (−0.95; 0.07) |
| Total LH | 0.016 | (−0.43; 0.46) | −0.743 | (−1.25; −0.21) |
| BB R | −0.152 | (−0.52; 0.22) | −0.375 | (−0.77; 0.02) |
| TB R | −0.141 | (−0.54; 0.26) | 0.086 | (−0.28; 0.45) |
| RF R | −0.072 | (−0.47; 0.33) | −0.262 | (−0.64; 0.12) |
| TA R | −0.135 | (−0.54; 0.27) | 0.171 | (−0.17; 0.51) |
| BB L | −0.172 | (−0.57; 0.23) | −0.385 | (−0.76; 0) |
| TB L | −0.092 | (−0.48; 0.3) | −0.39 | (−0.76; −0.02) |
| RF L | −0.521 | (−0.92; −0.12) | −0.389 | (−0.76; −0.01) |
| TA L | 0.443 | (0.03; 0.85) | 0.339 | (−0.04; 0.71) |
| Fasciculations | ||||
| BB R P1 | −0.205 | (−0.48; 0.07) | −0.238 | (−0.66; 0.19) |
| BB R P10 | 0.205 | (−0.25; 0.66) | −0.362 | (−0.74; 0.01) |
| BB L P1 | −0.005 | (−0.47; 0.46) | −0.584 | (−1.1; −0.07) |
| BB L P10 | 0.058 | (−0.46; 0.57) | −0.46 | (−1.01; 0.09) |
| TB R P1 | 0.377 | (−0.12; 0.87) | −0.201 | (−0.7; 0.3) |
| TB R P10 | 0.204 | (−0.19; 0.6) | −0.087 | (−0.49; 0.32) |
| TB L P1 | −0.172 | (−0.6; 0.25) | −0.519 | (−1.02; −0.02) |
| TB L P10 | −0.045 | (−0.61; 0.52) | −0.526 | (−0.91; −0.14) |
| RF R P1 | −0.638 | (−1.1; −0.17) | −0.499 | (−0.81; −0.19) |
| RF R P10 | −0.458 | (−0.87; −0.05) | −0.299 | (−0.67; 0.07) |
| RF L P1 | −0.105 | (−0.57; 0.36) | −0.203 | (−0.51; 0.11) |
| RF L P10 | −0.586 | (−1.1; −0.08) | −0.383 | (−0.92; 0.15) |
| TA R P1 | −0.143 | (−0.61; 0.33) | 0.049 | (−0.3; 0.4) |
| TA R P10 | −0.181 | (−0.87; 0.51) | 0.272 | (−0.12; 0.67) |
| TA L P1 | −0.446 | (−0.83; −0.07) | 0.202 | (−0.23; 0.63) |
| TA L P10 | 0.314 | (−0.26; 0.89) | 0.312 | (−0.13; 0.76) |
| Total Upper | −0.04 | (−0.96; 0.88) | −0.858 | (−1.56; −0.15) |
| Total Lower | −0.68 | (−1.44; 0.08) | −0.141 | (−0.69; 0.4) |
References
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [PubMed]
- Peters, T.L.; Weibull, C.E.; Fang, F.; Sandler, D.P.; Lambert, P.C.; Ye, W.; Kamel, F. Association of fractures with the incidence of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2017, 18, 419–425. [Google Scholar]
- Bucheli, M.E.; Campos, M.; Bermudes, D.B.; Chuquimarca, J.P.; Sambache, K.; Cheverrez, K.; Niato, J.F.; Montalvo, J.; Andino, A.; Calero, C.; et al. Esclerosis lateral amiotrófica: Criterios de El Escorial y la Electromiografía en su Temprano Diagnóstico. Rev. Ecuat. Neurol. 2012, 21, 61–68. [Google Scholar]
- Penedo-Vázquez, A.; Duran, X.; Mateu, J.; López-Postigo, A.; Barreiro, E. Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia. Molecules 2021, 26, 4904. [Google Scholar] [CrossRef]
- Finkelstein, J.S.; Yu, E.W.; Burnett-Bowie, S.M. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013, 369, 2457. [Google Scholar] [CrossRef]
- Morris, J. Amyotrophic Lateral Sclerosis (ALS) and Related Motor Neuron Diseases: An Overview. Neurodiagn. J. 2015, 55, 180–194. [Google Scholar]
- Joyce, N.C.; Carter, G.T. Electrodiagnosis in persons with amyotrophic lateral sclerosis. PM&R 2013, 5, S89–S95. [Google Scholar]
- Avidan, R.; Fainmesser, Y.; Drory, V.E.; Bril, V.; Abraham, A. Fasciculation frequency at the biceps brachii and brachialis muscles is associated with amyotrophic lateral sclerosis disease burden and activity. Muscle Nerve 2021, 63, 204–208. [Google Scholar] [CrossRef]
- Turner, M.R. Diagnosing ALS: The Gold Coast criteria and the role of EMG. Pract. Neurol. 2022, 22, 176–178. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Niu, J.; Zhang, L.; Fan, J.; Guan, Y.; Cui, L.; Liu, M. Fasciculation differences between ALS and non-ALS patients: An ultrasound study. BMC Neurol. 2021, 21, 441. [Google Scholar]
- Suzuki, Y.; Shibuya, K.; Misawa, S.; Suichi, T.; Tsuneyama, A.; Kojima, Y.; Nakamura, K.; Kano, H.; Prado, M.; Kuwabara, S. Fasciculation intensity and limb dominance in amyotrophic lateral sclerosis: A muscle ultrasonographic study. BMC Neurol. 2022, 22, 85. [Google Scholar]
- Masrori, P.; Van Damme, P. Amyotrophic lateral sclerosis: A clinical review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Bashford, J.A.; Wickham, A.; Iniesta, R.; Drakakis, E.M.; Boutelle, M.G.; Mills, K.R.; Shaw, C.E. The rise and fall of fasciculations in amyotrophic lateral sclerosis. Brain Commun. 2020, 2, fcaa018. [Google Scholar] [CrossRef]
- Duarte, M.L.; Iared, W.; Oliveira, A.S.B.; Dos Santos, L.R.; Peccin, M.S. Ultrasound versus electromyography for the detection of fasciculation in amyotrophic lateral sclerosis: Systematic review and meta-analysis. Radiol Bras. 2020, 53, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Rajula, R.R.; Saini, J.; Unnikrishnan, G.; Vengalil, S.; Nashi, S.; Bardhan, M.; Huddar, A.; Chawla, T.; Sindhu, D.M.; Ganaraja, V.H.; et al. Muscle ultrasonography in detecting fasciculations: A noninvasive diagnostic tool for amyotrophic lateral sclerosis. J. Clin. Ultrasound. 2022, 50, 286–291. [Google Scholar]
- Bokuda, K.; Shimizu, T.; Kimura, H.; Morishima, R.; Kamiyama, T.; Kawata, A.; Nakayama, Y.; Isozaki, E. Relationship between EMG-detected and ultrasound-detected fasciculations in amyotrophic lateral sclerosis: A prospective cohort study. Clin. Neurophysiol. 2020, 131, 259–264. [Google Scholar] [CrossRef]
- Bashford, J.; Mills, K.; Shaw, C. The evolving role of surface electromyography in amyotrophic lateral sclerosis: A systematic review. Clin. Neurophysiol. 2020, 131, 942–950. [Google Scholar] [CrossRef]
- Andersen, P.M.; Borasio, G.D.; Dengler, R.; Hardiman, O.; Kollewe, K.; Leigh, P.N.; Pradat, P.; Silani, V.; Tomik, B. Good practice in the management of amyotrophic lateral sclerosis: Clinical guidelines. An evidence-based review with good practice points. EALSC Working Group. Amyotroph. Lateral Scler. 2007, 8, 195–213. [Google Scholar]
- Soriani, M.-H.; Desnuelle, C. Care management in amyotrophic lateral sclerosis. Rev Neurol. 2017, 173, 288–299. [Google Scholar] [CrossRef]
- Novak, V.; Rogelj, B.; Župunski, V. Therapeutic Potential of Polyphenols in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Antioxidants 2021, 10, 1328. [Google Scholar] [CrossRef]
- Viegas, F.P.D.; Gontijo, V.S.; de Freitas Silva, M.; Ortiz, C.J.C.; Dos Reis Rosa Franco, G.; Ernesto, J.T.; Damasio, C.M.; Silva, I.M.F.; Campos, T.G.; Viegas, C. Curcumin, Resveratrol and Cannabidiol as Natural Key Prototypes in Drug Design for Neuroprotective Agents. Curr. Neuropharmacol. 2022, 20, 1297–1328. [Google Scholar] [PubMed]
- Awasthi, M.; Upadhyay, A.K.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Terpenoids as promising therapeutic molecules against Alzheimer’s disease: Amyloid beta-and acetylcholinesterase-directed pharmacokinetic and molecular docking analyses. Mol. Simul. 2018, 44, 1–11. [Google Scholar]
- Yin, W.; Li, Y. Curcumin upregulate expression of HO-1 and Nrf-2 in SHSY5Y cells. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar]
- Akinyemi, A.J.; Oboh, G.; Fadaka, A.O.; Olatunji, B.P.; Akomolafe, S. Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats. Neurotoxicology 2017, 62, 75–79. [Google Scholar] [PubMed]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar]
- Zhang, L.-N.; Hao, L.; Wang, H.-Y.; Su, H.-N.; Sun, Y.-J.; Yang, X.-Y.; Che, B.; Xue, J.; Gao, Z.B. Neuroprotective effect of resveratrol against glutamate-induced excitotoxicity. Adv. Clin. Exp. Med. 2015, 24, 161–165. [Google Scholar]
- Wang, J.; Zhang, Y.; Tang, L.; Zhang, N.; Fan, D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 2011, 503, 250–255. [Google Scholar]
- Mancuso, R.; Del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Núñez, O.; Pallás, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol improves motoneuron function and extends survival in SOD1G93A ALS mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar]
- Chico, L.; Ienco, E.C.; Bisordi, C.; Lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial After Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar]
- Kato, H.; Sato, H.; Okuda, M.; Wu, J.; Koyama, S.; Izumi, Y.; Waku, T.; Iino, M.; Aoki, M.; Arawaka, S.; et al. Therapeutic effect of a novel curcumin derivative GT863 on a mouse model of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 489–495. [Google Scholar]
- Liu, X.; Lin, L.; Hu, G. Meta-analysis of the effect of curcumin supplementation on skeletal muscle damage status. PLoS ONE 2024, 19, e0299135. [Google Scholar]
- Rakotoarisoa, M.; Angelova, A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines 2018, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Poltronieri, P.; Xu, B.; Giovinazzo, G. Resveratrol and other Stilbenes: Effects on Dysregulated Gene Expression in Cancers and Novel Delivery Systems. Anticancer Agents Med. Chem. 2021, 21, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-Esfahani, S. Nanotechnology-applied curcumin for different diseases therapy. BioMed Res. Int. 2014, 2014, 394264. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-On Therapy to Riluzole in Patients with Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef]
- Huang, M.; Liang, C.; Tan, C.; Huang, S.; Ying, R.; Wang, Y.; Wang, Z.; Zhang, Y. Liposome co-encapsulation as a strategy for the delivery of curcumin and resveratrol. Food Funct. 2019, 10, 6447–6458. [Google Scholar] [CrossRef]
- Andriole, G.L.; Kirby, R. Safety and tolerability of the dual 5α-reductase inhibitor dutasteride in the treatment of benign prostatic hyperplasia. Eur. Urol. 2003, 44, 82–88. [Google Scholar] [CrossRef]
- Jin, Y.; Penning, T.M. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: Key enzymes in androgen metabolism. Best. Pract. Res. Clin. Endocrinol. Metab. 2001, 15, 79–94. [Google Scholar] [CrossRef]
- Gargiulo Monachelli, G.; Meyer, M.; Rodríguez, G.E.; Garay, L.I.; Sica, R.E.P.; De Nicola, A.F.; Deniselle, M.C.G. Endogenous progesterone is associated to amyotrophic lateral sclerosis prognostic factors. Acta Neurol. Scand. 2011, 123, 60–67. [Google Scholar] [CrossRef]
- Merz, S.F.; Bengtson, C.P.; Tepohl, C.; Hagenston, A.M.; Bading, H.; Bas-Orth, C. A microscopy-based small molecule screen in primary neurons reveals neuroprotective properties of the FDA-approved anti-viral drug Elvitegravir. Mol. Brain 2020, 13, 124. [Google Scholar] [CrossRef]
- Cook, C.N.; Wu, Y.; Odeh, H.M.; Gendron, T.F.; Jansen-West, K.; Del Rosso, G.; Yue, M.; Jiang, P.; Gomes, E.; Tong, J.; et al. C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy. Sci. Transl. Med. 2020, 12, eabb3774. [Google Scholar] [PubMed]
- Luo, D.; Han, L.; Gao, S.; Xiao, Z.; Zhou, Q.; Cheng, X.; Zhang, Y.; Zhou, W. LINCS Dataset-Based Repositioning of Dutasteride as an Anti-Neuroinflammation Agent. Brain Sci. 2021, 11, 1411. [Google Scholar] [CrossRef] [PubMed]
- Meydan, S.; Kus, I.; Tas, U.; Ogeturk, M.; Sancakdar, E.; Dabak, D.O.; Zararsız, I.; Sarsılmaz, M. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J. Chem. Neuroanat. 2010, 40, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lee, J.; Kim, H.; Kim, D.; Ahn, Y.; Son, C. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J. Neurochem. 2016, 136, 106–117. [Google Scholar]
- Ota, H.; Akishita, M.; Akiyoshi, T.; Kahyo, T.; Setou, M.; Ogawa, S.; Iijima, K.; Eto, M.; Ouchi, Y. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: Protective role of eNOS and SIRT1. PLoS ONE 2012, 7, e29598. [Google Scholar]
- Storer, T.W.; Basaria, S.; Traustadottir, T.; Harman, S.M.; Pencina, K.; Li, Z.; Travison, T.G.; Miciek, R.; Tsitouras, P.; Hally, K.; et al. Effects of Testosterone Supplementation for 3 Years on Muscle Performance and Physical Function in Older Men. J. Clin. Endocrinol. Metab. 2017, 102, 583–593. [Google Scholar] [CrossRef]
- Huang, G.; Basaria, S.; Travison, T.G.; Ho, M.H.; Davda, M.; Mazer, N.A.; Miciek, R.; Knapp, P.E.; Zhang, A.; Collins, L.; et al. Testosterone dose-response relationships in hysterectomized women with or without oophorectomy: Effects on sexual function, body composition, muscle performance and physical function in a randomized trial. Menopause 2014, 21, 612–623. [Google Scholar]
- Hirschberg, A.L.; Elings Knutsson, J.; Helge, T.; Godhe, M.; Ekblom, M.; Bermon, S.; Ekblom, B. Effects of moderately increased testosterone concentration on physical performance in young women: A double blind, randomised, placebo controlled study. Br. J. Sports Med. 2020, 54, 599–604. [Google Scholar]
- Bermon, S.; Garnier, P. Serum androgen levels and their relation to performance in track and field: Mass spectrometry results from 2127 observations in male and female elite athletes. Br. J. Sports Med. 2017, 51, 1309–1314. [Google Scholar]
- Dam, T.V.; Dalgaard, L.B.; Ringgaard, S.; Johansen, F.T.; Bisgaard Bengtsen, M.; Mose, M.; Lauritsen, K.M.; Ørtenblad, N.; Gravholt, C.H.; Hansen, M. Transdermal Estrogen Therapy Improves Gains in Skeletal Muscle Mass After 12 Weeks of Resistance Training in Early Postmenopausal Women. Front. Physiol. 2020, 11, 596130. [Google Scholar]
- Hansen, M.; Skovgaard, D.; Reitelseder, S.; Holm, L.; Langbjerg, H.; Kjaer, M. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1005–1013. [Google Scholar] [PubMed]
- Inghilleri, M.; Iacovelli, E. Clinical neurophysiology in ALS. Arch. Ital. Biol. 2011, 149, 57–63. [Google Scholar] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [PubMed]
- BioMed Research International. Retracted: Resveratrol Ameliorates Motor Neuron Degeneration and Improves Survival in SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. BioMed Res. Int. 2019, 2019, 4729651. [Google Scholar]
- Neltner, T.J.; Anders, J.P.V.; Keller, J.L.; Smith, R.W.; Housh, T.J.; Schmidt, R.J.; Johnson, G.O. Velocity-Specific Coactivation and Neuromuscular Responses to Fatiguing, Reciprocal, Isokinetic, Forearm Flexion, and Extension Muscle Actions. J. Strength Cond. Res. 2022, 36, 649–660. [Google Scholar]
- Alarcon-Jimenez, J.; de la Rubia Orti, J.E.; Martin Ruiz, J.; de Bernardo, N.; Proano, B.; Villaron-Casales, C. Muscular Response in ALS Patients during Maximal Bilateral Isometric Work of the Biceps Brachii until Fatigue. Life 2022, 12, 1978. [Google Scholar] [CrossRef]
- Ortega-Hombrados, L.; Molina-Torres, G.; Galan-Mercant, A.; Sanchez-Guerrero, E.; Gonzalez-Sanchez, M.; Ruiz-Munoz, M. Systematic Review of Therapeutic Physical Exercise in Patients with Amyotrophic Lateral Sclerosis over Time. Int. J. Environ. Res. Public Health 2021, 18, 1074. [Google Scholar] [CrossRef]
- Proaño, B.; Casani-Cubel, J.; Benlloch, M.; Rodriguez-Mateos, A.; Navarro-Illana, E.; Lajara-Romance, J.M.; Ortí, J.E.d.l.R. Is Dutasteride a Therapeutic Alternative for Amyotrophic Lateral Sclerosis? Biomedicines 2022, 10, 2084. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Rizzi, L.; Bresciani, E.; Omeljaniuk, R.J.; Torsello, A. Androgen Therapy in Neurodegenerative Diseases. J. Endocr. Soc. 2020, 4, bvaa120. [Google Scholar]
- De Nicola, A.F.; Meyer, M.; Garay, L.; Kruse, M.S.; Schumacher, M.; Guennoun, R.; Deniselle, M.C.G. Progesterone and Allopregnanolone Neuroprotective Effects in the Wobbler Mouse Model of Amyotrophic Lateral Sclerosis. Cell. Mol. Neurobiol. 2022, 42, 23–40. [Google Scholar]
- Bhasin, S.; Calof, O.M.; Storer, T.W.; Lee, M.L.; Mazer, N.A.; Jasuja, R.; Montori, V.M.; Gao, W.; Dalton, J.T. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, Z.; Farahani, R.M.; Aliaghaei, A.; Khodagholi, F.; Meftahi, G.H.; Danyali, S.; Abdollahifar, M.A.; Daftari, M.; Boroujeni, M.E.; Sadeghi, Y. Resveratrol Protects Purkinje Neurons and Restores Muscle Activity in Rat Model of Cerebellar Ataxia. J. Mol. Neurosci. 2018, 65, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Bayat, A.; Boroujeni, M.E.; Abdollahifar, M.A.; Ebrahimi, V.; Danyali, S.; Heidari, M.H.; Aliaghaei, A. Curcumin protects purkinje neurons ameliorates motor function reduces cerebellar atrophy in rat model of cerebellar ataxia induced by 3-AP. J. Chem. Neuroanat. 2019, 102, 101706. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Kiernan, M.C.; Swash, M. Fasciculation in amyotrophic lateral sclerosis: Origin and pathophysiological relevance. J. Neurol. Neurosurg. Psychiatry 2017, 88, 773–779. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Holobar, A.; Watanabe, K.; Takahashi, T.; Ueno, H.; Maeda, N.; Maruyama, H.; Tanaka, S.; Hyngstrom, A.S. Detecting motor unit abnormalities in amyotrophic lateral sclerosis using high-density surface, E.M.G. Clin. Neurophysiol. 2022, 142, 262–272. [Google Scholar] [CrossRef]
- Takamatsu, N.; Nodera, H.; Mori, A.; Maruyama-Saladini, K.; Osaki, Y.; Shimatani, Y.; Oda, M.; Izumi, Y.; Kaji, R. Which muscle shows fasciculations by ultrasound in patients with ALS? J. Med. Investig. 2016, 63, 49–53. [Google Scholar]
- Mogyoros, I.; Kiernan, M.C.; Burke, D.; Bostock, H. Excitability changes in human sensory and motor axons during hyperventilation and ischaemia. Brain 1997, 120 Pt 2, 317–325. [Google Scholar] [CrossRef]
- Kanning, K.C.; Kaplan, A.; Henderson, C.E. Motor neuron diversity in development and disease. Annu. Rev. Neurosci. 2010, 33, 409–440. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, Y.; Yu, I.; Yeh, S.; Wu, C.; Miyamoto, H.; Merry, D.E.; Sobue, G.; Chen, L.-M.; Chang, S.-S.; et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 2007, 13, 348–353. [Google Scholar]
- Maset-Roig, R.; Caplliure-Llopis, J.; de Bernardo, N.; Privado, J.; Alarcón-Jiménez, J.; Martín-Ruiz, J.; Botella-Navas, M.; Villarón-Casales, C.; Sancho-Cantus, D.; de la Rubia Ortí, J.E. Analysis of Heart Rate Variability in Individuals Affected by Amyotrophic Lateral Sclerosis. Sensors 2024, 24, 2355. [Google Scholar] [CrossRef]
- Kollewe, K.; Mauss, U.; Krampfl, K.; Petri, S.; Dengler, R.; Mohammadi, B. ALSFRS-R score and its ratio: A useful predictor for ALS-progression. J. Neurol. Sci. 2008, 275, 69–73. [Google Scholar] [CrossRef]
- SENIAM Sensor Locations. 2017. Available online: http://seniam.org/sensor_location.htm (accessed on 26 December 2017).
- Crisswell, E. Cram’s Introduction to Surface Electromyography, 2nd ed.; SciTech Book News; Jones & Bartlett Publishers: Burlington, MA, USA, 2010; Volume 34, Issue 3. [Google Scholar]


| M ± SD | ||
|---|---|---|
| Age (years) | 56.74 ± 10.47 | |
| Women | 38.2% | |
| Diagnostic time (months) | 29.63 ± 27.35 | |
| ALSFRS-R (points) | ALSFRS-R | 29.16 ± 8.1 |
| Type of ALS (by body region) | Spinal | 80.9% |
| Bulbar | 19.1% |
| Control | Intervention | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | p * | g | Pre | Post | p * | g | |
| M ± SD | M ± SD | M ± SD | M ± SD | |||||
| Total (μV) | 34.32 ± 8.96 | 34.16 ± 12.51 | 0.041 | 0.01 | 21.31 ± 9.20 | 31.02 ± 14.59 | 0.035 | −0.65 |
| Total RH (μV) | 15.45 ± 7.61 | 16.87 ± 7.36 | 0.489 | −0.18 | 10.45 ± 5.51 | 13.90 ± 7.17 | 0.053 | −0.45 |
| Total LH (μV) | 16.74 ± 7.9 | 16.60 ± 7.53 | 0.944 | 0.02 | 12.74 ± 6.32 | 21.54 ± 11 | 0.004 | −0.74 |
| Control | Intervention | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | n | p-Value | Pre | Post | n | p-Value | |||||
| M | SD | M | SD | M | SD | M | SD | |||||
| BB-R P1 | 28.68 | 25.23 | 35.27 | 27.24 | 27 | 0.186 | 28.15 | 22.19 | 35.94 | 27.65 | 34 | 0.206 |
| BB-R P10 | 14.41 | 12.31 | 12.65 | 9.90 | 27 | 0.548 | 12.23 | 12.67 | 14.20 | 13.54 | 32 | 0.011 |
| BB-L P1 | 19.27 | 14.81 | 22.80 | 17.16 | 27 | 0.968 | 17.13 | 11.06 | 30.81 | 23.33 | 33 | 0.056 |
| BB-L P10 | 8.82 | 6.83 | 9.21 | 5.00 | 26 | 0.710 | 8.72 | 6.16 | 11.49 | 8.67 | 29 | 0.220 |
| TB-R P1 | 23.79 | 16.57 | 19.67 | 12.31 | 27 | 0.209 | 18.78 | 14.89 | 19.71 | 17.08 | 30 | 0.246 |
| TB-R P10 | 10.32 | 8.55 | 9.29 | 6.43 | 26 | 0.511 | 8.70 | 7.55 | 9.11 | 8.65 | 32 | 0.452 |
| TB-L P1 | 23.77 | 17.71 | 32.65 | 27.99 | 26 | 0.424 | 25.47 | 21.16 | 35.40 | 23.09 | 29 | 0.012 |
| TB-L P10 | 12.87 | 9.77 | 14.48 | 11.21 | 26 | 0.797 | 12.04 | 9.77 | 18.15 | 14.66 | 32 | 0.007 |
| Total upper | 483.79 | 244.07 | 585.23 | 295.34 | 22 | 0.477 | 446.07 | 243.69 | 611.73 | 374.29 | 22 | 0.017 |
| RF-R P1 | 9.00 | 5.69 | 17.56 | 14.04 | 26 | 0.001 | 11.25 | 9.03 | 20.81 | 18.54 | 32 | 0.003 |
| RF-R P10 | 4.90 | 3.76 | 7.38 | 5.94 | 27 | 0.055 | 5.34 | 4.47 | 7.66 | 7.33 | 31 | 0.076 |
| RF-L P1 | 12.25 | 8.89 | 13.65 | 8.87 | 26 | 0.501 | 13.98 | 10.43 | 16.98 | 14.01 | 32 | 0.313 |
| RF-L P10 | 3.1 | 2.40 | 6.38 | 5.19 | 26 | 0.004 | 5.33 | 4.41 | 8.12 | 6.25 | 31 | 0.118 |
| TA-R P1 | 17.56 | 14.81 | 19.69 | 18.04 | 25 | 0.710 | 16.53 | 12.11 | 16.82 | 16.30 | 32 | 0.381 |
| TA-R P10 | 4.37 | 3.96 | 5.28 | 4.38 | 25 | 0.689 | 5.87 | 4.43 | 4.42 | 3.93 | 30 | 0.265 |
| TA-L P1 | 12.62 | 9.45 | 20.90 | 18.27 | 27 | 0.036 | 20.45 | 18.33 | 17.02 | 14.26 | 34 | 0.281 |
| TA-L P10 | 7.03 | 6.31 | 4.90 | 3.79 | 23 | 0.372 | 7.36 | 7.22 | 5.02 | 4.36 | 33 | 0.240 |
| Total lower | 233.10 | 96.71 | 327.11 | 172.03 | 17 | 0.037 | 275.26 | 165.74 | 315.57 | 177.53 | 24 | 0.535 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Ruiz, J.; Maset-Roig, R.; Caplliure-Llopis, J.; Villarón-Casales, C.; Alarcón-Jiménez, J.; de Bernardo, N.; Proaño, B.; Menargues-Ramírez, R.; Selvi-Sabater, P.; de la Rubia-Ortí, J.E. Enhanced Acute Muscle Activation in ALS Patients Following Liposomal Curcumin, Resveratrol, and Dutasteride Administration. Pharmaceuticals 2025, 18, 497. https://doi.org/10.3390/ph18040497
Martín-Ruiz J, Maset-Roig R, Caplliure-Llopis J, Villarón-Casales C, Alarcón-Jiménez J, de Bernardo N, Proaño B, Menargues-Ramírez R, Selvi-Sabater P, de la Rubia-Ortí JE. Enhanced Acute Muscle Activation in ALS Patients Following Liposomal Curcumin, Resveratrol, and Dutasteride Administration. Pharmaceuticals. 2025; 18(4):497. https://doi.org/10.3390/ph18040497
Chicago/Turabian StyleMartín-Ruiz, Julio, Rosa Maset-Roig, Jordi Caplliure-Llopis, Carlos Villarón-Casales, Jorge Alarcón-Jiménez, Nieves de Bernardo, Belén Proaño, Rubén Menargues-Ramírez, Pablo Selvi-Sabater, and José Enrique de la Rubia-Ortí. 2025. "Enhanced Acute Muscle Activation in ALS Patients Following Liposomal Curcumin, Resveratrol, and Dutasteride Administration" Pharmaceuticals 18, no. 4: 497. https://doi.org/10.3390/ph18040497
APA StyleMartín-Ruiz, J., Maset-Roig, R., Caplliure-Llopis, J., Villarón-Casales, C., Alarcón-Jiménez, J., de Bernardo, N., Proaño, B., Menargues-Ramírez, R., Selvi-Sabater, P., & de la Rubia-Ortí, J. E. (2025). Enhanced Acute Muscle Activation in ALS Patients Following Liposomal Curcumin, Resveratrol, and Dutasteride Administration. Pharmaceuticals, 18(4), 497. https://doi.org/10.3390/ph18040497










