The In Vitro Pharmacokinetics of Medicinal Plants: A Review
Abstract
1. Introduction
2. Methods
2.1. Question and PICOS Strategy
2.2. Data Sources and Literature Search
2.3. Study Selection and Eligibility Criteria
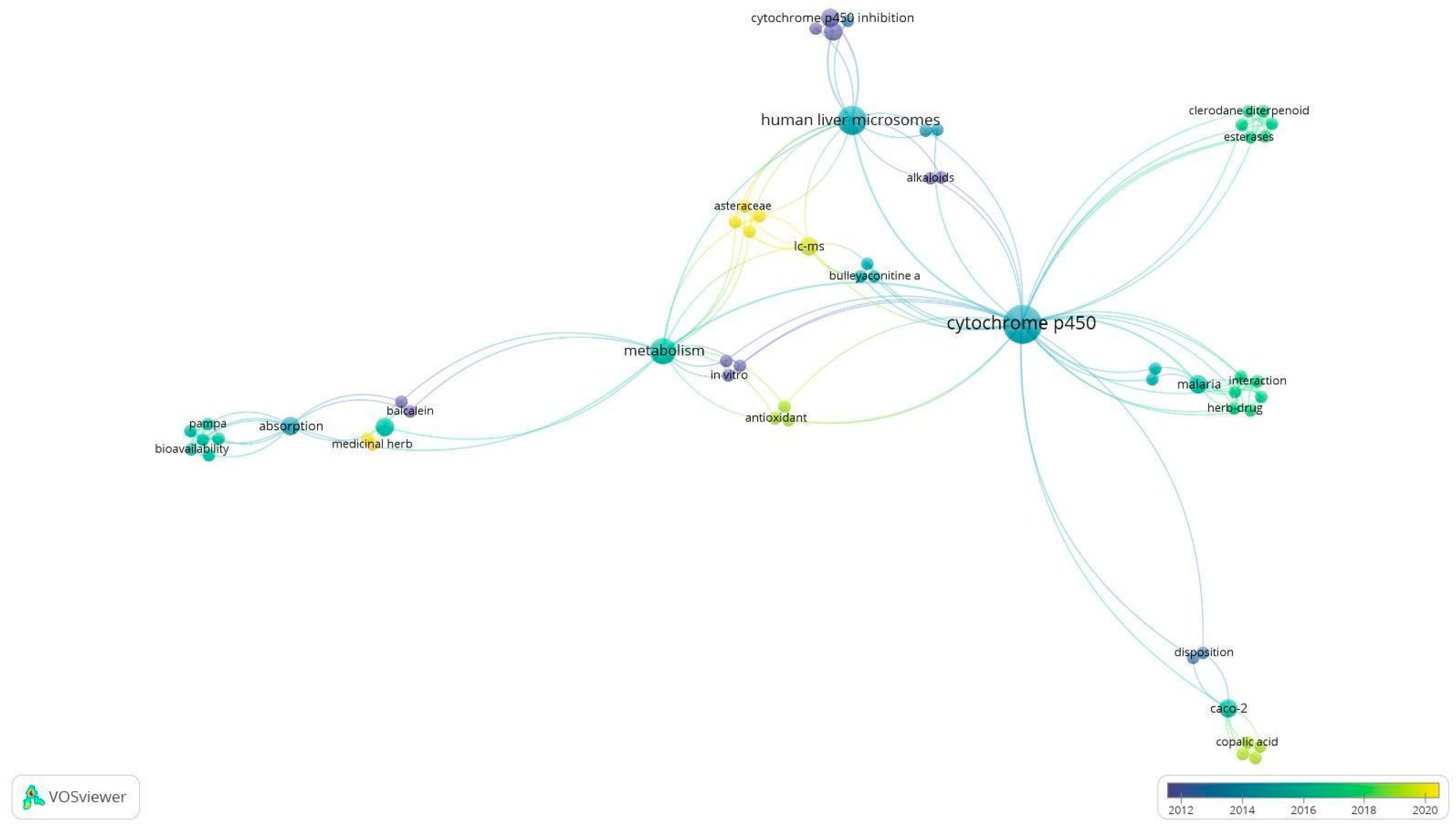
2.4. Data Extraction and Visualization
3. Results
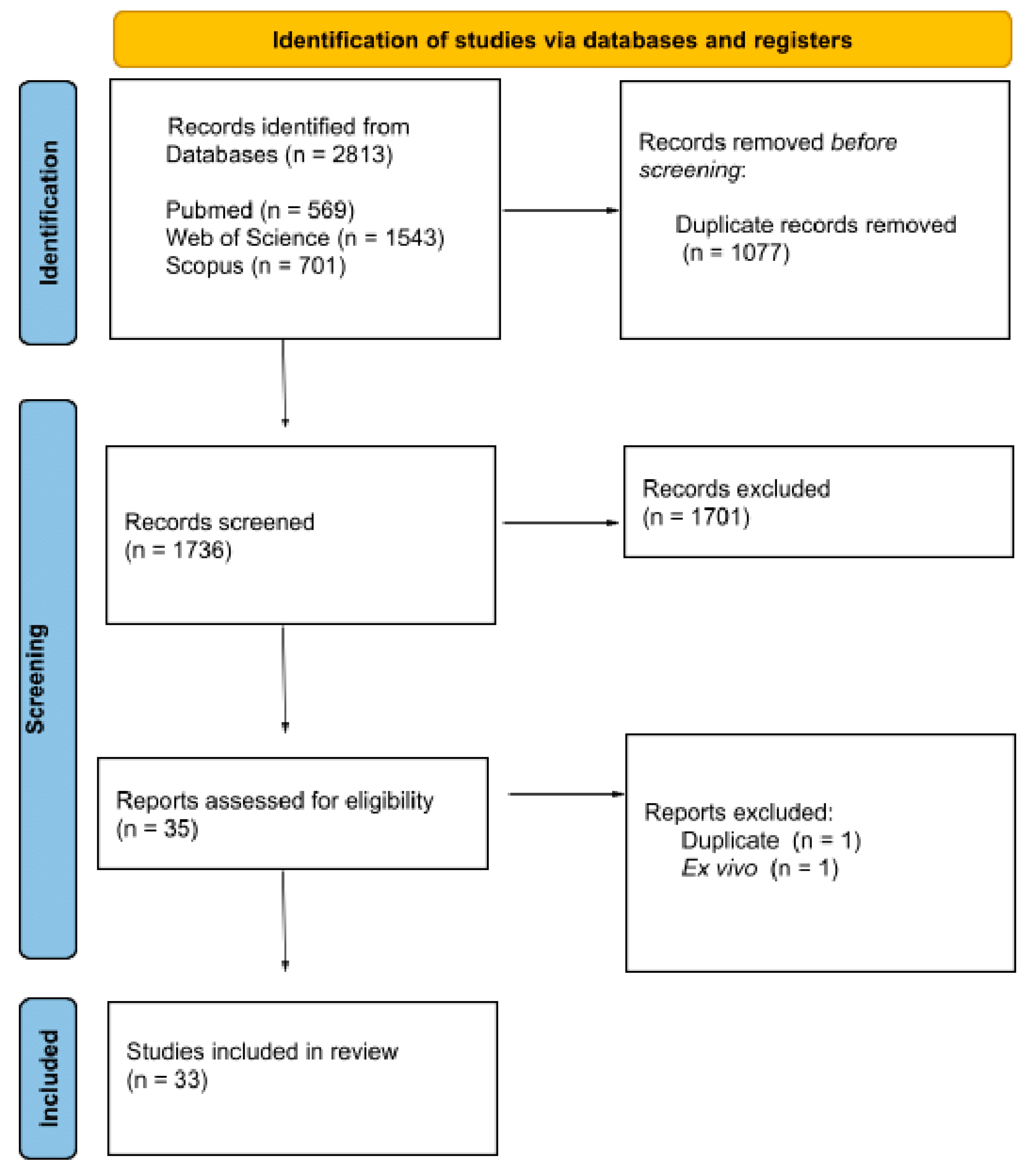
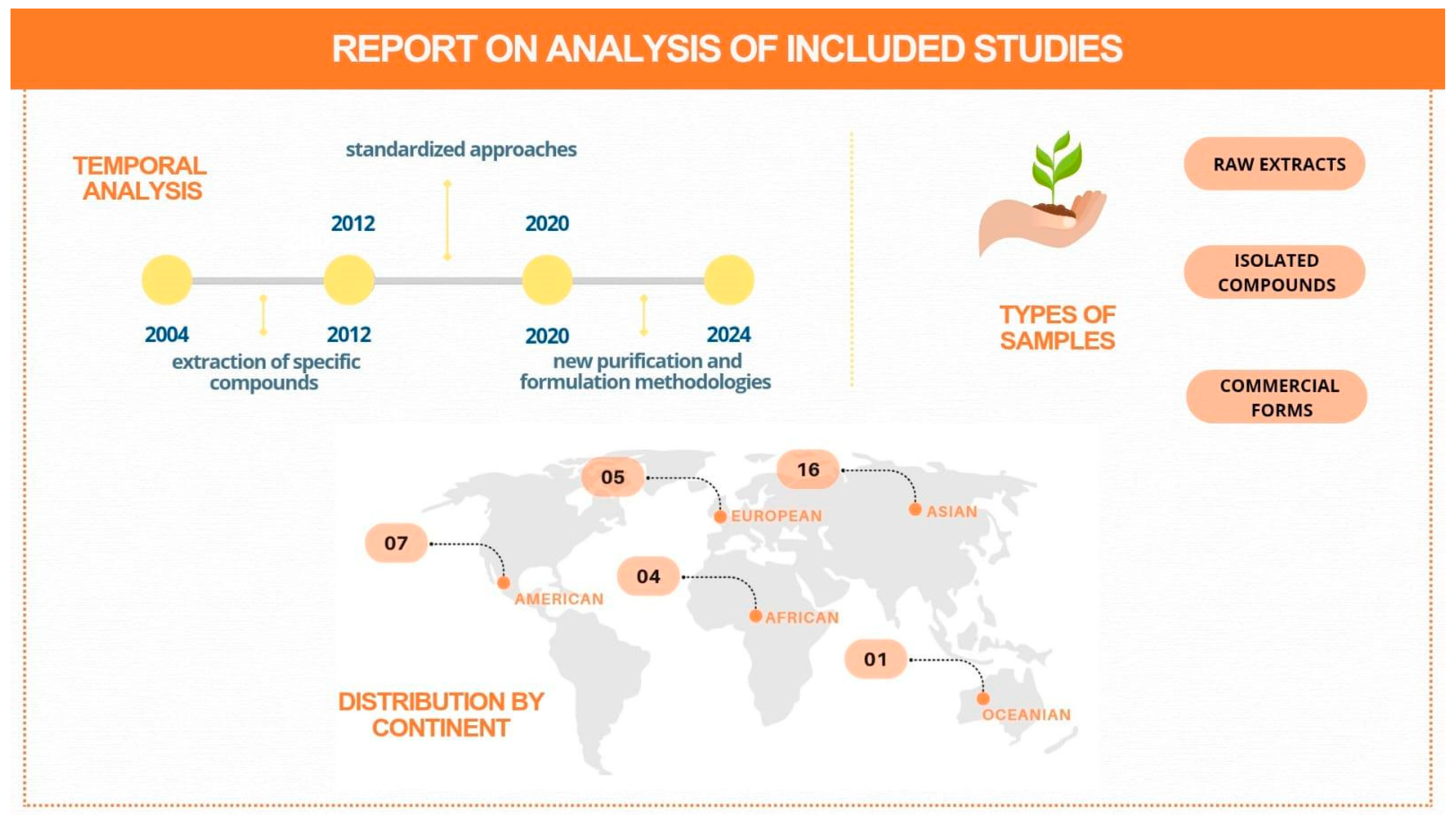
3.1. Search Results and Study Characteristics
3.2. Data Analysis
3.3. Natural Products and How to Prepare Them
3.4. Pharmacokinetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanda, S. A Review on Some Therapeutic Aspects of Phytochemicals Present in Medicinal Plants. J. Pharmacogn. Phytochem. 2019. [CrossRef]
- Ekiert, H.M.; Szopa, A. Biological activities of natural products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef]
- Askari, A. The sodium pump and digitalis drugs: Dogmas and fallacies. Pharmacol. Res. Perspect. 2019, 7, e00505. [Google Scholar] [CrossRef]
- Lal, R.K. The opium poppy (Papaver somniferum L.): Historical perspectives recapitulate and induced mutation towards latex less, low alkaloids in capsule husk mutant: A review. J. Med. Plants Stud. 2022, 10, 19–29. [Google Scholar]
- Shou, W.Z. Current status and future directions of high-throughput ADME screening in drug discovery. J. Pharm. Anal. 2020, 10, 201–208. [Google Scholar] [CrossRef]
- Contreras, A.; Pérez, C. Insomnia, in search of the ideal treatment: Drugs and non-pharmacological treatment. Rev. Medica Clin. Las Condes 2021, 32, 591–602. [Google Scholar] [CrossRef]
- Madden, J.C.; Enoch, S.J.; Paini, A.; Cronin, M.T.D. A Review of In Silico Tools as Alternatives to Animal Testing: Principles, Resources and Applications. Altern. Lab. Anim. 2020, 48, 146–172. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G.; Disclaimer Biometrics, P. The measurement of observer agreement for categorical data LinkOut-more resources Other Literature Sources The Lens-Patent Citations Database. Biometrics 1977, 33, 159–174. [Google Scholar]
- Fröhlich, E.; Loizou, G.D. Editorial: 3Rs—Strategies for reduction and refinement of animal studies. Front. Pharmacol. 2023, 14, 1200965. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, J.; Wu, D.; Xie, Y.; Li, J. Mapping the Research Trends by Co-word Analysis Based on Keywords from Funded Project. In Procedia Computer Science; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 91, pp. 547–555. [Google Scholar] [CrossRef]
- Lefebvre, T.; Foster, B.C.; Drouin, C.E.; Krantis, A.; Livesey, J.F.; Jordan, S.A. In Vitro Activity of Commercial Valerian Root Extracts Against Human Cytochrome P450 3A4. J. Pharm. Sci. 2004, 7, 265–273. [Google Scholar]
- Lee, S.K.; Jun, I.H.; Kang, M.J.; Jeon, T.W.; Kim, J.H.; Seo, Y.M.; Shin, S.; Choi, J.H.; Jeong, H.G.; Lee, S.H.; et al. Characterization of Deoxypodophyllotoxin Metabolism in Rat Liver Microsomes. Biomol. Ther. 2008, 16, 190–196. [Google Scholar] [CrossRef]
- Sun, D.X.; Lu, J.C.; Fang, Z.Z.; Zhang, Y.Y.; Cao, Y.F.; Mao, Y.X.; Zhu, L.L.; Yin, J.; Yang, L. Reversible inhibition of three important human liver cytochrome p450 enzymes by tiliroside. Phytother. Res. 2010, 24, 1670–1675. [Google Scholar] [CrossRef]
- Zhao, T.; He, Y.; Wang, J.; Ding, K.; Wang, C.; Wang, Z. Inhibition of Human Cytochrome P450 Enzymes 3A4 and 2D6 by β-Carboline Alkaloids, Harmine Derivatives. Phytother. Res. 2011, 25, 1671–1677. [Google Scholar] [CrossRef]
- Ji, H.Y.; Liu, K.H.; Lee, H.; Im, S.R.; Shim, H.J.; Son, M.; Lee, H.S. Corydaline Inhibits Multiple Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes. Molecules 2011, 16, 6591–6602. [Google Scholar] [CrossRef]
- Han, Y.L.; Yu, H.L.; Li, D.; Meng, X.L.; Zhou, Z.Y.; Yu, Q.; Zhang, X.Y.; Wang, F.J.; Guo, C. In Vitro Inhibition of Huanglian [Rhizoma coptidis (L.)] and its Six Active Alkaloids on Six Cytochrome P450 Isoforms in Human Liver Microsomes. Phytother. Res. 2011, 25, 1660–1665. [Google Scholar] [CrossRef]
- Fong, Y.K.; Li, C.R.; Wo, S.K.; Wang, S.; Zhou, L.; Zhang, L.; Lin, G.; Zuo, Z. In vitro and in situ evaluation of herb–drug interactions during intestinal metabolism and absorption of Baicalein. J. Ethnopharmacol. 2012, 141, 742–753. [Google Scholar] [CrossRef]
- Müller, A.C.; Patnala, S.; Kis, O.; Bendayan, R.; Kanfer, I. Interactions between Phytochemical Components of Sutherlandia Frutescens and the Antiretroviral, Atazanavir In Vitro: Implications for Absorption and Metabolism. J. Pharm. Pharm. Sci. 2012, 15, 221. [Google Scholar] [CrossRef]
- Cieniak, C.; Liu, R.; Fottinger, A.; Smiley, S.A.; Guerrero-Analco, J.A.; Bennett, S.A.; Haddad, P.S.; Cuerrier, A.; Saleem, A.; Arnason, J.T.; et al. In vitro inhibition of metabolism but not transport of gliclazide and repaglinide by Cree medicinal plant extracts. J. Ethnopharmacol. 2013, 150, 1087–1095. [Google Scholar] [CrossRef]
- Jeong, H.U.; Kong, T.Y.; Kwon, S.S.; Hong, S.W.; Yeon, S.H.; Choi, J.H.; Lee, J.Y.; Cho, Y.Y.; Lee, H.S. Effect of Honokiol on Cytochrome P450 and UDP-Glucuronosyltransferase Enzyme Activities in Human Liver Microsomes. Molecules 2013, 18, 10681–10693. [Google Scholar] [CrossRef]
- Bi, Y.; Zhuang, X.; Zhu, H.; Song, F.; Liu, Z.; Liu, S. Studies on metabolites and metabolic pathways of bulleyaconitine A in rat liver microsomes using LC-MSncombined with specific inhibitors. Biomed. Chromatogr. 2015, 29, 1027–1034. [Google Scholar] [CrossRef]
- Feng, R.; Zhou, X.; Tan, X.S.; Or, P.M.; Hu, T.; Fu, J.; Ma, J.Y.; Huang, M.; He, C.Y.; Shi, J.G.; et al. In vitro identification of cytochrome P450 isoforms responsible for the metabolism of 1-hydroxyl-2,3,5-trimethoxy-xanthone purified from Halenia elliptica, D. Don. Chem. Biol. Interact. 2014, 210, 12–19. [Google Scholar] [CrossRef]
- Sumsakul, W.; Mahavorasirikul, W.; Na-Bangchang, K. Inhibitory Activities of Thai Medicinal Plants with Promising Activities Against Malaria and Cholangiocarcinoma on Human Cytochrome P450. Phytother. Res. 2015, 29, 1926–1933. [Google Scholar] [CrossRef]
- Khadhri, A.; Bouali, I.; Belkhir, S.; Mokded, R.; Smiti, S.; Falé, P.; Araújo, M.E.; Serralheiro, M.L. In vitro digestion, antioxidant and antiacetylcholinesterase activities of two species of Ruta: Ruta chalepensis and Ruta montana. Pharm. Biol. 2017, 55, 101–107. [Google Scholar] [CrossRef]
- Kan, H.; Jiang, W.Y.; Ding, R.; Liu, Z.Y.; Pi, Z.F.; Liu, Z.Q. Studies of the intestinal absorption of the alkaloids in the Wu-tou decoction combined with different incompatible medicinal herbs in a Caco-2 cell culture system using UPLC–MS/MS. Chin. Chem. Lett. 2015, 26, 590–594. [Google Scholar] [CrossRef]
- Petit, C.; Bujard, A.; Skalicka-Woźniak, K.; Cretton, S.; Houriet, J.; Christen, P.; Carrupt, P.A.; Wolfender, J.L. Prediction of the Passive Intestinal Absorption of Medicinal Plant Extract Constituents with the Parallel Artificial Membrane Permeability Assay (PAMPA). Planta Med. 2016, 82, 424–431. [Google Scholar] [CrossRef]
- Thomford, N.E.; Awortwe, C.; Dzobo, K.; Adu, F.; Chopera, D.; Wonkam, A.; Skelton, M.; Blackhurst, D.; Dandara, C. Inhibition of CYP2B6 by Medicinal Plant Extracts: Implication for Use of Efavirenz and Nevirapine-Based Highly Active Anti-Retroviral Therapy (HAART) in Resource-Limited Settings. Molecules 2016, 21, 211. [Google Scholar] [CrossRef]
- Fasinu, P.; Manda, V.; Dale, O.; Egiebor, N.; Walker, L.; Khan, S. Modulation of Cytochrome P450, P-glycoprotein and Pregnane X Receptor by Selected Antimalarial Herbs—Implication for Herb-Drug Interaction. Molecules 2017, 22, 2049. [Google Scholar] [CrossRef]
- Bendikov, M.Y.; Miners, J.O.; Simpson, B.S.; Elliot, D.J.; Semple, S.J.; Claudie, D.J.; McKinnon, R.A.; Gillam, E.M.J.; Sykes, M.J. In vitro metabolism of the anti-inflammatory clerodane diterpenoid polyandric acid A and its hydrolysis product by human liver microsomes and recombinant cytochrome P450 and UDP-glucuronosyltransferase enzymes. Xenobiotica 2017, 47, 461–469. [Google Scholar] [CrossRef]
- Havenga, K.; Abay, E.; Wiesner, L.; Viljoen, A.; Steyn, D.; Hamman, J. The In Vitro and In Vivo Effects of Hypoxis hemerocallidea on Indinavir Pharmacokinetics: Modulation of Efflux. Planta Med. 2018, 84, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.S.; de Oliveira, T.L.; Kanunfre, C.C.; Paludo, K.S.; Minozzo, B.R.; Prestes, A.P.; Wang, M.; Fernandes, D.; Santos, F.A.D.; Manda, V.K. Pharmacokinetics and cytotoxic study of euphol from Euphorbia umbellata (Bruyns) Pax latex. Phytomedicine 2018, 47, 105–112. [Google Scholar] [CrossRef]
- Kolrep, F.; Numata, J.; Kneuer, C.; Preiss-Weigert, A.; Lahrssen-Wiederholt, M.; Schrenk, D.; These, A. In vitro biotransformation of pyrrolizidine alkaloids in different species. Part I: Microsomal degradation. Arch. Toxicol. 2018, 92, 1089–1097. [Google Scholar] [CrossRef]
- Varghese, A.; Saboo, P.; Wairkar, S. Bioactivity guided fractionation of methanolic extract of Terminalia arjuna for its CYP3A and CYP2D inhibition in rat liver microsomes. Biopharm. Drug Dispos. 2018, 39, 143–151. [Google Scholar] [CrossRef]
- Reid, A.M.; Juvonen, R.; Huuskonen, P.; Lehtonen, M.; Pasanen, M.; Lall, N. In Vitro Human Metabolism and Inhibition Potency of Verbascoside for CYP Enzymes. Molecules 2019, 24, 2191. [Google Scholar] [CrossRef]
- Bräuer, P.; Anielski, P.; Schwaiger, S.; Stuppner, H.; Tran, T.V.A.; Vollmer, G.; Zierau, O.; Thieme, D.; Keiler, A.M. In vitro metabolism of selected bioactive compounds of Eurycoma longifolia root extract to identify suitable markers in doping control. Drug Test. Anal. 2019, 11, 86–94. [Google Scholar] [CrossRef]
- Mauro, M.; De Grandis, R.A.; Campos, M.L.; Bauermeister, A.; Peccinini, R.G.; Pavan, F.R.; Lopes, N.P.; De Moraes, N.V. Acid diterpenes from Copaiba oleoresin (Copaifera langsdorffii): Chemical and plasma stability and intestinal permeability using Caco-2 cells. J. Ethnopharmacol. 2019, 235, 183–189. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, J.; Wang, G. Identification of cytochrome P450 isoenzymes involved in the metabolism of 23-hydroxybetulinic acid in human liver microsomes. Pharm. Biol. 2020, 58, 60–63. [Google Scholar] [CrossRef]
- Wang, B.; Lu, Y.; Wang, R.; Liu, S.; Hu, X.; Wang, H. Transport and metabolic profiling studies of amentoflavone in Caco-2 cells by UHPLC-ESI-MS/MS and UHPLC-ESI-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2020, 189, 113441. [Google Scholar] [CrossRef]
- Feng, L.; Xiao, X.; Liu, J.; Wang, J.; Zhang, N.; Bing, T.; Liu, X.; Zhang, Z.; Shangguan, D. Immunomodulatory Effects of Lycium barbarum Polysaccharide Extract and Its Uptake Behaviors at the Cellular Level. Molecules 2020, 25, 1351. [Google Scholar] [CrossRef]
- Chang, Y.; Li, C.; Wang, R.; Li, X.; Guo, S.; Zhang, W.; Liu, B. The metabolic profile elucidation of Lonicera japonica flos water extract and the metabolic characteristics evaluation of bioactive compounds in human gastrointestinal tract in vitro. J. Pharm. Biomed. Anal. 2022, 219, 114906. [Google Scholar] [CrossRef] [PubMed]
- Kane, N.F.; Kiani, B.H.; Desrosiers, M.R.; Towler, M.J.; Weathers, P.J. Artemisia extracts differ from artemisinin effects on human hepatic CYP450s 2B6 and 3A4 in vitro. J. Ethnopharmacol. 2022, 298, 115587. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Dale, O.R.; Manda, V.; Ali, Z.; Gurley, B.J.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Bulbine natalensis (currently Bulbine latifolia) and select bulbine knipholones modulate the activity of AhR, CYP1A2, CYP2B6, and P-gp. Planta Med. 2022, 88, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Grafakou, M.E.; Barda, C.; Skaltsa, H.; Heilmann, J. Study on the metabolism of natural sesquiterpene lactones in human liver microsomes using LC-Q-TOF-MS/MS. Nat. Prod. Res. 2024, 38, 1855–1863. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Xu, M.; Liu, H.; Li, L.; Xu, D. Molecular mechanism overview of metabolite biosynthesis in medicinal plants. Plant Physiol. Biochem. 2023, 204, 108125. [Google Scholar] [CrossRef]
- Donn, P.; Seyyedi-Mansour, S.; Chamorro, F.; Garcia-Oliveira, P.; Echave, J.; Perez-Vazquez, A.; Barciela, P.; Cassani, L.; Prieto, M. A Recent Advances in Understanding the Keys Factors Influencing Pressurized Liquid Extraction of Secondary Metabolites: A Comprehensive Review. Biol. Life Sci. Forum 2024, 35, 1. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Stupar, A.; Bulut, G.; Senkardes, I.; Dogan, A.; Sinan, K.I.; Uysal, S.; Aumeeruddy-Elalfi, Z.; et al. Modern and traditional extraction techniques affect chemical composition and bioactivity of Tanacetum parthenium (L.) Sch.Bip. Ind. Crops Prod. 2020, 146, 112202. [Google Scholar] [CrossRef]
- Wang, J.L.; Lai, C.C.; Ko, W.C.; Hsueh, P.R. Geographical patterns of in vitro susceptibilities to tigecycline and colistin among worldwide isolates of Acinetobacter baumannii, Escherichia coli and Klebsiella pneumoniae: Data from the Antimicrobial Testing Leadership and Surveillance (ATLAS) programme, 2016–2021. Int. J. Antimicrob. Agents 2023, 62, 106930. [Google Scholar] [CrossRef]
- Leitão, G.G.; Leal, C.M.; Mendonça, S.C.; Pereda-Miranda, R. Purification of Alkaloids by Countercurrent Chromatography. Rev. Bras. Farmacogn. 2021, 31, 625–647. [Google Scholar] [CrossRef]
- Afroz, N. Exploring Traditional Medicine in South Africa: A Review of Ethnobotanical Studies on Medicinal Plants. Plant Sci. Arch. 2022, 7, 14–18. [Google Scholar] [CrossRef]
- Bolleddula, J.; Brady, K.; Bruin, G.; Lee, A.; Martin, J.A.; Walles, M.; Xu, K.; Yang, T.Y.; Zhu, X.; Yu, H. Absorption, Distribution, Metabolism, and Excretion of Therapeutic Proteins: Current Industry Practices and Future Perspectives. Drug Metab. Dispos. 2022, 50, 837–845. [Google Scholar] [CrossRef]
- Sánchez-Gómez, T.; Santamaría, Ó.; Martín-García, J.; Poveda, J. Seed extracts as an effective strategy in the control of plant pathogens: Scalable industry bioactive compounds for sustainable agriculture. Biocatal. Agric. Biotechnol. 2024, 60, 103332. [Google Scholar] [CrossRef]
- Yu, C.; Wang, F.; Liu, X.; Miao, J.; Tang, S.; Jiang, Q.; Tang, X.; Gao, X. Corydalis Rhizoma as a model for herb-derived trace metabolites exploration: A cross-mapping strategy involving multiple doses and samples. J. Pharm. Anal. 2021, 11, 308–319. [Google Scholar] [CrossRef]
- Mabadahanye, K.; Bhembe, N.L.; Green, E. Crude extracts activity of three selected medicinal plants from the venda region against some pathogenic organisms. Afr. Health Sci. 2022, 22, 717–727. [Google Scholar] [CrossRef]
- Waweru, A.W.; Osoro, E.K.; Omolo, J.O. A review on the Phytochemistry and Pharmacological Activities of some Species from Genus Dodonaea (Sapindaceae Family). J. Phytopharm. 2024, 13, 70–76. [Google Scholar] [CrossRef]
- Ilmjärv, S.; Augsburger, F.; Bolleman, J.T.; Liechti, R.; Bridge, A.J.; Sandström, J.; Jaquet, V.; Xenarios, I.; Krause, K.H. Navigating in vitro bioactivity data by investigating available resources using model compounds. Sci. Data 2019, 6, 45. [Google Scholar] [CrossRef]
- Nuñez Santiago, I.; Machushynets, N.V.; Mladic, M.; van Bergeijk, D.A.; Elsayed, S.S.; Hankemeier, T.; van Wezel, G.P. nanoRAPIDS as an analytical pipeline for the discovery of novel bioactive metabolites in complex culture extracts at the nanoscale. Commun. Chem. 2024, 7, 71. [Google Scholar] [CrossRef]
- Peng, Y.; Cheng, Z.; Xie, F. Evaluation of pharmacokinetic drug-drug interactions: A review of the mechanisms, in vitro and in silico approaches. Metabolites 2021, 11, 75. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Ong, W.S.Y.; Subelzu, N.; Gleeson, J.P. Validation of a Caco-2 microfluidic Chip model for predicting intestinal absorption of BCS Class I-IV drugs. Int. J. Pharm. 2024, 656, 124089. [Google Scholar] [CrossRef]
- Innes, E.; Yiu, H.H.P.; McLean, P.; Brown, W.; Boyles, M. Simulated biological fluids–a systematic review of their biological relevance and use in relation to inhalation toxicology of particles and fibres. Crit. Rev. Toxicol. 2021, 51, 217–248. [Google Scholar] [CrossRef]
- Kus, M.; Ibragimow, I.; Piotrowska-Kempisty, H. Caco-2 Cell Line Standardization with Pharmaceutical Requirements and In Vitro Model Suitability for Permeability Assays. Pharmaceutics 2023, 15, 2523. [Google Scholar] [CrossRef] [PubMed]
- Horspool, A.M.; Wang, T.; Scaringella, Y.S.; Taub, M.E.; Chan, T.S. Human liver microsomes immobilized on magnetizable beads: A novel approach to study in vitro drug metabolism. Drug Metab. Dispos. 2020, 48, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Kim, G.J.; Park, S.Y.; Shon, J.C.; Liu, K.H.; Choi, H. In vitro metabolism study of seongsanamide a in human liver microsomes using non-targeted metabolomics and feature-based molecular networking. Pharmaceutics 2021, 13, 1031. [Google Scholar] [CrossRef]
- Mudyiwa, M.; Sharma, M.; Ray, S.K.; Masimirembwa, C.; Thelingwani, R.S. Inhibitory effects of herbal medicines with claimed anticancer indications on cytochrome P450—An evaluation of drug-herb interactions risk. Sci. Afr. 2023, 21, e01835. [Google Scholar] [CrossRef]
- Gardner, I.; Xu, M.; Han, C.; Wang, Y.; Jiao, X.; Jamei, M.; Khalidi, H.; Kilford, P.; Neuhoff, S.; Southall, R.; et al. Non-specific binding of compounds in in vitro metabolism assays: A comparison of microsomal and hepatocyte binding in different species and an assessment of the accuracy of prediction models. Xenobiotica 2022, 52, 943–956. [Google Scholar] [CrossRef]
- Murad, A.B.; Sousa, M.Q.; Correia, R.; Isidro, I.A.; Carrondo, M.J.T.; Roldão, A. Targeted Metabolic Analysis and MFA of Insect Cells Expressing Influenza HA-VLP. Processes 2022, 10, 2283. [Google Scholar] [CrossRef]
- Xavier, M.; Rodrigues, P.M.; Neto, M.D.; Guedes, M.I.; Calero, V.; Pastrana, L.; Gonçalves, C. From mouth to gut: Microfluidic in vitro simulation of human gastro-intestinal digestion and intestinal permeability. Analyst 2023, 148, 3193–3203. [Google Scholar] [CrossRef]
- Stanley, L.A.; Wolf, C.R. Through a glass, darkly? HepaRG and HepG2 cells as models of human phase I drug metabolism. Drug Metab. Rev. 2022, 54, 46–62. [Google Scholar] [CrossRef]
- Price, E.; Kalvass, J.C.; Degoey, D.; Hosmane, B.; Doktor, S.; Desino, K. Global Analysis of Models for Predicting Human Absorption: QSAR, in Vitro, and Preclinical Models. J. Med. Chem. 2021, 64, 9389–9403. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zhou, X.; Xu, Z.; Zhang, Y.; Ji, L.; Hong, C.; Li, C. Analyzing the metabolic fate of oral administration drugs: A review and state-of-the-art roadmap. Front. Pharmacol. 2022, 13, 962718. [Google Scholar] [CrossRef]
- Murata, Y.; Neuhoff, S.; Rostami-Hodjegan, A.; Takita, H.; Al-Majdoub, Z.M.; Ogungbenro, K. In Vitro to In Vivo Extrapolation Linked to Physiologically Based Pharmacokinetic Models for Assessing the Brain Drug Disposition. AAPS J. 2022, 24, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yang, Y.; Zhang, X.; Fan, J.; Grimstein, M.; Zhu, H.; Wang, Y. Usage of In Vitro Metabolism Data for Drug-Drug Interaction in Physiologically Based Pharmacokinetic Analysis Submissions to the US Food and Drug Administration. J. Clin. Pharmacol. 2021, 61, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yim, D.S.; Bae, S.H. Prediction of metabolizing enzyme-mediated clinical drug interactions using in vitro information. Transl. Clin. Pharmacol. 2022, 30, 1–12. [Google Scholar] [CrossRef]
- Saifudin, A.; Bahar, A.; Hidayatullah, M.H.; Norimoto, H.; Tezuka, Y.; Tanaka, K. Rethinking the basic action modes of herbal medicine and pondering classical standardization. J. Herbmed Pharmacol. 2024, 13, 163–175. [Google Scholar] [CrossRef]
| Author | Country | Plant | Metabolite | Form(s) of Preparation | Concentration or Dose |
|---|---|---|---|---|---|
| Lefebvre et al., 2004 [13] | Canada | Valeriana officinalis L. | Vairidoids, Monoterpenes And Sesquiterpenes | Commercial Product | Capsules, tablets and caplets were extracted at a concentration of 100 mg/mL. Teas were extracted at a concentration of 25 mg/mL |
| Lee et al., 2008 [14] | South Korea | Anthriscus sylvestris | Lignans | Were Isolated/Purified In The Laboratory Of The Research Institution | 50 µM |
| Sun et al., 2010 [15] | China | ** | Flavonoid | Were Isolated/Purified In The Laboratory Of The Research Institution | various concentrations |
| Zhao et al., 2011 [16] | China | Peganum harmala | Β-Carboline Alkaloids | Solutions Of Alkaloids | various concentrations |
| Ji et al., 2011 [17] | China | Aconitum bulleyanum | diester-diterpene alkaloid | Commercial Product | 50 μmol |
| Han et al., 2011 [18] | China | Huanglian (Rhizoma coptidis) | Alkaloids | Hot Water Extracts | various concentrations |
| Fong et al., 2012 [19] | China | Scutellaria baicalensis Georgi | Flavone Isolated | Solutions | final concentration of 5 μM |
| Müller et al., 2012 [20] | South Africa | Sutherlandia Frutescens | Triterpenoid Glycosides, Glycosides Of Flavonols, Non-Protein Amino Acid. | Aqueous And Methanolic Extracts | 10 mg/mL |
| 10 mg/mL | |||||
| Cieniak et al., 2013 [21] | Canada | Cree plants-list of 17 species | ** | Ethanolic And Methanolic Extracts | 100 μg/mL |
| 50 μg/mL | |||||
| 5 mg/mL | |||||
| 10 μL | |||||
| ** | |||||
| Jeong et al., 2013 [22] | South Korea | Magnolia officinalis, Magnolia grandiflora and other plants | ** | Commercial Product | Various Concentrations of honokiol (0.05–100 μM) |
| Various Concentrations of honokiol (1–200 μM for UGT1A1, UGT1A4, and UGT2B7; 0.01–2 μM for UGT1A9) | |||||
| Various Concentrations | |||||
| Bi et al., 2013 [23] | South Korea | Corydalis tubers | Alkaloid | Commercial Product | Final Concentrations of 1–200 μM |
| Final Concentrations of 1–1000 μM | |||||
| Various Concentrations | |||||
| Feng et al., 2014 [24] | China | Halenia elliptica D. Don | ** | Were Isolated/Purified In The Laboratory Of The Research Institution | 1–500 μM |
| Sumsakul et al., 2015 [25] | Thailand | Plumbago indica Linn., Garcinia mangostana Linn., Dracaena loureiri Gagnepv, Dioscorea membranacea Pierre and Myristica fragans Houtt. | ** | Ethanolic Extracts | 100 mg/mL |
| Khadhri et al., 2015 [26] | Tunisia | Ruta chalepensis L. and Ruta montana L. | Polyphenol | Ethanol Extracts | 4 mg/mL |
| Kan et al., 2015 [27] | China | Wu-tou decoction (Q) is composed of Aconiti Radix Cocta, Ephedrae Herba, Paeoniae Radix Alba, Astragali Radix and Glycyrrhiza Radix Preparata | Alkaloids | Commercial Product | various concentrations |
| Petit C et al., 2016 [28] | Switzerland | Angelica archangelica (L.) H.Karst. | Furanocoumarins | Methanolic Extracts | 10 mg/mL |
| Waltheria indica L. | Alkaloids | Aqueous Extract | |||
| Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep | Flavonoids | Hot Water Extracts | |||
| Thomfor et al., 2016 [29] | South Africa | Hyptis suaveolens, Boerhavia diffusa, Newbouldia laevis, Launaea taraxacifolia and Myrothamnus flabellifolius | ** | Aqueous Extracts | 10 µg/mL and 100 µg/mL |
| Fasinu et al., 2017 [30] | United States | Annona muricata, Argermone mexicana, Kalanchoe pinnata, Mangifera indica, Momordica charantia, Phyllanthus amarus and Tithonia diversifolia. | Alkaloids, Nematicidal Compounds, Phenolics | Methanolic Extracts | 2 µg/mL |
| Bendikov et al., 2017 [31] | Australia | Dodonaea polyandra | Clerodane Diterpenoids | Were Isolated/Purified In The Laboratory Of The Research Institution | 10 μM |
| Havenga et al., 2018 [32] | South Africa | Hypoxis hemerocallidea | ** | Reference Dried Plant Material | 500 µg/mL |
| Commercial Product | |||||
| Aqueous Extract | |||||
| Cruz et al., 2018 [33] | Brazil | Euphorbia umbellata (Pax) Bruyns | Diterpenes And Triterpenes | Suspension With Sulphoric Acid | 100 µM |
| 1.17 mM | |||||
| Kolrep et al., 2018 [34] | Germany | ** | Pyrrolizidine alkaloids | Commercial Product | 125 nM PA. |
| Varghese et al., 2018 [35] | India | Terminalia arjuna | ** | Methanolic Extract | various concentrations |
| Reid et al., 2019 [36] | Finland | Lippia scaberrima | Phenylethanoid | Commercial Product | 10 µM |
| 0.4, 2, 10, and 50 µM | |||||
| Bräuer et al., 2019 [37] | Germany | Eurycoma longifolia | Triterpenes, Alkaloids | Extracts | final concentration of 10 μM |
| Mauro et al., 2019 [38] | Brazil | Copaifera langsdorffii | Diterpenes | Commercial Product | ** |
| Zhou et al., 2020 [39] | China | Pulsatilla chinensis | Pentacyclic Triterpene | Were Isolated/Purified In The Laboratory Of The Research Institution | 0.5, 1, 2, 5, 10, 20, 50, 100 μM |
| 1 µM | |||||
| Wang et al., 2020 [40] | China | ** | Flavonoids | Commercial Product | Various Concentrations |
| Feng et al., 2020 [41] | China | Lycium barbarum L. | Polysaccharides | Ethanol Extracts | ** |
| various concentrations | |||||
| 200 μg/mL | |||||
| Chang et al., 2022 [42] | China | Lonicera japonica Flos | Flavones, Organic Acids And Iridoids | Aqueous Extract | 1 mL |
| Kane et al., 2022 [43] | United States | Artemisia annua L. cv. SAM (voucher MASS 317314) | Flavonoids | Hot Water Extracts | various concentrations |
| Artemisia afra SEN (voucher LG0019529) | |||||
| Artemisia afra MAL (voucher FTG, 181107) | |||||
| Husain et al., 2022 [44] | United States | Bulbine natalensis Baker | ** | Methanolic Extract | various concentrations |
| Grafakou et al., 2024 [45] | Greence | Tanacetum | Sesquiterpene Lactones | Were Isolated/Purified During Previous Work-University Laboratory | 10 μM test compound |
| parthenium | |||||
| Cynara spp., | |||||
| Crepis spp | |||||
| Calea spp. | |||||
| Centaurea spp. | |||||
| Artemisia | |||||
| dubia, Achillea | |||||
| coarctata |
| Technique/System | N° of Studies | Category | References |
|---|---|---|---|
| Caco-2 cells | 7 | Absorption | Havenga et al., 2018 [32]; Fong et al., 2012 [19]; Müller et al., 2012 [20]; Wang et al., 2020 [40]; Feng et al., 2020 [41]; Mauro et al., 2019 [38]; Kan et al., 2015 [27] |
| Simulated gastric juice | 1 | Absorption | Chang et al., 2022 [42] |
| Simulated gastric (SGF) and intestinal fluids (SIF) | 1 | Absorption | Cruz et al., 2018 [33] |
| Synthetic gastric and pancreatic juices | 1 | Absorption | Khadhri et al., 2015 [26] |
| C2BBe1 cell line | 1 | Absorption | Cieniak et al., 2013 [21] |
| PAMPA (Parallel Artificial Membrane Permeability Assay) | 1 | Absorption | Petit et al., 2016 [28] |
| Human liver microsomes-Incubation (phase I, II, or combined) | 1 | Metabolism | Grafakou et al., 2024 [45] |
| Human liver microsomes-Enzyme inhibition | 15 | Metabolism | Sun et al., 2010 [15]; Zhao et al., 2011 [16]; Zhou et al., 2020 [39]; Sumsakul et al., 2015 [25]; Bendikov et al., 2017 [31]; Jeong et al., 2013 [22]; Ji et al., 2013 [17]; Han et al., 2011 [18]; Kane et al., 2022 [43]; Cruz et al., 2018 [33] |
| UDGPA | 1 | Metabolism | Cieniak et al., 2013 [21] |
| Rat liver microsomes | 4 | Metabolism | Bräuer et al., 2019 [37]; Lee et al., 2008 [14]; Bi et al., 2011 [23]; Varghese et al., 2018 [35] |
| Placental microsomes | 1 | Metabolism | Reid et al., 2019 [36] |
| Rat intestinal S9 (RIs9) | 1 | Metabolism | Fong et al., 2012 [19] |
| CYP proteins | 1 | Metabolism | Fasinu et al., 2017 [30] |
| hMDR1-MDCKII cells | 1 | Metabolism | Fasinu et al., 2017 [30] |
| Baculovirus system in insect cells | 1 | Metabolism | Thomfor et al., 2016 [29] |
| HepaRG cells | 2 | Metabolism | Kane et al., 2022 [43]; Husain et al., 2022 [44] |
| Liver S9 fractions | 1 | Metabolism | Kolrep et al., 2018 [34] |
| Fluorometric microplates | 1 | Metabolism | Lefebvre et al., 2004 [13] |
| Fluorometric microplates | 1 | Distribution | Lefebvre et al., 2004 [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves de Jesus, P.; Rego Rodrigues Silva, D.M.; Macedo Moura, P.H.; Gopalsamy, R.G.; Dias Silva, E.E.; dos Santos Barreto, M.; Santana Santos, R.; Santos Martins, A.Y.; de Freitas Almeida, A.G.; Santana Corrêa, A.K.; et al. The In Vitro Pharmacokinetics of Medicinal Plants: A Review. Pharmaceuticals 2025, 18, 551. https://doi.org/10.3390/ph18040551
Chaves de Jesus P, Rego Rodrigues Silva DM, Macedo Moura PH, Gopalsamy RG, Dias Silva EE, dos Santos Barreto M, Santana Santos R, Santos Martins AY, de Freitas Almeida AG, Santana Corrêa AK, et al. The In Vitro Pharmacokinetics of Medicinal Plants: A Review. Pharmaceuticals. 2025; 18(4):551. https://doi.org/10.3390/ph18040551
Chicago/Turabian StyleChaves de Jesus, Pamela, Deise Maria Rego Rodrigues Silva, Pedro Henrique Macedo Moura, Rajiv Gandhi Gopalsamy, Eloia Emanuelly Dias Silva, Marina dos Santos Barreto, Ronaldy Santana Santos, Allec Yuri Santos Martins, Anne Gabriela de Freitas Almeida, Adriana Kelly Santana Corrêa, and et al. 2025. "The In Vitro Pharmacokinetics of Medicinal Plants: A Review" Pharmaceuticals 18, no. 4: 551. https://doi.org/10.3390/ph18040551
APA StyleChaves de Jesus, P., Rego Rodrigues Silva, D. M., Macedo Moura, P. H., Gopalsamy, R. G., Dias Silva, E. E., dos Santos Barreto, M., Santana Santos, R., Santos Martins, A. Y., de Freitas Almeida, A. G., Santana Corrêa, A. K., Alves da Mota Santana, L., Hariharan, G., Gibara Guimarães, A., & Pinto Borges, L. (2025). The In Vitro Pharmacokinetics of Medicinal Plants: A Review. Pharmaceuticals, 18(4), 551. https://doi.org/10.3390/ph18040551






