Advancements in Nanocarrier Systems for Nose-to-Brain Drug Delivery
Abstract
:1. Introduction
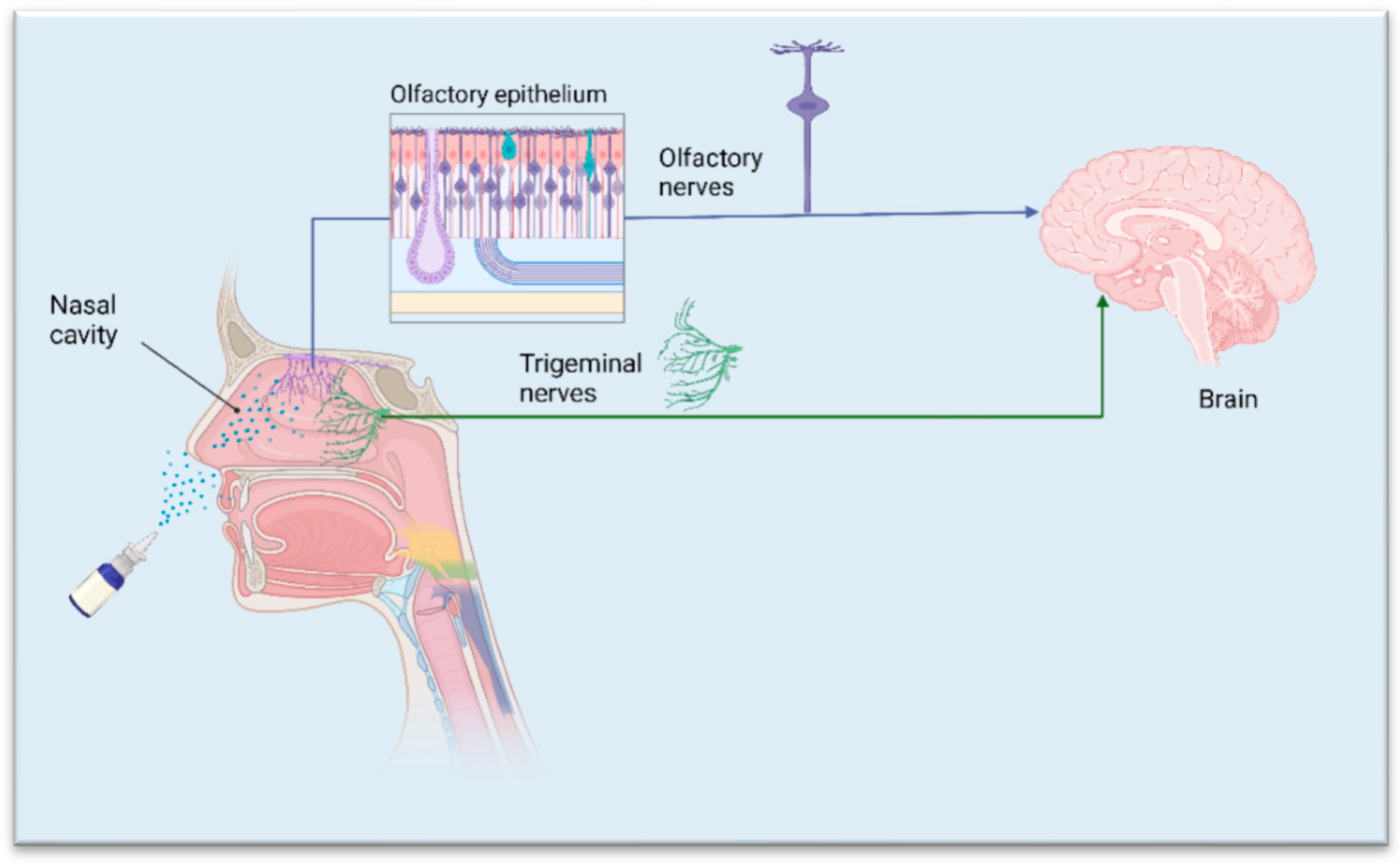
2. Structural Features Relevant to Drug Delivery and Pathways for Drug Transport to the Brain
3. Factors Affecting Nasal Absorption for Nose-to-Brain Delivery
3.1. Properties of Drugs
3.2. Formulation Properties
3.3. Nasal Cavity Conditions
3.4. Roles of Nano-Drug Delivery Systems in Nose-to-Brain Delivery
3.4.1. Enhanced Drug Retention and Penetration
3.4.2. Enhanced Stability and Reduced Side Effects
4. Evaluation of Intranasal Formulations for Nose-to-Brain Delivery
5. Nanocarriers for Nose-to-Brain Delivery
5.1. Micelles
5.2. Polymeric Nanoparticles
5.3. Lipid-Based Nanocarriers
5.4. Other Nanocarrier Systems
5.5. Material Used for Nanocarriers
6. Authors’ Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erdő, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018, 143, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Pardhi, D.M.; Rautio, J.; Rosenholm, J.M.; Pathak, K. Intranasal Nanoemulsions for Direct Nose-to-Brain Delivery of Actives for CNS Disorders. Pharmaceutics 2020, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. In Vitro Evaluation of Nasal Aerosol Depositions: An Insight for Direct Nose to Brain Drug Delivery. Pharmaceutics 2021, 13, 1079. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Napolitano, F.; Montesarchio, D.; Sampaolo, S.; Melone, M.A.B. Nanoparticle-Guided Brain Drug Delivery: Expanding the Therapeutic Approach to Neurodegenerative Diseases. Pharmaceutics 2021, 13, 1897. [Google Scholar] [CrossRef]
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049. [Google Scholar] [CrossRef]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Xie, H.; Wang, Y.; Gao, S.; Zhang, L.; Bo, F.; Yang, S.; Feng, A. Primary Studies on Construction and Evaluation of Ion-Sensitive in situ Gel Loaded with Paeonol-Solid Lipid Nanoparticles for Intranasal Drug Delivery. Int. J. Nanomed. 2020, 15, 3137–3160. [Google Scholar] [CrossRef]
- El-Setouhy, D.A.; Ibrahim, A.B.; Amin, M.M.; Khowessah, O.M.; Elzanfaly, E.S. Intranasal haloperidol-loaded miniemulsions for brain targeting: Evaluation of locomotor suppression and in-vivo biodistribution. Eur. J. Pharm. Sci. 2016, 92, 244–254. [Google Scholar] [CrossRef]
- El-Zaafarany, G.M.; Soliman, M.E.; Mansour, S.; Awad, G.A.S. Identifying lipidic emulsomes for improved oxcarbazepine brain targeting: In vitro and rat in vivo studies. Int. J. Pharm. 2016, 503, 127–140. [Google Scholar] [CrossRef]
- Boyuklieva, R.; Pilicheva, B. Micro- and Nanosized Carriers for Nose-to-Brain Drug Delivery in Neurodegenerative Disorders. Biomedicines 2022, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Deng, X.; Liu, M.; He, M.; Long, W.; Xu, Z.; Zhang, K.; Liu, T.; So, K.F.; Fu, Q.L.; et al. Intranasal delivery of BDNF-loaded small extracellular vesicles for cerebral ischemia therapy. J. Control. Release 2023, 357, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-Brain Delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in nose-to-brain drug delivery: A review of non-clinical brain targeting studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Duong, V.-A. A Review on Nanosystem-Based Delivery of Tofacitinib for Enhanced Treatment of Autoimmune Diseases and Inflammation. BioNanoScience 2024, 14, 2048–2064. [Google Scholar] [CrossRef]
- Deshmukh, V.; Pathan, N.S.; Haldar, N.; Nalawade, S.; Narwade, M.; Gajbhiye, K.R.; Gajbhiye, V. Exploring intranasal drug delivery via nanocarriers: A promising glioblastoma therapy. Colloids Surf. B Biointerfaces 2024, 245, 114285. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Duong, V.-A. Solid Lipid Nanoparticles. Encyclopedia 2022, 2, 952–973. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Jiao, X.; He, X.; Qin, S.; Yin, X.; Song, T.; Duan, X.; Shi, H.; Jiang, S.; Zhang, Y.; Song, X. Insights into the formulation of lipid nanoparticles for the optimization of mRNA therapeutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1992. [Google Scholar] [CrossRef]
- Kisku, A.; Nishad, A.; Agrawal, S.; Paliwal, R.; Datusalia, A.K.; Gupta, G.; Singh, S.K.; Dua, K.; Sulakhiya, K. Recent developments in intranasal drug delivery of nanomedicines for the treatment of neuropsychiatric disorders. Front. Med. 2024, 11, 1463976. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Younas, K.; Jamshaid, M.; Ereej, N.; Hameed, A.; Munir, R.; Khokhar, R. A comprehensive review on lipid-based nanoparticles via nose to brain targeting as a novel approach. J. Microencapsul. 2024, 41, 681–714. [Google Scholar] [CrossRef]
- Kumar, N.; Khurana, B.; Arora, D. Nose-to-brain drug delivery for the treatment of glioblastoma multiforme: Nanotechnological interventions. Pharm. Dev. Technol. 2023, 28, 1032–1047. [Google Scholar] [CrossRef]
- Du, L.; Chen, L.; Liu, F.; Wang, W.; Huang, H. Nose-to-brain drug delivery for the treatment of CNS disease: New development and strategies. Int. Rev. Neurobiol. 2023, 171, 255–297. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Ahmad, E.; Feng, Y.; Qi, J.; Fan, W.; Ma, Y.; He, H.; Xia, F.; Dong, X.; Zhao, W.; Lu, Y.; et al. Evidence of nose-to-brain delivery of nanoemulsions: Cargoes but not vehicles. Nanoscale 2017, 9, 1174–1183. [Google Scholar] [CrossRef]
- Nair, S.C.; Vinayan, K.P.; Mangalathillam, S. Nose to Brain Delivery of Phenytoin Sodium Loaded Nano Lipid Carriers: Formulation, Drug Release, Permeation and In Vivo Pharmacokinetic Studies. Pharmaceutics 2021, 13, 1640. [Google Scholar] [CrossRef]
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200. [Google Scholar] [CrossRef]
- Feng, Y.; He, H.; Li, F.; Lu, Y.; Qi, J.; Wu, W. An update on the role of nanovehicles in nose-to-brain drug delivery. Drug Discov. Today 2018, 23, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, Y.; Zhang, W.; Xia, X.; Li, H.; Qin, M.; Gao, H. Nanotechnology for enhanced nose-to-brain drug delivery in treating neurological diseases. J. Control. Release 2024, 366, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Feher, J. 4.6—The Chemical Senses. In Quantitative Human Physiology, 2nd ed.; Feher, J., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 427–439. [Google Scholar]
- Awad, R.; Avital, A.; Sosnik, A. Polymeric nanocarriers for nose-to-brain drug delivery in neurodegenerative diseases and neurodevelopmental disorders. Acta Pharm. Sinica. B 2023, 13, 1866–1886. [Google Scholar] [CrossRef]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef]
- Singh, A.P.; Saraf, S.K.; Saraf, S.A. SLN approach for nose-to-brain delivery of alprazolam. Drug Deliv. Transl. Res. 2012, 2, 498–507. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Maeng, H.-J. Pharmacokinetics and Pharmacodynamics of Intranasal Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 572. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Ritthidej, G.C. Chapter 3—Nasal Delivery of Peptides and Proteins with Chitosan and Related Mucoadhesive Polymers. In Peptide and Protein Delivery; Van Der Walle, C., Ed.; Academic Press: Boston, MA, USA, 2011; pp. 47–68. [Google Scholar]
- Jin, Z.; Han, Y.; Zhang, D.; Li, Z.; Jing, Y.; Hu, B.; Sun, S. Application of Intranasal Administration in the Delivery of Antidepressant Active Ingredients. Pharmaceutics 2022, 14, 2070. [Google Scholar] [CrossRef]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective-a review. Drug Deliv. Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef]
- England, R.J.; Homer, J.J.; Knight, L.C.; Ell, S.R. Nasal pH measurement: A reliable and repeatable parameter. Clin. Otolaryngol. Allied Sci. 1999, 24, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Ramvikas, M.; Arumugam, M.; Chakrabarti, S.R.; Jaganathan, K.S. Chapter Fifteen—Nasal Vaccine Delivery. In Micro and Nanotechnology in Vaccine Development; Skwarczynski, M., Toth, I., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 279–301. [Google Scholar]
- Koo, J.; Lim, C.; Oh, K.T. Recent Advances in Intranasal Administration for Brain-Targeting Delivery: A Comprehensive Review of Lipid-Based Nanoparticles and Stimuli-Responsive Gel Formulations. Int. J. Nanomed. 2024, 19, 1767–1807. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.; Paiva-Santos, A.C.; Veiga, F.; Pires, P.C. Lipid and Polymeric Nanoparticles: Successful Strategies for Nose-to-Brain Drug Delivery in the Treatment of Depression and Anxiety Disorders. Pharmaceutics 2022, 14, 2742. [Google Scholar] [CrossRef]
- Hussein, N.R.; Omer, H.K.; Elhissi, A.M.; Ahmed, W. Advances in nasal drug delivery systems. In Advances in Medical and Surgical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–311. [Google Scholar]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Rassu, G.; Ferraro, L.; Pavan, B.; Giunchedi, P.; Gavini, E.; Dalpiaz, A. The Role of Combined Penetration Enhancers in Nasal Microspheres on In Vivo Drug Bioavailability. Pharmaceutics 2018, 10, 206. [Google Scholar] [CrossRef]
- Madden, S.; Carrazana, E.; Rabinowicz, A.L. Optimizing Absorption for Intranasal Delivery of Drugs Targeting the Central Nervous System Using Alkylsaccharide Permeation Enhancers. Pharmaceutics 2023, 15, 2119. [Google Scholar] [CrossRef]
- Clementino, A.R.; Pellegrini, G.; Banella, S.; Colombo, G.; Cantù, L.; Sonvico, F.; Del Favero, E. Structure and Fate of Nanoparticles Designed for the Nasal Delivery of Poorly Soluble Drugs. Mol. Pharm. 2021, 18, 3132–3146. [Google Scholar] [CrossRef]
- Sosnowski, T.R.; Rapiejko, P.; Sova, J.; Dobrowolska, K. Impact of physicochemical properties of nasal spray products on drug deposition and transport in the pediatric nasal cavity model. Int. J. Pharm. 2020, 574, 118911. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, X.; Yu, S.; Gong, G.; Shu, H. Research progress in brain-targeted nasal drug delivery. Front. Aging Neurosci. 2023, 15, 1341295. [Google Scholar] [CrossRef]
- Gao, M.; Shen, X.; Mao, S. Factors influencing drug deposition in thenasal cavity upon delivery via nasal sprays. J. Pharm. Investig. 2020, 50, 251–259. [Google Scholar] [CrossRef]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef] [PubMed]
- Deruyver, L.; Rigaut, C.; Lambert, P.; Haut, B.; Goole, J. The importance of pre-formulation studies and of 3D-printed nasal casts in the success of a pharmaceutical product intended for nose-to-brain delivery. Adv. Drug Deliv. Rev. 2021, 175, 113826. [Google Scholar] [CrossRef] [PubMed]
- Ohwaki, T.; Ando, H.; Kakimoto, F.; Uesugi, K.; Watanabe, S.; Miyake, Y.; Kayano, M. Effects of Dose, pH, and Osmolarity on Nasal Absorption of Secretin in Rats II: Histological Aspects of the Nasal Mucosa in Relation to the Absorption Variation Due to the Effects of pH and Osmolarity. J. Pharm. Sci. 1987, 76, 695–698. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Parekh, H.S.; Pandey, P.; Siskind, D.J.; Falconer, J.R. Nose-to-brain delivery of antipsychotics using nanotechnology: A review. Expert. Opin. Drug Deliv. 2020, 17, 839–853. [Google Scholar] [CrossRef]
- Shrewsbury, S.B. The Upper Nasal Space: Option for Systemic Drug Delivery, Mucosal Vaccines and “Nose-to-Brain”. Pharmaceutics 2023, 15, 1720. [Google Scholar] [CrossRef]
- Fink, J.B.; Stapleton, K.W. Nebulizers. J. Aerosol Med. Pulm. Drug Deliv. 2024, 37, 140–156. [Google Scholar] [CrossRef]
- Khalili, S.; Tkachenko, N.; Rotenberg, B. A novel device for delivery of intranasal particulate medication: A pilot study. Int. Forum Allergy Rhinol. 2013, 3, 905–910. [Google Scholar] [CrossRef]
- Cooper, W.; Ray, S.; Aurora, S.K.; Shrewsbury, S.B.; Fuller, C.; Davies, G.; Hoekman, J. Delivery of Dihydroergotamine Mesylate to the Upper Nasal Space for the Acute Treatment of Migraine: Technology in Action. J. Aerosol Med. Pulm. Drug Deliv. 2022, 35, 321–332. [Google Scholar] [CrossRef]
- Trevino, J.T.; Quispe, R.C.; Khan, F.; Novak, V. Non-Invasive Strategies for Nose-to-Brain Drug Delivery. J. Clin. Trials 2020, 10, 439. [Google Scholar]
- Katare, P.; Pawar Medhe, T.; Nadkarni, A.; Deshpande, M.; Tekade, R.K.; Benival, D.; Jain, A. Nasal Drug Delivery System and Devices: An Overview on Health Effects. ACS Chem. Health Saf. 2024, 31, 127–143. [Google Scholar] [CrossRef]
- Keck, T.; Leiacker, R.; Heinrich, A.; Kühnemann, S.; Rettinger, G. Humidity and temperature profile in the nasal cavity. Rhinology 2000, 38, 167–171. [Google Scholar]
- Cingi, C.; Bayar Muluk, N.; Mitsias, D.I.; Papadopoulos, N.G.; Klimek, L.; Laulajainen-Hongisto, A.; Hytönen, M.; Toppila-Salmi, S.K.; Scadding, G.K. The Nose as a Route for Therapy: Part 1. Pharmacotherapy. Front. Allergy 2021, 2, 638136. [Google Scholar] [CrossRef]
- Ghadiri, M.; Young, P.M.; Traini, D. Strategies to Enhance Drug Absorption via Nasal and Pulmonary Routes. Pharmaceutics 2019, 11, 113. [Google Scholar] [CrossRef]
- Mistry, A.; Glud, S.Z.; Kjems, J.; Randel, J.; Howard, K.A.; Stolnik, S.; Illum, L. Effect of physicochemical properties on intranasal nanoparticle transit into murine olfactory epithelium. J. Drug Target. 2009, 17, 543–552. [Google Scholar] [CrossRef]
- Agbo, C.P.; Ugwuanyi, T.C.; Ugwuoke, W.I.; McConville, C.; Attama, A.A.; Ofokansi, K.C. Intranasal artesunate-loaded nanostructured lipid carriers: A convenient alternative to parenteral formulations for the treatment of severe and cerebral malaria. J. Control. Release 2021, 334, 224–236. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Khames, A.; Genedy, S.; Mostafa, S.; Khaleel, M.A.; Omar, M.M.; El Sisi, A.M. Sesame Oil-Based Nanostructured Lipid Carriers of Nicergoline, Intranasal Delivery System for Brain Targeting of Synergistic Cerebrovascular Protection. Pharmaceutics 2021, 13, 581. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Posadas, Y.; Miranda, J.; Uc, Y.P.; Ortega-Olvera, M.J.; Hernández, S. Strategies that Target Tight Junctions for Enhanced Drug Delivery. Curr. Pharm. Des. 2016, 22, 5313–5346. [Google Scholar] [CrossRef]
- Gabal, Y.M.; Kamel, A.O.; Sammour, O.A.; Elshafeey, A.H. Effect of surface charge on the brain delivery of nanostructured lipid carriers in situ gels via the nasal route. Int. J. Pharm. 2014, 473, 442–457. [Google Scholar] [CrossRef]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Agomelatine-based in situ gels for brain targeting via the nasal route: Statistical optimization, in vitro, and in vivo evaluation. Drug Deliv. 2017, 24, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.-A. Polymer Surface Treatments for Drug Delivery and Wound Healing. Appl. Sci. 2023, 13, 9054. [Google Scholar] [CrossRef]
- Ly, P.-D.; Ly, K.-N.; Phan, H.-L.; Nguyen, H.H.; Duong, V.-A.; Nguyen, H.V. Recent advances in surface decoration of nanoparticles in drug delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Kaur, R.; Gorki, V.; Singh, G.; Kaur, R.; Katare, O.P.; Nirmalan, N.; Singh, B. Intranasal delivery of polymer-anchored lipid nanoconstructs of artemether-lumefantrine in Plasmodium berghei ANKA murine model. J. Drug Deliv. Sci. Technol. 2021, 61, 102114. [Google Scholar] [CrossRef]
- Vieira, A.C.C.; Chaves, L.L.; Pinheiro, S.; Pinto, S.; Pinheiro, M.; Lima, S.C.; Ferreira, D.; Sarmento, B.; Reis, S. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int. J. Pharm. 2018, 536, 478–485. [Google Scholar] [CrossRef]
- Saini, S.; Sharma, T.; Jain, A.; Kaur, H.; Katare, O.P.; Singh, B. Systematically designed chitosan-coated solid lipid nanoparticles of ferulic acid for effective management of Alzheimer’s disease: A preclinical evidence. Colloids Surf. B Biointerfaces 2021, 205, 111838. [Google Scholar] [CrossRef]
- Singh, S.K.; Hidau, M.K.; Gautam, S.; Gupta, K.; Singh, K.P.; Singh, S.K.; Singh, S. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: Pharmacokinetic and teratogenic assessment. Int. J. Biol. Macromol. 2018, 108, 1092–1100. [Google Scholar] [CrossRef]
- Pokharkar, V.; Patil-Gadhe, A.; Palla, P. Efavirenz loaded nanostructured lipid carrier engineered for brain targeting through intranasal route: In-vivo pharmacokinetic and toxicity study. Biomed. Pharmacother. 2017, 94, 150–164. [Google Scholar] [CrossRef]
- Brunner, J.; Ragupathy, S.; Borchard, G. Target specific tight junction modulators. Adv. Drug Deliv. Rev. 2021, 171, 266–288. [Google Scholar] [CrossRef]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert. Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef]
- A, P.; Agrawal, M.; Dethe, M.R.; Ahmed, H.; Yadav, A.; Gupta, U.; Alexander, A. Nose-to-brain drug delivery for the treatment of Alzheimer’s disease: Current advancements and challenges. Expert Opin. Drug Deliv. 2022, 19, 87–102. [Google Scholar] [CrossRef]
- Gerber, W.; Svitina, H.; Steyn, D.; Peterson, B.; Kotzé, A.; Weldon, C.; Hamman, J.H. Comparison of RPMI 2650 cell layers and excised sheep nasal epithelial tissues in terms of nasal drug delivery and immunocytochemistry properties. J. Pharmacol. Toxicol. Methods 2022, 113, 107131. [Google Scholar] [CrossRef]
- Boyuklieva, R.; Zagorchev, P.; Pilicheva, B. Computational, In Vitro, and In Vivo Models for Nose-to-Brain Drug Delivery Studies. Biomedicines 2023, 11, 2198. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Duan, S.; Wang, W.; Ouyang, Q.; Qin, F.; Guo, P.; Hou, J.; He, Z.; Wei, W.; Qin, M. Nose-to-brain delivery of nanotherapeutics: Transport mechanisms and applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1956. [Google Scholar] [CrossRef]
- Alireza Mortazavi, S.; Smart, J.D. An in-vitro method for assessing the duration of mucoadhesion. J. Control. Release 1994, 31, 207–212. [Google Scholar] [CrossRef]
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological examination of the mucoadhesive/mucus interaction: The effect of mucoadhesive type and concentration. J. Control. Release 1998, 50, 167–178. [Google Scholar] [CrossRef]
- Li, L.; Wilkins, J.V.; Esmaeili, A.R.; Rahman, N.; Golshahi, L. In Vitro Comparison of Local Nasal Vaccine Delivery and Correlation with Device Spray Performance. Pharm. Res. 2023, 40, 537–550. [Google Scholar] [CrossRef]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, C.; Huang, Y.; Ma, Y.; Song, Q.; Chen, H.; Jiang, G.; Gao, X. Intranasal drug delivery: The interaction between nanoparticles and the nose-to-brain pathway. Adv. Drug Deliv. Rev. 2024, 207, 115196. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.N.; Sousa Lobo, J.M.; Silva, A.C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A current overview of in vivo studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Cagel, M.; Tesan, F.C.; Bernabeu, E.; Salgueiro, M.J.; Zubillaga, M.B.; Moretton, M.A.; Chiappetta, D.A. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur. J. Pharm. Biopharm. 2017, 113, 211–228. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Kanoujia, J.; Kishore, A.; Parashar, P. Progress in Polymeric Micelles as Viable Wagons for Brain Targeting. Curr. Pharm. Des. 2023, 29, 116–125. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Duong, V.-A.; Maeng, H.-J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Sipos, B.; Katona, G.; Csóka, I. Risperidone-Loaded Nasal Thermosensitive Polymeric Micelles: Quality by Design-Based Formulation Study. Pharmaceutics 2024, 16, 703. [Google Scholar] [CrossRef]
- Sipos, B.; Csóka, I.; Budai-Szűcs, M.; Kozma, G.; Berkesi, D.; Kónya, Z.; Balogh, G.T.; Katona, G. Development of dexamethasone-loaded mixed polymeric micelles for nasal delivery. Eur. J. Pharm. Sci. 2021, 166, 105960. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Huang, L.; Ho, P.C. Poly (ethylene glycol)-block-poly (D, L-lactide) (PEG-PLA) micelles for brain delivery of baicalein through nasal route for potential treatment of neurodegenerative diseases due to oxidative stress and inflammation: An in vitro and in vivo study. Int. J. Pharm. 2020, 591, 119981. [Google Scholar] [CrossRef]
- Wang, F.; Yang, Z.; Liu, M.; Tao, Y.; Li, Z.; Wu, Z.; Gui, S. Facile nose-to-brain delivery of rotigotine-loaded polymer micelles thermosensitive hydrogels: In vitro characterization and in vivo behavior study. Int. J. Pharm. 2020, 577, 119046. [Google Scholar] [CrossRef]
- Sayed, S.; Elsharkawy, F.M.; Amin, M.M.; Shamsel-Din, H.A.; Ibrahim, A.B. Brain targeting efficiency of intranasal clozapine-loaded mixed micelles following radio labeling with Technetium-99m. Drug Deliv. 2021, 28, 1524–1538. [Google Scholar] [CrossRef]
- Fatma, M.E.; Maha, M.A.; Hesham, A.S.-D.; Walla, I.; Ahmed, B.I.; Sinar, S. Self-Assembling Lecithin-Based Mixed Polymeric Micelles for Nose to Brain Delivery of Clozapine: In-vivo Assessment of Drug Efficacy via Radiobiological Evaluation. Int. J. Nanomed. 2023, 18, 1577–1595. [Google Scholar] [CrossRef]
- Sharifian, A.; Varshosaz, J.; Aliomrani, M.; Kazemi, M. Nose to brain delivery of ibudilast micelles for treatment of multiple sclerosis in an experimental autoimmune encephalomyelitis animal model. Int. J. Pharm. 2023, 638, 122936. [Google Scholar] [CrossRef]
- Pokharkar, V.; Suryawanshi, S.; Dhapte-Pawar, V. Exploring micellar-based polymeric systems for effective nose-to-brain drug delivery as potential neurotherapeutics. Drug Deliv. Transl. Res. 2020, 10, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Abo El-Enin, H.A.; Ahmed, M.F.; Naguib, I.A.; El-Far, S.W.; Ghoneim, M.M.; Alsalahat, I.; Abdel-Bar, H.M. Utilization of Polymeric Micelles as a Lucrative Platform for Efficient Brain Deposition of Olanzapine as an Antischizophrenic Drug via Intranasal Delivery. Pharmaceuticals 2022, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Sipos, B.; Bella, Z.; Gróf, I.; Veszelka, S.; Deli, M.A.; Szűcs, K.F.; Sztojkov-Ivanov, A.; Ducza, E.; Gáspár, R.; Kecskeméti, G.; et al. Soluplus® promotes efficient transport of meloxicam to the central nervous system via nasal administration. Int. J. Pharm. 2023, 632, 122594. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Pandey, P.; Falconer, J.R.; Siskind, D.J.; Balmanno, A.; Parekh, H.S. Clozapine-Encapsulated Binary Mixed Micelles in Thermosensitive Sol-Gels for Intranasal Administration. Gels 2022, 8, 38. [Google Scholar] [CrossRef]
- Sipos, B.; Szabó-Révész, P.; Csóka, I.; Pallagi, E.; Dobó, D.G.; Bélteky, P.; Kónya, Z.; Deák, Á.; Janovák, L.; Katona, G. Quality by Design Based Formulation Study of Meloxicam-Loaded Polymeric Micelles for Intranasal Administration. Pharmaceutics 2020, 12, 697. [Google Scholar] [CrossRef]
- Plapied, L.; Duhem, N.; des Rieux, A.; Préat, V. Fate of polymeric nanocarriers for oral drug delivery. Curr. Opin. Colloid. Interface Sci. 2011, 16, 228–237. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Raman, S.; Mahmood, S.; Hilles, A.R.; Javed, M.N.; Azmana, M.; Al-Japairai, K.A.S. Polymeric Nanoparticles for Brain Drug Delivery—A Review. Curr. Drug Metab. 2020, 21, 649–660. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Ma, C.; Li, T.; Yang, L. Preparation of baicalin-loaded ligand-modified nanoparticles for nose-to-brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022, 29, 1282–1298. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.T.; Li, N.; Zhu, X.; Li, Y.; Bansal, S.; Wang, Y.; Al-Jamal, K.T. Intranasal administration of edaravone nanoparticles improves its stability and brain bioavailability. J. Control. Release 2023, 359, 257–267. [Google Scholar] [CrossRef]
- Katona, G.; Sipos, B.; Budai-Szűcs, M.; Balogh, G.T.; Veszelka, S.; Gróf, I.; Deli, M.A.; Volk, B.; Szabó-Révész, P.; Csóka, I. Development of In Situ Gelling Meloxicam-Human Serum Albumin Nanoparticle Formulation for Nose-to-Brain Application. Pharmaceutics 2021, 13, 646. [Google Scholar] [CrossRef]
- Hard, S.; Shivakumar, H.N.; Redhwan, M.A.M. Development and optimization of in-situ gel containing chitosan nanoparticles for possible nose-to-brain delivery of vinpocetine. Int. J. Biol. Macromol. 2023, 253, 127217. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Wang, J.; Xu, X.; Ni, S.; Liu, M.; Hu, K. Nose to brain delivery of Astragaloside IV by β-Asarone modified chitosan nanoparticles for multiple sclerosis therapy. Int. J. Pharm. 2023, 644, 123351. [Google Scholar] [CrossRef]
- Huang, L.; Deng, M.; Zhang, S.; Lu, S.; Gui, X.; Fang, Y. β-asarone and levodopa coadministration increases striatal levels of dopamine and levodopa and improves behavioral competence in Parkinson’s rat by enhancing dopa decarboxylase activity. Biomed. Pharmacother. 2017, 94, 666–678. [Google Scholar] [CrossRef]
- Uppuluri, C.T.; Ravi, P.R.; Dalvi, A.V. Design and evaluation of thermo-responsive nasal in situ gelling system dispersed with piribedil loaded lecithin-chitosan hybrid nanoparticles for improved brain availability. Neuropharmacology 2021, 201, 108832. [Google Scholar] [CrossRef]
- Ullah, I.; Chung, K.; Bae, S.; Li, Y.; Kim, C.; Choi, B.; Nam, H.Y.; Kim, S.H.; Yun, C.O.; Lee, K.Y.; et al. Nose-to-Brain Delivery of Cancer-Targeting Paclitaxel-Loaded Nanoparticles Potentiates Antitumor Effects in Malignant Glioblastoma. Mol. Pharm. 2020, 17, 1193–1204. [Google Scholar] [CrossRef]
- Sharma, S.; Tyagi, A.; Dang, S. Nose to Brain Delivery of Transferrin conjugated PLGA nanoparticles for clonidine. Int. J. Biol. Macromol. 2023, 252, 126471. [Google Scholar] [CrossRef]
- Salem, H.F.; Ali, A.A.; Rabea, Y.K.; Abo El-Ela, F.I.; Khallaf, R.A. Optimization and Appraisal of Chitosan-Grafted PLGA Nanoparticles for Boosting Pharmacokinetic and Pharmacodynamic Effect of Duloxetine HCl Using Box-Benkhen Design. J. Pharm. Sci. 2023, 112, 544–561. [Google Scholar] [CrossRef]
- Sharma, S.; Gauba, P.; Tyagi, A.; Dang, S. Chitosan-modified polymeric nanoparticles for the nose-to-brain drug delivery of paroxetine: An in vitro and in vivo evaluation. Nanoscale 2025, 17, 1687–1702. [Google Scholar] [CrossRef]
- Elzayat, A.; Adam-Cervera, I.; Álvarez-Bermúdez, O.; Muñoz-Espí, R. Nanoemulsions for synthesis of biomedical nanocarriers. Colloids Surf. B Biointerfaces 2021, 203, 111764. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Ahmad, F.J.; Ahmad, W.; Alam, M.A.; Amir, M.; Ali, A. Poloxamer-chitosan-based Naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J. Biol. Sci. 2020, 27, 500–517. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, S.A.; Kokare, C.R.; Shrivastava, B.; Gorain, B.; Choudhury, H. Antipsychotic Potential and Safety Profile of TPGS-Based Mucoadhesive Aripiprazole Nanoemulsion: Development and Optimization for Nose-To-Brain Delivery. J. Pharm. Sci. 2021, 110, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.J.; Parikh, R.H. Intranasal delivery of topiramate nanoemulsion: Pharmacodynamic, pharmacokinetic and brain uptake studies. Int. J. Pharm. 2020, 585, 119486. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Kumar, S.; Ali, J.; Baboota, S. Intranasal delivery of tetrabenazine nanoemulsion via olfactory region for better treatment of hyperkinetic movement associated with Huntington’s disease: Pharmacokinetic and brain delivery study. Chem. Phys. Lipids 2020, 230, 104917. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bisht, B.; Dutta, S.; Paul, M.K. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol. Sin. 2022, 43, 2759–2776. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Saka, R.; Chella, N.; Khan, W. Development of Imatinib Mesylate-Loaded Liposomes for Nose to Brain Delivery: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 192. [Google Scholar] [CrossRef]
- Li, R.; Lu, F.; Sun, X.; He, L.; Duan, H.; Peng, W.; Wu, C. Development and in vivo Evaluation of Hydroxy-α-Sanshool Intranasal Liposomes as a Potential Remedial Treatment for Alzheimer’s Disease. Int. J. Nanomed. 2022, 17, 185–201. [Google Scholar] [CrossRef]
- Adnet, T.; Groo, A.-C.; Picard, C.; Davis, A.; Corvaisier, S.; Since, M.; Bounoure, F.; Rochais, C.; Le Pluart, L.; Dallemagne, P.; et al. Pharmacotechnical Development of a Nasal Drug Delivery Composite Nanosystem Intended for Alzheimer’s Disease Treatment. Pharmaceutics 2020, 12, 251. [Google Scholar] [CrossRef]
- Motawea, A.; Maria, S.N.; Maria, D.N.; Jablonski, M.M.; Ibrahim, M.M. Genistein transfersome-embedded topical delivery system for skin melanoma treatment: In vitro and ex vivo evaluations. Drug Deliv. 2024, 31, 2372277. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A.; Shahid, A.; Sardar, P.K.; Yeung, S.; Reyes, J.; Kim, J.; Parsa, C.; Orlando, R.; Wang, J.; Kelly, K.M.; et al. Transfersome Encapsulated with the R-carvedilol Enantiomer for Skin Cancer Chemoprevention. Nanomaterials 2023, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Aboud, H.M.; Abdellatif, M.M.; Abou-Taleb, H.A. Nose-to-Brain Targeted Delivery of Donepezil Hydrochloride via Novel Hyaluronic Acid-Doped Nanotransfersomes for Alzheimer’s Disease Mitigation. J. Pharm. Sci. 2024, 113, 1934–1945. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Zaki, R.M.; Alsaidan, O.A.; Elmowafy, M.; Zafar, A.; Shalaby, K.; Abdelgawad, M.A.; Abo El-Ela, F.I.; Rateb, M.E.; Naguib, I.A.; et al. Intranasal Nanotransferosomal Gel for Quercetin Brain Targeting: I. Optimization, Characterization, Brain Localization, and Cytotoxic Studies. Pharmaceutics 2023, 15, 1805. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Sedaghatnia, K.; Bourbour, M.; Fatemizadeh, M.; Salehi Moghaddam, Z.; Hejabi, F.; Heidari, F.; Quazi, S.; Farasati Far, B. Niosomes: A novel targeted drug delivery system for cancer. Med. Oncol. 2022, 39, 240. [Google Scholar] [CrossRef]
- Varshney, S.; Alam, M.A.; Kaur, A.; Dhoundiyal, S. Niosomes: A Smart Drug Delivery System for Brain Targeting. Pharm. Nanotechnol. 2024, 12, 108–125. [Google Scholar] [CrossRef]
- Bekhet, M.A.; Ali, A.A.; Kharshoum, R.M.; El-Ela, F.I.A.; Salem, H.F. Intranasal Niosomal In Situ Gel As A Novel Strategy for Improving Citicoline Efficacy and Brain Delivery in Treatment of Epilepsy: In Vitro and Ex Vivo Characterization and In Vivo Pharmacodynamics Investigation. J. Pharm. Sci. 2022, 111, 2258–2269. [Google Scholar] [CrossRef]
- Ourani-Pourdashti, S.; Mirzaei, E.; Heidari, R.; Ashrafi, H.; Azadi, A. Preparation and evaluation of niosomal chitosan-based in situ gel formulation for direct nose-to-brain methotrexate delivery. Int. J. Biol. Macromol. 2022, 213, 1115–1126. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J.; Chi, S.-C. Preparation of Ondansetron Hydrochloride-Loaded Nanostructured Lipid Carriers Using Solvent Injection Method for Enhancement of Pharmacokinetic Properties. Pharm. Res. 2019, 36, 138. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; Abd El Aziz, A.E.; Mohamed, S.A.; AbuelEzz, N.Z. The tiny big world of solid lipid nanoparticles and nanostructured lipid carriers: An updated review. J. Microencapsul. 2022, 39, 72–94. [Google Scholar] [CrossRef]
- Yadav, R.K.; Shah, K.; Dewangan, H.K. Intranasal drug delivery of sumatriptan succinate-loaded polymeric solid lipid nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2022, 48, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J.; Chi, S.-C. Nanostructured lipid carriers containing ondansetron hydrochloride by cold high-pressure homogenization method: Preparation, characterization, and pharmacokinetic evaluation. J. Drug Deliv. Sci. Technol. 2019, 53, 101185. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Masjedi, M.; Azadi, A.; Heidari, R.; Mohammadi-Samani, S. Nose-to-brain delivery of sumatriptan-loaded nanostructured lipid carriers: Preparation, optimization, characterization and pharmacokinetic evaluation. J. Pharm. Pharmacol. 2020, 72, 1341–1351. [Google Scholar] [CrossRef]
- Rojanaratha, T.; Tienthai, P.; Woradulayapinij, W.; Yimsoo, T.; Boonkanokwong, V.; Ritthidej, G.C. Preparation, physicochemical characterization, ex vivo, and in vivo evaluations of asiatic acid-loaded solid lipid nanoparticles formulated with natural waxes for nose-to-brain delivery. Eur. J. Pharm. Sci. 2024, 203, 106935. [Google Scholar] [CrossRef]
- Islamie, R.; Myint, S.L.L.; Rojanaratha, T.; Ritthidej, G.; Wanakhachornkrai, O.; Wattanathamsan, O.; Rodsiri, R. Neuroprotective effect of nose-to-brain delivery of Asiatic acid in solid lipid nanoparticles and its mechanisms against memory dysfunction induced by Amyloid Beta(1-42) in mice. BMC Complement. Med. Ther. 2023, 23, 294. [Google Scholar] [CrossRef]
- Khedkar, M.A.; Sharma, V.; Anjum, M.; Singh, S.; Shah, K.; Alam, P.; Dewangan, H.K. Paliperidone-loaded nose to brain targeted NLCS: Optimisation, evaluation, histopathology and pharmacokinetic estimation for schizophernia. J. Microencapsul. 2024, 41, 832–843. [Google Scholar] [CrossRef]
- Gadhave, D.; Rasal, N.; Sonawane, R.; Sekar, M.; Kokare, C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int. J. Biol. Macromol. 2021, 167, 906–920. [Google Scholar] [CrossRef]
- Wang, G.; Zhai, Z.; Wang, W.; Xia, X.; Guo, H.; Yue, X.; Wang, X.; Zhu, B.; Huang, Z.; Pan, X.; et al. Tailored Borneol-Modified Lipid Nanoparticles Nasal Spray for Enhanced Nose-to-Brain Delivery to Central Nervous System Diseases. ACS Nano 2024, 18, 23684–23701. [Google Scholar] [CrossRef]
- Vitorino, C.; Silva, S.; Gouveia, F.; Bicker, J.; Falcão, A.; Fortuna, A. QbD-driven development of intranasal lipid nanoparticles for depression treatment. Eur. J. Pharm. Biopharm. 2020, 153, 106–120. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Subhash Hinge, N.; Kathuria, H.; Monohar Pandey, M. Rivastigmine-DHA ion-pair complex improved loading in hybrid nanoparticles for better amyloid inhibition and nose-to-brain targeting in Alzheimer’s. Eur. J. Pharm. Biopharm. 2023, 190, 131–149. [Google Scholar] [CrossRef]
- Sánchez-Dengra, B.; Alfonso, M.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M.; Martínez-Máñez, R. Intranasal administration of molecular-gated mesoporous nanoparticles to increase ponatinib delivery to the brain. Nanomedicine 2023, 18, 1799–1813. [Google Scholar] [CrossRef]
- Patel, H.P.; Chaudhari, P.S.; Gandhi, P.A.; Desai, B.V.; Desai, D.T.; Dedhiya, P.P.; Vyas, B.A.; Maulvi, F.A. Nose to brain delivery of tailored clozapine nanosuspension stabilized using (+)-alpha-tocopherol polyethylene glycol 1000 succinate: Optimization and in vivo pharmacokinetic studies. Int. J. Pharm. 2021, 600, 120474. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
| Drug | Components | Outcome | Ref. |
|---|---|---|---|
| Risperidone | Poloxamer 407 and 188 | Size: 118 nm, PDI: 0.315 Increased permeation across a cellulose membrane | [98] |
| Dexamethasone | PCL-PVAc-PEG, TPGS | Size: 90 nm, PDI: 0.216, ZP: –21.1 mV, EE: 93.4% Increased aqueous solubility (14-fold), enhanced permeability (in vitro passive diffusion test and parallel artificial membrane permeability assay) | [99] |
| Baicalein | PEG-PLA | Size: 25 nm, PDI: 0.239, ZP: –7.3 mV, EE: 70%, DL: 1.39% Reduced drug toxicity, reduced inflammatory factors TNF-α and IL-6 Increased BA (inhalation, mice): 5.09-fold (plasma) and 1.50-fold (brain) | [100] |
| Rotigotine | mPEG-PLGA; poloxamer 407 and 188 (gelling agents) | Size: 88 nm, PDI: 0.237, EE: 94%, DL: 19.9% Sustained release, no obvious side effects on the nasal cilia and rat nasal mucosa Increased drug distribution in olfactory bulb (276.6%), cerebrum (170.5%), cerebellum (166.5%), and striatum (184.4%). | [101] |
| Clozapine | Tetronic® 904, Tetronic® 701, Synperonic® PE/F127 | Size: 217 nm, PDI: 0.24 Increased nasal permeation ex vivo (5-fold) Increased brain distribution in mice (vs. IV) with DTE = 396.5% | [102] |
| Clozapine | SPC, SDC | Size: 12.2 nm, PDI: 0.24, ZP: –38 mV, EE: 93%, DL: 6.47% Higher ex vivo permeation (3-fold) Rapid onset (15 min) and higher brain bioavailability (vs. IV) | [103] |
| Ibudilast | Surfactin, polydopamine (for coating) | Size: 175 nm, PDI: 0.3, ZP: –41 mV, EE: 87.6% Increased drug distribution to mouse brain Positive outcome in treating multiple sclerosis (anti-inflammation and neuroprotection) | [104] |
| Lurasidone | Gelucire 44/14, Poloxamer 407; carbopol 940 (gelling agent) | Size: 175 nm, EE: 97.8% Increased nasal permeation ex vivo (1.3-fold) Increased brain distribution in rat (vs. IV) with DTE = 394% and DTP = 74% | [105] |
| Olanzapine | Poloxamer 407, Pluronic P123, TPGS | Size: 39 nm, ZP: –15 mV, EE: 82% Increased brain distribution in rats with DTE = 535.9% and DTP = 81.3% Improved anti-schizophrenia-related deficits via the paw test and open field test Safe as indicated by histopathological examination | [106] |
| Meloxicam | PCL-PVAc-PEG | Size: 101 nm, PDI: 0.149, EE: 94%, ZP: –25.2 mV, EE: 89% Increase permeation across the culture model of the nasal mucosa barrier (5-fold) AUCbrain is only 0.65% of AUCplasma | [107,109] |
| Clozapine | Polysorbate 20 & 80, Poloxamer 407 | Size: 17–20 nm, PDI: 0.3, ZP: –2.7 mV Reduced permeation across nasal mucosal tissues No in vivo data | [108] |
| Drug | Components | Outcome | Ref. |
|---|---|---|---|
| Edaravone | PLGA, PLGA-PEG | Size: 90 nm, PDI: 0.214, ZP: –11.9 mV, EE: 20.58%, DL: 3.02% Reduced oxidative stress toxicity in mouse microglial cell line BV-2 Increased brain distribution in mice (vs. IV free drug and IV NPs) | [114] |
| Meloxicam | Human serum albumin; Poloxamer 407 (gelling agent) | Size: 176 nm, PDI: 0.205, ZP: –7.9 mV, EE: 81.64%, DL: 1.09% Increased drug permeation ex vivo | [115] |
| Baicalin | PEG-PLGA, RVG29 peptide | Size: 89–130 nm, PDI: 0.1–0.3 Reduced neurological dysfunction and oxidative stress in rats with ischemic brain injury | [113] |
| Vinpocetine | CS; Poloxamer 407 and Poloxamer 188 (gelling agent) | Size: 130.6 nm, PDI: 0.125, ZP: 40.81 mV, EE: 97.56% Increased Cmax (2.2-fold) and AUC (1.7-fold) in rat brain (vs. oral tablets) | [116] |
| Astragaloside IV | CS, β-asarone | Size: 118 nm, PDI: 0.253, ZP: 22.7 mV, DL: 0.14% Increased in vitro uptake (1.52-fold) and brain delivery (2.49-fold) (β-asarone-CS-NP vs. CS-NP). Reduced behavioral scores, decreased weight loss, suppressed inflammatory infiltration and astrocyte/microglial activation, reduced demyelination, and increased remyelination on an EAE mouse model. | [117] |
| Piribedil | CS, lecithin, methylcellulose in situ gel | Size: 147 nm, PDI: 0.29, ZP: 18.1 mV, EE: 53.5%, DL: 12.1% Increased brain bioavailability (IN NP gel > IN NP suspension > IN free drug suspension) with DTE = 228% and DTP = 56% for IN NP gel and DTE = 140% and DTP = 29% for IN NP suspension | [119] |
| Paclitaxel | PLGA, arginyl-glycyl-aspartic tripeptide | Size: 197 nm, PDI: 0.192, DL: 2.8% Increased brain accumulation in rats Reduced 72% tumor volume in rats implanted with C6 glioblastoma cells Reduced 75% tumor volume in mice implanted with U87MG glioblastoma cells | [120] |
| Clonidine | PLGA, transferrin | Size: 200 nm, PDI: 0.291, ZP: –17.4 mV, EE: 86.2%, DL: 7.8% Increased Neuro-2a cell uptake (97% vs. 82%) Increased drug concentration in mouse brain (2.4-fold vs. IN free drug) Improved behavioral responses | [121] |
| Paroxetine | CS, PLGA | Size: 182 nm, ZP: 36.3 mV, EE: 87.5%, DL: 13.4% Increased drug concentration in mouse brain Improved behavioral responses in forced swimming test and locomotor activity test | [123] |
| Duloxetine | PLGA, CS, PVA | Size: 122 nm, EE: 66.95% Increased ex vivo permeation (4-fold vs. drug solution) Improved behavior via the force-swimming test, tail suspension test, sucrose preference test, open field test, and novelty suppressed feeding Increased Cmax (3.33-fold), AUC (3.57-fold), t1/2 (1.76-fold), and MRT (1.43-fold) in rat brain vs. oral free drug | [122] |
| Nanocarrier | Drug | Components | Outcome | Ref. |
|---|---|---|---|---|
| NEs | Naringenin | Capmul MCM, Tween-80, PEG-400; Poloxamer 407 (gelling agent); CS (mucoadhesive agent) | Size: 98 nm, PDI: 0.386 Increased brain bioavailability with DTE = 1224% and DTP = 99.5% Improved locomotor activity and grip strength in rats Increased antioxidant enzyme levels (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase) | [125] |
| NEs | Aripiprazole | Capmul PG-8, TPGS, Transcutol-HP; Carbopol 971 (gelling agent) | Size: 140 nm, PDI: 0.401, ZP: −16.87 mV Increased ex vivo permeation (~2-fold vs. drug solution) Increased Cmax and AUC in rat brain. DTE = 974%, DTP = 89.73% Improved behaviors in rats (catalepsy, induced locomotor activity, and paw test) | [126] |
| NEs | Topiramate | Capmul MCM C8, Tween 20, Carbitol | Size: 4.73 nm, PDI: 0.206, ZP: 10.74 mV Decreased seizure duration (vs. oral NEs, IN free drug, and oral free drug) in rats Increased drug distribution to rat brains (vs. oral NEs, IN free drug, and oral dree drug) | [127] |
| NEs | Tetrabenazine | Capmul MCM, Tween 80, Transcutol P | Size: 106.8 nm, PDI: 0.198, ZP: –9.63 mV Increased ex vivo permeation (1.68-fold vs. free drug) Increased Cmax (4-fold) and AUC (6.07-fold) in rat brains vs. IV drug solution DTE = 1666%, DTP = 94% | [128] |
| Liposomes | Imatinib mesylate | Egg PC, cholesterol, and cardiolipin | Size: 102 nm, PDI: 0.28, ZP: −23 mV Increased AUC in rat brains (7-fold) vs. oral and IN-free drugs | [132] |
| Liposomes | Hydroxy-α-sanshool | Soybean lecithin, cholesterol | Size: 182 nm, PDI: 0.207, ZP: −54 mV, EE: 73% Increased mouse plasma (1.7-fold) and brain (2.1-fold) bioavailability (vs. free drug) | [133] |
| Transfersomes | Donepezil | PC, HA, Tween 80 | Size: 227.5 nm, EE: 75.8% Increased Cmax (4.12-fold), AUC (3.98-fold), t1/2 (1.89-fold), and MRT (1.82-fold) in rat brain vs. IV free drug | [137] |
| Transfersomes | Quercetin | Lecithin, sodium deoxycholate; Carbopol 971P, Poloxamer 188, Poloxamer 407 (gelling agents) | Size: 171 nm, ZP: −32.6 mV, EE: 78.2% Increased ex vivo permeation (2-fold vs. free drug gel) Increased drug accumulation in rat brain | [138] |
| Niosomes | Citicoline | Cholesterol, Span-60; Pluronic F-127, Pluronic F-68, HPMC K15M (gelling agents) | Size: 209 nm, ZP: −55.3 mV, EE: 36.65% Improved anticonvulsant activity against rats with pentylenetetrazole seizure induction (vs. oral free drug) | [141] |
| Niosomes | Methotrexate | Cholesterol, Span-60; Poloxamer 407, CS (gelling agents) | Size: 130.5 nm, PDI: 0.536, ZP: –38.5 mV, EE: 91.39% Increased brain-to-plasma concentration ratio (free drug < drug-loaded gel < drug-loaded niosomes < drug-loaded niosomal gel) | [142] |
| NLCs | Sumatriptan | Stearic acid, cholesterol, triolein, Brij 35 | Size: 101 nm, PDI: 0.27, EE: 91% Increased bain bioavailability with DTE = 258% and DTP = 61.2% in rats | [148] |
| SLNs | Asiatic acid | Rice bran wax, Tween 80, soybean lecithin | Size: 197 nm, PDI: 0.25, ZP: –31.6 mV, EE: 99.9% Increased brain distribution in mice (vs. IN solution and IV SLNs) | [149] |
| SLNs | Asiatic acid | N/A | Improved spatial memory dysfunction, recognition memory impairment, reduced tau hyperphosphorylation, inhibited glial activation and lipid peroxidation in Aβ1-42-injected rats. | [150] |
| NLCs | Paliperidone | Glyceryl monostearate, oleic acid, Tween 80 | Size: 129 nm, PDI: 0.304, ZP: −7.61 mV, EE: 58.16% Increased ex vivo drug permeation (3-fold vs. free drug) Increased drug delivery to rat brain | [151] |
| NLCs | Teriflunomide | Glyceryl di-behenate, glyceryl mono-linoleate, Gelucire 44/14; Carbopol 974P and gellan gum (gelling agents) | Size: 117.8 nm, PDI: 0.56, ZP: −21.86 mV, EE: 81.16% Increased permeability coefficient (1.53-fold vs. NPs) Increased Cmax (2-fold) and AUC (1.34-fold) in mouse brain (vs. IV and IN NPs) | [152] |
| SLNs | Sumatriptan | Soya lecithin, CS, tripalmitin | Size: 133 nm, ZP: –17.7 mV, EE: 75.4% Safety via histopathological evaluation of mucosal tissue | [145] |
| LNPs | Rizatriptan | EPC, cholesterol | Size: ~100 nm, PDI: ~0.25, ZP: ~ –23 mV Quick onset (5 min), higher Cmax and AUC in rats (vs. oral tablets and IV) Prolonged drug concentration in the brain for 120 min Reduced abnormal behavior duration by 32.04%. | [153] |
| LNPs | Rizatriptan | EPC, cholesterol, borneol | Size: ~120 nm, PDI: ~0.2, ZP: ~ –20 mV Increased drug absorption in nasal mucosa (1.37-fold), AUCbrain (1.23-fold) vs. non-modified LNPs. Reduced abnormal behavior duration by 56.64%. Alleviated symptoms of neuroinflammation-induced hyperalgesia | [153] |
| NLCs | Fluoxetine | Precirol™ ATO 5, Lauroglycol™ 90, Tween® 80 | Size: 154 nm, PDI: 0.514, ZP: 19.7 mV, EE: 74%, DL: 12.9% Reduced depressive and anxiety-like behaviors of mice in the marble-burying test and forced swimming test | [154] |
| SLNs | Asiatic acid | Rice bran wax, Tween 80, soybean lecithin | Size: 197 nm, PDI: 0.25, ZP: –31.6 mV, EE: 99.9% Increased brain distribution in mice (vs. IN solution and IV SLNs) | [149] |
| Nanocarrier | Drug | Components | Outcome | Ref. |
|---|---|---|---|---|
| PLNs | Rivastigmine-DHA | PLGA, stearyl amine, Miglyol 812, Span-80; Poloxamer 407 and 188 (gelling agents) | Size: 132 nm, PDI: 0.284, ZP: 36.4 mV, EE: 83.6% Increased ex vivo nasal permeation (4.07-fold vs. free drug gel) Increased mucociliary time (2-fold vs. free drug gel) Increased Cmax (2.37-fold), MRT (9.26-fold), and AUCbrain (7.67-fold) (vs. IN free drug gel) DTE = 792.5%, DTP = 87.4% | [156] |
| PLNs | Rivastigmine-DHA | PLGA, glyceryl monostearate, PEG-32-stearate; Poloxamer 407 and 188 (gelling agents) | Size: 160 nm, PDI: 0.254, ZP: –39.3 mV, EE: 88.2% Increased ex vivo nasal permeation (3.18-fold vs. free drug gel) Increased mucociliary time (1.4-fold vs. free drug gel) Increased Cmax (1.99-fold), MRT (5.63-fold), and AUCbrain (5.18-fold) (vs. IN free drug gel) DTE = 672.3%, DTP = 85.1% | [156] |
| Silica NPs | Ponatinib | Cetyltrimethylammonium bromide, diethanolamine, tetraethylorthosilicate | Increased in vitro BBB permeability (vs. free drug) Increased drug concentration in rat brains at 48 h (vs. free drug) | [157] |
| Nanosuspension | Clozapine | TPGS, PVP K-30 | Size: 281 nm Increased drug concentration in rat brains (3.56-fold with a 528-fold lower dose vs. conventional suspension) | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.-T.-L.; Duong, V.-A. Advancements in Nanocarrier Systems for Nose-to-Brain Drug Delivery. Pharmaceuticals 2025, 18, 615. https://doi.org/10.3390/ph18050615
Nguyen T-T-L, Duong V-A. Advancements in Nanocarrier Systems for Nose-to-Brain Drug Delivery. Pharmaceuticals. 2025; 18(5):615. https://doi.org/10.3390/ph18050615
Chicago/Turabian StyleNguyen, Thi-Thao-Linh, and Van-An Duong. 2025. "Advancements in Nanocarrier Systems for Nose-to-Brain Drug Delivery" Pharmaceuticals 18, no. 5: 615. https://doi.org/10.3390/ph18050615
APA StyleNguyen, T.-T.-L., & Duong, V.-A. (2025). Advancements in Nanocarrier Systems for Nose-to-Brain Drug Delivery. Pharmaceuticals, 18(5), 615. https://doi.org/10.3390/ph18050615








