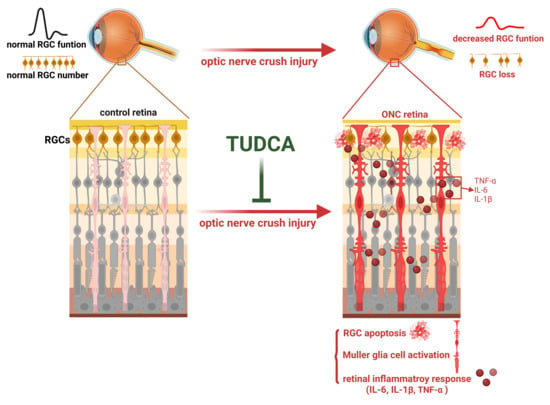
Tauroursodeoxycholic Acid Protects Retinal Ganglion Cells and Reduces Inflammation in Mice Following Optic Nerve Crush
Abstract
1. Introduction
2. Results
2.1. TUDCA Treatment Preserves RGC Function After Optic Nerve Injury
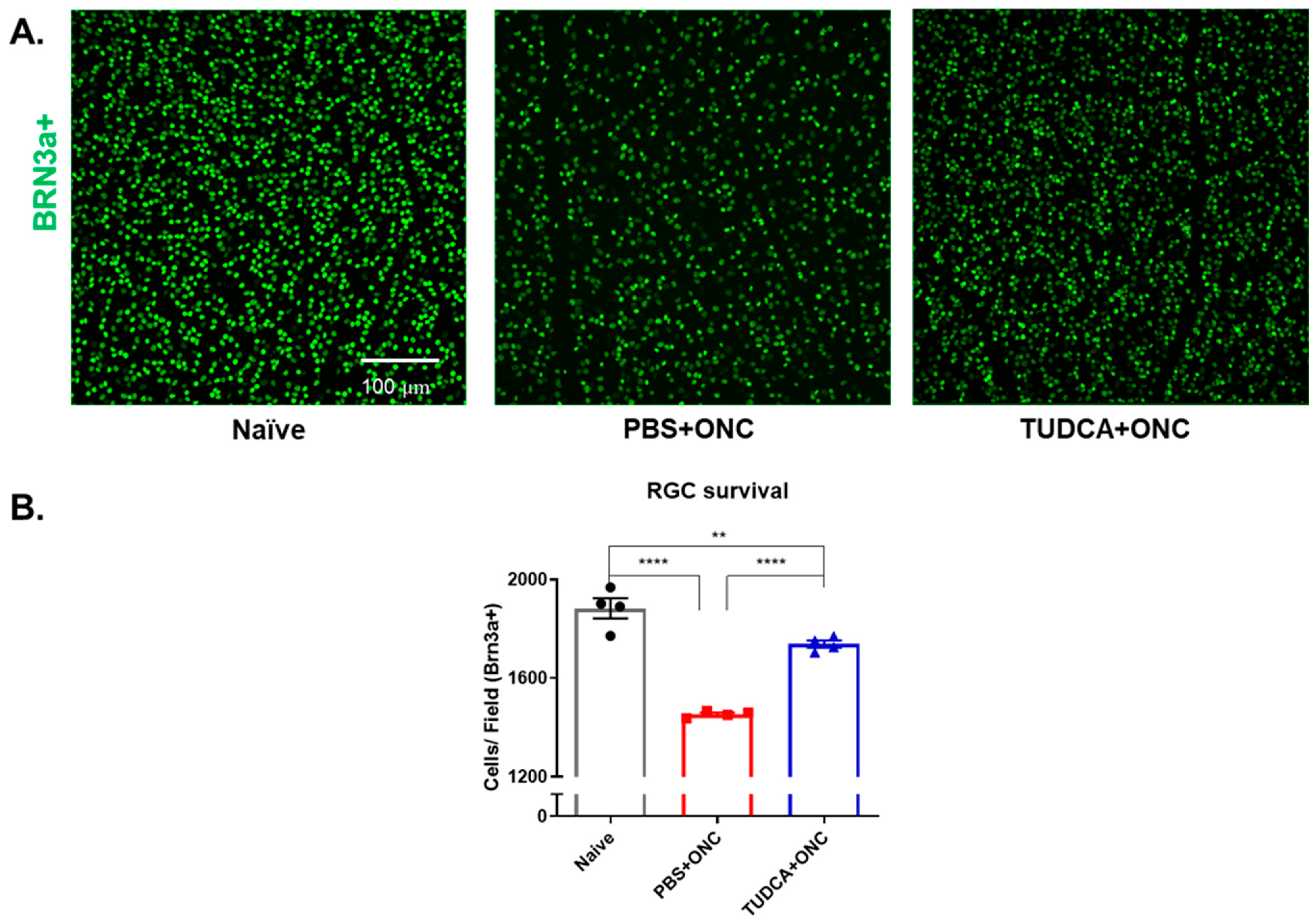
2.2. TUDCA Treatment Maintains RGC Survival After Optic Nerve Injury
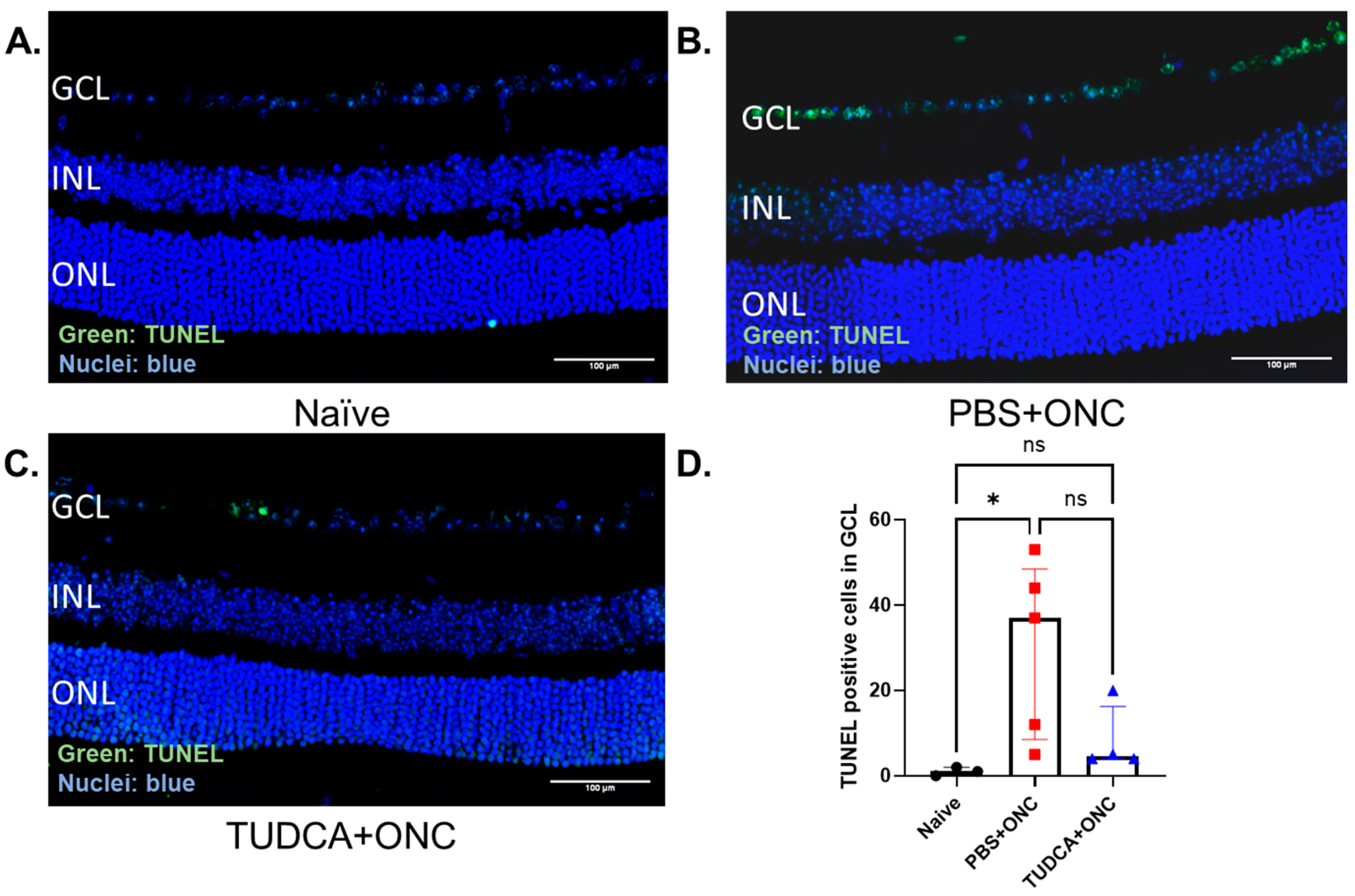
2.3. TUDCA Treatment Prevents ONC-Induced RGC Apoptosis
2.4. TUDCA Treatment Prevents Müller Cell Activation After Optic Nerve Injury
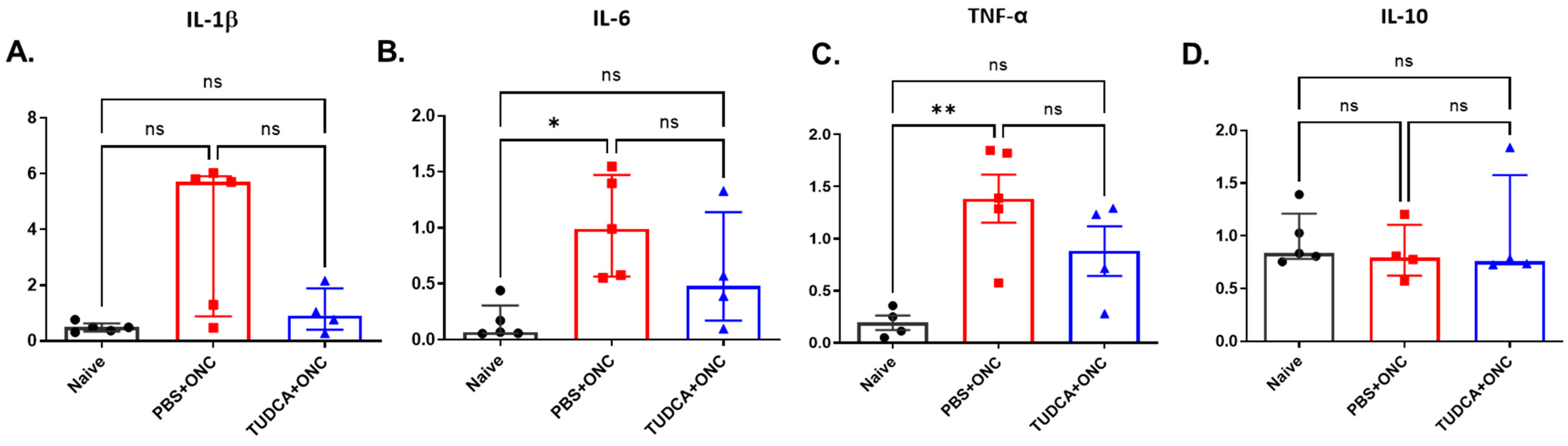
2.5. TUDCA Inhibits Inflammatory Response After Injury
3. Discussion
4. Materials and Methods
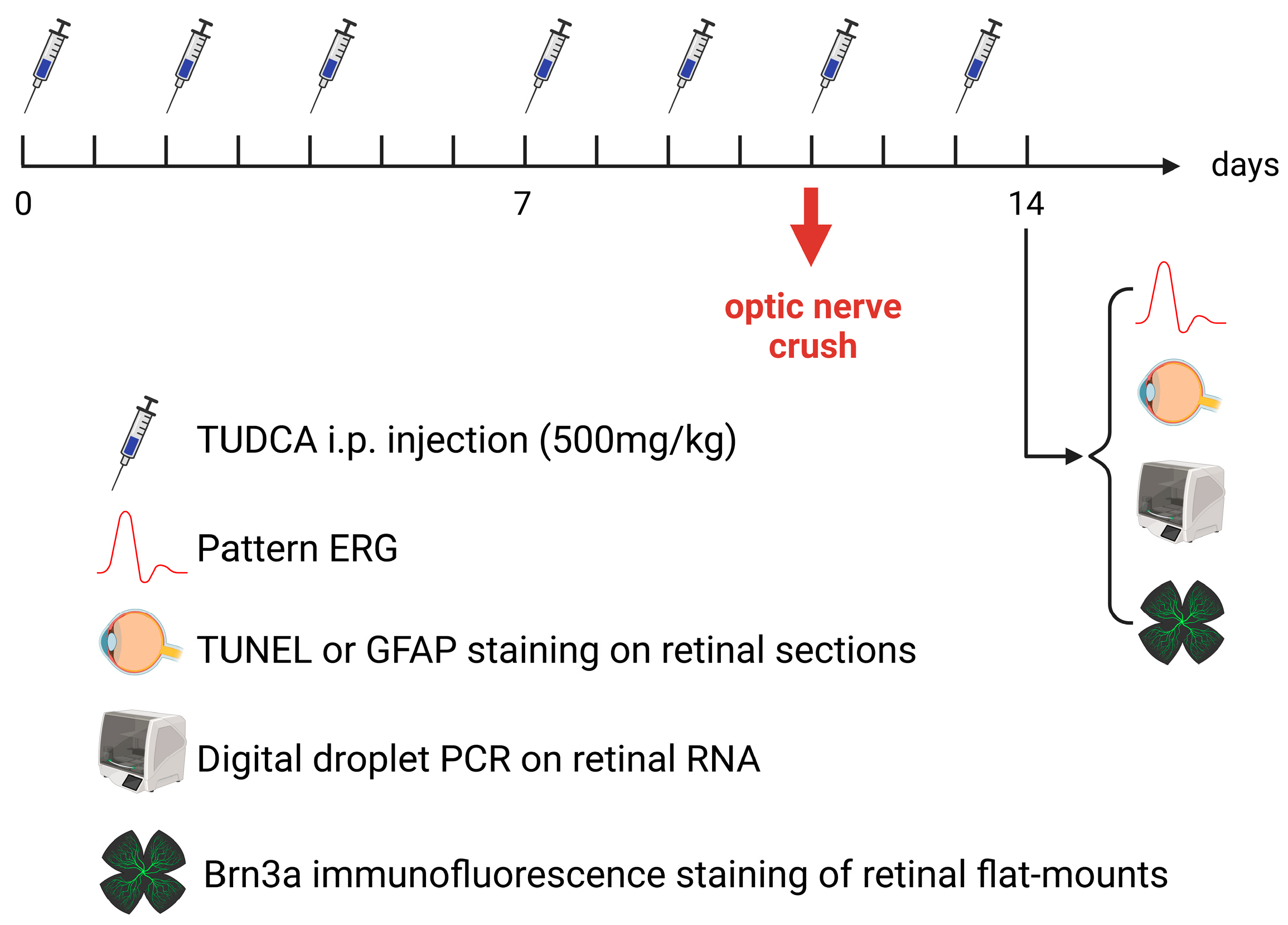
4.1. Experimental Design
4.2. Animals
4.3. Drugs
4.4. Optic Nerve Crush
4.5. Pattern ERG
4.6. RGC Quantification
4.7. Immunochemistry
4.8. TUNEL Assay
4.9. RNA Extraction and Digital Droplet PCR
4.10. Masking and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Gupta, D.; Chen, P.P. Glaucoma. Am. Fam. Physician 2016, 93, 668–674. [Google Scholar] [PubMed]
- Almasieh, M.; Levin, L.A. Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu. Rev. Vis. Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A. Glaucoma. Lancet 2011, 377, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Stankowska, D.L.; Ellis, D.Z.; Krishnamoorthy, R.R.; Yorio, T. Targets of Neuroprotection in Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 85–106. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Y.; Yao, K. Protection of retinal ganglion cells in glaucoma: Current status and future. Exp. Eye Res. 2021, 205, 108506. [Google Scholar] [CrossRef]
- Sarikcioglu, L.; Demir, N.; Demirtop, A. A standardized method to create optic nerve crush: Yasargil aneurysm clip. Exp. Eye Res. 2007, 84, 373–377. [Google Scholar] [CrossRef]
- Nuschke, A.C.; Farrell, S.R.; Levesque, J.M.; Chauhan, B.C. Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: Axon transport, injury and soma loss. Exp. Eye Res. 2015, 141, 111–124. [Google Scholar] [CrossRef]
- Cameron, E.G.; Xia, X.; Galvao, J.; Ashouri, M.; Kapiloff, M.S.; Goldberg, J.L. Optic Nerve Crush in Mice to Study Retinal Ganglion Cell Survival and Regeneration. Bio Protoc. 2020, 10, e3559. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, R.; Wu, J.; Xia, F.; Sun, Q.; Xu, J.; Liu, L. Low-dose carbon monoxide inhalation protects neuronal cells from apoptosis after optic nerve crush. Biochem. Biophys. Res. Commun. 2016, 469, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Qijun, Z.; Huan, Z.; Ling, G.; Kaijian, C.; Wei, L.; Shuxing, J.; Xiang, C.; Rongdi, Y.; Jian, Y. The levels and significance of inflammasomes in the mouse retina following optic nerve crush. Int. Immunopharmacol. 2019, 71, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Mac Nair, C.E.; Schlamp, C.L.; Montgomery, A.D.; Shestopalov, V.I.; Nickells, R.W. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J. Neuroinflamm. 2016, 13, 93. [Google Scholar] [CrossRef]
- Li, H.Y.; Ruan, Y.W.; Ren, C.R.; Cui, Q.; So, K.F. Mechanisms of secondary degeneration after partial optic nerve transection. Neural Regen. Res. 2014, 9, 565–574. [Google Scholar] [CrossRef]
- Tang, F.; Xu, F.; Cui, L.; Huang, W.; Jiang, L.; Chen, L.; Yan, W.; He, W.; Shen, C.; Huang, H.; et al. The expression and role of PIDD in retina after optic nerve crush. J. Mol. Histol. 2020, 51, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M. Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef]
- Khalaf, K.; Tornese, P.; Cocco, A.; Albanese, A. Tauroursodeoxycholic acid: A potential therapeutic tool in neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 33. [Google Scholar] [CrossRef]
- Keene, C.D.; Rodrigues, C.M.; Eich, T.; Chhabra, M.S.; Steer, C.J.; Low, W.C. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10671–10676. [Google Scholar] [CrossRef]
- Castro-Caldas, M.; Carvalho, A.N.; Rodrigues, E.; Henderson, C.J.; Wolf, C.R.; Rodrigues, C.M.; Gama, M.J. Tauroursodeoxycholic acid prevents MPTP-induced dopaminergic cell death in a mouse model of Parkinson’s disease. Mol. Neurobiol. 2012, 46, 475–486. [Google Scholar] [CrossRef]
- Pardue, M.T.; Allen, R.S. Neuroprotective strategies for retinal disease. Prog. Retin. Eye Res. 2018, 65, 50–76. [Google Scholar] [CrossRef]
- Daruich, A.; Picard, E.; Boatright, J.H.; Behar-Cohen, F. Review: The bile acids urso- and tauroursodeoxycholic acid as neuroprotective therapies in retinal disease. Mol. Vis. 2019, 25, 610–624. [Google Scholar] [PubMed]
- Zangerolamo, L.; Vettorazzi, J.F.; Solon, C.; Bronczek, G.A.; Engel, D.F.; Kurauti, M.A.; Soares, G.M.; Rodrigues, K.S.; Velloso, L.A.; Boschero, A.C.; et al. The bile acid TUDCA improves glucose metabolism in streptozotocin-induced Alzheimer’s disease mice model. Mol. Cell. Endocrinol. 2021, 521, 111116. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. TUDCA-Treated Mesenchymal Stem Cells Protect against ER Stress in the Hippocampus of a Murine Chronic Kidney Disease Model. Int. J. Mol. Sci. 2019, 20, 613. [Google Scholar] [CrossRef]
- Gao, X.; Fu, L.; Xiao, M.; Xu, C.; Sun, L.; Zhang, T.; Zheng, F.; Mei, C. The nephroprotective effect of tauroursodeoxycholic acid on ischaemia/reperfusion-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Basic Clin. Pharmacol. Toxicol. 2012, 111, 14–23. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Drack, A.V.; Dumitrescu, A.V.; Bhattarai, S.; Gratie, D.; Stone, E.M.; Mullins, R.; Sheffield, V.C. TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice. Investig. Ophthalmol. Vis. Sci. 2012, 53, 100–106. [Google Scholar] [CrossRef]
- Oveson, B.C.; Iwase, T.; Hackett, S.F.; Lee, S.Y.; Usui, S.; Sedlak, T.W.; Snyder, S.H.; Campochiaro, P.A.; Sung, J.U. Constituents of bile, bilirubin and TUDCA, protect against oxidative stress-induced retinal degeneration. J. Neurochem. 2011, 116, 144–153. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, L.; Bravo-Osuna, I.; Lax, P.; Arranz-Romera, A.; Maneu, V.; Esteban-Perez, S.; Pinilla, I.; Puebla-Gonzalez, M.D.M.; Herrero-Vanrell, R.; Cuenca, N. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS ONE 2017, 12, e0177998. [Google Scholar] [CrossRef]
- Tao, Y.; Dong, X.; Lu, X.; Qu, Y.; Wang, C.; Peng, G.; Zhang, J. Subcutaneous delivery of tauroursodeoxycholic acid rescues the cone photoreceptors in degenerative retina: A promising therapeutic molecule for retinopathy. Biomed. Pharmacother. 2019, 117, 109021. [Google Scholar] [CrossRef]
- Ketabforoush, A.; Faghihi, F.; Azedi, F.; Ariaei, A.; Habibi, M.A.; Khalili, M.; Ashtiani, B.H.; Joghataei, M.T.; Arnold, W.D. Sodium Phenylbutyrate and Tauroursodeoxycholic Acid: A Story of Hope Turned to Disappointment in Amyotrophic Lateral Sclerosis Treatment. Clin. Drug Investig. 2024, 44, 495–512. [Google Scholar] [CrossRef]
- Setchell, K.D.; Rodrigues, C.M.; Podda, M.; Crosignani, A. Metabolism of orally administered tauroursodeoxycholic acid in patients with primary biliary cirrhosis. Gut 1996, 38, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Aung, M.H.; Prunty, M.C.; Hanif, A.M.; Hutson, L.M.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic Acid Protects Retinal and Visual Function in a Mouse Model of Type 1 Diabetes. Pharmaceutics 2021, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.J.; Walker, T.A.; Choi, H.Y.; Faulkner, A.E.; Kim, M.K.; Sidney, S.S.; Boyd, A.P.; Nickerson, J.M.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic acid preservation of photoreceptor structure and function in the rd10 mouse through postnatal day 30. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.C.; Bhatia, S.K.; Han, M.K.; Aung, M.H.; Ciavatta, V.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic Acid Protects Retinal Function and Structure in rd1 Mice. Adv. Exp. Med. Biol. 2016, 854, 431–436. [Google Scholar] [CrossRef]
- Kitamura, Y.; Bikbova, G.; Baba, T.; Yamamoto, S.; Oshitari, T. In vivo effects of single or combined topical neuroprotective and regenerative agents on degeneration of retinal ganglion cells in rat optic nerve crush model. Sci. Rep. 2019, 9, 101. [Google Scholar] [CrossRef]
- Gomez-Vicente, V.; Lax, P.; Fernandez-Sanchez, L.; Rondon, N.; Esquiva, G.; Germain, F.; de la Villa, P.; Cuenca, N. Neuroprotective Effect of Tauroursodeoxycholic Acid on N-Methyl-D-Aspartate-Induced Retinal Ganglion Cell Degeneration. PLoS ONE 2015, 10, e0137826. [Google Scholar] [CrossRef]
- Xia, H.; Nan, Y.; Huang, X.; Gao, J.; Pu, M. Effects of Tauroursodeoxycholic Acid and Alpha-Lipoic-Acid on the Visual Response Properties of Cat Retinal Ganglion Cells: An In Vitro Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6638–6645. [Google Scholar] [CrossRef]
- Boatright, J.H.; Boyd, A.P.; Garcia–Valenzuela, E. Effect of Tauroursodeoxycholic Acid on Mouse Retinal Ganglion Cell Degeneration Following Optic Nerve Transection. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3180. [Google Scholar]
- Li, P.; Fu, D.; Sheng, Q.; Yu, S.; Bao, X.; Lv, Z. TUDCA attenuates intestinal injury and inhibits endoplasmic reticulum stress-mediated intestinal cell apoptosis in necrotizing enterocolitis. Int. Immunopharmacol. 2019, 74, 105665. [Google Scholar] [CrossRef]
- Deng, T.; Xie, J.; Ge, H.; Liu, Q.; Song, X.; Hu, L.; Meng, L.; Zhang, C. Tauroursodeoxycholic acid (TUDCA) enhanced intracytoplasmic sperm injection (ICSI) embryo developmental competence by ameliorating endoplasmic reticulum (ER) stress and inhibiting apoptosis. J. Assist. Reprod. Genet. 2020, 37, 119–126. [Google Scholar] [CrossRef]
- Yanguas-Casas, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramirez, L. TUDCA: An Agonist of the Bile Acid Receptor GPBAR1/TGR5 With Anti-Inflammatory Effects in Microglial Cells. J. Cell. Physiol. 2017, 232, 2231–2245. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Ma, J.; Feng, B.; Zhang, H.; Diehl, J.A.; Chin, Y.E.; Yan, W.; Xu, H. FFA-induced adipocyte inflammation and insulin resistance: Involvement of ER stress and IKKbeta pathways. Obesity 2011, 19, 483–491. [Google Scholar] [CrossRef]
- Yanguas-Casas, N.; Barreda-Manso, M.A.; Nieto-Sampedro, M.; Romero-Ramirez, L. Tauroursodeoxycholic acid reduces glial cell activation in an animal model of acute neuroinflammation. J. Neuroinflamm. 2014, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- Noailles, A.; Fernandez-Sanchez, L.; Lax, P.; Cuenca, N. Microglia activation in a model of retinal degeneration and TUDCA neuroprotective effects. J. Neuroinflamm. 2014, 11, 186. [Google Scholar] [CrossRef]
- Xing, C.; Huang, X.; Wang, D.; Yu, D.; Hou, S.; Cui, H.; Song, L. Roles of bile acids signaling in neuromodulation under physiological and pathological conditions. Cell Biosci. 2023, 13, 106. [Google Scholar] [CrossRef]
- Ramalho, R.M.; Ribeiro, P.S.; Sola, S.; Castro, R.E.; Steer, C.J.; Rodrigues, C.M. Inhibition of the E2F-1/p53/Bax pathway by tauroursodeoxycholic acid in amyloid beta-peptide-induced apoptosis of PC12 cells. J. Neurochem. 2004, 90, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ge, Z.; Li, N.; Yu, Z.; Wu, R.; Zhao, Y.; He, X.; Cai, G. TUDCA protects against tunicamycin-induced apoptosis of dorsal root ganglion neurons by suppressing activation of ER stress. Exp. Ther. Med. 2022, 24, 509. [Google Scholar] [CrossRef]
- Wu, H.; Yu, N.; Wang, X.; Yang, Y.; Liang, H. Tauroursodeoxycholic acid attenuates neuronal apoptosis via the TGR5/ SIRT3 pathway after subarachnoid hemorrhage in rats. Biol. Res. 2020, 53, 56. [Google Scholar] [CrossRef]
- Garcia-Valenzuela, E.; Gorczyca, W.; Darzynkiewicz, Z.; Sharma, S.C. Apoptosis in adult retinal ganglion cells after axotomy. J. Neurobiol. 1994, 25, 431–438. [Google Scholar] [CrossRef]
- Donahue, R.J.; Maes, M.E.; Grosser, J.A.; Nickells, R.W. BAX-Depleted Retinal Ganglion Cells Survive and Become Quiescent Following Optic Nerve Damage. Mol. Neurobiol. 2020, 57, 1070–1084. [Google Scholar] [CrossRef]
- Lewis, G.P.; Fisher, S.K. Up-regulation of glial fibrillary acidic protein in response to retinal injury: Its potential role in glial remodeling and a comparison to vimentin expression. Int. Rev. Cytol. 2003, 230, 263–290. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Muller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef]
- Zhao, T.T.; Tian, C.Y.; Yin, Z.Q. Activation of Muller cells occurs during retinal degeneration in RCS rats. Adv. Exp. Med. Biol. 2010, 664, 575–583. [Google Scholar] [CrossRef]
- Augustine, J.; Pavlou, S.; Ali, I.; Harkin, K.; Ozaki, E.; Campbell, M.; Stitt, A.W.; Xu, H.; Chen, M. IL-33 deficiency causes persistent inflammation and severe neurodegeneration in retinal detachment. J. Neuroinflamm. 2019, 16, 251. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Chrenek, M.A.; Girardot, P.E.; Wang, J.; Sellers, J.T.; Geisert, E.E.; Brenner, C.; Nickerson, J.M.; Boatright, J.H.; et al. Systemic Treatment with Nicotinamide Riboside Is Protective in Two Mouse Models of Retinal Ganglion Cell Damage. Pharmaceutics 2021, 13, 893. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.; Hoffmann, M.B. Update on the pattern electroretinogram in glaucoma. Optom. Vis. Sci. 2008, 85, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrosio, A.F.; Santiago, A.R. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef]
- Adornetto, A.; Russo, R.; Parisi, V. Neuroinflammation as a target for glaucoma therapy. Neural Regen. Res. 2019, 14, 391–394. [Google Scholar] [CrossRef]
- Wei, X.; Cho, K.S.; Thee, E.F.; Jager, M.J.; Chen, D.F. Neuroinflammation and microglia in glaucoma: Time for a paradigm shift. J. Neurosci. Res. 2019, 97, 70–76. [Google Scholar] [CrossRef]
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef]
- Wilson, G.N.; Inman, D.M.; Dengler Crish, C.M.; Smith, M.A.; Crish, S.D. Early pro-inflammatory cytokine elevations in the DBA/2J mouse model of glaucoma. J. Neuroinflamm. 2015, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.F.; Lin, S.; Geng, Z.; Luo, L.L.; Liu, Y.J.; Zhang, Z.; Liu, W.Y.; Chen, X.; Li, X.; Yan, J.; et al. Integrin CD11b Deficiency Aggravates Retinal Microglial Activation and RGCs Degeneration After Acute Optic Nerve Injury. Neurochem. Res. 2020, 45, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hondur, G.; Tezel, G. Antioxidant Treatment Limits Neuroinflammation in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2344–2354. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wu, S.; Zeng, W.; Chen, X.; Zheng, T.; Ren, J.; Ke, M. Protective Effects of Rapamycin on Trabecular Meshwork Cells in Glucocorticoid-Induced Glaucoma Mice. Front. Pharmacol. 2020, 11, 1006. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, R.K.; Miller, L.J.; Singh, P.K.; Kanwar, M. Muller glia in retinal innate immunity: A perspective on their roles in endophthalmitis. Crit. Rev. Immunol. 2013, 33, 119–135. [Google Scholar] [CrossRef]
- Zhang, T.; Baehr, W.; Fu, Y. Chemical chaperone TUDCA preserves cone photoreceptors in a mouse model of Leber congenital amaurosis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3349–3356. [Google Scholar] [CrossRef]
- Parrilla, G.E.; Vander Wall, R.; Chitranshi, N.; Basavarajappa, D.; Gupta, V.; Graham, S.L.; You, Y. RXR agonist, 9-cis-13,14-dihydroretinoic acid (9CDHRA), reduces damage and protects from demyelination in transsynaptic degeneration model. Neuroscience 2024, 559, 91–104. [Google Scholar] [CrossRef]
- Maciulaitiene, R.; Kalesnykas, G.; Pauza, D.H.; Januleviciene, I. A combination of topical and systemic administration of brimonidine is neuroprotective in the murine optic nerve crush model. PLoS ONE 2024, 19, e0308671. [Google Scholar] [CrossRef]
- Taniguchi, T.; Sharif, N.A.; Ota, T.; Farjo, R.A.; Rausch, R. Assessment of Brain-Derived Neurotrophic Factor on Retinal Structure and Visual Function in Rodent Models of Optic Nerve Crush. Pharmaceuticals 2024, 17, 798. [Google Scholar] [CrossRef]
- Boatright, J.H.; Moring, A.G.; McElroy, C.; Phillips, M.J.; Do, V.T.; Chang, B.; Hawes, N.L.; Boyd, A.P.; Sidney, S.S.; Stewart, R.E.; et al. Tool from ancient pharmacopoeia prevents vision loss. Mol. Vis. 2006, 12, 1706–1714. [Google Scholar] [PubMed]
- Li, Y.; Struebing, F.L.; Wang, J.; King, R.; Geisert, E.E. Different Effect of Sox11 in Retinal Ganglion Cells Survival and Axon Regeneration. Front. Genet. 2018, 9, 633. [Google Scholar] [CrossRef] [PubMed]
- Talla, V.; Koilkonda, R.; Porciatti, V.; Chiodo, V.; Boye, S.L.; Hauswirth, W.W.; Guy, J. Complex I subunit gene therapy with NDUFA6 ameliorates neurodegeneration in EAE. Invest. Ophthalmol. Vis. Sci. 2015, 56, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Miura, G.; Wang, M.H.; Ivers, K.M.; Frishman, L.J. Retinal pathway origins of the pattern ERG of the mouse. Exp. Eye Res. 2009, 89, 49–62. [Google Scholar] [CrossRef]
- Carpenter, A.E.; Jones, T.R.; Lamprecht, M.R.; Clarke, C.; Kang, I.H.; Friman, O.; Guertin, D.A.; Chang, J.H.; Lindquist, R.A.; Moffat, J.; et al. CellProfiler: Image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006, 7, R100. [Google Scholar] [CrossRef]
- Dordea, A.C.; Bray, M.A.; Allen, K.; Logan, D.J.; Fei, F.; Malhotra, R.; Gregory, M.S.; Carpenter, A.E.; Buys, E.S. An open-source computational tool to automatically quantify immunolabeled retinal ganglion cells. Exp. Eye Res. 2016, 147, 50–56. [Google Scholar] [CrossRef]
- Mary-Sinclair, M.N.; Wang, X.; Swanson, D.J.; Sung, C.Y.; Mendonca, E.A.; Wroblewski, K.; Baumer, S.H.; Goldowitz, D.; Jablonski, M.M.; Skapek, S.X. Varied manifestations of persistent hyperplastic primary vitreous with graded somatic mosaic deletion of a single gene. Mol. Vis. 2014, 20, 215–230. [Google Scholar]
- Zhang, X.; Henneman, N.F.; Girardot, P.E.; Sellers, J.T.; Chrenek, M.A.; Li, Y.; Wang, J.; Brenner, C.; Nickerson, J.M.; Boatright, J.H. Systemic Treatment With Nicotinamide Riboside Is Protective in a Mouse Model of Light-Induced Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 47. [Google Scholar] [CrossRef]
- Sellers, J.T.; Chrenek, M.A.; Girardot, P.E.; Nickerson, J.M.; Pardue, M.T.; Boatright, J.H. Initial Assessment of Lactate as Mediator of Exercise-Induced Retinal Protection. Adv. Exp. Med. Biol. 2019, 1185, 451–455. [Google Scholar]
- Mees, L.M.; Coulter, M.M.; Chrenek, M.A.; Motz, C.T.; Landis, E.G.; Boatright, J.H.; Pardue, M.T. Low-Intensity Exercise in Mice Is Sufficient to Protect Retinal Function During Light-Induced Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1328–1335. [Google Scholar] [CrossRef]
- Getz, T.E.; Chrenek, M.A.; Papania, J.T.; Shelton, D.A.; Markand, S.; Iuvone, P.M.; Kozmik, Z.; Boatright, J.H.; Nickerson, J.M. Conditional Knockouts of Interphotoreceptor Retinoid Binding Protein Suggest Two Independent Mechanisms for Retinal Degeneration and Myopia. Investig. Ophthalmol. Vis. Sci. 2024, 65, 32. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Li, Y.; Zhang, X.; Chrenek, M.A.; Wang, J.; Girardot, P.E.; Sellers, J.T.; Geisert, E.E.; Nickerson, J.M.; Boatright, J.H. Tauroursodeoxycholic Acid Protects Retinal Ganglion Cells and Reduces Inflammation in Mice Following Optic Nerve Crush. Pharmaceuticals 2025, 18, 569. https://doi.org/10.3390/ph18040569
Zhang N, Li Y, Zhang X, Chrenek MA, Wang J, Girardot PE, Sellers JT, Geisert EE, Nickerson JM, Boatright JH. Tauroursodeoxycholic Acid Protects Retinal Ganglion Cells and Reduces Inflammation in Mice Following Optic Nerve Crush. Pharmaceuticals. 2025; 18(4):569. https://doi.org/10.3390/ph18040569
Chicago/Turabian StyleZhang, Nan, Ying Li, Xian Zhang, Micah A. Chrenek, Jiaxing Wang, Preston E. Girardot, Jana T. Sellers, Eldon E. Geisert, John M. Nickerson, and Jeffrey H. Boatright. 2025. "Tauroursodeoxycholic Acid Protects Retinal Ganglion Cells and Reduces Inflammation in Mice Following Optic Nerve Crush" Pharmaceuticals 18, no. 4: 569. https://doi.org/10.3390/ph18040569
APA StyleZhang, N., Li, Y., Zhang, X., Chrenek, M. A., Wang, J., Girardot, P. E., Sellers, J. T., Geisert, E. E., Nickerson, J. M., & Boatright, J. H. (2025). Tauroursodeoxycholic Acid Protects Retinal Ganglion Cells and Reduces Inflammation in Mice Following Optic Nerve Crush. Pharmaceuticals, 18(4), 569. https://doi.org/10.3390/ph18040569