Investigation of c-Fos/c-Jun Signaling Pathways in Periostracum Cicadae’s Inhibition of EMT in Gastric Tissue
Abstract
1. Introduction
2. Results
2.1. UHPLC-Q-Orbitrap-MS/MS Analysis of the PC Extract-Containing Serum
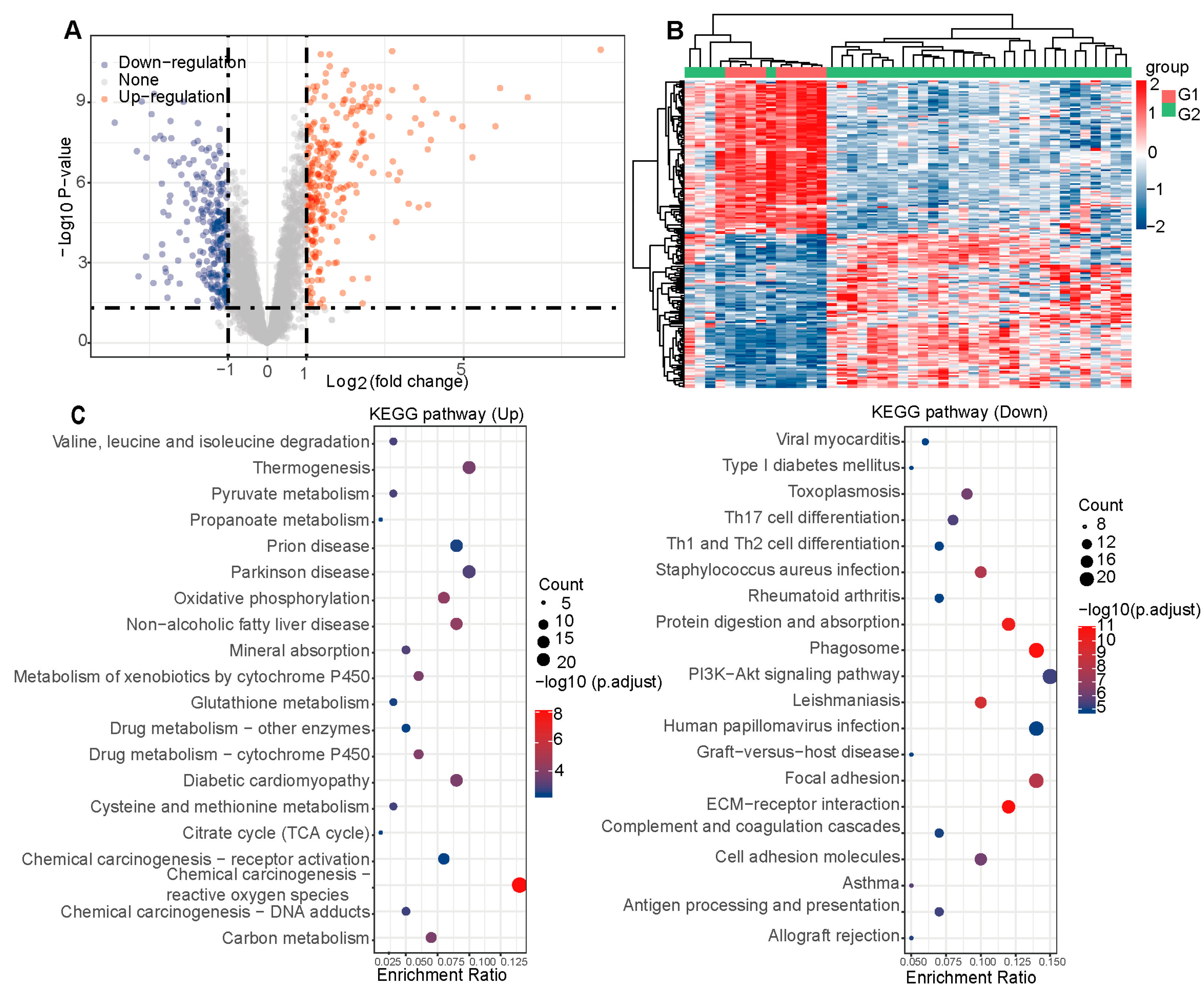
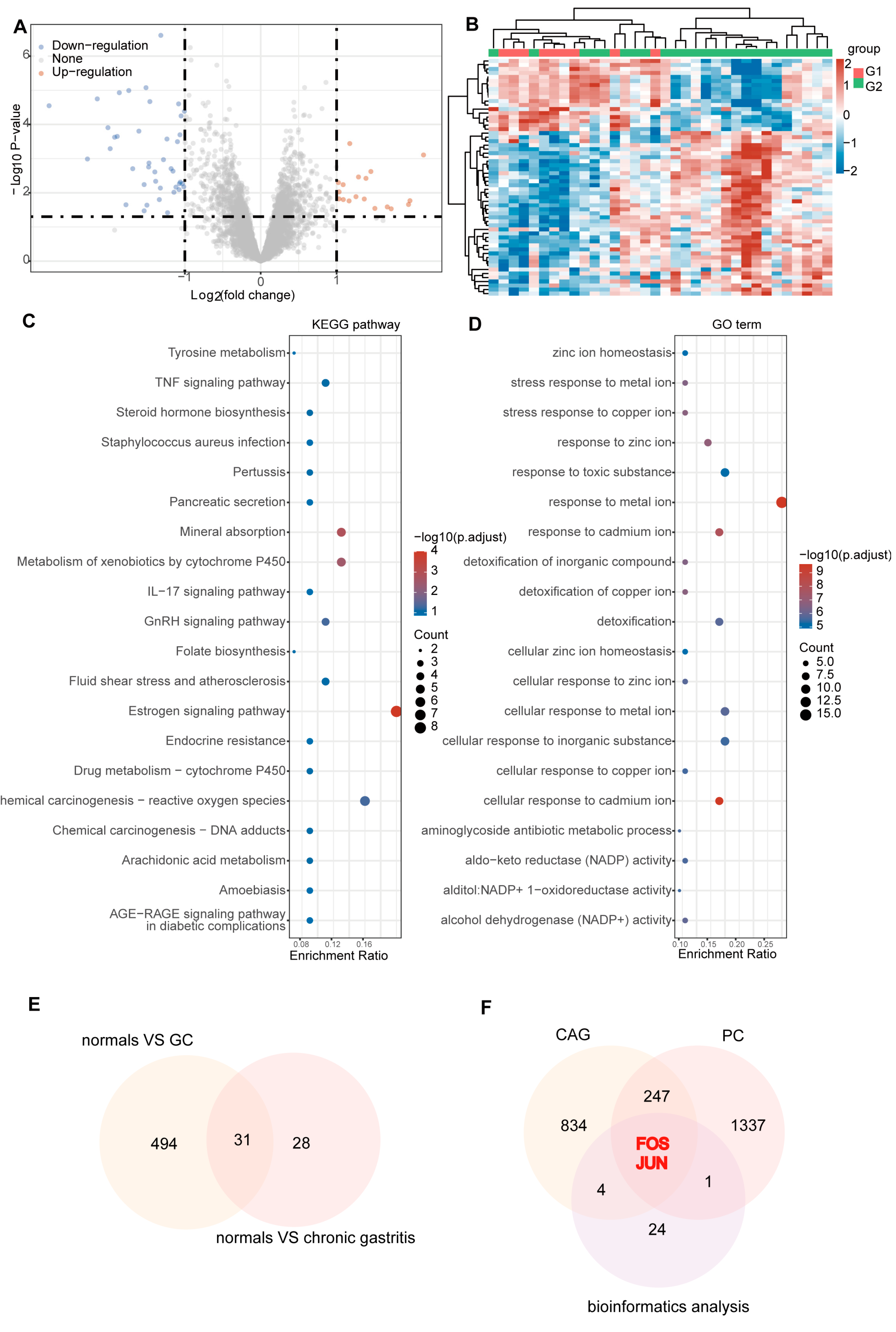
2.2. Hub Targets for PC Therapy of CAG
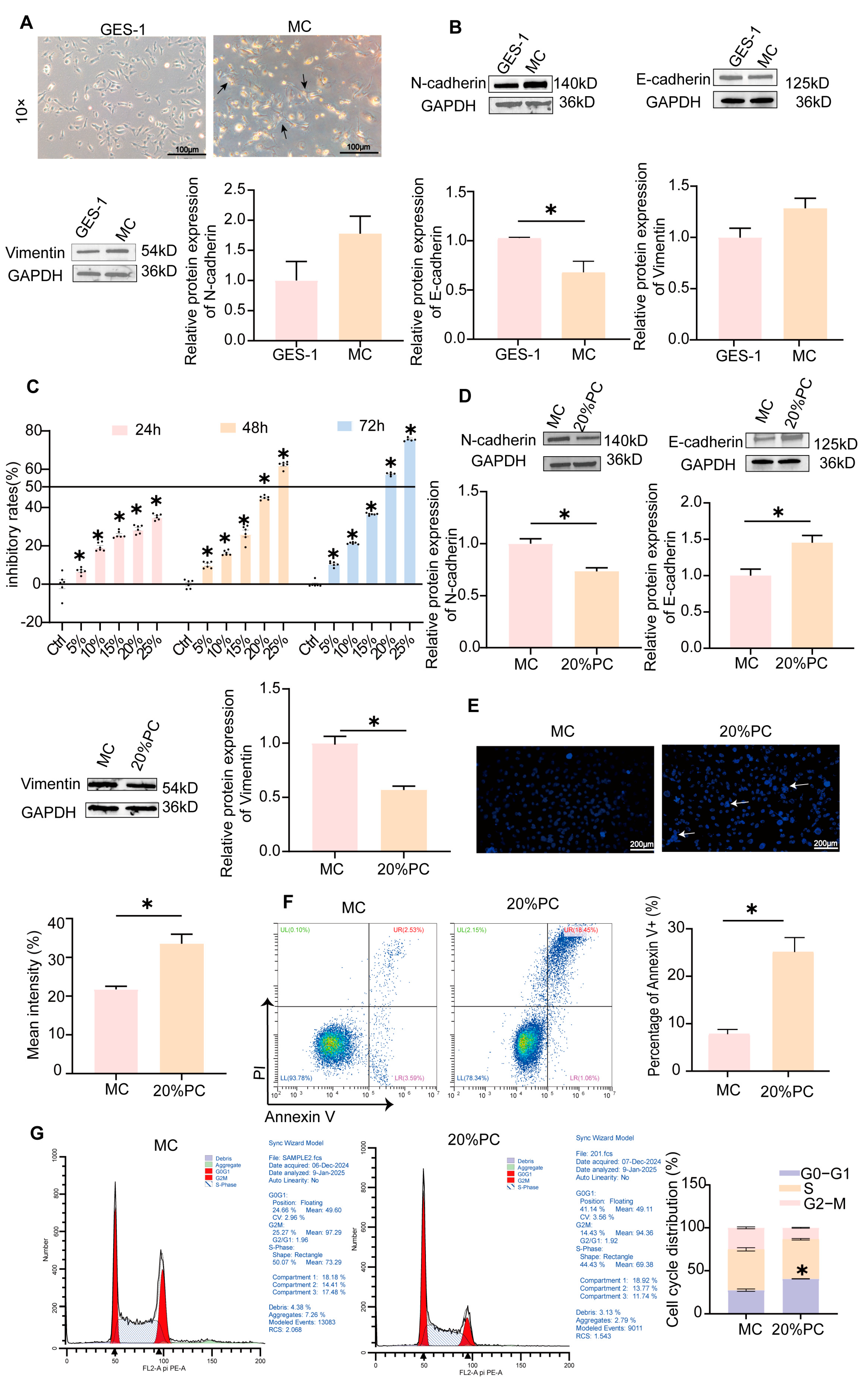
2.3. MC Cells Model Establishment and Validation
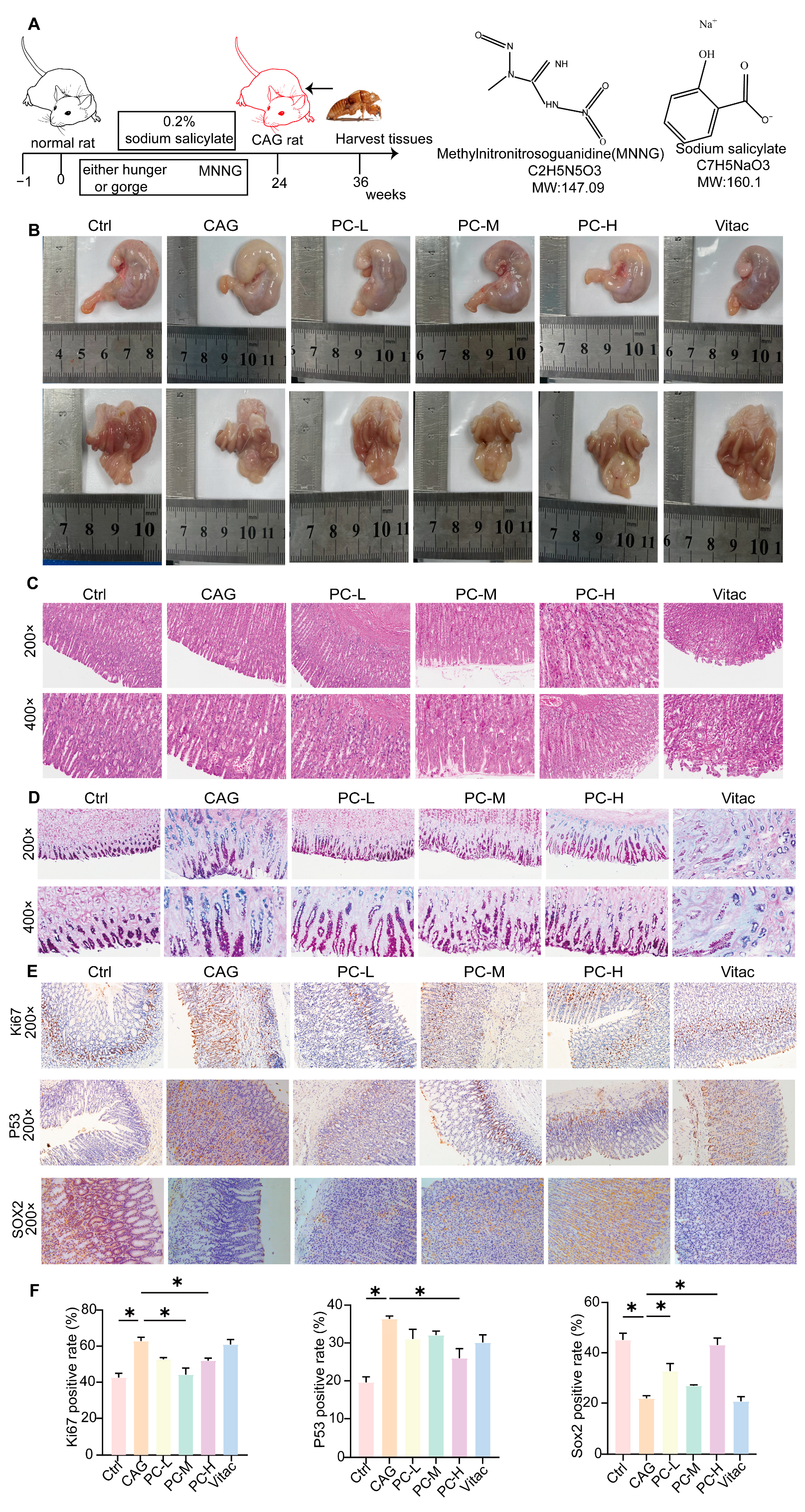
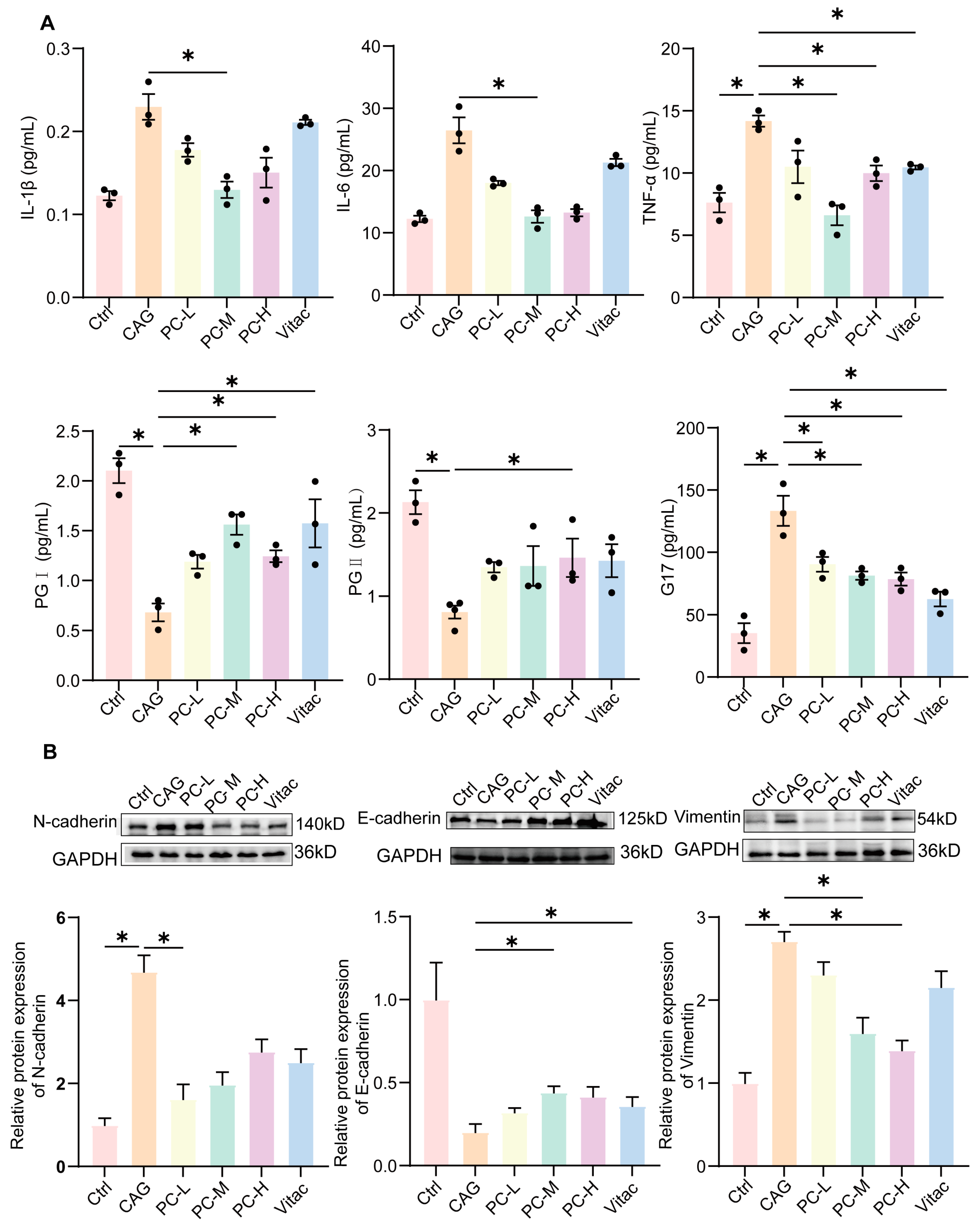
2.4. PC Exhibited Therapeutic Effects in CAG Rats
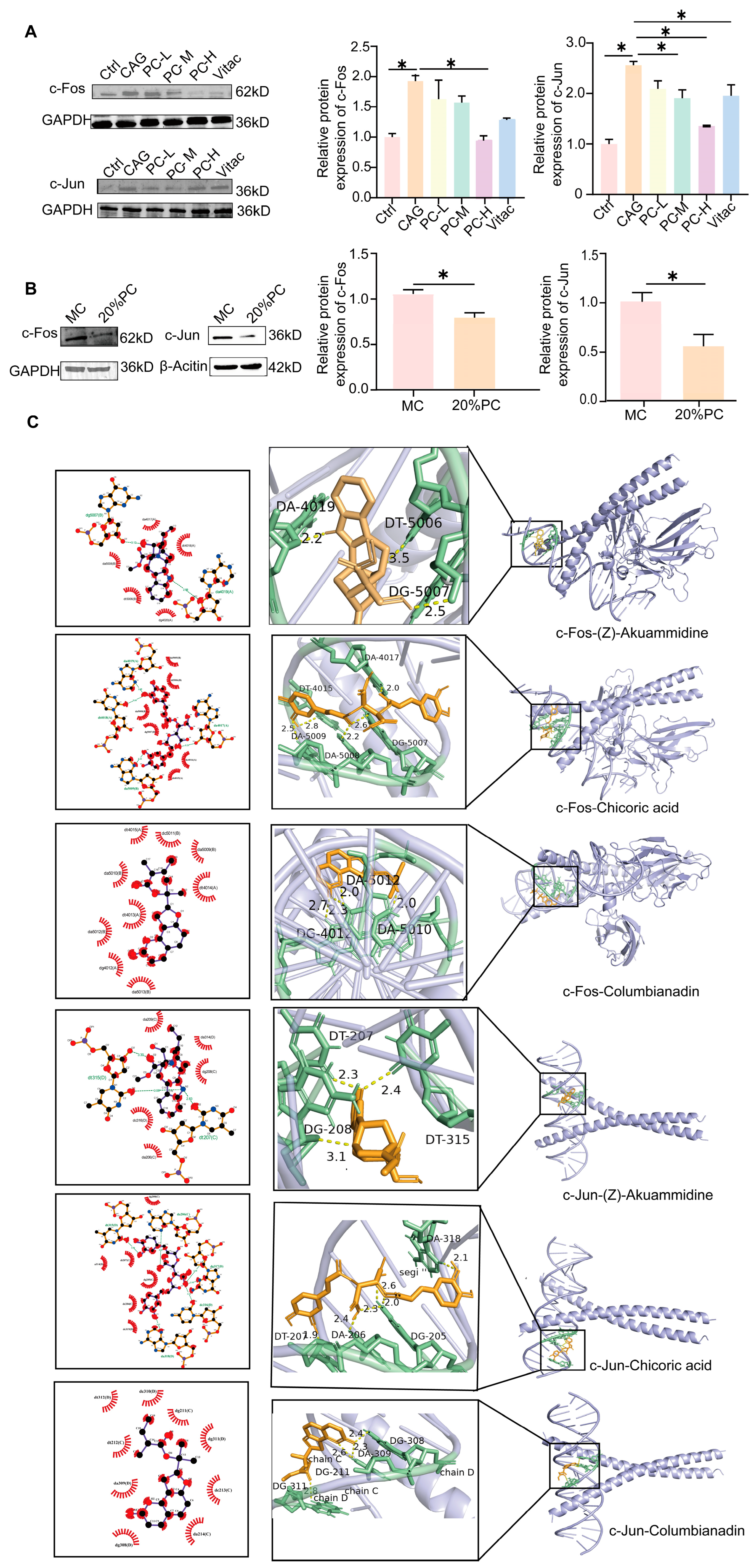
2.5. PC Improves the Symptoms of CAG Through c-Fos/c-Jun
3. Discussion
4. Materials and Methods
4.1. Preparation of PC Extract
4.2. Chemical Composition of PC Extract-Containing Serum Based on UPLC-Q-Extractive Orbitrap MS/MS
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Flow Cytometry Analysis of Apoptosis and Cell Cycle
4.6. Cell Wound Scrape Assay
4.7. Morphological Analysis of Apoptosis
4.8. Transwell® Migration and Invasion Assay
4.9. Colony Formation Assay
4.10. Animals
4.11. Enzyme-Linked Immunosorbent Assay
4.12. HE, AB-PAS, and IHC Staining
4.13. Western Blot Analysis
4.14. Network Pharmacology Analysis and Molecular Docking
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Wang, Q.; Tang, W.; Ma, Z.; Yang, Z.; Li, X.; Chen, W.; Ma, H.; Ye, X. Palmatine ameliorates N-methyl-N’-nitrosoguanidine-induced chronic atrophic gastritis through the STAT1/CXCL10 axis. FASEB J. 2024, 38, e70037. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Xu, J.; Xu, F.; Huang, W.; Majid, M.; Shi, H.; Yuan, X.; Ruan, Y.; Hu, X. Current study of pathogenetic mechanisms and therapeutics of chronic atrophic gastritis: A comprehensive review. Front. Cell Dev. Biol. 2024, 12, 1513426. [Google Scholar] [CrossRef]
- Hou, D.; Yang, M.; Hu, Z.; Yang, L. Effects of rebamipide for chronic atrophic gastritis: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e20620. [Google Scholar] [CrossRef]
- Goni, E.; Tammer, I.; Schütte, K.; Thon, C.; Jechorek, D.; Mahajan, U.M.; Vasapolli, R.; Macke, L.; Aulinger, B.; Selgrad, M.; et al. The influence of gastric atrophy on Helicobacter pylori antibiotics resistance in therapy-naïve patients. Front. Microbiol. 2022, 13, 938676. [Google Scholar] [CrossRef]
- Glaviano, A.; Lau, H.S.-H.; Carter, L.M.; Lee, E.H.C.; Lam, H.Y.; Okina, E.; Tan, D.J.J.; Tan, W.; Ang, H.L.; Carbone, D.; et al. Harnessing the tumor microenvironment: Targeted cancer therapies through modulation of epithelial-mesenchymal transition. J. Hematol. Oncol. 2025, 18, 6. [Google Scholar] [CrossRef]
- Guinn, S.; Kinny-Köster, B.; Tandurella, J.A.; Mitchell, J.T.; Sidiropoulos, D.N.; Loth, M.; Lyman, M.R.; Pucsek, A.B.; Zabransky, D.J.; Lee, J.W.; et al. Transfer Learning Reveals Cancer-Associated Fibroblasts Are Associated with Epithelial–Mesenchymal Transition and Inflammation in Cancer Cells in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2024, 84, 1517–1533. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, L.; Li, Q.; Yao, L.; Wang, X.; Liu, H.; Shi, J. Design of an In Vitro Model for Epithelial-to-Mesenchymal Transition in Gastric Cancer. Biochem. Genet. 2024, 63, 698–712. [Google Scholar] [CrossRef]
- Yu, W.; Chen, S.; Guan, X.; He, G.; Zhang, W.; Zhang, H.; Huang, S.; Ye, Z.; Pan, H.; Zhong, Z. Yiqi Huayu Jiedu formula inhibits JAK2/STAT3-mediated partial EMT in treating chronic atrophic gastritis. Phytomedicine 2024, 137, 156356. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, T.; Wang, L.; Wang, Y.; Liu, Y.; Bai, J.; Wen, X.; Li, Y.; Long, K.; Zhang, H. Traditional Chinese Medicine in the treatment of chronic atrophic gastritis, precancerous lesions and gastric cancer. J. Ethnopharmacol. 2024, 337, 118812. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, H.; Wang, X.; Kang, J.; Guo, W.; Zhou, L.; Liu, H.; Wang, M.; Jia, R.; Du, X.; et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine 2022, 95, 153837. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhong, J.; Song, Y.; Li, M.-F.; Song, S.-Y.; Li, C.-R.; Hou, W.-W.; Li, Q.-J. Chinese herbal formula shen-ling-bai-zhu-san to treat chronic gastritis: Clinical evidence and potential mechanisms. World J. Gastroenterol. 2022, 28, 4890–4908. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Wu, C.; Li, J.; Liang, B.; Zhang, F.; Fan, X.; Li, Z.; Wang, Y.; Li, Z.; Liu, D.; et al. Network pharmacology based investigation into the effect and mechanism of Modified Sijunzi Decoction against the subtypes of chronic atrophic gastritis. Pharmacol. Res. 2019, 144, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chen, X.; Xiong, M.; Wang, H.; Sun, C.; Tang, W.; Li, J.; Li, X.; Ma, H.; Ye, X. Cyberpharmacology uncover the mechanism of the total Rhizoma Coptidis extracts ameliorate chronic atrophic gastritis. J. Ethnopharmacol. 2024, 335, 118644. [Google Scholar] [CrossRef]
- Wen, J.; Wu, S.; Ma, X.; Zhao, Y. Zuojin Pill attenuates Helicobacter pylori-induced chronic atrophic gastritis in rats and improves gastric epithelial cells function in GES-1 cells. J. Ethnopharmacol. 2022, 285, 114855. [Google Scholar] [CrossRef]
- Wang, D.; Wu, T.; Jin, J.; Si, Y.; Wang, Y.; Ding, X.; Guo, T.; Wei, W. Periostracum Cicadae Extract and N-Acetyldopamine Regulate the Sleep-Related Neurotransmitters in PCPA-Induced Insomnia Rats. Molecules 2024, 29, 3638. [Google Scholar] [CrossRef]
- Thapa, P.; Gu, Y.; Kil, Y.-S.; Baek, S.C.; Kim, K.H.; Han, A.-R.; Seo, E.K.; Choi, H.; Chang, J.-H.; Nam, J.-W. N-Acetyldopamine derivatives from Periostracum Cicadae and their regulatory activities on Th1 and Th17 cell differentiation. Bioorganic Chem. 2020, 102, 104095. [Google Scholar] [CrossRef]
- Hsieh, M.-T.; Peng, W.-H.; Yeh, F.-T.; Tsai, H.-Y.; Chang, Y.-S. Studies on the anticonvulsive, sedative and hypothermic effects of Periostracum Cicadae extracts. J. Ethnopharmacol. 1991, 35, 83–90. [Google Scholar] [CrossRef]
- Kim, S.-H.; Hong, J.-H.; Yang, W.-K.; Kim, H.-J.; An, H.-J.; Lee, Y.-C. Cryptotympana pustulata Extract and Its Main Active Component, Oleic Acid, Inhibit Ovalbumin-Induced Allergic Airway Inflammation through Inhibition of Th2/GATA-3 and Interleukin-17/RORγt Signaling Pathways in Asthmatic Mice. Molecules 2021, 26, 1854. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, R.-L.; Tao, T.; Sun, J.-Y.; Liu, J.; Peng, W.; Wu, C.-J.; Zhang, T. Antiepileptic Effects of Cicadae Periostracum on Mice and Its Antiapoptotic Effects in H2O2-Stimulated PC12 Cells via Regulation of PI3K/Akt/Nrf2 Signaling Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 5598818. [Google Scholar] [CrossRef]
- Huang, L.-J.; Wang, Y.-M.; Gong, L.-Q.; Hu, C.; Gui, Y.; Zhang, C.; Tan, X.; Yu, X.-K.; Liao, Y.-L.; Luo, Y.; et al. N-Acetyldopamine Dimer Attenuates DSS-Induced Ulcerative Colitis by Suppressing NF-κB and MAPK Pathways. Front. Pharmacol. 2022, 13, 842730. [Google Scholar] [CrossRef]
- Li, D.; Hu, J.; Zhang, L.; Li, L.; Yin, Q.; Shi, J.; Guo, H.; Zhang, Y.; Zhuang, P. Deep learning and machine intelligence: New computational modeling techniques for discovery of the combination rules and pharmacodynamic characteristics of Traditional Chinese Medicine. Eur. J. Pharmacol. 2022, 933, 175260. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xiang, M.; Zhou, M.; Li, C.; Xin, H.; Zhang, S.; Lin, J. Pharmacological effects and mechanisms of danlong oral liquid in asthma airway remodeling: Insights from serum medicinal chemistry, network pharmacology, and experimental validation. J. Ethnopharmacol. 2024, 340, 119259. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wen, Y.; Wang, J.; Zhao, M.; Lv, S.; Chen, N.; Li, Y.; Wan, L.; Zheng, Q.; Mou, Y.; et al. Gallic acid alleviates gastric precancerous lesions through inhibition of epithelial mesenchymal transition via Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 302, 115885. [Google Scholar] [CrossRef]
- Cai, J.; Wang, M.; Zhu, M.; Zhang, Q.; Zhang, X.; Yan, Y.; Qian, H.; Xu, W. N-methyl-N-nitro-N′-nitrosoguanidine induces the expression of CCR2 in human gastric epithelial cells promoting CCL2-mediated migration. Mol. Med. Rep. 2015, 13, 1083–1090. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamada, M.; Mushiake, M.; Tsuda, M.; Miwa, M. Elucidating Differences in Early-Stage Centrosome Amplification in Primary and Immortalized Mouse Cells. Int. J. Mol. Sci. 2023, 25, 383. [Google Scholar] [CrossRef]
- Luo, T.; Pan, Y.; Liu, Y.; Zheng, J.; Zhuang, Z.; Ren, Z.; Zhu, J.; Gu, Y.; Zeng, Y. LANA regulates miR-155/GATA3 signaling axis by enhancing c-Jun/c-Fos interaction to promote the proliferation and migration of KSHV-infected cells. J. Med. Virol. 2022, 95, e28255. [Google Scholar] [CrossRef]
- Suzuki, S.; Satoh, K.; Nakano, H.; Hatayama, I.; Sato, K.; Tsuchida, S. Lack of correlated expression between the glutathione S-transferase P-form and the oncogene products c-Jun and c-Fos in rat tissues and preneoplastic hepatic foci. Carcinogenesis 1995, 16, 567–571. [Google Scholar] [CrossRef]
- Wang, L.; Lian, Y.-J.; Dong, J.-S.; Liu, M.-K.; Liu, H.-L.; Cao, Z.-M.; Wang, Q.-N.; Lyu, W.-L.; Bai, Y.-N. Traditional Chinese medicine for chronic atrophic gastritis: Efficacy, mechanisms and targets. World J. Gastroenterol. 2025, 31, 102053. [Google Scholar] [CrossRef]
- Liu, X.; Bai, F.; Wang, Y.; Wang, C.; Chan, H.L.; Zheng, C.; Fang, J.; Zhu, W.-G.; Pei, X.-H. Loss of function of GATA3 regulates FRA1 and c-FOS to activate EMT and promote mammary tumorigenesis and metastasis. Cell Death Dis. 2023, 14, 370. [Google Scholar] [CrossRef]
- Razavi-Mohseni, M.; Huang, W.; Guo, Y.A.; Shigaki, D.; Ho, S.W.T.; Tan, P.; Skanderup, A.J.; Beer, M.A. Machine learning identifies activation of RUNX/AP-1 as drivers of mesenchymal and fibrotic regulatory programs in gastric cancer. Genome Res. 2024, 34, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Tang, S.; Liu, L.; Zhao, M.; Ma, X.; Zhao, Y.; Shen, C.; Liu, Q.; Tang, J.; Zeng, J.; et al. Tanshinone I attenuates gastric precancerous lesions by inhibiting epithelial mesenchymal transition through the p38/STAT3 pathway. Int. Immunopharmacol. 2023, 124, 110902. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, Y.; Wang, X.; Du, X.; Li, G.; Luo, L.; Yao, B.; Zhang, J.; Zhao, F.; Liu, D. The fruit of Rosa odorata sweet var. gigantea (Coll. et Hemsl.) Rehd. et Wils attenuates chronic atrophic gastritis induced by MNNG and its potential mechanism. J. Ethnopharmacol. 2024, 337, 118876. [Google Scholar] [CrossRef]
- Shaebani, M.R.; Stankevicins, L.; Vesperini, D.; Urbanska, M.; Flormann, D.A.; Terriac, E.; Gad, A.K.; Cheng, F.; Eriksson, J.E.; Lautenschläger, F. Effects of vimentin on the migration, search efficiency, and mechanical resilience of dendritic cells. Biophys. J. 2022, 121, 3950–3961. [Google Scholar] [CrossRef] [PubMed]
- Gustems, M.; Woellmer, A.; Rothbauer, U.; Eck, S.H.; Wieland, T.; Lutter, D.; Hammerschmidt, W. c-Jun/c-Fos heterodimers regulate cellular genes via a newly identified class of methylated DNA sequence motifs. Nucleic Acids Res. 2013, 42, 3059–3072. [Google Scholar] [CrossRef]
- Bakiri, L.; Hasenfuss, S.C.; Guío-Carrión, A.; Thomsen, M.K.; Hasselblatt, P.; Wagner, E.F. Liver cancer development driven by the AP-1/c-Jun~Fra-2 dimer through c-Myc. Proc. Natl. Acad. Sci. USA 2024, 121, e2404188121. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z.-G.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Ming, S.; Zheng, H.; Hua, B.; Yang, H.-S. Platycodin D induces apoptosis through JNK1/AP-1/PUMA pathway in non-small cell lung cancer cells: A new mechanism for an old compound. Front. Pharmacol. 2022, 13, 1045375. [Google Scholar] [CrossRef]
- Mishra, A.; Bharti, A.C.; Saluja, D.; Das, B.C. Transactivation and expression patterns of Jun and Fos/AP-1 super-family proteins in human oral cancer. Int. J. Cancer 2009, 126, 819–829. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y.; Zhou, L.; Lin, F.; Wan, M.; Gan, A.; Wu, B.; Yan, T.; Jia, Y. Costunolide ameliorates MNNG-induced chronic atrophic gastritis through inhibiting oxidative stress and DNA damage via activation of Nrf2. Phytomedicine 2024, 130, 155581. [Google Scholar] [CrossRef]
- Tong, Y.; Liu, L.; Wang, R.; Yang, T.; Wen, J.; Wei, S.; Jing, M.; Zou, W.; Zhao, Y. Berberine Attenuates Chronic Atrophic Gastritis Induced by MNNG and Its Potential Mechanism. Front. Pharmacol. 2021, 12, 644638. [Google Scholar] [CrossRef]
- Burke, E.; Harkins, P.; Arumugasamy, M. Incidence of Gastric Adenocarcinoma in Those With Gastric Atrophy: A Systematic Review. Cureus 2024, 16, e71768. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-S.; Zhi, Y.; Shao, C.-M.; Zeng, B.-F. Yangyin Huowei mixture alleviates chronic atrophic gastritis by inhibiting the IL-10/JAK1/STAT3 pathway. World J. Gastrointest. Surg. 2024, 16, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Abbate, J.M.; Arfuso, F.; Riolo, K.; Giudice, E.; Brunetti, B.; Lanteri, G. Upregulation of miR-21 and pro-inflammatory cytokine genes IL-6 and TNF-α in promoting a pro-tumorigenic microenvironment in canine mammary carcinomas. Res. Veter-Sci. 2023, 164, 105014. [Google Scholar] [CrossRef]
- Bao, C.; Tourdot, R.W.; Brunette, G.J.; Stewart, C.; Sun, L.; Baba, H.; Watanabe, M.; Agoston, A.T.; Jajoo, K.; Davison, J.M.; et al. Genomic signatures of past and present chromosomal instability in Barrett’s esophagus and early esophageal adenocarcinoma. Nat. Commun. 2023, 14, 6203. [Google Scholar] [CrossRef]
- Anjorin, A.O.; Olaofe, O.O.; Anjorin, A.O.; Omoniyi-Esan, G.O.; Komolafe, A.O. P53 marker expression in epithelial ovarian tumours in a centre in Nigeria—A descriptive study. BMC Women’s Health. 2024, 24, 639. [Google Scholar] [CrossRef]
- Oh, Y.S.; Kwak, M.-K.; Kim, K.; Cho, E.-H.; Jang, S.-E. Development and application of an antibody that binds to interleukin-1β of various mammalian species for the treatment of inflammatory diseases. Biochem. Biophys. Res. Commun. 2020, 527, 751–756. [Google Scholar] [CrossRef]
- Wang, P.; Xu, T.; Yan, Z.; Zheng, X.; Zhu, F. Jian-Pi-Yi-Qi-Fang ameliorates chronic atrophic gastritis in rats through promoting the proliferation and differentiation of gastric stem cells. Ann. Transl. Med. 2022, 10, 932. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Chen, G.; Yang, J.; Jiang, Y.; Li, F.; Hua, H. Jianwei Xiaoyan granule ameliorates chronic atrophic gastritis by regulating HIF-1α-VEGF pathway. J. Ethnopharmacol. 2024, 334, 118591. [Google Scholar] [CrossRef]
- Wang, J.-X.; Cao, Y.-P.; Su, P.; He, W.; Li, X.-P.; Zhu, Y.-M. Serum gastrin-17 concentration for prediction of upper gastrointestinal tract bleeding risk among peptic ulcer patients. World J. Clin. Cases 2021, 9, 10948–10955. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Li, J.; Zhang, L.; Wu, W.; Wei, S.; Zou, W.; Zhao, Y. Elucidation of the mechanism of berberine against gastric mucosa injury in a rat model with chronic atrophic gastritis based on a combined strategy of multi-omics and molecular biology. Front. Pharmacol. 2025, 15, 1499753. [Google Scholar] [CrossRef]


| No. | Name | Formula | Annot. DeltaMass [ppm] | m/z | RT [min] | Adducts | Ion Mode |
|---|---|---|---|---|---|---|---|
| MOL01 | (+)-Afzelechin | C15H14O5 | 0.78 | 319.0825 | 6.29 | M+FA-H | NEG |
| MOL02 | (Z)-Akuammidine | C21H24N2O4 | −3.48 | 386.2062 | 3.96 | M+NH4 | POS |
| MOL03 | 2,6-Dimethoxyphenol | C14H18O10 | 0.95 | 327.0725 | 2.59 | M-H2O-H | NEG |
| MOL04 | 3′,4′-Dihydroxyacetophenone | C14H16O9 | 0.77 | 327.0724 | 3.77 | M-H | NEG |
| MOL05 | 4,5-Dimethoxycanthin-6-one | C14H8N2O6S | −0.15 | 331.003 | 5.04 | M-H | NEG |
| MOL06 | 7-Hydroxyisoflavone | C16H12O7S | 0.43 | 697.0683 | 5.51 | 2M+H | POS |
| MOL07 | 15-Hydroxydehydroabietic acid | C20H28O4 | 0.04 | 350.2326 | 6.48 | M+NH4 | POS |
| MOL08 | Alfacalcidol | C27H44O3 | −0.37 | 417.3362 | 11.29 | M+H | POS |
| MOL09 | Angelol A | C26H32O13 | 0.08 | 570.2182 | 5.76 | M+NH4 | POS |
| MOL10 | Chicoric acid | C9H10O7S | 0.47 | 261.0076 | 3.76 | M-H | NEG |
| MOL11 | Columbianadin | C19H20O6 | 0.71 | 343.119 | 8.07 | M-H | NEG |
| MOL12 | Guaiacin | C26H32O11 | −3.47 | 559.1558 | 5.23 | M+K | POS |
| MOL13 | Icaritin | C27H28O12 | −4.46 | 527.1524 | 8.83 | M+H-H2O | POS |
| MOL14 | Kaurenoic acid | C20H30O3 | 0.43 | 301.2163 | 9.99 | M+H-H2O | POS |
| MOL15 | Methyl indole-3-carboxylate | C16H17NO9 | 0.38 | 368.0977 | 4.35 | M+H | POS |
| MOL16 | m-Methoxyphenol | C7H8O5S | 1.04 | 203.0022 | 4.52 | M-H | NEG |
| MOL17 | Nuciferine | C19H21NO3 | 2.12 | 623.3129 | 5.62 | 2M+H | POS |
| MOL18 | 6-hydroxy-7-(hydroxymethyl)-3-(1H-pyrrol-3-yl) isochromen-1-one | C14H11NO4 | 1.32 | 302.0674 | 4.66 | M+FA-H | NEG |
| MOL19 | Proxyphylline | C10H12N4O3 | −4.95 | 217.0719 | 4.18 | M-H2O-H | NEG |
| MOL20 | Trametenolic acid | C30H48O4 | −4.02 | 495.3426 | 11.92 | M+Na | POS |
| MOL21 | Gamma-l-glutamyl-l-tyrosine | C14H18N2O6 | −1.03 | 311.1234 | 2.47 | M+H | POS |
| MOL22 | 2-Heptadecanone | C17H34O | 0.08 | 299.2592 | 13.19 | M+FA-H | NEG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Jin, X.; He, T.; Zhou, X.; Liu, Z.; Gao, W. Investigation of c-Fos/c-Jun Signaling Pathways in Periostracum Cicadae’s Inhibition of EMT in Gastric Tissue. Pharmaceuticals 2025, 18, 537. https://doi.org/10.3390/ph18040537
Liang H, Jin X, He T, Zhou X, Liu Z, Gao W. Investigation of c-Fos/c-Jun Signaling Pathways in Periostracum Cicadae’s Inhibition of EMT in Gastric Tissue. Pharmaceuticals. 2025; 18(4):537. https://doi.org/10.3390/ph18040537
Chicago/Turabian StyleLiang, Hua, Xiaofei Jin, Tongtong He, Xiaohong Zhou, Zhenyi Liu, and Weijuan Gao. 2025. "Investigation of c-Fos/c-Jun Signaling Pathways in Periostracum Cicadae’s Inhibition of EMT in Gastric Tissue" Pharmaceuticals 18, no. 4: 537. https://doi.org/10.3390/ph18040537
APA StyleLiang, H., Jin, X., He, T., Zhou, X., Liu, Z., & Gao, W. (2025). Investigation of c-Fos/c-Jun Signaling Pathways in Periostracum Cicadae’s Inhibition of EMT in Gastric Tissue. Pharmaceuticals, 18(4), 537. https://doi.org/10.3390/ph18040537






