Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies
Abstract
1. Introduction
1.1. Overview of Osteosarcoma
1.2. Standard Treatment Modalities
1.3. The Need for New Treatment Approaches
2. Chemotherapy Resistance in Osteosarcoma: Therapeutic Implications
2.1. Mechanisms of Chemotherapy Resistance
2.1.1. Drug Efflux Transporters
2.1.2. Altered Drug Metabolism
2.1.3. Enhanced DNA Repair Mechanisms
2.1.4. Apoptosis Resistance
2.1.5. Autophagy and Cell Survival
2.1.6. Epigenetic Modifications
2.1.7. Cancer Stem Cells
2.2. Current Therapeutic Approaches to Overcome Resistance
2.2.1. Combination Chemotherapy Regimens
2.2.2. Dose Intensification and Modification
2.2.3. Novel Drug Delivery Systems
3. Targeted Therapies Based on Molecular Abnormalities
3.1. Genetic and Molecular Targets in Osteosarcoma
3.1.1. Receptor Tyrosine Kinases (RTKs)
3.1.2. Multi-Kinase Inhibitors (MKIs)
3.1.3. Signal Transduction Pathways
3.1.4. MicroRNA-Based Therapies
4. Immunotherapy in Osteosarcoma Treatment
4.1. The Tumor Immune Microenvironment (TIME)
4.1.1. Immune Cell Infiltration
4.1.2. Prognostic Significance
4.2. Immune Evasion Mechanisms
4.3. Expression of Immune Checkpoint Molecules
4.4. Immunotherapeutic Strategies
4.5. Immune Checkpoint Inhibitors
4.5.1. Immune Checkpoint Inhibitors and Osteosarcoma
4.5.2. CAR T-Cell Therapy Targeting Osteosarcoma Antigens
4.6. Adoptive Cell Therapy
Natural Killer (NK) Cell Therapy
4.7. Cancer Vaccines
Development of Peptide-Based and Dendritic Cell Vaccines
4.8. Oncolytic Viruses
Mechanism and Therapeutic Potential
5. Novel Therapeutic Approaches and Clinical Trials
5.1. Epigenetic Therapy
5.2. Gene Therapy
5.3. Personalized Medicine Approaches
5.4. New Therapies, Challenges, and Limitations
6. Summary and Future Directions
6.1. Summary of Therapeutic Advancements
6.2. Future Perspectives
6.3. Call to Action
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. BioMed. Eng. OnLine 2021, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Davis, L.E.; Albert, C.M.; Samuels, B.; Roberts, J.L.; Wagner, M.J. Osteosarcoma in Pediatric and Adult Populations: Are Adults Just Big Kids? Cancers 2023, 15, 5044. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.R.; Mandelker, D.L. Tumor Syndromes Predisposing to Osteosarcoma. Adv. Anat. Pathol. 2018, 25, 217–222. [Google Scholar] [CrossRef]
- Beaury, M.W.; Kelly-Beaury, M.L.; Sharp, G.; Cottrell, J. A Review of Osteosarcoma Therapeutics. J. Cancer Treat. Diagn. 2018, 2, 21–29. [Google Scholar] [CrossRef]
- Menendez, N.; Epelman, M.; Shao, L.; Douglas, D.; Meyers, A.B. Pediatric Osteosarcoma: Pearls and Pitfalls. Semin. Ultrasound CT MRI 2022, 43, 97–114. [Google Scholar] [CrossRef]
- DePalma, M.; Gupta, S.; Nguyen, J.; Talwar, D.; Arkader, A.; Wells, L. Do Not Miss the Tumor: A Novel Presentation of Osteosarcoma. Case Rep. Pediatr. 2021, 2021, 5531238. [Google Scholar] [CrossRef]
- Crombé, A.; Simonetti, M.; Longhi, A.; Hauger, O.; Fadli, D.; Spinnato, P. Imaging of Osteosarcoma: Presenting Findings, Metastatic Patterns, and Features Related to Prognosis. J. Clin. Med. 2024, 13, 5710. [Google Scholar] [CrossRef]
- Rothzerg, E.; Xu, J.; Wood, D. Different Subtypes of Osteosarcoma: Histopathological Patterns and Clinical Behaviour. J. Mol. Pathol. 2023, 4, 99–108. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Liu, J.-B.; Wang, X.-F.; Ma, Y.-S.; Fu, D. Current Status and Prospects of Clinical Treatment of Osteosarcoma. Technol. Cancer Res. Treat. 2022, 21, 15330338221124696. [Google Scholar] [CrossRef]
- Evans, D.R.; Lazarides, A.L.; Visgauss, J.D.; Somarelli, J.A.; Blazer, D.G.; Brigman, B.; Eward, W.C. Limb salvage versus amputation in patients with osteosarcoma of the extremities: An update in the modern era using the National Cancer Database. BMC Cancer 2020, 20, 995. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Stamatopoulos, A.; I Athanasiadis, D.; Kenanidis, E.; Potoupnis, M.; Haidich, A.-B.; Tsiridis, E. Limb-salvage surgery offers better five-year survival rate than amputation in patients with limb osteosarcoma treated with neoadjuvant chemotherapy. A systematic review and meta-analysis. J. Bone Oncol. 2020, 25, 100319. [Google Scholar]
- Meazza, C.; Asaftei, S.D. State-of-the-art, approved therapeutics for the pharmacological management of osteosarcoma. Expert. Opin. Pharmacother. 2021, 22, 1995–2006. [Google Scholar] [PubMed]
- Hurkmans, E.G.E.; Brand, A.C.A.M.; Verdonschot, J.A.J.; te Loo, D.M.W.M.; Coenen, M.J.H. Pharmacogenetics of chemotherapy treatment response and -toxicities in patients with osteosarcoma: A systematic review. BMC Cancer 2022, 22, 1326. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, I.; Herold, N. Targeting Molecular Mechanisms Underlying Treatment Efficacy and Resistance in Osteosarcoma: A Review of Current and Future Strategies. Int. J. Mol. Sci. 2020, 21, 6885. [Google Scholar] [CrossRef]
- Panez-Toro, I.; Muñoz-García, J.; Vargas-Franco, J.W.; Renodon-Cornière, A.; Heymann, M.F.; Lézot, F.; Heymann, D. Advances in Osteosarcoma. Curr. Osteoporos. Rep. 2023, 21, 330–343. [Google Scholar] [CrossRef]
- Argenziano, M.; Tortora, C.; Pota, E.; Di Paola, A.; di Martino, M.; Di Leva, C.; Di Pinto, D.; Rossi, F. Osteosarcoma in Children: Not Only Chemotherapy. Pharmaceuticals 2021, 14, 923. [Google Scholar] [CrossRef]
- Sheng, G.; Gao, Y.; Yang, Y.; Wu, H. Osteosarcoma and Metastasis. Front. Oncol. 2021, 11, 780264. [Google Scholar]
- García-Ortega, D.Y.; Cabrera-Nieto, S.A.; Caro-Sánchez, H.S.; Cruz-Ramos, M. An overview of resistance to chemotherapy in osteosarcoma and future perspectives. Cancer Drug Resist. 2022, 5, 762–793. [Google Scholar]
- Marchandet, L.; Lallier, M.; Charrier, C.; Baud’huin, M.; Ory, B.; Lamoureux, F. Mechanisms of Resistance to Conventional Therapies for Osteosarcoma. Cancers 2021, 13, 683. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Zeng, J.-W.; Peng, Y.; Wang, D.; Ayesha, K.; Chen, S. The interaction between osteosarcoma and other cells in the bone microenvironment: From mechanism to clinical applications. Front. Cell Dev. Biol. 2023, 11, 1123065. [Google Scholar]
- Biffi, G.; Tuveson, D.A. Diversity and biology of cancer-associated fibroblasts. Physiol. Rev. 2020, 101, 147–176. [Google Scholar] [PubMed]
- Orrapin, S.; Moonmuang, S.; Udomruk, S.; Yongpitakwattana, P.; Pruksakorn, D.; Chaiyawat, P. Unlocking the tumor-immune microenvironment in osteosarcoma: Insights into the immune landscape and mechanisms. Front. Immunol. 2024, 15, 1394284. [Google Scholar] [CrossRef]
- Wu, P.-C.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 641469. [Google Scholar]
- Ji, Z.; Shen, J.; Lan, Y.; Yi, Q.; Liu, H. Targeting signaling pathways in osteosarcoma: Mechanisms and clinical studies. MedComm 2023, 4, e308. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, Q.; Li, G.; Zhao, X.; Zhao, X.; Zhang, Z. The Targeted Therapies for Osteosarcoma via Six Major Pathways. Curr. Mol. Pharmacol. 2023, 17, e210823220109. [Google Scholar]
- Assi, A.; Farhat, M.; Catherine Rita Hachem, M.; Zalaquett, Z.; Aoun, M.; Daher, M.; Sebaaly, A.; Kourie, H.R. Tyrosine kinase inhibitors in osteosarcoma: Adapting treatment strategiesa. J. Bone Oncol. 2023, 43, 100511. [Google Scholar]
- Just, M.A.; Van Mater, D.; Wagner, L.M. Receptor tyrosine kinase inhibitors for the treatment of osteosarcoma and Ewing sarcoma. Pediatr. Blood Cancer 2021, 68, e29084. [Google Scholar] [CrossRef]
- Miwa, S.; Shirai, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Igarashi, K.; Tsuchiya, H. Current and Emerging Targets in Immunotherapy for Osteosarcoma. J. Oncol. 2019, 2019, 7035045. [Google Scholar]
- Li, S.; Zhang, H.; Shang, G. Current status and future challenges of CAR-T cell therapy for osteosarcoma. Front. Immunol. 2023, 14, 1290762. [Google Scholar] [CrossRef]
- Zhu, J.; Simayi, N.; Wan, R.; Huang, W. CAR T targets and microenvironmental barriers of osteosarcoma. Cytotherapy 2022, 24, 567–576. [Google Scholar] [PubMed]
- Poboży, K.; Domański, P.; Domańska, J.; Konarski, W.; Poboży, T. Advances in immunotherapy for osteosarcoma: A review of emerging treatment strategies. OncoReview 2023, 13, 75–84. [Google Scholar]
- Liang, H.; Cui, M.; Tu, J.; Chen, X. Advancements in osteosarcoma management: Integrating immune microenvironment insights with immunotherapeutic strategies. Front. Cell Dev. Biol. 2024, 12, 1394339. [Google Scholar] [CrossRef]
- Desai, S.A.; Manjappa, A.S.; Khulbe, P. Drug delivery nanocarriers and recent advances ventured to improve therapeutic efficacy against osteosarcoma: An overview. J. Egypt. Natl. Cancer Inst. 2021, 33, 4. [Google Scholar]
- Luo, Y.; Sun, M.; Tan, L.; Li, T.; Min, L. Nano-Based Drug Delivery Systems: Potential Developments in the Therapy of Metastatic Osteosarcoma—A Narrative Review. Pharmaceutics 2023, 15, 2717. [Google Scholar] [CrossRef]
- Li, K.; Li, D.; Zhao, L.; Chang, Y.; Zhang, Y.; Cui, Y.; Zhang, Z. Calcium-mineralized polypeptide nanoparticle for intracellular drug delivery in osteosarcoma chemotherapy. Bioact. Mater. 2020, 5, 721–731. [Google Scholar] [CrossRef]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qian, C.; Ren, P.; Yu, H.; Kong, X.; Huang, C.; Luo, H.; Chen, G. Light-Responsive Micelles Loaded with Doxorubicin for Osteosarcoma Suppression. Front. Pharmacol. 2021, 12, 679610. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Shao, N.; Hu, Z.; Chen, H.; Xu, L.; Wang, C.; Cheng, Y.; Xiao, J. Triazine-modified dendrimer for efficient TRAIL gene therapy in osteosarcoma. Acta Biomater. 2015, 17, 115–124. [Google Scholar] [CrossRef]
- Shi, P.; Cheng, Z.; Zhao, K.; Chen, Y.; Zhang, A.; Gan, W.; Zhang, Y. Active targeting schemes for nano-drug delivery systems in osteosarcoma therapeutics. J. Nanobiotechnol. 2023, 21, 103. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved Nanomedicine against Diseases. Pharmaceutics 2023, 15, 774. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, A.; Kukreti, G.; Bharkatiya, M.; Dobhal, K.; Parashar, T.; Suyal, J.; Jakhmola, V. Nanoparticles—A Booming Drug Delivery System in Chemotherapy. Biomed. Pharmacol. J. 2023, 16, 1785–1790. [Google Scholar]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose-Response 2020, 18, 1559325820936161. [Google Scholar]
- Sahu, K.; Pathak, R.; Agrawal, N.; Banjare, P.; Sharma, H.K.; Sahu, G.K. A Review of the Novel Drug Delivery System used in the Treatment of Cancer. Res. J. Pharm. Dos. Forms Technol. 2019, 11, 199–205. [Google Scholar]
- Roszkowska, M. Multilevel Mechanisms of Cancer Drug Resistance. Int. J. Mol. Sci. 2024, 25, 12402. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Pote, M.S.; Gacche, R.N. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef]
- Barata, I.S.; Gomes, B.C.; Rodrigues, A.S.; Rueff, J.; Kranendonk, M.; Esteves, F. The Complex Dynamic of Phase I Drug Metabolism in the Early Stages of Doxorubicin Resistance in Breast Cancer Cells. Genes 2022, 13, 1977. [Google Scholar] [CrossRef]
- Tippett, V.L.; Tattersall, L.; Ab Latif, N.B.; Shah, K.M.; Lawson, M.A.; Gartland, A. The strategy and clinical relevance of in vitro models of MAP resistance in osteosarcoma: A systematic review. Oncogene 2022, 42, 259–277. [Google Scholar]
- Zhou, L.; Tang, J.; Hu, F.; Liao, Y.; Li, R.-C.; Zhou, Y.; Yao, Z.; Geng, Z.; Yang, Z.; Zhang, X.; et al. Effects of different levels of TGF-β expression and tumor cell necrosis rates in osteosarcoma on the chemotherapy resistance of osteosarcoma. J. Bone Oncol. 2020, 23, 100299. [Google Scholar]
- Tsukamoto, S.; Errani, C.; Angelini, A.; Mavrogenis, A.F. Current Treatment Considerations for Osteosarcoma Metastatic at Presentation. Orthopedics 2020, 43, e345–e358. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Van Tine, B.A. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. J. Clin. Med. 2021, 10, 1182. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, S.; Feng, A.; Xu, D.; Zhu, Q.; Mao, Y.; Zhao, Y.; Lv, Y.; Han, C.; Liu, R.; et al. Methotrexate, doxorubicin, and cisplatinum regimen is still the preferred option for osteosarcoma chemotherapy: A meta-analysis and clinical observation. Medicine 2019, 98, e15582. [Google Scholar] [CrossRef]
- Saito, A.; Kitayama, J.; Nagai, R.; Aizawa, K. Anatomical Targeting of Anticancer Drugs to Solid Tumors Using Specific Administration Routes: Review. Pharmaceutics 2023, 15, 1664. [Google Scholar] [CrossRef]
- Kortam, S.; Lu, Z.; Zreiqat, H. Recent advances in drug delivery systems for osteosarcoma therapy and bone regeneration. Commun. Mater. 2024, 5, 168. [Google Scholar] [CrossRef]
- Ma, H.; He, C.; Cheng, Y.; Yang, Z.; Zang, J.; Liu, J.; Chen, X. Localized Co-delivery of Doxorubicin, Cisplatin, and Methotrexate by Thermosensitive Hydrogels for Enhanced Osteosarcoma Treatment. ACS Appl. Mater. Interfaces 2015, 7, 27040–27048. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, S.; Sohi, H.; Dang, S. Advances in Delivery of Chemotherapeutic Agents for Cancer Treatment. AAPS PharmSciTech 2021, 23, 25. [Google Scholar] [PubMed]
- Tian, Y.; Lei, Y.; Wang, Y.; Lai, J.; Wang, J.; Xia, F. Mechanism of multidrug resistance to chemotherapy mediated by P-glycoprotein (Review). Int. J. Oncol. 2023, 63, 119. [Google Scholar] [CrossRef]
- He, C.; Sun, Z.; Hoffman, R.M.; Yang, Z.; Jiang, Y.; Wang, L.; Hao, Y. P-Glycoprotein Overexpression Is Associated with Cisplatin Resistance in Human Osteosarcoma. Anticancer Res. 2019, 39, 1711–1718. [Google Scholar] [CrossRef]
- Serra, M.; Picci, P.; Ferrari, S.; Bacci, G. Prognostic Value of P-Glycoprotein in High-Grade Osteosarcoma. J. Clin. Oncol. 2007, 25, 4858–4860. [Google Scholar] [CrossRef]
- Schwartz, C.L.; Gorlick, R.; Teot, L.; Krailo, M.; Chen, Z.; Goorin, A.; Grier, H.E.; Bernstein, M.L.; Meyers, P. Multiple Drug Resistance in Osteogenic Sarcoma: INT0133 From the Children’s Oncology Group. J. Clin. Oncol. 2007, 25, 2057–2062. [Google Scholar] [CrossRef] [PubMed]
- Belisario, D.C.; Akman, M.; Godel, M.; Campani, V.; Patrizio, M.P.; Scotti, L.; Hattinger, C.M.; De Rosa, G.; Donadelli, M.; Serra, M.; et al. ABCA1/ABCB1 Ratio Determines Chemo- and Immune-Sensitivity in Human Osteosarcoma. Cells 2020, 9, 647. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/Akt Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Jabbarzadeh Kaboli, P.; Salimian, F.; Aghapour, S.; Xiang, S.; Zhao, Q.; Li, M.; Wu, X.; Du, F.; Zhao, Y.; Shen, J.; et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—A comprehensive review from chemotherapy to immunotherapy. Pharmacol. Res. 2020, 156, 104806. [Google Scholar] [CrossRef]
- Qin, T.; Zhu, W.; Kan, X.; Li, L.; Wu, D. Luteolin attenuates the chemoresistance of osteosarcoma through inhibiting the PTN/β-catenin/MDR1 signaling axis by upregulating miR-384. J. Bone Oncol. 2022, 34, 100429. [Google Scholar]
- Chen, Y.; Zhang, K.; Li, Y.; Guo, R.; Zhang, K.; Zhong, G.; He, Q. Oestrogen-related receptor alpha mediates chemotherapy resistance of osteosarcoma cells via regulation of ABCB1. J. Cell. Mol. Med. 2019, 23, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Hossein Gholami, M.; Hashemi, F.; Zabolian, A.; Vasheghani Farahani, M.; Hushmandi, K.; Zarrabi, A.; Goldman, A.; Ashrafizadeh, M.; Orive, G. Advances in understanding the role of P-gp in doxorubicin resistance: Molecular pathways, therapeutic strategies, and prospects. Drug Discov. Today 2021, 27, 436–455. [Google Scholar]
- Ji, Y.; Liu, J.; Zhu, W.; Ji, J. circ_0002060 Enhances Doxorubicin Resistance in Osteosarcoma by Regulating the miR-198/ABCB1 Axis. Cancer Biother. Radiopharm. 2020, 38, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, L.; Yan, X.; Wang, C.; Wang, Y.; Han, K.; Lin, S.; Gan, Z.; Min, D. Pleiotrophin promotes chemoresistance to doxorubicin in osteosarcoma by upregulating P-glycoprotein. Oncotarget 2017, 8, 63857–63870. [Google Scholar] [CrossRef]
- Xia, Y.-Z.; Yang, L.; Xue, G.-M.; Zhang, C.; Guo, C.; Yang, Y.-W.; Li, S.-S.; Zhang, L.-Y.; Guo, Q.-L.; Kong, L.-Y. Combining GRP78 suppression and MK2206-induced Akt inhibition decreases doxorubicin-induced P-glycoprotein expression and mitigates chemoresistance in human osteosarcoma. Oncotarget 2016, 7, 56371. [Google Scholar]
- Haddadi, N.; Lin, Y.; Travis, G.; Simpson, A.M.; McGowan, E.M.; Nassif, N.T. PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol. Cancer 2018, 17, 37. [Google Scholar] [PubMed]
- Trujillo-Paolillo, A.; Tesser-Gamba, F.; Petrilli, A.S.; de Seixas Alves, M.T.; Garcia Filho, R.J.; de Oliveira, R.; de Toledo, S.R.C. CYP genes in osteosarcoma: Their role in tumorigenesis, pulmonary metastatic microenvironment and treatment response. Oncotarget 2017, 8, 38530–38540. [Google Scholar] [CrossRef]
- Huang, G.; Mills, L.; Worth, L.L. Expression of human glutathione S-transferase P1 mediates the chemosensitivity of osteosarcoma cells. Mol. Cancer Ther. 2007, 6, 1610–1619. [Google Scholar] [CrossRef]
- Dhaini, H.R.; Thomas, D.G.; Giordano, T.J.; Johnson, T.D.; Biermann, J.S.; Leu, K.; Hollenberg, P.F.; Baker, L.H. Cytochrome P450 CYP3A4/5 expression as a biomarker of outcome in osteosarcoma. J. Clin. Oncol. 2003, 21, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.W.; Yang, Z.M.; Li, J.; Xu, B. Predictive role of Glutathione S-transferases (GSTs) on the prognosis of osteosarcoma patients treated with chemotherapy. Pak. J. Med. Sci. 2013, 29, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, F.; Wang, X.; Gou, S. Multifunctional Pt(iv) complexes containing a glutathione S-transferase inhibitor lead to enhancing anticancer activity and preventing metastasis of osteosarcoma cells. Met. Integr. Biometal Sci. 2019, 11, 317–326. [Google Scholar] [CrossRef]
- Moynihan, E.; Bassi, G.; Ruffini, A.; Panseri, S.; Montesi, M.; Velasco-Torrijos, T.; Montagner, D. Click Pt(IV)-Carbohydrates Pro-Drugs for Treatment of Osteosarcoma. Front. Chem. 2021, 9, 795997. [Google Scholar]
- Lv, N.; Huang, C.; Huang, H.; Dong, Z.; Chen, X.; Lu, C.; Zhang, Y. Overexpression of Glutathione S-Transferases in Human Diseases: Drug Targets and Therapeutic Implications. Antioxidants 2023, 12, 1970. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Kim, H.; Moustafa, A.A.; Datta, A.; Barata, P.C.; Boulares, A.H.; Abdel-Mageed, A.B.; Krane, L.S. Repurposing ketoconazole as an exosome directed adjunct to sunitinib in treating renal cell carcinoma. Sci. Rep. 2021, 11, 10200. [Google Scholar]
- Prado-Carrillo, O.; Arenas-Ramírez, A.; Llaguno-Munive, M.; Jurado, R.; Pérez-Rojas, J.M.; Cervera-Ceballos, E.E.; Garcia-Lopez, P. Ketoconazole Reverses Imatinib Resistance in Human Chronic Myelogenous Leukemia K562 Cells. Int. J. Mol. Sci. 2022, 23, 7715. [Google Scholar] [CrossRef]
- Amato, R.J.; Saxena, S.; Stepankiw, M. Phase II Trial Assessing Granulocyte-macrophage—Colony Stimulating Factor, Ketoconazole Plus Mitoxantrone in Metastatic Castration-resistant Prostate Cancer Progressing After Docetaxel Treatments. Cancer Investig. 2013, 31, 177–182. [Google Scholar]
- Mao, K.; Liu, C.; Tang, Z.; Rao, Z.; Wen, J. Advances in drug resistance of osteosarcoma caused by pregnane X receptor. Drug Metab. Rev. 2024, 56, 385–398. [Google Scholar] [PubMed]
- Lim, Y.P.; Kuo, S.C.; Lai, M.L.; Huang, J.D. Inhibition of CYP3A4 expression by ketoconazole is mediated by the disruption of pregnane X receptor, steroid receptor coactivator-1, and hepatocyte nuclear factor 4alpha interaction. Pharmacogenet. Genom. 2009, 19, 11–24. [Google Scholar] [CrossRef]
- Greenblatt, D.J.; Zhao, Y.; Venkatakrishnan, K.; Duan, S.X.; Harmatz, J.S.; Parent, S.J.; Court, M.H.; von Moltke, L.L. Mechanism of cytochrome P450-3A inhibition by ketoconazole. J. Pharm. Pharmacol. 2011, 63, 214–221. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar]
- Sadoughi, F.; Dana, P.M.; Asemi, Z.; Yousefi, B. DNA damage response and repair in osteosarcoma: Defects, regulation and therapeutic implications. DNA Repair 2021, 102, 103105. [Google Scholar] [CrossRef] [PubMed]
- Dianov, G.L.; Hübscher, U. Mammalian Base Excision Repair: The Forgotten Archangel. Nucleic Acids Res. 2013, 41, 3483–3490. [Google Scholar]
- Kim, Y.J.; Wilson, D.M. Overview of base excision repair biochemistry. Curr. Mol. Pharmacol. 2012, 5, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hindi, N.N.; Elsakrmy, N.; Ramotar, D. The base excision repair process: Comparison between higher and lower eukaryotes. Cell. Mol. Life Sci. CMLS 2021, 78, 7943–7965. [Google Scholar] [CrossRef]
- Spiegel, J.O.; Van Houten, B.; Durrant, J.D. PARP1: Structural insights and pharmacological targets for inhibition. DNA Repair 2021, 103, 103125. [Google Scholar] [CrossRef]
- Li, S.; Cui, Z.; Meng, X. Knockdown of PARP-1 Inhibits Proliferation and ERK Signals, Increasing Drug Sensitivity in Osteosarcoma U2OS Cells. Oncol. Res. 2016, 24, 279–286. [Google Scholar]
- Goričar, K.; Kovač, V.; Jazbec, J.; Zakotnik, B.; Lamovec, J.; Dolžan, V. Genetic variability of DNA repair mechanisms and glutathione-S-transferase genes influences treatment outcome in osteosarcoma. Cancer Epidemiol. 2015, 39, 182–188. [Google Scholar] [PubMed]
- Zh, C.; Hp, Y.; Jiang, N.; Yu, B. Association between ERCC1 and ERCC2 gene polymorphisms and chemotherapy response and overall survival in osteosarcoma. Genet. Mol. Res. GMR 2015, 14, 10145–10151. [Google Scholar]
- Zhang, H.; Ge, J.-B.; Hong, H.; Bi, L.; Sun, Z. Genetic polymorphisms in ERCC1 and ERCC2 genes are associated with response to chemotherapy in osteosarcoma patients among Chinese population: A meta-analysis. World J. Surg. Oncol. 2017, 15, 75. [Google Scholar] [CrossRef][Green Version]
- Sun, Y.; Wu, Y.; Li, W.; Kong, Z.; Zou, X. Genetic polymorphisms in nucleotide excision repair pathway influences response to chemotherapy and overall survival in osteosarcoma. Int. J. Clin. Exp. Pathol. 2015, 8, 7905–7912. [Google Scholar] [PubMed]
- Zhang, Q.; Ly, L.; Li, B.J.; Zhang, J.; Wei, F. Investigation of ERCC1 and ERCC2 gene polymorphisms and response to chemotherapy and overall survival in osteosarcoma. Genet. Mol. Res. GMR 2015, 14, 11235–11241. [Google Scholar] [PubMed]
- Igarashi, K.; Yamamoto, N.; Shirai, T.; Hayashi, K.; Nishida, H.; Kimura, H.; Takeuchi, A.; Miwa, S.; Inatani, H.; Shimozaki, S.; et al. Impact of excision repair cross-complementation group 1 (ERCC1) protein on survival of patients with osteosarcoma treated with cisplatin-based chemotherapy. J. Clin. Oncol. 2014, 32, 10535. [Google Scholar]
- Alkan, O.; Schoeberl, B.; Shah, M.; Koshkaryev, A.; Heinemann, T.; Drummond, D.C.; Yaffe, M.B.; Raue, A. Modeling chemotherapy-induced stress to identify rational combination therapies in the DNA damage response pathway. Sci. Signal. 2018, 11, eaat0229. [Google Scholar]
- Fanelli, M.; Tavanti, E.; Patrizio, M.P.; Vella, S.; Fernandez-Ramos, A.; Magagnoli, F.; Luppi, S.; Hattinger, C.M.; Serra, M. Cisplatin Resistance in Osteosarcoma: In vitro Validation of Candidate DNA Repair-Related Therapeutic Targets and Drugs for Tailored Treatments. Front. Oncol. 2020, 10, 331. [Google Scholar] [CrossRef]
- Ma, W.; Yang, L.; Liu, H.; Chen, P.; Ren, H.; Ren, P. PAXX is a novel target to overcome resistance to doxorubicin and cisplatin in osteosarcoma. Biochem. Biophys. Res. Commun. 2020, 521, 204–211. [Google Scholar] [CrossRef]
- Park, H.J.; Bae, J.S.; Kim, K.M.; Moon, Y.J.; Park, S.-H.; Ha, S.H.; Hussein, U.K.; Zhang, Z.; Park, H.S.; Park, B.-H.; et al. The PARP inhibitor olaparib potentiates the effect of the DNA damaging agent doxorubicin in osteosarcoma. J. Exp. Clin. Cancer Res. 2018, 37, 107. [Google Scholar] [CrossRef]
- Engert, F.; Kovác, M.; Baumhoer, D.; Nathrath, M.; Fulda, S. Osteosarcoma cells with genetic signatures of BRCAness are susceptible to the PARP inhibitor talazoparib alone or in combination with chemotherapeutics. Oncotarget 2016, 8, 48794–48806. [Google Scholar]
- Alemi, F.; Malakoti, F.; Vaghari-Tabari, M.; Soleimanpour, J.; Shabestani, N.; Sadigh, A.R.; Khelghati, N.; Asemi, Z.; Ahmadi, Y.; Yousefi, B. DNA damage response signaling pathways as important targets for combination therapy and chemotherapy sensitization in osteosarcoma. J. Cell. Physiol. 2022, 237, 2374–2386. [Google Scholar] [PubMed]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting p53 pathways: Mechanisms, structures and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, M.L. TP53 mutations in cancer: Molecular features and therapeutic opportunities (Review). Int. J. Mol. Med. 2024, 55, 7. [Google Scholar]
- Wong, R.P.C.; Tsang, W.P.; Chau, P.Y.; Co, N.N.; Tsang, T.Y.; Kwok, T.T. p53-R273H gains new function in induction of drug resistance through down-regulation of procaspase-3. Mol. Cancer Ther. 2007, 6, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Huang, C.Y.; Su, H.-L.; Tang, C.H. CCN2 Enhances Resistance to Cisplatin-Mediating Cell Apoptosis in Human Osteosarcoma. PLoS ONE 2014, 9, e90159. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Ding, C.; Liu, P.; Wang, R.; Ding, W.; Tong, D.; Wu, D.; Cheng, L.; Wei, Q.; et al. Increased Circular RNA UBAP2 Acts as a Sponge of miR-143 to Promote Osteosarcoma Progression. Oncotarget 2017, 8, 61687–61697. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Zhang, B. 6-Gingerol Inhibits Osteosarcoma Cell Proliferation Through Apoptosis and AMPK Activation. Tumor Biol. 2014, 36, 1135–1141. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, Q.; Han, J.; Chen, J. Alantolactone Suppresses Human Osteosarcoma Through the PI3K/Akt Signaling Pathway. Mol. Med. Rep. 2020, 21, 675–684. [Google Scholar] [CrossRef]
- Ning, R.; Chen, G.; Fang, R.; Zhang, Y.; Zhao, W.; Qian, F. Diosmetin Inhibits Cell Proliferation and Promotes Apoptosis Through STAT3/c-Myc Signaling Pathway in Human Osteosarcoma Cells. Biol. Res. 2021, 54, 40. [Google Scholar] [CrossRef]
- Jiang, C.; Ma, S.; Hu, R.; Wang, X.; Li, M.; Tian, F.; Jiang, W.; Zhu, L.; Bian, Z. Effect of CXCR4 on Apoptosis in Osteosarcoma Cells via the PI3K/Akt/NF-κβ Signaling Pathway. Cell. Physiol. Biochem. 2018, 46, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.J.; Liu, Y.-C.; Hsu, F.T. Protein Kinase B and Extracellular Signal-Regulated Kinase Inactivation Is Associated with Regorafenib-Induced Inhibition of Osteosarcoma Progression in Vitro and in Vivo. J. Clin. Med. 2019, 8, 900. [Google Scholar] [CrossRef]
- Chao, C.C.; Hou, S.M.; Huang, C.-C.; Hou, C.H.; Chen, P.; Liu, J.F. Plumbagin Induces Apoptosis in Human Osteosarcoma Through ROS Generation, Endoplasmic Reticulum Stress and Mitochondrial Apoptosis Pathway. Mol. Med. Rep. 2017, 16, 5480–5488. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, H.; Li, M.; Wu, Y.; Liu, Y.; Zhao, Y.; Chen, X.; Ma, M. Honokiol Induces Autophagy and Apoptosis of Osteosarcoma Through PI3K/Akt/mTOR Signaling Pathway. Mol. Med. Rep. 2018, 17, 2719–2723. [Google Scholar] [CrossRef]
- Liu, K.; Ren, T.; Huang, Y.; Sun, K.; Bao, X.; Wang, S.; Zheng, B.; Guo, W. Apatinib Promotes Autophagy and Apoptosis Through VEGFR2/STAT3/BCL-2 Signaling in Osteosarcoma. Cell Death Dis. 2017, 8, e3015. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.-C.; Wang, J.; Wang, S. Role of autophagy in drug resistance and regulation of osteosarcoma (Review). Mol. Clin. Oncol. 2022, 16, 72. [Google Scholar] [CrossRef]
- Ji, G.; Yu, N.; Xue, X.; Li, Z.-g. PERK-mediated Autophagy in Osteosarcoma Cells Resists ER Stress-Induced Cell Apoptosis. Int. J. Biol. Sci. 2015, 11, 803–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Hong, H.; Wang, L.; Zhou, Y.; Lang, Y. JNK Pathway Mediates Curcumin-Induced Apoptosis and Autophagy in Osteosarcoma MG63 Cells. Exp. Ther. Med. 2017, 14, 593–599. [Google Scholar] [CrossRef]
- Xu, R.; Liu, S.; Chen, H.; Lao, L. MicroRNA-30a Downregulation Contributes to Chemoresistance of Osteosarcoma Cells Through Activating Beclin-1-Mediated Autophagy. Oncol. Rep. 2015, 35, 1757–1763. [Google Scholar] [CrossRef]
- Ren, C.; Li, X.; He, B.; Hu, W. MicroRNA-410 Regulates Autophagy-Related Gene ATG16L1 Expression and Enhances Chemosensitivity via Autophagy Inhibition in Osteosarcoma. Mol. Med. Rep. 2017, 15, 1326–1334. [Google Scholar]
- Bai, Y.-j.; Chen, Y.; Chen, X.; Jiang, J.; Wang, X.; Wang, L.; Wang, J.; Gao, L. Trichostatin a Activates FOXO1 and Induces Autophagy in Osteosarcoma. Arch. Med. Sci. 2019, 15, 204–213. [Google Scholar] [CrossRef]
- Horie, R.; Nakamura, O.; Yamagami, Y.; Mori, M.; Nishimura, H.; Fukuoka, N.; Yamamoto, T. Apoptosis and Antitumor Effects Induced by the Combination of an mTOR Inhibitor and an Autophagy Inhibitor in Human Osteosarcoma MG63 Cells. Int. J. Oncol. 2015, 48, 37–44. [Google Scholar] [CrossRef]
- Zeng, K.; Jin, L.; Yang, X.; Yang, Z.; Zhu, G. Suppression of Autophagy Sensitizes Osteosarcoma to mTOR Inhibition. Res. Sq. 2020. preprint. [Google Scholar] [CrossRef]
- Pires, S.F.; de Barros, J.S.; da Costa, S.S.; de Oliveira Scliar, M.; van Helvoort Lengert, A.; Boldrini, É.; da Silva, S.R.M.; Tasić, L.; Vidal, D.O.; Krepischi, A.C.V.; et al. DNA methylation patterns suggest the involvement of DNMT3B and TET1 in osteosarcoma development. Mol. Genet. Genom. 2023, 298, 721–733. [Google Scholar]
- Wang, T.; Tan, W.-L.; Huang, J.; Cui, Z.; Liang, R.; Li, Q.-C.; Lu, H.-L. Identification of aberrantly methylated differentially expressed genes targeted by differentially expressed miRNA in osteosarcoma. Ann. Transl. Med. 2020, 8, 373. [Google Scholar]
- Shao, D.; Liu, C.; Wang, Y.; Lin, J.; Cheng, X.; Han, P.; Li, Z.; Jian, D.; Nie, J.; Jiang, M.; et al. DNMT1 determines osteosarcoma cell resistance to apoptosis by associatively modulating DNA and mRNA cytosine-5 methylation. FASEB J. 2023, 37, e23284. [Google Scholar] [CrossRef]
- Chaiyawat, P.; Pruksakorn, D.; Phanphaisarn, A.; Teeyakasem, P.; Klangjorhor, J.; Settakorn, J. Expression Patterns of Class I Histone Deacetylases in Osteosarcoma: A Novel Prognostic Marker with Potential Therapeutic Implications. Mod. Pathol. 2018, 31, 264–274. [Google Scholar] [PubMed]
- La Noce, M.; Paino, F.; Mele, L.; Papaccio, G.; Regad, T.; Lombardi, A.; Papaccio, F.; Desiderio, V.; Tirino, V. HDAC2 depletion promotes osteosarcoma’s stemness both in vitro and in vivo: A study on a putative new target for CSCs directed therapy. J. Exp. Clin. Cancer Res. 2018, 37, 296. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Liu, C.; Jiang, Y.; Hao, Y. Elevated H3K27me3 levels sensitize osteosarcoma to cisplatin. Clin. Epigenet. 2019, 11, 8. [Google Scholar]
- Wirries, A.; Jabari, S.; Jansen, E.P.; Roth, S.; Figueroa-Juárez, E.; Wissniowski, T.T.; Neureiter, D.; Klieser, E.; Lechler, P.; Ruchholtz, S.; et al. Panobinostat Mediated Cell Death: A Novel Therapeutic Approach for Osteosarcoma. Oncotarget 2018, 9, 32997–33010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, W.; Li, W.; Yin, C.; Feng, C.; Liu, B.; Xu, H.; Jin, X.; Tu, C.; Li, Z. PRKDC Induces Chemoresistance in Osteosarcoma by Recruiting GDE2 to Stabilize GNAS and Activate AKT. Cancer Res. 2024, 84, 2873–2887. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ding, L.; Chen, Z. Potential Therapeutic Implications of miRNAs in Osteosarcoma Chemotherapy. Tumor Biol. 2017, 39, 101042831770576. [Google Scholar] [CrossRef]
- Elahi, M.A.; Tariq, A.; Malik, A.; Zhra, M. Role of Hypoxia-Associated Long Noncoding RNAs in Cancer Chemo-Therapy Resistance. Int. J. Mol. Sci. 2025, 26, 936. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.; Xiao, H. Identification of Epigenetic-Dysregulated lncRNAs Signature in Osteosarcoma by Multi-Omics Data Analysis. Front. Med. 2022, 9, 892593. [Google Scholar] [CrossRef]
- Polvani, S.; Martignano, F.; Scoccianti, G.; Pasqui, A.; Palomba, A.R.; Conticello, S.G.; Galli, A.; Palchetti, I.; Caporalini, C.; Antonuzzo, L.; et al. Growth Arrest-Specific 5 lncRNA as a Valuable Biomarker of Chemoresistance in Osteosarcoma. Anti-Cancer Drugs 2022, 33, 278–285. [Google Scholar] [CrossRef]
- Liu, C.; Han, X.; Li, B.; Huang, S.; Zhong, Z.; Wang, Z.; Wang, W. MALAT-1 Is Associated With the Doxorubicin Resistance in U-2os Osteosarcoma Cells. Cancer Manag. Res. 2021, 13, 6879–6889. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Sampaio-Ribeiro, G.; Gomes, C.M.F. Self-Renewal and Pluripotency in Osteosarcoma Stem Cells’ Chemoresistance: Notch, Hedgehog, and Wnt/β-Catenin Interplay with Embryonic Markers. Int. J. Mol. Sci. 2023, 24, 8401. [Google Scholar] [CrossRef]
- Cavalcanti, A.d.S.; Meohas, W.; Ribeiro, G.d.O.; Lopes, A.; Gholamin, S.; Razavi, S.M.; Kasai-Brunswick, T.H.; Avan, A.; Guimarães, J.A.M.; Duarte, M.E.L.; et al. Patient-Derived Osteosarcoma Cells Are Resistant to Methotrexate. PLoS ONE 2017, 12, e0184891. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Z.; Wang, Y.; Li, W.; Qiao, S.; Jia, Q.; Zhang, J.; Zhang, X.; Shen, J.; Yin, J. Dioscin Inhibits Stem-Cell-Like Properties and Tumor Growth of Osteosarcoma Through Akt/Gsk3/Β-Catenin Signaling Pathway. Cell Death Dis. 2018, 9, 343. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, S.; Wang, Y.; Yang, S.-B.; Huang, Z.; Zhu, X.; Cai, S.; Guo, Q.-F.; Zhong, W.; Liu, S. EID3 Promotes Cancer Stem Cell-Like Phenotypes in Osteosarcoma Through the Activation of PI3K-AKT Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 5941562. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Fabbrocini, G.; Cappello, M.; Costa, C.; Scalvenzi, M. Real-Life Effectiveness of Vismodegib in Patients With Metastatic and Advanced Basal Cell Carcinoma: Characterization of Adverse Events and Assessment of Health-Related Quality of Life Using the Dermatology Life Quality Index (DLQI) Test. Dermatol. Ther. 2019, 9, 505–510. [Google Scholar] [CrossRef]
- Qü, W.; Wang, Y.; Wu, Q.; Hao, D.; Li, D. Emodin Impairs Radioresistance of Human Osteosarcoma Cells by Suppressing Sonic Hedgehog Signaling. Med. Sci. Monit. 2017, 23, 5767–5773. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kang, X.; Li, Z.; Chen, L.; Ma, Q.; Fan, P. Hedgehog/Gli1 Signaling Pathway Regulates the Resistance to Cisplatin in Human Osteosarcoma. J. Cancer 2021, 12, 6676–6684. [Google Scholar] [CrossRef]
- Verma, P.; Jain, S.; Kapoor, G.; Tripathi, R.; Sharma, P.; Doval, D.C. IAP Chemotherapy Regimen Is a Viable and Cost-Effective Option in Children and Adolescents With Osteosarcoma: A Comparative Analysis with MAP Regimen on Toxicity and Survival. J. Pediatr. Hematol./Oncol. 2020, 43, e466–e471. [Google Scholar] [CrossRef]
- Sirikul, W.; Buawangpong, N.; Pruksakorn, D.; Charoentum, C.; Teeyakasem, P.; Koonrungsesomboon, N. The Survival Outcomes, Prognostic Factors and Adverse Events following Systemic Chemotherapy Treatment in Bone Sarcomas: A Retrospective Observational Study from the Experience of the Cancer Referral Center in Northern Thailand. Cancers 2023, 15, 1979. [Google Scholar] [CrossRef]
- Harris, M.A.; Hawkins, C.J. Recent and Ongoing Research into Metastatic Osteosarcoma Treatments. Int. J. Mol. Sci. 2022, 23, 3817. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.-K.; Su, Z.; Jin, Q.; Deng, L.; Huang, G.; Shen, J. Cordycepin Augments the Chemosensitivity of Osteosarcoma to Cisplatin by Activating AMPK and Suppressing the AKT Signaling Pathway. Cancer Cell Int. 2021, 21, 706. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, H.; Shou, T.; Tang, C.; Miao, K.; Wang, P. Effectiveness of multi-drug regimen chemotherapy treatment in osteosarcoma patients: A network meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2017, 12, 52. [Google Scholar] [CrossRef]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet. Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef]
- Higuchi, T.; Sugisawa, N.; Miyake, K.; Oshiro, H.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Igarashi, K.; Bouvet, M.; et al. The Combination of Olaratumab with Doxorubicin and Cisplatinum Regresses a Chemotherapy-Resistant Osteosarcoma in a Patient-Derived Orthotopic Xenograft Mouse Model. Transl. Oncol. 2019, 12, 1257–1263. [Google Scholar] [CrossRef]
- Xian, M.; Cao, H.; Cao, J.; Shao, X.; Zhu, D.; Zhang, N.; Huang, P.; Li, W.; Yang, B.; Ying, M.; et al. Bortezomib Sensitizes Human Osteosarcoma Cells to Adriamycin-induced Apoptosis Through ROS-dependent Activation of P-eIF2α/Atf4/Chop Axis. Int. J. Cancer 2017, 141, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, J.; Chen, T.; Zhou, Q. Curcumol Synergizes With Cisplatin in Osteosarcoma by Inhibiting M2-Like Polarization of Tumor-Associated Macrophages. Molecules 2022, 27, 4345. [Google Scholar] [CrossRef]
- Low, K.; Foulkes, P.; Hills, F.; Roberts, H.C.; Stordal, B. The Efficacy of Gemcitabine and Docetaxel Chemotherapy for the Treatment of Relapsed and Refractory Osteosarcoma: A Systematic Review and Pre-clinical Study. Cancer Med. 2024, 13, e70248. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Li, Y.; Nelson, S.D.; Dry, S.; Singh, A.S.; Elliott, I.A.; et al. Temozolomide Combined with Irinotecan Regresses a Cisplatinum-Resistant Relapsed Osteosarcoma in a Patient-Derived Orthotopic Xenograft (PDOX) Precision-Oncology Mouse Model. Oncotarget 2017, 9, 7774–7781. [Google Scholar] [CrossRef][Green Version]
- Isakoff, M.S.; Bielack, S.; Meltzer, P.S.; Görlick, R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J. Clin. Oncol. 2015, 33, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, J.; Chandrashekharan, A.; Banavali, S.; Gupta, S. Osteosarcoma Journey in India: Each Step Reveals a New Horizon! Indian J. Med. Paediatr. Oncol. 2020, 41, 4–6. [Google Scholar] [CrossRef]
- Silva, M.; Renata Rodrigues da Cunha Colombo, B.; Matos, G.D.R.; Toloi, D.d.A.; Nardo, M.; Munhoz, R.R.; Zampieri, C.; Reboledo, D.; Correa, L.; Baptista, A.M.; et al. Treatment Outcomes for Adult Patients with Localized Osteosarcoma Treated with Chemotherapy Without Methotrexate. medRxiv 2020. [Google Scholar] [CrossRef]
- Suárez-Mattos, A.; Arroyave, F.; Infante, A.M.; Narváez, C.F.; Soto, C.; Gómez, L.; Amaya-Nieto, J. Response to Neoadjuvant Chemotherapy and Survival of Children and Adolescents with High-Grade Osteosarcoma Treated Based on the EURAMOS-1 Protocol. Bol. Méd. Hosp. Infant. Méx. 2022, 79, 17–25. [Google Scholar] [CrossRef]
- Ismail, M.D.; Wiratnaya, I.G.E.; Raditya, R.H. Evaluating the Outcome and Patient Safety of Methotrexate, Doxorubicin, and Cisplatin Regimen for Chemotherapy in Osteosarcoma: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2024, 25, 1497–1505. [Google Scholar] [CrossRef]
- Ferrari, S.; Bielack, S.; Smeland, S.; Longhi, A.; Egerer, G.; Hall, K.S.; Donati, D.M.; Kevric, M.; Brosjö, O.; Comandone, A.; et al. EURO-B.O.S.S.: A European Study on Chemotherapy in Bone-Sarcoma Patients Aged over 40: Outcome in Primary High-Grade Osteosarcoma. Tumori J. 2018, 104, 30–36. [Google Scholar] [CrossRef]
- Xi, Q.; Ma, J. Polypeptide N-Acetylgalactosaminyltransferase 14 (GALNT14) as a Chemosensitivity-Related Biomarker for Osteosarcoma. J. Oncol. 2023, 2023, 1083423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhi-min, Z.; Zhang, L.; Zhong, Z. Cytoplasmic APE1 Promotes Resistance Response in Osteosarcoma Patients with Cisplatin Treatment. Cell Biochem. Funct. 2020, 38, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, F.; Zhang, T.; Yu, M.; Sun, Y. Recent advances in anti-multidrug resistance for nano-drug delivery system. Drug Deliv. 2022, 29, 1684–1697. [Google Scholar]
- Wu, K.-Z.; Yu, B.; Li, D.; Tian, Y.; Liu, Y.; Jiang, J. Recent Advances in Nanoplatforms for the Treatment of Osteosarcoma. Front. Oncol. 2022, 12, 805978. [Google Scholar] [CrossRef]
- Deng, J.; Liu, S.; Li, G.; Zheng, Y.; Zhang, W.; Lin, J.; Yu, F.; Weng, J.; Liu, P.; Zeng, H. pH-sensitive Charge-Conversion Cinnamaldehyde Polymeric Prodrug Micelles for Effective Targeted Chemotherapy of Osteosarcoma in Vitro. Front. Chem. 2023, 11, 1190596. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; You, X.; Yi, T.; Lu, B.; Liu, J.; Lu, G.; Ma, M.; Zou, C.; Wu, J.; et al. Nanoparticle Enhanced Combination Therapy for Stem-Like Progenitors Defined by Single-Cell Transcriptomics in Chemotherapy-Resistant Osteosarcoma. Signal Transduct. Target. Ther. 2020, 5, 196. [Google Scholar] [CrossRef]
- Qiu, R.; Sun, D.; Bai, Y.; Li, J.; Wang, L. Application of tumor-targeting peptide-decorated polypeptide nanoparticles with doxorubicin to treat osteosarcoma. Drug Deliv. 2020, 27, 1704–1717. [Google Scholar]
- Zhao, J.; Mu, X.; Hou, X.; Zhang, X.; Li, P.; Jiang, J. Synergistic treatment of osteosarcoma with biomimetic nanoparticles transporting doxorubicin and siRNA. Front. Oncol. 2023, 13, 1111855. [Google Scholar]
- Liang, W.; Dong, Y.; Shao, R.; Zhang, S.; Wu, X.; Huang, X.; Sun, B.; Zeng, B.; Zhao, J. Application of nanoparticles in drug delivery for the treatment of osteosarcoma: Focussing on the liposomes. J. Drug Target. 2021, 30, 463–475. [Google Scholar]
- Wang, S.Y.; Hu, H.-Z.; Qing, X.; Zhang, Z.-C.; Shao, Z.-W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Luo, Y.; Li, B.; Wu, C.; Wang, D.; Xin, Y.; Xu, W.; Xiao, J. IL-11-Engineered Macrophage Membrane-Coated Reactive Oxygen Species-Responsive Nanoparticles for Targeted Delivery of Doxorubicin to Osteosarcoma. ACS Appl. Mater. Interfaces 2024, 16, 55981–55995. [Google Scholar] [PubMed]
- Yang, P.; Zhang, L.; Wang, T.; Liu, Q.; Wang, J.; Wang, Y.; Tu, Z.; Lin, F. Doxorubicin and Edelfosine Combo-Loaded Lipid–Polymer Hybrid Nanoparticles for Synergistic Anticancer Effect Against Drug-Resistant Osteosarcoma. Oncotargets Ther. 2020, 13, 8055–8067. [Google Scholar] [CrossRef]
- Kendre, P.N.; Kayande, D.R.; Pote, A.K.; Kanthale, S.B.; Prajapati, B.G.; Kendre, Y.; Jain, S. Emerging Lipid-based Carriers for Systematic Utilization in the Pharmaceutical and Biomedical Sciences: A Review. Pharm. Nanotechnol. 2024, 13, 2–21. [Google Scholar] [CrossRef]
- Gerardo-Ramírez, M.; Keggenhoff, F.; Giam, V.; Becker, D.; Groth, M.; Hartmann, N.; Straub, B.K.; Morrison, H.; Galle, P.R.; Marquardt, J.U.; et al. CD44 Contributes to the Regulation of MDR1 Protein and Doxorubicin Chemoresistance in Osteosarcoma. Int. J. Mol. Sci. 2022, 23, 8616. [Google Scholar] [CrossRef]
- Feng, S.; Wu, Z.-X.; Zhao, Z.; Liu, J.; Sun, K.; Guo, C.; Wang, H.; Wu, Z.-X. Engineering of Bone- and CD44-Dual-Targeting Redox-Sensitive Liposomes for the Treatment of Orthotopic Osteosarcoma. ACS Appl. Mater. Interfaces 2019, 11, 7357–7368. [Google Scholar] [CrossRef]
- Wu, F.; Xu, J.; Chen, Z.; Jin, M.; Li, X.; Li, J.; Wang, Z.; Li, J. Macrophage Membrane-Coated Liposomes as Controlled Drug Release Nanocarriers for Precision Treatment of Osteosarcoma. ACS Appl. Nano Mater. 2022, 5, 18396–18408. [Google Scholar] [CrossRef]
- Zhong, J.; Wen, W.; Wang, J.; Zhang, M.; Jia, Y.; Ma, X.; Su, Y.X.; Wang, Y.; Lan, X. Bone-Targeted Dual Functional Lipid-Coated Drug Delivery System for Osteosarcoma Therapy. Pharm. Res. 2022, 40, 231–243. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xia, G.; Adilijiang, N.; Li, Y.; Hou, Z.; Fan, Z.; Li, J. Recent Advances in Targeted Drug Delivery Strategy for Enhancing Oncotherapy. Pharmaceutics 2023, 15, 2233. [Google Scholar] [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front. Bioeng. Biotechnol. 2023, 11, 1177151. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Abdolahinia, E.D.; Nakhlband, A.; Aslzad, S.; Fathi, M.; Barar, J.; Omidi, Y. Smart chitosan-folate hybrid magnetic nanoparticles for targeted delivery of doxorubicin to osteosarcoma cells. Colloids Surf. B Biointerfaces 2022, 220, 112911. [Google Scholar]
- Pereira-Silva, M.; Alvarez-Lorenzo, C.; Concheiro, A.; Cláudia Santos, A.; Veiga, F.J.; Figueiras, A. Nanomedicine in osteosarcoma therapy: Micelleplexes for delivery of nucleic acids and drugs for osteosarcoma-targeted therapy. Eur. J. Pharm. Biopharm. 2020, 148, 88–106. [Google Scholar]
- Shaikh, A.B.; Li, F.; Li, M.; He, B.; He, X.; Chen, G.; Guo, B.; Li, D.; Jiang, F.; Dang, L.; et al. Present Advances and Future Perspectives of Molecular Targeted Therapy for Osteosarcoma. Int. J. Mol. Sci. 2016, 17, 506. [Google Scholar] [CrossRef]
- Hassan, S.E.; Bekarev, M.; Kim, M.Y.; Lin, J.; Piperdi, S.; Gorlick, R.; Geller, D.S. Cell surface receptor expression patterns in osteosarcoma. Cancer 2012, 118, 740–749. [Google Scholar] [CrossRef]
- Suehara, Y.; Alex, D.; Bowman, A.; Middha, S.; Zehir, A.; Chakravarty, D.; Wang, L.; Jour, G.; Nafa, K.; Hayashi, T.; et al. Clinical Genomic Sequencing of Pediatric and Adult Osteosarcoma Reveals Distinct Molecular Subsets with Potentially Targetable Alterations. Clin. Cancer Res. 2019, 25, 6346–6356. [Google Scholar] [CrossRef]
- Celik, B.; Cicek, K.; Leal, A.F.; Tomatsu, S. Regulation of Molecular Targets in Osteosarcoma Treatment. Int. J. Mol. Sci. 2022, 23, 12583. [Google Scholar] [CrossRef] [PubMed]
- Galifi, C.A.; Wood, T.L. IGF1R crosstalk with integrins, cadherins, and the tumor microenvironment: Sticking points in understanding IGF1R function in cancer. Endocr.-Relat. Cancer 2023, 30, e230031. [Google Scholar]
- Fleuren, E.D.G.; Versleijen-Jonkers, Y.M.H.; Boerman, O.C.; van der Graaf, W.T.A. Targeting receptor tyrosine kinases in osteosarcoma and Ewing sarcoma: Current hurdles and future perspectives. Biochim. Biophys. Acta (BBA) Rev. Cancer 2014, 1845, 266–276. [Google Scholar]
- Subbiah, V.; Kurzrock, R. Phase 1 clinical trials for sarcomas: The cutting edge. Curr. Opin. Oncol. 2011, 23, 352–360. [Google Scholar]
- Chen, X.; Liu, L.; Liu, P.; Chen, Y.; Lin, D.; Yan, H.; Yan, Q.; Wang, Y.; Qiu, Y.; Fang, B.; et al. Discovery of Potent and Orally Bioavailable Platelet-Derived Growth Factor Receptor (PDGFR) Inhibitors for the Treatment of Osteosarcoma. J. Med. Chem. 2022, 65, 5374–5391. [Google Scholar] [CrossRef]
- Gobin, B.; Moriceau, G.; Ory, B.; Charrier, C.; Brion, R.; Blanchard, F.; Redini, F.; Heymann, D. Imatinib mesylate exerts anti-proliferative effects on osteosarcoma cells and inhibits the tumour growth in immunocompetent murine models. PLoS ONE 2014, 9, e90795. [Google Scholar] [CrossRef]
- Li, C.J.; Liu, X.Z.; Zhang, L.; Chen, L.B.; Shi, X.; Wu, S.J.; Zhao, J.N. Advances in Bone-targeted Drug Delivery Systems for Neoadjuvant Chemotherapy for Osteosarcoma. Orthop. Surg. 2016, 8, 105–110. [Google Scholar] [CrossRef]
- Higuchi, T.; Igarashi, K.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Bouvet, M.; Tsuchiya, H.; Hoffman, R.M. Multikinase-Inhibitor Screening in Drug-resistant Osteosarcoma Patient-derived Orthotopic Xenograft Mouse Models Identifies the Clinical Potential of Regorafenib. Cancer Genom. Proteom. 2021, 18, 637–643. [Google Scholar] [CrossRef]
- Nirala, B.K.; Yamamichi, T.; Yustein, J.T. Deciphering the Signaling Mechanisms of Osteosarcoma Tumorigenesis. Int. J. Mol. Sci. 2023, 24, 11367. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Niu, X.; Yao, W. Receptor Tyrosine Kinases in Osteosarcoma Treatment: Which Is the Key Target? Front. Oncol. 2020, 10, 1642. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Jing, J. Cell Cycle Kinases in Osteosarcoma: Potential for Therapeutic Intervention. Curr. Pharm. Des. 2016, 22, 4830–4834. [Google Scholar] [PubMed]
- Yang, C.; Ji, D.; Weinstein, E.J.; Choy, E.; Hornicek, F.J.; Wood, K.B.; Liu, X.; Mankin, H.; Duan, Z. The kinase Mirk is a potential therapeutic target in osteosarcoma. Carcinogenesis 2009, 31, 552–558. [Google Scholar] [CrossRef]
- Chou, Y.-S.; Yen, C.-C.; Chen, W.-M.; Lin, Y.-C.; Wen, Y.-S.; Ke, W.-T.; Wang, J.-Y.; Liu, C.-Y.; Yang, M.-H.; Chen, T.-H.; et al. Cytotoxic mechanism of PLK1 inhibitor GSK461364 against osteosarcoma: Mitotic arrest, apoptosis, cellular senescence, and synergistic effect with paclitaxel. Int. J. Oncol. 2016, 48, 1187–1194. [Google Scholar] [CrossRef]
- Albarrán, V.; Villamayor, M.L.; Chamorro, J.; Rosero, D.I.; Pozas, J.; San Román, M.; Calvo, J.C.; Pérez de Aguado, P.; Moreno, J.; Guerrero, P.; et al. Receptor Tyrosine Kinase Inhibitors for the Treatment of Recurrent and Unresectable Bone Sarcomas. Int. J. Mol. Sci. 2022, 23, 13784. [Google Scholar] [CrossRef]
- Pignochino, Y.; Grignani, G.; Cavalloni, G.; Motta, M.; Tapparo, M.; Bruno, S.; Bottos, A.; Gammaitoni, L.; Migliardi, G.; Camussi, G.; et al. Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol. Cancer 2009, 8, 118. [Google Scholar] [CrossRef]
- Pignochino, Y.; Dell’aglio, C.; Basiricò, M.; Capozzi, F.; Soster, M.; Marchiò, S.; Bruno, S.; Gammaitoni, L.; Sangiolo, D.; Torchiaro, E.; et al. The Combination of Sorafenib and Everolimus Abrogates mTORC1 and mTORC2 Upregulation in Osteosarcoma Preclinical Models. Clin. Cancer Res. 2013, 19, 2117–2131. [Google Scholar] [PubMed]
- Fioramonti, M.; Fausti, V.; Pantano, F.; Iuliani, M.; Ribelli, G.; Lotti, F.; Pignochino, Y.; Grignani, G.; Santini, D.; Tonini, G.; et al. Cabozantinib Affects Osteosarcoma Growth Through A Direct Effect On Tumor Cells and Modifications In Bone Microenvironment. Sci. Rep. 2018, 8, 4177. [Google Scholar] [CrossRef]
- Cren, P.-Y.; Lebellec, L.; Ryckewaert, T.; Penel, N. Anti-Angiogenic Agents in Management of Sarcoma Patients: Overview of Published Trials. Front. Oncol. 2020, 10, 594445. [Google Scholar] [CrossRef]
- Chen, C.; Shi, Q.; Xu, J.; Ren, T.; Huang, Y.; Guo, W. Current progress and open challenges for applying tyrosine kinase inhibitors in osteosarcoma. Cell Death Discov. 2022, 8, 488. [Google Scholar] [CrossRef]
- Jin, R.; Jin, Y.Y.; Tang, Y.L.; Yang, H.J.; Zhou, X.Q.; Lei, Z. GPNMB silencing suppresses the proliferation and metastasis of osteosarcoma cells by blocking the PI3K/Akt/mTOR signaling pathway. Oncol. Rep. 2018, 39, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yu, W.; Liu, B.; Yang, M.; Tao, H. Estrogen receptor β induces autophagy of osteosarcoma through the mTOR signaling pathway. J. Orthop. Surg. Res. 2020, 15, 50. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, C.; Shi, J.; Wen, K.; Wang, X. AIM2 inhibits the proliferation, invasion and migration, and promotes the apoptosis of osteosarcoma cells by inactivating the PI3K/Akt/mTOR signaling pathway. Mol. Med. Rep. 2022, 25, 53. [Google Scholar] [CrossRef]
- Ma, H.; Su, R.; Feng, H.; Guo, Y.; Su, G. Long noncoding RNA UCA1 promotes osteosarcoma metastasis through CREB1-mediated epithelial-mesenchymal transition and activating PI3K/Akt/mTOR pathway. J. Bone Oncol. 2019, 16, 100228. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Chen, J.; Liu, H.; Lu, J.; Jiang, H.; Huang, A.; Chen, Y. Fibulin-4 promotes osteosarcoma invasion and metastasis by inducing epithelial to mesenchymal transition via the PI3K/Akt/mTOR pathway. Int. J. Oncol. 2017, 50, 1513–1530. [Google Scholar] [CrossRef]
- Gupte, A.; Baker, E.K.; Wan, S.S.; Stewart, E.; Loh, A.; Shelat, A.A.; Gould, C.M.; Chalk, A.M.; Taylor, S.; Lackovic, K.; et al. Systematic Screening Identifies Dual PI3K and mTOR Inhibition as a Conserved Therapeutic Vulnerability in Osteosarcoma. Clin. Cancer Res. 2015, 21, 3216–3229. [Google Scholar] [CrossRef]
- Moriceau, G.; Ory, B.; Mitrofan, L.; Riganti, C.; Blanchard, F.; Brion, R.; Charrier, C.; Battaglia, S.; Pilet, P.; Denis, M.G.; et al. Zoledronic acid potentiates mTOR inhibition and abolishes the resistance of osteosarcoma cells to RAD001 (Everolimus): Pivotal role of the prenylation process. Cancer Res. 2010, 70, 10329–10339. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, J.; Xu, B.; He, S.; Yang, H.; Liu, L. Long non-coding RNA XIST serves an oncogenic role in osteosarcoma by sponging miR-137. Exp. Ther. Med. 2019, 17, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhu, Y.; Fan, G.; Hu, S. MicroRNA-2682-3p inhibits osteosarcoma cell proliferation by targeting CCND2, MMP8 and Myd88. Oncol. Lett. 2018, 16, 3359–3364. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, S.T.; Wang, X.; Deng, J.; Li, W.H.; Zhang, P.; Liu, B.S. MiR-100 Inhibits Osteosarcoma Cell Proliferation, Migration, and Invasion and Enhances Chemosensitivity by Targeting IGFIR. Technol. Cancer Res. Treat. 2016, 15, Np40–Np48. [Google Scholar] [CrossRef]
- Zu, D.; Dong, Q.; Yao, J.; Chen, S.; Fang, B.; Ma, J.; Wu, B. miRNA-23b-5p affects the proliferation, migration and invasion of osteosarcoma by targeting TMEM127. Discov. Oncol. 2022, 13, 71. [Google Scholar] [CrossRef]
- Ziyan, W.; Shuhua, Y.; Xiufang, W.; Xiaoyun, L. MicroRNA-21 is involved in osteosarcoma cell invasion and migration. Med. Oncol. 2011, 28, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, L.; Lu, Y.; Yu, X.; Chen, H.; Yin, Q.; Zhang, Y. miRNA-21 inhibition inhibits osteosarcoma cell proliferation by targeting PTEN and regulating the TGF-β1 signaling pathway. Oncol. Lett. 2018, 16, 4337–4342. [Google Scholar] [CrossRef]
- Geng, X.; Wang, H.; Xu, L.; Han, Y.; Liu, Y. MicroRNA-140-5p is Downregulated in Osteosarcoma and Overexpression of MicroRNA-140-5p Inhibits Cancer Cell Proliferation by Downregulating GLUT-1. OncoTargets Ther. 2021, 14, 995–1002. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Xu, J.; Luo, Y.; Xin, Y.; Wang, Y. Downregulation of microRNA-95-3p suppresses cell growth of osteosarcoma via CDKN1A/p21 expression. Oncol. Rep. 2018, 39, 289–297. [Google Scholar] [CrossRef]
- Tiram, G.; Segal, E.; Krivitsky, A.; Shreberk-Hassidim, R.; Ferber, S.; Ofek, P.; Udagawa, T.; Edry, L.; Shomron, N.; Roniger, M.; et al. Identification of Dormancy-Associated MicroRNAs for the Design of Osteosarcoma-Targeted Dendritic Polyglycerol Nanopolyplexes. ACS Nano 2016, 10, 2028–2045. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhang, Z.C.; Han, G.S.; Han, J.Z.; Qiu, D.P. Overexpression of miR-214 promotes the progression of human osteosarcoma by regulating the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2017, 15, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Cuscino, N.; Raimondi, L.; De Luca, A.; Carcione, C.; Russelli, G.; Conti, L.; Baldi, J.; Conaldi, P.G.; Giavaresi, G.; Gallo, A. Gathering Novel Circulating Exosomal microRNA in Osteosarcoma Cell Lines and Possible Implications for the Disease. Cancers 2019, 11, 1924. [Google Scholar] [CrossRef]
- Song, Q.C.; Shi, Z.B.; Zhang, Y.T.; Ji, L.; Wang, K.Z.; Duan, D.P.; Dang, X.Q. Downregulation of microRNA-26a is associated with metastatic potential and the poor prognosis of osteosarcoma patients. Oncol. Rep. 2014, 31, 1263–1270. [Google Scholar] [CrossRef]
- Hua, J.; Liu, D.; Cao, L.; Wang, D.; Wu, T.; Lin, F.; Su, P.; Niu, Y.; Sun, Y. Diagnostic and prognostic values of blood microRNA-Let7A for osteosarcoma. J. Bone Oncol. 2018, 12, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, S.; Xu, B.; Luo, H. Cancer evolution: A means by which tumors evade treatment. Biomed. Pharmacother. 2021, 133, 111016. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, J.H.; Lin, Z.H.; Lv, H.Y.; Ye, Z.M.; Chen, Y.P.; Zhang, X.Y. Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of osteosarcoma. Aging 2020, 12, 3486–3501. [Google Scholar] [CrossRef]
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 5825401. [Google Scholar] [CrossRef]
- Singh, T.; Bhattacharya, M.; Mavi, A.K.; Gulati, A.; Rakesh; Sharma, N.K.; Gaur, S.; Kumar, U. Immunogenicity of cancer cells: An overview. Cell. Signal. 2024, 113, 110952. [Google Scholar] [CrossRef]
- Evdokimova, V.; Gassmann, H.; Radvanyi, L.; Burdach, S.E.G. Current State of Immunotherapy and Mechanisms of Immune Evasion in Ewing Sarcoma and Osteosarcoma. Cancers 2022, 15, 272. [Google Scholar] [CrossRef]
- Wahid, B.; Ali, A.; Rafique, S.; Waqar, M.; Wasim, M.; Wahid, K.; Idrees, M. An overview of cancer immunotherapeutic strategies. Immunotherapy 2018, 10, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, E.; Chorya, H.; Muili, A.; Moradeyo, A.; Kayode, A.; Naik, A.; Odedele, T.; Opabode, M. Chemotherapy, immunotherapy, and targeted therapy for osteosarcoma: Recent advancements. Crit. Rev. Oncol. Hematol. 2025, 206, 104575. [Google Scholar] [CrossRef]
- Hemminki, O.; Dos Santos, J.M.; Hemminki, A. Oncolytic viruses for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Min, L.; Seebacher, N.A.; Li, X.; Zhou, Y.; Hornicek, F.J.; Wei, Y.; Tu, C.; Duan, Z. Targeting mutant TP53 as a potential therapeutic strategy for the treatment of osteosarcoma. J. Orthop. Res. 2019, 37, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiong, W.; Li, S.; Wang, G.; Zhou, J.; Li, H. CRISPR-Cas9 screening identified lethal genes enriched in necroptosis pathway and of prognosis significance in osteosarcoma. J. Gene Med. 2023, 25, e3563. [Google Scholar] [CrossRef]
- Liang, C.; Li, F.; Wang, L.; Zhang, Z.K.; Wang, C.; He, B.; Li, J.; Chen, Z.; Shaikh, A.B.; Liu, J.; et al. Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials 2017, 147, 68–85. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Lin, B.; Zhang, W.; Ji, G. Applications of CRISPR/Cas9 in the research of malignant musculoskeletal tumors. BMC Musculoskelet. Disord. 2021, 22, 149. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.V. Promise and Challenges of T Cell Immunotherapy for Osteosarcoma. Int. J. Mol. Sci. 2023, 24, 12520. [Google Scholar] [CrossRef]
- Hidalgo, L.; Somovilla-Crespo, B.; Garcia-Rodriguez, P.; Morales-Molina, A.; Rodriguez-Milla, M.A.; Garcia-Castro, J. Switchable CAR T cell strategy against osteosarcoma. Cancer Immunol. Immunother. 2023, 72, 2623–2633. [Google Scholar] [CrossRef]
- Hui, X.; Farooq, M.A.; Chen, Y.; Ajmal, I.; Ren, Y.; Xue, M.; Ji, Y.; Du, B.; Wu, S.; Jiang, W. A novel strategy of co-expressing CXCR5 and IL-7 enhances CAR-T cell effectiveness in osteosarcoma. Front. Immunol. 2024, 15, 1462076. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Zhu, H.; Ivanovic, M.; Nguyen, A.; Nguyen, P.K.; Wu, S.M. Immune checkpoint inhibitor cardiotoxicity: Breaking barriers in the cardiovascular immune landscape. J. Mol. Cell. Cardiol. 2021, 160, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, M.; Zhang, B.; Yang, C.; Wang, Y.; Zheng, S.; Tang, L.; Zhou, C.; Qian, G.; Huang, Y.; et al. A pilot study of multi-antigen stimulated cell therapy-I plus camrelizumab and apatinib in patients with advanced bone and soft-tissue sarcomas. BMC Med. 2023, 21, 470. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowska, S.; Murty, T.; Alimadadi, A.; Contreras, C.F.; Duault, C.; Subrahmanyam, P.B.; Reynolds, W.; Gutierrez, N.A.; Baskar, R.; Wu, C.J.; et al. Immune determinants of CAR-T cell expansion in solid tumor patients receiving GD2 CAR-T cell therapy. Cancer Cell 2024, 42, 35–51.e8. [Google Scholar] [CrossRef]
- Al-Haideri, M.; Tondok, S.B.; Safa, S.H.; Maleki, A.H.; Rostami, S.; Jalil, A.T.; Al-Gazally, M.E.; Alsaikhan, F.; Rizaev, J.A.; Mohammad, T.A.M.; et al. CAR-T cell combination therapy: The next revolution in cancer treatment. Cancer Cell Int. 2022, 22, 365. [Google Scholar] [CrossRef]
- Wudhikarn, K.; Soh, S.Y.; Huang, H.; Perales, M.A. Toxicity of Chimeric Antigen Receptor T Cells and its Management. Blood Cell Ther. 2021, 4, S1–S7. [Google Scholar] [CrossRef]
- Moghanloo, E.; Mollanoori, H.; Talebi, M.; Pashangzadeh, S.; Faraji, F.; Hadjilooei, F.; Mahmoodzadeh, H. Remote controlling of CAR-T cells and toxicity management: Molecular switches and next generation CARs. Transl. Oncol. 2021, 14, 101070. [Google Scholar] [CrossRef]
- Nishimoto, M.; Takakuwa, T.; Kuno, M.; Makuuchi, Y.; Okamura, H.; Nakashima, Y.; Koh, H.; Namba, H.; Itoh, Y.; Hino, M.; et al. Recapitulated Late-Onset Inflammatory Toxicities and Progressive Dysautonomia with Persistence of Central Memory CD4+ Chimeric Antigen Receptor T Cells in a Case of Transformed Follicular Lymphoma: Case Report. Acta Haematol. 2023, 146, 338–342. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Z.; Cui, D.; Bian, L.; Zheng, Z.; Zhu, J.; Geng, H.; Sun, Z.; Pan, Y.; Shi, Y.; et al. Triple knockdown of CD11a, CD49d, and PSGL1 in T cells reduces CAR-T cell toxicity but preserves activity against solid tumors in mice. Sci. Transl. Med. 2025, 17, eadl6432. [Google Scholar] [CrossRef]
- Zhang, E.; Xu, H. A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J. Hematol. Oncol. 2017, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Tang, D.; Han, Y.; Shen, E.; Abdihamid, O.; Guo, C.; Shen, H.; Zeng, S. A comprehensive analysis of the fatal toxic effects associated with CD19 CAR-T cell therapy. Aging 2020, 12, 18741–18753. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hu, J.; Jia, Z.; Wang, Q.; Wang, J.; Dao, L.H.; Zhang, W.; Zhang, S.; Xia, X.; Gorlick, R.; et al. Membrane-Anchored and Tumor-Targeted IL12 (attIL12)-PBMC Therapy for Osteosarcoma. Clin. Cancer Res. 2022, 28, 3862–3873. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Deng, C.; Zheng, X.; Huang, Y.; Chen, C.; Gu, H. Identification of a novel cellular senescence-related lncRNA signature for prognosis and immune response in osteosarcoma. Transl. Cancer Res. 2024, 13, 3742–3759. [Google Scholar] [CrossRef]
- Luo, Z.W.; Liu, P.P.; Wang, Z.X.; Chen, C.Y.; Xie, H. Macrophages in Osteosarcoma Immune Microenvironment: Implications for Immunotherapy. Front. Oncol. 2020, 10, 586580. [Google Scholar] [CrossRef]
- Wu, C.C.; Beird, H.C.; Andrew Livingston, J.; Advani, S.; Mitra, A.; Cao, S.; Reuben, A.; Ingram, D.; Wang, W.L.; Ju, Z.; et al. Immuno-genomic landscape of osteosarcoma. Nat. Commun. 2020, 11, 1008. [Google Scholar] [CrossRef]
- Park, J.A.; Cheung, N.V. GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma. J. Hematol. Oncol. 2020, 13, 172. [Google Scholar] [CrossRef]
- Ligon, J.A.; Choi, W.; Cojocaru, G.; Fu, W.; Hsiue, E.H.; Oke, T.F.; Siegel, N.; Fong, M.H.; Ladle, B.; Pratilas, C.A.; et al. Pathways of immune exclusion in metastatic osteosarcoma are associated with inferior patient outcomes. J. Immunother. Cancer 2021, 9, e001772. [Google Scholar] [CrossRef]
- Palmerini, E.; Agostinelli, C.; Picci, P.; Pileri, S.; Marafioti, T.; Lollini, P.L.; Scotlandi, K.; Longhi, A.; Benassi, M.S.; Ferrari, S. Tumoral immune-infiltrate (IF), PD-L1 expression and role of CD8/TIA-1 lymphocytes in localized osteosarcoma patients treated within protocol ISG-OS1. Oncotarget 2017, 8, 111836–111846. [Google Scholar] [CrossRef]
- Peng, L.; Fang, H.; Yang, X.; Zeng, X. Analysis of combination therapy of immune checkpoint inhibitors in osteosarcoma. Front. Chem. 2022, 10, 847621. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, J.; Duan, C.; Liu, Y. Retrospective Analysis of Adoptive TIL Therapy plus Anti-PD1 Therapy in Patients with Chemotherapy-Resistant Metastatic Osteosarcoma. J. Immunol. Res. 2020, 2020, 7890985. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, Q.; Fan, Y.; Li, J.; Zhang, J.; Wang, W.; Fan, J.; Guo, Y.; Liu, S.; Hao, D.; et al. MYC inhibition reprograms tumor immune microenvironment by recruiting T lymphocytes and activating the CD40/CD40L system in osteosarcoma. Cell Death Discov. 2022, 8, 117. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Wang, H.; Yu, Z.; Zhu, Y.; Gao, Y. Specific inhibition of PI3Kδ/γ enhances the efficacy of anti-PD1 against osteosarcoma cancer. J. Bone Oncol. 2019, 16, 100206. [Google Scholar] [CrossRef]
- Ratti, C.; Botti, L.; Cancila, V.; Galvan, S.; Torselli, I.; Garofalo, C.; Manara, M.C.; Bongiovanni, L.; Valenti, C.F.; Burocchi, A.; et al. Trabectedin Overrides Osteosarcoma Differentiative Block and Reprograms the Tumor Immune Environment Enabling Effective Combination with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2017, 23, 5149–5161. [Google Scholar] [CrossRef]
- Hennessy, M.; Wahba, A.; Felix, K.; Cabrera, M.; Segura, M.G.; Kundra, V.; Ravoori, M.K.; Stewart, J.; Kleinerman, E.S.; Jensen, V.B.; et al. Bempegaldesleukin (BEMPEG; NKTR-214) efficacy as a single agent and in combination with checkpoint-inhibitor therapy in mouse models of osteosarcoma. Int. J. Cancer 2021, 148, 1928–1937. [Google Scholar] [CrossRef]
- McEachron, T.A.; Triche, T.J.; Sorenson, L.; Parham, D.M.; Carpten, J.D. Profiling targetable immune checkpoints in osteosarcoma. Oncoimmunology 2018, 7, e1475873. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Li, B.; Wang, S.; Chen, T.; Ye, Z. Innate Immune Cells: A Potential and Promising Cell Population for Treating Osteosarcoma. Front. Immunol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Tullius, B.P.; Setty, B.A.; Lee, D.A. Natural Killer Cell Immunotherapy for Osteosarcoma. Adv. Exp. Med. Biol. 2020, 1257, 141–154. [Google Scholar] [CrossRef]
- Kisseberth, W.C.; Lee, D.A. Adoptive Natural Killer Cell Immunotherapy for Canine Osteosarcoma. Front. Vet. Sci. 2021, 8, 672361. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Lorentzen, C.L.; Haanen, J.B.; Met, Ö.; Svane, I.M. Clinical advances and ongoing trials on mRNA vaccines for cancer treatment. Lancet Oncol. 2022, 23, e450–e458. [Google Scholar] [CrossRef] [PubMed]
- Omole, R.K.; Oluwatola, O.; Akere, M.T.; Eniafe, J.; Agboluaje, E.O.; Daramola, O.B.; Ayantunji, Y.J.; Omotade, T.I.; Torimiro, N.; Ayilara, M.S.; et al. Comprehensive assessment on the applications of oncolytic viruses for cancer immunotherapy. Front. Pharmacol. 2022, 13, 1082797. [Google Scholar] [CrossRef]
- Asano, N.; Takeshima, H.; Yamashita, S.; Takamatsu, H.; Hattori, N.; Kubo, T.; Yoshida, A.; Kobayashi, E.; Nakayama, R.; Matsumoto, M.; et al. Epigenetic reprogramming underlies efficacy of DNA demethylation therapy in osteosarcomas. Sci. Rep. 2019, 9, 20360. [Google Scholar] [CrossRef]
- Wang, R.; Wang, D.; Bai, X.; Guo, J.; Xia, S.; Cheng, Y.; Gu, Y.; Wang, Q.; Nie, J.; Chen, D.; et al. Kinome-wide CRISPR-Cas9 knockout screens revealed PLK1 as a therapeutic target for osteosarcoma. Cell Death Discov. 2023, 9, 231. [Google Scholar] [CrossRef]
- Mohr, A.; Marques Da Costa, M.E.; Fromigue, O.; Audinot, B.; Balde, T.; Droit, R.; Abbou, S.; Khneisser, P.; Berlanga, P.; Perez, E.; et al. From biology to personalized medicine: Recent knowledge in osteosarcoma. Eur. J. Med. Genet. 2024, 69, 104941. [Google Scholar] [CrossRef]
- Twenhafel, L.; Moreno, D.; Punt, T.; Kinney, M.; Ryznar, R. Epigenetic Changes Associated with Osteosarcoma: A Comprehensive Review. Cells 2023, 12, 1595. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA methyltransferase inhibitors combination therapy for the treatment of solid tumor: Mechanism and clinical application. Clin. Epigenet. 2021, 13, 166. [Google Scholar] [CrossRef]
- Manara, M.C.; Valente, S.; Cristalli, C.; Nicoletti, G.; Landuzzi, L.; Zwergel, C.; Mazzone, R.; Stazi, G.; Arimondo, P.B.; Pasello, M.; et al. A Quinoline-Based DNA Methyltransferase Inhibitor as a Possible Adjuvant in Osteosarcoma Therapy. Mol. Cancer Ther. 2018, 17, 1881–1892. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, L.; Liu, Z.; He, S.; Wang, C.Z.; Wu, Y.; Han, L.; Liu, Z.; Fu, Z.; Tu, C.; et al. Integrated multiomic analysis and high-throughput screening reveal potential gene targets and synergetic drug combinations for osteosarcoma therapy. MedComm 2023, 4, e317. [Google Scholar] [CrossRef]
- Murahari, S.; Jalkanen, A.L.; Kulp, S.K.; Chen, C.S.; Modiano, J.F.; London, C.A.; Kisseberth, W.C. Sensitivity of osteosarcoma cells to HDAC inhibitor AR-42 mediated apoptosis. BMC Cancer 2017, 17, 67. [Google Scholar] [CrossRef]
- Zhou, M.; Yuan, M.; Zhang, M.; Lei, C.; Aras, O.; Zhang, X.; An, F. Combining histone deacetylase inhibitors (HDACis) with other therapies for cancer therapy. Eur. J. Med. Chem. 2021, 226, 113825. [Google Scholar] [CrossRef]
- Hontecillas-Prieto, L.; Flores-Campos, R.; Silver, A.; de Álava, E.; Hajji, N.; García-Domínguez, D.J. Synergistic Enhancement of Cancer Therapy Using HDAC Inhibitors: Opportunity for Clinical Trials. Front. Genet. 2020, 11, 578011. [Google Scholar] [CrossRef]
- Chaiyawat, P.; Sirikaew, N.; Budprom, P.; Klangjorhor, J.; Phanphaisarn, A.; Teeyakasem, P.; Settakorn, J.; Pruksakorn, D. Expression profiling of DNA methyl transferase I (DNMT1) and efficacy of a DNA-hypomethylating agent (decitabine) in combination with chemotherapy in osteosarcoma. J. Bone Oncol. 2020, 25, 100321. [Google Scholar] [CrossRef]
- Capobianco, E.; Mora, A.; La Sala, D.; Roberti, A.; Zaki, N.; Badidi, E.; Taranta, M.; Cinti, C. Separate and Combined Effects of DNMT and HDAC Inhibitors in Treating Human Multi-Drug Resistant Osteosarcoma HosDXR150 Cell Line. PLoS ONE 2014, 9, e95596. [Google Scholar] [CrossRef]
- Thayanithy, V.; Park, C.; Sarver, A.L.; Kartha, R.V.; Korpela, D.M.; Graef, A.J.; Steer, C.J.; Modiano, J.F.; Subramanian, S. Combinatorial Treatment of DNA and Chromatin-Modifying Drugs Cause Cell Death in Human and Canine Osteosarcoma Cell Lines. PLoS ONE 2012, 7, e43720. [Google Scholar] [CrossRef]
- Cain, J.E.; McCaw, A.; Jayasekara, W.S.N.; Rossello, F.J.; Marini, K.D.; Irving, A.T.; Kansara, M.; Thomas, D.M.; Ashley, D.M.; Watkins, D.N. Sustained Low-Dose Treatment with the Histone Deacetylase Inhibitor LBH589 Induces Terminal Differentiation of Osteosarcoma Cells. Sarcoma 2013, 2013, 608964. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, X.; Jin, J.; Xu, H.; Gao, Q.; Wang, Y.; Zhao, J. Histone Deacetylase Inhibitor Trichostatin a Promotes the Apoptosis of Osteosarcoma Cells through p53 Signaling Pathway Activation. Int. J. Biol. Sci. 2016, 12, 1298–1308. [Google Scholar]
- McGuire, J.J.; Nerlakanti, N.; Lo, C.H.; Tauro, M.; Utset-Ward, T.J.; Reed, D.R.; Lynch, C.C. Histone deacetylase inhibition prevents the growth of primary and metastatic osteosarcoma. Int. J. Cancer 2020, 147, 2811–2823. [Google Scholar] [CrossRef]
- Liu, T.; Yan, Z.; Liu, Y.; Choy, E.; Hornicek, F.J.; Mankin, H.; Duan, Z. CRISPR-Cas9-Mediated Silencing of CD44 in Human Highly Metastatic Osteosarcoma Cells. Cell. Physiol. Biochem. 2018, 46, 1218–1230. [Google Scholar] [CrossRef]
- Feng, Y.; Sassi, S.; Shen, J.K.; Yang, X.; Gao, Y.; Osaka, E.; Zhang, J.; Yang, S.; Yang, C.; Mankin, H.J.; et al. Targeting CDK11 in osteosarcoma cells using the CRISPR-Cas9 system. J. Orthop. Res. 2015, 33, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Z.; Zhang, Q.; De Amorim Bernstein, K.; Lozano-Calderon, S.; Choy, E.; Hornicek, F.J.; Duan, Z. Targeting ABCB1 (MDR1) in multi-drug resistant osteosarcoma cells using the CRISPR-Cas9 system to reverse drug resistance. Oncotarget 2016, 7, 83502–83513. [Google Scholar] [CrossRef]
- Mirgayazova, R.; Khadiullina, R.; Chasov, V.; Mingaleeva, R.; Miftakhova, R.; Rizvanov, A.; Bulatov, E. Therapeutic Editing of the TP53 Gene: Is CRISPR/Cas9 an Option? Genes 2020, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M.D. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA 2020, 11, e1594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Zhu, K.P.; Ma, X.L. Antisense lncRNA FOXC2-AS1 promotes doxorubicin resistance in osteosarcoma by increasing the expression of FOXC2. Cancer Lett. 2017, 396, 66–75. [Google Scholar] [CrossRef]
- Ameline, B.; Kovac, M.; Nathrath, M.; Barenboim, M.; Witt, O.; Krieg, A.H.; Baumhoer, D. Overactivation of the IGF signalling pathway in osteosarcoma: A potential therapeutic target? J. Pathol. Clin. Res. 2021, 7, 165–172. [Google Scholar] [CrossRef]
- Gagliardi, M.; Ashizawa, A.T. The Challenges and Strategies of Antisense Oligonucleotide Drug Delivery. Biomedicines 2021, 9, 433. [Google Scholar] [CrossRef]
- Gazouli, I.; Kyriazoglou, A.; Kotsantis, I.; Anastasiou, M.; Pantazopoulos, A.; Prevezanou, M.; Chatzidakis, I.; Kavourakis, G.; Economopoulou, P.; Kontogeorgakos, V.; et al. Systematic Review of Recurrent Osteosarcoma Systemic Therapy. Cancers 2021, 13, 1757. [Google Scholar] [CrossRef]
- Song, X.; Liu, C.; Wang, N.; Huang, H.; He, S.; Gong, C.; Wei, Y. Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy. Adv. Drug Deliv. Rev. 2021, 168, 158–180. [Google Scholar] [CrossRef]
- Shen, C.C.; Hsu, M.N.; Chang, C.W.; Lin, M.W.; Hwu, J.R.; Tu, Y.; Hu, Y.C. Synthetic switch to minimize CRISPR off-target effects by self-restricting Cas9 transcription and translation. Nucleic Acids Res. 2019, 47, e13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, H.; Wang, H.; Liu, J.; Jiang, W.; Zhou, F.; Zhang, J. DNA nanotechnology-based strategies for minimising hybridisation-dependent off-target effects in oligonucleotide therapies. Mater. Horiz. 2024, 12, 1388–1412. [Google Scholar] [CrossRef]
- Sayles, L.C.; Breese, M.R.; Koehne, A.L.; Leung, S.G.; Lee, A.G.; Liu, H.Y.; Spillinger, A.; Shah, A.T.; Tanasa, B.; Straessler, K.; et al. Genome-Informed Targeted Therapy for Osteosarcoma. Cancer Discov. 2019, 9, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Sun, X.; Wang, X.; Ren, T.; Huang, Y.; Zhang, R.; Zheng, B.; Guo, W. Exosomal PD-L1 and N-cadherin predict pulmonary metastasis progression for osteosarcoma patients. J. Nanobiotechnol. 2020, 18, 151. [Google Scholar] [CrossRef]
- Scotlandi, K.; Hattinger, C.M.; Pellegrini, E.; Gambarotti, M.; Serra, M. Genomics and Therapeutic Vulnerabilities of Primary Bone Tumors. Cells 2020, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Tang, F.; Tu, C.; Hornicek, F.; Duan, Z.; Min, L. Immune checkpoints in osteosarcoma: Recent advances and therapeutic potential. Cancer Lett. 2022, 547, 215887. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Fantoni, L.; Casotti, C.; Riganti, C.; Serra, M. Drug Resistance in Osteosarcoma: Emerging Biomarkers, Therapeutic Targets and Treatment Strategies. Cancers 2021, 13, 2878. [Google Scholar] [CrossRef]
- Vos, H.I.; Coenen, M.J.; Guchelaar, H.J.; Te Loo, D.M. The role of pharmacogenetics in the treatment of osteosarcoma. Drug Discov. Today 2016, 21, 1775–1786. [Google Scholar] [CrossRef]
- Hattinger, C.M.; Patrizio, M.P.; Luppi, S.; Serra, M. Pharmacogenomics and Pharmacogenetics in Osteosarcoma: Translational Studies and Clinical Impact. Int. J. Mol. Sci. 2020, 21, 4659. [Google Scholar] [CrossRef]
- Salazar, J.; Arranz, M.J.; Martin-Broto, J.; Bautista, F.; Martínez-García, J.; Martínez-Trufero, J.; Vidal-Insua, Y.; Echebarria-Barona, A.; Díaz-Beveridge, R.; Valverde, C.; et al. Pharmacogenetics of Neoadjuvant MAP Chemotherapy in Localized Osteosarcoma: A Study Based on Data from the GEIS-33 Protocol. Pharmaceutics 2024, 16, 1585. [Google Scholar] [CrossRef]
- Ji, W.P.; He, N.B. Investigation on the DNA repaired gene polymorphisms and response to chemotherapy and overall survival of osteosarcoma. Int. J. Clin. Exp. Pathol. 2015, 8, 894–899. [Google Scholar]
- Serra, M.; Hattinger, C.M. Polymorphisms of genes related to metotrexate response and toxicity in high-grade osteosarcoma. Expert Opin. Drug Metab. Toxicol. 2017, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Paolillo, A.; Tesser-Gamba, F.; Seixas Alves, M.T.; Filho, R.J.G.; Oliveira, R.; Petrilli, A.S.; Toledo, S.R.C. Pharmacogenetics of the Primary and Metastatic Osteosarcoma: Gene Expression Profile Associated with Outcome. Int. J. Mol. Sci. 2023, 24, 5607. [Google Scholar] [CrossRef] [PubMed]
- Obiedat, H.; Alrabadi, N.; Sultan, E.; Al Shatti, M.; Zihlif, M. The effect of ERCC1 and ERCC2 gene polymorphysims on response to cisplatin based therapy in osteosarcoma patients. BMC Med. Genet. 2018, 19, 112. [Google Scholar] [CrossRef]
- Campbell, K.; Posner, A.; Chen, N.; Cavanaugh, K.L.; Bhushan, K.; Janeway, K.A.; Shulman, D.S.; George, S.; Klega, K.S.; Crompton, B.D.; et al. Phase 1 study of cabozantinib in combination with topotecan–cyclophosphamide for patients with relapsed Ewing sarcoma or osteosarcoma. Pediatr. Blood Cancer 2023, 70, e30681. [Google Scholar] [PubMed]
- Le Cesne, A.; Marec-Berard, P.; Blay, J.Y.; Gaspar, N.; Bertucci, F.; Penel, N.; Bompas, E.; Cousin, S.; Toulmonde, M.; Bessede, A.; et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: Results from the PEMBROSARC study. Eur. J. Cancer 2019, 119, 151–157. [Google Scholar] [CrossRef]
- Chou, A.; Kleinerman, E.S.; Krailo, M.; Chen, Z.; Betcher, D.L.; Healey, J.H.; Conrad, E.U.; Nieder, M.L.; Weiner, M.A.; Wells, R.J.; et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma. Cancer 2009, 115, 5339–5348. [Google Scholar]
- Chou, A.; Gupta, R.; Bell, M.D.; Riewe, K.O.D.; Meyers, P.A.; Gorlick, R.G. Inhaled lipid cisplatin (ILC) in the treatment of patients with relapsed/progressive osteosarcoma metastatic to the lung. Pediatr. Blood Cancer 2013, 60, 580–586. [Google Scholar]
- Forrest, S.J.; Kinnaman, M.D.; Livingston, J.A.; Vo, K.T.; Merriam, P.; Clinton, C.M.; Desmith, K.; Cavanaugh, K.L.; Felicetti, B.; Smith, S.A.; et al. Phase II trial of olaparib in combination with ceralasertib in patients with recurrent osteosarcoma. J. Clin. Oncol. 2021, 39, TPS11575. [Google Scholar]
- Xie, L.; Xu, J.; Sun, X.; Li, X.; Liu, K.; Liang, X.; Zhou, Z.; Zhuang, H.; Sun, K.; Wu, Y.; et al. Apatinib plus ifosfamide and etoposide for relapsed or refractory osteosarcoma: A retrospective study in two centres. Oncol. Lett. 2021, 22, 552. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, X.; Zhu, X.; Xu, H.; Song, G.; Lu, J.; Wu, H.; Deng, C.; Ai, F.; Zhang, Y.; et al. Camrelizumab in combination with doxorubicin, cisplatin, ifosfamide, and methotrexate in neoadjuvant treatment of resectable osteosarcoma: A prospective, single-arm, exploratory phase II trial. Cancer Med. 2024, 13, e70206. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, W.; Chen, Z.; Jiang, D.; Zhou, S.; Lv, S.; Cui, H. Tumor microenvironment phenotypes and prognostic evaluation tools for osteosarcoma characterized by different prognostic outcomes and immunotherapy responses. J. Gene Med. 2024, 26, e3572. [Google Scholar] [CrossRef]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef]
- Nathenson, M.J.; Conley, A.P.; Sausville, E. Immunotherapy: A New (and Old) Approach to Treatment of Soft Tissue and Bone Sarcomas. Oncologist 2018, 23, 71–83. [Google Scholar]
- Pahl, J.H.; Ruslan, S.E.; Buddingh, E.P.; Santos, S.J.; Szuhai, K.; Serra, M.; Gelderblom, H.; Hogendoorn, P.C.; Egeler, R.M.; Schilham, M.W.; et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin. Cancer Res. 2012, 18, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhu, X.; Sun, L.; Yuan, L.; Zhang, J.; Li, H.; Ye, Z. Induction of a specific CD8+ T-cell response to cancer/testis antigens by demethylating pre-treatment against osteosarcoma. Oncotarget 2014, 5, 10791–10802. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Wu, S.; Cao, X.; Cai, T.; Hu, H. Antigen processing and presentation-related signature-derived BNIP3 is a novel oncogene and immunotherapy determinant in osteosarcoma based on machine learning and in vitro validation. J. Gene Med. 2024, 26, e3586. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Zhang, Q.M.; Kou, Q.C.; Tang, B. In vitro generation of cytotoxic T lymphocyte response using dendritic cell immunotherapy in osteosarcoma. Oncol. Lett. 2016, 12, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Dou, X.; Li, H.; Sun, Z.; Li, H.; Shen, Y.; Weng, W.; Min, J. Identification of Cell Subpopulations and Interactive Signaling Pathways From a Single-Cell RNA Sequencing Dataset in Osteosarcoma: A Comprehensive Bioinformatics Analysis. Front. Oncol. 2022, 12, 853979. [Google Scholar] [CrossRef]
- Zhang, Y.; Zvi, Y.S.; Batko, B.; Zaphiros, N.; O’Donnell, E.F.; Wang, J.; Sato, K.; Yang, R.; Geller, D.S.; Koirala, P.; et al. Down-regulation of Skp2 expression inhibits invasion and lung metastasis in osteosarcoma. Sci. Rep. 2018, 8, 14294. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, T.; Li, Z.; Luo, C.; Wu, Y.; Zhang, J.; Zhang, X.; Fan, W. Hyaluronate-Based Self-Stabilized Nanoparticles for Immunosuppression Reversion and Immunochemotherapy in Osteosarcoma Treatment. ACS Biomater. Sci. Eng. 2021, 7, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, R.; Wang, W.; Wang, C.; Zhang, N.; Shao, X.; He, Q.; Ying, M. Advances in targeted therapy for osteosarcoma based on molecular classification. Pharmacol. Res. 2021, 169, 105684. [Google Scholar] [CrossRef] [PubMed]
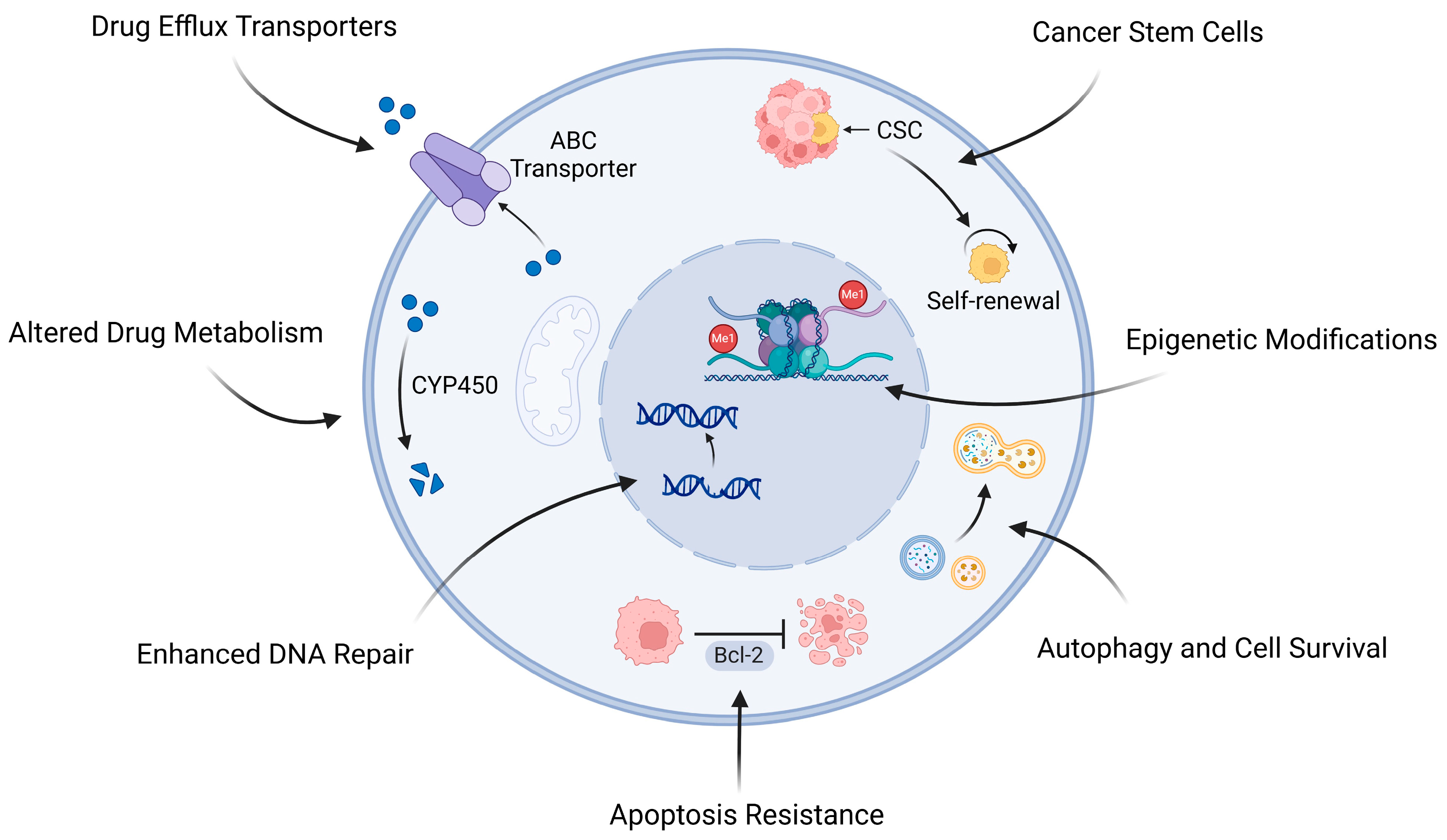
| Drug Type | Epigenetic Approach | Study Type | Treatment Protocol | Treatment Response | Synergistic Effects | Ref. |
|---|---|---|---|---|---|---|
| Decitabine (5-aza-2′-deoxycytidine) | DNA Methyltransferase (DNMT) inhibition | In vitro cell line study | Decitabine alone or in combination with chemotherapy (doxorubicin or cisplatin) | Low potency as a single agent | Synergistic with cisplatin in high-DNMT1-expressing cells, while doxorubicin showed consistent effects across all cell lines | [284] |
| MC3343 | DNA Methyltransferase (DNMT) inhibition | In vitro (cell lines) and in vivo (patient-derived xenograft model) | MC3343 alone or in combination with chemotherapy (doxorubicin or cisplatin) | Inhibits cell proliferation, induces osteoblastic differentiation, cytostatic in vivo | Synergistic effects with doxorubicin and cisplatin | [279] |
| 5-Azacytidine | DNA Methyltransferase (DNMT) inhibition | In vitro cell line study | 5-Aza-dC (2.5 µM) alone or combined with TSA (300 nM) | Induced growth arrest and reprogramming of MDR OS cells | Synergistic effects with TSA (HDAC inhibitor) | [285] |
| Zebularine (Zeb) | DNA Methyltransferase (DNMT) inhibition | In vitro cell line study | Zebularine (1, 10, or 100 μM) for 48 h. | Inhibits cell growth, cytotoxic effects | Synergistic effects with SAHA (HDAC inhibitor) | [286] |
| LBH589 (Panobinostat) | Histone Deacetylase (HDAC) inhibition | In vitro (cell lines) and in vivo (mouse xenograft model) | Continuous exposure to LBH589 (0.5–500 nM in vitro, 2–10 mg/kg in vivo) | Induced differentiation and senescence; sustained cytostatic response in vivo | N/A | [287] |
| Trichostatin A (TSA) | Histone Deacetylase (HDAC) inhibition | In vitro cell line study | TSA treatment with varying concentrations (0–200 nM) | Inhibition of cell growth Induced apoptosis, increased p53 acetylation. | N/A | [288] |
| Romidepsin | Histone Deacetylase (HDAC) inhibition | In vitro and in vivo preclinical study | In vitro: Dose range of 5.9–150 nM in cell lines. In vivo: Administered subcutaneously (2.4 mg/kg) twice weekly | Reduced lung metastatic growth and improved survival | N/A | [289] |
| SAHA (Vorinostat) | Histone Deacetylase (HDAC) inhibition | In vitro cell line study | SAHA (1, 5, or 10 μM) for 48 h. | Inhibits cell growth, cytotoxic effects | Synergistic with Zebularine (DNMT inhibitor) | [286] |
| AR-42 | Histone Deacetylase (HDAC) inhibition | In vitro cell line study | AR-42 (0.1–10 μM) for 24–72 h | Induced apoptosis, reduced cell viability | Synergistic with doxorubicin | [281] |
| Clinical Trial Identification | Study Phase | Population | Treatment Protocol | Primary Outcomes | Novel Strategy | Type of Targeted Therapy | Ref. |
|---|---|---|---|---|---|---|---|
| NCT04661852 | Phase I study | Relapsed osteosarcoma and Ewing sarcoma patients (n = 12) | Cabozantinib + topotecan + cyclophosphamide | Safety and toxicity | Multi-agent targeted therapy | Receptor tyrosine kinases (RTKs) inhibitor | [315] |
| NCT02406781 | Phase II study | Patients with advanced osteosarcomas (n = 17) | Pembrolizumab + metronomic cyclophosphamide | Objective response rate and 6-month non-progression rate | Immunotherapy + chemotherapy | PD-1 inhibitor (Pembrolizumab) | [316] |
| NCT00631631 | Phase III trial | Patients < 30 years with metastatic osteosarcoma (n = 91) | L-MTP-PE + standard chemotherapy vs. chemotherapy alone | Event-Free Survival (EFS) and Overall Survival (OS) | Immunotherapy combined with standard chemotherapy | N/A | [317] |
| NCT01650090 | Phase Ib/IIa study | Recurrent osteosarcoma patients with pulmonary metastases (n = 19) | Inhaled lipid cisplatin (ILC) | Safety and efficacy | Novel delivery of Cisplatin (Inhaled Lipid) | N/A | [318] |
| NCT04417062 | Phase II trial | Patients aged 12–40 with recurrent osteosarcoma | Olaparib + ceralasertib | 4-month event-free rate (Cohort 1), tumor sample submission (Cohort 2) | Dual inhibition of DNA repair pathways | PARP inhibitor (Olaparib), ATR inhibitor (Ceralasertib)ibitors | [319] |
| NCT04690231 | Phase II study | Patients aged 2–25 years with refractory or relapsed osteosarcoma (n = 33) | Apatinib (500 mg/day) + Ifosfamide and Etoposide (IE) | Objective responses, event-free survival, overall survival | Combination of TKI with chemotherapy | Multi-kinase inhibitor (Apatinib) | [320] |
| NCT04294511 | Phase II trial | Resectable osteosarcoma patients (n = 75) | Camrelizumab + doxorubicin/liposomal doxorubicin + cisplatin + methotrexate + ifosfamide | Rate of good tumor necrosis (TNR ≥ 90%) | Combination of immunotherapy and multiple chemotherapeutics | PD-1 inhibitor (Camrelizumab)r | [321] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhra, M.; Akhund, S.A.; Mohammad, K.S. Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies. Pharmaceuticals 2025, 18, 520. https://doi.org/10.3390/ph18040520
Zhra M, Akhund SA, Mohammad KS. Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies. Pharmaceuticals. 2025; 18(4):520. https://doi.org/10.3390/ph18040520
Chicago/Turabian StyleZhra, Mahmoud, Shahid Akhtar Akhund, and Khalid S. Mohammad. 2025. "Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies" Pharmaceuticals 18, no. 4: 520. https://doi.org/10.3390/ph18040520
APA StyleZhra, M., Akhund, S. A., & Mohammad, K. S. (2025). Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies. Pharmaceuticals, 18(4), 520. https://doi.org/10.3390/ph18040520








