Vacuum-Free Quantum Dots Planar Hybrid Solar Cells: Improving Charge Transport Using Reduced Graphene Oxide and PEO as the Buffer Layer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PbS Nanoparticles
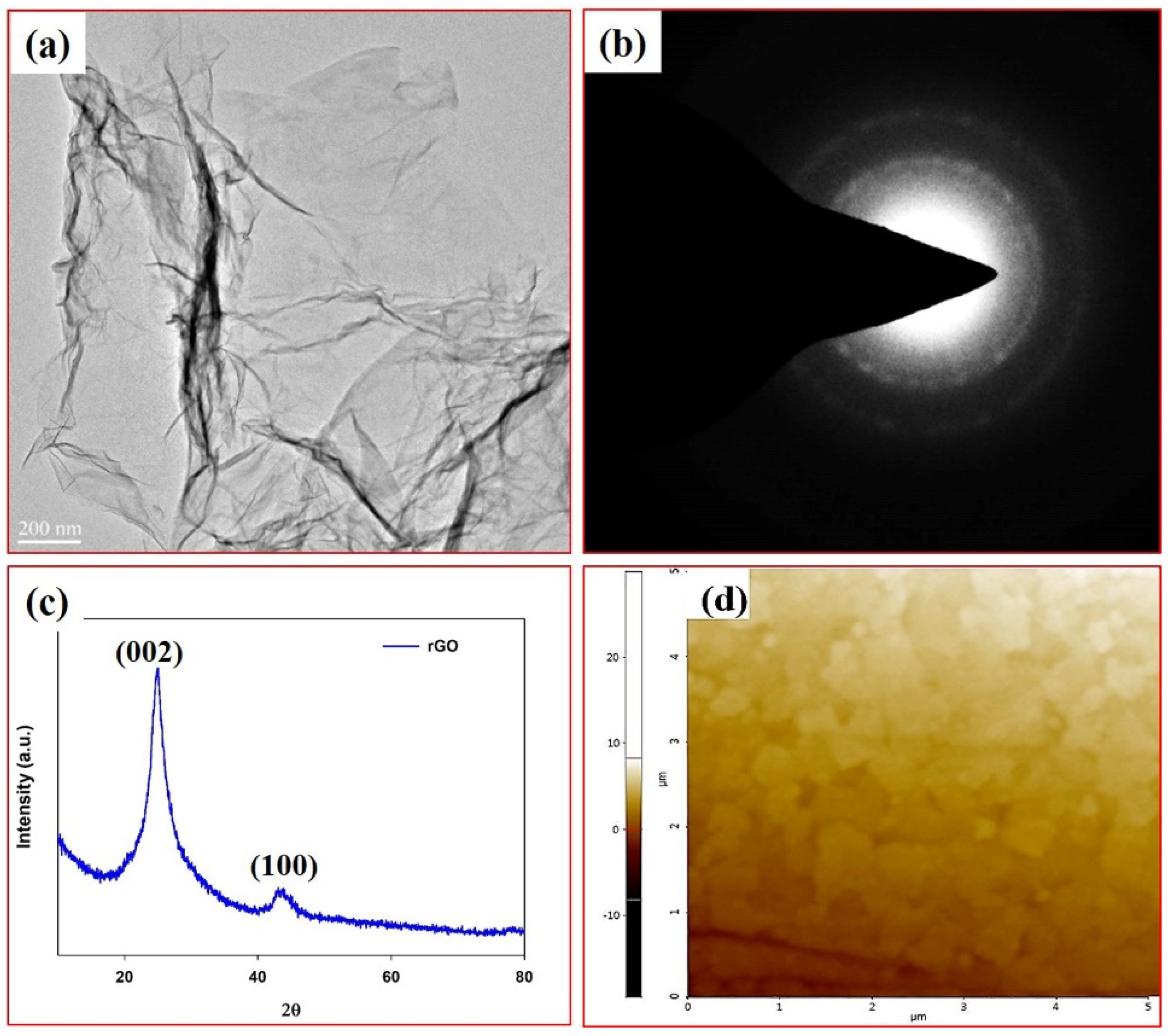
2.2. Preparation of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO)
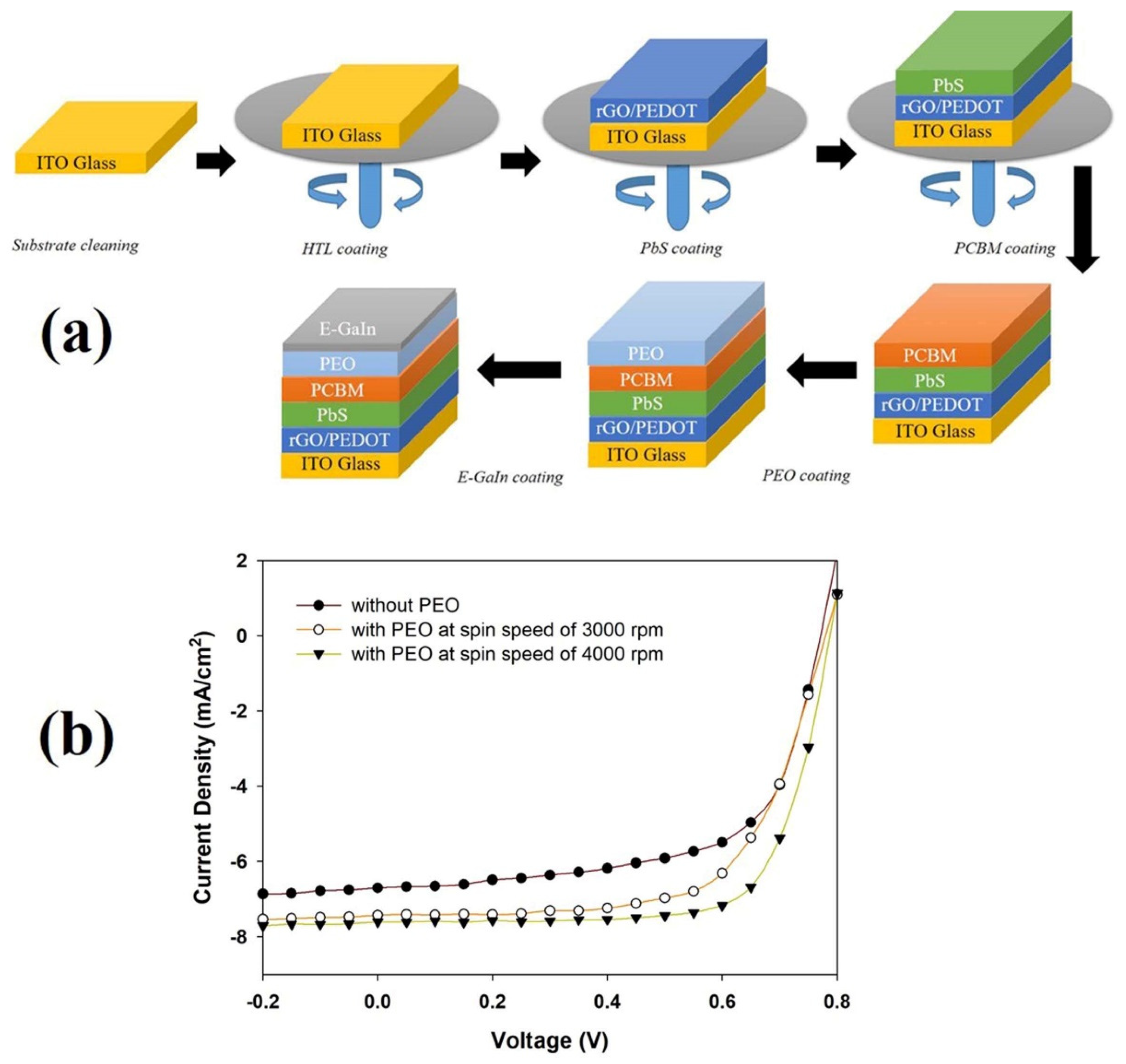
2.3. Solar Cell Fabrication
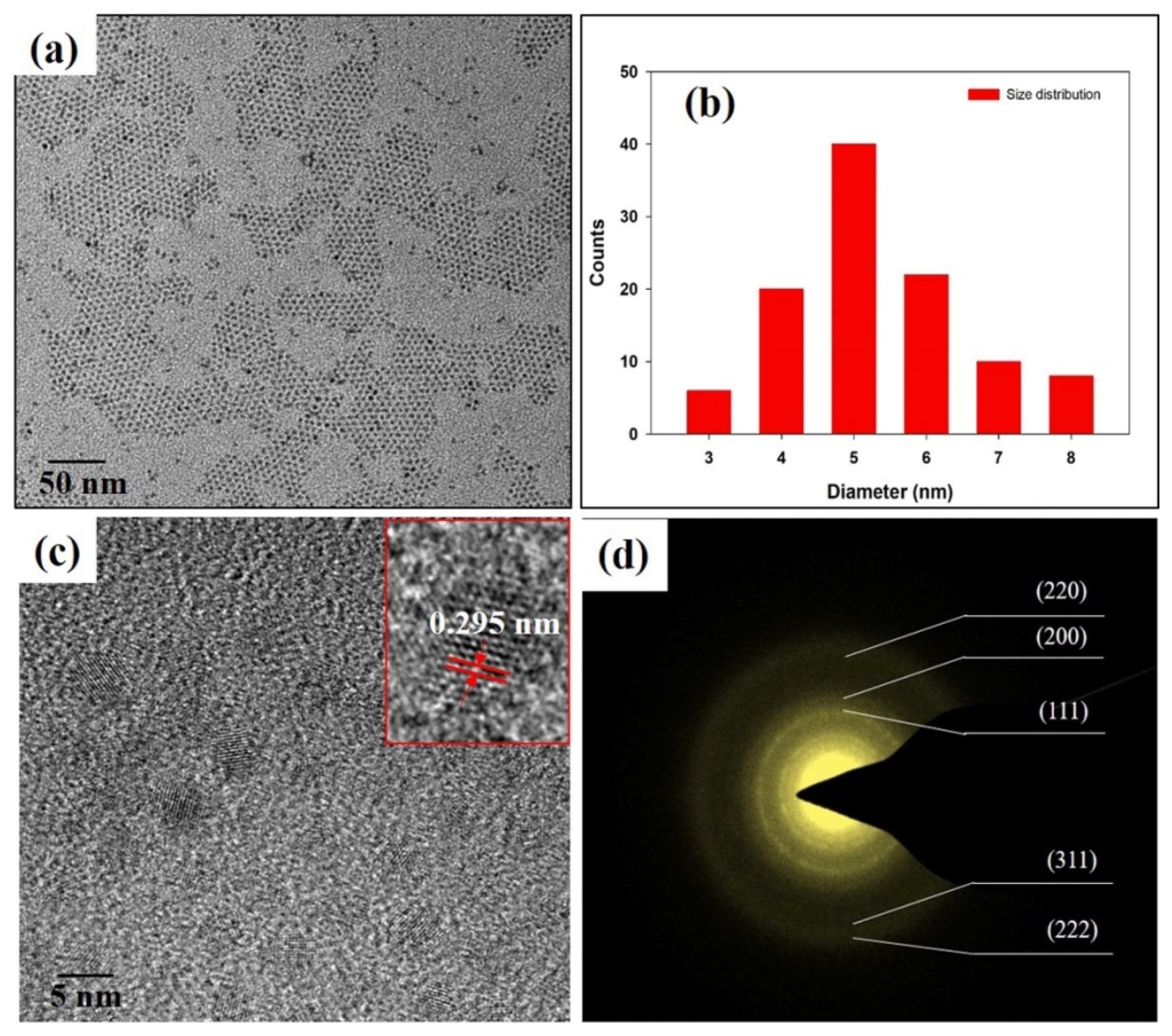
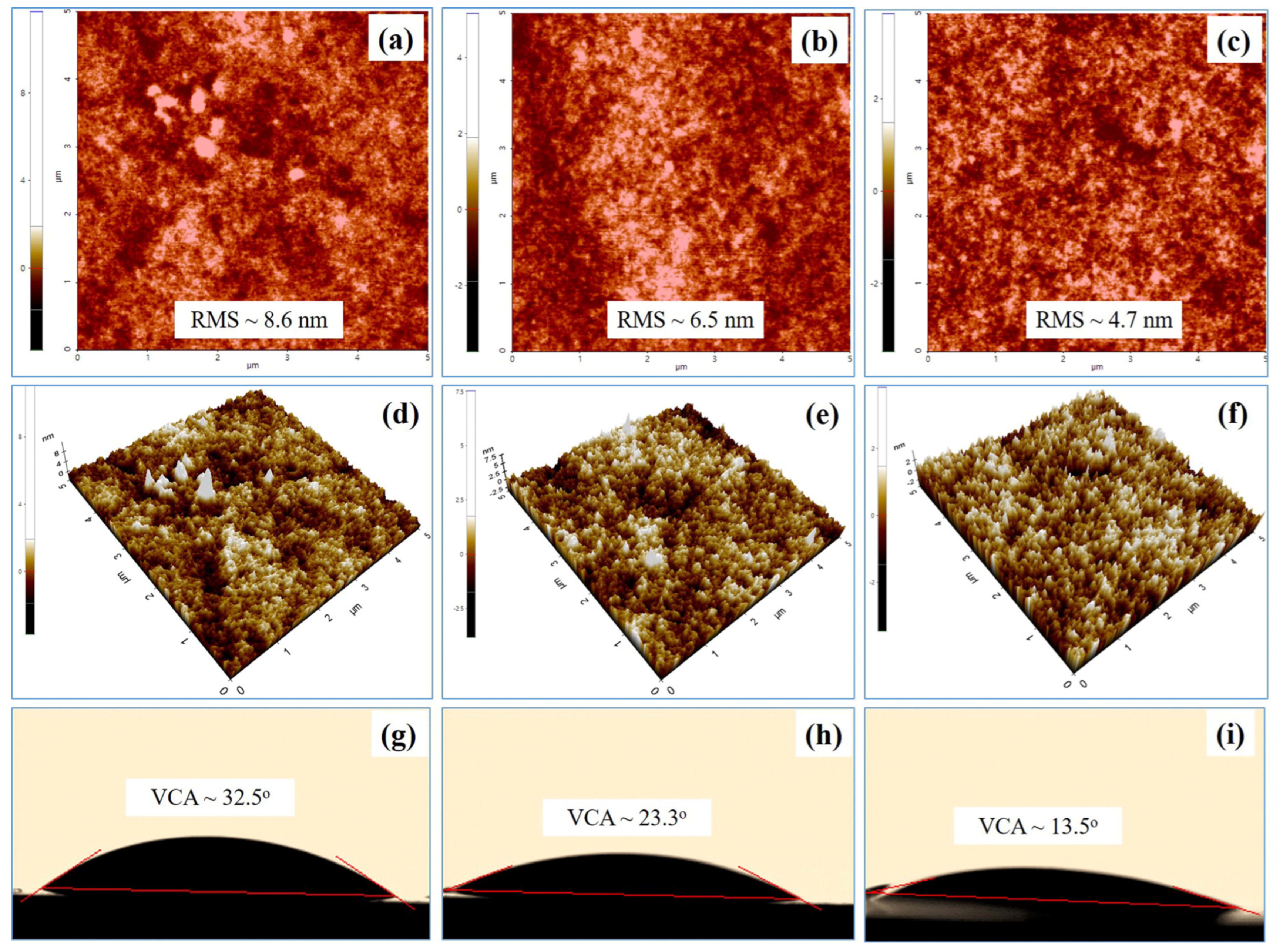
2.4. Characterization
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-Stable All-Inorganic Nanocrystal Solar Cells Processed from Solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid Nanorod Polymer Solar Cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Luther, J.M.; Law, M.; Beard, M.C.; Song, Q.; Reese, M.O.; Ellingson, R.J.; Nozik, A.J. Schottky Solar Cells Based on Colloidal Nanocrystal Films. Nano Lett. 2008, 8, 3488–3492. [Google Scholar] [CrossRef]
- Barkhouse, D.A.R.; Pattantyus-Abraham, A.G.; Levina, L.; Sargent, E.H. Thiols Passivate Recombination Centers in Colloidal Quantum Dots Leading to Enhanced Photovoltaic Device Efficiency. ACS Nano 2008, 2, 2356–2362. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; Smeets, D.; Muynck, D.; Nollet, T.; Martins, J.C.; Vanhaecke, F.; Vantomme, A.; Delerue, C.; Allan, G.; et al. Size-dependent optical properties of colloidal PbS quantum dots. ACS Nano 2009, 3, 3023–3030. [Google Scholar] [CrossRef] [Green Version]
- Chang, I.; Waclawik, E.R. Colloidal semiconductor nanocrystals: Controlled synthesis and surface chemistry in organic media. RCS Adv. 2014, 4, 23505–23527. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Tsang, S.W. Infrared colloidal lead chalcogenide nanocrystals: Synthesis, properties, and photovoltaic applications. Nanoscale 2012, 4, 2187–2201. [Google Scholar] [CrossRef]
- Szendrei, K.; Gomulya, W.; Yarema, M.; Heiss, W.; Loi, M.A. PbS nanocrystal solar cells with high efficiency and fill factor. Appl. Phys. Lett. 2010, 97, 203501. [Google Scholar] [CrossRef] [Green Version]
- Zhai, G.M.; Bezryadina, A.; Breeze, A.J.; Zhang, D.L.; Alersand, G.B.; Carter, S.A. Air stability of TiO2/PbS colloidal nanoparticle solar cells and its impact on power efficiency. Appl. Phys. Lett. 2011, 99, 063512. [Google Scholar] [CrossRef]
- Choi, J.J.; Wenger, W.N.; Hoffman, R.S.; Lim, Y.F.; Luria, J.; Jasieniak, J.; Marohn, J.A.; Hanrath, T. Solution-Processed Nanocrystal Quantum Dot Tandem Solar Cells. Adv. Mater. 2011, 23, 3144–3148. [Google Scholar] [CrossRef] [PubMed]
- Xiea, Q.; Ming, S.; Chena, L.; Wub, Y.; Zhangb, W.; Liu, X.; Cao, M.; Wang, H.Q.; Fang, J. Parameters in planar quantum dot-polymer solar cell: Tuned by QD Eg, ligand exchange and fabrication process. Org. Electron. 2019, 69, 1–6. [Google Scholar] [CrossRef]
- Trinh, T.K.; Truong, N.T.N.; Pham, V.T.H.; Park, C. Simple and Green Synthesis of Lead Sulfide Nanocrystals for Hybrid Bulk Hetero-Junction Solar Cells. Sci. Adv. Mater. 2016, 8, 601–606. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ryu, M.S.; Jang, J. Effect of solution processed graphene oxide/nickel oxide bilayer on cell performance of bulk-heterojunction organic photovoltaic. Sol. Energy Mater. Sol. Cells 2011, 95, 2893–2896. [Google Scholar] [CrossRef]
- Chen, D.; Li, L.; Guo, L. An environmental-friendly preparation of reduced graphene oxide nanosheets via amino acid. Nanotechnology 2011, 22, 32567–325601. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Bai, H.; Du, C.; Wei, D.; Su, Y.; Wang, Y.; Yang, L. Incorporation of reduced graphene oxide into faceted flower-like {001} TiO2 for enhanced photocatalytic activity. R. Soc. Open Sci. 2018, 5, 180613. [Google Scholar] [CrossRef] [Green Version]
- Khenfouch, M.; Buttner, U.; Baitoul, M.; Maaza, M. Synthesis and characterization of mass produced high quality few layered graphene sheets via a chemical method. Graphene 2014, 3, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Meng, F.; Lei, Y.; Zhao, R.; Zhong, J.; Liu, X. Solid state NMR of immobilized catalysts and nanocatalysts. Mater. Lett. 2011, 65, 1737–1740. [Google Scholar] [CrossRef]
- Chen, T.L.; Chen, J.J.A.; Catane, L.; Ma, B. Fully solution processed pin organic solar cells with an industrial pigment—Quinacridone. Org. Electron. 2011, 12, 1126–1131. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, Y.; Xu, G.; Xu, X. The inverse correlation between series resistance and parallel resistance of small molecule organic solar cells. Prog. Nat. Sci. 2015, 25, 323–326. [Google Scholar] [CrossRef] [Green Version]
- Rusu, M.; Wiesner, S.; Lauermann, I.; Fischer, C.H.; Fostiropoulos, K.; Audinot, J.N.; Fleming, Y.; Steiner, M.C.L. Formation of charge-selective Mg–Ag electrodes to CuPc:C60 blend layers. Appl. Phys. Lett. 2010, 97, 073504. [Google Scholar] [CrossRef]
- Lloyd, M.T.; Mayer, A.C.; Tayi, A.S.; Bowen, A.M.; Kasen, T.G.; Herman, D.J.; Mourey, D.A.; Anthony, J.E.; Malliaras, G.G. Photovoltaic cells from a soluble pentacene derivative. Org. Electron. 2006, 7, 243–248. [Google Scholar] [CrossRef]
- Colinge, J.P.; Colinge, C.A. Physics of Semiconductor Devices, 3rd ed.; Kluwer Academic Publishers: New York, NY, USA, 2007; pp. 77–133. [Google Scholar]
- Servaites, J.D.; Yeganeh, S.; Marks, T.J.; Ratner, M.A. Efficiency enhancement in organic photovoltaic cells: Consequences of optimizing series resistance. Adv. Funct. Mater. 2010, 20, 97–104. [Google Scholar] [CrossRef]
- Park, Y.; Choong, V.; Gao, Y.; Hsieh, B.R.; Tang, C.W. Work function of indium tin oxide transparent conductor meas-ured by photoelectron spectroscopy. Appl. Phys. Lett. 1996, 68, 2699–2701. [Google Scholar] [CrossRef]
- Mengistie, D.A.; Ibrahem, M.A.; Wang, P.C.; Chu, C.W. Highly Conductive PEDOT:PSS Treated with Formic Acid for ITO-Free Polymer Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 2290–2297. [Google Scholar] [CrossRef]
- Chiechi, R.C.; Weiss, E.A.; Dickey, M.D.; Whitesides, G.M. Eutectic Gallium–Indium (EGaIn): A Mold-able Liquid Metal for Electrical Characterization of Self-Assembled Monolayers. Angew. Chem. Int. Ed. 2008, 47, 142–144. [Google Scholar] [CrossRef]
- Schmiedova, V.; Pospisil, J.; Kovalenko, A.; Ashcheulov, P.; Fekete, L.; Cubon, T.; Peter, K.; Oldrich, Z.; Weiter, M. Physical properties investigation of reduced graphene oxide thin films prepared by material inkjet printing. J. Nanomater. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Osedach, T.P.; Chang, L.Y.; Geyer, S.M.; Wanger, D.; Binda, M.T.; Alexi, C.A.; Moungi, G.B.; Bulovic, V. Colloidal PbS quantum dot solar cells with high fill factor. ACS Nano 2010, 4, 3743–3752. [Google Scholar] [CrossRef]
- Kim, H.P.; Yusoff, A.R.; Jang, J. Organic solar cells using a reduced graphene oxide anode buffer layer. Sol. Energy Mater. Sol. Cells 2013, 110, 87–93. [Google Scholar] [CrossRef]
- Bordi, F.; Cametti, C.; Di Biasio, A. Electrical conductivity behavior of poly (ethylene oxide) in aqueous electrolyte solutions. Am. J. Phys. Chem. 1988, 92, 4772–4777. [Google Scholar] [CrossRef]
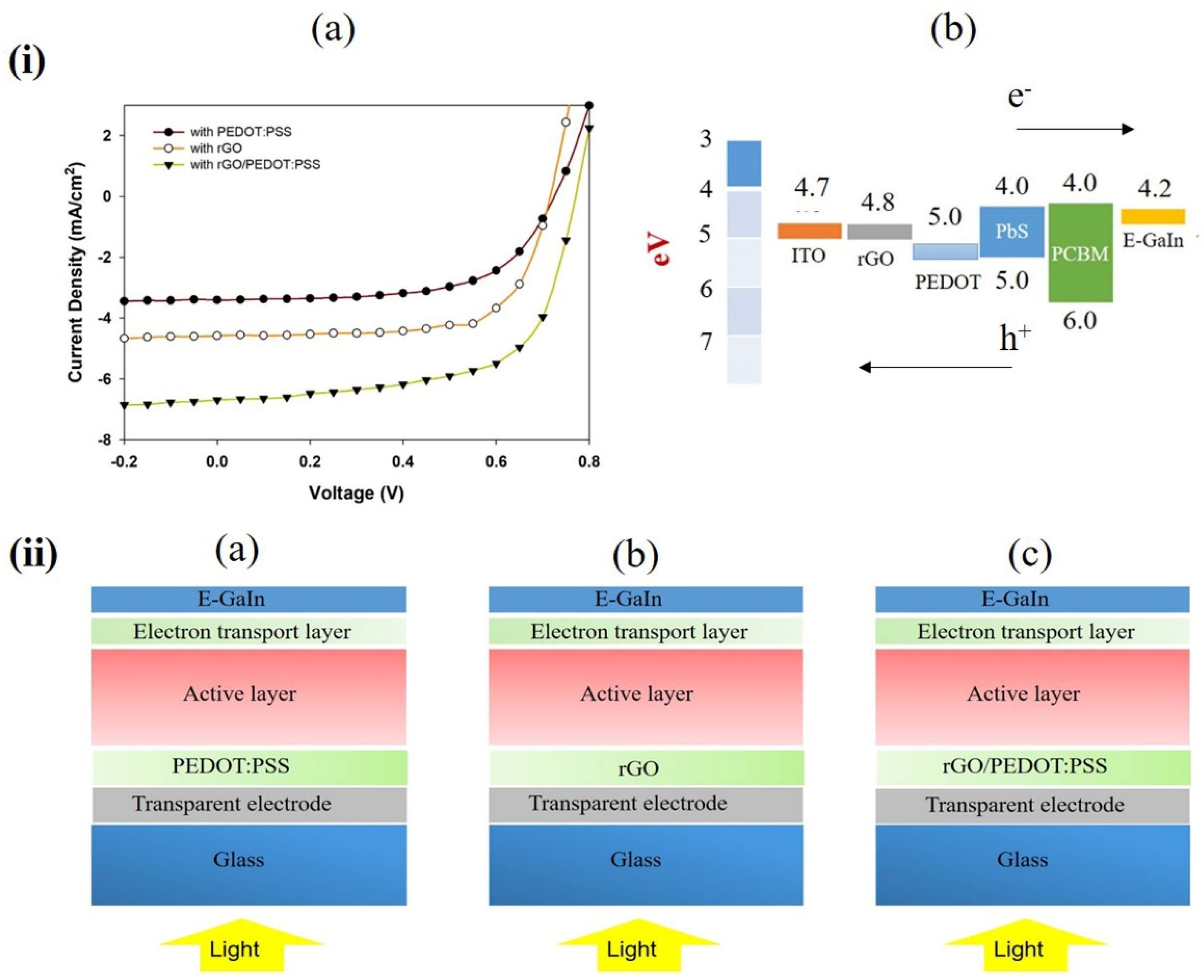
| Device Structure | Jsc (mA/cm2) | Voc (V) | FF (%) | Eff (%) |
|---|---|---|---|---|
| With PEDOT:PSS | 3.41 ± 0.02 | 0.67 ± 0.03 | 62 ± 4 | 1.52 ± 0.02 |
| With rGO | 4.58 ± 0.01 | 0.70 ± 0.02 | 67 ± 2 | 2.14 ± 0.01 |
| With rGO/PEDOT:PSS | 6.69 ± 0.01 | 0.70 ± 0.00 | 70 ± 2 | 3.27 ± 0.01 |
| Device Structure | Jsc (mA/cm2) | Voc (V) | FF (%) | Eff (%) |
|---|---|---|---|---|
| Without PEO | 6.69 ± 0.01 | 0.70 ± 0.00 | 70 ± 2 | 3.27 ± 0.01 |
| With PEO (SS-3000 rpm) | 7.42 ± 0.02 | 0.75 ± 0.02 | 65 ± 2 | 3.78 ± 0.02 |
| With PEO (SS-4000 rpm) | 7.61 ± 0.02 | 0.75 ± 0.00 | 72 ± 2 | 4.34 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Truong, N.T.N.; Lam, N.H.; Le, N.; Tamboli, A.M.; Tamboli, M.S.; Vu, T.N.L.; Jung, J.H. Vacuum-Free Quantum Dots Planar Hybrid Solar Cells: Improving Charge Transport Using Reduced Graphene Oxide and PEO as the Buffer Layer. Appl. Sci. 2022, 12, 1185. https://doi.org/10.3390/app12031185
Kim M, Truong NTN, Lam NH, Le N, Tamboli AM, Tamboli MS, Vu TNL, Jung JH. Vacuum-Free Quantum Dots Planar Hybrid Solar Cells: Improving Charge Transport Using Reduced Graphene Oxide and PEO as the Buffer Layer. Applied Sciences. 2022; 12(3):1185. https://doi.org/10.3390/app12031185
Chicago/Turabian StyleKim, Minsu, Nguyen Tam Nguyen Truong, Nguyen Hoang Lam, Nam Le, Asiya M. Tamboli, Mohaseen S. Tamboli, Truong Nguyen Luan Vu, and Jae Hak Jung. 2022. "Vacuum-Free Quantum Dots Planar Hybrid Solar Cells: Improving Charge Transport Using Reduced Graphene Oxide and PEO as the Buffer Layer" Applied Sciences 12, no. 3: 1185. https://doi.org/10.3390/app12031185






