Efficacy of RADA16-Based Self-Assembling Peptides on Wound Healing: A Meta-Analysis of Preclinical Animal Studies
Abstract
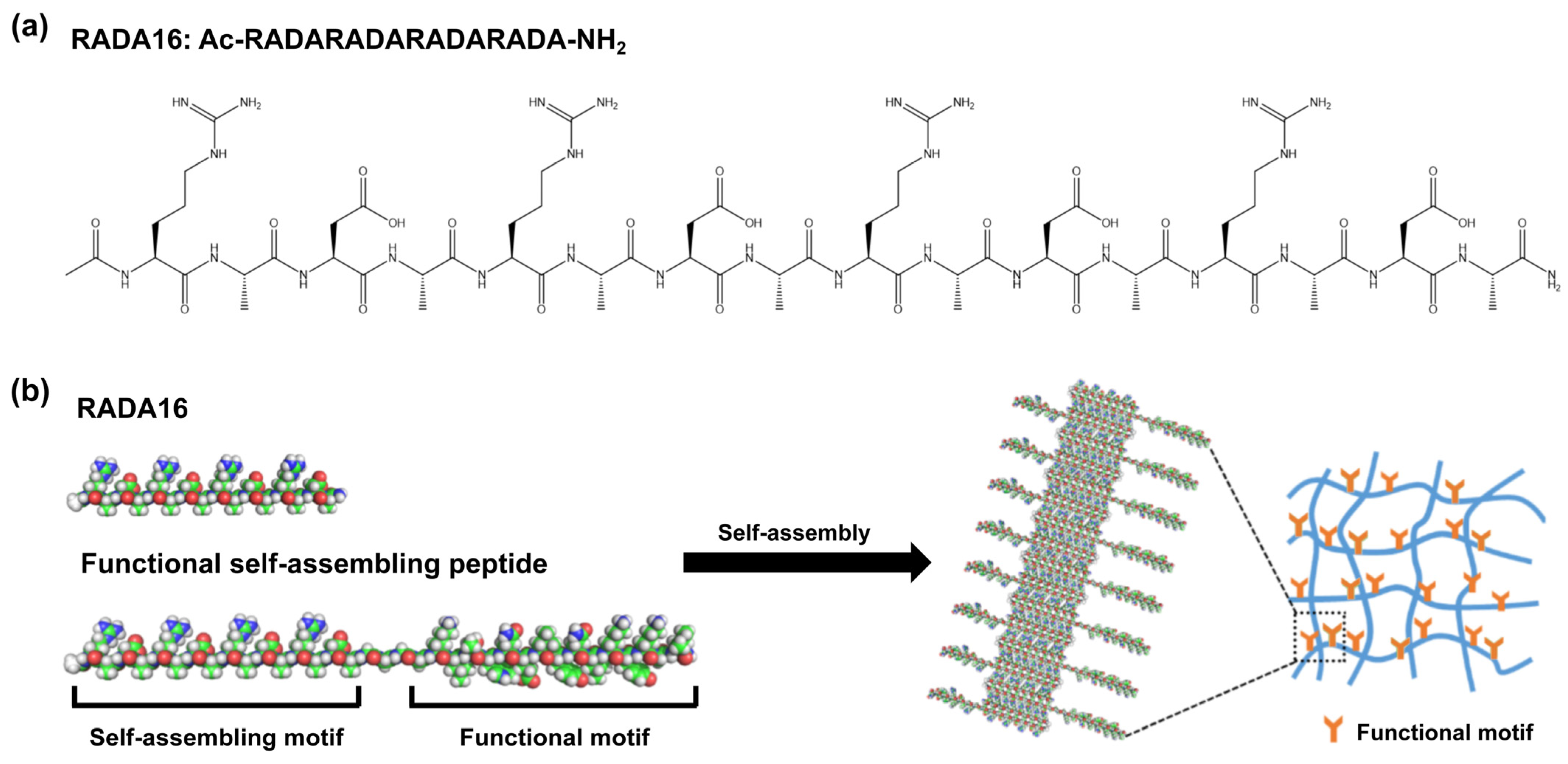
1. Introduction
2. Results
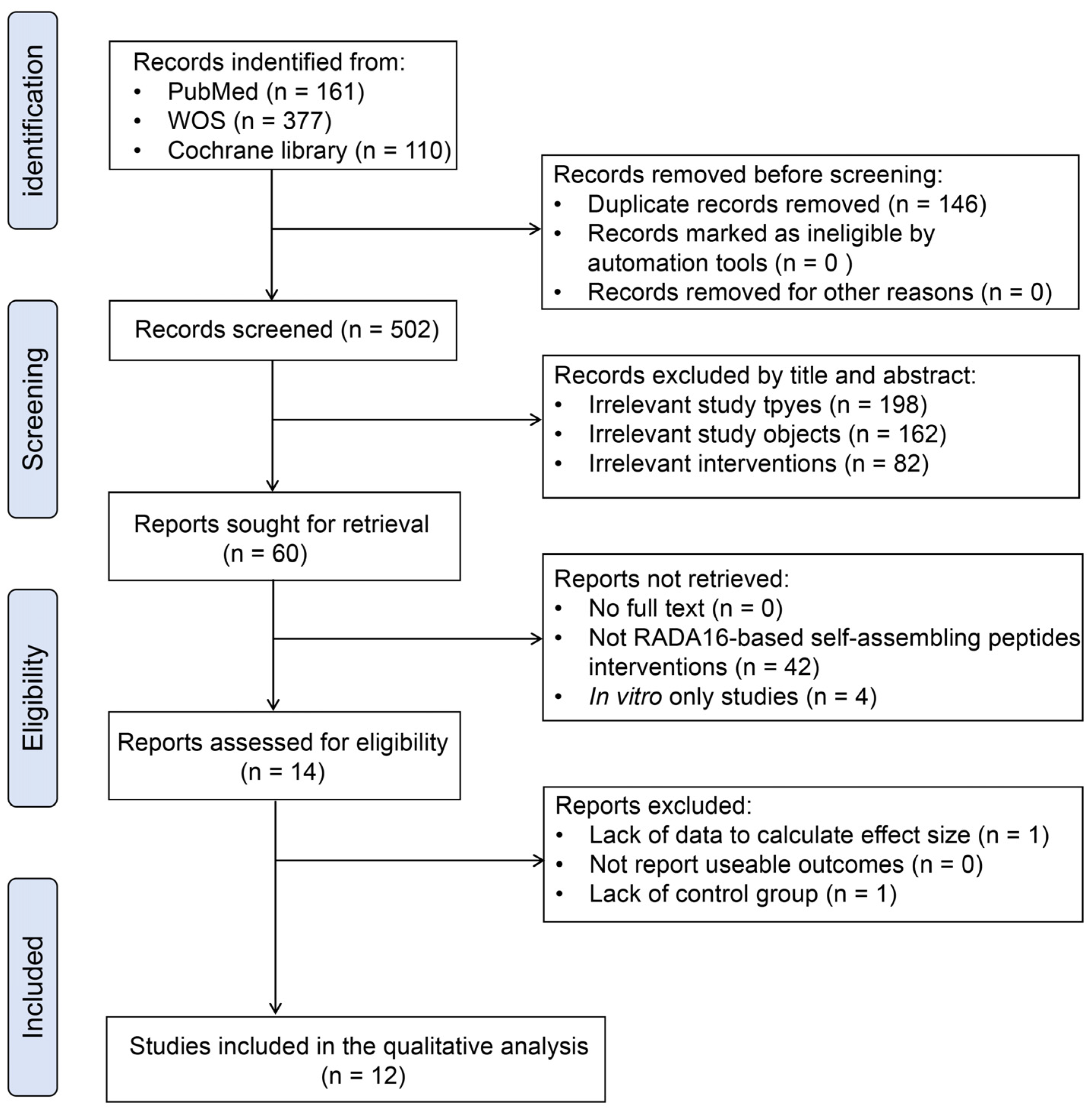
2.1. Search Results
2.2. Study Characteristics
2.3. ROB Assessment of the Included Articles
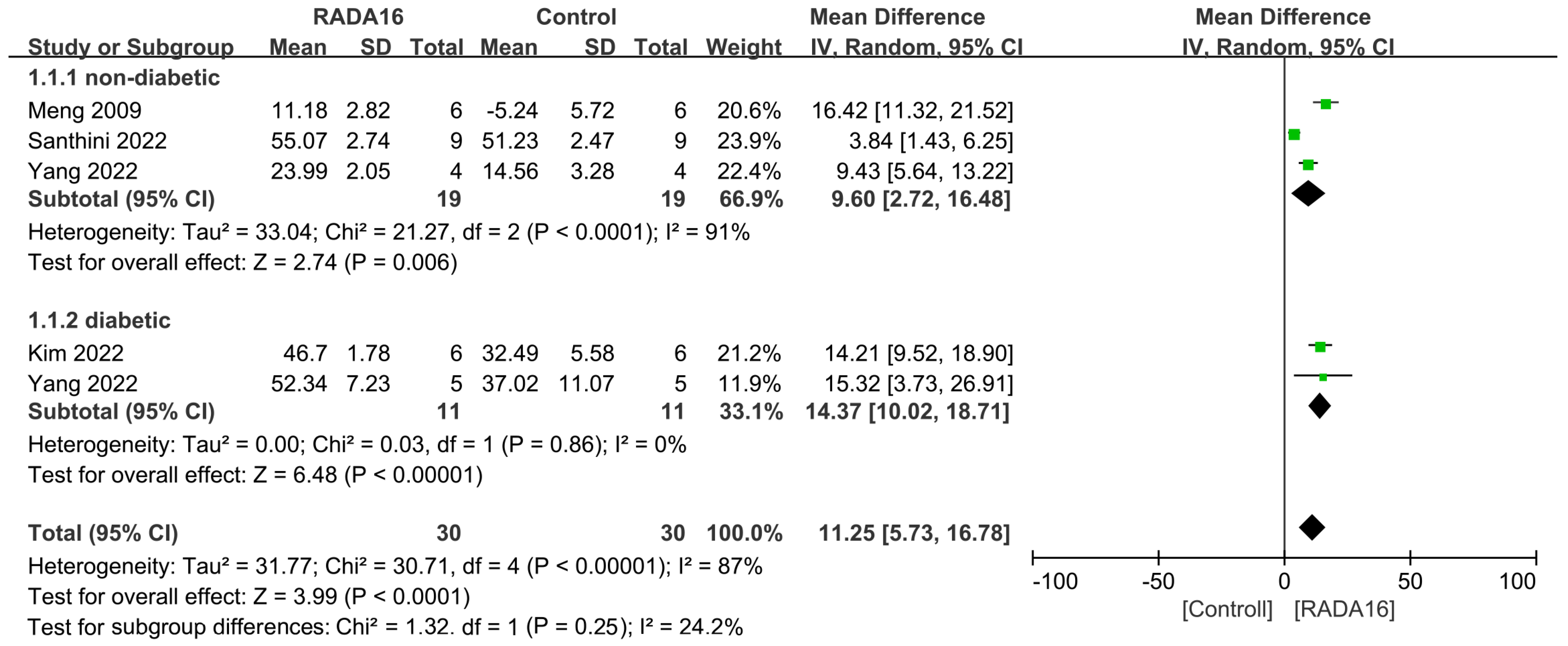
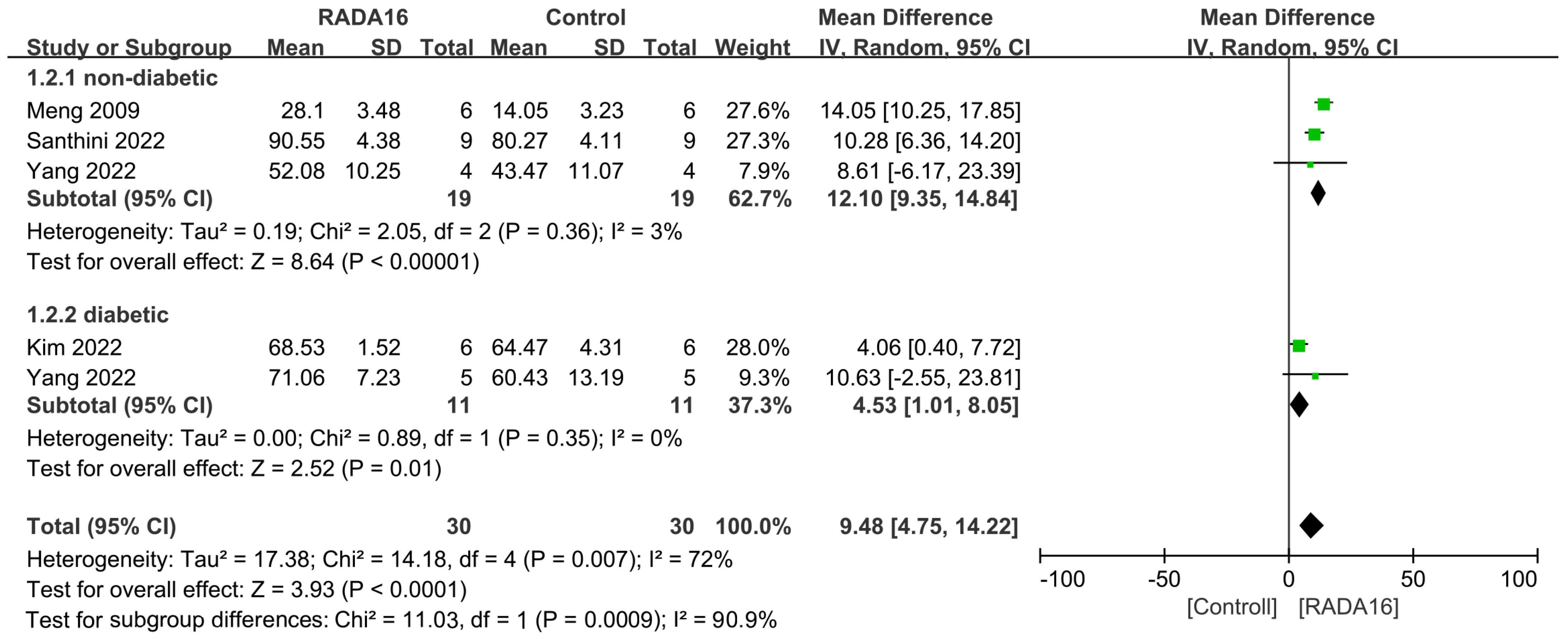
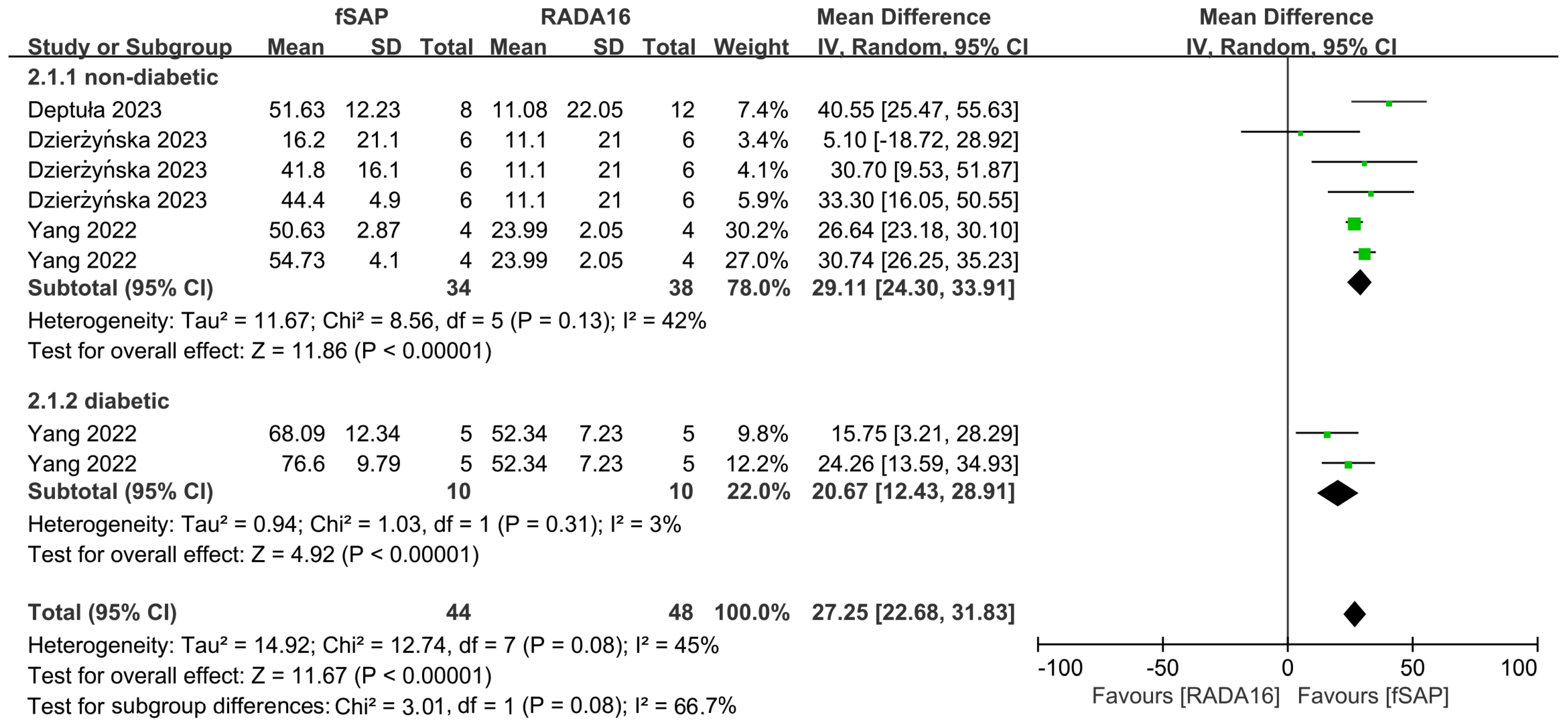
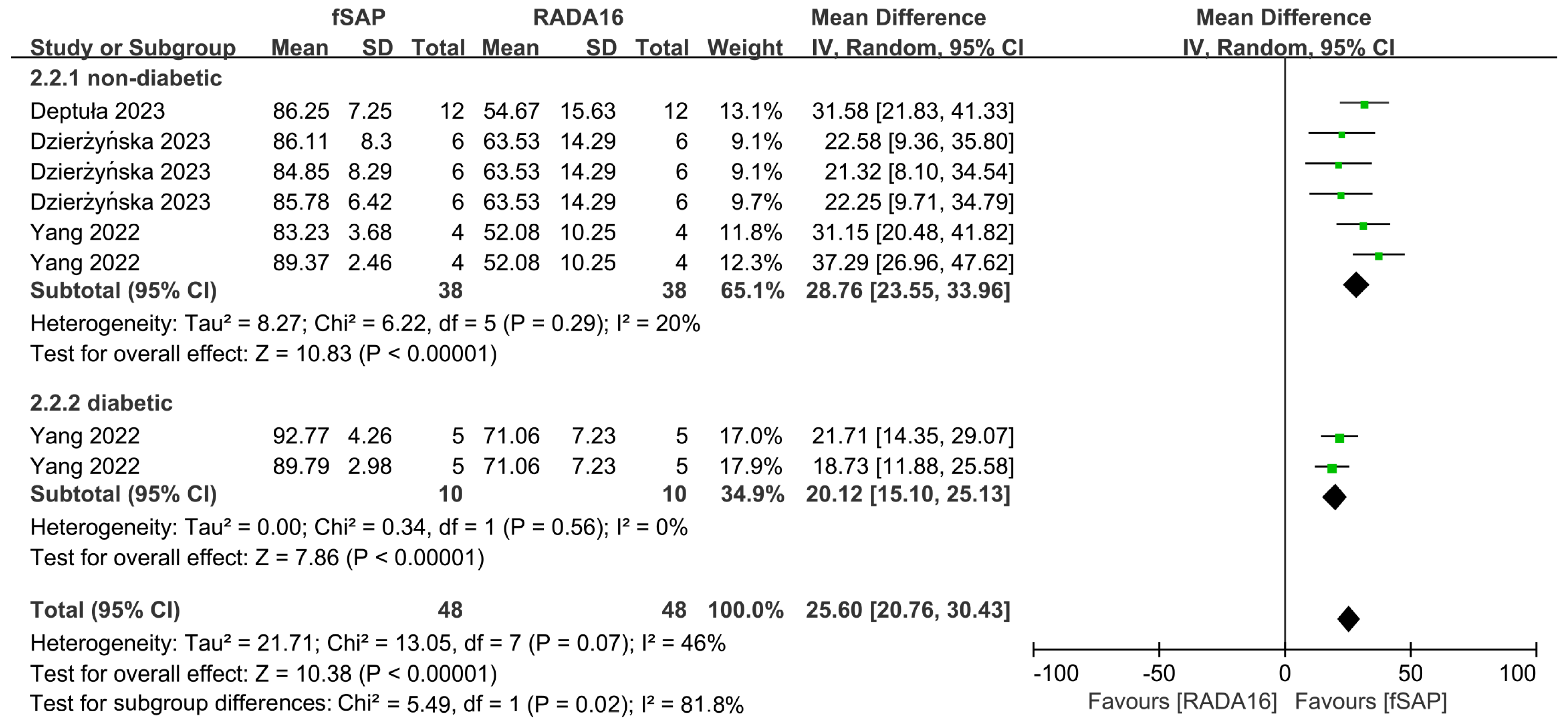
2.4. Outcomes of Meta-Analysis
3. Discussion
4. Materials and Methods
4.1. Protocol and Registration
4.2. Eligibility Criteria
4.3. Search Strategy
4.4. Study Selection
4.5. Assessment of Risk of Bias (ROB)
4.6. Data Extraction and Synthesis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROB | Risk of bias |
| SYRCLE | Systematic Review Centre for Laboratory animal Experimentation |
| MD | Mean Difference |
| CI | Confidence Interval |
| RADA16 | RADARADARADARADA |
| 3D | Three-dimensional |
| ECM | Extracellular matrix |
| PROSPERO | International Prospective Register of Systematic Reviews |
| PICOS | Population, intervention, comparison, outcome, and study design |
| MeSH | Medical subject heading |
References
- Harris-Tryon, T.A.; Grice, E.A. Microbiota and maintenance of skin barrier function. Science 2022, 376, 940–945. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Li, J.; Chen, C.; Liu, Y. Self-Assembling Peptide-Based Hydrogels for Wound Tissue Repair. Adv. Sci. 2022, 9, e2104165. [Google Scholar] [CrossRef]
- Holmes, T.C. Novel peptide-based biomaterial scaffolds for tissue engineering. Trends Biotechnol. 2002, 20, 16–21. [Google Scholar] [CrossRef]
- Amit, M.; Yuran, S.; Gazit, E.; Reches, M.; Ashkenasy, N. Tailor-Made Functional Peptide Self-Assembling Nanostructures. Adv. Mater. 2018, 30, e1707083. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Matson, J.B. pH-Responsive Self-Assembling Peptide-Based Biomaterials: Designs and Applications. ACS Appl. Bio Mater. 2022, 5, 4635–4651. [Google Scholar] [CrossRef]
- Gelain, F.; Luo, Z.; Zhang, S. Self-Assembling Peptide EAK16 and RADA16 Nanofiber Scaffold Hydrogel. Chem. Rev. 2020, 120, 13434–13460. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Guo, Y.; Li, H.; Chen, Z. Design of a RADA16-based self-assembling peptide nanofiber scaffold for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 713–736. [Google Scholar] [CrossRef]
- Cormier, A.R.; Pang, X.; Zimmerman, M.I.; Zhou, H.-X.; Paravastu, A.K. Molecular Structure of RADA16-I Designer Self-Assembling Peptide Nanofibers. ACS Nano 2013, 7, 7562–7572. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, X. Biomimetic Self-Assembling Peptide Hydrogels for Tissue Engineering Applications. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Singapore, 2018; pp. 297–312. [Google Scholar]
- Guo, H.-d.; Cui, G.-h.; Yang, J.-j.; Wang, C.; Zhu, J.; Zhang, L.-s.; Jiang, J.; Shao, S.-j. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 2012, 424, 105–111. [Google Scholar] [CrossRef]
- Zhang, W.; Mo, S.; Liu, M.; Liu, L.; Yu, L.; Wang, C. Rationally Designed Protein Building Blocks for Programmable Hierarchical Architectures. Front. Chem. 2020, 8, 587975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiong, N.; Liu, Y.; Gan, L. Biomimetic cell-adhesive ligand-functionalized peptide composite hydrogels maintain stemness of human amniotic mesenchymal stem cells. Regen. Biomater. 2021, 8, rbaa057. [Google Scholar] [CrossRef]
- Wang, X.; Horii, A.; Zhang, S. Designer functionalized self-assembling peptide nanofiber scaffolds for growth, migration, and tubulogenesis of human umbilical vein endothelial cells. Soft Matter 2008, 4, 2388–2395. [Google Scholar] [CrossRef]
- Bradshaw, M.; Ho, D.; Fear, M.W.; Gelain, F.; Wood, F.M.; Iyer, K.S. Designer self-assembling hydrogel scaffolds can impact skin cell proliferation and migration. Sci. Rep. 2014, 4, 6903. [Google Scholar] [CrossRef]
- Schneider, A.; Garlick, J.A.; Egles, C. Self-Assembling Peptide Nanofiber Scaffolds Accelerate Wound Healing. PLoS ONE 2008, 3, e1410. [Google Scholar] [CrossRef]
- Feng, T.; Wu, H.; Ma, W.; Wang, Z.; Wang, C.; Wang, Y.; Wang, S.; Zhang, M.; Hao, L. An injectable thermosensitive hydrogel with a self-assembled peptide coupled with an antimicrobial peptide for enhanced wound healing. J. Mater. Chem. B 2022, 10, 6143–6157. [Google Scholar] [CrossRef]
- Meng, H.; Chen, L.; Ye, Z.; Wang, S.; Zhao, X. The effect of a self-assembling peptide nanofiber scaffold (peptide) when used as a wound dressing for the treatment of deep second degree burns in rats. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 379–391. [Google Scholar] [CrossRef]
- Wang, W.; Liu, G.; Liu, M.; Li, X. Mechanisms underlying the action of self-assembling short-peptide nano-fiber gel scaffold materials in the aesthetic repair of burn wounds. Mater. Express 2020, 10, 454–459. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Yuan, J.; Wang, X.; Tao, K.; Yan, J. A Bionic Self-Assembly Hydrogel Constructed by Peptides With Favorable Biosecurity, Rapid Hemostasis and Antibacterial Property for Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 901534. [Google Scholar] [CrossRef]
- Xue, J.; Sun, N.; Liu, Y. Self-Assembled Nano-Peptide Hydrogels with Human Umbilical Cord Mesenchymal Stem Cell Spheroids Accelerate Diabetic Skin Wound Healing by Inhibiting Inflammation and Promoting Angiogenesis. Int. J. Nanomed. 2022, 17, 2459–2474. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Huang, C.; Lu, L.; Chen, J.; Weng, Y. Biomimetic Hydrogel Scaffolds with Copper Peptide-Functionalized RADA16 Nanofiber Improve Wound Healing in Diabetes. Macromol. Biosci. 2022, 22, e2200019. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.B.; Conway, W.; Tschabrunn, C.M.; Mehta, M.; Perez-Cuevas, M.B.; Zhang, S.; Hammond, P.T. Clotting Mimicry from Robust Hemostatic Bandages Based on Self-Assembling Peptides. ACS Nano 2015, 9, 9394–9406. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Siddiqui, Z.; Acevedo-Jake, A.M.; Roy, A.; Choudhury, M.; Grasman, J.; Kumar, V. Angiogenic Hydrogels to Accelerate Early Wound Healing. Macromol. Biosci. 2022, 22, e2200067. [Google Scholar] [CrossRef]
- Deptula, M.; Sawicka, J.; Sass, P.; Sosnowski, P.; Karpowicz, P.; Zawrzykraj, M.; Wardowska, A.; Tyminska, A.; Dzierzynska, M.; Pietralik-Molinska, Z.; et al. Development and evaluation of RADA-PDGF2 self-assembling peptide hydrogel for enhanced skin wound healing. Front. Pharmacol. 2023, 14, 1293647. [Google Scholar] [CrossRef]
- Dzierżyńska, M.; Sawicka, J.; Deptuła, M.; Sosnowski, P.; Sass, P.; Peplińska, B.; Pietralik-Molińska, Z.; Fularczyk, M.; Kasprzykowski, F.; Zieliński, J.; et al. Release systems based on self-assembling RADA16-I hydrogels with a signal sequence which improves wound healing processes. Sci. Rep. 2023, 13, 6273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, J.H.; Kim, S.H.; Jung, Y. Skin Regeneration with Self-Assembled Peptide Hydrogels Conjugated with Substance P in a Diabetic Rat Model. Tissue Eng. Part A 2018, 24, 21–33. [Google Scholar] [CrossRef]
- Santhini, E.; Parthasarathy, R.; Shalini, M.; Dhivya, S.; Mary, L.A.; Padma, V.V. Bio inspired growth factor loaded self assembling peptide nano hydrogel for chronic wound healing. Int. J. Biol. Macromol. 2022, 197, 77–87. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Broussard, K.C.; Powers, J.G. Wound Dressings: Selecting the Most Appropriate Type. Am. J. Clin. Dermatol. 2013, 14, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Griffith, M.; Phopase, J. Applications of self-assembling peptide scaffolds in regenerative medicine: The way to the clinic. J. Mater. Chem. B 2014, 2, 8466–8478. [Google Scholar] [CrossRef]
- Yao, X.; Yicun, H.; Maoqiang, L.; Kaichen, P.; Peng, W.; Yanbing, G.; Xidan, G.; Taowen, G.; Xiaobo, Z.; Zhou, H. Self-Assembling Peptide RADA16: A Promising Scaffold for Tissue Engineering and Regenerative Medicine. Nanomedicine 2023, 18, 1305–1326. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; O’Neill, K.; Bagot D’Arc, M.; Rebeca, F.; Buffier, M.; Aleksi, E.; Fan, M.; Matsuda, N.; Gil, E.S.; Spirio, L. Clinical Use of the Self-Assembling Peptide RADA16: A Review of Current and Future Trends in Biomedicine. Front. Bioeng. Biotechnol. 2021, 9, 679525. [Google Scholar] [CrossRef]
- Stenson, K.M.; Loftus, I.M.; Chetter, I.; Fourneau, I.; Cavanagh, S.; Bicknell, C.; Loftus, P. A Multi-Centre, Single-Arm Clinical Study to Confirm Safety and Performance of PuraStat®, for the Management of Bleeding in Elective Carotid Artery Surgery. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221144307. [Google Scholar] [CrossRef]
- Branchi, F.; Klingenberg-Noftz, R.; Friedrich, K.; Bürgel, N.; Daum, S.; Buchkremer, J.; Sonnenberg, E.; Schumann, M.; Treese, C.; Tröger, H.; et al. PuraStat in gastrointestinal bleeding: Results of a prospective multicentre observational pilot study. Surg. Endosc. 2022, 36, 2954–2961. [Google Scholar] [CrossRef]
- Wong, E.; Ho, J.; Smith, M.; Sritharan, N.; Riffat, F.; Smith, M.C. Use of Purastat, a novel haemostatic matrix based on self-assembling peptides in the prevention of nasopharyngeal adhesion formation. Int. J. Surg. Case Rep. 2020, 70, 227–229. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, X.; Wang, S.; Lv, F.; Zhao, X. Molecular mechanisms of RADA16-1 peptide on fast stop bleeding in rat models. Int. J. Mol. Sci. 2012, 13, 15279–15290. [Google Scholar] [CrossRef]
- Guo, W.; Ma, Y.; Hu, L.; Feng, Y.; Liu, Y.; Yi, X.; Zhang, W.; Tang, F. Modification Strategies for Ionic Complementary Self-Assembling Peptides: Taking RADA16-I as an Example. Polymers 2022, 14, 5221. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, S.; Zhao, Y.; Ao, N.; Ramakrishna, S.; He, L. The effects of motif net charge and amphiphilicity on the self-assembly of functionally designer RADA16-I peptides. Biomed. Mater. 2018, 13, 035011. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Tian, L.; Zhao, Y.; Wu, D.; Xue, W.; Ramakrishna, S.; Wu, W.; He, L. Self-assembly behaviors of molecular designer functional RADA16-I peptides: Influence of motifs, pH, and assembly time. Biomed. Mater. 2017, 12, 015007. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]

| Study/Year/ Ref./Country | Animal Type | Strain | Sex | Age | Weight | Sample Size | Wound Model | Wound Size | Intervention | Control Group | Follow-up Period | In Vivo Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deptuła, 2023 [27] Poland | Mouse | BALB/c | Female | 8-week-old | -- | Ne = 8; Nc = 12 | Full-thickness dorsal skin wound | 6.0 mm | RADA-PDGF2 | RADA16 | At 2, 4, 7, 9, 11, 14, 18, and 21 days | RADA-PDGF2 accelerated wound closure in the mouse model compared to RADA16 |
| Dzierżyńska 2023 [28] Poland | Mouse | BALB/c | Female | 8–10-week-old | -- | Ne = 18; Nc = 6 | Full-thickness dorsal skin wound | 6.0 mm | RADA-IM, RADA-GHK, and RADA-KGHK | RADA16 | At 2, 4, 7, 9, 11, 14, and 18 days | RADA-GHK and RADA-KGHK peptide hybrids improved skin wound healing; RADA-IM stimulates the growth of hair follicles |
| Feng, 2022 [19] China | Rat | Sprague Dawley | Male | -- | 200–250 g | Ne = 12; Nc = 4 | Full-thickness dorsal skin wound | 10 mm | PNI/RA-Amps3 group, PNI/RA-Amps3/E group, and commercial dressing group | Normal saline | At 1, 3, 5, 7, 9, 11, and 13 days | PNI/RA-Amps/E hydrogel accelerated wound healing significantly compared with commercial dressing |
| Hsu, 2015 [25] USA | Swine | Yorkshire | Male | -- | 42 kg | Ne = 7; Nc = 6 | Porcine skin injury | 8 mm | (RADA16/HA)200 group | Wounds without treatment | 2 min time period | (RADA16/HA)200 group accelerated hemostasis in porcine skin wounds as compared to plain gauze. |
| Kim, 2018 [29] Korea | Rat | Sprague–Dawley | -- | -- | 200–250 g | At 7 and 21 days: Ne = 15; Nc = 5 At 14 days: Ne = 9; Nc = 3 | Full-thickness skin wounds, with STZ-induced diabetes | 10 mm | RADA, RADA and soluble substance P, RADA and substance P conjugated RADA | PBS | At 7, 14, and 21 days | RADA with substance P promoted wound healing to enhance skin regeneration without cell transplantation in a diabetic model |
| Kim, 2022 [26] USA | Rat | Sprague–Dawley | -- | -- | -- | Ne = 24; Nc = 6 | Full-thickness skin wounds, with STZ-induced diabetes | 8 mm | RADA16, Slan low, Slan high, and K2 group | PBS | At 3, 7, 10, 14, 17, 21, 24, and 28 days | SLan groups showed similar wound contraction as control groups (RADA16, PBS, and K2), but increased deposition of new mature blood vessels. |
| Meng, 2009 [20] China | Rat | Sprague–Dawley | Female | -- | 250–290 g | Ne = 24; Nc = 6 | Deep second degree burn wound model | 3.0 cm | RADA16, Chitosan, PDLA, and Collagen | Saline | At 4, 7, 10, 14, 18, and 21 days | RADA16 dressings reduced the edema of the burn wound, speed up the beginning and disappearance of eschar and accelerate wound contraction |
| Santhini, 2022 [30] India | Rat | Rattus norvegicus | -- | -- | 250–300 g | Ne = 9; Nc = 9 | Excision wounds infected with S. aureus (1 × 105 CFU/mL) | 1.5 cm (width) × 0.2 cm (depth) | SAP nanohydrogel | Wounds without treatment | At 7, 14, and 21 days | SAP-GF nano hydrogel completely healed the infected wounds compared to the control |
| Wang, 2020 [21] China | Rat | Sprague–Dawley | Female | -- | -- | -- | Burn wound model | -- | RADA16 | NaCl | At 5, 10, 15, 30, 40, 50, and 90 days | RADA16 greatly promoted the healing of burn wounds |
| Wang, 2022 [22] China | Rat | Sprague–Dawley | -- | -- | 200 g | Ne = 3; Nc = 3 | Full-thickness skin wounds model infected with 1×1010 E. coli and 1 × 1010 S. aureus | 8 mm | BASP hydrogel | Wounds without treatment | At 2, 6, 10, and 14 days | BSAP hydrogel had remarkably antibacterial ability and accelerate the wound-healing |
| Xue, 2022 [23] China | Mouse | NOD/SCID | -- | 6–8 weeks old | 20–25 g | Ne = 20; Nc = 5 | Full-thickness diabetic skin wounds | 8 mm | hUC-MSCs, hUC-MSCsp, hUC-MSCs +hydrogel, and hUC-MSCsp+hydrogel groups | PBS | At 3, 7, 10, 14, and 21 days | Nanopeptide hydrogels loaded with hUC-MSCsp accelerated diabetic skin wound healing by inhibiting inflammation and promoting angiogenesis compared with conventional stem cell transplantation. |
| Yang, 2022 [24] China | Model 1 and 2: mouse | Model 1 and 2: Sprague–Dawley | Model 1 and 2: male | Model 1: 7-week-old; Model 2: Eight to ten-week-old | Not mentioned | Model 1: Ne = 12; Nc = 4 Model 2: Ne = 15; Nc = 5 | Model 1: full-thickness dorsal wounds; Model 2: full-thickness skin wounds, with STZ-induced diabetes | Model 1 and 2:10 mm | Model 1 and 2: RADA16, 5% R-GHK-Cu, and 10%R-GHK-Cu | Model 1 and 2: PBS | At 3, 6, 9, 12, and 15 days | The functionalized nanofiber scaffolds significantly accelerated wound closure, collagen deposition, and tissue remodeling both in healthy and diabetic mice |
| Study/Year/Source | Name | Peptide Sequence | Description |
|---|---|---|---|
| Deptuła, 2023 [27] | RADA-PDGF2 | Ac-(RADA)4-GGG-AAPV-GGG-RLIDRTNANFL-NH2 | From platelet-derived growth factor BB (PDGF-BB) |
| Dzierżyńska, 2023 [28] | RADA-IM | Ac-(RADA)4-GGG-AAPV-GGG-RDKVYR-NH2 | Imunofan (IM) that stimulates migration of keratinocytes |
| RADA-GHK | Ac-(RADA)4-GGG-AAPV-GG-GHK-NH2 | A primary regulatory factors of metalloproteinases and their inhibitors | |
| RADA-KGHK | Ac-(RADA)4-GGG-AAPV-GGG-KGHK-NH2 | A primary regulatory factors of metalloproteinases and their inhibitors | |
| Feng, 2022 [19] | RA-Amps3 | Ac-RADARADARADARADA-Acp-RRWRVIVKW | An antibacterial peptide |
| Kim, 2018 [29] | RADA-SP | Ac-RARADADARARADADA-GG-RPKPQQFFGLM-NH2 | Substance P secreted from the peripheral terminals of sensory nerve fibers as a neurotransmitter or hormone |
| Wang, 2022 [22] | ERC | RADARADARADARADA-GGQQLK | Enzyme-reaction chain |
| CBC | RADARADARADARADA-GSVLGYIQIR | Calcium binding chain | |
| Xue, 2022 [23] | KLT | GGGKLTWQELYQLKYKGI-RADARADARADARADA-NH2 | From a VEGF mimetic fragment that activates VEGF receptors and VEGF-related cellular signaling pathways, to activate endothelial cell proliferation |
| RGD | RGDRADARADARADA-NH2 | The RGD polypeptide family is thought to have a specific recognition site for integrin receptors | |
| Yang, 2022 [24] | R-GHK | Ac-(RADA)4-GG-GHK | GHK tripeptide (copper peptide) presents a strong affinity for copper ion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Chen, L.; Sun, Z.; Yang, Z. Efficacy of RADA16-Based Self-Assembling Peptides on Wound Healing: A Meta-Analysis of Preclinical Animal Studies. Pharmaceuticals 2025, 18, 526. https://doi.org/10.3390/ph18040526
Lu J, Chen L, Sun Z, Yang Z. Efficacy of RADA16-Based Self-Assembling Peptides on Wound Healing: A Meta-Analysis of Preclinical Animal Studies. Pharmaceuticals. 2025; 18(4):526. https://doi.org/10.3390/ph18040526
Chicago/Turabian StyleLu, Jiaju, Liuting Chen, Zeyue Sun, and Zhimou Yang. 2025. "Efficacy of RADA16-Based Self-Assembling Peptides on Wound Healing: A Meta-Analysis of Preclinical Animal Studies" Pharmaceuticals 18, no. 4: 526. https://doi.org/10.3390/ph18040526
APA StyleLu, J., Chen, L., Sun, Z., & Yang, Z. (2025). Efficacy of RADA16-Based Self-Assembling Peptides on Wound Healing: A Meta-Analysis of Preclinical Animal Studies. Pharmaceuticals, 18(4), 526. https://doi.org/10.3390/ph18040526









