Influence of Antibiotics on Functionality and Viability of Liver Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Treatment
2.2. Cell Proliferation and Death
2.3. Enzyme Activity Assays and Albumin Release
2.4. Statistical Analysis
3. Results
3.1. Rifampicin and Cefuroxime Significantly Reduce Vitality and Cell Proliferation in a Dose-Dependent Manner
3.2. Cefuroxime Causes the Highest Dose-Dependent Loss of Cell Integrity (LDH)
3.3. Effects of Antibiotics on the Activity of Mitochondrial Dehydrogenase in Hepatocytes
3.4. Decrease in Cytochrome 1A2 Activity
3.5. Tigecycline Led to a Noticeable Dose-Dependent Impairment of Albumin-Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luna, C.M.; Aruj, P.; Niederman, M.S.; Garzón, J.; Violi, D.; Prignoni, A.; Ríos, F.; Baquero, S.; Gando, S. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur. Respir. J. 2006, 27, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Curcio, D.; Alí, A.; Duarte, A.; Defilippi Pauta, A.; Ibáñez-Guzmán, C.; Chung Sang, M.; Valencia, E.; Plano, F.; Paredes Oña, F.; Arancibia, F.; et al. Prescription of antibiotics in intensive care units in Latin America: An observational study. J. Chemother. 2009, 21, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control 1999, 27, 97–132. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Li, D.X.; Dzintars, K.; Cosgrove, S.E. Association of Adverse Events with Antibiotic Use in Hospitalized Patients. JAMA Intern. Med. 2017, 177, 1308–1315. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.J.; Sweitzer, B.J.; Bates, D.W.; Burdick, E.; Edmondson, A.; Leape, L.L. Preventable adverse drug events in hospitalized patients: A comparative study of intensive care and general care units. Crit. Care Med. 1997, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, D.C.; Bonten, M.J.; Gaillard, C.A.; Van Tiel, F.H.; Van Der Geest, S.; De Leeuw, P.W.; Stobberingh, E.E. Indications for antibiotic use in ICU patients: A one-year prospective surveillance. J. Antimicrob. Chemother. 1997, 39, 527–535. [Google Scholar] [CrossRef][Green Version]
- David, S.; Hamilton, J.P. Drug-induced Liver Injury. US Gastroenterol. Hepatol. Rev. 2010, 6, 73–80. [Google Scholar]
- Sundaram, V.; Björnsson, E.S. Drug-induced cholestasis. Hepatol. Commun. 2017, 1, 726–735. [Google Scholar] [CrossRef]
- Aithal, G.P.; Watkins, P.B.; Andrade, R.J.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.M.; Wilke, R.A.; Avigan, M.; Kaplowitz, N.; et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef]
- Thiim, M.; Friedman, L.S. Hepatotoxicity of antibiotics and antifungals. Clin. Liver Dis. 2003, 7, 381–399. [Google Scholar] [CrossRef]
- Polson, J.E. Hepatotoxicity due to antibiotics. Clin. Liver Dis. 2007, 11, 549–561. [Google Scholar] [CrossRef]
- Tillmann, H.L.; Suzuki, A.; Barnhart, H.X.; Serrano, J.; Rockey, D.C. Tools for causality assessment in drug-induced liver disease. Curr. Opin. Gastroenterol. 2019, 35, 183–190. [Google Scholar] [CrossRef]
- García-Cortés, M.; Stephens, C.; Lucena, M.I.; Fernández-Castañer, A.; Andrade, R.J. Causality assessment methods in drug induced liver injury: Strengths and weaknesses. J. Hepatol. 2011, 55, 683–691. [Google Scholar] [CrossRef]
- Andrade, R.J.; Tulkens, P.M. Hepatic safety of antibiotics used in primary care. J. Antimicrob. Chemother. 2011, 66, 1431–1446. [Google Scholar] [CrossRef]
- Shanks, N.; Greek, R.; Greek, J. Are animal models predictive for humans? Philos. Ethics Humanit. Med. 2009, 4, 2. [Google Scholar] [CrossRef]
- Hartung, T. Toxicology for the twenty-first century. Nature 2009, 460, 208–212. [Google Scholar] [CrossRef]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef]
- Stephens, C.; Robles-Diaz, M.; Medina-Caliz, I.; Garcia-Cortes, M.; Ortega-Alonso, A.; Sanabria-Cabrera, J.; Gonzalez-Jimenez, A.; Alvarez-Alvarez, I.; Slim, M.; Jimenez-Perez, M.; et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J. Hepatol. 2021, 75, 86–97. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Joseph, T.; Sunil Kumar, N.; Rathi, C.; Thomas, V.; Prasad Singh, S.; Sawant, P.; Goel, A.; Eapen, C.E.; Rai, P.; et al. The Indian Network of Drug-Induced Liver Injury: Etiology, Clinical Features, Outcome and Prognostic Markers in 1288 Patients. J. Clin. Exp. Hepatol. 2021, 11, 288–298. [Google Scholar] [CrossRef]
- Bessone, F.; Hernandez, N.; Mendizabal, M.; Sanchez, A.; Paraná, R.; Arrese, M.; Tagle, M.; Girala, M.; Lizarzabal, M.; Carrera, E.; et al. When the Creation of a Consortium Provides Useful Answers: Experience of The Latin American DILI Network (LATINDILIN). Clin. Liver Dis. 2019, 13, 51–57. [Google Scholar] [CrossRef]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015, 148, 1340–1352. [Google Scholar] [CrossRef]
- Friis, H.; Andreasen, P.B. Drug-induced hepatic injury: An analysis of 1100 cases reported to the Danish Committee on Adverse Drug Reactions between 1978 and 1987. J. Intern. Med. 1992, 232, 133–138. [Google Scholar] [CrossRef]
- Sauer, M.; Haubner, C.; Mencke, T.; Noldge-Schomburg, G.; Mitzner, S.; Altrichter, J.; Stange, J. Impaired cell functions of hepatocytes incubated with plasma of septic patients. Inflamm. Res. 2012, 61, 609–616. [Google Scholar] [CrossRef]
- Sauer, M.; Altrichter, J.; Haubner, C.; Pertschy, A.; Wild, T.; Doss, F.; Mencke, T.; Thomsen, M.; Ehler, J.; Henschel, J.; et al. Bioartificial Therapy of Sepsis: Changes of Norepinephrine-Dosage in Patients and Influence on Dynamic and Cell Based Liver Tests during Extracorporeal Treatments. Biomed Res. Int. 2016, 2016, 7056492. [Google Scholar] [CrossRef]
- Doß, S.; Potschka, H.; Doß, F.; Mitzner, S.; Sauer, M. Hepatotoxicity of Antimycotics Used for Invasive Fungal Infections: In Vitro Results. Biomed Res. Int. 2017, 2017, 9658018. [Google Scholar] [CrossRef]
- Sauer, M. Use of Human Hepatocytes for Determining Liver Function and Liver Regeneration. U.S. Patent US20090061470A1, 5 March 2009. [Google Scholar]
- Sombetzki, M.; Koslowski, N.; Doss, S.; Loebermann, M.; Trauner, M.; Reisinger, E.C.; Sauer, M. Biosensor for Hepatocellular Injury Corresponds to Experimental Scoring of Hepatosplenic Schistosomiasis in Mice. Biomed Res. Int. 2016, 2016, 1567254. [Google Scholar] [CrossRef]
- Wu, H.; Liu, M.; Wang, S.; Feng, W.; Yao, W.; Zhao, H.; Wei, M. Pharmacokinetic properties and bioequivalence of two compound formulations of 1500 mg ampicillin (1167 mg)/probenecid (333 mg): A randomized-sequence, single-dose, open-label, two-period crossover study in healthy Chinese male volunteers. Clin. Ther. 2010, 32, 597–606. [Google Scholar] [CrossRef]
- Pichardo, C.; Conejo, M.D.C.; Bernabéu-Wittel, M.; Pascual, A.; Jiménez-Mejías, M.E.; de Cueto, M.; Pachón-Ibáñez, M.E.; García, I.; Pachón, J.; Martínez-Martínez, L. Activity of cefepime and carbapenems in experimental pneumonia caused by porin-deficient Klebsiella pneumoniae producing FOX-5 beta-lactamase. Clin. Microbiol. Infect. 2005, 11, 31–38. [Google Scholar] [CrossRef]
- Whited, L.; Grove, M.; Rose, D.; Rhodes, N.J.; Scheetz, M.H.; O’Donnell, J.N.; Neeb, J.; Thoele, K.; Jones, D.R.; Lowe, C.; et al. Pharmacokinetics of Cefepime in Patients with Cancer and Febrile Neutropenia in the Setting of Hematologic Malignancies or Hematopoeitic Cell Transplantation. Pharmacotherapy 2016, 36, 1003–1010. [Google Scholar] [CrossRef]
- Zhao, L.-S.; Yin, R.; Wei, B.-B.; Li, Q.; Jiang, Z.-Y.; Chen, X.-H.; Bi, K.-S. Comparative pharmacokinetics of cefuroxime lysine after single intravenous, intraperitoneal, and intramuscular administration to rats. Acta Pharmacol. Sin. 2012, 33, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Tøttrup, M.; Bibby, B.M.; Hardlei, T.F.; Bue, M.; Kerrn-Jespersen, S.; Fuursted, K.; Søballe, K.; Birke-Sørensen, H. Continuous versus short-term infusion of cefuroxime: Assessment of concept based on plasma, subcutaneous tissue, and bone pharmacokinetics in an animal model. Antimicrob. Agents Chemother. 2015, 59, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Furlanut, M. Pharmacokinetic aspects of levofloxacin 500 mg once daily during sequential intravenous/oral therapy in patients with lower respiratory tract infections. J. Antimicrob. Chemother. 2002, 51, 101–106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGee, B.; Dietze, R.; Hadad, D.J.; Molino, L.P.D.; Maciel, E.L.N.; Boom, W.H.; Palaci, M.; Johnson, J.L.; Peloquin, C.A. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 2009, 53, 3981–3984. [Google Scholar] [CrossRef]
- De Bus, L.; Depuydt, P.; Libbrecht, L.; Vandekerckhove, L.; Nollet, J.; Benoit, D.; Vogelaers, D.; Van Vlierberghe, H. Severe drug-induced liver injury associated with prolonged use of linezolid. J. Med. Toxicol. 2010, 6, 322–326. [Google Scholar] [CrossRef]
- Maglio, D.; Teng, R.; Thyrum, P.T.; Nightingale, C.H.; Nicolau, D.P. Pharmacokinetic profile of meropenem, administered at 500 milligrams every 8 hours, in plasma and cantharidin-induced skin blister fluid. Antimicrob. Agents Chemother. 2003, 47, 1771–1773. [Google Scholar] [CrossRef]
- Seth, V.; Beotra, A.; Seth, S.D.; Semwal, O.P.; Kabra, S.; Jain, Y.; Mukhopadhya, S. Serum concentrations of rifampicin and isoniazid in tuberculosis. Indian Pediatr. 1993, 30, 1091–1098. [Google Scholar]
- Muralidharan, G.; Micalizzi, M.; Speth, J.; Raible, D.; Troy, S. Pharmacokinetics of tigecycline after single and multiple doses in healthy subjects. Antimicrob. Agents Chemother. 2005, 49, 220–229. [Google Scholar] [CrossRef]
- Gu, Q.; Zhu, Z.-H.; Ge, M.; Ge, W.-H. Pharmacokinetics of vancomycin in continuous veno-venous hemofiltration. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2003, 15, 114–116. [Google Scholar]
- Lorentz, K.; Klauke, R.; Schmidt, E. Recommendation for the determination of the catalytic concentration of lactate dehydrogenase at 37 degrees C. Standardization Committee of the German Society for Clinical Chemistry, Enzyme Working Group of the German Society for Clinical Chemistry. Eur. J. Clin. Chem. Clin. Biochem. 1993, 31, 897–899. [Google Scholar]
- De Logu, A.; Borgna, R.; Uda, P.; Sanna, A.; Pellerano, M.L.; Saddi, B. The 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay as rapid colorimetric method for determination of antibiotic susceptibility of clinical Mycobacterium tuberculosis isolates in liquid medium. Clin. Lab. 2003, 49, 357–365. [Google Scholar]
- Donato, M.T.; Gomez-Lechon, M.J.; Castell, J.V. A microassay for measuring cytochrome P450IA1 and P450IIB1 activities in intact human and rat hepatocytes cultured on 96-well plates. Anal. Biochem. 1993, 213, 29–33. [Google Scholar] [CrossRef]
- Krasteva, N.; Groth, T.H.; Fey-Lamprecht, F.; Altankov, G. The role of surface wettability on hepatocyte adhesive interactions and function. J. Biomater. Sci. Polym. Ed. 2001, 12, 613–627. [Google Scholar] [CrossRef]
- Zimmerman, H.J. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; ISBN 0781719526. [Google Scholar]
- Sundar, S.; Chakravarty, J. Liposomal amphotericin B and leishmaniasis: Dose and response. J. Glob. Infect. Dis. 2010, 2, 159–166. [Google Scholar] [CrossRef]
- Driscoll, T.A.; Frangoul, H.; Nemecek, E.R.; Murphey, D.K.; Yu, L.C.; Blumer, J.; Krance, R.A.; Baruch, A.; Liu, P. Comparison of pharmacokinetics and safety of voriconazole intravenous-to-oral switch in immunocompromised adolescents and healthy adults. Antimicrob. Agents Chemother. 2011, 55, 5780–5789. [Google Scholar] [CrossRef]
- Kami, M.; Sawada, Y.; Mori, S.-I.; Hirate, J.; Kojima, N.; Kanda, Y.; Moriya, A.; Yuji, K.; Saito, T.; Chiba, S.; et al. Serum levels of fluconazole in patients after cytotoxic chemotherapy for hematological malignancy. Am. J. Hematol. 2001, 66, 85–91. [Google Scholar] [CrossRef]
- Krishna, G.; Vickery, D.; Ma, L.; Yu, X.; Noren, C.; Power, E.; Beresford, E.; Medlock, M. Lack of pharmacokinetic drug interaction between oral posaconazole and caspofungin or micafungin. J. Clin. Pharmacol. 2011, 51, 84–92. [Google Scholar] [CrossRef]
- Dowell, J.A.; Stogniew, M.; Krause, D.; Henkel, T.; Weston, I.E. Assessment of the safety and pharmacokinetics of anidulafungin when administered with cyclosporine. J. Clin. Pharmacol. 2005, 45, 227–233. [Google Scholar] [CrossRef]
- LeCluyse, E.L.; Witek, R.P.; Andersen, M.E.; Powers, M.J. Organotypic liver culture models: Meeting current challenges in toxicity testing. Crit. Rev. Toxicol. 2012, 42, 501–548. [Google Scholar] [CrossRef]
- Donato, M.T.; Lahoz, A.; Castell, J.V.; Gómez-Lechón, M.J. Cell lines: A tool for in vitro drug metabolism studies. Curr. Drug Metab. 2008, 9, 1–11. [Google Scholar] [CrossRef]
- Prot, J.M.; Aninat, C.; Griscom, L.; Razan, F.; Brochot, C.; Guillouzo, C.G.; Legallais, C.; Corlu, A.; Leclerc, E. Improvement of HepG2/C3a cell functions in a microfluidic biochip. Biotechnol. Bioeng. 2011, 108, 1704–1715. [Google Scholar] [CrossRef]
- Rodríguez-Antona, C.; Donato, M.T.; Boobis, A.; Edwards, R.J.; Watts, P.S.; Castell, J.V.; Gómez-Lechón, M.-J. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: Molecular mechanisms that determine lower expression in cultured cells. Xenobiotica 2002, 32, 505–520. [Google Scholar] [CrossRef]
- Elkayam, T.; Amitay-Shaprut, S.; Dvir-Ginzberg, M.; Harel, T.; Cohen, S. Enhancing the drug metabolism activities of C3A—A human hepatocyte cell line—By tissue engineering within alginate scaffolds. Tissue Eng. 2006, 12, 1357–1368. [Google Scholar] [CrossRef]
- Ellis, A.J.; Hughes, R.D.; Wendon, J.A.; Dunne, J.; Langley, P.G.; Kelly, J.H.; Gislason, G.T.; Sussman, N.L.; Williams, R. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology 1996, 24, 1446–1451. [Google Scholar] [CrossRef]
- Ramirez, T.; Strigun, A.; Verlohner, A.; Huener, H.-A.; Peter, E.; Herold, M.; Bordag, N.; Mellert, W.; Walk, T.; Spitzer, M.; et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch. Toxicol. 2018, 92, 893–906. [Google Scholar] [CrossRef]
- Paech, F.; Mingard, C.; Grünig, D.; Abegg, V.F.; Bouitbir, J.; Krähenbühl, S. Mechanisms of mitochondrial toxicity of the kinase inhibitors ponatinib, regorafenib and sorafenib in human hepatic HepG2 cells. Toxicology 2018, 395, 34–44. [Google Scholar] [CrossRef]
- Flynn, T.J.; Ferguson, M.S. Multiendpoint mechanistic profiling of hepatotoxicants in HepG2/C3A human hepatoma cells and novel statistical approaches for development of a prediction model for acute hepatotoxicity. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2008, 22, 1618–1631. [Google Scholar] [CrossRef]
- Robles, M.; Andrade, R.J. Hepatotoxicidad por antibióticos: Actualización en 2008. Rev. Esp. Quimioter. 2008, 21, 224–233. [Google Scholar]
- Björnsson, E.S.; Hoofnagle, J.H. Categorization of drugs implicated in causing liver injury: Critical assessment based on published case reports. Hepatology 2016, 63, 590–603. [Google Scholar] [CrossRef]
- Meier, Y.; Cavallaro, M.; Roos, M.; Pauli-Magnus, C.; Folkers, G.; Meier, P.J.; Fattinger, K. Incidence of drug-induced liver injury in medical inpatients. Eur. J. Clin. Pharmacol. 2005, 61, 135–143. [Google Scholar] [CrossRef]
- Long, S.S.; Prober, C.G.; Fischer, M. (Eds.) Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Elsevier: Philadelphia, PA, USA, 2018; ISBN 9780323401814. [Google Scholar]
- Scott, L.J.; Ormrod, D.; Goa, K.L. Cefuroxime axetil: An updated review of its use in the management of bacterial infections. Drugs 2001, 61, 1455–1500. [Google Scholar] [CrossRef] [PubMed]
- Niriella, M.A.; Kumarasena, R.S.; Dassanayake, A.S.; Pathirana, A.; Hewavisenthi, J.D.S.; De Silva, H.J. Worsening cholestasis and possible cefuroxime-induced liver injury following “successful” therapeutic endoscopic retrograde cholangiopancreatography for a distal common bile duct stone: A case report. J. Med. Case Rep. 2016, 10, 371. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kunze, W.; Streidl, J.-P.; Klemm, T.; Lutze, J. Cefuroxime-Induced Hepatocellular-Cholestatic Hepatitis with Pancytopenia. OALib 2019, 6, 1–7. [Google Scholar] [CrossRef]
- Ekiz, F.; Usküdar, O.; Simsek, Z.; Yüksel, I.; Basar, O.; Altinbas, A.; Yüksel, O. Cefuroxime axetil-induced liver failure. Ann. Hepatol. 2010, 9, 306. [Google Scholar] [CrossRef]
- Asai, H.; Imaoka, S.; Kuroki, T.; Monna, T.; Funae, Y. Microsomal ethanol oxidizing system activity by human hepatic cytochrome P450s. J. Pharmacol. Exp. Ther. 1996, 277, 1004–1009. [Google Scholar]
- Bibi, Z. Role of cytochrome P450 in drug interactions. Nutr. Metab. 2008, 5, 27. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Rifampicin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548314/ (accessed on 22 August 2022).
- Ansari, J.A.; Sayyed, M.; Sayeed, F. Management of Non Alcoholic Fatty Liver Diseases and their Complications. Int. J. Pharmacol. 2011, 7, 579–588. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Vancomycin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548881/ (accessed on 22 August 2022).
- Launay-Vacher, V.; Izzedine, H.; Mercadal, L.; Deray, G. Clinical review: Use of vancomycin in haemodialysis patients. Crit. Care 2002, 6, 313–316. [Google Scholar] [CrossRef]
- Martí, R.; Rosell, M.; Pou, L.; García, L.; Pascual, C. Influence of biochemical parameters of liver function on vancomycin pharmacokinetics. Pharmacol. Toxicol. 1996, 79, 55–59. [Google Scholar] [CrossRef]
- Aldaz, A.; Ortega, A.; Idoate, A.; Giráldez, J.; Brugarolas, A. Effects of hepatic function on vancomycin pharmacokinetics in patients with cancer. Ther. Drug Monit. 2000, 22, 250–257. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Tigecycline. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547888/ (accessed on 22 August 2022).
- Jing, L.; Kai, Z.; Liqin, Z.; Yihe, L. Tigecycline Induced Cholestatic Liver Injury: A Case Report. J. Infect. Dis. Epidemiol. 2018, 4, 60. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Lee, C.-H.; Lee, K.-Y.; Jung, S.-H.; Lee, B.-H. Increased hepatic Fatty Acid uptake and esterification contribute to tetracycline-induced steatosis in mice. Toxicol. Sci. 2015, 145, 273–282. [Google Scholar] [CrossRef]
- Pessayre, D.; Fromenty, B.; Berson, A.; Robin, M.-A.; Lettéron, P.; Moreau, R.; Mansouri, A. Central role of mitochondria in drug-induced liver injury. Drug Metab. Rev. 2012, 44, 34–87. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vardakas, K.Z.; Tsiveriotis, K.P.; Triarides, N.A.; Tansarli, G.S. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int. J. Antimicrob. Agents 2014, 44, 1–7. [Google Scholar] [CrossRef]
- Kaewpoowat, Q.; Ostrosky-Zeichner, L. Tigecycline: A critical safety review. Expert Opin. Drug Saf. 2015, 14, 335–342. [Google Scholar] [CrossRef]
- Alraish, R.; Wicha, S.G.; Frey, O.R.; Roehr, A.C.; Pratschke, J.; Stockmann, M.; Wuensch, T.; Kaffarnik, M. Pharmacokinetics of tigecycline in critically ill patients with liver failure defined by maximal liver function capacity test (LiMAx). Ann. Intensive Care 2020, 10, 106. [Google Scholar] [CrossRef]
- Carrascosa, M.F.; Lucena, M.I.; Andrade, R.J.; Caviedes, J.R.S.; Lavín, A.C.; Mones, J.C.; Rivero, A.P.; Serrano, V.B. Fatal acute hepatitis after sequential treatment with levofloxacin, doxycycline, and naproxen in a patient presenting with acute Mycoplasma pneumoniae infection. Clin. Ther. 2009, 31, 1014–1019. [Google Scholar] [CrossRef]
- Spahr, L.; Rubbia-Brandt, L.; Marinescu, O.; Armenian, B.; Hadengue, A. Acute fatal hepatitis related to levofloxacin. J. Hepatol. 2001, 35, 308–309. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Sci. Transl. Med. 2013, 5, 192ra85. [Google Scholar] [CrossRef]
- Wang, T.; Weinman, S.A. Interactions Between Hepatitis C Virus and Mitochondria: Impact on Pathogenesis and Innate Immunity. Curr. Pathobiol. Rep. 2013, 1, 179–187. [Google Scholar] [CrossRef]
- Nishikawa, T.; Bellance, N.; Damm, A.; Bing, H.; Zhu, Z.; Handa, K.; Yovchev, M.I.; Sehgal, V.; Moss, T.J.; Oertel, M.; et al. A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J. Hepatol. 2014, 60, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Levofloxacin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548357/ (accessed on 22 August 2022).
- Liao, P.-F.; Wu, Y.-K.; Huang, K.-L.; Chen, H.-Y. A rare case of cefepime-induced cholestatic liver injury. Ci Ji Yi Xue Za Zhi 2019, 31, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wynalda, M.A.; Hauer, M.J.; Wienkers, L.C. Oxidation of the novel oxazolidinone antibiotic linezolid in human liver microsomes. Drug Metab. Dispos. 2000, 28, 1014–1017. [Google Scholar] [PubMed]
- Zhang, L.; Wei, M.-J.; Zhao, C.-Y.; Qi, H.-M. Determination of the inhibitory potential of 6 fluoroquinolones on CYP1A2 and CYP2C9 in human liver microsomes. Acta Pharmacol. Sin. 2008, 29, 1507–1514. [Google Scholar] [CrossRef]
- Zhu, Q.; Liao, J.; Xie, L.; Wang, G.J.; Liu, X.D. Mechanism-based inhibition of CYP1A2 by antofloxacin, an 8-NH2 derivative of levofloxacin in rats. Xenobiotica 2009, 39, 293–301. [Google Scholar] [CrossRef]
- Backman, J.T.; Granfors, M.T.; Neuvonen, P.J. Rifampicin is only a weak inducer of CYP1A2-mediated presystemic and systemic metabolism: Studies with tizanidine and caffeine. Eur. J. Clin. Pharmacol. 2006, 62, 451–461. [Google Scholar] [CrossRef]
- Genilloud, O.; Vicente, F. Tetracycline Antibiotics and Novel Analogs. In Antimicrobials; Marinelli, F., Genilloud, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 231–245. ISBN 978-3-642-39967-1. [Google Scholar]
- Ye, L.; Zhang, J.; Xiao, W.; Liu, S. Efficacy and mechanism of actions of natural antimicrobial drugs. Pharmacol. Ther. 2020, 216, 107671. [Google Scholar] [CrossRef]
- Pankey, G.A. Tigecycline. J. Antimicrob. Chemother. 2005, 56, 470–480. [Google Scholar] [CrossRef]
- Ogu, C.C.; Maxa, J.L. Drug interactions due to cytochrome P450. Proc. Bayl. Univ. Med. Cent. 2000, 13, 421–423. [Google Scholar] [CrossRef]
- Lněničková, K.; Skálová, L.; Stuchlíková, L.; Szotáková, B.; Matoušková, P. Induction of xenobiotic-metabolizing enzymes in hepatocytes by beta-naphthoflavone: Time-dependent changes in activities, protein and mRNA levels. Acta Pharm. 2018, 68, 75–85. [Google Scholar] [CrossRef]
- El-Saadany, M.A.; Rawel, H.M.; Raila, J.; El-Dashloty, M.S.; Schweigert, F.J. Antioxidants modulate the IL-6 induced inhibition of negative acute-phase protein secretion in HepG2 cells. Cell Biochem. Funct. 2008, 26, 95–101. [Google Scholar] [CrossRef]
- Thyrum, P.T.; Yeh, C.; Birmingham, B.; Lasseter, K. Pharmacokinetics of meropenem in patients with liver disease. Clin. Infect. Dis. 1997, 24, S184–S190. [Google Scholar] [CrossRef]
- Mishra, R.; Patel, H.; Goel, B.; Vakde, T. A Case of Linezolid Toxicity Presenting as a Sepsis Mimic. Case Rep. Crit. Care 2019, 2019, 2157674. [Google Scholar] [CrossRef]
- Santini, A.; Ronchi, D.; Garbellini, M.; Piga, D.; Protti, A. Linezolid-induced lactic acidosis: The thin line between bacterial and mitochondrial ribosomes. Expert Opin. Drug Saf. 2017, 16, 833–843. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Linezolid. Available online: https://www.ncbi.nlm.nih.gov/books/NBK548245/ (accessed on 22 August 2022).
- De Vriese, A.S.; Van Coster, R.; Smet, J.; Seneca, S.; Lovering, A.; Van Haute, L.L.; Vanopdenbosch, L.J.; Martin, J.-J.; Groote, C.C.-D.; Vandecasteele, S.; et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 2006, 42, 1111–1117. [Google Scholar] [CrossRef]
- Carson, J.; Cerda, J.; Chae, J.-H.; Hirano, M.; Maggiore, P. Severe lactic acidosis associated with linezolid use in a patient with the mitochondrial DNA A2706G polymorphism. Pharmacotherapy 2007, 27, 771–774. [Google Scholar] [CrossRef]
- Shaikh, A.; McHugh, J. Linezolid use and drug-induced liver injury. Proc. Bayl. Univ. Med. Cent. 2020, 34, 316–317. [Google Scholar] [CrossRef]
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Ampicillin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547894/ (accessed on 22 August 2022).
- Berg, P.; Hahn, E.G. The Action of Amoxicillin-Clavulanate on Hepatocytes: Hepatotoxic reactions induced by beta-lactamase inhibitors. Eur. J. Med. Res. 2001, 17, 535–542. [Google Scholar]
- Sánchez-Ruiz-Granados, E.; Bejarano-García, A.; Uceda-Torres, E. Recurrent cholestasis by amoxicillin-clavulanic acid: The importance of a correct diagnosis of hepatotoxicity. Rev. Esp. Enferm. Dig. 2012, 104, 616–617. [Google Scholar] [CrossRef][Green Version]
- Demir, M.; Lang, S.; Hartmann, P.; Duan, Y.; Martin, A.; Miyamoto, Y.; Bondareva, M.; Zhang, X.; Wang, Y.; Kasper, P.; et al. The fecal mycobiome in non-alcoholic fatty liver disease. J. Hepatol. 2022, 76, 788–799. [Google Scholar] [CrossRef]
- Serras, A.S.; Rodrigues, J.S.; Cipriano, M.; Rodrigues, A.V.; Oliveira, N.G.; Miranda, J.P. A Critical Perspective on 3D Liver Models for Drug Metabolism and Toxicology Studies. Front. Cell Dev. Biol. 2021, 9, 626805. [Google Scholar] [CrossRef]
- Li, D.-D.; Tang, X.-L.; Tan, H.-L.; Liang, Q.-d.; Wang, Y.-G.; Ma, Z.-C.; Xiao, C.-R.; Gao, Y. 3D evaluation model for drug hepatotoxicity testing on HepG2 cells and its application in drug safety evaluation. Zhongguo Zhong Yao Za Zhi 2016, 41, 1313–1317. [Google Scholar] [CrossRef]
- Corbett, J.L.; Duncan, S.A. iPSC-Derived Hepatocytes as a Platform for Disease Modeling and Drug Discovery. Front. Med. 2019, 6, 265. [Google Scholar] [CrossRef]
- Sirenko, O.; Hesley, J.; Rusyn, I.; Cromwell, E.F. High-content assays for hepatotoxicity using induced pluripotent stem cell-derived cells. Assay Drug Dev. Technol. 2014, 12, 43–54. [Google Scholar] [CrossRef]

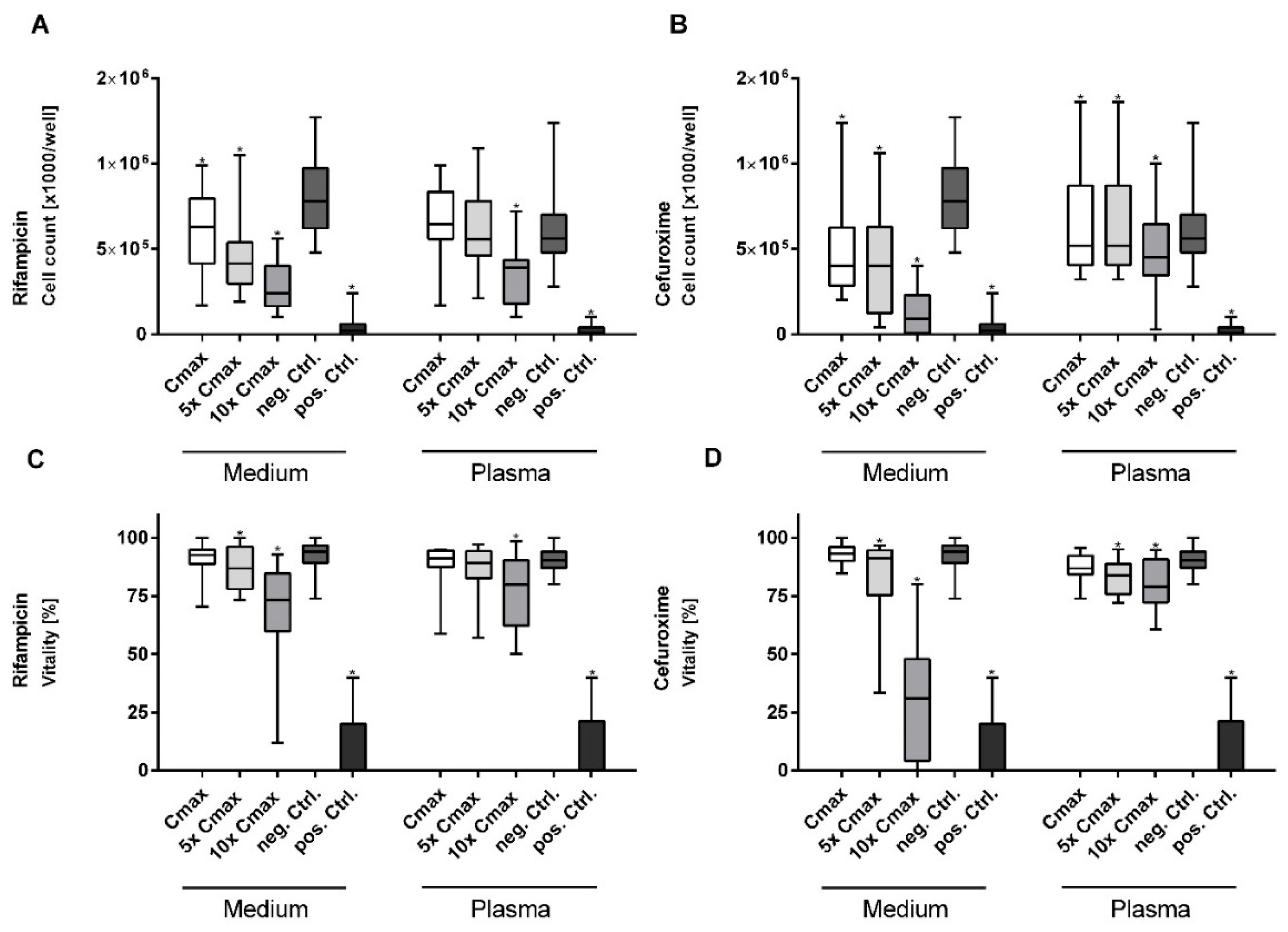

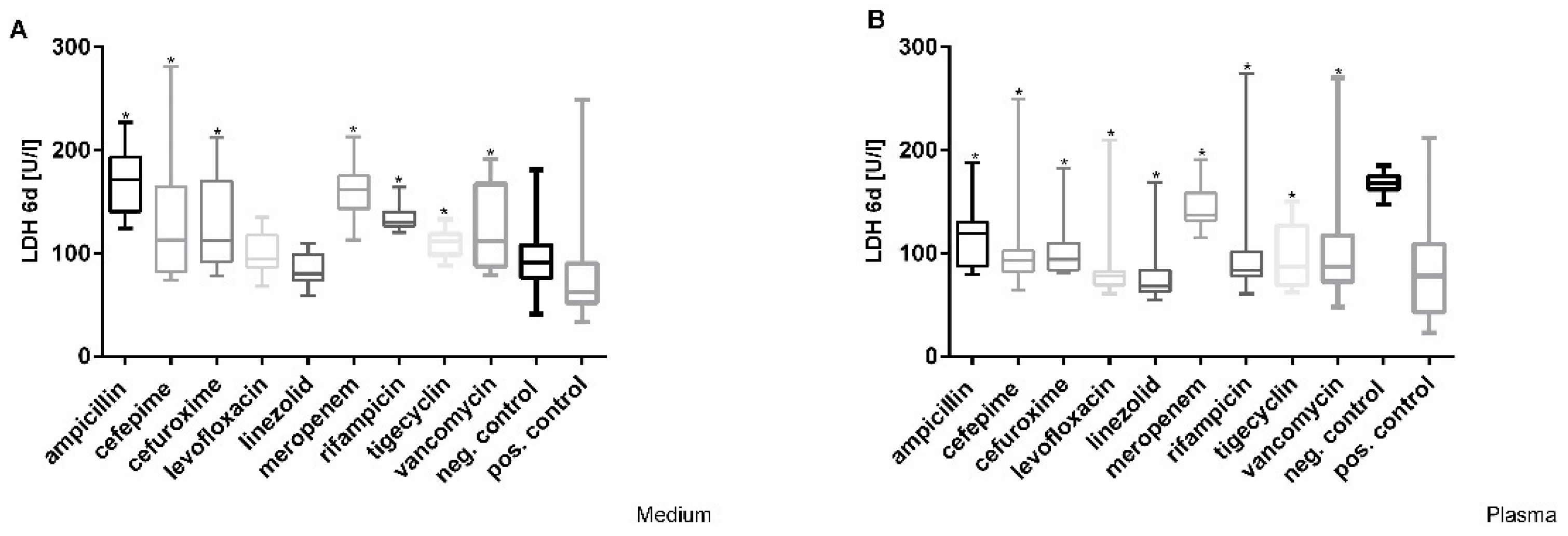

| Parameter | Microalbumin [mg/L]. Medium | Microalbumin [mg/L]. Plasma | ||||
|---|---|---|---|---|---|---|
| Testsubstance | Cmax | 5x Cmax | 10x Cmax | Cmax | 5x Cmax | 10x Cmax |
| Ampicillin | 9.37 * 8.64/9.96 | 5.21 * 5.01/5.41 | 4.66 * 4.56/4.84 | 57.65 * 55.83/62.43 | 31.65 18.00/38.43 | 30.15 23.35/48.93 |
| Cefepim | 9.23 * 6.93/12.15 | 5.69 4.99/7.83 | 5.44 * 4.78/6.63 | 94.90 * 80.90/106.00 | 85.15 * 72.88/106.80 | 96.90 * 85.40/109.80 |
| Cefuroxime | 4.26 * 2.31/5.01 | 1.01 * 0.00/2.77 | 2.16 * 0.00/2.54 | 6.73 * 5.33/7.67 | 5.71 * 5.31/6.63 | 5.88 * 5.23/10.26 |
| Levofloxacin | 8.55 6.43/11.93 | 13.45 * 11.98/20.15 | 11.30 * 10.30/14.10 | 134.50 * 99.58/146.80 | 129.00 * 101.00/135.80 | 98.10 * 36.95/147.50 |
| Linezolid | 8.87 * 7.85/11.02 | 6.47 5.71/8.91 | 4.35 * 3.03/5.97 | 90.95 * 86.15/108.20 | 94.05 * 79.75/104.70 | 73.80 * 59.98/87.65 |
| Meropenem | 9.68 * 8.87/11.60 | 5.43 * 4.83/7.78 | 4.97 * 4.00/6.17 | 21.40 11.58/65.65 | 17.80 10.15/78.20 | 24.90 14.25/66.70 |
| Rifampicin | 7.49 5.30/12.43 | 7.49 5.30/12.45 | 6.48 0.00/8.35 | 106.00 * 34.78/136.00 | 32.60 * 19.08/102.50 | 38.65 * 14.95/99.00 |
| Tigecyline | 5.57 * 4.48/6.99 | 2.86 * 0.55/3.05 | 0.00 * 0.00/0.00 | 27.95 14.10/60.05 | 35.15 11.43/68.02 | 20.85 6.76/48.50 |
| Vancomycin | 7.28 6.35/8.57 | 5.99 * 5.04/6.93 | 6.61 4.99/7.15 | 10.45 8.96/11.55 | 9.97 7.81/13.80 | 12.30 10.28/13.68 |
| pos.Ctrl | 0.00 0.00/3.15 | 2.96 3.73/4.95 | ||||
| neg. Ctrl | 6.59 5.68/8.78 | 20.40 7.93/50.70 | ||||
| neg. Ctrl | Ampicillin | Cefepime | Cefuroxime | Levofloxacin | Linezolid | Meropenem | Rifampicin | Tigecycline | Vancomycin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein binding [%] | 20 | 20 | 50–70 | 30–40 | 30 | 15 | 75–80 | 90 | 50 | |||||||||||
| Medium/Plasma | M | P | M | P | M | P | M | P | M | P | M | P | M | P | M | P | M | P | M | P |
| Cell Count (×100,000) | 7.8 | 5.6 | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||
| Vitality [%] | 94 | 91 | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||
| LDH 6d [U/L] | 91 | 168 | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||||||||||
| XTT (OD) | 1.38 | 1.26 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||||
| MA [mg/L] | 6.6 | 33.1 | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||||
| CYP1A2 [pmol/L] | 8.77 | 5.3 | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ||||
| Hepatotoxic-Potential | ** | * | ** | * | *** | ** | *** | ** | ** | * | ** | ** | **** | * | ***** | **** | **** | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doß, S.; Blessing, C.; Haller, K.; Richter, G.; Sauer, M. Influence of Antibiotics on Functionality and Viability of Liver Cells In Vitro. Curr. Issues Mol. Biol. 2022, 44, 4639-4657. https://doi.org/10.3390/cimb44100317
Doß S, Blessing C, Haller K, Richter G, Sauer M. Influence of Antibiotics on Functionality and Viability of Liver Cells In Vitro. Current Issues in Molecular Biology. 2022; 44(10):4639-4657. https://doi.org/10.3390/cimb44100317
Chicago/Turabian StyleDoß, Sandra, Corinne Blessing, Katharina Haller, Georg Richter, and Martin Sauer. 2022. "Influence of Antibiotics on Functionality and Viability of Liver Cells In Vitro" Current Issues in Molecular Biology 44, no. 10: 4639-4657. https://doi.org/10.3390/cimb44100317
APA StyleDoß, S., Blessing, C., Haller, K., Richter, G., & Sauer, M. (2022). Influence of Antibiotics on Functionality and Viability of Liver Cells In Vitro. Current Issues in Molecular Biology, 44(10), 4639-4657. https://doi.org/10.3390/cimb44100317






