SWEEPS-Assisted Antibacterial Photodynamic Therapy Against Dual-Species Biofilms in Mandibular Molars: An In Vitro Study
Abstract
:1. Introduction
2. Results
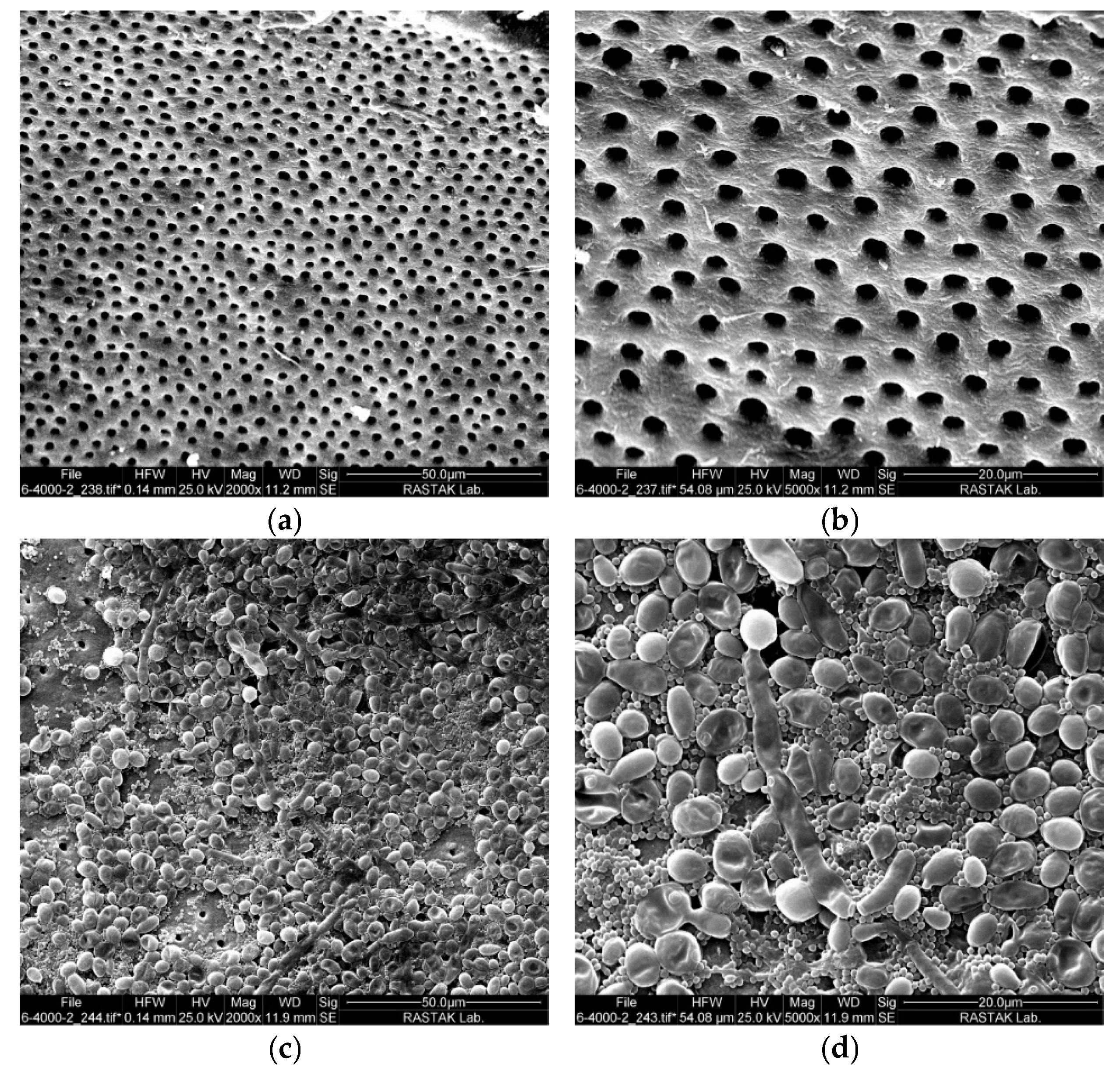
2.1. Intracanal Biofilm Formation
2.2. Antimicrobial Efficacy of aPDT
2.3. Outcomes of Tooth Discoloration
3. Discussion
4. Materials and Methods
4.1. Materials and Instruments
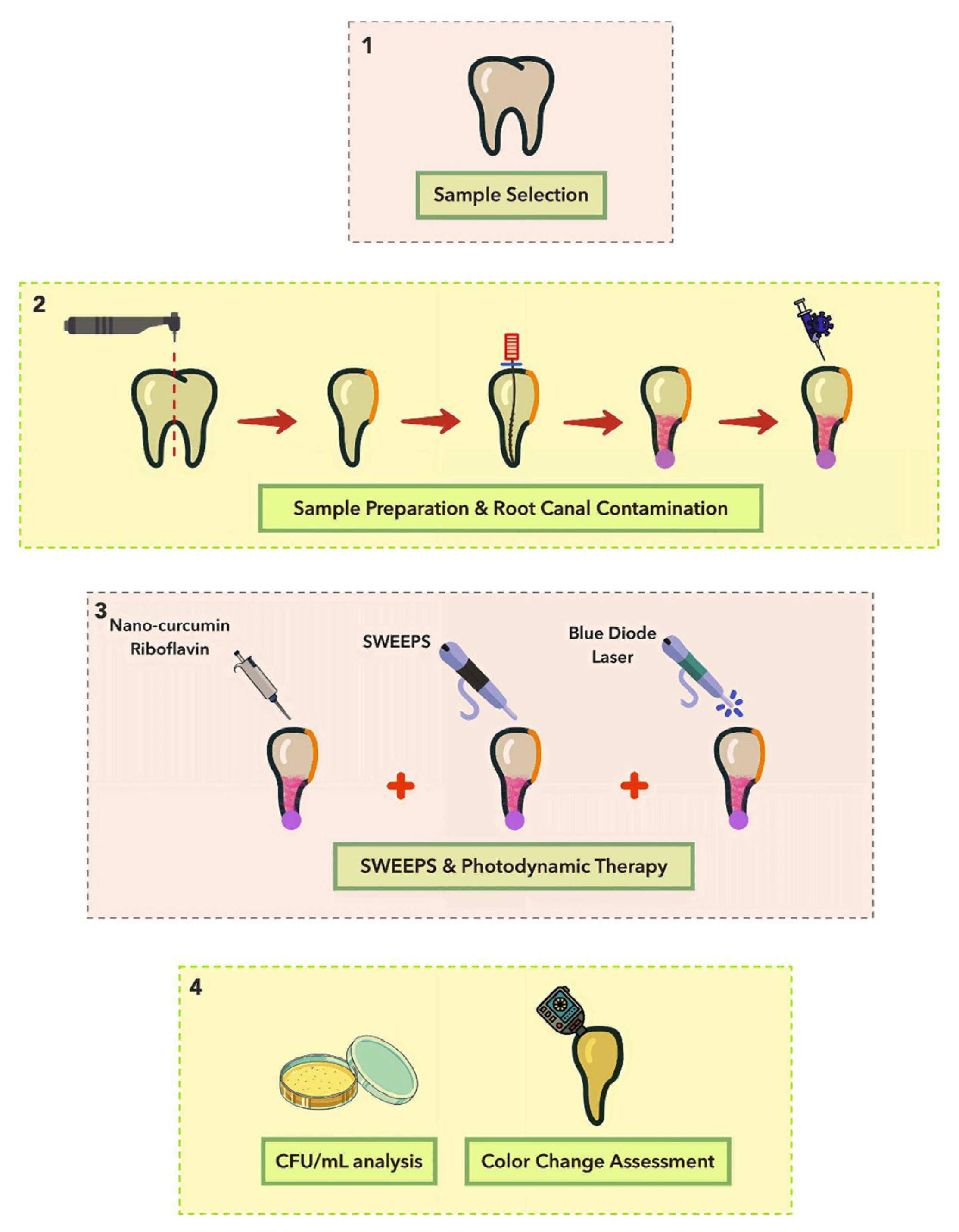
4.2. Sample Selection and Preparation
4.3. Root Canal Contamination
4.4. Control of Intracanal Biofilm Formation
4.5. Light Sources and Photosensitizers
4.6. Experimental Design
- Normal saline (negative control): 10 μL of normal saline was injected into the canal using a micropipette, with samples kept in the dark for 5 min [74].
- NaOCl (positive control): 10 μL of 5.25% NaOCl was injected using a micropipette, with samples kept in the dark for 5 min.
- Normal saline + SWEEPS: the normal saline was applied like group 1 and then the SWEEPS technique was performed.
- Nano-curcumin: 10 μL of nano-curcumin was injected into the canal using a micropipette, with samples kept in the dark for 5 min.
- Riboflavin: 10 μL of riboflavin was injected into the canal using a micropipette, with samples kept in the dark for 5 min.
- Nano-curcumin + SWEEPS: the root canal was filled by nano-curcumin (like group 5). Then, the SWEEPS technique was performed.
- Riboflavin + SWEEPS: the root canal was filled by riboflavin (like group 6). Then, the SWEEPS technique was performed.
- Nano-curcumin + BDL: the root canal was filled by nano-curcumin (like group 5). Then, according to the specified parameters, the BDL was irradiated.
- Riboflavin + BDL: the root canal was filled by riboflavin (like group 6). Then, according to the specified parameters, the BDL was irradiated.
- Nano-curcumin + BDL + SWEEPS: the application of nano-curcumin and SWEEPS technique was conducted like group 7 and then, the BDL was irradiated.
- Riboflavin + BDL + SWEEPS: the application of riboflavin and SWEEPS technique was performed like group 8, and then the BDL was irradiated. A schematic illustration of the different phases of the study is shown in Figure 3.
4.7. Assessment of Antimicrobial Efficacy
4.8. Assessment of Color Change
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamberi, B.; Hoxha, V.; Stavileci, M.; Dragusha, E.; Kuci, A.; Kqiku, L. Prevalence of apical periodontitis and endodontic treatment in a Kosovar adult population. BMC Oral. Health 2011, 11, 32. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55 (Suppl. S3), 512–530. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef]
- Jenkinson, H.F.; Lamont, R.J. Oral microbial communities in sickness and in health. Trends Microbiol. 2005, 13, 589–595. [Google Scholar] [CrossRef]
- Kuramitsu, H.K.; He, X.; Lux, R.; Anderson, M.H.; Shi, W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 2007, 71, 653–670. [Google Scholar] [CrossRef]
- Gomes, B.P.; Pinheiro, E.T.; Jacinto, R.C.; Zaia, A.A.; Ferraz, C.C.; Souza-Filho, F.J. Microbial analysis of canals of root-filled teeth with periapical lesions using polymerase chain reaction. J. Endod. 2008, 34, 537–540. [Google Scholar] [CrossRef]
- Cheung, G.S.; Ho, M.W. Microbial flora of root canal-treated teeth associated with asymptomatic periapical radiolucent lesions. Oral. Microbiol. Immunol. 2001, 16, 332–337. [Google Scholar] [CrossRef]
- Egan, M.W.; Spratt, D.A.; Ng, Y.L.; Lam, J.M.; Moles, D.R.; Gulabivala, K. Prevalence of yeasts in saliva and root canals of teeth associated with apical periodontitis. Int. Endod. J. 2002, 35, 321–329. [Google Scholar] [CrossRef]
- Brasil, S.C.; Marceliano-Alves, M.F.; Marques, M.L.; Grillo, J.P.; Lacerda, M.; Alves, F.R.F.; Siqueira, J.F., Jr.; Provenzano, J.C. Canal Transportation, Unprepared Areas, and Dentin Removal after Preparation with BT-RaCe and ProTaper Next Systems. J. Endod. 2017, 43, 1683–1687. [Google Scholar] [CrossRef]
- Harris, S.P.; Bowles, W.R.; Fok, A.; McClanahan, S.B. An anatomic investigation of the mandibular first molar using micro-computed tomography. J. Endod. 2013, 39, 1374–1378. [Google Scholar] [CrossRef]
- Song, M.; Kim, H.C.; Lee, W.; Kim, E. Analysis of the cause of failure in nonsurgical endodontic treatment by microscopic inspection during endodontic microsurgery. J. Endod. 2011, 37, 1516–1519. [Google Scholar] [CrossRef]
- Gaeta-Araujo, H.; Fontenele, R.C.; Nascimento, E.H.L.; Nascimento, M.; Freitas, D.Q.; de Oliveira-Santos, C. Association between the Root Canal Configuration, Endodontic Treatment Technical Errors, and Periapical Hypodensities in Molar Teeth: A Cone-beam Computed Tomographic Study. J. Endod. 2019, 45, 1465–1471. [Google Scholar] [CrossRef]
- Vivacqua, F.D.; Hungaro Duarte, M.A.; Vivan, R.R.; Alcalde, M.P.; Furlan, R.D.; Bramante, C.M. Analysis of Instrumentation Protocols Regarding the Quality of Mesial Canal Preparation in Mandibular Molars: A Micro-computed Tomographic Study. J. Endod. 2021, 47, 1481–1486. [Google Scholar] [CrossRef]
- Siqueira Junior, J.F.; Rocas, I.D.N.; Marceliano-Alves, M.F.; Perez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral. Res. 2018, 32, e65. [Google Scholar] [CrossRef]
- Almeida, B.M.; Provenzano, J.C.; Marceliano-Alves, M.F.; Rocas, I.N.; Siqueira, J.F., Jr. Matching the Dimensions of Currently Available Instruments with the Apical Diameters of Mandibular Molar Mesial Root Canals Obtained by Micro-computed Tomography. J. Endod. 2019, 45, 756–760. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55 (Suppl. S3), 588–612. [Google Scholar] [CrossRef]
- Fedorowicz, Z.; Nasser, M.; Sequeira-Byron, P.; de Souza, R.F.; Carter, B.; Heft, M. Irrigants for non-surgical root canal treatment in mature permanent teeth. Cochrane Database Syst. Rev. 2012, 2012, CD008948. [Google Scholar] [CrossRef]
- Susila, A.V.; Sai, S.; Sharma, N.; Balasubramaniam, A.; Veronica, A.K.; Nivedhitha, S. Can natural irrigants replace sodium hypochlorite? A systematic review. Clin. Oral. Investig. 2023, 27, 1831–1849. [Google Scholar] [CrossRef]
- Jiang, L.M.; Verhaagen, B.; Versluis, M.; Langedijk, J.; Wesselink, P.; van der Sluis, L.W. The influence of the ultrasonic intensity on the cleaning efficacy of passive ultrasonic irrigation. J. Endod. 2011, 37, 688–692. [Google Scholar] [CrossRef]
- Rhodes, S.C. Ultrasonic Device Complications in Endodontics: An Analysis of Adverse Events From the Food and Drug Administration Manufacturer and User Facility Device Experience. J. Patient Saf. 2022, 18, 269–275. [Google Scholar] [CrossRef]
- Retsas, A.; Koursoumis, A.; Tzimpoulas, N.; Boutsioukis, C. Uncontrolled Removal of Dentin during In Vitro Ultrasonic Irrigant Activation in Curved Root Canals. J. Endod. 2016, 42, 1545–1549. [Google Scholar] [CrossRef]
- Meire, M.; De Moor, R.J.G. Principle and antimicrobial efficacy of laser-activated irrigation: A narrative review. Int. Endod. J. 2024, 57, 841–860. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Dust, P.C.; Schafer, E.; Burklein, S. Comparative Analysis of Irrigation Techniques for Cleaning Efficiency in Isthmus Structures. J. Endod. 2024, 50, 644–650.e1. [Google Scholar] [CrossRef]
- Swimberghe, R.C.D.; De Clercq, A.; De Moor, R.J.G.; Meire, M.A. Efficacy of sonically, ultrasonically and laser-activated irrigation in removing a biofilm-mimicking hydrogel from an isthmus model. Int. Endod. J. 2019, 52, 515–523. [Google Scholar] [CrossRef]
- Lei, L.; Wang, F.; Wang, Y.; Li, Y.; Huang, X. Laser activated irrigation with SWEEPS modality reduces concentration of sodium hypochlorite in root canal irrigation. Photodiagn. Photodyn. Ther. 2022, 39, 102873. [Google Scholar] [CrossRef]
- Lukac, N.; Jezersek, M. Amplification of pressure waves in laser-assisted endodontics with synchronized delivery of Er:YAG laser pulses. Lasers Med. Sci. 2018, 33, 823–833. [Google Scholar] [CrossRef]
- Sathorn, C.; Parashos, P.; Messer, H. The prevalence of postoperative pain and flare-up in single- and multiple-visit endodontic treatment: A systematic review. Int. Endod. J. 2008, 41, 91–99. [Google Scholar] [CrossRef]
- Erkan, E.; Gundogar, M.; Uslu, G.; Ozyurek, T. Postoperative pain after SWEEPS, PIPS, sonic and ultrasonic-assisted irrigation activation techniques: A randomized clinical trial. Odontology 2022, 110, 786–794. [Google Scholar] [CrossRef]
- Petricevic, G.K.; Katic, M.; Anic, I.; Salaric, I.; Vrazic, D.; Bago, I. Efficacy of different Er:YAG laser-activated photoacoustic streaming modes compared to passive ultrasonic irrigation in the retreatment of curved root canals. Clin. Oral. Investig. 2022, 26, 6773–6781. [Google Scholar] [CrossRef]
- Baraba, A.; Rajda, M.; Barsic, G.; Jukic Krmek, S.; Snjaric, D.; Miletic, I. Efficacy of Shock Wave-Enhanced Emission Photoacoustic Streaming (SWEEPS) in the Removal of Different Combinations of Sealers Used with Two Obturation Techniques: A Micro-CT Study. Materials 2023, 16, 3273. [Google Scholar] [CrossRef]
- Chrepa, V.; Kotsakis, G.A.; Pagonis, T.C.; Hargreaves, K.M. The effect of photodynamic therapy in root canal disinfection: A systematic review. J. Endod. 2014, 40, 891–898. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Hatamipour, M.; Sahebkar, A.; Alavizadeh, S.H.; Dorri, M.; Jaafari, M.R. Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iran. J. Basic. Med. Sci. 2019, 22, 282–289. [Google Scholar] [CrossRef]
- Zendedel, E.; Gheybi, F.; Chamani, J.; Jaafari, M.R. Cytotoxic effects investigation of nanomicelle and free curcuminoids against cancer and normal cells. Nanomed. Res. J. 2019, 4, 63–68. [Google Scholar] [CrossRef]
- Cusicanqui Mendez, D.A.; Gutierres, E.; Jose Dionisio, E.; Afonso Rabelo Buzalaf, M.; Cardoso Oliveira, R.; Andrade Moreira Machado, M.A.; Cruvinel, T. Curcumin-mediated antimicrobial photodynamic therapy reduces the viability and vitality of infected dentin caries microcosms. Photodiagn. Photodyn. Ther. 2018, 24, 102–108. [Google Scholar] [CrossRef]
- Hosseinpour-Nader, A.; Karimi, N.; Ghafari, H.A.; Ghorbanzadeh, R. Effect of nanomicelle curcumin-based photodynamic therapy on the dynamics of white spot lesions and virulence of Streptococcus mutans in patients undergoing fixed orthodontic treatment: A randomized double-blind clinical trial. Photodiagn. Photodyn. Ther. 2022, 40, 103183. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in Human Health: A Review of Current Evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar] [CrossRef]
- Zempleni, J.; Galloway, J.R.; McCormick, D.B. Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. Am. J. Clin. Nutr. 1996, 63, 54–66. [Google Scholar] [CrossRef]
- Kubrak, T.P.; Kolodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef]
- Hersek, N.; Canay, S.; Uzun, G.; Yildiz, F. Color stability of denture base acrylic resins in three food colorants. J. Prosthet. Dent. 1999, 81, 375–379. [Google Scholar] [CrossRef]
- Pauli, H. Proposed extension of the CIE recommendation on “Uniform color spaces, color difference equations, and metric color terms”. J. Opt. Soc. Am. 1976, 66, 866–867. [Google Scholar] [CrossRef]
- Imirzalioglu, P.; Karacaer, O.; Yilmaz, B.; Ozmen Msc, I. Color stability of denture acrylic resins and a soft lining material against tea, coffee, and nicotine. J. Prosthodont. 2010, 19, 118–124. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Rocas, I.N.; Siqueira, J.F., Jr.; Santos, K.R. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef]
- Miranda, T.T.; Vianna, C.R.; Rodrigues, L.; Rosa, C.A.; Correa, A., Jr. Differential Proteinase Patterns among Candida albicans Strains Isolated from Root Canal and Lingual Dorsum: Possible Roles in Periapical Disease. J. Endod. 2015, 41, 841–845. [Google Scholar] [CrossRef]
- Mergoni, G.; Percudani, D.; Lodi, G.; Bertani, P.; Manfredi, M. Prevalence of Candida Species in Endodontic Infections: Systematic Review and Meta-analysis. J. Endod. 2018, 44, 1616–1625.e9. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. In vitro growth and analysis of Candida biofilms. Nat. Protoc. 2008, 3, 1909–1924. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, X.; Lin, D.; Chen, Y.; Tong, Z. The Starvation Resistance and Biofilm Formation of Enterococcus faecalis in Coexistence with Candida albicans, Streptococcus gordonii, Actinomyces viscosus, or Lactobacillus acidophilus. J. Endod. 2016, 42, 1233–1238. [Google Scholar] [CrossRef]
- St Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef]
- Ozok, A.R.; Wu, M.K.; Luppens, S.B.; Wesselink, P.R. Comparison of growth and susceptibility to sodium hypochlorite of mono- and dual-species biofilms of Fusobacterium nucleatum and Peptostreptococcus (micromonas) micros. J. Endod. 2007, 33, 819–822. [Google Scholar] [CrossRef]
- Jiang, Q.; Jing, Q.; Ren, B.; Cheng, L.; Zhou, X.; Lai, W.; He, J.; Li, M. Culture Supernatant of Enterococcus faecalis Promotes the Hyphal Morphogenesis and Biofilm Formation of Candida albicans. Pathogens 2022, 11, 1177. [Google Scholar] [CrossRef]
- Neelakantan, P.; Vishwanath, V.; Taschieri, S.; Corbella, S. Present status and future directions: Minimally invasive root canal preparation and periradicular surgery. Int. Endod. J. 2022, 55 (Suppl. S4), 845–871. [Google Scholar] [CrossRef]
- Călin, C.; Focșăneanu, A.M.; Paulsen, F.; Didilescu, A.C.; Niță, T. Shaping Efficiency of Rotary and Reciprocating Kinematics of Engine-driven Nickel-Titanium Instruments in Moderate and Severely curved Root Canals Using Microcomputed Tomography: A Systematic Review of Ex Vivo Studies. J. Endod. 2024, 50, 907–924. [Google Scholar] [CrossRef]
- Stringheta, C.P.; Bueno, C.E.S.; Kato, A.S.; Freire, L.G.; Iglecias, E.F.; Santos, M.; Pelegrine, R.A. Micro-computed tomographic evaluation of the shaping ability of four instrumentation systems in curved root canals. Int. Endod. J. 2019, 52, 908–916. [Google Scholar] [CrossRef]
- Chaniotis, A.; Ordinola-Zapata, R. Present status and future directions: Management of curved and calcified root canals. Int. Endod. J. 2022, 55 (Suppl. S3), 656–684. [Google Scholar] [CrossRef]
- Fan, B.; Pan, Y.; Gao, Y.; Fang, F.; Wu, Q.; Gutmann, J.L. Three-dimensional morphologic analysis of isthmuses in the mesial roots of mandibular molars. J. Endod. 2010, 36, 1866–1869. [Google Scholar] [CrossRef]
- Vera, J.; Hernandez, E.M.; Romero, M.; Arias, A.; van der Sluis, L.W. Effect of maintaining apical patency on irrigant penetration into the apical two millimeters of large root canals: An in vivo study. J. Endod. 2012, 38, 1340–1343. [Google Scholar] [CrossRef]
- Ruksakiet, K.; Hanak, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-Ngoen, T.; Garami, A.; Miko, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041.e7. [Google Scholar] [CrossRef]
- Goncalves, L.S.; Rodrigues, R.C.; Andrade Junior, C.V.; Soares, R.G.; Vettore, M.V. The Effect of Sodium Hypochlorite and Chlorhexidine as Irrigant Solutions for Root Canal Disinfection: A Systematic Review of Clinical Trials. J. Endod. 2016, 42, 527–532. [Google Scholar] [CrossRef]
- Dotto, L.; Sarkis Onofre, R.; Bacchi, A.; Rocha Pereira, G.K. Effect of Root Canal Irrigants on the Mechanical Properties of Endodontically Treated Teeth: A Scoping Review. J. Endod. 2020, 46, 596–604.e3. [Google Scholar] [CrossRef]
- Mostafa, M.; El-Shrief, Y.A.I.; Anous, W.I.O.; Hassan, M.W.; Salamah, F.T.A.; El Boghdadi, R.M.; El-Bayoumi, M.A.A.; Seyam, R.M.; Abd-El-Kader, K.G.; Amin, S.A.W. Postoperative pain following endodontic irrigation using 1.3% versus 5.25% sodium hypochlorite in mandibular molars with necrotic pulps: A randomized double-blind clinical trial. Int. Endod. J. 2020, 53, 154–166. [Google Scholar] [CrossRef]
- Abuhaimed, T.S.; Abou Neel, E.A. Sodium Hypochlorite Irrigation and Its Effect on Bond Strength to Dentin. Biomed. Res. Int. 2017, 2017, 1930360. [Google Scholar] [CrossRef]
- Sharma, S.; Sonali, A.; Udhey, C.; Kour, P.; Chauchan, M. A literature review on complications and management of use sodium hypochlorite in endodontics. IP Indian. J. Conserv. Endod. 2021, 6, 191–193. [Google Scholar] [CrossRef]
- Dutner, J.; Mines, P.; Anderson, A. Irrigation trends among American Association of Endodontists members: A web-based survey. J. Endod. 2012, 38, 37–40. [Google Scholar] [CrossRef]
- Malki, M.; Verhaagen, B.; Jiang, L.M.; Nehme, W.; Naaman, A.; Versluis, M.; Wesselink, P.; van der Sluis, L. Irrigant flow beyond the insertion depth of an ultrasonically oscillating file in straight and curved root canals: Visualization and cleaning efficacy. J. Endod. 2012, 38, 657–661. [Google Scholar] [CrossRef]
- Wieliczka, D.M.; Weng, S.; Querry, M.R. Wedge shaped cell for highly absorbent liquids: Infrared optical constants of water. Appl. Opt. 1989, 28, 1714–1719. [Google Scholar] [CrossRef]
- Puleio, F.; Lizio, A.S.; Coppini, V.; Lo Giudice, R.; Lo Giudice, G. CBCT-Based Assessment of Vapor Lock Effects on Endodontic Disinfection. Appl. Sci. 2023, 13, 9542. [Google Scholar] [CrossRef]
- Beck, F.; Ilie, N. Riboflavin and Its Effect on Dentin Bond Strength: Considerations for Clinical Applicability-An In Vitro Study. Bioengineering 2022, 9, 34. [Google Scholar] [CrossRef]
- Eusufzai, S.Z.; Barman, A.; Jamayet, N.B.; Ahmad, W.; Mahdi, S.S.; Sheikh, Z.; Daood, U. Effects of Riboflavin Collagen Crosslinker on Dentin Adhesive Bonding Efficiency: A Systematic Review and Meta-Analysis. Materials 2023, 16, 1701. [Google Scholar] [CrossRef]
- Alkahtany, M.F. Efficacy of curcumin-mediated photodynamic therapy for root canal therapy procedures: A systematic review. Photodiagn. Photodyn. Ther. 2023, 41, 103252. [Google Scholar] [CrossRef]
- Moradi, M.; Fazlyab, M.; Pourhajibagher, M.; Chiniforush, N. Antimicrobial action of photodynamic therapy on Enterococcus faecalis biofilm using curing light, curcumin and riboflavin. Aust. Endod. J. 2022, 48, 274–282. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Parker, S.; Chiniforush, N. Synergistic Antimicrobial Effect of Photodynamic Inactivation and SWEEPS in Combined Treatment against Enterococcus faecalis in a Root Canal Biofilm Model: An In Vitro Study. Appl. Sci. 2023, 13, 5668. [Google Scholar] [CrossRef]
- Rostami, G.; Afrasiabi, S.; Benedicenti, S.; Signore, A.; Chiniforush, N. The Evaluation of SWEEPS Plus Antimicrobial Photodynamic Therapy with Indocyanine Green in Eliminating Enterococcus faecalis Biofilm from Infected Root Canals: An In Vitro Study. Biomedicines 2023, 11, 1850. [Google Scholar] [CrossRef]
- Costa, L.M.; Matos Fde, S.; Correia, A.M.; Carvalho, N.C.; Faria, E.S.A.L.; Paranhos, L.R.; Ribeiro, M.A. Tooth color change caused by photosensitizers after photodynamic therapy: An in vitro study. J. Photochem. Photobiol. B 2016, 160, 225–228. [Google Scholar] [CrossRef]
- Noie, F.; O’Keefe, K.L.; Powers, J.M. Color stability of resin cements after accelerated aging. Int. J. Prosthodont. 1995, 8, 51–55. [Google Scholar]
- Johnston, W.M.; Kao, E.C. Assessment of appearance match by visual observation and clinical colorimetry. J. Dent. Res. 1989, 68, 819–822. [Google Scholar] [CrossRef]
- Kuehni, R.G.; Marcus, R.T. An Experiment in Visual Scaling of Small Color Differences. Color. Res. Appl. 1979, 4, 83–91. [Google Scholar] [CrossRef]
- de Araujo, L.P.; Marchesin, A.R.; Gobbo, L.B.; da Rosa, W.L.O.; Soares, A.J.; de Almeida, J.F.A.; Gomes, B.; Ferraz, C.C.R. Tooth color change after photodynamic therapy in endodontics: A systematic review. Photodiagn. Photodyn. Ther. 2023, 42, 103626. [Google Scholar] [CrossRef]
- Schneider, S.W. A comparison of canal preparations in straight and curved root canals. Oral. Surg. Oral. Med. Oral. Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef]
- Mustafa, R.; Al Omari, T.; Al-Nasrawi, S.; Al Fodeh, R.; Dkmak, A.; Haider, J. Evaluating In Vitro Performance of Novel Nickel-Titanium Rotary System (TruNatomy) Based on Debris Extrusion and Preparation Time from Severely Curved Canals. J. Endod. 2021, 47, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Sen, B.H.; Piskin, B.; Demirci, T. Observation of bacteria and fungi in infected root canals and dentinal tubules by SEM. Endod. Dent. Traumatol. 1995, 11, 6–9. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Chiniforush, N. Antibacterial potential of riboflavin mediated blue diode laser photodynamic inactivation against Enterococcus faecalis: A laboratory investigation. Photodiagn. Photodyn. Ther. 2023, 41, 103291. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Figueiredo, R.A.; Anami, L.C.; Mello, I.; Carvalho Edos, S.; Habitante, S.M.; Raldi, D.P. Tooth discoloration induced by endodontic phenothiazine dyes in photodynamic therapy. Photomed. Laser Surg. 2014, 32, 458–462. [Google Scholar] [CrossRef]
- Westland, S. Review of the CIE system of colorimetry and its use in dentistry. J. Esthet. Restor. Dent. 2003, 15 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef]

| Sample | Before Treatment | After Treatment | ΔE | |
|---|---|---|---|---|
| Rib 1 | L | 74.92 ± 1.78 | 75.48 ± 0.86 | 1.39 ± 0.67 |
| a | 1.98 ± 0.42 | 2.34 ± 0.34 | ||
| b | 17.38 ± 1.70 | 18.86 ± 1.52 | ||
| Rib 2 | L | 75.30 ± 1.06 | 77.10 ± 2.05 | 1.93 ± 0.99 |
| a | 0.10 ± 0.02 | 0.40 ± 0.04 | ||
| b | 19.00 ± 0.81 | 21.30 ± 1.80 | ||
| Rib 3 | L | 76.90 ± 0.67 | 75.20 ± 1.45 | 2.07 ± 0.79 |
| a | 0.70 ± 0.12 | 0.90 ± 0.25 | ||
| b | 19.80 ± 1.87 | 25.70 ± 1.05 | ||
| Rib 4 | L | 73.50 ± 1.34 | 75.30 ± 1.43 | 1.80 ± 0.14 |
| a | 2.70 ± 0.83 | 2.90 ± 0.26 | ||
| b | 13.50 ± 0.74 | 13.25 ± 0.92 | ||
| Rib 5 | L | 72.30 ± 0.92 | 73.80 ± 1.44 | 1.85 ± 0.52 |
| a | 2.70 ± 0.26 | 2.10 ± 0.51 | ||
| b | 16.30 ± 1.21 | 18.10 ± 1.48 | ||
| nCur 1 | L | 77.30 ± 1.38 | 77.10 ± 1.01 | 0.78 ± 0.37 |
| a | 0.10 ± 0.05 | 0.60 ± 0.03 | ||
| b | 17.10 ± 0.38 | 18.60 ± 0.79 | ||
| nCur 2 | L | 77.30 ± 0.84 | 77.00 ± 0.65 | 0.37 ± 0.19 |
| a | 0.90 ± 0.12 | 1.20 ± 0.08 | ||
| b | 17.50 ± 1.10 | 18.20 ± 1.08 | ||
| nCur 3 | L | 74.70 ± 1.76 | 76.40 ± 1.41 | 1.74 ± 0.35 |
| a | 2.20 ± 0.09 | 2.40 ± 0.05 | ||
| b | 16.50 ± 1.60 | 14.70 ± 1.03 | ||
| nCur 4 | L | 74.30 ± 1.19 | 74.40 ± 1.34 | 0.63 ± 0.16 |
| a | 2.80 ± 0.44 | 2.50 ± 0.65 | ||
| b | 17.10 ± 1.07 | 15.30 ± 0.88 | ||
| nCur 5 | L | 74.00 ± 1.84 | 75.90 ± 0.99 | 1.92 ± 0.86 |
| a | 2.50 ± 0.45 | 2.00 ± 0.24 | ||
| b | 13.70 ± 0.68 | 14.2 ± 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guity, P.; Afrasiabi, S.; Shahi Ardakani, A.; Benedicenti, S.; Signore, A.; Chiniforush, N.; Nazari Moghaddam, K. SWEEPS-Assisted Antibacterial Photodynamic Therapy Against Dual-Species Biofilms in Mandibular Molars: An In Vitro Study. Pharmaceuticals 2025, 18, 558. https://doi.org/10.3390/ph18040558
Guity P, Afrasiabi S, Shahi Ardakani A, Benedicenti S, Signore A, Chiniforush N, Nazari Moghaddam K. SWEEPS-Assisted Antibacterial Photodynamic Therapy Against Dual-Species Biofilms in Mandibular Molars: An In Vitro Study. Pharmaceuticals. 2025; 18(4):558. https://doi.org/10.3390/ph18040558
Chicago/Turabian StyleGuity, Pargol, Shima Afrasiabi, Ali Shahi Ardakani, Stefano Benedicenti, Antonio Signore, Nasim Chiniforush, and Kiumars Nazari Moghaddam. 2025. "SWEEPS-Assisted Antibacterial Photodynamic Therapy Against Dual-Species Biofilms in Mandibular Molars: An In Vitro Study" Pharmaceuticals 18, no. 4: 558. https://doi.org/10.3390/ph18040558
APA StyleGuity, P., Afrasiabi, S., Shahi Ardakani, A., Benedicenti, S., Signore, A., Chiniforush, N., & Nazari Moghaddam, K. (2025). SWEEPS-Assisted Antibacterial Photodynamic Therapy Against Dual-Species Biofilms in Mandibular Molars: An In Vitro Study. Pharmaceuticals, 18(4), 558. https://doi.org/10.3390/ph18040558









