Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium sidoides (EPs® 7630) in the Context of Health Promotion
Abstract
: Pelargonium species contribute significantly to the health care of a large population in the Southern African region, as part of a long-standing medical system intimately linked to traditional healing practices. Most notably, extracts of the roots of P. sidoides have commonly been applied for the treatment of dysentery and diarrhoea but only occasionally for respiratory complaints. Clinical trials have shown that a modern aqueous-ethanolic formulation of P. sidoides extracts (EPs® 7630) is an efficacious treatment for disorders of the respiratory tract, for example bronchitis and sinusitis. It should be noted that EPs® 7630 is the most widely investigated extract and therefore is the focus of this review. In order to provide a rationale for its therapeutic activity extracts have been evaluated for antibacterial activity and for their effects on non-specific immune functions. Only moderate direct antibacterial capabilities against a spectrum of bacteria, including Mycobacteria strains, have been noted. In contrast, a large body of in vitro studies has provided convincing evidence for an anti-infective principle associated with activation of the non-specific immune system. Interestingly, significant inhibition of interaction between bacteria and host cells, a key to the pathogenesis of respiratory tract infections, has emerged from recent studies. In addition, antiviral effects have been demonstrated, including inhibition of the replication of respiratory viruses and the enzymes haemagglutinin and neuraminidase. Besides, an increase of cilliary beat frequency of respiratory cells may contribute to the beneficial effects of P. sidoides extracts. This example provides a compelling argument for continuing the exploration of Nature and traditional medical systems as a source of therapeutically useful herbal medicines.1. Introduction
Infectious diseases have been and continue to be an ever-present threat to mankind. Despite medical advances, infectious diseases are still a cause of death for millions around the World. Of the estimated 57 million deaths that occur throughout the World each year, more than 25 percent are directly caused by infectious diseases [1]. More than 90 percent of the worldwide mortality from infectious diseases is caused by only a handful of diseases, including lower respiratory infections, HIV/AIDS, diarrheal diseases, tuberculosis, malaria and measles (Figure 1) [1]. New targets for novel anti-infective drugs need to be identified, particularly in the light of the emergence of drug resistant strains.
Many herbal medicines have ethnomedicinal uses for the treatment of infectious conditions such as respiratory ailments. However, research programmes are important for validating the traditional use, providing evidence for the safety and the quality of the plant material. That traditionally used herbal medicines may well provide the basis for the development of a modern and highly successful phytopharmaceutical that meets the internationally required criteria of quality, safety and efficacy for an evidence-based therapy is exemplarily shown for Pelargonium sidoides. Members of the genus Pelargonium are highly valued by traditional healers and the South African native population for their curative and palliative effects in the treatment of gastrointestinal disorders, while interest in P. sidoides has been heightened by reports of therapeutic benefits in infectious conditions of the respiratory tract such as tuberculosis and related diseases. The interesting ethnobotanical and commercial history, as well as the phytochemical profile of this medicinal plant, have been reviewed in detail elsewhere and are thus not reiterated (vide infra). This report is aimed at reviewing the current knowledge on the mechanisms of the underlying beneficial effects of P. sidoides and the related herbal drug preparation EPs® 7630. Notably, this special extract has been the subject of 20 clinical studies involving more than 9,000 patients, including 3,900 children as young as 1 year, reviewed repeatedly elsewhere (vide infra). The objective of this review is to show the potential of a traditional plant, P. sidoides, for the defence against pathogens and the improvement of immune functions at various levels and, thus, the invaluable role that natural products continue to play in the drug discovery process in the areas of infectious and other diseases.
2. Discussion
2.1. Traditional and Therapeutic Uses of Pelargonium Sidoides
Historically, the ethnomedicinal use of P. sidoides in the traditional medical systems of southern Africa is mainly associated with gastrointestinal disorders (diarrhoea and dysentery), whereas only some records exist for the treatment of respiratory conditions (tuberculosis, coughs, colds, sore throat) and sources of this information are not always provided [2-5]. In this context it appears important to note the general problems surrounding the taxonomic classification of Pelargonium species, as reflected in the past by numerous revisions. In southern Africa, tuberculosis is one of the most commonly notified diseases. In the absence of an effective chemotherapy in earlier days, traditional healers were widely consulted and many local plants were used to alleviate tuberculosis-related symptoms. As for P. sidoides, a key role in the successful introduction of a profound traditional herbal remedy in modern phytotherapy in Europe is linked to Charles Henry Stevens, who believed he was cured by taking a brew prepared from the roots and subsequently tried to introduce “Stevens' Consumption Cure” on the market in Great Britain [5-8].
This herbal treatment is seeing increasing popularity, not only in southern Africa but also in European countries, in the Commonwealth of Independent States, the Baltic States and in Mexico [4,9]. Following the well-documented therapeutic benefits in infectious conditions of the respiratory tract, a modern formulation of the roots of P. sidoides, EPs® 7630 (exclusively contained in Umckaloabo®, marketed by Spitzner Arzneimittel, Ettlingen, Germany), has been elaborated from this traditional herbal medicine. EPs® 7630 is a special aqueous ethanolic extract. Results from a number of clinical studies including several randomised, double-blind and placebo-controlled trials, support the use of Umckaloabo® in respiratory tract infections such as bronchitis, sinusitis and tonsillitis. Several post-marketing surveillance studies are available, demonstrating the safety and tolerability of the traded product [10-18]. Umckaloabo® was approved in Germany as a drug for treating acute bronchitis. This illness is one of the most frequent diagnoses in medical practice and predominantly caused by RNA viruses. Only in less than 10% of cases non-viral agents play a role in its etiology [19]. Surprisingly, prescription of antibiotics for infections of viral origin is still wide-spread even though their efficacy is limited to bacterial infections [20]. One purpose might be to avoid a secondary bacterial superinfection with group A β-hemolytic streptococci. Development of resistant bacteria is a disadvantage of non-indicated antibiotic therapy. Based on clinical studies, the Pelargonium-based preparation offers an alternative therapeutic agent to otherwise not indicated antibiotic treatment.
2.2. Antimycobacterial and Respiratory Tract Related Antibacterial Activity
Following earlier claims of antimycobacterial efficacy of P. sidoides, methanol extracts of the roots and some of its characteristic phenol and coumarin constituents were tested for this particular biological activity. Using the radiorespirometric BACTEC method, the extracts showed antimycobacterial activity against Mycobacterium tuberculosis (96% at 12.5 μg/mL), while none of the purified compounds tested exhibited any antimycobacterial activities under these experimental conditions. The same extract exhibited weak activities against M. tuberculosis (MIC of 100 μg/mL) as assessed in a broth microdilution alamar blue assay. Rifampicin, used as reference, showed an MIC of 0.06 μg/mL under the experimental conditions used. Independent support of the weak antimycobacterial activity of P. sidoides was provided by similar studies of acetone and ethanol root extracts using M. tuberculosis and M. smegmatis as test organisms, while coumarins and flavonoids isolated from this plant source again proved inactive [4]. With MICs of >1,024 μg/mL, an aqueous acetone extract of P. sidoides showed negligible antimycobacterial activities against the rapidly growing avirulent M. smegmatis in a broth microdilution assay [21]. Similar results were obtained from testing the extracts against M. aurum, a species reported to be predictive of activity against M. tuberculosis because of their comparable drug sensitivity profiles. This finding suggests that direct antimycobacterial activity apparently depends not only on the strain of mycobacterium, but also strongly on extract preparation and composition.
In the course of a search for antimycobacterial constituents some straight-chain fatty acids present in n-hexane root extracts with activity against a panel of rapidly growing mycobacteria were identified [22,23]. Interestingly, all the saturated fatty acids except lauric acid were devoid of any antimycobacterial activity, whereas unsaturated analogues, including oleic acid and linoleic acid, were active, with the latter being the most potent one (MIC of 2 μg/mL against M. aurum). However, these data do not provide convincing evidence for an antimycobacterial principle of P. sidoides. In traditional medicine decoctions and infusions are used, thus putting into question the presence of effective levels of lipophilic constituents in aqueous preparations. Furthermore, it should be noted that Mycobacteria are intracellularly residing pathogens, while the in vitro protocols conducted reflect a direct action on these pathogens. That P. sidoides root extracts apparently show limited antimycobacterial activity under these test conditions is reflected in further studies [24,25]. Taking into account the overall moderate antimycobacterial activity in experiments carried out so far, it is highly likely that the claimed effective use of P. sidoides in the treatment of tuberculosis may be due to stimulation of the non-specific immune system rather than any direct action on Mycobacteria pathogens. The antimycobacterial principle of this popular Pelargonium species remains to be identified.
Parallel work focused on the evaluation of the antibacterial activity of P. sidoides against a panel of microorganisms (Staphylococcus aureus, Streptococcus pneumoniae, ß-hemolytic Streptococcus, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Haemophilus influenzae) associated with respiratory diseases [26]. However, the in vitro antibacterial effects of an aqueous acetone extract, fractions obtained thereof from successive partitions between water and organic solvents (ethyl acetate, n-butanol, MICs of 600–7,500 μg/mL), and a range of isolated compounds (MICs of 200–1,000 μg/mL) have been shown to be only modest compared with classic antibiotics. It should be noted that the standardized root extract EPs® 7630 has hitherto not been subjected to similar antibacterial studies, though moderate activities are commonly noted. Independent support of weak direct antibacterial activities followed from studies using the liquid preparation Umca® and isolates (Streptococcus pneumonia, S. pyogenes, S. viridans, Staphylococcus aureus, S. epidermis, Neisseria spp., Moraxella catarrhalis, Haemophilus influenza) obtained from the throat cultures of patients [27]. Employing a microdilution broth method, MIC values were in the range of 200–1,600 μg/mL, with MICs >800 μg/mL for most of the bacteria tested.
These disappointing findings prompted a series of studies attempting to demonstrate indirect antibacterial mechanisms that could provide a clue to the anti-infective properties of P. sidoides-based herbal medicines [28]. Adhesion of pathogenic bacteria to the host cell surface is a crucial event in colonization and infection. Inhibition of the microbial docking process to epithelial cells at an early stage would be effective in protecting host cells from infection. Using fluorescent-labelled group A-streptococci and viable human laryngeal cells (HEp-2) as a model, flow cytometric measurements showed prominent (by up to ca. 45% compared with untreated cells) anti-adhesive and anti-invasive capabilities of EPs® 7630 in a concentration dependent manner (< 30 μg/mL). Notably, inhibition of streptococcal adhesion to host cells was only evident when the bacteria were pre-treated with EPs® 7630, indicating that the interaction of the anti-adhesive principle occurred only with the bacterial outer membane surface and not with binding sides at the epithelial surface.
Recent experiments revealed that proanthocyanidins (Figure 2) play a decisive role in the observed inhibition of group A-streptococci docking process to HEp-2 cells, as concluded from the apparent ‘missing’ activity of phenolic-free extracts [29]. The picture that emerged from these anti-adhesive studies including homogeneous epicatechin- and catechin-based polyflavans, a ‘mixed’ procyanidin sample, a prodelphinidin test substance as well as an A-type proanthocyanidin provided evidence that the anti-streptococcal activity of EPs® 7630 polyphenols appeared to be due to the prodelphinidin-type substructure (epigallocatechin and gallocatechin extender units). Pre-treatment experiments indicated that the interaction of the anti-adhesive proanthocyanidins occurred only with binding factors on the bacterial surface. The blocked molecules, for example fibronectin- and collagen-binding adhesions [30], remain to be identified.
Interestingly, bacterial attachment was enhanced ca. 7-fold under the influence of EPs® when using buccal epithelial cells that, noticeably, are mostly dead [28]. Inactivation of the trapped microorganisms may be explained by the fact that the decaying buccal cells are physiologically sloughed off and swallowed with the saliva. In this way the intercepted pathogens are not available for adhesion to intact cells. Taking into account the fairly high inhibition of bacterial adhesion to viable epithelial cells, these indirect antibacterial activities have beneficial effects on infectious conditions. The impact of EPs® 7630 on microbial adhesion was also demonstrated in studies with Helicobacter pylori [31,32]. Again, pre-treatment of fluorescent-labelled H. pylori with the P. sidoides extract inhibited the adherence to human gastric mucosa cells in a dose-dependent manner. However, the underlying biologically active principle remains to be identified. Taken together, the demonstrated anti-adhesive mechanism may well contribute to the anti-infective activity of EPs® 7630 at an early time point of a bacterial infection.
Also worthy of mention is the potential of the P. sidoides extract to significantly inhibit streptococcal invasion into HEp-2 cells. Similarly to the anti-adhesion activity, polyphenols may interfere with extracellular proteins produced by the bacteria to facilitate invasiveness. Clearly, further studies are needed to get insight into the underlying principle that bacteria employ to invade our cells. From a more medicinal viewpoint, inhibition of the bacterial invasion of epithelial cells protects the host from pathogens that evade host defences and antibiotic therapy. Once inside the host cells, recurrent streptococcal infections may well occur. The demonstrated inhibition of the streptococcal invasion thus prevents recurrent infections.
Although the above findings have provided evidence for both direct and indirect antibacterial properties of P. sidoides, the pronounced anti-infective capabilities of this herbal medicine cannot adequately be explained merely on the basis of the observed antibacterial activities.
2.3. Effect on the Mucociliary System of Respiratory Cells
The mucociliary system represents a defence mechanism of the nasal cavity and the bronchial tree for cleaning the air of bacteria and foreign particles, with the ciliary beat frequency (CBF) being an important parameter for determining its efficacy. EPs® 7630 significantly and concentration-dependently increased CBF of an adherent monolayer culture of human nasal epithelium cells. At 30 μg/mL, the increase was ca. 125% compared to the equilibrium phase [33]. After washing procedures the CBF returned to that of the equilibration period. However, subsequent examinations have to prove the effect under in vivo conditions with emphasis on the mucociliary clearance.
2.5. Role of the Non-Specific Immune System
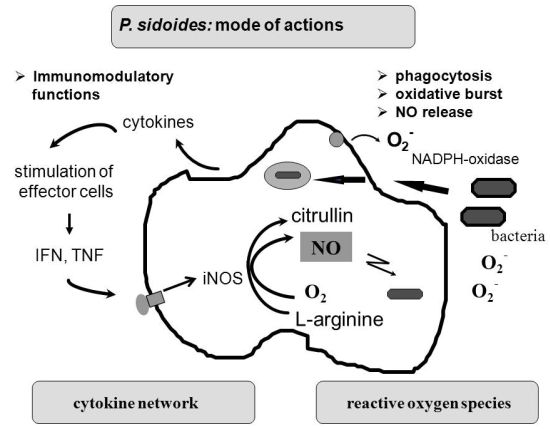
To understand the many effects of P. sidoides on the non-specific immune system and its potential role in host response to pathogens, it seems appropriate to illustrate the key components involved in the clearing of microbial agents. Since several immunological factors are associated with anti-infective responses, only those parameters are briefly described which primarily imply stimulation of the non-specific immune system and are closely related to the hitherto known anti-infective principle detected in P. sidoides based medicines. A simplified overview of induced cytotoxic defence mechanisms is shown in Figure 3.
Macrophages are extremely versatile cells involved in a number of complex processes in immune responses. When activated they acquire microbicidal effector functions and secrete cytokines, resulting in recruitment of immune cells and subsequent elimination of the pathogen by phagocytosis or release of reactive oxygen and nitrogen species. Generation of reactive oxygen species is initiated by NADPH oxidase. The primary product is superoxide, which can be converted to H2O2 by superoxide dismutase and subsequently to hydroxyl radicals and anions.
Production of nitric oxide and its many congeners (NO) is another key macrophage antimicrobial response. NO are important intra- and intercellular regulatory molecules of multiple biological functions, including macrophage-mediated cytotoxicity [49-52]. Endogenously derived NO is generated enzymatically from L-arginine by constitutive (c) or inducible (i) nitric oxide synthases (NOS) present in different cell types and released constantly at physiological levels under the influence of cNOS [53]. All NO synthases convert L-arginine and molecular oxygen to L-citrulline and NO. However, expression of iNOS in activated macrophages in response to immunological stimuli induces the release of large amounts of NO for long periods. Although NO species function beneficially as antimicrobial effector molecules in the immune protection against bacteria, parasites and viruses and regulate cell survival, the relatively high and sustained level of inducible NO produced during the immune response may be harmful, as dramatically shown in septic shock. Accordingly, regulation of NO production is important for human health. In the presence of superoxide anion, a series of NO effector molecules might prevail that are also involved in the host defence mechanism.
Although the production of reactive oxygen and nitrogen species considerably contributes to the killing of infectious pathogens, the secretion of cytokines from activated macrophages and related cells is also an integral component of an effective immune response to a viral or bacterial infection, with regulatory functions on the production of these cytotoxic effector species. They act at all levels of the immune response and form a complex network of molecules. Among the macrophage-associated cytokines, release of tumour necrosis factor (TNF)-α is an essential early step in a signalling cascade leading to production of antimicrobial NO [54,55]. Also, TNF-α synergizes with IFN-γ in the induction of iNOS and NO production by macrophages, though IFN-γ alone was shown to be capable of independently enhancing iNOS transcription and NO release [56,57]. The role that these cytokines play in NO-mediated destruction of microbial pathogens prompted in the beginning exploration of Pelargonium-induced immune modulatory effects on macrophage functions using functional assays such as a fibroblast-lysis assay for release of TNF and a cytopathic effect inhibition assay for IFN-like properties (vide infra). Importantly, control of viruses is achieved through IFN-〈 and IFN-ß which are produced by host cells, while IFN-γ mediates macrophage activation.
2.6. Immunomodulatory Activities
The destabilized defence mechanisms resulting from a viral infection can clear the way for a secondary bacterial infection. Recalling the ethnomedicinal use of the roots of P. sidoides in the treatment of tuberculosis, it is appropriate to stress that pathogenic mycobacteria have developed multiple mechanisms for entering macrophages by a receptor-mediated pathway that is not coupled to the activation of macrophage cytotoxic defence mechanisms to ensure their own survival [58]. The fact that mycobacteria reside within cells and the less effective direct antibacterial potencies (vide supra) suggested that stimulation of the non-specific immune system may contribute to the anti-infective activity of P. sidoides extracts. The immediate question thus concerned the factors that contribute to the immune control of microbial pathogens and possibly viruses associated with respiratory tract infections.
2.6.1. Phagocytic Activity and Oxidative Burst
Phagocytosis and related activity of phagocytes play a crucial role in the innate immunity of host defence against invading pathogens including bacteria and viruses. Circulating blood phagocytes are rapidly recruited to sites of infection. Activated by attached pathogen-associated molecular patterns, the microorganisms are ingested by the phagocytes (phagocytosis) followed by the production and release of reactive oxygen species (oxidative burst) that contribute to the destruction of pathogens (intracellular killing). Using a whole blood-based, flow cytometric assay with Candida albicans as the target organism, EPs® 7630 significantly increased the number of phagocytosing cells (maximal enhanced by 56% after 2 min upon addition of 30 μg/mL of the extract sample) in a concentration-dependent manner. With a target to effector cell ratio of 1:1, all yeast cells were ingested after 30 min [59].
As for the oxidative burst, the application of 30 μg/mL extract similarly led to a marked increase of burst-active blood phagocytes for all time points observed beyond 2 min, with a maximum augmentation of 120% at 4 min when compared with controls. The stimulation of the burst activity was still detectable even when all Candida organisms have already been ingested. Although phagocytic activity may strongly depend on the kind of microorganism, this finding clearly demonstrated that the Pelargonium extract effectively improved phagocytic activity. Furthermore, results from a microbiological intracellular killing assay indicated that the number of surviving yeast cells were reduced by ca. 30% at 120 min compared with controls. The benefit of the oral application may be seen in local effects on both the resident and invading phagocytes at the site of infection.
2.6.2. Intracellular Killing of Pathogens and Release of Nitric Oxides
Experimental infection of macrophages constitutes a particularly versatile model for assessing the immunoregulation that occurs during a cell-mediated response to an intracellular pathogen, consistent with the previous antitubercular usage of P. sidoides in traditional medicine. For safety reasons and convenience, an established in vitro model for infectious diseases was selected in which murine macrophages were infected with obligate intracellular Leishmania parasites rather than pathogenic Mycobacteria strains [60]. A colorimetric assay was used for assessing the activation of macrophages against the intracellular parasites, based on infection of macrophages in suspension culture, exposure to the Pelargonium extract, and lysis of the host cells with sodium dodecyl sulfate (SDS) [61]. Surviving Leishmania organisms were then quantitated by their conversion of yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into a blue formazan product. While Pelargonium extracts, including parent aqueous acetone and methanol extracts as well as organic fractions (petroleum ether, ethyl acetate, n-butanol), proved to be inactive against extracellular promastigote forms of Leishmania species, pronounced antileishmanial effects were observed against intracellular amastigotes that are of clinical and pharmacological importance, with EC50 values ranging from < 0.1 to 3.3 μg/mL compared with that of 7.9 μg/mL for Pentostam® as positive control [60]. Confirmatory evidence was recently provided by fluorescence activated cell scanning (FACS) analysis using transgenic L. major expressing green fluorescent protein (GFP) [62]. This method has significant advantages over those involving counting parasites in microscopic preparations and the SDS lysis/MTT protocol. Treatment of infected BMMΦ with EPs® 7630 resulted in a significant decrease of the intracellular GFP signal in a dose dependent manner and reaffirmed previous results concerning the activation of immune defence mechanisms but at single cell levels (Figure 4). That reduction of the parasite load was not due to general cytotoxicity followed from addition of propidium iodide before FACS measurement in order to discriminate dead (PI-positive cells< 10% in all experiments) from living cells. Exposure of infected cells to EPs® 7630 in combination with IFN-γ markedly enhanced the effects. For example, the combination of EPs® 7630 (10 μg/mL) plus IFN-γ (100 U/mL) was almost as effective as IFN-γ (100 U/mL) plus LPS (10 ng/mL) in killing intracellular parasites [36].
Given the crucial role of NO as cytotoxic effector molecule (vide supra), the NO inducing potential of Pelargonium extracts and their constituents was determined using the supernatants of sample treated macrophage (BMMΦ, RAW 264.7) cultures as a source of secreted NO which was quantitated by determining the nitrite concentration using the Griess assay. That the immune response was considerably more expressed in infected cells suggests effective stimulation of the non-specific immune system when needed. Compared with the positive control (100 U/mL IFN-γ + 10 ng/mL LPS), the NO inducing potential of hydrophilic Pelargonium extracts was significantly less prominent. However, it should be noted that the induced NO production strongly depended on the kind of macrophages (BMMΦ; RAW 264.7) used in experimental models [63]. Inhibition of iNOS by addition of L-NMMA produced significantly lower NO levels and concomitantly increased the GFP signal, providing strong evidence for the crucial role of NO as toxic effector molecule in the host defence to microbial infections and the pronounced NO-inducing capabilities of EPs® 7630. Although a strong inverse correlation between NO production and parasite kill was observed, the involvement of additional NO-independent defence mechanisms cannot be ruled out.
The above promising results prompted studies with an additional infection model using intracellular Listeria monocytogenes bacteria and motivated the pursuit of more relevant studies at the cellular and molecular level [64]. This experimental infection model is also commonly used for studying antimicrobial defence [65]. Consistent with the Leishmania model, exposure of infected cells to EPs® 7630 significantly enhanced NO production in a concentration dependent manner when compared to just infected cells. Conspicuously, NO production was already increased at an early time point post infection (6 h). Although the survival rate was not measured in this FACS-based assay due to non-availability of labelled L. monocytogenes organisms, an efficient elimination of the invading pathogens may be anticipated. The data suggest that EPs® 7630 induces NO release in a broad spectrum of infectious conditions and, importantly, in therapeutically relevant doses (≤ 30 μg/mL). Accordingly, stimulation of the non-specific immune system may be anticipated for intracellular residing mycobacteria. Although this still remains to be demonstrated, the present data provide a clue for the claimed efficacy of this herbal medicine in the earlier treatment of tuberculosis and related diseases in traditional medicine. Interestingly, when tested against macrophages as a mammalian host cell control, all samples revealed no or only moderate cytotoxicity.
2.6.3. Induction of the Release of Tumour Necrosis Factor, IL-1 and IL-12
Macrophage activation usually is a polyphenotypic event, with TNF release during the early response to infection [54]. Measurement of TNF-activity induced by the above mentioned Pelargonium extracts was therefore another important immunological parameter in the evaluation of the anti-infective potential of this herbal medicine. Initially, TNF-release was assessed in a functional assay [66], in which TNF-sensitive ML929F treated with supernatants of sample-activated BMMΦ were rapidly lysed in its presence. Surviving cells incorporate crystal violet dye and their relative number was spectrophotometrically determined. At the concentration of ≤ 25 μg/mL, the ethyl acetate (ca. 20 U/mL) and the n-butanol phase (ca. 19 U/mL) were found to possess a moderate TNF-inducing potential. All remaining samples showed only a negligible effect (< 5 U/mL) compared to the LPS stimulus (10 ng/mL; 184 U/mL) as positive control [60]. Having in mind that systemic TNF can be fatal the moderate TNF-inducing capability of the Pelargonium extracts in non-infected cells may be beneficial. This functional bioassay has hitherto not been extended to infected macrophages. However, gene expression analysis clearly showed augmented and prolonged up-regulation of TNF- α transcripts in infected cells (vide supra). Confirmatory evidence of increased intracellular TNF- α levels induced by EPs® 7630 in infectious conditions compared to non-infected cells was recently obtained from FACS analysis at single cell levels [64]. Conspicuously, the accumulation of this membrane-bound cytokine in stimulated infected cells (ca. 35%) was less pronounced than that in untreated cultures (ca. 45%). This phenomenon may be explained by an enhanced activity of the TNF-α converting enzyme mediated by the herbal extract. This conjecture found support by increased soluble TNF-α protein levels detected by ELISA in the supernatant of infected cells treated with EPs® 7630. The TNF-α titers (ca. 10–16 ng/mL; EPs® 7630 test concentrations 1–30 μg/mL) were conspicuously above those of both the positive LPS control and infected cells (ca. 10 ng/mL in each case).
Besides TNF-α, early macrophage-associated cytokine production includes interleukin (IL)-1 and IL-12. Analyses of the intracellular/membrane-bound cytokines were again carried out in parallel in non-infected and in infected cells in the absence and presence of EPs® 7630, respectively, at single cell levels using flow cytometry. ELISA was used for monitoring secreted cytokines in cell supernatants. Using BMMΦ experimentally infected with L. monocytogenes, incubation with EPs® 7630 increased the production of intra- and extracellular IL-1 and IL-12 when compared to non-infected and just infected cells [64].
The demonstrated augmented productions of IL-1, IL-12 and TNF-α confirmed once more the potential of EPs® 7630 to induce host defence mechanisms for controlling infectious agents at single cell level. It is known that IL-1 synergizes with TNF-α in the induction of IL-12 production which, in turn, induces IFN-γ release from both NK cells and T cells. IFN-γ in turn mediates protection by inducing NO production. Also, the importance of IL-12 in Th1 maturation has been demonstrated, thus providing a link between innate and adaptive immunity.
2.7. Gene Expression Experiments
Induction of protective immunity against Leishmania infection and other pathogenic agents is closely linked to the production of cytokines. The analysis of the data obtained from densitometric measurements revealed that the iNOS and cytokine (IL-1, IL-10, IL-12, IL-18, IFN-α, IFN-γ and TNF-α) mRNA levels of infected RAW 264.7 cells were significantly enhanced in response to LPS plus IFN-γ when compared with those of non-infected cells. Notably, EPs® 7630 produced similar transcript profiles with considerably increased and prolonged up-regulations distinctly expressed under infectious conditions (Figure 5) [67-69].
Transcripts of IL-10, a cytokine associated with downregulatory functions [70], were clearly expressed later. This finding independently supported the anti-infective potential of P. sidoides observed at functional levels (vide supra). Interestingly, this herbal medicine also stimulated infected cells to produce IFN-γ transcripts. The expression of IFN-γ itself in cells of monocytic lineage has merely been noted under certain physiological and pathological conditions [71,72]. Also worthy of mention is the up-regulation of IL-12 and IL-18 mRNA levels in that both cytokines are critical to host defence against a variety of pathogens and are involved in the production of IFN-γ [73,74]. Subsequent studies suggested that IFN-γ mRNA expression appeared to be a unique feature of P. sidoides among herbal extracts [75]. The gene expression experiments thus provided evidence for macrophage activation at the transcriptional level.
2.8. Expression of Surface Markers
Recently, some surface markers became a focus of research interest [64]. CD40 is a member of the TNF receptor superfamily, expressed primarily on antigen presenting cells including macrophages. This surface marker was found to be an important regulatory receptor for IFN-γ dependent and independent host defence mechanisms [76,77] and to be essential for the development of a CD4+ T cell mediated immune response [78]. Stimulation of non-infected and Listeria-infected BMMΦ with EPs® 7630 moderately enhanced the expression of CD40 [63]. However, it was suggested that this herbal medicine may sustain up-regulated CD40 expression in infected conditions as concluded from prominent expressions at the very low concentration of 1 μg/mL.
Stimulation of BMMΦ with LPS + IFN-γ or EPs® 7630 (30 μg/mL) resulted in a dramatic down-regulation of the IFN-γ receptor subunit CD119 after 6 h post infection, which may be rationalized by internalisation of the receptor. The down-regulation of this receptor under infectious conditions was consistent with similar effects in L. monocytogenes infected BMMΦ [79]. Changes in the expression of CD119 are suggested to affect the sensitivity of the cell in responding to IFN-γ [80]. These limited molecular studies do not allow definite statements, but, at the very least, they do provide encouragement for further study of the P. sidoides extract's mode of action in anti-infective assays.
2.9. Release of Antimicrobial Peptides
Polymorphonuclear neutrophils (PMNs) play a key role in the front-line defence against pathogens. In addition to recruiting and activating other immune cells, PMNs have been demonstrated to release soluble peptides with broad-spectrum antimicrobial activity such as the bactericidal/permeability-increasing protein (BPI) and the defensins, human neutrophil peptides (HNP) 1–3. Recently EPs® 7630 was shown to enhance the release of these antimicrobial peptides in a concentration-dependent manner [81]. At 30 μg/mL EPs® 7630 increased the levels of HNP 1–3 and BPI by 150% and 127%, respectively. It should be noted that the extract was a much better inducer for the release of the defensins than LPS (82%, 10 ng/mL), whereas LPS proved to be more effective for BPI (356%). Interestingly, the combination of both stimuli enhanced the release of the studied antimicrobial peptides disproportionately (531% and 294% for BPI and HNP 1–3, respectively). This finding provided further evidence that the immune system reacts faster and most likely more effective against pathogens under the treatment of EPs® 7630 than in the absence of this herbal medicine.
3. Conclusions
A traditional South African herbal medicine that has been used for centuries in the empirical treatment of respiratory tract diseases has found its way into European laboratories. Recent chemical, pharmacological and clinical studies of a special extract, referred to as EPs® 7630 and developed from this traditional medicine, proved its efficacy in these conditions. Studying herbs such as P. sidoides for effects on the non-specific immune system may lead to the identification of novel chemical structures on which newer therapeutic agents can be derived and provide additional insight into the mode of action at the molecular level. This example provides a compelling argument for continuing the exploration of Nature and traditional medical systems as a source of therapeutically useful herbal medicines which satisfy current criteria of quality, safety and efficacy for an evidence-based therapy.





Acknowledgments
The unflagging efforts of the co-workers listed on the project's publications over the past 20 years have proven to be an inspiration, and the author is grateful to them all.
Conflict of Interest
The author's work was supported in part by the pharmaceutical company Willmar Schwabe GmbH & Co, Karlsruhe, Germany.
References
- WHO. Health statistics and health information systems, Available online: http://www.who.int/healthinfo/statistics (accessed on 22 June 2011).
- Watt, J.M.; Breyer-Brandwyk, M.G. Medicinal and Poisonous Plants of Southern and Eastern Africa; Livingstone: Edinburgh/London, UK, 1962; pp. 449–455. [Google Scholar]
- Hutchings, A. Zulu Medicinal Plants; Natal University Press: Pietermaritzburg, South Africa; p. 1996.
- Kolodziej, H.; Kiderlen, A.F. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs® 7630. Phytomedicine 2007, 14, 18–26. [Google Scholar]
- Brendler, T.; van Wyk, B.E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J. Ethnopharmacol. 2008, 119, 420–433. [Google Scholar]
- Helmstädter, A. Umckaloabo – Late vindication of a secret remedy. Pharm. Historian 1996, 26, 2–4. [Google Scholar]
- Taylor, P.W.; Maalim, S.; Coleman, S. The strange story of Umckaloabo. Pharmac. J. 2005, 275, 790–792. [Google Scholar]
- Kolodziej, H. Aqueous ethanolic extract of the roots of Pelargonium sidoides – New scientific evidence for an old anti-infective phytopharmaceutica. Planta Med. 2008, 74, 661–666. [Google Scholar]
- Kolodziej, H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo®. Phytomedicine 2007, 14, 9–17. [Google Scholar]
- Chuchalin, A.G.; Berman, B.; Lehmacher, W. Treatment of acute bronchitis in adults with a Pelargonium sidoides preparation (EPs® 7630): A randomized, double-blind, placebo-controlled trial. Explore 2005, 1, 437–445. [Google Scholar]
- Haidvogl, M.; Heger, M. Treatment effect and safety of EPs® 7630-solution in acute bronchitis in childhood: Report of a multicentre observational study. Phytomedicine 2007, 14, 60–64. [Google Scholar]
- Matthys, H.; Heger, M. Treatment of acute bronchitis with a liquid herbal drug preparation from Pelargonium sidoides (EPs® 7630): A randomised, double-blind, placebo-controlled multicentre study. Curr. Med. Res. Opin. 2007, 23, 323–331. [Google Scholar]
- Lizogub, V.G.; Riley, D.S.; Heger, M. Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore 2007, 3, 573–584. [Google Scholar]
- Matthys, H.; Funk, P. EPs® 7630 improves acute bronchitis symptoms and shortens time to remission. Results of a randomized, double-blind, placebo-controlled, multicentre trial. Planta Med. 2008, 7, 686–692. [Google Scholar]
- Agbabiaka, T.B.; Guo, R.; Ernst, E. Pelargonium sidoides for acute bronchitis: A systematic review and meta-analysis. Phytomedicine 2008, 15, 378–385. [Google Scholar]
- Bachert, C.; Schapowal, A.; Funk, P.; Kieser, M. Treatment of acute rhinosinusitis with the preparation from Pelargonium sidoides EPs 7630: A randomized, double-blind, placebo-controlled trial. Rhinology 2009, 47, 51–55. [Google Scholar]
- Kamin, W.; Maydannik, V.G.; Malek, F.A.; Kieser, M. Efficacy and tolerability of EPs 7630 in children and adolescents with acute bronchitis: A randomized, double-blind, placebo-controlled multicenter trial with a herbal drug preparation from Pelargonium sidoides roots. Int. J. Clin. Pharmacol. Ther. 2010, 48, 184–191. [Google Scholar]
- Kirk, K.M.; Garbes Netto, P.G. Post-marketing surveillance of Pelargonium sidoides for treatment of presumably viral acute community acquired tonsillopharyngitis. Rev. Panam. Infectol. 2007, 9, 15–24. [Google Scholar]
- Gonzales, R.; Sande, A.M. Uncomplicated acute bronchitis. Ann. Intern. Med. 2000, 133, 981–991. [Google Scholar]
- Gonzales, R.; Bartlett, J.G.; Besser, R.E.; Cooper, R.J.; Hickner, J.M.; Hoffmann, J.R.; Sande, A.M. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: Background. Ann. Emerg. Med. 2001, 37, 720–727. [Google Scholar]
- Gödecke, T. Phytochemische und Pharmakologische Untersuchungen an Pelargonium sidoides DC. PhD. Thesis, Freie Universität, Berlin: Berlin, Germany, 2005. [Google Scholar]
- Taylor, P.W. Antimycobacterial activity of indigenous South African plants. S. Afr. Med. J. 2003, 93, 904–907. [Google Scholar]
- Seidel, V.; Taylor, P.W. In vitro activity of extracts and constituents of Pelargonium against rapidly growing mycobacteria. Int. J. Antimicrob. Agents 2004, 23, 613–619. [Google Scholar]
- Mativandlela, S.N.P.; Lall, N.; Meyer, J.J.M. Antibacterial, antifungal and antitubercular activity of Pelargonium reniforme Curtis and Pelargonium sidoides DC. (Geraniaceae) root extracts. S. Afr. J. Bot. 2006, 72, 232–237. [Google Scholar]
- Mativandlela, S.N.P.; Meyer, J.J.M.; Hussein, A.A.; Lall, N. Antitubercular activity of compounds isolated from Pelargonium sidoides. Pharm. Biol. 2007, 45, 645–650. [Google Scholar]
- Kayser, O.; Kolodziej, H. Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 1997, 63, 508–510. [Google Scholar]
- Uslu, H.; Yoruk, O.; Ayyildiz, A.; Aktan, B. Antibacterial spectrum of Umckaloabo (Pelargonium sidoides) on upper airway infection agents. Eur. J. Gen. Med. 2009, 6, 245–248. [Google Scholar]
- Conrad, A.; Jung, I.; Tioua, D.; Lallemand, C.; Carrapatoso, F.; Engels, I.; Daschner, F.D.; Frank, U. Extract of Pelargonium sidoides (EPs® 7630) inhibits the interactions of group A-streptococci and host epithelia in vivo. Phytomedicine 2007, 14, 52–59. [Google Scholar]
- Janecki, A.; Conrad, A.; Engels, I.; Frank, U.; Kolodziej, H. Evaluation of an aqueous-ethanolic extract from Pelargonium sidoides (EPs® 7630) for its activity against group A-streptococci adhesion to human HEp-2 cells. J. Ethnopharmacol. 2011, 133, 147–152. [Google Scholar]
- Podbielski, A.; Kreikemeyer, B. The background of tissue specificity of Streptococcus pyogenes. Bioforum 2009, 29, 32–34. [Google Scholar]
- Beil, W.; Kilian, P. EPs® 7630, an extract from Pelargonium sidoides roots inhibits adherence of Helicobacter pylori to gastric epithelial cells. Phytomedicine 2007, 14, 5–8. [Google Scholar]
- Wittschier, N.; Faller, G.; Hensel, A. An extract of Pelargonium sidoides (EPs® 7630) inhibits in situ adhesion of Helicobacter pylori to human stomach. Phytomedicine 2007, 14, 285–288. [Google Scholar]
- Neugebauer, P.; Mickenhagen, A.; Siefer, O.; Walger, M. A new approach to pharmacological effects on ciliary beat frequency in cell cultures – Exemplary measurements under Pelargonium sidoides extract. Phytomedicine 2005, 12, 47–52. [Google Scholar]
- Marcucci, F.; Klein, B.; Kirchner, M.; Zawatzky, R. Production of high titers of interferon gamma by prestimulated spleen cells. Eur. J. Immunol. 1982, 12, 787–790. [Google Scholar]
- Kolodziej, H.; Kayser, O.; Radtke, O.A.; Kiderlen, A.F.; Koch, E. Pharmacological profile of extracts of Pelargonium sidoides and their constituents. Phytomedicine 2003, 10, 18–24. [Google Scholar]
- Thäle, C.; Kiderlen, A.F.; Kolodziej, H. Anti-infective activities of Pelargonium sidoides (EPs® 7630): Effects of induced NO-production on Leismania major in infected macrophages and antiviral effects as assessed in a fibroblast-virus protection assay. Planta Med 2011, 77, 718–725. [Google Scholar]
- Schroder, K.; Hertzog, P.J.; Ravani, T.; Hume, D.A. Interferon-γ: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar]
- Ank, N.; Iversen, M.B.; Bartholdy, C.; Staeheli, P.; Hartmann, R.; Jensen, U.B.; Dagnaes-Hansen, F.; Thomsen, A.R.; Chen, Z.; Haugen, H.; et al. An important role for type III interferon (IFN-lamba/IL-2) in TLR-induced antiviral activity. J. Immunol. 2008, 180, 2474–2485. [Google Scholar]
- Koch, E.; Augustin, R.; Wohn, C.; Erdelmeier, C.A.J. Inhibition of mediator release from RBL-2H3 cells and human granulocytes by the Pelargonium sidoides root extract EPs® 7630. Planta Med. 2010, 76, 1291. [Google Scholar]
- Kolodziej, H.; Kayser, O.; Kiderlen, A.F.; Ito, H.; Hatano, T.; Yoshida, T.; Foo, L.Y. Proanthocyanidins and related compounds: Antileishmanial activity and modulatory effects on nitric oxide and tumour necrosis factor α release in the murine macrophage-like cell line RAW 264.7. Biol. Pharm. Bull. 2001, 24, 1016–1021. [Google Scholar]
- Biron, C.A. Interferons α and ß as immune regulators – A new look. Immunity 2001, 41, 661–664. [Google Scholar]
- Schnitzler, P.; Schneider, S.; Stintzing, F.C.; Carle, R.; Reichling, J. Efficacy of an aqueous Pelargonium sidoides extract against herpesvirus. Phytomedicine 2008, 15, 1108–1116. [Google Scholar]
- Michaelis, M.; Doerr, H.W.; Cinatl, J., Jr. Investigation of the influence of EPs® 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine 2011, 18, 384–386. [Google Scholar]
- von Itzstein, M. The war against influenza: Discovery and development of sialidase inhibitors. Nat. Rev. Drug Discov. 2007, 6, 967–974. [Google Scholar]
- Potier, M.; Mameli, L.; Belisle, M.; Dallaire, L.; Melancon, S.B. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-α-D-N-acetylneuraminate) substrate. Anal. Biochem. 1979, 94, 287–296. [Google Scholar]
- Janecki, A.J.; Kiderlen, A.F.; Kolodziej, H. In vitro evaluation of EPs® 7630 for its ability to inhibit neuraminidase using sodium (4-methyl-umbelliferyl)-α-D-N-acetylneuraminate as substrate. Planta Med. 2009, 75, 989. [Google Scholar]
- Muller, C.P. Institute of Immunology, Centre de Recherche Public-Santé/Laboratoire National de Santè: Luxembourg; Personal communication; 2011. [Google Scholar]
- Moncada, S.; Palmer, R.M.J.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Nathan, C.F.; Hibbs, J. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991, 3, 65–70. [Google Scholar]
- Liew, F.Y.; Wei, X.Q.; Proudfoot, L. Cytokines and nitric oxide as effector molecules against parasitic infections. Phil. Trans. Royal Soc. Lond. B 1997, 352, 1311–1315. [Google Scholar]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr. Opin. Immunol. 2000, 12, 64–76. [Google Scholar]
- Bredt, D.S. Endogeneous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar]
- Reiner, S.L.; Locksley, R.M. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 1995, 13, 151–177. [Google Scholar]
- Roach, T.I.; Kiderlen, A.F.; Blackwell, J.M. Role of inorganic nitrogen oxides and tumor necrosis factor alpha in killing Leishmania donovani amastigotes in gamma interferon-lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect. Immun. 1991, 59, 3935–3944. [Google Scholar]
- Deng, W.; Thiel, B.; Tannenbau, C.S.; Hamilton, T.A.; Stuehr, D.J. Synergistic cooperation between T cell lymphokines for induction of the nitric oxide synthase gene in murine peritoneal macrophages. J. Immunol. 1993, 151, 322–329. [Google Scholar]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar]
- Ernst, J.D. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 1998, 66, 1277–1281. [Google Scholar]
- Conrad, A.; Hansmann, C.; Engels, I.; Daschner, F.D.; Frank, U. Extract of Pelargonium sidoides (EPs® 7630) improves phagocytosis, oxidative burst, and intracellular killing of human peripheral blood phagocytes in vitro. Phytomedicine 2007, 14, 46–51. [Google Scholar]
- Kayser, O.; Kolodziej, H.; Kiderlen, A.F. Immunomodulatory principles of Pelargonium sidoides. Phytother. Res. 2001, 15, 122–126. [Google Scholar]
- Kiderlen, A.F.; Kaye, P.M. A modified colorimetric assay of macrophage activation for intracellular cytotoxicity against Leishmania parasites. J. Immunol. Methods 1990, 127, 11–18. [Google Scholar]
- Kram, D.; Thäle, C.; Kolodziej, H.; Kiderlen, A.F. Intracellular parasite kill: Flow cytometry and NO detection for rapid discrimination between anti-leishmanial activity and macrophage activation. J. Immunol. Methods 2008, 333, 79–88. [Google Scholar]
- Kiderlen, A.F.; Kayser, O.; Ferreira, D.; Kolodziej, H. Tannins and related compounds: Killing of amastigotes of Leishmania donovani and release of nitric oxide and tumour necrosis factor α in macrophages in vitro. Z. Naturforsch. 2001, 56c, 444–454. [Google Scholar]
- Thäle, C.; Kiderlen, A.; Kolodziej, H. Anti-infective mode of action of EPs® 7630 at the molecular level. Planta Med. 2008, 74, 675–681. [Google Scholar]
- North, R.J.; Dunn, P.L.; Conlan, J.W. Murine listeriosis as a model for antimicrobial defense. Immunol. Rev. 1997, 158, 27–36. [Google Scholar]
- Wagner, H.; Jurcic, K. Methods in Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: London, UK, 1991; Volume 6, pp. 195–217. [Google Scholar]
- Radtke, O.A.; Kiderlen, A.F.; Kayser, O.; Kolodziej, H. Gene expression profiles of inducible nitric oxide synthase and cytokines in Leishmania major-infected macrophage-like RAW 264.7 cells treated with gallic acid. Planta Med. 2004, 70, 924–928. [Google Scholar]
- Kolodziej, H.; Burmeister, A.; Trun, W.; Radtke, O.A.; Kiderlen, A.F.; Ito, H.; Hatano, T.; Yoshida, T.; Foo, L.Y. Tannins and related compounds induce nitric oxide synthase and cytokines gene expressions in Leishmania major-infected macrophage-like RAW 264.7 cells. Bioorg. Med. Chem. 2005, 13, 6470–6476. [Google Scholar]
- Trun, W.; Kiderlen, A.F.; Kolodziej, H. Nitric oxide synthase and cytokines gene expression analyses in Leishmania-infected RAW 264.7 cells treated with an extract of Pelargonium sidoides (EPs® 7630). Phytomedicine 2006, 13, 570–575. [Google Scholar]
- Mocellin, S.; Panelli, M.C.; Wang, E.; Nagorsen, D.; Marincola, F.M. The dual role of IL-10. Trends Immunol. 2003, 24, 36–38. [Google Scholar]
- Mogensen, S.C.; Virelizier, J.L. The interferon-macrophage alliance. Interferon 1987, 8, 55–84. [Google Scholar]
- Gessani, S.; Belardelli, F. IFN-γ expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 1998, 9, 117–123. [Google Scholar]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen specific adaptive immunity. Annu. Rev. Immunol. 1995, 13, 251–276. [Google Scholar]
- Sugawara, I. Interleukin-18 (IL-18) and infectious diseases, with special emphasis on diseases induced by intracellular pathogens. Microbes Infect. 2000, 2, 1257–1263. [Google Scholar]
- Kolodziej, H.; Kiderlen, A.F. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry 2005, 66, 2056–2071. [Google Scholar]
- Andrade, R.M.; Portillo, J.A.; Wessendarp, M.; Subauste, C.S. CD40 signalling in macrophages induces activity against an intracellular pathogen independently of gamma interferon and reactive nitrogen intermediates. Infect. Immun. 2005, 73, 3115–3123. [Google Scholar]
- Andrade, R.M.; Wessendar, M.; Subauste, C.S. CD154 activates macrophage antimicrobial activity in the absence of IFN-gamma through a TNF-alpha-dependent mechanism. J. Immunol. 2003, 171, 6750–6756. [Google Scholar]
- van Kooten, C.; Banchereau, J. CD40-CD40 ligand. J. Leukoc. Biol. 2000, 67, 2–17. [Google Scholar]
- Demuth, A.; Goebel, W.; Beuscher, H.U.; Kuhn, M. Differential regulation of cytokine and cytokine receptor mRNA expression upon infection of bone marrow-derived macrophages with Listeria monocytogenes. Infect. Immun. 1996, 64, 3475–3483. [Google Scholar]
- Tau, G.; Rothman, P. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar]
- Koch, E.; Wohn, C. Pelargonium sidoides root extract EPs® 7630 stimulates release of antimicrobial peptides from neutrophil granulocytes in human whole blood. Planta Med. 2007, 73. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kolodziej, H. Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium sidoides (EPs® 7630) in the Context of Health Promotion . Pharmaceuticals 2011, 4, 1295-1314. https://doi.org/10.3390/ph4101295
Kolodziej H. Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium sidoides (EPs® 7630) in the Context of Health Promotion . Pharmaceuticals. 2011; 4(10):1295-1314. https://doi.org/10.3390/ph4101295
Chicago/Turabian StyleKolodziej, Herbert. 2011. "Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium sidoides (EPs® 7630) in the Context of Health Promotion " Pharmaceuticals 4, no. 10: 1295-1314. https://doi.org/10.3390/ph4101295
APA StyleKolodziej, H. (2011). Antimicrobial, Antiviral and Immunomodulatory Activity Studies of Pelargonium sidoides (EPs® 7630) in the Context of Health Promotion . Pharmaceuticals, 4(10), 1295-1314. https://doi.org/10.3390/ph4101295