Electrospun Nanofiber-Scaffold-Loaded Levocetirizine Dihydrochloride Cerosomes for Combined Management of Atopic Dermatitis and Methicillin-Resistant Staphylococcus Aureus (MRSA) Skin Infection: In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Analysis of 21.31 Factorial-Design
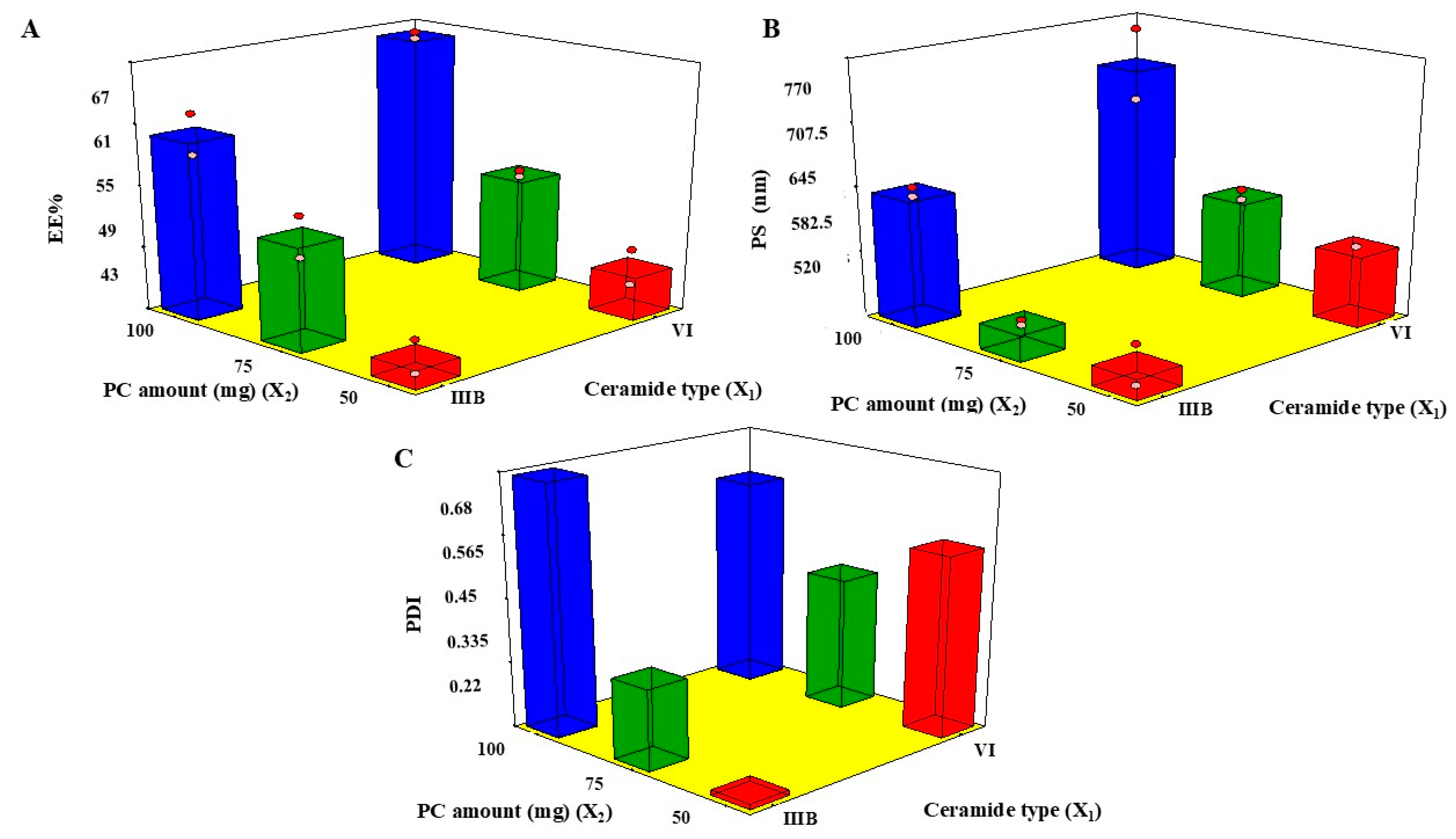
2.2. EE%
2.3. PS
2.4. PDI
2.5. Determination of the Selected CERs
2.6. Effect of Storage
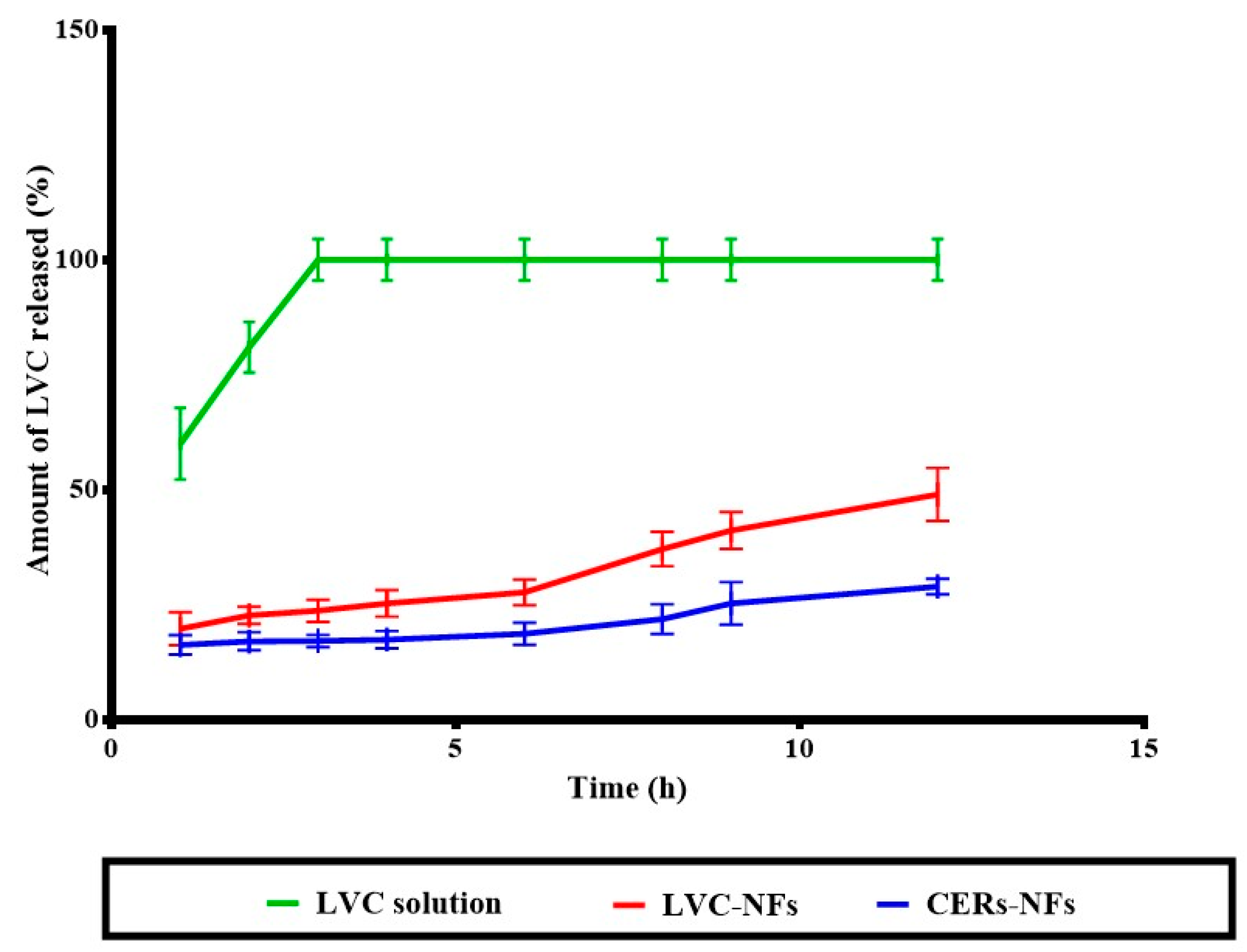
2.7. In Vitro Release
2.8. Morphological Assessment
2.8.1. Scanning Electron Microscopy
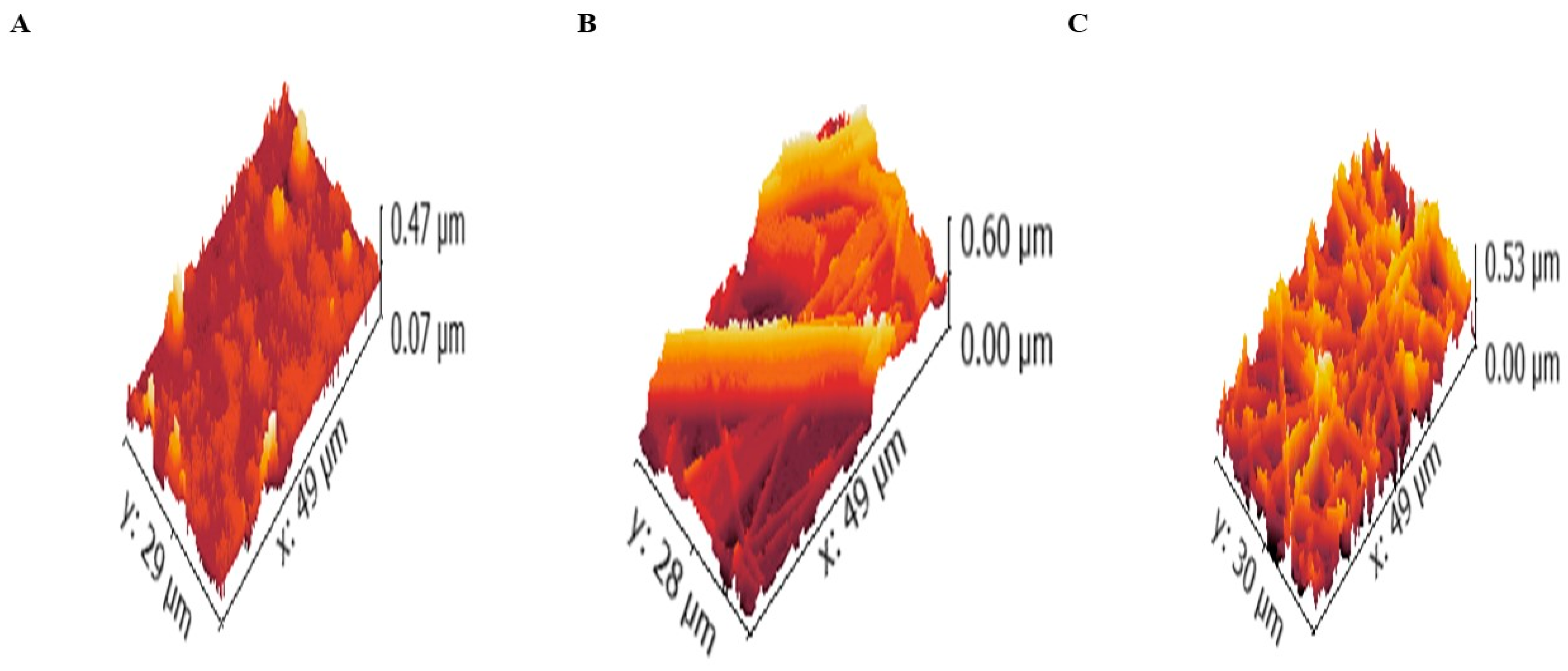
2.8.2. Atomic Force Microscopy (AFM)
2.8.3. Transmission Electron Microscopy (TEM)
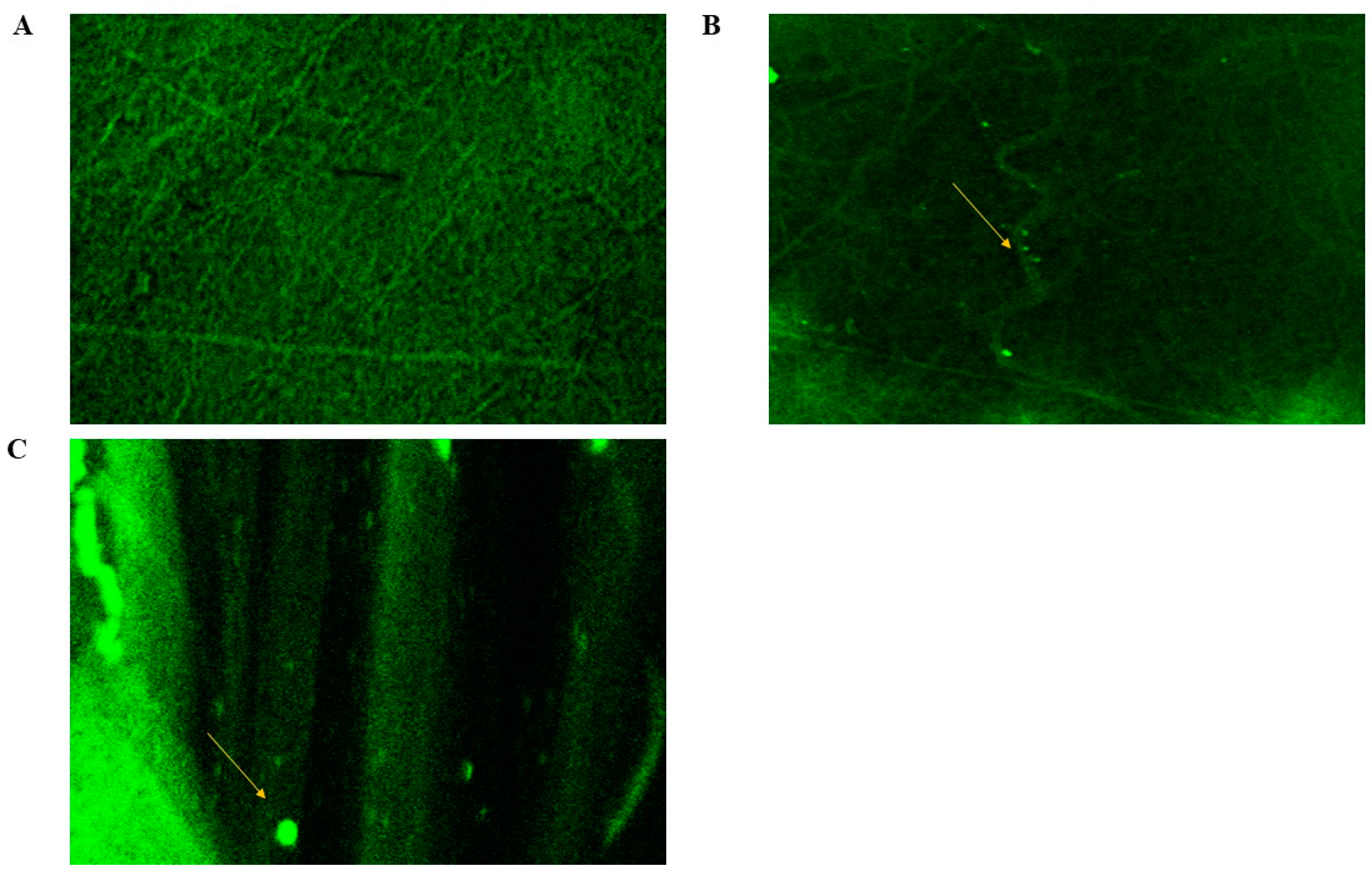
2.8.4. Confocal Laser Scanning Microscopy (CLSM)
2.9. In Vivo Studies
2.9.1. Dermatokinetic Study
2.9.2. Histopathological Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of LVC-CERs
3.2.2. Characterization of LVC-CERs
EE%
PS and PDI
3.2.3. Experimental Design
3.2.4. Effect of Storage
3.2.5. Preparation of the Prepared NFs
3.2.6. In Vitro Release
3.2.7. Morphological Assessment of NFs
SEM
AFM
TEM
CLSM
3.2.8. In Vivo Studies
Animals
In Vivo Dermatokinetic Assessment
Histopathologic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simpson, E.L. Atopic dermatitis: A review of topical treatment options. Curr. Med. Res. Opin. 2010, 26, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Leung, D.Y.M.; Taylor, P.A.; Li, H. Methicillin-Resistant Staphylococcus aureus Colonization Is Associated with Decreased Skin Commensal Bacteria in Atopic Dermatitis. J. Investig. Dermatol. 2018, 138, 1668–1671. [Google Scholar] [CrossRef]
- Imokawa, G. A possible mechanism underlying the ceramide deficiency in atopic dermatitis: Expression of a deacylase enzyme that cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. J. Dermatol. Sci. 2009, 55, 1–9. [Google Scholar] [CrossRef]
- Asano, S.; Ichikawa, Y.; Kumagai, T.; Kawashima, M.; Imokawa, G. Microanalysis of an antimicrobial peptide, β-defensin-2, in the stratum corneum from patients with atopic dermatitis. Br. J. Dermatol. 2008, 159, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Tan, G.; Zhou, J.; He, J.; Lawson, L.B.; Mcpherson, G.L.; John, V.T. Undulating tubular liposomes through incorporation of a synthetic skin ceramide into phospholipid bilayers. Langmuir 2009, 25, 10422–10425. [Google Scholar] [CrossRef]
- Fathi-azarbayjani, A.; Ng, K.X.; Chan, Y.W.; Chan, S.Y. Lipid Vesicles for the Skin Delivery of Diclofenac: Cerosomes vs. Other Lipid Suspensions. Adv. Pharm. Bull. 2015, 5, 25–33. [Google Scholar] [PubMed]
- Abdelgawad, R.; Nasr, M.; Moftah, N.H.; Hamza, M.Y. Phospholipid membrane tubulation using ceramide doping “Cerosomes”: Characterization and clinical application in psoriasis treatment. Eur. J. Pharm. Sci. 2017, 101, 258–268. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Manta, K.; Kostantini, C.; Kikionis, S.; Banella, S.; Ioannou, E.; Christodoulou, E.; Rekkas, D.M.; Dallas, P.; Vertzoni, M.; et al. Nasal powders of quercetin-β-cyclodextrin derivatives complexes with mannitol/lecithin microparticles for Nose-to-Brain delivery: In vitro and ex vivo evaluation. Int. J. Pharm. 2021, 607, 121016. [Google Scholar] [CrossRef]
- Elsadek, N.E.; Nagah, A.; Ibrahim, T.M.; Chopra, H.; Ghonaim, G.A.; Emam, S.E.; Cavalu, S.; Attia, M.S. Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials 2022, 15, 1934. [Google Scholar] [CrossRef]
- Eltabeeb, M.A.; Hamed, R.R.; El-Nabarawi, M.A.; Teaima, M.H.; Hamed, M.I.A.; Darwish, K.M.; Hassan, M.; Abdellatif, M.M. Nanocomposite alginate hydrogel loaded with propranolol hydrochloride kolliphor® based cerosomes as a repurposed platform for Methicillin-Resistant Staphylococcus aureus-(MRSA)-induced skin infection; in-vitro, ex-vivo, in-silico, and in-vivo evaluation. Drug Deliv. Transl. Res. 2024, 15, 556–576. [Google Scholar] [CrossRef]
- Albash, R.; Fahmy, A.M.; Hamed, M.I.A.; Darwish, K.M.; El-Dahmy, R.M. Spironolactone hyaluronic acid enriched cerosomes (HAECs) for topical management of hirsutism: In silico studies, statistical optimization, ex vivo, and in vivo studies. Drug Deliv. 2021, 28, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Goindi, S.; Kumar, G.; Kaur, A. Novel flexible vesicles based topical formulation of levocetirizine: In vivo evaluation using oxazolone-induced atopic dermatitis in murine model. J. Liposome Res. 2014, 24, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Amelian, A.; Wasilewska, K.; Wesoły, M.; Ciosek-Skibińska, P.; Winnicka, K. Taste-masking assessment of orally disintegrating tablets and lyophilisates with cetirizine dihydrochloride microparticles. Saudi Pharm. J. 2017, 25, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Alomar, A.; Bieber, T.; Deleuran, M.; Fink-Wagner, A.; Gelmetti, C.; Gieler, U.; Lipozencic, J.; Luger, T.; Oranje, A.; et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1176–1193. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; El-Nabarawi, M.A.; Teaima, M.H.; Hassan, M.; Hamed, M.I.A.; Elrashedy, A.A.; Albash, R. Integration of terpesomes loaded Levocetrizine dihydrochloride gel as a repurposed cure for Methicillin-Resistant Staphylococcus aureus (MRSA)-Induced skin infection; D-optimal optimization, ex-vivo, in-silico, and in-vivo studies. Int. J. Pharm. 2023, 633, 122621. [Google Scholar] [CrossRef]
- Esentürk, İ.; Balkan, T.; Özhan, G.; Döşler, S.; Güngör, S.; Erdal, M.S.; Sarac, A.S. Voriconazole incorporated nanofiber formulations for topical application: Preparation, characterization and antifungal activity studies against Candida species. Pharm. Dev. Technol. 2020, 25, 440–453. [Google Scholar] [CrossRef]
- Esenturk, I.; Gumrukcu, S.; Sert, A.B.Ö.; Kök, F.N.; Döşler, S.; Gungor, S.; Erdal, M.S.; Sarac, A.S. Silk-fibroin-containing nanofibers for topical sertaconazole delivery: Preparation, characterization, and antifungal activity. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 605–622. [Google Scholar] [CrossRef]
- Ali, I.H.; Khalil, I.A.; El-Sherbiny, I.M. Single-Dose Electrospun Nanoparticles-in-Nanofibers Wound Dressings with Enhanced Epithelialization, Collagen Deposition, and Granulation Properties. ACS Appl. Mater. Interfaces 2016, 8, 14453–14469. [Google Scholar] [CrossRef]
- Bhagwat, G.S.; Athawale, R.B.; Gude, R.P.; Alhakamy, N.A. Formulation and Development of Transferrin Targeted Solid Lipid Nanoparticles for Breast Cancer Therapy. Front. Pharmacol. 2020, 11, 614290. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Buffa, F.; Thomas, V.; Vohra, Y.K.; Abraham, G.A. Biodegradable Polyurethanes: Comparative Study of Electrospun Scaffolds and Films. J. Appl. Polym. Sci. 2011, 121, 3292–3299. [Google Scholar] [CrossRef]
- Akduman, C.; Özgüney, I.; Kumbasar, E.P.A. Preparation and characterization of naproxen-loaded electrospun thermoplastic polyurethane nanofibers as a drug delivery system. Mater. Sci. Eng. C 2016, 64, 383–390. [Google Scholar] [CrossRef]
- Qin, X. Coaxial electrospinning of nanofibers. In Electrospun Nanofibers; Woodhead Publishing: Cambridge, UK, 2017; pp. 41–71. [Google Scholar]
- Jiang, H.; Wang, L.; Zhu, K. Coaxial Electrospinning for Encapsulation and Controlled Release of Fragile Water-soluble Bioactive Agents. J. Control. Release 2014, 193, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203–5221. [Google Scholar] [CrossRef]
- Albash, R.; El-Nabarawi, M.A.; Refai, H.; Abdelbary, A.A. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: Invitro characterization, ex-vivo permeation and in-vivo assessment. Int. J. Nanomed. 2019, 14, 6555–6574. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Tadros, M.I.; Salama, N.M.; Ghoneim, A.M. Ethosome-derived invasomes as a potential transdermal delivery system for vardenafil hydrochloride: Development, optimization and application of physiologically based pharmacokinetic modeling in adults and geriatrics. Int. J. Nanomed. 2020, 15, 5671–5685. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo, and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are nanostructured lipid carriers (NLCs) better than solid lipid nanoparticles (SLNs): Development, characterizations and comparative evaluations of clotrimazole-loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Kassem, A.A.; Abd El-Alim, S.H.; Asfour, M.H. Enhancement of 8-methoxypsoralen topical delivery via nanosized niosomal vesicles: Formulation development, in vitro and in vivo evaluation of skin deposition. Int. J. Pharm. 2017, 517, 256–268. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, M.; Zhang, L.; Feng, L.; Zhang, N. Hyaluronic acid-coated nanostructured lipid carriers for targeting paclitaxel to cancer. Cancer Lett. 2013, 334, 338–345. [Google Scholar] [CrossRef]
- Naveen, N.R.; Kurakula, M.; Gowthami, B. Proceedings Process optimization by response surface methodology for preparation and evaluation of methotrexate loaded chitosan nanoparticles. Mater. Today Proc. 2020, 33, 2716–2724. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of levofloxacin polyethylene glycol decorated nanoliposomes for enhanced management of acute otitis media: Statistical optimization, trans-tympanic permeation and in vivo evaluation. Int. J. Pharm. 2019, 559, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Sarieddine, R.; Alwattar, J.K.; Chouaib, R.; Gali-Muhtasib, H. Anticancer Activity of Thymoquinone Cubic Phase Nanoparticles Against Human Breast Cancer: Formulation, Cytotoxicity and Subcellular Localization. Int. J. Nanomed. 2020, 15, 9557–9570. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xie, J.; Tong, Y.W.; Wang, C.H. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. J. Control. Release 2007, 118, 7–17. [Google Scholar] [CrossRef]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Abdelnaby, F.A.; Fadel, M.; El-Nabarawi, M.A.; Shoueir, K.R. Synthesis of biocompatible and environmentally nanofibrous mats loaded with moxifloxacin as a model drug for biomedical applications. Pharmaceutics 2020, 12, 1029. [Google Scholar] [CrossRef]
- Elsayed, I.; Sayed, S. Tailored nanostructured platforms for boosting transcorneal permeation: Box—Behnken statistical optimization, comprehensive in vitro, ex vivo and in vivo characterization. Int. J. Nanomed. 2023, 12, 7949–7962. [Google Scholar] [CrossRef]
- Shoueir, K.; Ahmed, M.K.; Abdel Gaber, S.A.; El-Kemary, M. Microstructural features and in vitro lung cancer activity of thallium and selenite doped carbonated hydroxyapatite Kamel. Ceram. Int. 2019, 33142-6. [Google Scholar] [CrossRef]
- Jatoi, A.W. Polyurethane nanofibers incorporated with ZnAg composite nanoparticles for antibacterial wound dressing applications. Compos. Commun. 2020, 19, 103–107. [Google Scholar] [CrossRef]
- Shen, L.N.; Zhang, Y.T.; Wang, Q.; Xu, L.; Feng, N.P. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int. J. Pharm. 2014, 460, 280–288. [Google Scholar] [CrossRef]
- Refai, H.; El-Gazar, A.A.; Ragab, G.M.; Hassan, D.H.; Ahmed, O.S.; Hussein, R.A.; Shabana, S.; Waffo-Téguo, P.; Valls, J.; Al-Mokaddem, A.K.; et al. Enhanced Wound Healing Potential of Spirulina platensis Nanophytosomes: Metabolomic Profiling, Molecular Networking, and Modulation of HMGB-1 in an Excisional Wound Rat Model. Mar. Drugs 2023, 21, 149. [Google Scholar] [CrossRef]
- Ahmed, S.; Attia, H.; Saher, O.; Fahmy, A.M. Augmented glycerosomes as a promising approach against fungal ear infection: Optimization and microbiological, ex vivo and in vivo assessments. Int. J. Pharm. X 2024, 8, 100295. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.M.; Balkhi, B.; Sadek, M.A.; ElBishbishy, R.M.; Ahmed, S. PEGylated terpesomes of curcumin for prominent hepatoprotective activity: Fabrication, optimization, biochemical analysis and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2025, 108, 106876. [Google Scholar] [CrossRef]
- Ahmed, S.; Farag, M.M.; Attia, H.; Balkhi, B.; Adel, I.M.; Nemr, A.A. Terconazole loaded edge-activated hybrid elastosome for revamped corneal permeation in ocular mycosis: In-vitro characterization, statistical optimization, microbiological assessment, and in-vivo evaluation. Int. J. Pharm. X 2025, 9, 100333. [Google Scholar] [CrossRef]
- Teaima, M.H.; Eltabeeb, M.A.; El-Nabarawi, M.A.; Abdellatif, M.M. Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting contraceptive: In-vitro, ex-vivo, and in-vivo evaluation. Drug Deliv. 2022, 31, 2549–2560. [Google Scholar] [CrossRef]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/shell nanofibers with embedded liposomes as a drug delivery system. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef]
- Aydogdu, A.; Sumnu, G.; Sahin, S. A novel electrospun hydroxypropyl methylcellulose/polyethylene oxide blend nanofibers: Morphology and physicochemical properties. Carbohydr. Polym. 2018, 181, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdelbari, M.A.; Elbesh, R.M.; Khaleel, E.F.; Badi, R.M.; Eldehna, W.M.; Elkaeed, E.B.; El Hassab, M.A.; Ahmed, S.M.; Mosallam, S. Sonophoresis mediated diffusion of caffeine loaded Transcutol® enriched cerosomes for topical management of cellulite. Eur. J. Pharm. Sci. 2024, 201, 106875. [Google Scholar] [CrossRef]
- Elmahboub, Y.; Albash, R.; Magdy William, M.; Rayan, A.H.; Hamed, N.O.; Ousman, M.S.; Raslan, N.A.; Mosallam, S. Metformin Loaded Zein Polymeric Nanoparticles to Augment Antitumor Activity against Ehrlich Carcinoma via Activation of AMPK Pathway: D-Optimal Design Optimization, In Vitro Characterization, and In Vivo Study. Molecules 2024, 29, 1614. [Google Scholar] [CrossRef]
- Eltabeeb, M.A.; Abdellatif, M.M.; El-Nabarawi, M.A.; Teaima, M.H.; AHamed, M.I.; Darwish, K.M.; Hassan, M.; Hamdan, A.M.; Hamed, R.R. Chitosan decorated oleosomes loaded propranolol hydrochloride hydrogel repurposed for Candida albicans-vaginal infection. Nanomedicine 2024, 19, 1369–1388. [Google Scholar] [CrossRef]
- Arayne, M.S.; Sultana, N.; Nawaz, M. Simultaneous quantification of cefpirome and cetirizine or levocetirizine in pharmaceutical formulations and human plasma by RP-HPLC. J. Anal. Chem. 2008, 63, 881–887. [Google Scholar] [CrossRef]
- Albash, R.; Yousry, C.; Al-Mahallawi, A.M.; Alaa-Eldin, A.A. Utilization of PEGylated cerosomes for effective topical delivery of fenticonazole nitrate: In-vitro characterization, statistical optimization, and in-vivo assessment. Drug Deliv. 2021, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]




| Responses | EE% | PS (nm) | PDI |
|---|---|---|---|
| Adjusted R2 | 0.92 | 0.86 | 0.80 |
| Predicted R2 | 0.83 | 0.71 | 0.62 |
| Adequate precision | 13.88 | 10.59 | 8.73 |
| Predicted value of CER6 | 65.62 | 689 | 0.49 |
| Observed value of CER6 | 65.03 | 680 | 0.51 |
| Ceramide Type | PC Amount (mg) | EE% | PS (nm) | PDI | Drug Loading (%) | |
|---|---|---|---|---|---|---|
| CER1 | IIIB | 50 | 43.15 ± 3.23 | 531.66 ± 19.39 | 0.229 ± 0.0005 | 14.38 ± 0.01 |
| CER2 | IIIB | 75 | 51.66 ± 2.49 | 540.66 ± 7.13 | 0.366 ± 0.067 | 12.29 ± 0.23 |
| CER3 | IIIB | 100 | 59.05 ± 2.32 | 648.33 ± 7.76 | 0.675 ± 0.025 | 11.81 ± 0.67 |
| CER4 | VI | 50 | 46.60 ± 1.67 | 589.85 ± 0.15 | 0.548 ± 0.011 | 15.53 ± 0.98 |
| CER5 | VI | 75 | 53.44 ± 1.01 | 615.00 ± 5.00 | 0.460 ± 0.017 | 13.36 ± 0.78 |
| CER6 | VI | 100 | 65.03 ± 1.07 | 680.00 ± 39.50 | 0.600 ± 0.100 | 13.00 ± 0.02 |
| Samples | Ra | Rq | Rt | Rp | Rtm |
|---|---|---|---|---|---|
| Fiber without LVC or CERs | 49.57 ± 6.40 | 64.62 ± 11.50 | 532.59 ± 147.50 | 305.64 ± 71.80 | 410.89 ± 106.90 |
| LVC-NFs | 46.27 ± 5.40 | 60.32 ± 10.10 | 511.39 ± 140.40 | 285.64 ± 65.20 | 403.27 ± 104.40 |
| CERs-NFs | 42.06 ± 4.00 | 54.37 ± 8.10 | 473.42 ± 127.80 | 168.95 ± 36.30 | 385.42 ± 98.40 |
| Factors | Levels |
|---|---|
| X1: Ceramide type | IIIB VI |
| X2: PC amount (mg) | 50 75 100 |
| Responses | Constraints |
| Y1: EE (%) | Maximize |
| Y2: PS (nm) | Maximize |
| Y3: PDI | Minimize |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albash, R.; Ali, S.K.; Abdelmonem, R.; Agiba, A.M.; Aldhahri, R.; Saleh, A.; Kassem, A.B.; Abdellatif, M.M. Electrospun Nanofiber-Scaffold-Loaded Levocetirizine Dihydrochloride Cerosomes for Combined Management of Atopic Dermatitis and Methicillin-Resistant Staphylococcus Aureus (MRSA) Skin Infection: In Vitro and In Vivo Studies. Pharmaceuticals 2025, 18, 633. https://doi.org/10.3390/ph18050633
Albash R, Ali SK, Abdelmonem R, Agiba AM, Aldhahri R, Saleh A, Kassem AB, Abdellatif MM. Electrospun Nanofiber-Scaffold-Loaded Levocetirizine Dihydrochloride Cerosomes for Combined Management of Atopic Dermatitis and Methicillin-Resistant Staphylococcus Aureus (MRSA) Skin Infection: In Vitro and In Vivo Studies. Pharmaceuticals. 2025; 18(5):633. https://doi.org/10.3390/ph18050633
Chicago/Turabian StyleAlbash, Rofida, Samer Khalid Ali, Rehab Abdelmonem, Ahmed M. Agiba, Renad Aldhahri, Asmaa Saleh, Amira B. Kassem, and Menna M. Abdellatif. 2025. "Electrospun Nanofiber-Scaffold-Loaded Levocetirizine Dihydrochloride Cerosomes for Combined Management of Atopic Dermatitis and Methicillin-Resistant Staphylococcus Aureus (MRSA) Skin Infection: In Vitro and In Vivo Studies" Pharmaceuticals 18, no. 5: 633. https://doi.org/10.3390/ph18050633
APA StyleAlbash, R., Ali, S. K., Abdelmonem, R., Agiba, A. M., Aldhahri, R., Saleh, A., Kassem, A. B., & Abdellatif, M. M. (2025). Electrospun Nanofiber-Scaffold-Loaded Levocetirizine Dihydrochloride Cerosomes for Combined Management of Atopic Dermatitis and Methicillin-Resistant Staphylococcus Aureus (MRSA) Skin Infection: In Vitro and In Vivo Studies. Pharmaceuticals, 18(5), 633. https://doi.org/10.3390/ph18050633








