Antipyretic Mechanism of Bai Hu Tang on LPS-Induced Fever in Rat: A Network Pharmacology and Metabolomics Analysis
Abstract
1. Introduction
2. Results
2.1. Active Compounds and Targets of BHT and Targets of PMD
2.2. GO and KEGG Analysis of BHT Antipyretic Targets
2.3. Screening of DEGs (Differential Genes)
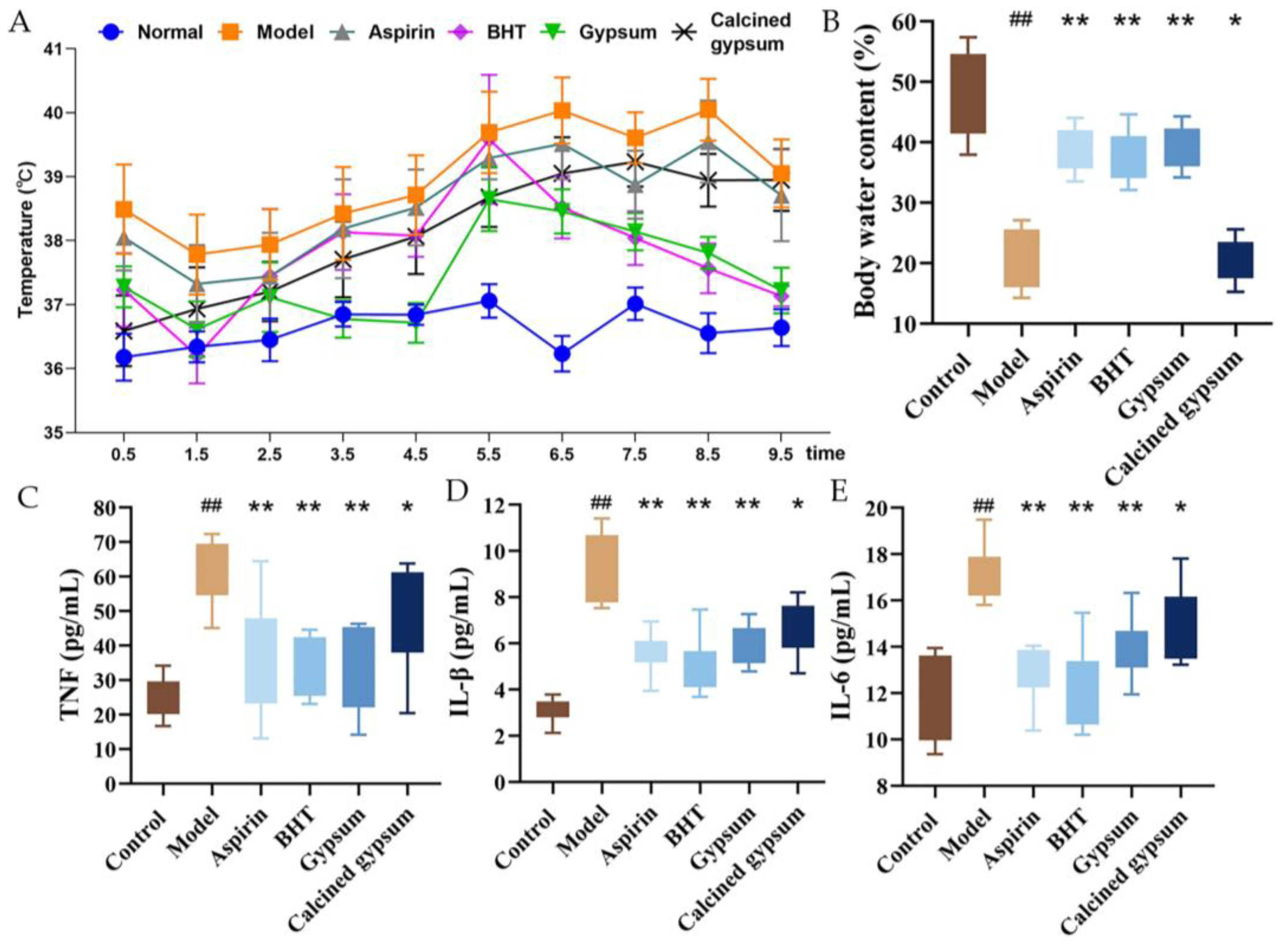
2.4. Effect of BHT on Body Temperature and Serum Levels of Inflammatory Factors in Model Rats
2.5. Effect of BHT on Serum and CFS Levels of Ca2+, AVP, cAMP, PGE2 and CRH
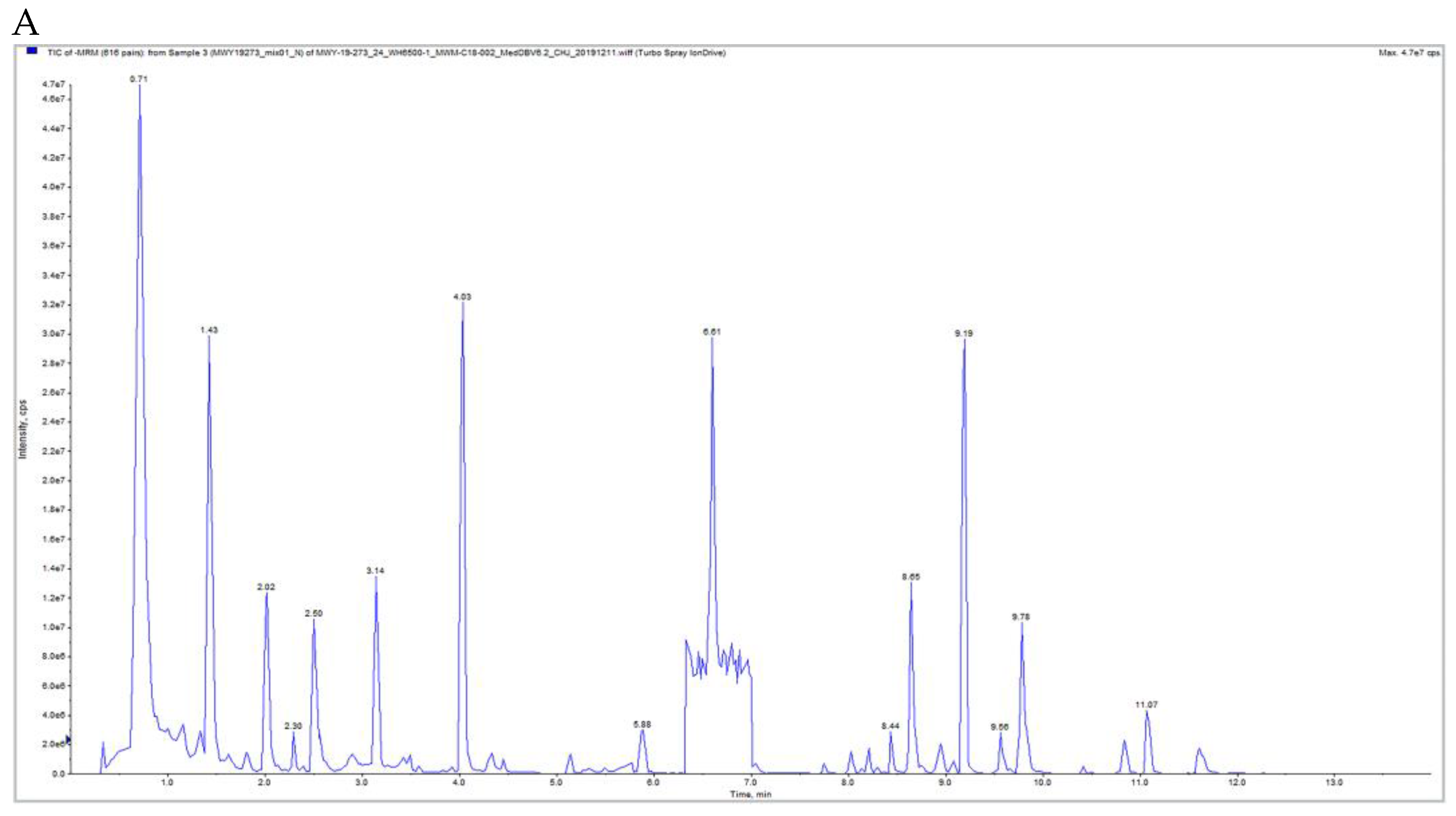
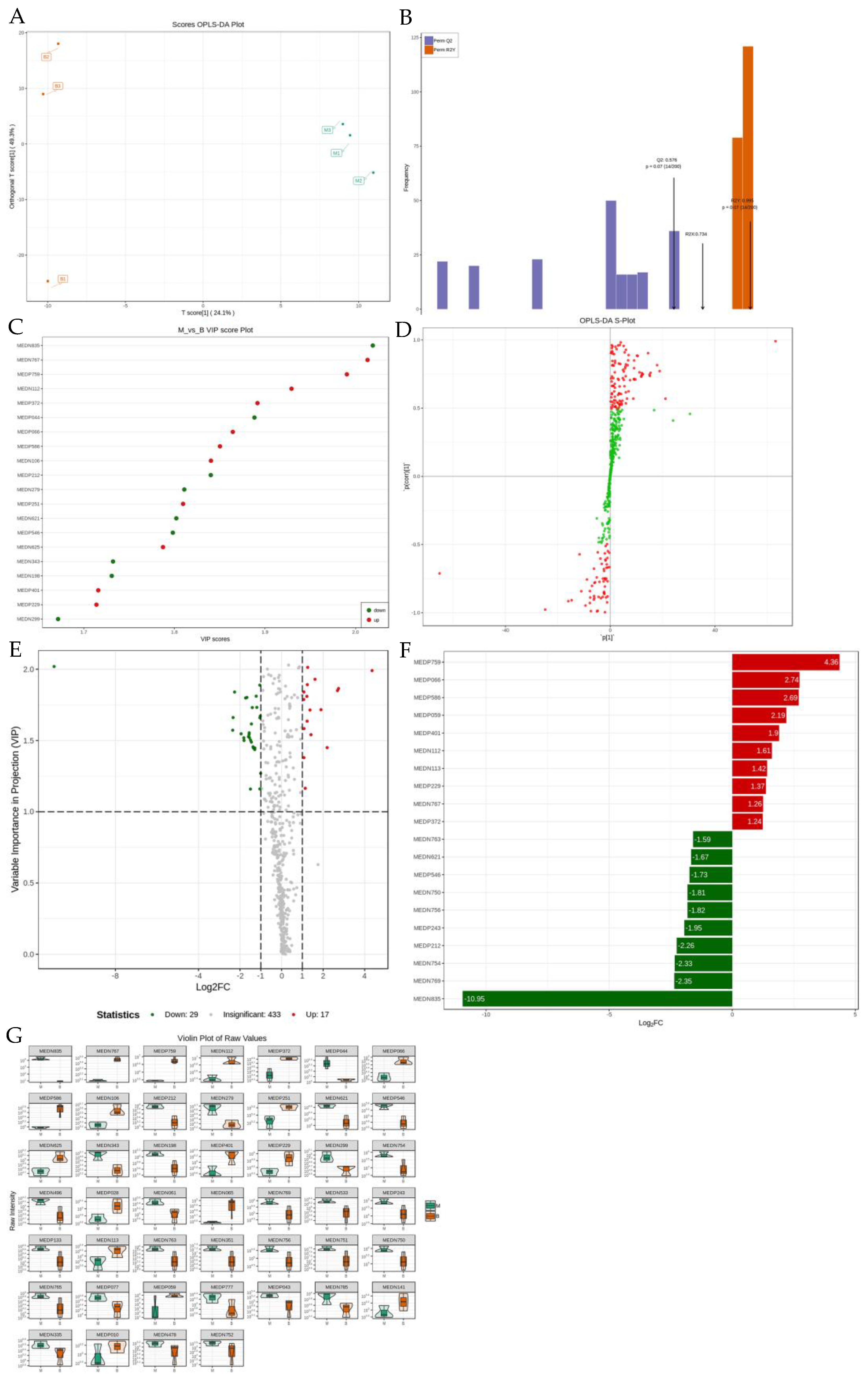
2.6. Qualitative and Quantitative Metabolite and Quality Control (QC) Analyses
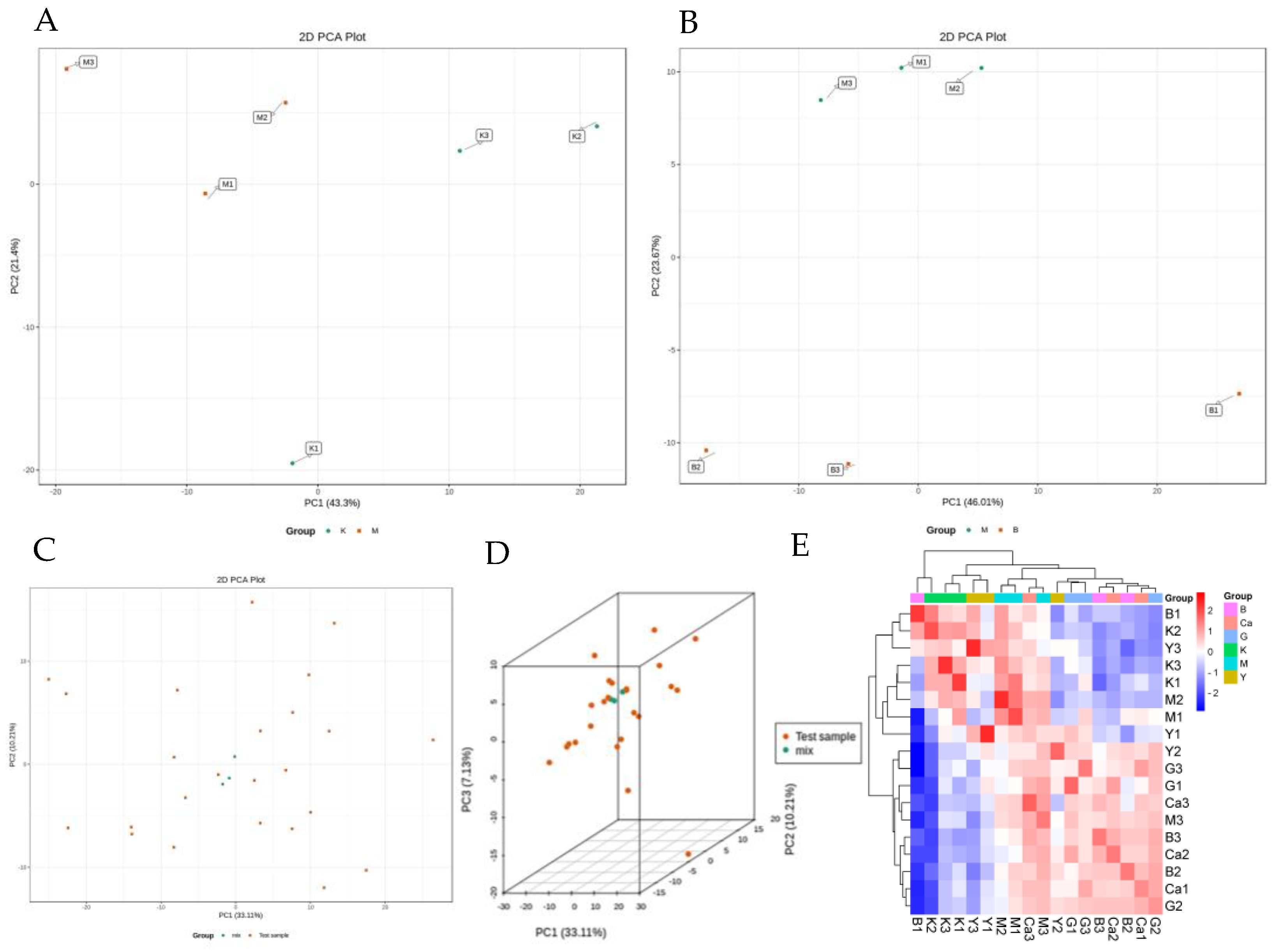
2.7. Cluster Principal Component Analysis (PCA)
2.8. Differential Metabolite Screening
2.9. GO and KEGG Pathway Enrichment Analysis for Differential Metabolites
2.10. Effect of BHT on Brain Expression of TNF-α, IL6R, PTGS2 in Fever Rats
3. Discussion
4. Materials and Methods
4.1. Liquid Preparation
4.2. Chemicals and Reagents
4.3. Animals and Treatments
4.4. Monitor Body Temperature
4.5. Acquisition of Blood and Tissue Samples
4.6. Network Pharmacology Analysis
4.7. GEO Sample Acquisition and Processing
4.8. Metabolomic Analysis
4.9. Molecular Docking
4.10. Detection of Biochemical Indicators
4.11. Western Blot
4.12. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BHT | Bai Hu Tang |
| TCM | Traditional Chinese Medicine |
| BP | Biological Processes |
| MF | Molecular Functions |
| CC | Cellular Components |
| TNF-α | Tumour Necrosis Factor |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| AVP | Argipressin |
| cAMP | Phosphoadenosine |
| PGE2 | Prostaglandin E2 |
| CRH | Corticotropin-releasing Hormone |
| LPS | Lipopolysaccharide |
| CFS | Cerebrospinal Fluid |
References
- Cock, I.E.; Luwaca, N.; Van Vuuren, S.F. The traditional use of Southern African medicinal plants to alleviate fever and their antipyretic activities. J. Ethnopharmacol. 2023, 303, 115850. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.K.; Kim, H.W.; Park, S.H.; Park, J.H.; Kim, S.M.; Lee, M.J. The role of zinc sulfate in enhancing cellular and humoral immune responses to foot-and-mouth disease vaccine. Virus Res. 2023, 335, 199189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, D.; Wang, X.; Li, S.; Li, J.; Li, H.; Yan, Z. Aqueous extract of Bai-Hu-Tang, a classical Chinese herb formula, prevents excessive immune response and liver injury induced by LPS in rabbits. J. Ethnopharmacol. 2013, 149, 321–327. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X.; Lin, Y.; Zheng, X.; Yan, M.; Li, Y.; Shi, D.; Guo, S.; Liu, C. The mixture of Radix isatidis, Forsythiae, and Gypsum alleviates lipopolysaccharide-induced fever in broilers by inhibition of TLR4/NF-κB signaling pathway. Poult. Sci. 2023, 102, 103032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, D.; Dong, S.; Yang, Z.; Yan, Z. iTRAQ-based quantitative proteomic analysis reveals Bai-Hu-Tang enhances phagocytosis and cross-presentation against LPS fever in rabbit. J. Ethnopharmacol. 2017, 207, 1–7. [Google Scholar] [CrossRef]
- Lin, C.J.; Su, Y.C.; Lee, C.H.; Li, T.C.; Chen, Y.A.; Lin, S.J. Bai-hu-tang, ancient chinese medicine formula, may provide a new complementary treatment option for sepsis. Evid. Based Complement. Altern. Med. 2013, 2013, 193084. [Google Scholar] [CrossRef]
- Feng, M.; Wang, D.; Wang, X.; Yang, Y.; Zhang, S. Bai-Hu-Tang regulates endothelin-1 and its signalling pathway in vascular endothelial cells. J. Ethnopharmacol. 2022, 284, 114812. [Google Scholar] [CrossRef]
- Ye, H.B.; Zuo, J.H.; Ma, C.; Lin, H.; Xu, Y.; Sun, J.M.; Ye, D.D.; Lv, G.F.; Lin, Z. Study on the antipyretic effect of gypsum and its compatibility on yeast-induced pyrexia rats based on NF-κB signaling pathway. Mater. Express 2020, 10, 748–755. [Google Scholar] [CrossRef]
- Liu, T.; Li, R.; Cui, Y.; Yu, Z.; Zhao, Y. Metabonomic analysis of plasma biochemical changes in pyrexia rats after treatment with Gegenqinlian decoction, aspirin and itraconazole by UHPLC-FT-ICR-MS. J. Pharm. Anal. 2020, 10, 581–587. [Google Scholar] [CrossRef]
- Gao, X.; Huang, C.; Geng, T.; Chen, X.; Wang, J.; Liu, J.; Duan, K.; Cao, L.; Wang, Z.; Xiao, W. Serum and urine metabolomics based on UPLC-Q-TOF/MS reveals the antipyretic mechanism of Reduning injection in a rat model. J. Ethnopharmacol. 2020, 250, 112429. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, A.-H.; Yan, G.-L.; Liu, L.; Wang, X.-J. Technological advances in current metabolomics and its application in tradition Chinese medicine. RSC Adv. 2017, 7, 53516–53524. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Yang, B.; Zhao, S.; Kuang, H. The Progress of Metabolomics Study in Traditional Chinese Medicine Research. Am. J. Chin. Med. 2015, 43, 1281–1310. [Google Scholar] [CrossRef]
- Ju, S.; He, J.; Wang, H.; Yang, L.; Guo, A.; Guo, Y.; Qi, M.; Wang, H.; Ai, L. Potential therapeutic drug targets and pathways prediction for premature ovarian insufficiency -Based on network pharmacologic method. J. Ethnopharmacol. 2023, 304, 116054. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.-B.; Wang, Z.-X.; Liu, H.-B.; Jia, Y.-J.; Wang, Y.-P.; Xu, X.; Zhang, Y.; Qi, X.-D.; Hu, F.-D. Study on the mechanism of Fufang E’jiao Jiang on precancerous lesions of gastric cancer based on network pharmacology and metabolomics. J. Ethnopharmacol. 2023, 304, 116030. [Google Scholar] [CrossRef]
- Beeson, P.B. Temperature-elevating effect of a substance obtained from polymorphonuclear leucocytes. J. Clin. Investig. 1948, 27, 524. [Google Scholar] [PubMed]
- Nilsson, A.; Wilhelms, D.B.; Mirrasekhian, E.; Jaarola, M.; Blomqvist, A.; Engblom, D. Inflammation-induced anorexia and fever are elicited by distinct prostaglandin dependent mechanisms, whereas conditioned taste aversion is prostaglandin independent. Brain Behav. Immun. 2017, 61, 236–243. [Google Scholar] [CrossRef]
- Li, X.; Wei, S.; Ma, X.; Li, H.; Jing, M.; Liu, H.; Niu, S.; Tong, Y.; Chen, L.; Wei, Y.; et al. Huanglian Jiedu Decoction Exerts Antipyretic Effect by Inhibiting MAPK Signaling Pathway. Evid. Based Complement. Altern. Med. 2021, 2021, 2209574. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Ogata, A.; Kishimoto, T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin. Immunol. 2014, 26, 88–96. [Google Scholar] [CrossRef]
- Gupta, P.; Barthwal, M.K. IL-1 β genesis: The art of regulating the regulator. Cell Mol. Immunol. 2018, 15, 998–1000. [Google Scholar] [CrossRef]
- van Miert, A.S.; van Duin, C.T. Wensing. Fever and acute phase response induced in dwarf goats by endotoxin and bovine and human recombinant tumour necrosis factor alpha. J. Vet. Pharmacol. Ther. 1992, 15, 332–342. [Google Scholar] [CrossRef]
- Cabrera, R.A.; Dozier, B.L.; Duffy, D.M. Prostaglandin-endoperoxide synthase (PTGS1 and PTGS2) expression and prostaglandin production by normal monkey ovarian surface epithelium. Fertil. Steril. 2006, 86, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; He, Z.; Liu, X.; Liu, H.; Wang, X. Analgesic and Anxiolytic Effects of Gastrodin and Its Influences on Ferroptosis and Jejunal Microbiota in Complete Freund’s Adjuvant-Injected Mice. Front. Microbiol. 2022, 13, 841662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Wang, N.; Song, H.X.; Shen, Z.L.; Sun, B.; Tang, Y. Simultaneous telemetric monitoring of the circadian changes in core and BAT temperature in rats: Endogenous vasopressin may contribute to reduced BAT themogenesis and body temperature in the light phase of the circadian cycle. J. Therm. Biol. 2012, 37, 316–322. [Google Scholar] [CrossRef]
- Yang, Y.L.; Hu, X.S.; Zan, W.; Sheng, Z.L.; Huang, T.; Wei, Y.J. Arginine vasopressin does not mediate heat loss in the tail of the rat. J. Therm. Biol. 2013, 38, 247–254. [Google Scholar] [CrossRef]
- Roth, J.; Zeisberger, E.; Vybíral, S.; Janský, L. Endogenous antipyretics: Neuropeptides and glucocorticoids. Front. Biosci. 2004, 9, 816–826. [Google Scholar] [CrossRef]
- Boulant, J.A. Neuronal basis of Hammel’s model for set-point thermoregulation. J. Appl. Physiol. (1985) 2006, 100, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Ranels, H.J.; Griffin, J.D. The effects of prostaglandin E2 on the firing rate activity of thermosensitive and temperature insensitive neurons in the ventromedial preoptic area of the rat hypothalamus. Brain Res. 2003, 964, 42–50. [Google Scholar] [CrossRef]
- Gordon, C.J.; Heath, J.E. Effects of prostaglandin E2 on the activity of thermosensitive and insensitive single units in the preoptic/anterior hypothalamus of unanesthetized rabbits. Brain Res. 1980, 183, 113–121. [Google Scholar] [CrossRef]
- Fleisher-Berkovich, S.; Kagan, E.; Grossman, N.; Danon, A. Multiple effects of arginine vasopressin on prostaglandin E2 synthesis in fibroblasts. Eur. J. Pharmacol. 2004, 485, 53–59. [Google Scholar]
- Morrison, S.F. Central neural control of thermoregulation and brown adipose tissue. Auton. Neurosci. 2016, 196, 14–24. [Google Scholar] [CrossRef]
- Gupta, M.; Mazumder, U.K.; Kumar, R.S.; Gomathi, P.; Rajeshwar, Y.; Kakoti, B.B.; Selven, V.T. Anti-inflammatory, analgesic and antipyretic effects of methanol extract from Bauhinia racemosa stem bark in animal models. J. Ethnopharmacol. 2005, 98, 267–273. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.P.; Liu, R.; Jin, Y.; Guo, J.M.; Zhao, J.L.; Ding, S.X.; Lin, X.Z.; Lin, R.R.; Duan, J.A. Antipyretic and anti-inflammatory activities of Thais luteostoma extracts and underlying mechanisms. Chin. J. Nat. Med. 2015, 13, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Hou, X.Y.; Tang, Y.; Luo, R.; Zhang, J.; Liu, C.; Yang, Y.L. Arginine vasopressin antagonizes the effects of prostaglandin E(2) on the spontaneous activity of warm-sensitive and temperature-insensitive neurons in the medial preoptic area in rats. Neurosci. Lett. 2018, 662, 59–64. [Google Scholar] [CrossRef]
- Kovacs, G.L.; Baars, A.M.; De Wied, D. Antipyretic effect of central arginine8-vasopressin treatment: V1 receptors specifically involved? Life Sci. 1992, 50, 1625–1630. [Google Scholar] [CrossRef]
- Dinarello, C.A. Infection, fever, and exogenous and endogenous pyrogens: Some concepts have changed. J. Endotoxin Res. 2004, 10, 201–222. [Google Scholar] [PubMed]
- Satriotomo, I.; Nichols, N.L.; Dale, E.A.; Emery, A.T.; Dahlberg, J.M.; Mitchell, G.S. Repetitive acute intermittent hypoxia increases growth/neurotrophic factor expression in non-respiratory motor neurons. Neuroscience 2016, 322, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.; Simpson, C.; Higgins, D.; Nattermann, R.; Rice, J.; Redgrave, P.; Metcalf, G. Hypothalamic Na+ and Ca++ ions and temperature set-point: New mechanisms of action of a central or peripheral thermal challenge and intrahypothalamic 5-HT, NE, PGEi and pyrogen. Brain Res. Bull. 1976, 1, 301–327. [Google Scholar]
- Myers, R.D.; Brophy, P.D. Temperature changes in the rat produced by altering the sodium-calcium ratio in the cerebral ventricles. Neuropharmacology 1972, 11, 351–361. [Google Scholar] [CrossRef]
- Gangane, P.; Sharma, V.; Selokar, M.; Vidhate, D.; Pawar, K.; Mahajan, N. A Review of Anti-Inflammatory Phytoconstituents Used in Herbal Cosmeceuticals for the Treatment of Atopic Dermatitis. Curr. Drug Deliv. 2024, 21, 312–325. [Google Scholar]
- Mohammed, E.A.H.; Peng, Y.; Wang, Z.; Qiang, X.; Zhao, Q. Synthesis, Antiviral, and Antibacterial Activity of the Glycyrrhizic Acid and Glycyrrhetinic Acid Derivatives. Russ. J. Bioor. Chem. 2022, 48, 906–918. [Google Scholar] [CrossRef]
- Kozłowski, H.M.; Sobocińska, J.; Jędrzejewski, T.; Maciejewski, B.; Dzialuk, A.; Wrotek, S. Fever-range whole body hyperthermia leads to changes in immune-related genes and miRNA machinery in Wistar rats. Int. J. Hyperth. 2023, 40, 2216899. [Google Scholar] [CrossRef]
- Colares, J.R.; Hartmann, R.M.; Schemitt, E.G.; Fonseca, S.R.B.; Brasil, M.S.; Picada, J.N.; Dias, A.S.; Bueno, A.F.; Marroni, C.A.; Marroni, N.P. Melatonin prevents oxidative stress, inflammatory activity, and DNA damage in cirrhotic rats. World J. Gastroenterol. 2022, 28, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Nava, F.; Calapai, G.; Facciolà, G.; Cuzzocrea, S.; Giuliani, G.; De Sarro, A.; Caputi, A.P. Melatonin effects on inhibition of thirst and fever induced by lipopolysaccharide in rat. Eur. J. Pharmacol. 1997, 331, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.M.; Saed, S.A.A.; Moawad, M.H.-E.; Moawad, W.A.E.-T.; Al-Hejazi, T.; Mousa, Y.; Sharma, R.; Reiter, R.J. Safety and efficacy of melatonin as an adjuvant therapy in COVID-19 patients: Systematic review and meta-analysis. Adv. Med. Sci. 2023, 68, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahat, A.; Hulail, M.A.E.; Soliman, N.M.M.; Khamis, T.; Fericean, L.M.; Arisha, A.H.; Moawad, R.S. Melatonin Mitigates Cisplatin-Induced Ovarian Dysfunction via Altering Steroidogenesis, Inflammation, Apoptosis, Oxidative Stress, and PTEN/PI3K/Akt/mTOR/AMPK Signaling Pathway in Female Rats. Pharmaceutics 2022, 14, 2769. [Google Scholar] [CrossRef]
- Harding, E.C.; Ba, W.; Zahir, R.; Yu, X.; Yustos, R.; Hsieh, B.; Lignos, L.; Vyssotski, A.L.; Merkle, F.T.; Constandinou, T.G.; et al. Nitric Oxide Synthase Neurons in the Preoptic Hypothalamus Are NREM and REM Sleep-Active and Lower Body Temperature. Front. Neurosci. 2021, 15, 709825. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.W.; Zhang, X.; Zhuo, Q.Y.; Chen, M.X.; Yang, C.; Chen, Z.J.; Chen, Y.; Liao, Y.Q.; Wang, L.L. Metabolomics and integrated network pharmacology analysis reveal attenuates cardiac hypertrophic mechanisms of HuoXin pill. J. Ethnopharmacol. 2022, 292, 115150. [Google Scholar] [CrossRef]
- Jiahang, Z.; Hongbo, Y.; He, L.; Guangfu, L.; Yuchen, W.; Xiaowei, H.; Zhe, L. Study on the Antipyretic Mechanism of Baihu Decoction: Network Pharmacology Prediction and Experimental Verification. J. Biobased Mater. Bio 2021, 15, 334–341. [Google Scholar]






| K vs. M | M vs. B | ||
|---|---|---|---|
| Index | Compounds | Index | Compounds |
| MEND019 | Glycocholic Acid | MEND044 | Diethyl Malonate |
| MEND043 | L-Theanine | MEND066 | D-glucuronolactone |
| MEND061 | Tryptophan Betaine | MEND106 | Uridine 5-Monophosphate |
| MEND097 | Glycocholic Acid | MEND112 | Glycyrrhetinic Acid |
| MEND112 | Glycyrrhetinic Acid | MEND198 | Phenylacetyl-L-Glutamine |
| MEND113 | L-arginine | MEND212 | Fenugreek Alkaloids |
| MEND176 | N-Phenylacetylglycine | MEND229 | N-acetylphenylalanine |
| MEND324 | 19Z-docosahexaenoic Acid | MEND251 | L-O-phosphatidylserine |
| MEND334 | Xanthosine | MEND279 | Glutathione (Reduced Form) |
| MEND338 | 4-Oxoretinol | MEND299 | N-acetylpententrone |
| MEND356 | Glycyrrhizic Acid | MEND343 | Oxidised Lipid |
| MEND394 | 14Z-eicosatetraenoic Acid | MEND372 | Epinephrine |
| MEND416 | 17Z-eicosapentaenoic Acid | MEND401 | 2′-Deoxyuridine |
| MEND440 | 8Z,14Z-eicosatrienoic Acid | MEND546 | Phenols and its Derivatives |
| MEND454 | 10E,14Z-eicosatetraenoic Acid | MEND586 | Uridine 5-Monophosphate |
| MEND509 | Methylmalonic Acid | MEND624 | Organic Acid and its Derivatives |
| MEND523 | Aminomalonic Acid | MEND625 | Trigonelline |
| MEND531 | Glycochenodeoxycholic Acid | MEND759 | Lactose |
| MEND611 | Cortisol 21-Acetate | MEND767 | L-Theanine |
| MEND720 | Tryptophan Betaine | MEND835 | Amino Acid Metabolomics |
| Ligand Name | Receptor | Receptor Residue | Type of Interaction | Binding Energy (kcal/mol) |
|---|---|---|---|---|
| Glycyrrhetinic acid | IL6R | GLN147 (A chain) | Conventional Hydrogen Bond | −9.79 |
| Glycyrrhetinic acid | IL6R | ASN226 (A chain) | Conventional Hydrogen Bond | −8 |
| Glycyrrhetinic acid | IL6R | ASN110 (A chain) | Conventional Hydrogen Bond | −7.91 |
| Glycyrrhetinic acid | TNF-α | CYS77 (A chain) | Covalent bond | −6.99 |
| Glycyrrhetinic acid | TNF-α | LEU75 (A chain) | Covalent bond | −8.81 |
| Glycyrrhetinic acid | TNF-α | ILE97 (A chain) | Covalent bond | −7.25 |
| Glycyrrhetinic acid | PTGS2 | LYS28 (A chain) | Salt Bridge | −9.21 |
| Glycyrrhetinic acid | PTGS2 | LYS179 (A chain) | Salt Bridge | −8.26 |
| Glycyrrhetinic acid | PTGS2 | ARG183 (A chain) | Attractive charge | −7.59 |
| Glycyrrhetinic acid | PTGS2 | SER142 (A chain) | Conventional Hydrogen Bond | −7.99 |
| Glycyrrhetinic acid | PTGS2 | HOH392 (A chain) | Water Hydrogen bond | −6.91 |
| Glycyrrhetinic acid | PTGS2 | HOH444 (A chain) | Water Hydrogen bond | −5.86 |
| Glycyrrhetinic acid | PTGS2 | HOH510 (A chain) | Water Hydrogen bond | −8.36 |
| Glycyrrhetinic acid | PTGS2 | PHE214 (A chain) | Pi Alkyl | −10.59 |
| Glycyrrhetinic acid | PTGS2 | PHE207 (A chain) | Pi-Pi Stacked | −12.12 |
| Glycyrrhetinic acid | PTGS2 | MET34 (A chain) | Alkyl | −10.05 |
| Glycyrrhetinic acid | PTGS2 | VAL146 (A chain) | Alkyl | −8.02 |
| Glycyrrhetinic acid | PTGS2 | TYR134 (A chain) | Conventional Hydrogen Bond | −7.32 |
| Glycyrrhetinic acid | PTGS2 | ALA28 (A chain) | Pi Alkyl | −5.98 |
| Glycyrrhetinic acid | PTGS2 | LEU147 (A chain) | Alkyl | −5.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, K.; Wang, Y.; Guo, W.; Lin, H.; Lin, Z.; Lv, G. Antipyretic Mechanism of Bai Hu Tang on LPS-Induced Fever in Rat: A Network Pharmacology and Metabolomics Analysis. Pharmaceuticals 2025, 18, 610. https://doi.org/10.3390/ph18050610
Pei K, Wang Y, Guo W, Lin H, Lin Z, Lv G. Antipyretic Mechanism of Bai Hu Tang on LPS-Induced Fever in Rat: A Network Pharmacology and Metabolomics Analysis. Pharmaceuticals. 2025; 18(5):610. https://doi.org/10.3390/ph18050610
Chicago/Turabian StylePei, Ke, Yuchen Wang, Wentao Guo, He Lin, Zhe Lin, and Guangfu Lv. 2025. "Antipyretic Mechanism of Bai Hu Tang on LPS-Induced Fever in Rat: A Network Pharmacology and Metabolomics Analysis" Pharmaceuticals 18, no. 5: 610. https://doi.org/10.3390/ph18050610
APA StylePei, K., Wang, Y., Guo, W., Lin, H., Lin, Z., & Lv, G. (2025). Antipyretic Mechanism of Bai Hu Tang on LPS-Induced Fever in Rat: A Network Pharmacology and Metabolomics Analysis. Pharmaceuticals, 18(5), 610. https://doi.org/10.3390/ph18050610






