Clove (Syzygium aromaticum) Pods: Revealing Their Antioxidant Potential via GC-MS Analysis and Computational Insights
Abstract
1. Introduction
2. Results
2.1. Antioxidant Properties Results
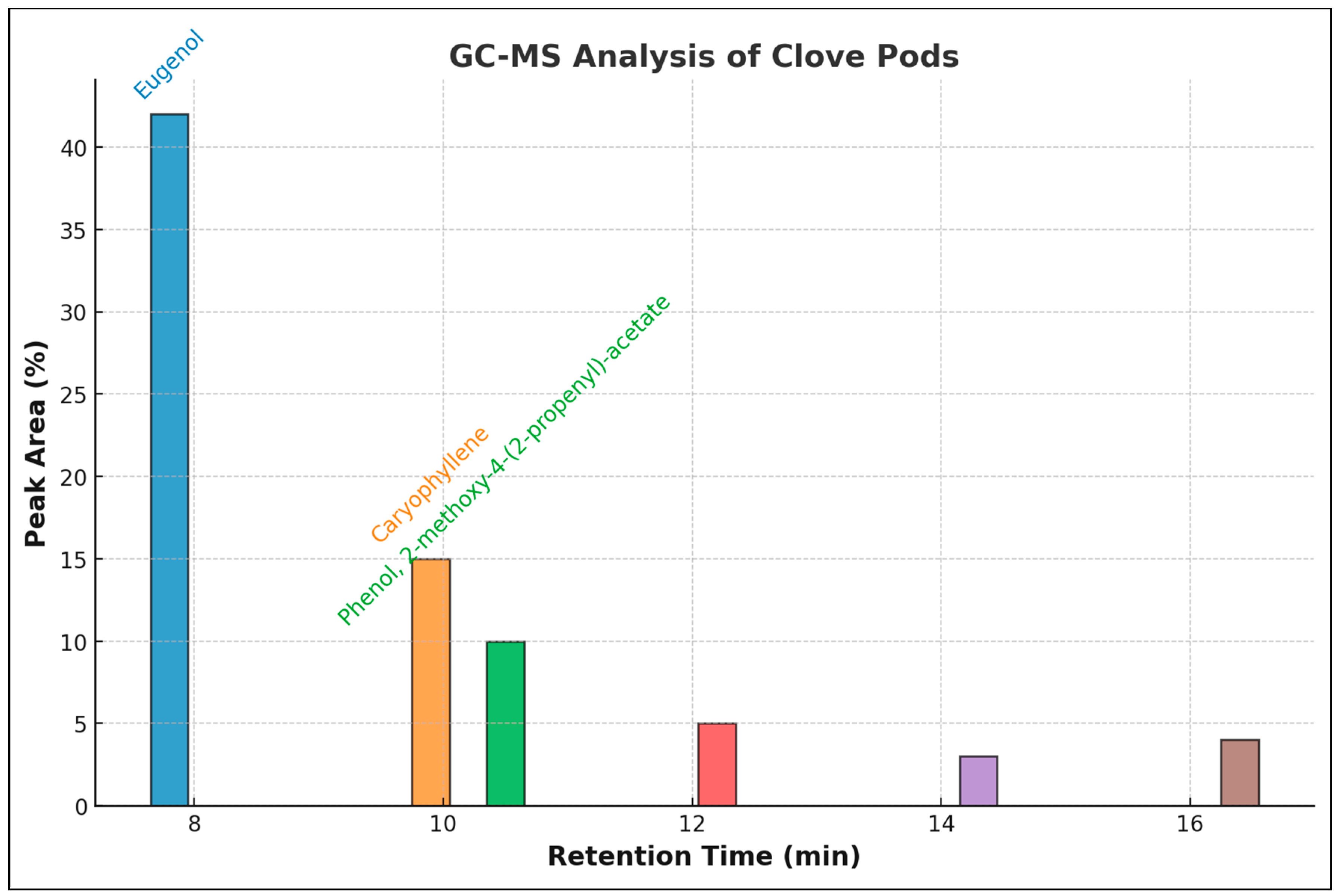
2.2. GC-MS of Clove Pods
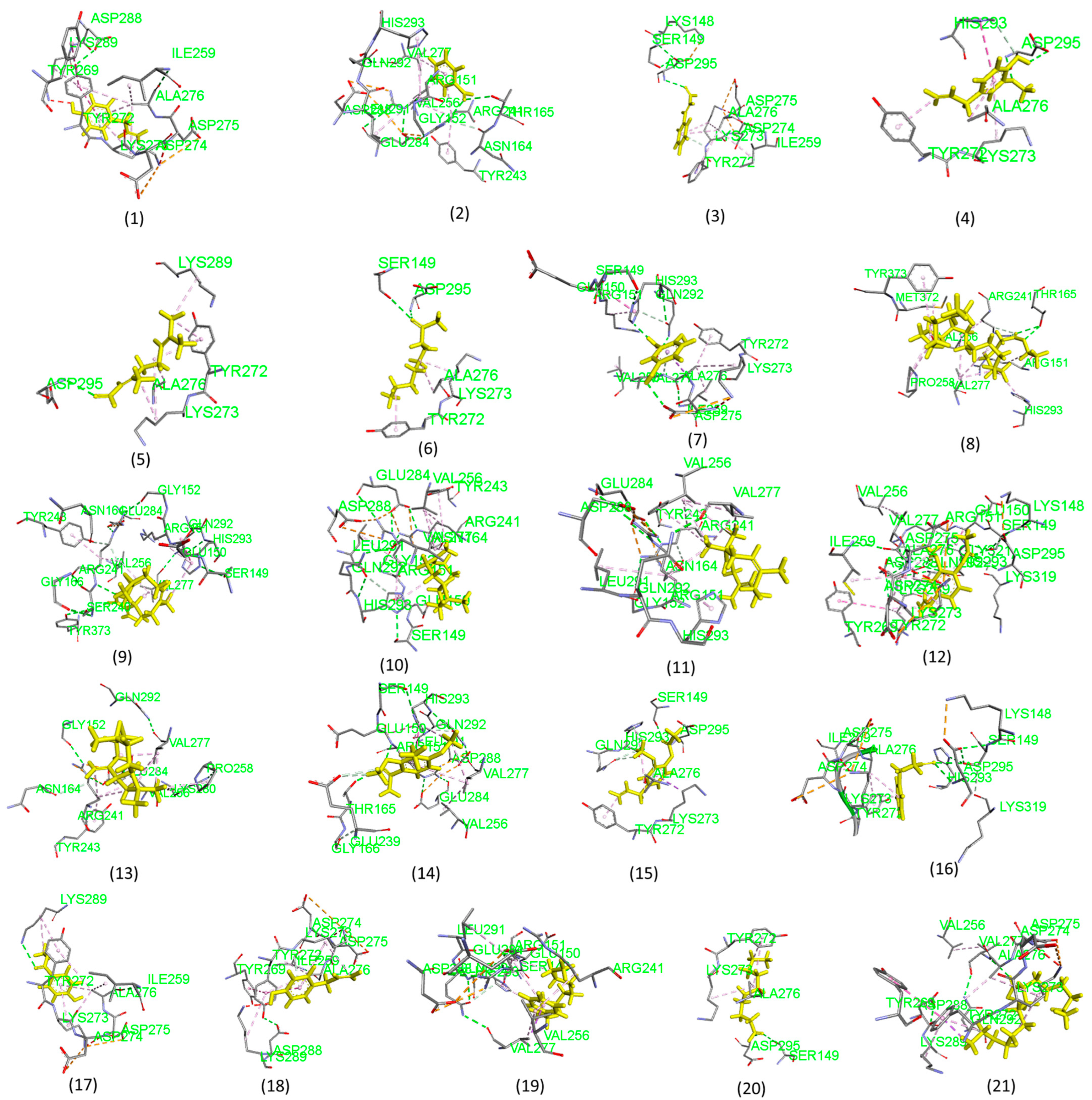
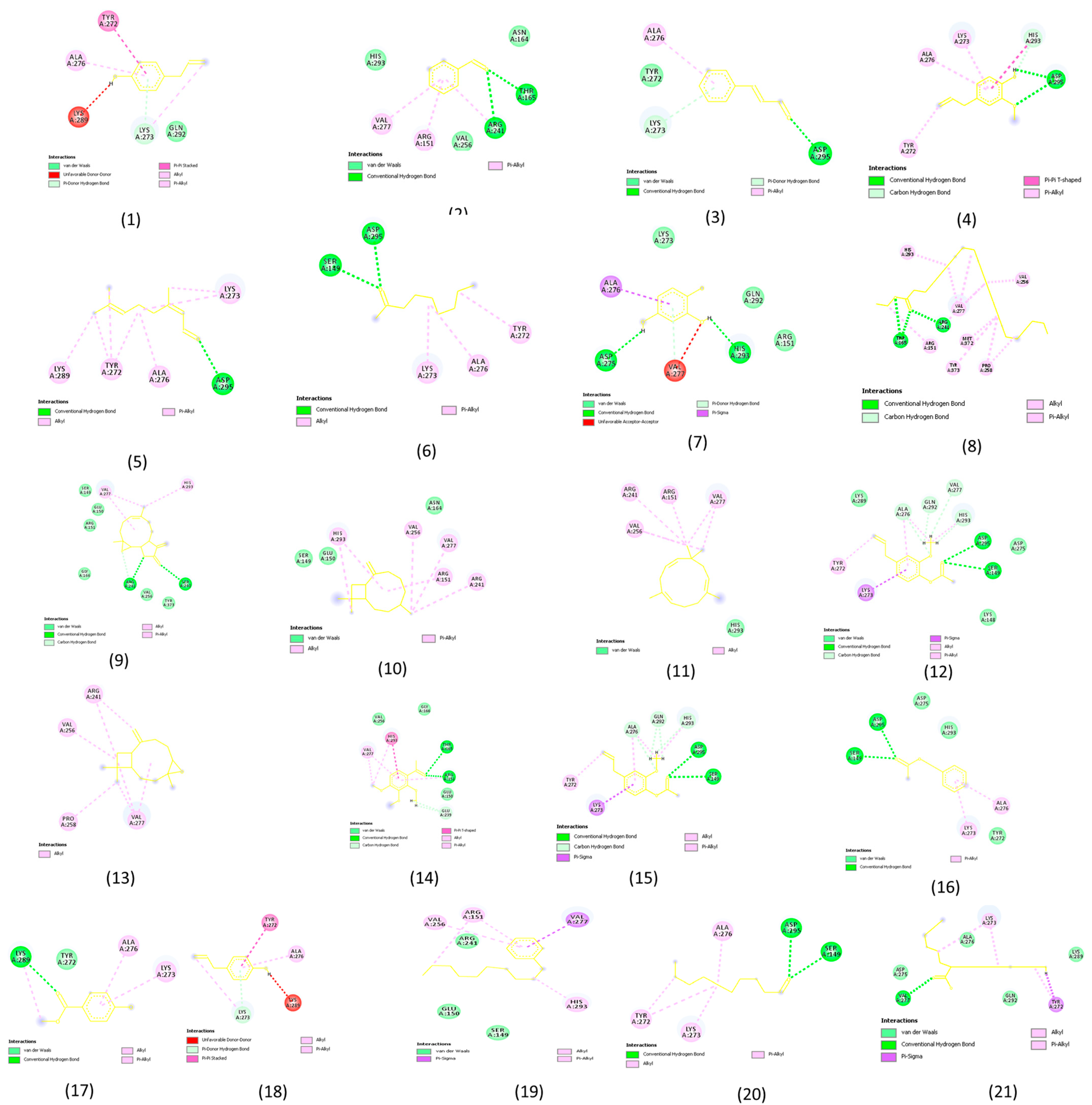
2.3. Molecular Docking Analysis Results
3. Discussion
3.1. Antioxidant Properties of Clove Pods
3.2. GC-MS Constituents
3.3. Molecular Docking Analysis
4. Materials and Methods
4.1. Collection and Preparation of the Plant Extract
4.2. Antioxidant Properties
4.2.1. Total Phenolics Measurement
4.2.2. Calculating Total Flavonoids
4.2.3. DPPH Radical Test
4.2.4. ABTS Radical Scavenging Activity Assay
4.3. β-Carotene/Linoleic Acid Method
4.4. GC-MS Analysis
4.5. Molecular Docking
4.5.1. Protein Structure Preparation
4.5.2. Performing Molecular Docking
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, A.; del Carmen Flores-Vallejo, R.; Cardoso-Taketa, A.; Villarreal, M.L. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017, 208, 264–329. [Google Scholar] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar]
- Sulieman, A.M.E.; Shaarawy, S.M.; Alghamdi, A.A.; Veettil, V.N.; Abdelgadir, M.; Ibrahim, N.A. Evaluation of antimicrobial and synergistic effects of selected medicinal plants of Hail area with antibiotics. Biosci. Biotechnol. Res. Commun. 2017, 10, 44–50. [Google Scholar]
- Sulieman, A.M.E.; Alanaizy, E.; Alanaizy, N.A.; Abdallah, E.M.; Idriss, H.; Salih, Z.A.; Abd El Hakeem, B.S. Unveiling chemical, antioxidant and antibacterial properties of fagonia indica grown in the hail mountains, Saudi arabia. Plants 2023, 12, 1354. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, S.; Dash, K.K.; Singh, R.; Dar, A.H.; Singh, T.; Farooqui, A.; Shaikh, A.M.; Kovacs, B. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: A comprehensive review. Heliyon 2023, 10, e22437. [Google Scholar]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular Basis of the Therapeutical Potential of Clove (Syzygium aromaticum L.) and Clues to Its Anti-COVID-19 Utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Dey, B.K.; Mukherjee, S.S. Potential of clove and its nutritional benefits in physiological perspective: A review. Int. J. Physiol. Nutr. Phys. Educ. 2021, 6, 103–106. [Google Scholar]
- Daniel, A.N.; Sartoretto, S.M.; Schmidt, G.; Caparroz-Assef, S.M.; Bersani Amado, C.A.; Cuman, R.K.N. Anti inflammatory and antino ciceptive activities of eugenol essential oil in experimental animals models. Rev. Bras. Farmacogn. 2009, 19, 212–217. [Google Scholar] [CrossRef]
- Idowu, S.; Adekoya, A.E.; Igiehon, O.O.; Idowu, A.T. Clove (Syzygium aromaticum) spices: A review on their bioactivities, current use, and potential application in dairy products. J. Food Meas. Charact. 2021, 15, 3419–3435. [Google Scholar]
- Haro-González, J.N.; Barbosa-Nuñez, J.A.; Castillo-Herrera, G.A.; Estarrón-Espinosa, M.; Herrera-Rodríguez, S.E.; Espinosa-Andrews, H.; Martínez-Velázquez, M. Clove essential oil and its major component, eugenol: A comparative study of their in vitro antioxidant and anticancer properties. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Zahid, M.A.; Seo, J.K.; Parvin, R.; Ko, J.; Yang, H.S. Comparison of butylated hydroxytoluene, ascorbic acid, and clove extract as antioxidants in fresh beef patties at refrigerated storage. Food Sci. Anim. Resour. 2019, 39, 768. [Google Scholar] [PubMed]
- Ricardo-Rodrigues, S.; Rouxinol, M.I.; Agulheiro-Santos, A.C.; Potes, M.E.; Laranjo, M.; Elias, M. The Antioxidant and Antibacterial Potential of Thyme and Clove Essential Oils for Meat Preservation—An Overview. Appl. Biosci. 2024, 3, 87–101. [Google Scholar] [CrossRef]
- Yuwono, M.; Hafid, A.F.; Poernomo, A.T.; Agil, M.; Indrayanto, G.; Ebel, S. Eugenol. In Analytical Profiles of Drug Substances and Excipients; Academic Press: Cambridge, MA, USA, 2002; Volume 29, pp. 149–177. [Google Scholar]
- Gengatharan, A.; Abd Rahim, M.H. The application of clove extracts as a potential functional component in active food packaging materials and model food systems: A mini-review. Appl. Food Res. 2023, 3, 100283. [Google Scholar]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Chalcone scaffolds as anti-infective agents: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar]
- Ahmed, D.M.; Mohsen, A.E.A.M.; El-Deeb, M.A.; Alkhedaide, A.; El-Tahan, A.M.; Metwally, E.S.M. The larvicidal effect of neemazal T/S, clove oil and ginger oil on tomato leafminer, Tuta absoluta compared to coragen. Saudi J. Biol. Sci. 2022, 29, 1447–1455. [Google Scholar] [PubMed]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar]
- Park, B.S.; Choi, W.S.; Kim, J.H.; Kim, K.H. Monoterpenes from clove bud essential oil as potential insecticidal agents. Pest Manag. Sci. 2017, 73, 222–228. [Google Scholar]
- Afrendi, E.; Prastya, M.E.; Astuti, R.I.; Wahyuni, W.T.; Batubara, I. Bioactivity of the Ethanol Extract of Clove (Syzygium aromaticum) as Antitoxin. Int. J. Food Sci. 2023, 2023, 3245210. [Google Scholar]
- Rayess, Y.E.; Nehme, L.; Ghanem, C.; Beyrouthy, M.E.; Sadaka, C.; Azzi-Achkouty, S.; Sharifi-Rad, J. Phenolic content, antioxidant and antimicrobial activities evaluation and relationship of commercial spices in the lebanese market. BMC Chem. 2023, 17, 157. [Google Scholar]
- Bao, Y.; Ren, X.; Zhu, Y.; Zhang, Y.; Peng, Z.; Zhou, G. Comparison of lipid radical scavenging capacity of spice extract in situ in roast beef with DPPH and peroxy radical scavenging capacities in vitro models. Lwt 2020, 130, 109626. [Google Scholar]
- Charles, D.J.; Charles, D.J. Clove. In Antioxidant Properties of Spices, Herbs and Other Sources; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2023; pp. 245–253. [Google Scholar]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar]
- Młynarska, E.; Hajdys, J.; Czarnik, W.; Fularski, P.; Leszto, K.; Majchrowicz, G.; Lisińska, W.; Rysz, J.; Franczyk, B. The Role of Antioxidants in the Therapy of Cardiovascular Diseases—A Literature Review. Nutrients 2024, 16, 2587. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Aruoma, O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003, 523, 9–20. [Google Scholar]
- Silva, M.V.; de Lima, A.D.C.A.; Silva, M.G.; Caetano, V.F.; de Andrade, M.F.; da Silva, R.G.C.; Vinhas, G.M. Clove essential oil and eugenol: A review of their significance and uses. Food Biosci. 2024, 62, 105112. [Google Scholar]
- Bechelli, S.; Delhommelle, J. AI’s role in pharmaceuticals: Assisting drug design from protein interactions to drug development. Artif. Intell. Chem. 2024, 2, 100038. [Google Scholar]
- Shahrajabian, M.H.; Marmitt, D.J.; Cheng, Q.; Sun, W. Natural antioxidants of the underutilized and neglected plant species of Asia and South America. Lett. Drug Des. Discov. 2023, 20, 1512–1537. [Google Scholar]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.P.V.; Olika Keyata, E. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Sci. World J. 2022, 23, 9901018. [Google Scholar]
- Sharma, C.M.; Al Kaabi, J.M.; Nurulain, S.N.; Goyal, S.; Amjad Kamal, M.; Ojha, S. Polypharmacological properties and therapeutic potential of β-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar]
- Rasool, N.; Saeed, Z.; Pervaiz, M.; Ali, F.; Younas, U.; Bashir, R.; Bukhari, S.M.; Khan, R.R.M.; Jelani, S.; Sikandar, R. Evaluation of essential oil extracted from ginger, cinnamon and lemon for therapeutic and biological activities. Biocatal. Agric. Biotechnol. 2020, 44, 102470. [Google Scholar]
- Ansari, K.; Goodarznia, I. Optimization of supercritical carbon dioxide extraction of essential oil from spearmint (Mentha spicata L.) leaves by using Taguchi methodology. J. Supercrit. Fluids 2012, 67, 123–130. [Google Scholar]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Ahamad, J.; Omer, A.Y.; Majid, D.A.; Khidr, T.M.; Jameel, S.Y. Essential Oil Characterization in Clove (Syzigium aromaticum Linn.) by GC-MS and Detection of Its Adulteration by ATR-FTIR Method. Indian J. 2022, 24, 309–316. [Google Scholar]
- Hassan, W.H.; Abdel-Ghany, A.E.; Afifi, S.I.; Sedik, S.H. Genotypic characterization of Campylobacter species isolated from livestock and poultry and evaluation of some herbal oil antimicrobial effect against selected Campylobacter species. Adv. Anim. Vet. Sci. 2019, 7, 1083–1092. [Google Scholar]
- Hu, Q.; Zhou, M.; Wei, S. Progress on the antimicrobial activity research of clove oil and eugenol in the food antisepsis field. J. Food Sci. 2018, 83, 1476–1483. [Google Scholar]
- Momo, E.J.; Nguimatsia, F.; Ateufouet Ngouango, L.; Lunga, P.K.; Pone Kamdem, B.; Jazet Dongmo, P.M. Eugenol-Rich Essential Oils from Flower Buds and Leaves of Syzygium aromaticum Show Antifungal Activity against Candida and Cryptococcus Species. Future Pharmacol. 2024, 4, 449–465. [Google Scholar] [CrossRef]
- Liñán-Atero, R.; Aghababaei, F.; García, S.R.; Hasiri, Z.; Ziogkas, D.; Moreno, A.; Hadidi, M. Clove Essential Oil: Chemical Profile, Biological Activities, Encapsulation Strategies, and Food Applications. Antioxidants 2024, 13, 488. [Google Scholar] [CrossRef]
- Malaisamy, A.; Eswaran, M.; Meyyazhagan, A.; Arumugam, V.A.; Rengasamy, K.R.R.; Balasubramanian, B.; Liu, W.C. Evaluation of Clove Phytochemicals as Potential Antiviral Drug Candidates Targeting SARS-CoV-2 Main Protease: Computational Docking, Molecular Dynamics Simulation, and Pharmacokinetic Profiling. Front. Mol. Biosci. 2022, 9, 918101. [Google Scholar]
- Coelho, J.R.A.; Vieira, T.F.; Pereira, R.B.; Pereira, D.M.; Castanheira, E.M.S.; Fortes, A.G.; Sousa, S.F.; Fernandes, M.J.G.; Gonçalves, M.S.T. Eugenol Ester Derivatives: Synthesis, Insecticidal Activity and Computational Studies. Chem. Proc. 2022, 8, 83. [Google Scholar]
- Kalemba, D.; Kunicka, A. Antibacterial and Antifungal Properties of Essential Oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Ahuja, A.; Singh, S. Impact of the current scenario and future perspectives for the management of oral diseases: Remarkable contribution of herbs in dentistry. Anti Infect. Agents 2022, 20, 27–45. [Google Scholar] [CrossRef]
- Sulieman, A.M.E.; Ibrahim, S.M.; Alshammari, M.; Abdulaziz, F.; Idriss, H.; Alanazi, N.A.H.; Abdallah, E.M.; Siddiqui, A.J.; Shommo, S.A.M.; Jamal, A.; et al. Zingiber officinale Uncovered: Integrating Experimental and Computational Approaches to Antibacterial and Phytochemical Profiling. Pharmaceuticals 2024, 17, 1551. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Teitelbaum, S.L.; Pinney, S.M.; Windham, G.; Liao, L.; Biro, F. Breast Cancer and Environment Research Centers. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ. Health Perspect. 2010, 118, 1039–1046. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef]
- Ikram, E.H.K.; Eng, K.H.; Jalil, A.M.M.; Ismail, A.; Idris, S.; Azlan, A.; Mokhtar, R.A.M. Antioxidant capacity and total phenolic content of Malaysian underutilized fruits. J. Food Compos. Anal. 2009, 22, 388–393. [Google Scholar] [CrossRef]
- Aggarwal, B.; Sharma, P.; Lamba, H.S. Gas Chromatography-Mass Spectrometry Characterization of Bioactive Compounds from Ziziphus nummularia (Burm. F.) Stem Bark with Promising In Vitro Antiplasmodial Activity. J. Pharm. Bioallied Sci. 2020, 12, 42–47. [Google Scholar] [CrossRef]
| Test System | Extract | Butylated Hydroxytoluene | Ascorbic Acid |
|---|---|---|---|
| Phytochemical screening | |||
| 1. Total Phenols (mg GAE/g Extract) | 7.25 ± 0.12 | - | - |
| 2. Total Flavonoids (mg QE/g Extract) | 57.22 ± 0.41 | - | - |
| Antioxidant Assays | |||
| 1. DPPH IC50 (mg/mL) | 0.08 ± 0.01 | 0.024 ± 2 × 10−4 | 0.021 ± 5 × 10−4 |
| 2. ABTS IC50 (mg/mL) | 0.18 ± 0.01 | 0.017 ± 3 × 10−4 | 0.022 ± 0.001 |
| 3. β-carotene IC50 (mg/mL) | 1.78 ± 0.11 | 0.044 ± 3.2 × 10−3 | 0.019 ± 0.001 |
| Peak | R. Time | Area % | Compound Name | Mol. Form. |
|---|---|---|---|---|
| 1 | 7.564 | 0.39 | Phenol,4-(2-propenyl)- | C9H10O |
| 2 | 7.810 | 0.53 | Benzaldehyde | C7H6O |
| 3 | 8.029 | 0.31 | 2-propenal, 3-phenyl- | C9H8O |
| 4 | 8.231 | 41.4 | Eugenol | C10H12O2 |
| 5 | 8.633 | 1.13 | Bicycle [3,1-1]heptan-3-ol | C10H16O |
| 6 | 9.120 | 0.32 | 2-nonanone | C9H18O |
| 7 | 9.166 | 0.68 | 1,2,3-benzeneetriol | C6H6O |
| 8 | 9.566 | 0.11 | Docosanoic acid, ethyl ester | C24H34O2 |
| 9 | 9.599 | 0.14 | Naphtho [2,3-c]furan-1,3-dione | C12H6O3 |
| 10 | 10.050 | 10.42 | Caryophyllene | C15H24 |
| 11 | 10.385 | 2.73 | Humulene | C15H24 |
| 12 | 10.702 | 7.21 | Phenol, 2-methoxy-4-(2-propenyl)-acetate | C12H14O3 |
| 13 | 11.584 | 0.78 | Caryophyllene oxide | C15H24O |
| 14 | 12.337 | 0.69 | 2,3,4-trimethoxyacetophenone | C11H14O4 |
| 15 | 12.541 | 2.22 | Eugenyl acetate | C12H14O3 |
| 16 | 13.045 | 0.54 | Acetic acid, phenylmethyl ester | C9H10O2 |
| 17 | 13.641 | 0.17 | Methy salicylate | C8H8O3 |
| 18 | 14.326 | 0.36 | Chavicol | C10H18O |
| 19 | 14.675 | 1.54 | 1-propyl-3(popen-1-yl)adamantine | C16H26 |
| 20 | 16.283 | 0.76 | Decanal | C10H20O |
| 21 | 17.023 | 2.14 | n-hexadecanoic acid, methyl ester | C16H32O2 |
| Total | 100 | |||
| SN. NO. | Receptor | Compound Name | Binding Energy (kcal/mol) |
|---|---|---|---|
| 1 | Staphylococcus aureus (PDB ID: 5M18) | Phenol,4-(2-propenyl)- | −5.1 |
| 2 | Benzaldehyde | −4.7 | |
| 3 | 2-propenal, 3-phenyl- | −5.2 | |
| 4 | Eugenol | −5.4 | |
| 5 | Bicycle [3,1-1]heptan-3-ol | −5.6 | |
| 6 | 2-nonanone | −4.7 | |
| 7 | 1,2,3-benzeneetriol | −5.0 | |
| 8 | Docosanoic acid, ethyl ester | −4.6 | |
| 9 | Naphtho [2,3-b]furan-2-one,3-[[2-(4-methoxy | −7.2 | |
| 10 | Caryophyllene | −6.8 | |
| 11 | Humulene | −6.5 | |
| 12 | Phenol, 2-methoxy-4-(2-propenyl)- acetate | −5.6 | |
| 13 | Caryophyllene oxide | −6.8 | |
| 14 | 2,3,4-trimethoxyacetophenone | −5.2 | |
| 15 | Eugenyl acetate | −6.0 | |
| 16 | Acetic acid, phenylmethyl ester | −5.4 | |
| 17 | Methy salicylate | −5.3 | |
| 18 | Chavicol | −5.1 | |
| 19 | 1-propyl-3(popen-1-yl)adamantine | −5.0 | |
| 20 | Decanal | −4.9 | |
| 21 | n-hexadecanoic acid, methyl ester | −5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmuhsin, A.A.; Sulieman, A.M.E.; Salih, Z.A.; Al-Azmi, M.; Alanaizi, N.A.; Goniem, A.E.; Alam, M.J. Clove (Syzygium aromaticum) Pods: Revealing Their Antioxidant Potential via GC-MS Analysis and Computational Insights. Pharmaceuticals 2025, 18, 504. https://doi.org/10.3390/ph18040504
Abdelmuhsin AA, Sulieman AME, Salih ZA, Al-Azmi M, Alanaizi NA, Goniem AE, Alam MJ. Clove (Syzygium aromaticum) Pods: Revealing Their Antioxidant Potential via GC-MS Analysis and Computational Insights. Pharmaceuticals. 2025; 18(4):504. https://doi.org/10.3390/ph18040504
Chicago/Turabian StyleAbdelmuhsin, Abdelmuhsin Abdegadir, Abdel Moniem Elhadi Sulieman, Zakaria Ahmed Salih, Meshari Al-Azmi, Naimah Asid Alanaizi, Ahmed Eisa Goniem, and Mohammad Jahoor Alam. 2025. "Clove (Syzygium aromaticum) Pods: Revealing Their Antioxidant Potential via GC-MS Analysis and Computational Insights" Pharmaceuticals 18, no. 4: 504. https://doi.org/10.3390/ph18040504
APA StyleAbdelmuhsin, A. A., Sulieman, A. M. E., Salih, Z. A., Al-Azmi, M., Alanaizi, N. A., Goniem, A. E., & Alam, M. J. (2025). Clove (Syzygium aromaticum) Pods: Revealing Their Antioxidant Potential via GC-MS Analysis and Computational Insights. Pharmaceuticals, 18(4), 504. https://doi.org/10.3390/ph18040504






