Silkworm Cocoon—Derived Carbon Dots for Post-Trauma Hemostasis and Tissue Repair
Abstract
:1. Introduction
2. Results
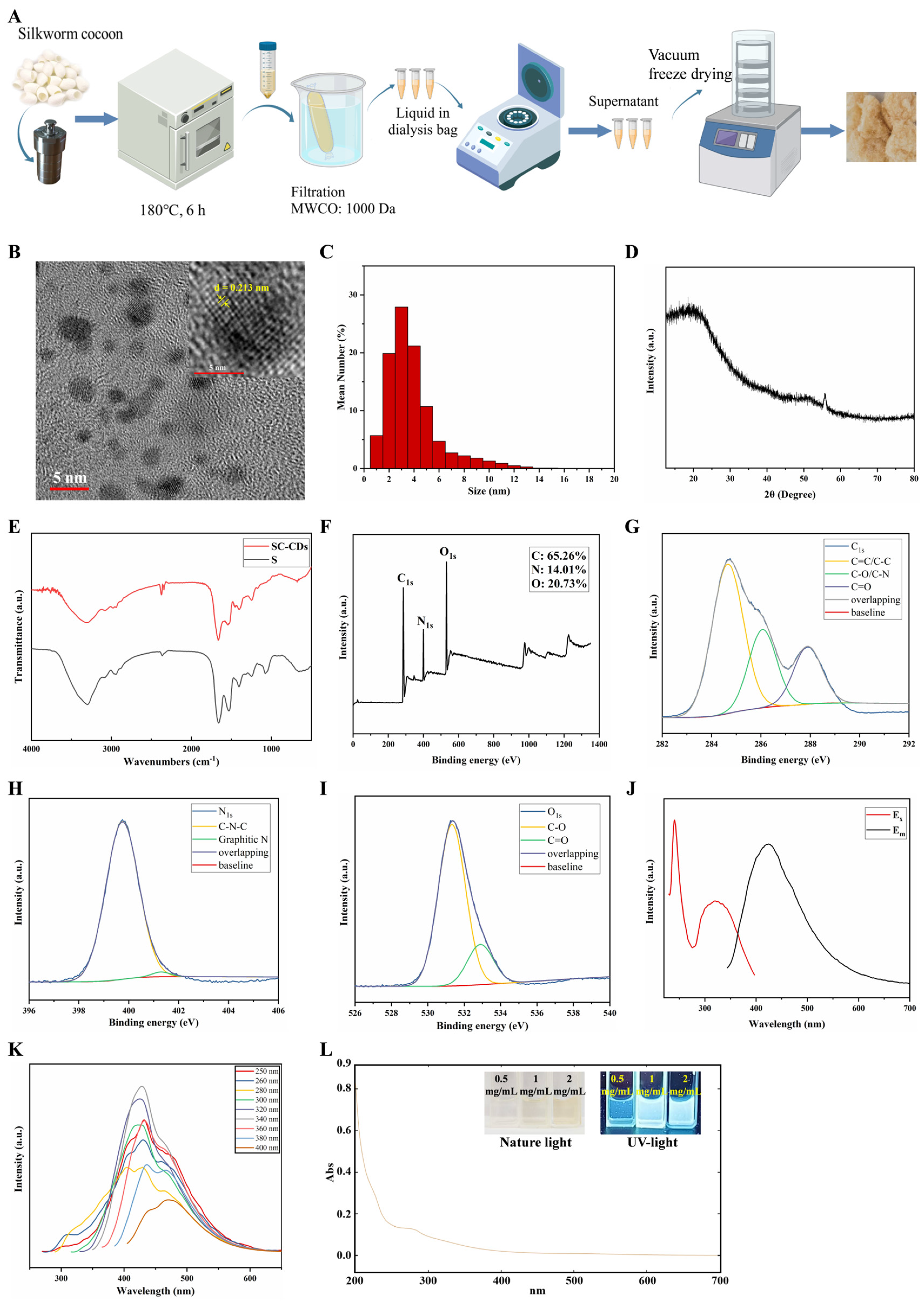
2.1. Synthesis and Characterization of SC-CDs
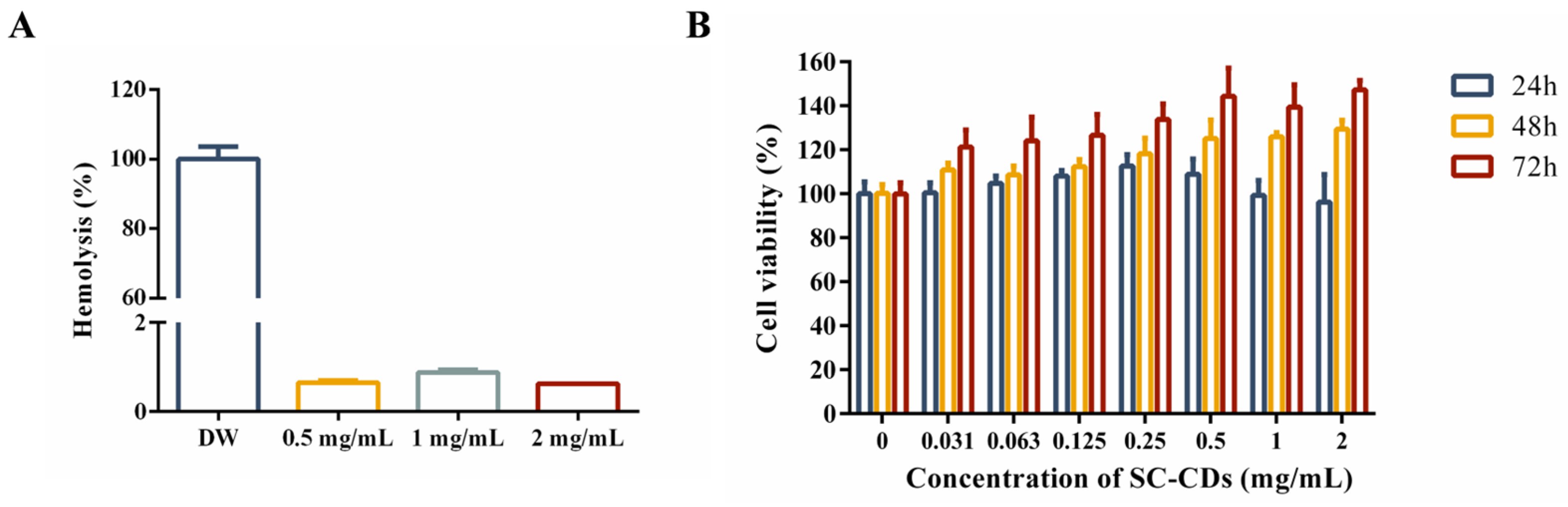
2.2. In Vitro Biocompatibility of SC-CDs
2.2.1. Hemocompatibility of SC-CDs
2.2.2. Cytotoxicity of SC-CDs in L929 Cells
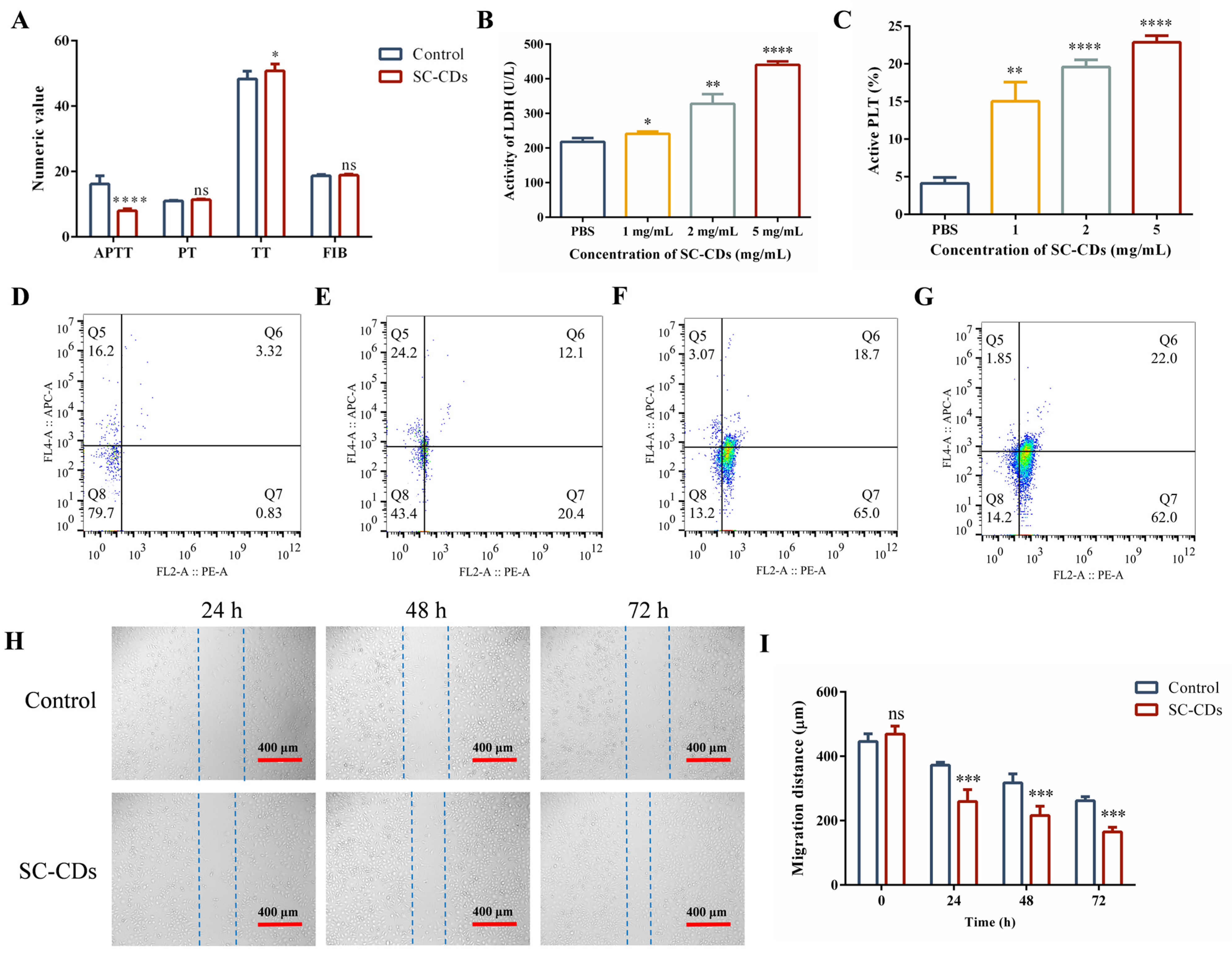
2.3. In Vitro Bioactivity of SC-CDs
2.3.1. In Vitro Hemostatic Activity
2.3.2. Cell Wound Scratch Assay
2.4. In Vivo Hemostatic Activity of SC-CDs
2.4.1. Hemostatic Effect of SC-CDs on Acute Hemorrhage in Rats
2.4.2. Coagulation Capacity in a Rat Model of Tail Amputation Hemorrhage
2.4.3. Hemostasis of SC-CDs in Snake Venom-Induced Bleeding
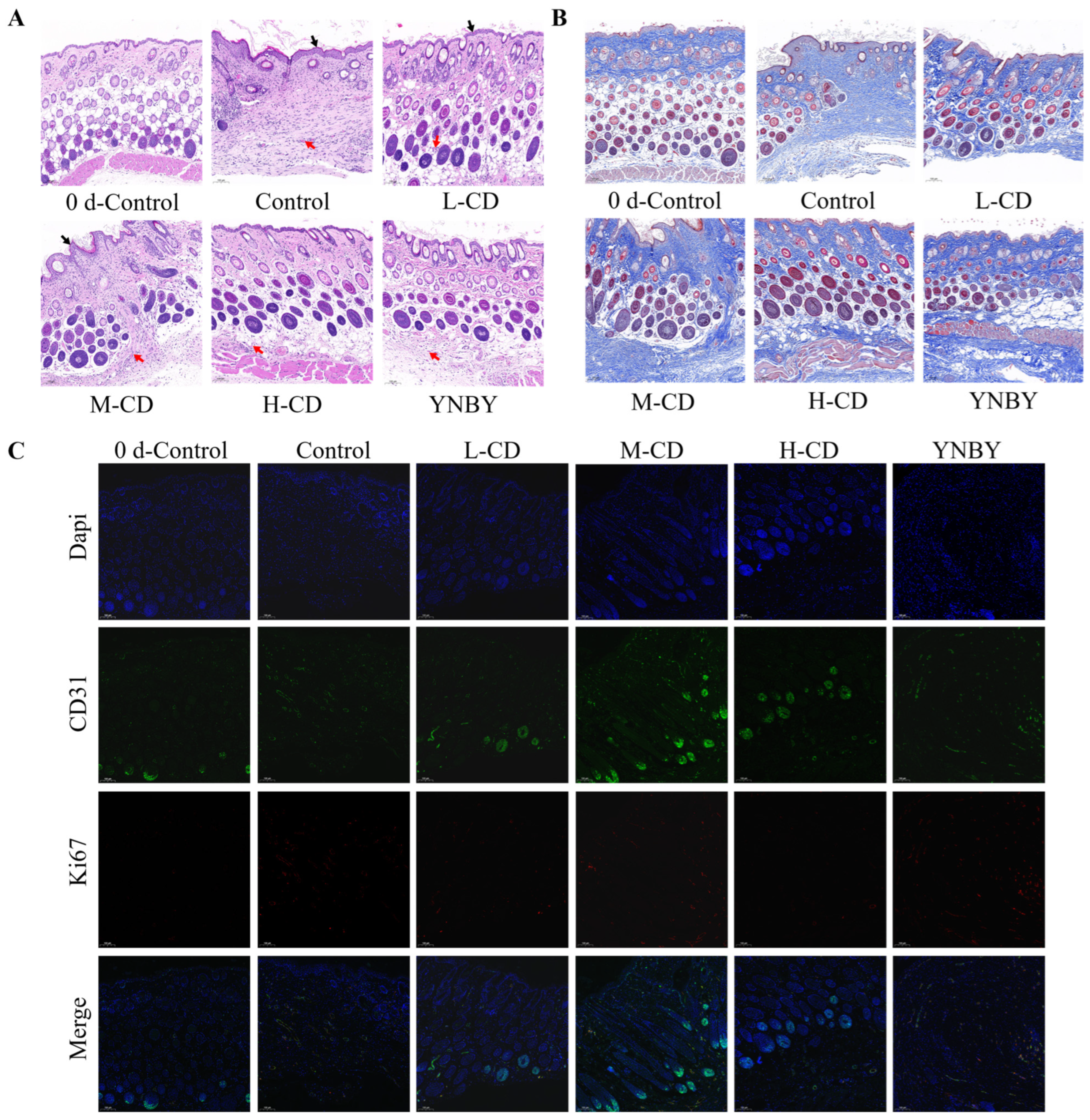
2.5. Wound-Healing Effect of SC-CDs
2.5.1. Change in Wound Area in a Mouse Model
2.5.2. HE Staining and Histopathological Scoring of the Wound Surface
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells and Animals
4.3. Preparation of SC-CDs
4.4. Characterization of SC-CDs
4.5. In Vitro Biocompatibility of SC-CDs
4.5.1. Hemocompatibility of SC-CDs
4.5.2. Cytotoxicity of SC-CDs in L929 Cells
4.6. In Vitro Bioactivity
4.6.1. In Vitro Hemostatic Activity
4.6.2. Cell Scratch Assay
4.7. In Vivo Hemostatic Activity
4.7.1. Rat Liver Injury Bleeding Model
4.7.2. Rat Tail Amputation Bleeding Model
4.7.3. Mouse Snake Venom Bleeding Model
4.8. In Vivo Wound-Healing Effect of SC-CDs
4.8.1. Complete Cortical Defect Model Construction
4.8.2. Wound Area Change in the Mouse Model
4.8.3. HE Staining and Histopathological Scoring of the Wound Surface
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SC-CDs | silkworm cocoon-derived carbon dots |
| APTT | activated partial thromboplastin time |
| PT | prothrombin time |
| TT | thrombin time |
| FIB | fibrinogen |
| UV | ultraviolet |
| FTIR | Fourier-transform infrared spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| LDH | lactate dehydrogenase |
| YNBY | Yunnan Baiyao |
| SV | snake venom |
| HC | hemocoagulase |
| HR-TEM | high-resolution transmission electron microscopy |
| QY | quantum yield |
| ROS | reactive oxygen species |
| DMEM | Dulbecco’s Modified Eagle Medium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DMSO | dimethyl sulfoxide |
References
- Shamloo, A.; Sarmadi, M.; Aghababaie, Z.; Vossoughi, M. Accelerated full-thickness wound healing via sustained bFGF delivery based on a PVA/chitosan/gelatin hydrogel incorporating PCL microspheres. Int. J. Pharm. 2018, 537, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Liao, S.; Kong, Y.; Wang, Q.; Wei, Q. In situ biosynthetic BC/zeolite hybrid hemostat for quick clot. J. Appl. Polym. Sci. 2023, 140, e54315. [Google Scholar] [CrossRef]
- Liang, Y.P.; Xu, C.C.; Li, G.F.; Liu, T.C.; Liang, J.F.; Wang, X. Graphene-kaolin composite sponge for rapid and riskless hemostasis. Colloids Surf. B-Biointerfaces 2018, 169, 168–175. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.J.; Chen, S.Q. Effect of chitosan molecular weight and deacetylation degree on Hemostasis. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2008, 84B, 131–137. [Google Scholar] [CrossRef]
- Battistini, A.; Gottlieb, L.J.; Vrouwe, S.Q. Topical Hemostatic Agents in Burn Surgery: A Systematic Review. J. Burn Care Res. 2023, 44, 262–273. [Google Scholar] [CrossRef]
- Wu, G.J.; Mazzitelli, B.A.; Quek, A.J.; Veldman, M.J.; Conroy, P.J.; Caradoc-Davies, T.T.; Ooms, L.M.; Tuck, K.L.; Schoenecker, J.G.; Whisstock, J.C.; et al. Tranexamic acid is an active site inhibitor of urokinase plasminogen activator. Blood Adv. 2019, 3, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Damén, T.; Dellborg, M.; Jeppsson, A.; Nygren, A. Intraoperative infusion of noradrenaline improves platelet aggregation in patients undergoing coronary artery bypass grafting: A randomized controlled trial. J. Thromb. Haemost. 2019, 17, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The Effect of Comorbidities on Wound Healing. Surg. Clin. North Am. 2020, 100, 695–705. [Google Scholar] [CrossRef]
- Miao, R.P.; Meng, Q.G.; Wang, C.N.; Yuan, W.J. Bibliometric Analysis of Network Pharmacology in Traditional Chinese Medicine. Evid.-Based Complement. Altern. Med. 2022, 2022, 1583773. [Google Scholar] [CrossRef]
- Brouns, S.L.N.; van Geffen, J.P.; Campello, E.; Swieringa, F.; Spiezia, L.; van Oerle, R.; Provenzale, I.; Verdoold, R.; Farndale, R.W.; Clemetson, K.J.; et al. Platelet-primed interactions of coagulation and anticoagulation pathways in flow-dependent thrombus formation. Sci. Rep. 2020, 10, 11910. [Google Scholar] [CrossRef]
- Latha, B.; Ramakrishnan, M.; Jayaraman, V.; Babu, M. Physicochemical properties of extracellular matrix proteins in post-burn human granulation tissue. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 1999, 124, 241–249. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Yazdi, M.K.; Ghavami, S.B.; Farokhimanesh, S.; Amirabad, L.M.; Zarrintaj, P.; Saeb, M.R.; Hamblin, M.R.; Zare, M.; Mozafari, M. Mesenchymal Stem Cell Spheroids Embedded in an Injectable Thermosensitive Hydrogel: An In Situ Drug Formation Platform for Accelerated Wound Healing. ACS Biomater. Sci. Eng. 2020, 6, 5096–5109. [Google Scholar] [CrossRef] [PubMed]
- Heilborn, J.D.; Nilsson, M.F.; Kratz, G.; Weber, G.; Sorensen, O.; Borregaard, N.; Ståhle-Bäckdahl, M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Investig. Dermatol. 2003, 120, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, L.; Rybka, M.; Jurak, J.; Frankowski, J.; Konop, M. Silk Sericin and Its Effect on Skin Wound Healing: A State of the Art. Macromol. Biosci. 2024, 24, e2400145. [Google Scholar] [CrossRef]
- Sultan, M.T.; Hong, H.; Lee, O.J.; Ajiteru, O.; Lee, Y.J.; Lee, J.S.; Lee, H.; Kim, S.H.; Park, C.H. Silk Fibroin-Based Biomaterials for Hemostatic Applications. Biomolecules 2022, 12, 660. [Google Scholar] [CrossRef]
- Kong, Y.; Sun, Q.; Zhao, Q.; Zhang, Y.Q. Purification and Characterization of a Novel Antiplatelet Peptide from Deinagkistrodon acutus Venom. Toxins 2018, 10, 332. [Google Scholar] [CrossRef]
- Li, Y.W.; Wu, T.F.; Zhang, G.Z.; Fang, A.; Li, Y.R.; Wang, S.S.; Yan, H.; Liang, P.S.; Lian, J.L.; Zhang, Y.S. A native sericin wound dressing spun directly from silkworms enhances wound healing. Colloids Surf. B-Biointerfaces 2023, 225, 113228. [Google Scholar] [CrossRef]
- Yang, X.; Li, P.L.; Tang, W.T.; Du, S.K.; Yu, M.Z.; Lu, H.J.; Tan, H.P.; Xing, X.D. A facile injectable carbon dot/oxidative polysaccharide hydrogel with potent self-healing and high antibacterial activity. Carbohydr. Polym. 2021, 251, 117040. [Google Scholar] [CrossRef]
- Li, M.; Gong, J.J.; Yu, Y.X.; Xu, J.M.; Yin, Y.; Wang, A.Q.; Wang, J.N. Sericin/silk fibroin composite aerogel for hemostatic application. Appl. Mater. Today 2024, 41, 102514. [Google Scholar] [CrossRef]
- Chang, J.J.; Zhao, Y.; Xu, J.W. Conformational, physiochemical, and functional properties of quinoa protein isolate influenced by thermal treatment. J. Food Sci. 2025, 90, e70051. [Google Scholar] [CrossRef]
- Ge, W.X.; Xiao, Z.X.; Ding, X.R.; Bi, W.T.; Chen, D.D.Y. Deep eutectic system enhanced oat protein extraction. J. Food Sci. 2025, 90, e17645. [Google Scholar] [CrossRef] [PubMed]
- Aad, R.; Dragojlov, I.; Vesentini, S. Sericin Protein: Structure, Properties, and Applications. J. Funct. Biomater. 2024, 15, 322. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.Z.; Chen, H.L.; Shen, H.M.; Wang, Z.; Zhang, C. Functionalization and Application of Polymer-Modified Proteins. Prog. Chem. 2024, 36, 416–429. [Google Scholar] [CrossRef]
- Tang, X.X.; Gong, Z.; Lang, Y.; Chen, H.Y.; Huang, S.Q.; Lv, Y.G. Research Progress Towards and Prospects of Carbon Dots Derived from Tea and Chinese Medicinal Materials. Nanomaterials 2025, 15, 171. [Google Scholar] [CrossRef]
- Li, X.Y.; Kang, C.Y.; Tao, S.Y.; Yang, B. A new nanomaterial-Carbonized polymer dots and their applications: A review. Carbon 2025, 237, 120122. [Google Scholar] [CrossRef]
- Liu, Y.S.; Li, W.; Wu, P.; Liu, S.X. Preparation and Applications of Carbon Quantum Dots Prepared via Hydrothermal Carbonization Method. Prog. Chem. 2018, 30, 349–364. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.L.; Cai, H.J.; Qu, X.L.; Chang, J.B.; Waterhouse, G.I.N.; Lu, S.Y. Biomass-derived carbon dots with pharmacological activity for biomedicine: Recent advances and future perspectives. Sci. Bull. 2024, 69, 3127–3149. [Google Scholar] [CrossRef]
- Shen, L.M.; Liu, J. New development in carbon quantum dots technical applications. Talanta 2016, 156, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.T.; Wei, J.F.; Qiang, L.; Chen, X.; Meng, X.W. Fluorescent Carbon Dots for Bioimaging and Biosensing Applications. J. Biomed. Nanotechnol. 2014, 10, 2677–2699. [Google Scholar] [CrossRef]
- Ali, H.; Ghosh, S.; Jana, N.R. Fluorescent carbon dots as intracellular imaging probes. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2020, 12, e1617. [Google Scholar] [CrossRef]
- Bian, Z.Y.; Gomez, E.; Gruebele, M.; Levine, B.G.; Link, S.; Mehmood, A.; Nie, S.M. Bottom-up carbon dots: Purification, single-particle dynamics, and electronic structure. Chem. Sci. 2025, 16, 4195–4212. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.K.; Zhong, Y.S.; Fu, G.R.; Lai, W.X.; Pan, Z.W.; Yang, Y.L.; Chen, F.Y.; Yan, H.C. Microwave-Assisted Synthesis of Luminescent Carbonaceous Nanoparticles as Silkworm Feed for Fabricating Fluorescent Silkworm Silk. Coatings 2023, 13, 31. [Google Scholar] [CrossRef]
- Liang, P.; Bi, T.; Zhou, Y.; Wang, C.; Ma, Y.; Xu, H.; Shen, H.; Ren, W.; Yang, S. Carbonized Platycladus orientalis Derived Carbon Dots Accelerate Hemostasis through Activation of Platelets and Coagulation Pathways. Small 2023, 19, 2303498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, Y.; Yan, S.; Qian, S.; Wang, Y.; Ju, E.; Zhang, C. Herbal Medicine-Inspired Carbon Quantum Dots with Antibiosis and Hemostasis Effects for Promoting Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 8527–8537. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, M.; Zheng, X.; Tao, S.Y.; Zhang, Z.Q.; Sun, M.D.; Song, Y.B.; Zhang, J.; Shao, D.; He, K.; et al. Berberine-based carbon dots for selective and safe cancer theranostics. RSC Adv. 2018, 8, 1168–1173. [Google Scholar] [CrossRef]
- Du, T.; Liang, J.G.; Dong, N.; Liu, L.; Fang, L.R.; Xiao, S.B.; Han, H.Y. Carbon dots as inhibitors of virus by activation of type I interferon response. Carbon 2016, 110, 278–285. [Google Scholar] [CrossRef]
- Wang, M.; Wan, Y.Y.; Zhang, K.L.; Fu, Q.F.; Wang, L.J.; Zeng, J.; Xia, Z.N.; Gao, D. Green synthesis of carbon dots using the flowers of Osmanthus fragrans (Thunb.) Lour. as precursors: Application in Fe3+ and ascorbic acid determination and cell imaging. Anal. Bioanal. Chem. 2019, 411, 2715–2727. [Google Scholar] [CrossRef]
- Mei, S.; Fu, B.; Su, X.; Chen, H.; Lin, H.; Zheng, Z.; Dai, C.; Yang, D.P. Developing silk sericin-based and carbon dots reinforced bio-nanocomposite films and potential application to litchi fruit. LWT-Food Sci. Technol. 2022, 164, 113630. [Google Scholar] [CrossRef]
- Wang, X.K.; Zhang, Y.; Kong, H.; Cheng, J.J.; Zhang, M.L.; Sun, Z.W.; Wang, S.N.; Liu, J.X.; Qu, H.H.; Zhao, Y. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif. Cells Nanomed. Biotechnol. 2020, 48, 68–76. [Google Scholar] [CrossRef]
- Wang, X.K.; Wu, T.; Yang, Y.X.; Zhou, L.; Wang, S.X.; Liu, J.X.; Zhao, Y.F.; Zhang, M.L.; Zhao, Y.; Qu, H.H.; et al. Ultrasmall and highly biocompatible carbon dots derived from natural plant with amelioration against acute kidney injury. J. Nanobiotechnol. 2023, 21, 63. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, Y.; Luo, J.; Xiong, W.; Liu, X.M.; Cheng, J.J.; Wang, Y.Z.; Zhang, M.L.; Qu, H.H. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J. Nanobiotechnol. 2017, 15, 60. [Google Scholar] [CrossRef]
- Xue, M.Y.; Zou, M.B.; Zhao, J.J.; Zhan, Z.H.; Zhao, S.L. Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells. J. Mater. Chem. B 2015, 3, 6783–6789. [Google Scholar] [CrossRef]
- Yu, L.D.; Li, X.T.; He, M.Y.; Wang, Q.C.; Chen, C.; Li, F.S.; Li, B.S.; Li, L. Antioxidant Carboxymethyl Chitosan Carbon Dots with Calcium Doping Achieve Ultra-Low Calcium Concentration for Iron-Induced Osteoporosis Treatment by Effectively Enhancing Calcium Bioavailability in Zebrafish. Antioxidants 2023, 12, 583. [Google Scholar] [CrossRef]
- Miao, C.; Zhang, Y.; Liu, G.; Yang, J.; Yu, K.; Lv, J.; Liu, R.; Yao, Z.; Niu, Y.; Wang, X.; et al. Multi-step strategies for synergistic treatment of urinary tract infections based on D-xylose-decorated antimicrobial peptide carbon dots. Biomaterials 2024, 308, 122547. [Google Scholar] [CrossRef]
- Jian, K.; Fu, L.M.; Zhang, Y.J.; Zhang, H.M.; Guo, X.J.; Zhao, X.H. Microwave synthesis of chitosan-based carbon dots for Al3+ detection and biological application. Int. J. Biol. Macromol. 2024, 260, 129413. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, D.; Yin, H.G.; Zhang, K.L.; Zeng, J.; Wang, L.J.; Xia, L.; Zhou, K.; Xia, Z.N.; Fu, Q.F. Facile, green and energy-efficient preparation of fluorescent carbon dots from processed traditional Chinese medicine and their applications for on-site semi-quantitative visual detection of Cr(VI). Sens. Actuators B-Chem. 2020, 324, 128722. [Google Scholar] [CrossRef]
- Li, P.F.; Xue, S.S.; Sun, L.; Ma, X.B.; Liu, W.N.; An, L.; Liu, Y.C.; Qu, D.; Sun, Z.C. Formation and Fluorescent Mechanism of Multiple Color Emissive Carbon Dots from o-Phenylenediamine. Small 2024, 20, e2310563. [Google Scholar] [CrossRef]
- Zhang, W.K.; Shi, L.J.; Liu, Y.Q.; Meng, X.R.; Xu, H.; Xu, Y.Q.; Liu, B.Y.; Fang, X.M.; Li, H.B.; Ding, T. Supramolecular interactions via hydrogen bonding contributing to citric-acid derived carbon dots with high quantum yield and sensitive photoluminescence. Rsc Adv. 2017, 7, 20345–20353. [Google Scholar] [CrossRef]
- Gu, W.W.; Dong, Z.F.; Zhang, A.Y.; Ma, T.Y.; Hu, Q.; Wei, J.F.; Wang, R. Functionalization of PET with carbon dots as copolymerizable flame retardants for the excellent smoke suppressants and mechanical properties. Polym. Degrad. Stab. 2022, 195, 109766. [Google Scholar] [CrossRef]
- Manchala, S.; Gandamalla, A.; Vempuluru, N.R.; Venkatakrishnan, S.M.; Shanker, V. High potential and robust ternary LaFeO3/CdS/carbon quantum dots nanocomposite for photocatalytic H2 evolution under sunlight illumination. J. Colloid Interface Sci. 2021, 583, 255–266. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, X.; Wu, F.; Liu, H.Z.; Liang, E.Y.; He, R.R.; Liu, M.X. Systematic studies on blood coagulation mechanisms of halloysite nanotubes-coated PET dressing as superior topical hemostatic agent. Chem. Eng. J. 2022, 428, 132049. [Google Scholar] [CrossRef]
- Das, B.; Dadhich, P.; Pal, P.; Srivas, P.K.; Bankoti, K.; Dhara, S. Carbon nanodots from date molasses: New nanolights for the in vitro scavenging of reactive oxygen species. J. Mater. Chem. B 2014, 2, 6839–6847. [Google Scholar] [CrossRef]
- Liu, X.M.; Wang, Y.Z.; Yan, X.; Zhang, M.L.; Zhang, Y.; Cheng, J.J.; Lu, F.; Qu, H.H.; Wang, Q.G.; Zhao, Y. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine 2018, 13, 391–405. [Google Scholar] [CrossRef]
- Zhu, S.J.; Meng, Q.N.; Wang, L.; Zhang, J.H.; Song, Y.B.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.Y.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem.-Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Mitropoulos, K.A.; Martin, J.C.; Reeves, B.E.; Esnouf, M.P. The activation of the contact phase of coagulation by physiologic surfaces in plasma: The effect of large negatively charged liposomal vesicles. Blood 1989, 73, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Brass, L.F.; Diamond, S.L. Transport physics and biorheology in the setting of hemostasis and thrombosis. J. Thromb. Haemost. 2016, 14, 906–917. [Google Scholar] [CrossRef]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Li, Q.B.; Yu, Q.S.; Huang, G.W.; Tokeshi, Y.; Nakamura, M.; Kinjoh, K.; Kosugi, T. Hemostatic disturbances observed in patients with snakebite in south China. Toxicon 2000, 38, 1355–1366. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.L.; Zhao, B.H.; Lu, Y.F.; Huang, S.R.; Yuan, Z.Q.; Luo, G.X.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Cai, H.; Li, Y.; Wu, X.; Yang, Y.; Tedesco, A.C.; Li, Z.; Bi, H. Two Birds with One Stone: Guanidyl Carbon Dots with Enhanced Antioxidative and Lipolytic Functions in Metabolic Associated Fatty Liver. Adv. Funct. Mater. 2024, 34, 2406096. [Google Scholar] [CrossRef]
- Lin, L.S.; Wu, M.Y.; Zhao, J.H. The initiation and effects of plasma contact activation: An overview. Int. J. Hematol. 2017, 105, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Chang, B.T.; Chen, R.; Wei, Y.J.; Gong, Q.J.; Yu, D.; Zhang, Y.; Han, X.; Yang, H.B.; Tang, S.J.; et al. Research Advances in Pharmacology, Safety, and Clinical Applications of Yunnan Baiyao, a Traditional Chinese Medicine Formula. Front. Pharmacol. 2021, 12, 773185. [Google Scholar] [CrossRef] [PubMed]
- Gowda, B.H.J.; Mohanto, S.; Singh, A.; Bhunia, A.; Abdelgawad, M.A.; Ghosh, S.; Ansari, M.J.; Pramanik, S. Nanoparticle-based therapeutic approaches for wound healing: A review of the state-of-the-art. Mater. Today Chem. 2023, 27, 101319. [Google Scholar] [CrossRef]
- Xu, L.L.; Wu, D.J. Prolonged Hemocoagulase Agkistrodon Halys Pallas Administration Induces Hypofibrinogenemia in Patients with Hematological Disorders: A Clinical Analysis of 11 Patients. Indian J. Hematol. Blood Transfus. 2018, 34, 322–327. [Google Scholar] [CrossRef]
- He, L.; Li, Z.; Gu, M.Q.; Li, Y.F.; Yi, C.L.; Jiang, M.; Yu, X.; Xu, L. Intelligent Carbon Dots with Switchable Photo-Activated Oxidase-Mimicking Activity and pH Responsive Antioxidant Activity Adaptive to the Wound Microenvironment for Selective Antibacterial Therapy. Adv. Sci. 2024, 11, e2406681. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Y.; Zeng, W.H.; Cheng, J.J.; Zhang, M.L.; Kong, H.; Qu, H.H.; Lu, T.; Zhao, Y. Fluorescence Imaging, Metabolism, and Biodistribution of Biocompatible Carbon Dots Synthesized Using Punica granatum L. Peel. J. Biomed. Nanotechnol. 2022, 18, 381–393. [Google Scholar] [CrossRef]


| Number | Precursor | Synthesis | Application | Ref. |
|---|---|---|---|---|
| 1 | Platycladus orientalis | Hydrothermal methods | Hemostasis | [33] |
| 2 | Ligusticum wallichii | Hemostasis, wound healing | [34] | |
| 3 | Berberine | Anticancer | [35] | |
| 4 | Ascorbic acid | Antibacterial | [36] | |
| 5 | Osmanthus fragrans lours | Biosensing | [37] | |
| 6 | Degummed silk | Antibacterial, antioxidant | [38] | |
| 7 | Silkworm cocoon | High-temperature pyrolysis methods | Anti-inflammatory | [39] |
| 8 | Pollen typhae | Anti-inflammatory, antioxidant | [40] | |
| 9 | Hemostasis | [41] | ||
| 10 | Lychee seeds | Bioimaging | [42] | |
| 11 | Poly-L-lysine and oxidized dextran | Antibacterial, wound healing | [18] | |
| 12 | Carboxymethyl chitosan | Microwave methods | Antioxidant | [43] |
| 13 | Elaeagnus angustifolia | Antibacterial | [44] | |
| 14 | Chitosan and o-phenylenediamine | Bioimaging | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Yao, M.; Qiao, X.; Li, L.; Meng, Z.; Liu, S.; Sun, Y.; Gan, H.; Zhu, X.; Wu, Z.; et al. Silkworm Cocoon—Derived Carbon Dots for Post-Trauma Hemostasis and Tissue Repair. Pharmaceuticals 2025, 18, 603. https://doi.org/10.3390/ph18050603
Wu X, Yao M, Qiao X, Li L, Meng Z, Liu S, Sun Y, Gan H, Zhu X, Wu Z, et al. Silkworm Cocoon—Derived Carbon Dots for Post-Trauma Hemostasis and Tissue Repair. Pharmaceuticals. 2025; 18(5):603. https://doi.org/10.3390/ph18050603
Chicago/Turabian StyleWu, Xinru, Miaomiao Yao, Xuan Qiao, Lintao Li, Zhiyun Meng, Shuchen Liu, Yunbo Sun, Hui Gan, Xiaoxia Zhu, Zhuona Wu, and et al. 2025. "Silkworm Cocoon—Derived Carbon Dots for Post-Trauma Hemostasis and Tissue Repair" Pharmaceuticals 18, no. 5: 603. https://doi.org/10.3390/ph18050603
APA StyleWu, X., Yao, M., Qiao, X., Li, L., Meng, Z., Liu, S., Sun, Y., Gan, H., Zhu, X., Wu, Z., Gu, R., & Dou, G. (2025). Silkworm Cocoon—Derived Carbon Dots for Post-Trauma Hemostasis and Tissue Repair. Pharmaceuticals, 18(5), 603. https://doi.org/10.3390/ph18050603







