Physiologically Based Pharmacokinetic Modeling to Predict Lamotrigine Exposure in Special Populations to Facilitate Therapeutic Drug Monitoring and Guide Dosing Regimens
Abstract
:1. Introduction
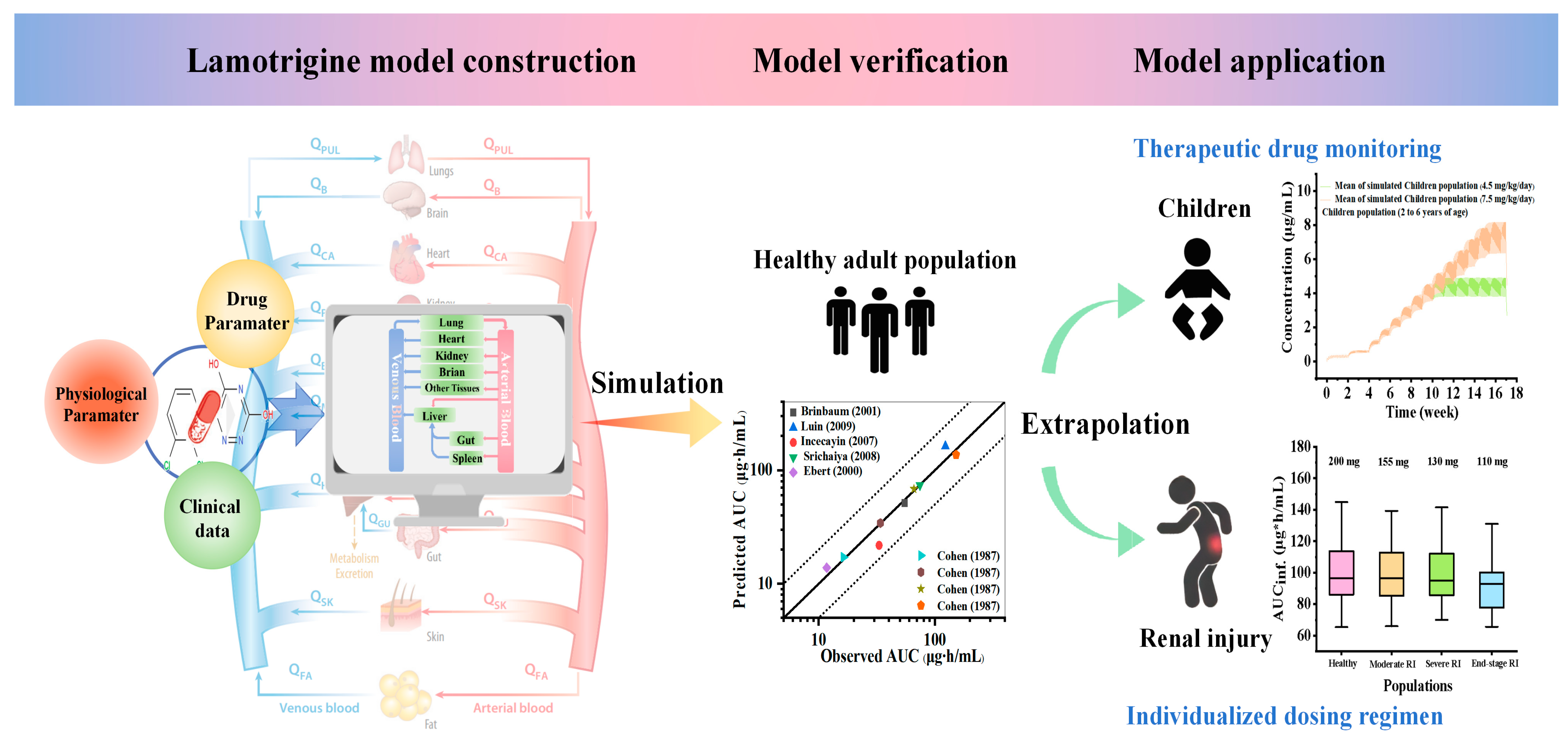
2. Results
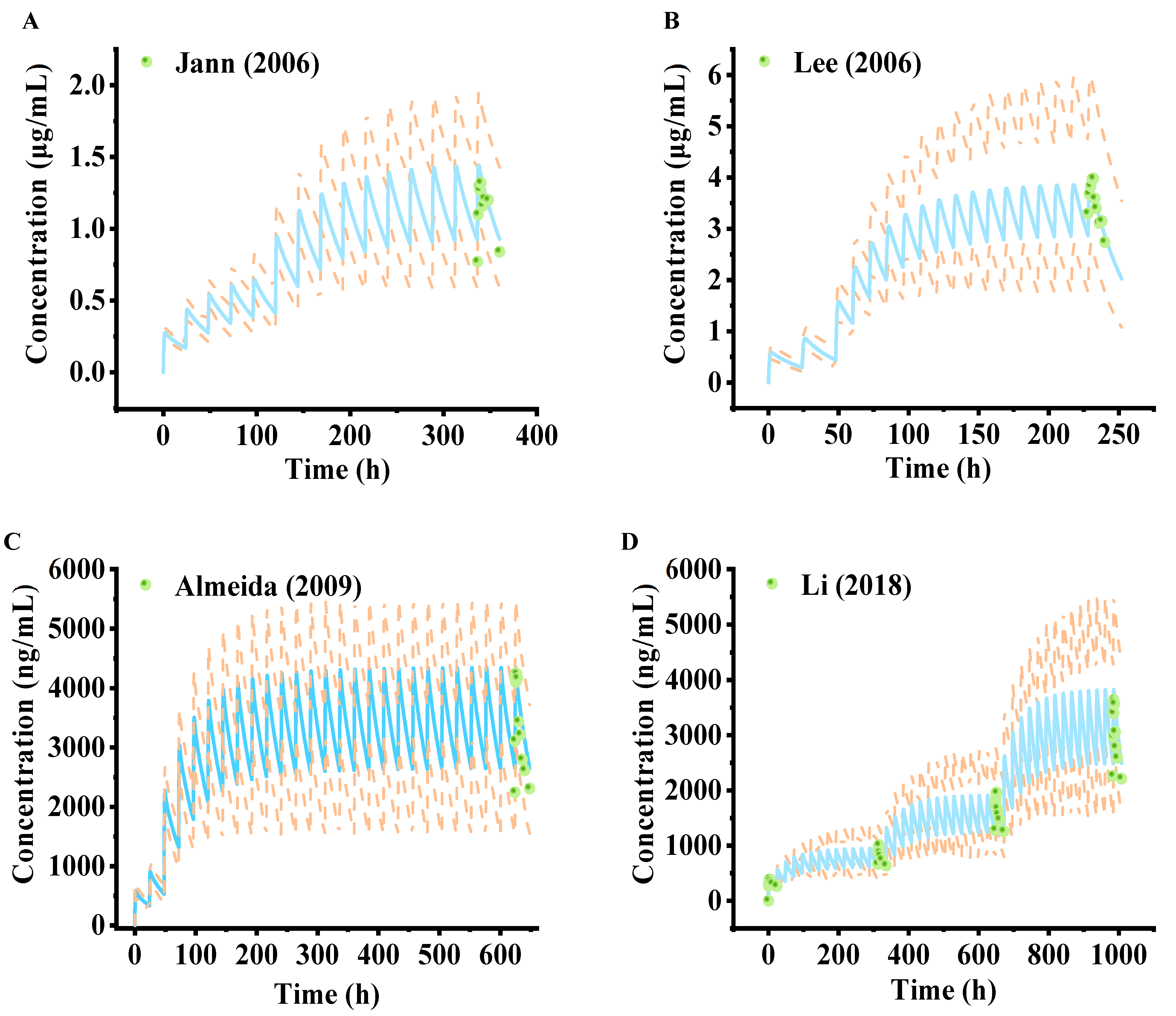
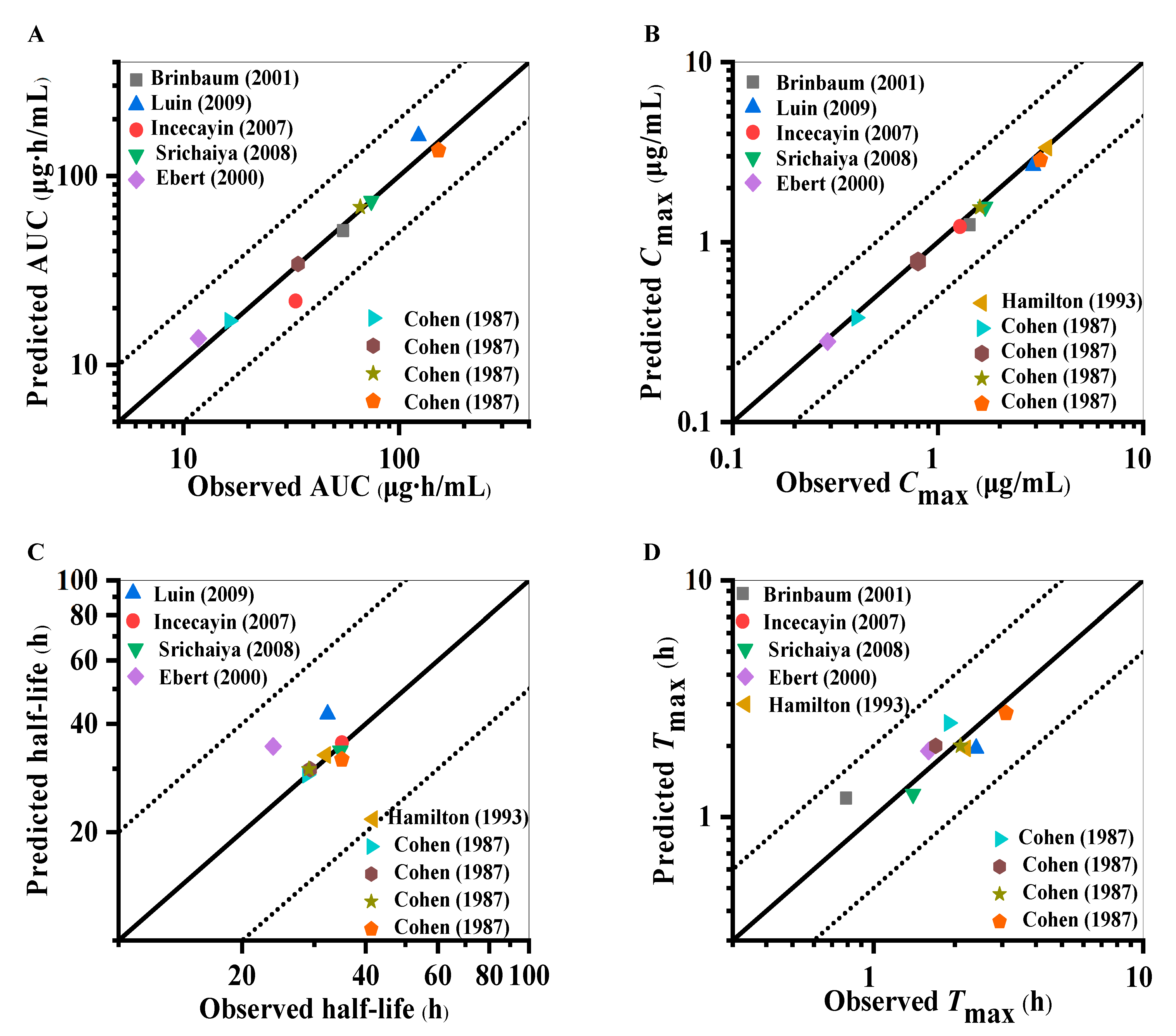
2.1. Establishment and Evaluation of Lamotrigine PBPK Model in Healthy Adults
2.2. Sensitivity Analysis
2.3. Establishment and Assessment of the Lamotrigine PBPK Model in Pediatrics
2.4. Application of the PBPK Model of Lamotrigine for Facilitating Therapeutic Drug Monitoring in Clinical Treatment
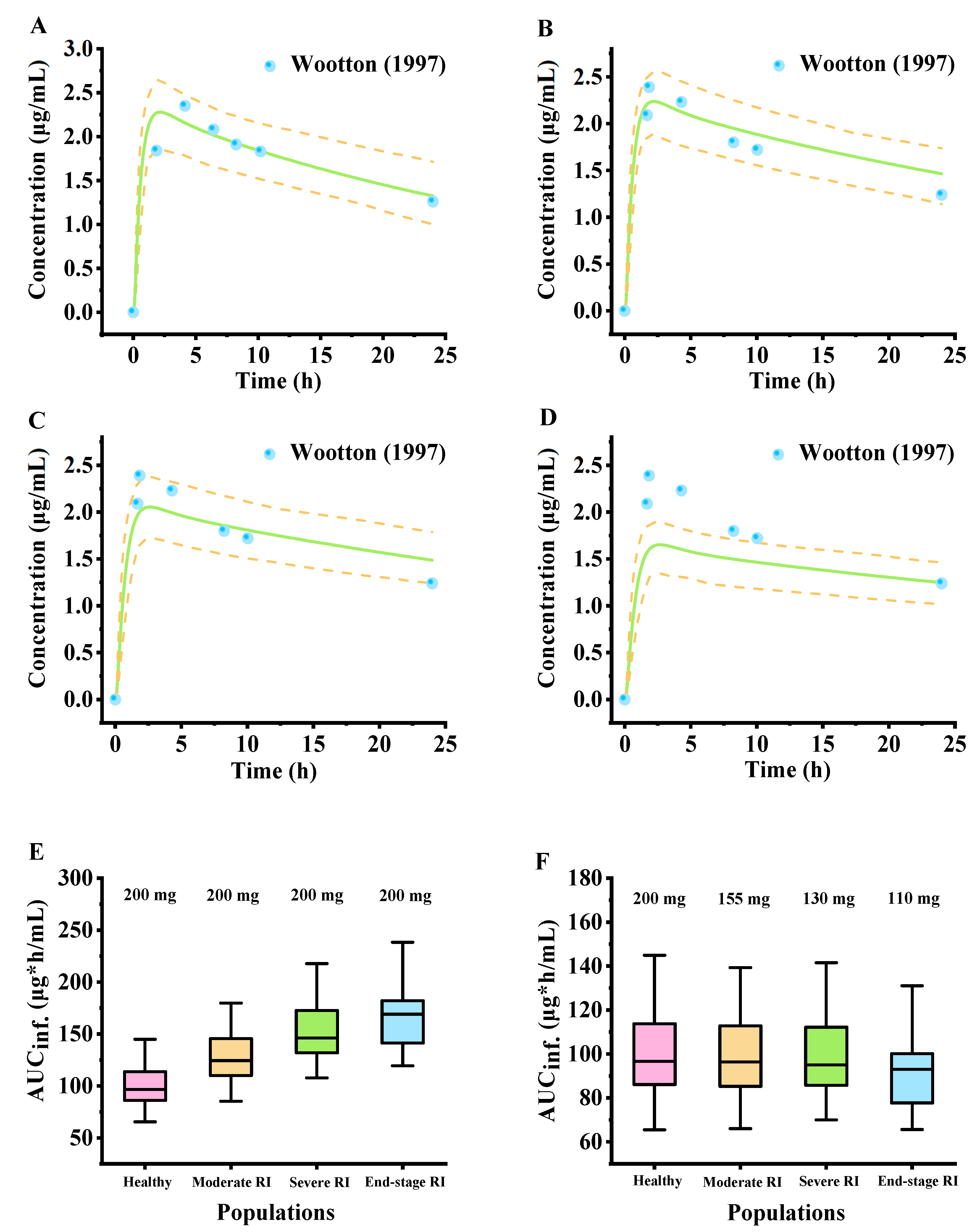
2.5. Simulated Pharmacokinetic Profiles in Populations with Renal Impairment
3. Discussion
4. Materials and Methods
4.1. Software and Data
4.2. Establishment of PBPK Model in Adults After Oral Administration of Lamotrigine
4.3. Development and Validation of PBPK Models for Pediatric Population Oral Lamotrigine Pharmacokinetics
4.4. PBPK Model Scaling for the Renal Impairment Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karoly, P.J.; Rao, V.R.; Gregg, N.M.; Worrell, G.A.; Bernard, C.; Cook, M.J.; Baud, M.O. Cycles in epilepsy. Nat. Rev. Neurol. 2021, 17, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Lybrand, Z.R.; Goswami, S.; Zhu, J.; Jarzabek, V.; Merlock, N.; Aktar, M.; Smith, C.; Zhang, L.; Varma, P.; Cho, K.-O.; et al. A critical period of neuronal activity results in aberrant neurogenesis rewiring hippocampal circuitry in a mouse model of epilepsy. Nat. Commun. 2021, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Foiling epilepsy in a brain at risk. Science 2019, 366, 1304. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Jones, N.A.; Cunningham, M.O.; Jayasekera, B.A.P.; Devore, S.; Whalley, B.J. Cannabinoid treatments in epilepsy and seizure disorders. Physiol. Rev. 2024, 104, 591–649. [Google Scholar] [CrossRef]
- Schevon, C.; Michalak, A. Demystifying interictal discharges and seizure initiation in focal epilepsy. Brain 2023, 146, 1734–1736. [Google Scholar] [CrossRef]
- Hullett, P.W.; Lowenstein, D.H. Major advances in epilepsy research in 2023. Lancet Neurol. 2024, 23, 19–20. [Google Scholar] [CrossRef]
- Johannesen, K.M. From precision diagnosis to precision treatment in epilepsy. Nat. Rev. Neurol. 2023, 19, 69–70. [Google Scholar] [CrossRef]
- Husein, N.; Thijs, R.D.; Bunschoten, J.W.; Keezer, M.R.; Sander, J.W. Concerns about lamotrigine. Lancet Neurol. 2021, 20, 418–419. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Q.; Ou, S.; Wang, Y.; Li, Y.; Xia, L.; Pan, S. Comparison of long-term efficacy, tolerability, and safety of oxcarbazepine, lamotrigine, and levetiracetam in patients with newly diagnosed focal epilepsy: An observational study in the real world. Epilepsy Res. 2020, 166, 106408. [Google Scholar] [CrossRef]
- Mitra-Ghosh, T.; Callisto, S.P.; Lamba, J.K.; Remmel, R.P.; Birnbaum, A.K.; Barbarino, J.M.; Klein, T.E.; Altman, R.B. PharmGKB summary: Lamotrigine pathway, pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2020, 30, 81–90. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Zeng, L.; Jiang, Z.; Xiong, M.; Jia, Z.-J.; Cheng, G.; Miao, L.; Zhao, L.; Zhang, L. The reference range of lamotrigine in the treatment of epilepsy in children: A systematic review. Eur. J. Clin. Pharmacol. 2024, 80, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.S.; Kodagali, R.; Mathew, B.S.; Thomas, M.; Prabha, R.; Mathew, V.; Fleming, D.H. Therapeutic drug monitoring of levetiracetam and lamotrigine: Is there a need? Ther. Drug Monit. 2015, 37, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Pirie, D.A.; Al Wattar, B.H.; Pirie, A.M.; Houston, V.; Siddiqua, A.; Doug, M.; Bagary, M.; Greenhill, L.; Khan, K.S.; McCorry, D.; et al. Effects of monitoring strategies on seizures in pregnant women on lamotrigine: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 172, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Small, B.G.; Johnson, T.N.; Rowland Yeo, K. Another step toward qualification of pediatric physiologically based pharmacokinetic models to facilitate inclusivity and diversity in pediatric clinical studies. Clin. Pharmacol. Ther. 2023, 113, 735–745. [Google Scholar] [CrossRef]
- Heimbach, T.; Chen, Y.; Chen, J.; Dixit, V.; Parrott, N.; Peters, S.A.; Poggesi, I.; Sharma, P.; Snoeys, J.; Shebley, M.; et al. Physiologically-based pharmacokinetic modeling in renal and hepatic impairment populations: A pharmaceutical industry perspective. Clin. Pharmacol. Ther. 2021, 110, 297–310. [Google Scholar] [CrossRef]
- Verscheijden, L.F.M.; Koenderink, J.B.; Johnson, T.N.; de Wildt, S.N.; Russel, F.G.M. Physiologically-based pharmacokinetic models for children: Starting to reach maturation? Pharmacol. Ther. 2020, 211, 107541. [Google Scholar] [CrossRef]
- Dallmann, A.; Pfister, M.; van den Anker, J.; Eissing, T. Physiologically based pharmacokinetic modeling in pregnancy: A systematic review of published models. Clin. Pharmacol. Ther. 2018, 104, 1110–1124. [Google Scholar] [CrossRef]
- Luzon, E.; Blake, K.; Cole, S.; Nordmark, A.; Versantvoort, C.; Berglund, E.G. Physiologically based pharmacokinetic modeling in regulatory decision-making at the European Medicines Agency. Clin. Pharmacol. Ther. 2017, 102, 98–105. [Google Scholar] [CrossRef]
- Caleffi-Marchesini, E.R.; Borghi-Pangoni, F.B.; Macente, J.; Chiamulera-Mantovani, P.; Mazucheli, J.; Cristofoletti, R.; Diniz, A. Exploring in vitro solubility of lamotrigine in physiologically mimetic conditions to prospect the in vivo dissolution in pediatric population. Biopharm. Drug Dispos. 2023, 44, 147–156. [Google Scholar] [CrossRef]
- Caleffi-Marchesini, E.R.; Herling, A.A.; Macente, J.; Bonan, R.H.; Lima, P.d.F.; Moreno, R.; Alexandre, V.; Charbe, N.B.; Borghi-Pangoni, F.B.; Cristofoletti, R.; et al. Adult and pediatric physiologically-based biopharmaceutics modeling to explain lamotrigine immediate release absorption process. CPT Pharmacomet. Syst. Pharmacol. 2024, 13, 208–221. [Google Scholar] [CrossRef]
- Yeung, C.H.T.; Ito, S.; Autmizguine, J.; Edginton, A.N. Incorporating breastfeeding-related variability with physiologically based pharmacokinetic modeling to predict infant exposure to maternal medication through breast milk: A workflow applied to lamotrigine. AAPS J. 2021, 23, 93. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, A.K.; Kriel, R.L.; Im, Y.; Remmel, R.P. Relative bioavailability of lamotrigine chewable dispersible tablets administered rectally. Pharmacotherapy 2001, 21, 158–162. [Google Scholar] [CrossRef] [PubMed]
- van Luin, M.; Colbers, A.; Verwey-van Wissen, C.P.; van Ewijk-Beneken-Kolmer, E.W.; van der Kolk, M.; Hoitsma, A.; da Silva, H.G.; Burger, D.M. The effect of raltegravir on the glucuronidation of lamotrigine. J. Clin. Pharmacol. 2009, 49, 1220–1227. [Google Scholar] [CrossRef]
- Incecayir, T.; Agabeyoglu, I.; Gucuyener, K. Comparison of plasma and saliva concentrations of lamotrigine in healthy volunteers. Arzneimittelforschung 2007, 57, 517–521. [Google Scholar] [CrossRef]
- Srichaiya, A.; Longchoopol, C.; Oo-Puthinan, S.; Sayasathid, J.; Sripalakit, P.; Viyoch, J. Bioequivalence of generic lamotrigine 100-mg tablets in healthy Thai male volunteers: A randomized, single-dose, two-period, two-sequence crossover study. Clin Ther. 2008, 30, 1844–1851. [Google Scholar] [CrossRef]
- Ebert, U.; Thong, N.Q.; Oertel, R.; Kirch, W. Effects of rifampicin and cimetidine on pharmacokinetics and pharmacodynamics of lamotrigine in healthy subjects. Eur. J. Clin. Pharmacol. 2000, 56, 299–304. [Google Scholar] [CrossRef]
- Cohen, A.F.; Land, G.S.; Breimer, D.D.; Yuen, W.C.; Winton, C.; Peck, A.W. Lamotrigine, a new anticonvulsant: Pharmacokinetics in normal humans. Clin. Pharmacol. Ther. 1987, 42, 535–541. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Cohen, A.F.; Yuen, A.W.; Harkin, N.; Land, G.; Weatherley, B.C.; Peck, A.W. Carbamazepine and lamotrigine in healthy volunteers: Relevance to early tolerance and clinical trial dosage. Epilepsia 1993, 34, 166–173. [Google Scholar] [CrossRef]
- Jann, M.W.; Hon, Y.Y.; Shamsi, S.A.; Zheng, J.; Awad, E.A.; Spratlin, V. Lack of pharmacokinetic interaction between lamotrigine and olanzapine in healthy volunteers. Pharmacotherapy 2006, 26, 627–633. [Google Scholar] [CrossRef]
- Van Der Lee, M.J.; Dawood, L.; Ter Hofstede, H.J.; Degraaffteulen, M.; Vanewijkbenekenkolmer, E.; Caliskanyassen, N.; Koopmans, P.; Burger, D. Lopinavir/ritonavir reduces lamotrigine plasma concentrations in healthy subjects. Clin. Pharmacol. Ther. 2006, 80, 159–168. [Google Scholar] [CrossRef]
- Almeida, L.; Nunes, T.; Sicard, E.; Rocha, J.-F.; Falcão, A.; Brunet, J.-S.; Lefebvre, M.; Soares-Da-Silva, P. Pharmacokinetic interaction study between eslicarbazepine acetate and lamotrigine in healthy subjects. Acta Neurol. Scand. 2010, 121, 257–264. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Xu, Y.; Hu, J.; Li, H. Pharmacokinetics, safety, and tolerability of lamotrigine chewable/dispersible tablet following repeat-dose administration in healthy chinese volunteers. Clin. Pharmacol. Drug Dev. 2018, 7, 627–633. [Google Scholar] [CrossRef]
- Chien, S.; Yao, C.; Mertens, A.; Verhaeghe, T.; Solanki, B.; Doose, D.R.; Novak, G.; Bialer, M. An interaction study between the new antiepileptic and CNS drug carisbamate (RWJ-333369) and lamotrigine and valproic acid. Epilepsia 2007, 48, 1328–1338. [Google Scholar] [CrossRef]
- Sidhu, J.; Job, S.; Bullman, J.; Francis, E.; Abbott, R.; Ascher, J.; Theis, J.G. Pharmacokinetics and tolerability of lamotrigine and olanzapine coadministered to healthy subjects. Br. J. Clin. Pharmacol. 2006, 61, 420–426. [Google Scholar] [CrossRef]
- Colucci, R.; Glue, P.; Holt, B.; Banfield, C.; Reidenberg, P.; Meehan, J.W.; Pai, S.; Nomeir, A.; Lim, J.; Lin, C.C.; et al. Effect of felbamate on the pharmacokinetics of lamotrigine. J. Clin. Pharmacol. 1996, 36, 634–638. [Google Scholar] [CrossRef]
- Gastrup, S.; Stage, T.B.; Fruekilde, P.B.; Damkier, P. Paracetamol decreases steady-state exposure to lamotrigine by induction of glucuronidation in healthy subjects. Br. J. Clin. Pharmacol. 2016, 81, 735–741. [Google Scholar] [CrossRef]
- Chen, C.; Casale, E.J.; Duncan, B.; Culverhouse, E.H.; Gilman, J. Pharmacokinetics of lamotrigine in children in the absence of other antiepileptic drugs. Pharmacotherapy 1999, 19, 437–441. [Google Scholar] [CrossRef]
- Wootton, R.; Soul-Lawton, J.; Rolan, P.E.; Sheung, C.T.; Cooper, J.D.; Posner, J. Comparison of the pharmacokinetics of lamotrigine in patients with chronic renal failure and healthy volunteers. Br. J. Clin. Pharmacol. 1997, 43, 23–27. [Google Scholar] [CrossRef]
- Johnson, T.N.; Small, B.G.; Rowland Yeo, K. Increasing application of pediatric physiologically based pharmacokinetic models across academic and industry organizations. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 373–383. [Google Scholar] [CrossRef]
- Meesters, K.; Balbas-Martinez, V.; Allegaert, K.; Downes, K.J.; Michelet, R. Personalized dosing of medicines for children: A primer on pediatric pharmacometrics for clinicians. Paediatr. Drugs 2024, 26, 365–379. [Google Scholar] [CrossRef]
- Salerno, S.N.; Capparelli, E.V.; McIlleron, H.; Gerhart, J.G.; Dumond, J.B.; Kashuba, A.D.M.; Denti, P.; Gonzalez, D. Leveraging physiologically based pharmacokinetic modeling to optimize dosing for lopinavir/ritonavir with rifampin in pediatric patients. Pharmacotherapy 2023, 43, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, H.; European Paediatric Formulation Initiative (EUPFI). Paediatric biopharmaceutics classification system: Current status and future decisions. Int. J. Pharm. 2014, 469, 251–253. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic drug monitoring of antiepileptic drugs in epilepsy: A 2018 update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Berry, D.J.; Bourgeois, B.F.; Cloyd, J.C.; Glauser, T.A.; Johannessen, S.I.; Leppik, I.E.; Tomson, T.; Perucca, E. Antiepileptic drugs—Best practice guidelines for therapeutic drug monitoring: A position paper by the subcommission on therapeutic drug monitoring, ILAE commission on therapeutic strategies. Epilepsia 2008, 49, 1239–1276. [Google Scholar] [CrossRef]
- Bohnert, A.S.B.; Walton, M.A.; Cunningham, R.M.; Ilgen, M.A.; Barry, K.; Chermack, S.T.; Blow, F.C. Overdose and adverse drug event experiences among adult patients in the emergency department. Addict. Behav. 2018, 86, 66–72. [Google Scholar] [CrossRef]
- Ni, J.; Tang, X.; Chen, L. Medication overdose data analysis: A review of medication error reports in the FDA adverse event reporting system (FAERS). BMC Pharmacol. Toxicol. 2023, 24, 41. [Google Scholar] [CrossRef]
- Zamir, A.; Alqahtani, F.; Rasool, M.F. Chronic kidney disease and physiologically based pharmacokinetic modeling: A critical review of existing models. Expert Opin. Drug Metab. Toxicol. 2024, 20, 95–105. [Google Scholar] [CrossRef]
- Franchetti, Y.; Nolin, T.D. Dose optimization in kidney disease: Opportunities for PBPK modeling and mimulation. J. Clin. Pharmacol. 2020, 60, 36–51. [Google Scholar] [CrossRef]
- Argikar, U.A.; Remmel, R.P. Variation in glucuronidation of lamotrigine in human liver microsomes. Xenobiotica 2009, 39, 355–363. [Google Scholar] [CrossRef]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef]
- Rowland Yeo, K.; Aarabi, M.; Jamei, M.; Rostami-Hodjegan, A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert. Rev. Clin. Pharmacol. 2011, 4, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.R.V.; Yeung, C.H.T.; Ismaeil, S.; Advani, U.; Djie, S.; Edginton, A.N. A physiological approach to pharmacokinetics in chronic kidney disease. J. Clin. Pharmacol. 2020, 60, S52–S62. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-C.; Miao, C.-F.; Lei, Y.; Liu, A.-L. Physiologically Based Pharmacokinetic Modeling to Predict Lamotrigine Exposure in Special Populations to Facilitate Therapeutic Drug Monitoring and Guide Dosing Regimens. Pharmaceuticals 2025, 18, 637. https://doi.org/10.3390/ph18050637
Li J-C, Miao C-F, Lei Y, Liu A-L. Physiologically Based Pharmacokinetic Modeling to Predict Lamotrigine Exposure in Special Populations to Facilitate Therapeutic Drug Monitoring and Guide Dosing Regimens. Pharmaceuticals. 2025; 18(5):637. https://doi.org/10.3390/ph18050637
Chicago/Turabian StyleLi, Ji-Cheng, Chen-Fang Miao, Yun Lei, and Ai-Lin Liu. 2025. "Physiologically Based Pharmacokinetic Modeling to Predict Lamotrigine Exposure in Special Populations to Facilitate Therapeutic Drug Monitoring and Guide Dosing Regimens" Pharmaceuticals 18, no. 5: 637. https://doi.org/10.3390/ph18050637
APA StyleLi, J.-C., Miao, C.-F., Lei, Y., & Liu, A.-L. (2025). Physiologically Based Pharmacokinetic Modeling to Predict Lamotrigine Exposure in Special Populations to Facilitate Therapeutic Drug Monitoring and Guide Dosing Regimens. Pharmaceuticals, 18(5), 637. https://doi.org/10.3390/ph18050637





