Identifying Circulating MicroRNA in Kawasaki Disease by Next-Generation Sequencing Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples and RNA Extraction
2.2. Generation of Small RNA Profiles through NGS
2.3. Mapping Clean Reads to Pre-miRNAs
2.4. Identifying Differentially Expressed miRNAs and Pathway Enrichment Analysis
2.5. TaqMan Real-Time PCR Assay
2.6. Statistical Analysis
3. Results
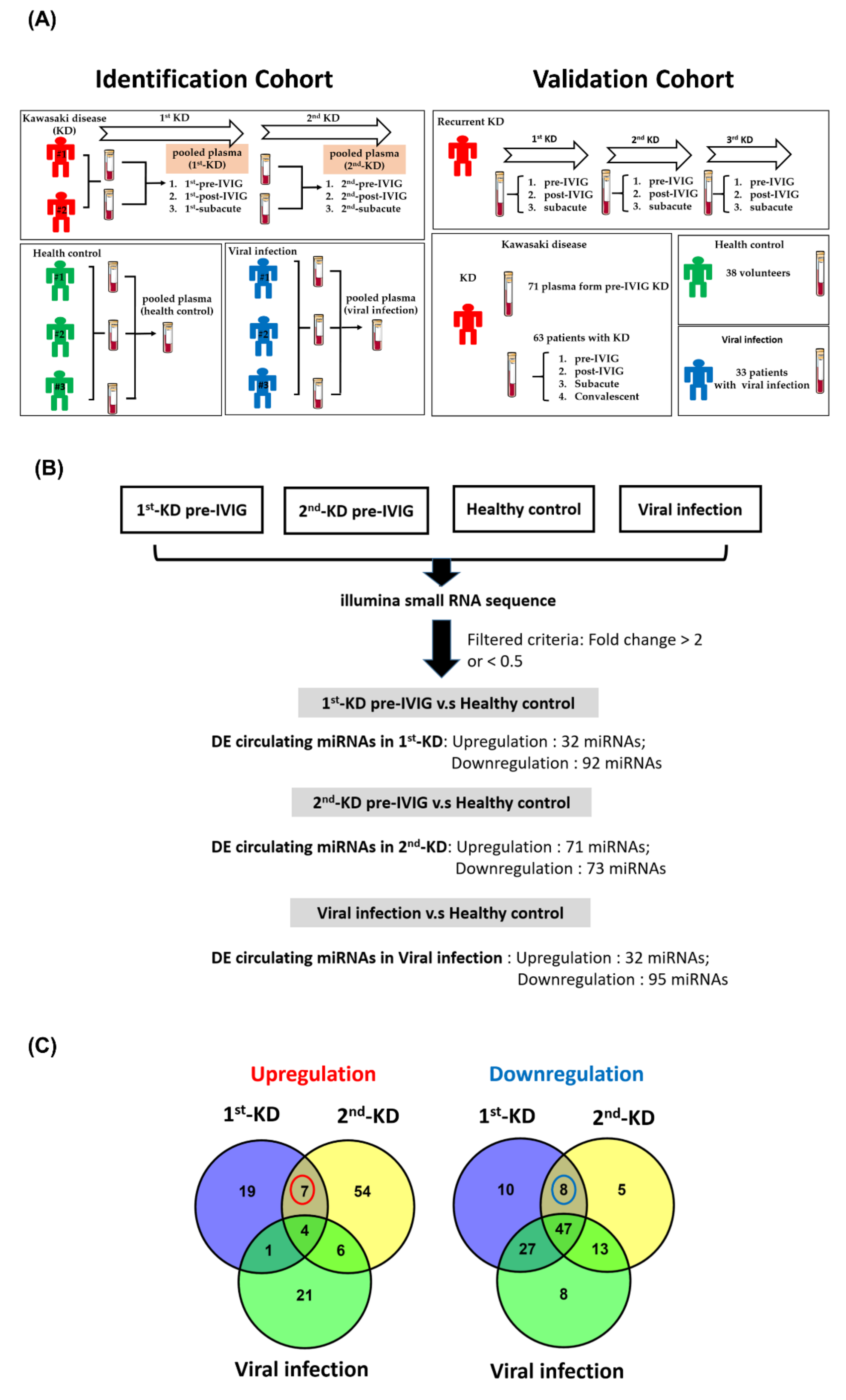
3.1. Generating miRNA Profiles of Plasma from Recurrent KD and Control Groups
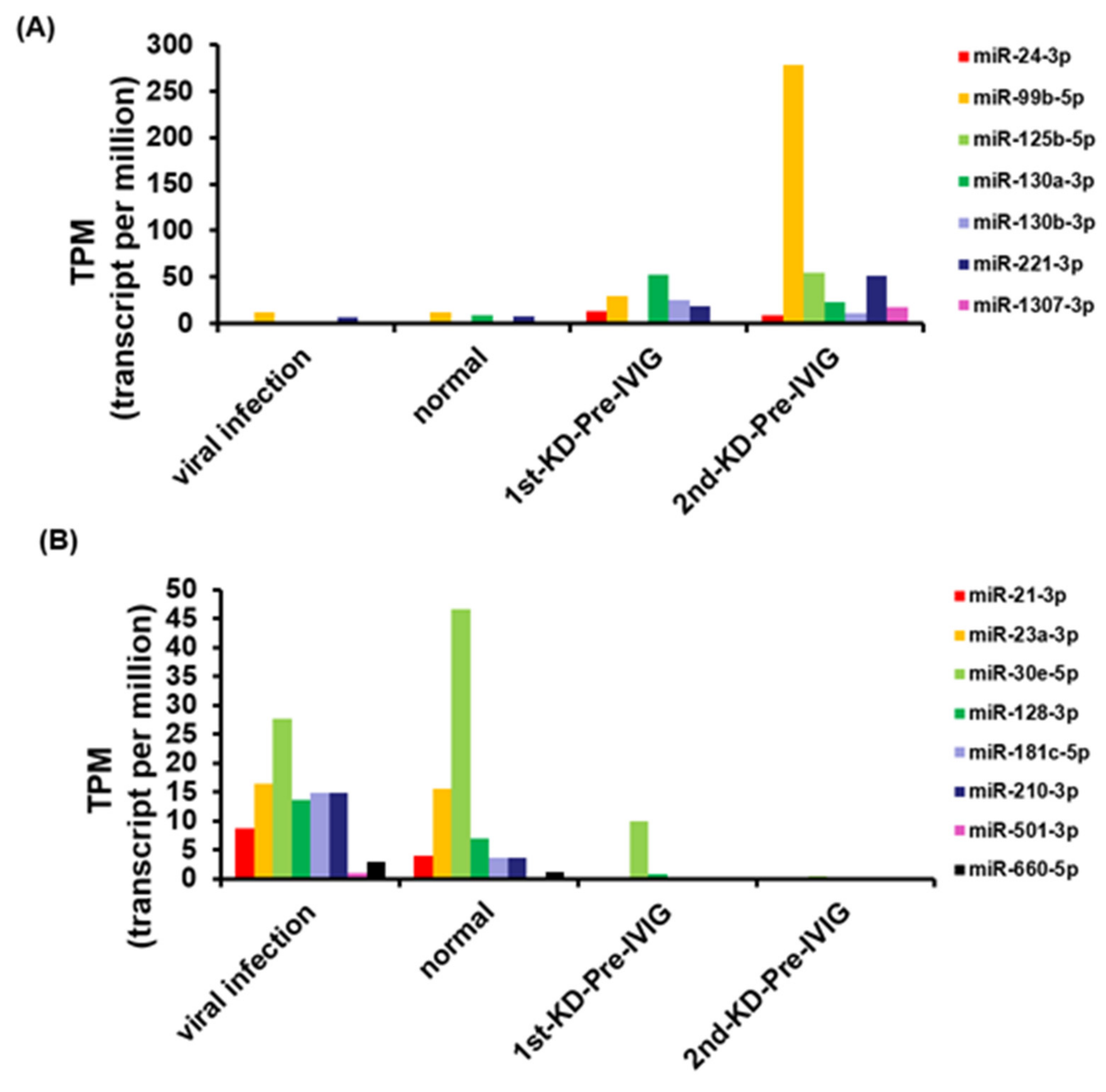
3.2. Circulating miRNAs in Recurrent KD
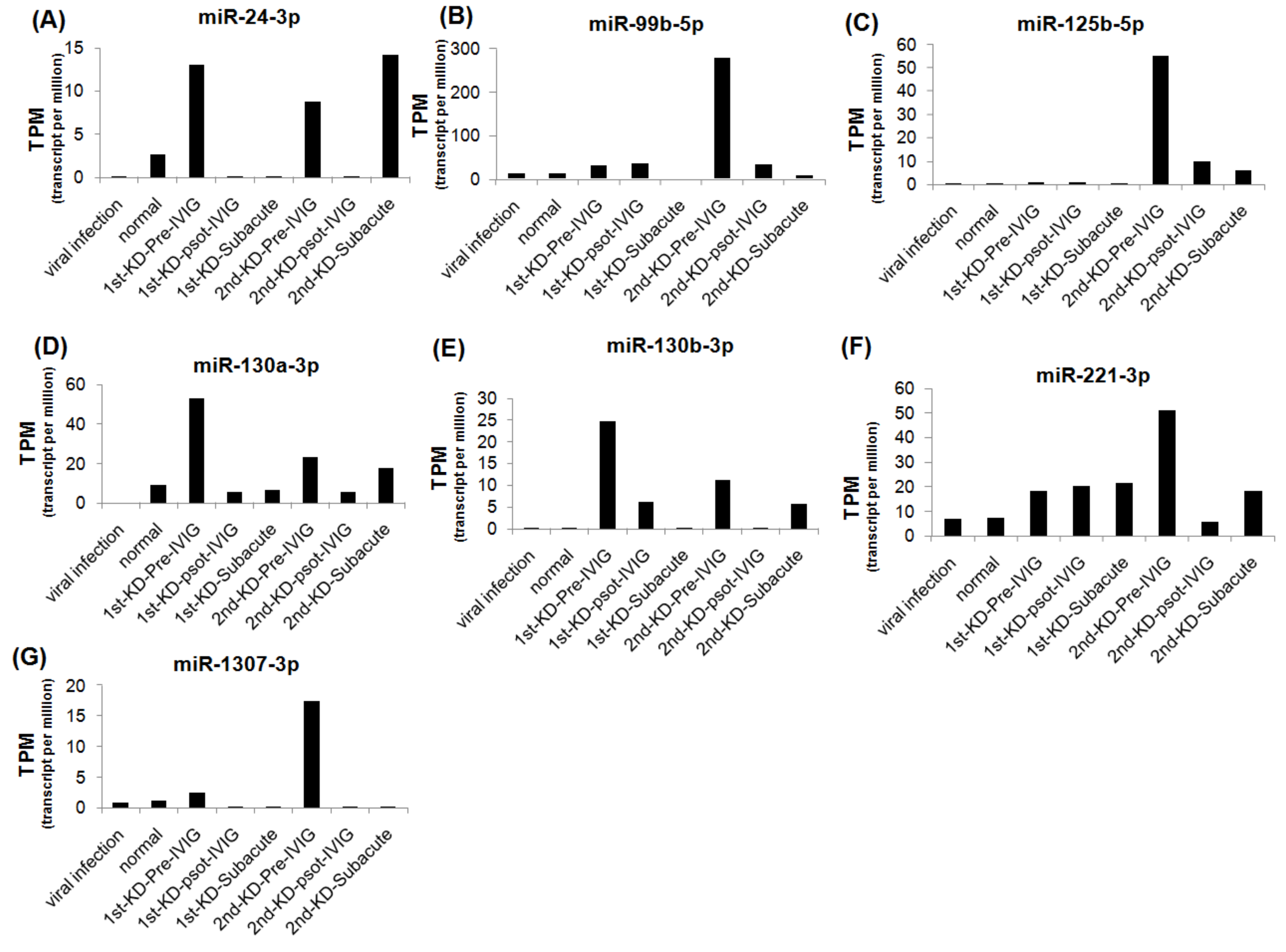
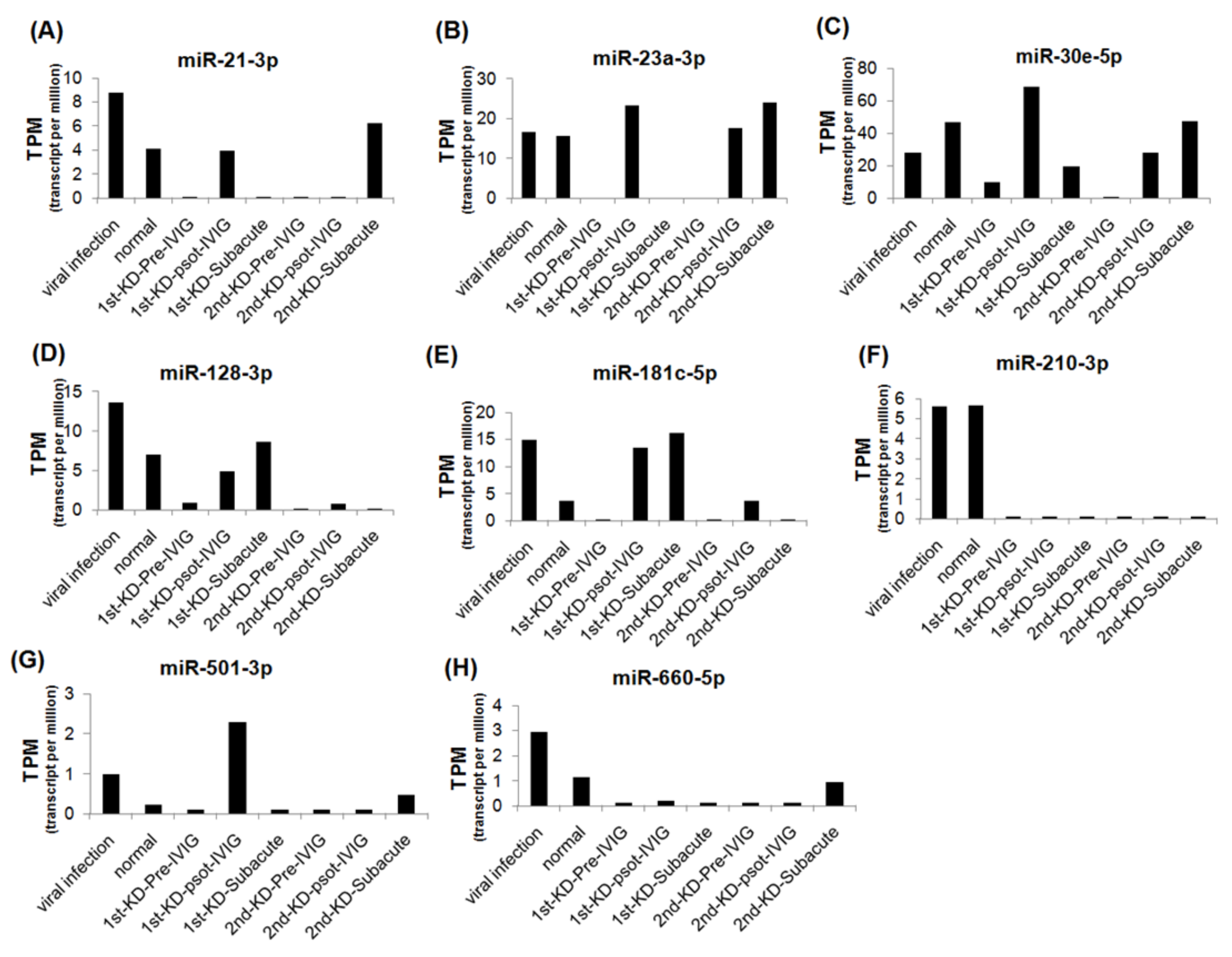
3.3. Circulating MiRNAs during KD Progression
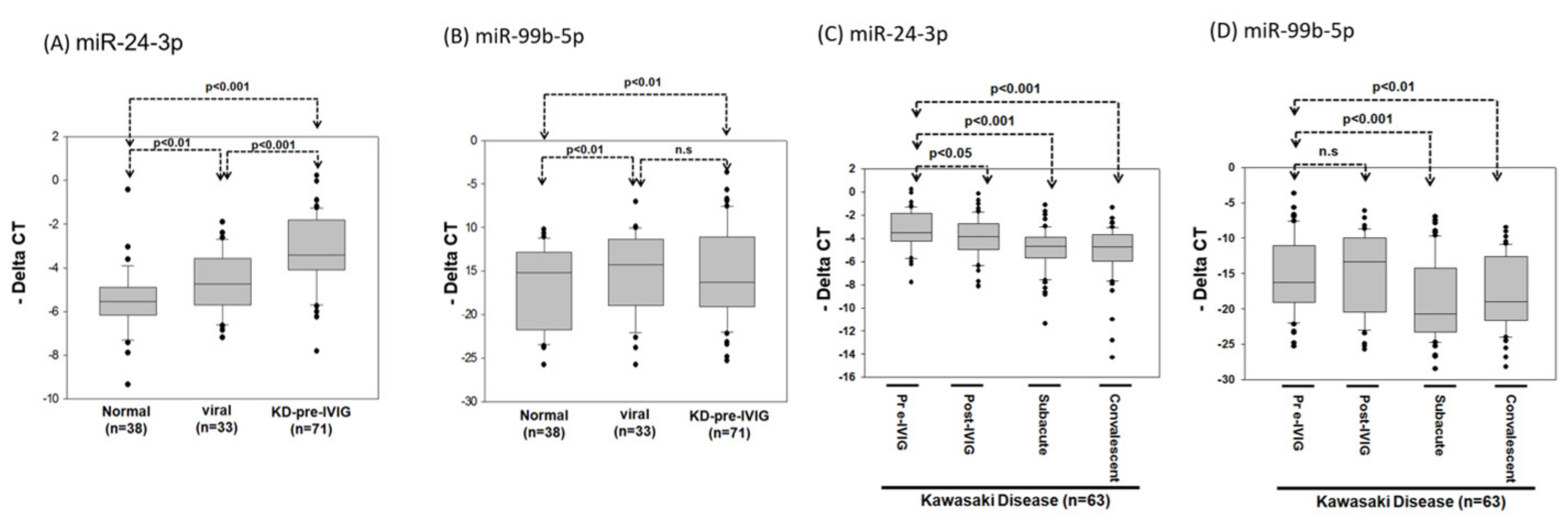
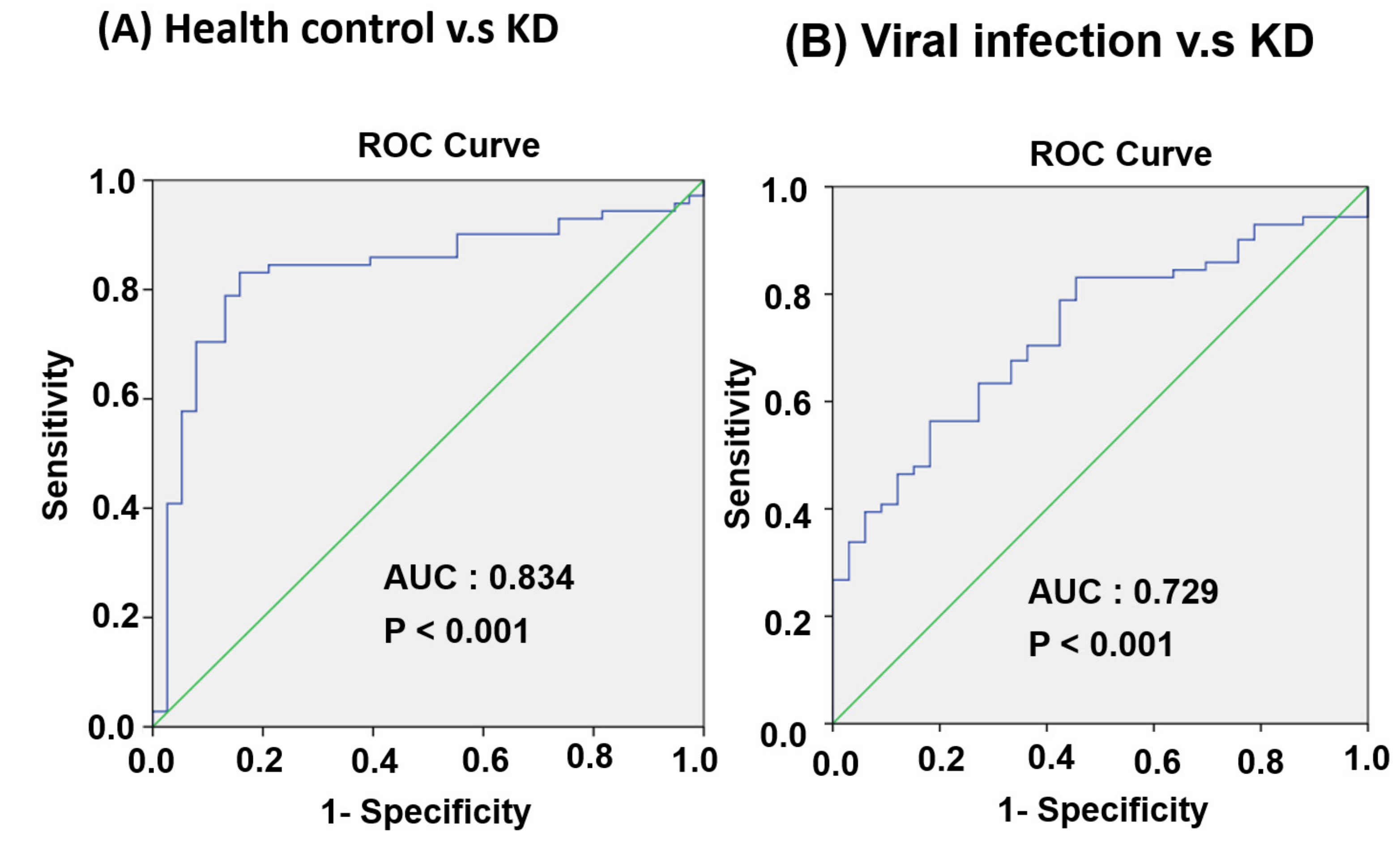
3.4. Circulating MiR-24-3p Levels in Patients with KD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newburger, J.W.; Takahashi, M.; Gerber, M.A.; Gewitz, M.H.; Tani, L.Y.; Burns, J.C.; Shulman, S.T.; Bolger, A.F.; Ferrieri, P.; Baltimore, R.S.; et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004, 110, 2747–2771. [Google Scholar] [CrossRef] [Green Version]
- Rife, E.; Gedalia, A. Kawasaki Disease: An Update. Curr. Rheumatol. Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Kim, G.B.; Eun, L.Y.; Han, J.W.; Kim, S.H.; Yoon, K.L.; Han, M.Y.; Yu, J.J.; Choi, J.W.; Rhim, J.W. Epidemiology of Kawasaki Disease in South Korea: A Nationwide Survey 2015–2017. Pediatr. Infect. Dis. J. 2020, 39, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Lin, K.M.; Ho, S.C.; Yan, J.H.; Lo, M.H.; Kuo, H.C. Increased Incidence of Kawasaki Disease in Taiwan in Recent Years: A 15 Years Nationwide Population-Based Cohort Study. Front. Pediatr. 2019, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Yashiro, M.; Yamashita, M.; Aoyama, N.; Otaki, U.; Ozeki, Y.; Sano, T.; Kojo, T.; Ae, R.; Aoyama, Y.; et al. Cumulative incidence of Kawasaki disease in Japan. Pediatr. Int. 2018, 60, 19–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, H.; Ichinose, E.; Yoshioka, F.; Takechi, T.; Matsunaga, S.; Suzuki, K.; Rikitake, N. Fate of coronary aneurysms in Kawasaki disease: Serial coronary angiography and long-term follow-up study. Am. J. Cardiol. 1982, 49, 1758–1766. [Google Scholar] [CrossRef]
- Kato, H.; Ichinose, E.; Kawasaki, T. Myocardial infarction in Kawasaki disease: Clinical analyses in 195 cases. J. Pediatr. 1986, 108, 923–927. [Google Scholar] [CrossRef]
- Suzuki, A.; Kamiya, T.; Kuwahara, N.; Ono, Y.; Kohata, T.; Takahashi, O.; Kimura, K.; Takamiya, M. Coronary arterial lesions of Kawasaki disease: Cardiac catheterization findings of 1100 cases. Pediatr. Cardiol. 1986, 7, 3–9. [Google Scholar] [CrossRef]
- Hsieh, K.S.; Weng, K.P.; Lin, C.C.; Huang, T.C.; Lee, C.L.; Huang, S.M. Treatment of acute Kawasaki disease: aspirin’s role in the febrile stage revisited. Pediatrics 2004, 114, e689–e693. [Google Scholar] [CrossRef] [Green Version]
- Senzaki, H. Long-term outcome of Kawasaki disease. Circulation 2008, 118, 2763–2772. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, H.; Nameirakpam, J.; Kumrah, R.; Pandiarajan, V.; Suri, D.; Rawat, A.; Singh, S. Biomarkers for Kawasaki Disease: Clinical Utility and the Challenges Ahead. Front. Pediatr. 2019, 7, 242. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020, 16, 391–405. [Google Scholar] [CrossRef]
- Weng, K.P.; Hsieh, K.S.; Huang, S.H.; Ou, S.F.; Lai, T.J.; Tang, C.W.; Lin, C.C.; Ho, T.Y.; Liou, H.H.; Ger, L.P. Interleukin-18 and coronary artery lesions in patients with Kawasaki disease. J. Chin. Med. Assoc. 2013, 76, 438–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirao, J.; Hibi, S.; Andoh, T.; Ichimura, T. High levels of circulating interleukin-4 and interleukin-10 in Kawasaki disease. Int. Arch. Allergy Immunol. 1997, 112, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.K.; Noh, G.W.; Lee, S.I.; Lee, K.Y. Increased serum interleukin-10 level in Kawasaki disease. Yonsei Med. J. 1996, 37, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lin, C.C.; Hwang, B.; Chiang, B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J. Pediatr. 1992, 121, 924–926. [Google Scholar] [CrossRef]
- Matsubara, T.; Furukawa, S.; Yabuta, K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin. Immunol. Immunopathol. 1990, 56, 29–36. [Google Scholar] [CrossRef]
- Maury, C.P.; Salo, E.; Pelkonen, P. Circulating interleukin-1 beta in patients with Kawasaki disease. N. Engl. J. Med. 1988, 319, 1670–1671. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Takahashi, M.; Beiser, A.S.; Burns, J.C.; Bastian, J.; Chung, K.J.; Colan, S.D.; Duffy, C.E.; Fulton, D.R.; Glode, M.P.; et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N. Engl. J. Med. 1991, 324, 1633–1639. [Google Scholar] [CrossRef]
- Yekta, S.; Shih, I.H.; Bartel, D.P. MicroRNA-directed cleavage of HOXB8 mRNA. Science 2004, 304, 594–596. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilad, S.; Meiri, E.; Yogev, Y.; Benjamin, S.; Lebanony, D.; Yerushalmi, N.; Benjamin, H.; Kushnir, M.; Cholakh, H.; Melamed, N.; et al. Serum microRNAs are promising novel biomarkers. PLoS ONE 2008, 3, e3148. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.H.; Lin, W.C.; Tsai, K.W. Advances in molecular biomarkers for gastric cancer: miRNAs as emerging novel cancer markers. Exp. Rev. Mol. Med. 2014, 16, e1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, Y.; Yao, L.; Song, G.; Teng, C. Circulating microRNAs: Promising Biomarkers Involved in Several Cancers and Other Diseases. DNA Cell Biol. 2017, 36, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Lu, R.B.; Wang, L.J.; Chang, C.H.; Lu, T.; Wang, T.Y.; Tsai, K.W. Serum miRNA as a possible biomarker in the diagnosis of bipolar II disorder. Sci. Rep. 2020, 10, 1131. [Google Scholar] [CrossRef] [Green Version]
- Yun, K.W.; Lee, J.Y.; Yun, S.W.; Lim, I.S.; Choi, E.S. Elevated serum level of microRNA (miRNA)-200c and miRNA-371-5p in children with Kawasaki disease. Pediatr. Cardiol. 2014, 35, 745–752. [Google Scholar] [CrossRef]
- Ni, F.F.; Li, C.R.; Li, Q.; Xia, Y.; Wang, G.B.; Yang, J. Regulatory T cell microRNA expression changes in children with acute Kawasaki disease. Clin. Exp. Immunol. 2014, 178, 384–393. [Google Scholar] [CrossRef]
- Shimizu, C.; Kim, J.; Stepanowsky, P.; Trinh, C.; Lau, H.D.; Akers, J.C.; Chen, C.; Kanegaye, J.T.; Tremoulet, A.; Ohno-Machado, L.; et al. Differential expression of miR-145 in children with Kawasaki disease. PLoS ONE 2013, 8, e58159. [Google Scholar] [CrossRef] [Green Version]
- Rowley, A.H.; Pink, A.J.; Reindel, R.; Innocentini, N.; Baker, S.C.; Shulman, S.T.; Kim, K.Y. A study of cardiovascular miRNA biomarkers for Kawasaki disease. Pediatr. Infect. Dis. J. 2014, 33, 1296–1299. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Nakaoka, H.; Takasaki, I.; Hirono, K.; Yamamoto, S.; Kinoshita, K.; Miyao, N.; Ibuki, K.; Ozawa, S.; Watanabe, K.; et al. MicroRNA-93 may control vascular endothelial growth factor A in circulating peripheral blood mononuclear cells in acute Kawasaki disease. Pediatr. Res. 2016, 80, 425–432. [Google Scholar] [CrossRef]
- Maddox, R.A.; Holman, R.C.; Uehara, R.; Callinan, L.S.; Guest, J.L.; Schonberger, L.B.; Nakamura, Y.; Yashiro, M.; Belay, E.D. Recurrent Kawasaki disease: USA and Japan. Pediatr. Int. 2015, 57, 1116–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, D.; Nakamura, Y. Nationwide surveys show that the incidence of recurrent Kawasaki disease in Japan has hardly changed over the last 30 years. Acta Paediatr. 2017, 106, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Guleria, S.; Pilania, R.K.; Jindal, A.K.; Bhattarai, D.; Suri, D.; Singh, S. Recurrent Kawasaki disease at a tertiary care center in Chandigarh, North West India: 24 years of clinical experience. Int. J. Rheum. Dis. 2019, 22, 1183–1187. [Google Scholar] [CrossRef]
- Sudo, D.; Makino, N.; Nakamura, Y. Recurrent Kawasaki disease and cardiac complications: Nationwide surveys in Japan. Arch. Dis. Child 2020, 105, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Oki, I.; Tanihara, S.; Ojima, T.; Yanagawa, H. Cardiac sequelae in recurrent cases of Kawasaki disease: A comparison between the initial episode of the disease and a recurrence in the same patients. Pediatrics 1998, 102, E66. [Google Scholar] [CrossRef] [Green Version]
- Leung, C.M.; Li, S.C.; Chen, T.W.; Ho, M.R.; Hu, L.Y.; Liu, W.S.; Wu, T.T.; Hsu, P.C.; Chang, H.T.; Tsai, K.W. Comprehensive microRNA profiling of prostate cancer cells after ionizing radiation treatment. Oncol. Rep. 2014, 31, 1067–1078. [Google Scholar] [CrossRef] [Green Version]
- Hackenberg, M.; Sturm, M.; Langenberger, D.; Falcon-Perez, J.M.; Aransay, A.M. miRanalyzer: A microRNA detection and analysis tool for next-generation sequencing experiments. Nucleic Acids Res. 2009, 37, W68–W76. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.T.; Tsai, K.W.; Hung, T.M.; Lin, W.C.; Pan, C.Y.; Yu, H.R.; Li, S.C. miRSeq: A user-friendly standalone toolkit for sequencing quality evaluation and miRNA profiling. BioMed. Res. Int. 2014, 2014, 462135. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Xiang, M.; Zeng, Y.; Yang, R.; Xu, H.; Chen, Z.; Zhong, J.; Xie, H.; Xu, Y.; Zeng, X. U6 is not a suitable endogenous control for the quantification of circulating microRNAs. Biochem. Biophys. Res. Commun. 2014, 454, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, C.; Oharaseki, T.; Takahashi, K.; Kottek, A.; Franco, A.; Burns, J.C. The role of TGF-beta and myofibroblasts in the arteritis of Kawasaki disease. Hum. Pathol. 2013, 44, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissmann, A.; Pieper, S.; Adams, I.; Brune, T.; Wiemann, D.; Reinhold, D. Increased blood plasma concentrations of TGF-beta1 and TGF-beta2 after treatment with intravenous immunoglobulins in childhood autoimmune diseases. Pediatr. Allergy Immunol. 2009, 20, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, W.; Xu, R.; Nie, Y.; Cao, X.; Meng, J.; Xu, X.; Hu, S.; Zheng, Z. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J. Cell. Mol. Med. 2012, 16, 2150–2160. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, S.; Xu, M.; Liu, P.; Ren, R.; Ma, W. Role of miR-24, Furin, and Transforming Growth Factor-beta1 Signal Pathway in Fibrosis After Cardiac Infarction. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 65–70. [Google Scholar]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J. Cell. Physiol. 2011, 226, 1407–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, H.C.; Hsieh, K.S.; Ming-Huey Guo, M.; Weng, K.P.; Ger, L.P.; Chan, W.C.; Li, S.C. Next-generation sequencing identifies micro-RNA-based biomarker panel for Kawasaki disease. J. Allergy Clin. Immunol. 2016, 138, 1227–1230. [Google Scholar] [CrossRef] [Green Version]

| Sample Name | Total Illumina Reads | # Clean (% Percentage) | Detected miRNAs# |
|---|---|---|---|
| Viral infection | 6,689,522 | 6,444,268 (98.03%) | 117 |
| Healthy control | 7,616,599 | 7,460,596 (98.88%) | 155 |
| 1st KD-pre-IVIG | 7,945,909 | 7,442,444 (94.6%) | 123 |
| 1st KD-post-IVIG | 7,881,043 | 7,658,251 (98.15%) | 137 |
| 1st KD-subacute | 8,056,044 | 7,841,016 (98.23%) | 127 |
| 2nd KD-pre-IVIG | 6,912,925 | 6,468,229 (94.67%) | 123 |
| 2nd KD-post-IVIG | 6,734,488 | 6,457,836 (97.034%) | 146 |
| 2nd KD-subacute | 7,230,145 | 7,032,058 (98.19%) | 162 |
| Pathway Maps | Total | p-Value | FDR | Genes from Active Data |
|---|---|---|---|---|
| 1. Signal transduction_AKT signaling | 43 | 3.540 × 10−12 | 1.349 × 10−9 | p21, Bim, FOXO3A, PTEN, mTOR, p27KIP1, c-Myc, MDM2, PI3K reg class IA, HGF receptor (Met) |
| 2. Apoptosis and survival_p53-dependent apoptosis | 29 | 1.289 × 10−10 | 2.455 × 10−8 | Bim, Apaf-1, Bcl-2, MEK4(MAP2K4), p38alpha (MAPK14), BMF, MDM2, p14ARF |
| 3. Apoptosis and survival_Cytoplasmic/mitochondrial transport of proapoptotic proteins Bid, Bmf and Bim | 34 | 5.254 × 10−10 | 6.672 × 10−8 | TRAF2, Bim, Apaf-1, Bcl-2, MEK4(MAP2K4), BMF, MKK7 (MAP2K7), TNF-alpha |
| 4. Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling | 111 | 4.303 × 10−9 | 4.099 × 10−7 | p21, p38 MAPK, FOXO3A, mTOR, ROCK, NLK, c-Myc, MDM2, PI3K reg class IA, Dsh, TGF-beta receptor type II |
| 5. Cell cycle_Regulation of G1/S transition (part 1) | 38 | 4.209 × 10−8 | 3.207 × 10−6 | p21, CDC25A, p27KIP1, SMAD4, TGF-beta receptor type II, CDK6, p16INK4 |
| 6. Translation_Non-genomic (rapid) action of Androgen Receptor | 40 | 6.127 × 10−8 | 3.891 × 10−6 | ErbB2, FOXO3A, PTEN, mTOR, PI3K reg class IA (p85-alpha), MDM2, Dsh |
| 7. Immune response_IL-15 signaling | 64 | 1.027 × 10−7 | 5.589 × 10−6 | p38 MAPK, TRAF2, Bcl-2, mTOR, MEK4(MAP2K4), c-Myc, ETS1, PI3K reg class IA (p85) |
| 8. Apoptosis and survival_Regulation of Apoptosis by Mitochondrial Proteins | 33 | 4.490 × 10−7 | 1.595 × 10−5 | Bak, Bim, Apaf-1, Bcl-2, BMF, PUMA |
| 9. Cell cycle_ESR1 regulation of G1/S transition | 33 | 4.490 × 10−7 | 1.595 × 10−5 | p21, ESR1 (nuclear), CDC25A, p27KIP1, c-Myc, CDK6 |
| 10. Apoptosis and survival_Endoplasmic reticulum stress response pathway | 53 | 4.604 × 10−7 | 1.595 × 10−5 | Bak, TRAF2, Bim, Apaf-1, Bcl-2, MEK4(MAP2K4), p38alpha (MAPK14) |
| Pathway Maps | Total | p-Value | FDR | Genes from Active Data |
|---|---|---|---|---|
| 1. Development_Regulation of epithelial-to-mesenchymal transition (EMT) | 64 | 9.407 × 10−13 | 3.612 × 10−10 | SNAIL1, IL-1 beta, SMAD2, NOTCH4, Jagged1, Bcl-2, TGF-beta 1, SIP1 (ZFHX1B), WNT, SP1, TNF-alpha, TGF-beta receptor type II, Tropomyosin-1 |
| 2. Development_TGF-beta-dependent induction of EMT via SMADs | 35 | 1.120 × 10−11 | 2.150 × 10−9 | HMGA2, SNAIL1, SMAD2, SMAD4, Jagged1, TGF-beta 1, SIP1 (ZFHX1B), TGF-beta, SP1, TGF-beta receptor type II |
| 3. Development_TGF-beta receptor signaling | 50 | 5.340 × 10−10 | 6.835 × 10−8 | Ski, XIAP, SMAD2, NFKBIA, SMAD4, MEK3(MAP2K3), TGF-beta 1, SMAD7, SP1, TGF-beta receptor type II |
| 4. Cytoskeleton remodeling_TGF, WNT and cytoskeletal remodeling | 111 | 1.306 × 10−9 | 1.254 × 10−7 | NLK, XIAP, SMAD2, PLAT (TPA), MEK3(MAP2K3), DOCK1, FOXO3A, Cofilin, TGF-beta 1, WNT, SP1, TGF-beta receptor type II, Collagen IV |
| 5. Cell adhesion_Plasmin signaling | 35 | 1.003 × 10−8 | 7.701 × 10−7 | XIAP, PLAT (TPA), TGF-beta R III (betaglycan), MEK3(MAP2K3), TGF-beta 1, Neuroserpin, TGF-beta receptor type II, Collagen IV |
| 6. Immune response_HMGB1/RAGE signaling pathway | 53 | 1.873 × 10−8 | 1.199 × 10−6 | K-RAS, ICAM1, IL-1 beta, NFKBIA, PLAT (TPA), I-kB, MEF2C, SP1, TNF-alpha |
| 7. Cytoskeleton remodeling_Cytoskeleton remodeling | 102 | 6.356 × 10−7 | 3.487 × 10−5 | PTEN, XIAP, PLAT (TPA), MEK3(MAP2K3), DOCK1, Cofilin, TGF-beta 1, MyHC, TGF-beta receptor type II, Collagen IV |
| 8. Possible pathway of TGF-beta 1-dependent inhibition of CFTR expression | 27 | 9.252 × 10−7 | 4.441 × 10−5 | XIAP, SMAD4, MEK3(MAP2K3), TGF-beta 1, SMAD7, TGF-beta receptor type II |
| 9. Apoptosis and survival_FAS signaling cascades | 44 | 1.225 × 10−6 | 5.225 × 10−5 | Bim, Lamin B, XIAP, FasL(TNFSF6), Apaf-1, Bcl-2, DAXX |
| 10. Development_BMP signaling | 33 | 3.252 × 10−6 | 1.249 × 10−4 | Ski, XIAP, SMAD4, MEK3(MAP2K3), BMP receptor 2, SMAD7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, K.-P.; Cheng, C.-F.; Chien, K.-J.; Ger, L.-P.; Huang, S.-H.; Tsai, K.-W. Identifying Circulating MicroRNA in Kawasaki Disease by Next-Generation Sequencing Approach. Curr. Issues Mol. Biol. 2021, 43, 485-500. https://doi.org/10.3390/cimb43020037
Weng K-P, Cheng C-F, Chien K-J, Ger L-P, Huang S-H, Tsai K-W. Identifying Circulating MicroRNA in Kawasaki Disease by Next-Generation Sequencing Approach. Current Issues in Molecular Biology. 2021; 43(2):485-500. https://doi.org/10.3390/cimb43020037
Chicago/Turabian StyleWeng, Ken-Pen, Ching-Feng Cheng, Kuang-Jen Chien, Luo-Ping Ger, Shih-Hui Huang, and Kuo-Wang Tsai. 2021. "Identifying Circulating MicroRNA in Kawasaki Disease by Next-Generation Sequencing Approach" Current Issues in Molecular Biology 43, no. 2: 485-500. https://doi.org/10.3390/cimb43020037






