Design and Synthesis of Thionated Levofloxacin: Insights into a New Generation of Quinolones with Potential Therapeutic and Analytical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. General
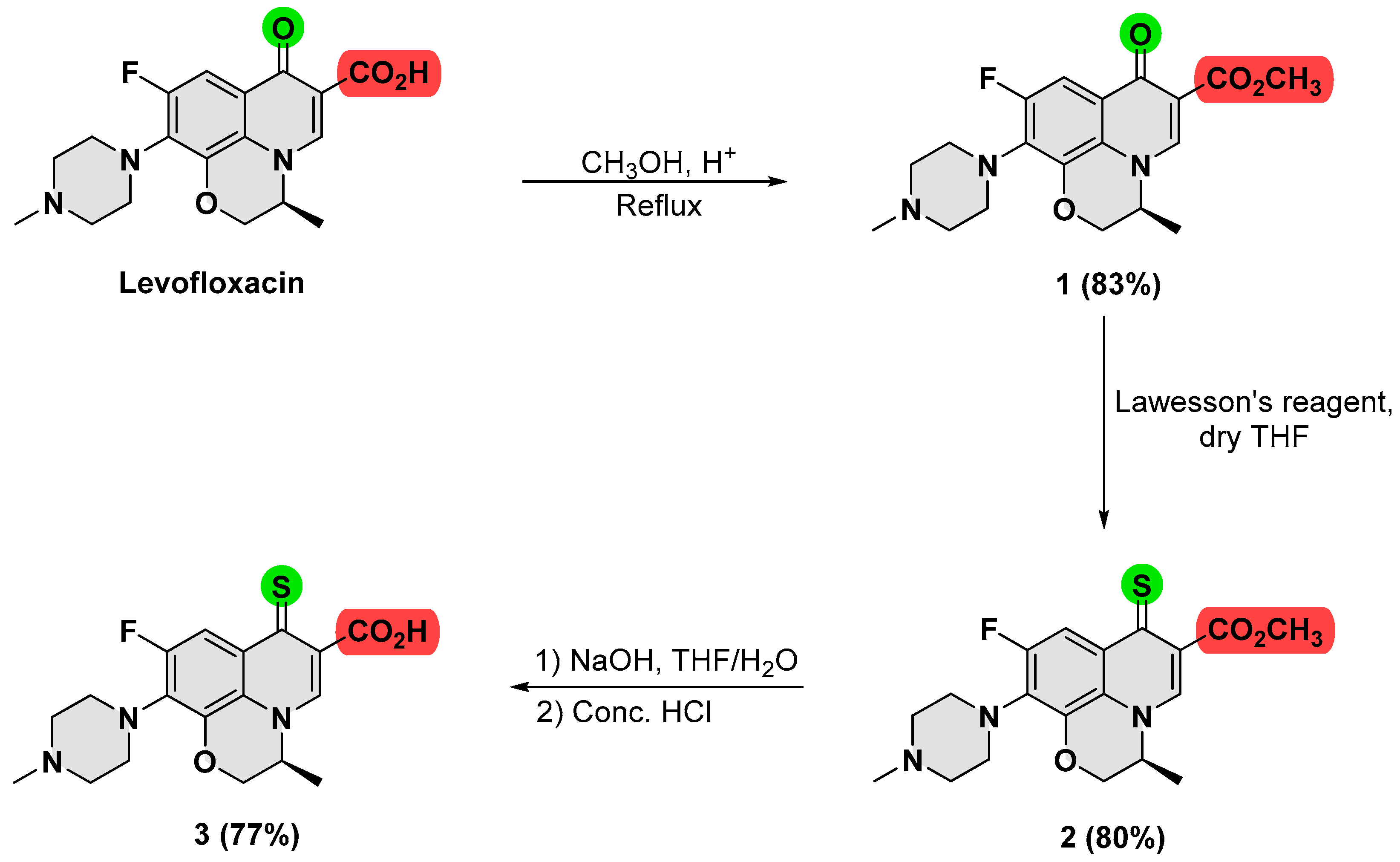
2.2. Synthesis and Characterization
2.2.1. Methyl (3R)-8-Fluoro-3-methyl-9-(4-methylpiperazin-1-yl)-6-oxo-2,3,3a,6-tetrahydrobenzo[de]chromene-5-carboxylate (Compound 1)
2.2.2. Methyl (3R)-8-Fluoro-3-methyl-9-(4-methylpiperazin-1-yl)-6-thioxo-2,3,3a,6-tetrahydrobenzo[de]chromene-5-carboxylate (Compound 2)
2.2.3. (3R)-8-Fluoro-3-methyl-9-(4-methylpiperazin-1-yl)-6-thioxo-2,3,3a,6-tetrahydrobenzo[de]chromene-5-carboxylic Acid (Compound 3)
2.3. Microbiological Procedure
2.3.1. Microorganisms
2.3.2. Well Diffusion Methods
2.3.3. Minimum Inhibitory Concentration (MIC)
2.3.4. Minimum Bactericidal Concentrations (MBC)
2.4. Biocompatibility Assay
2.5. Cytotoxicity Assay
2.6. Molecular Modeling
2.6.1. Protein and Ligand Preparation
2.6.2. Molecular Docking
2.6.3. GLIDE 5.0
3. Results and Discussion
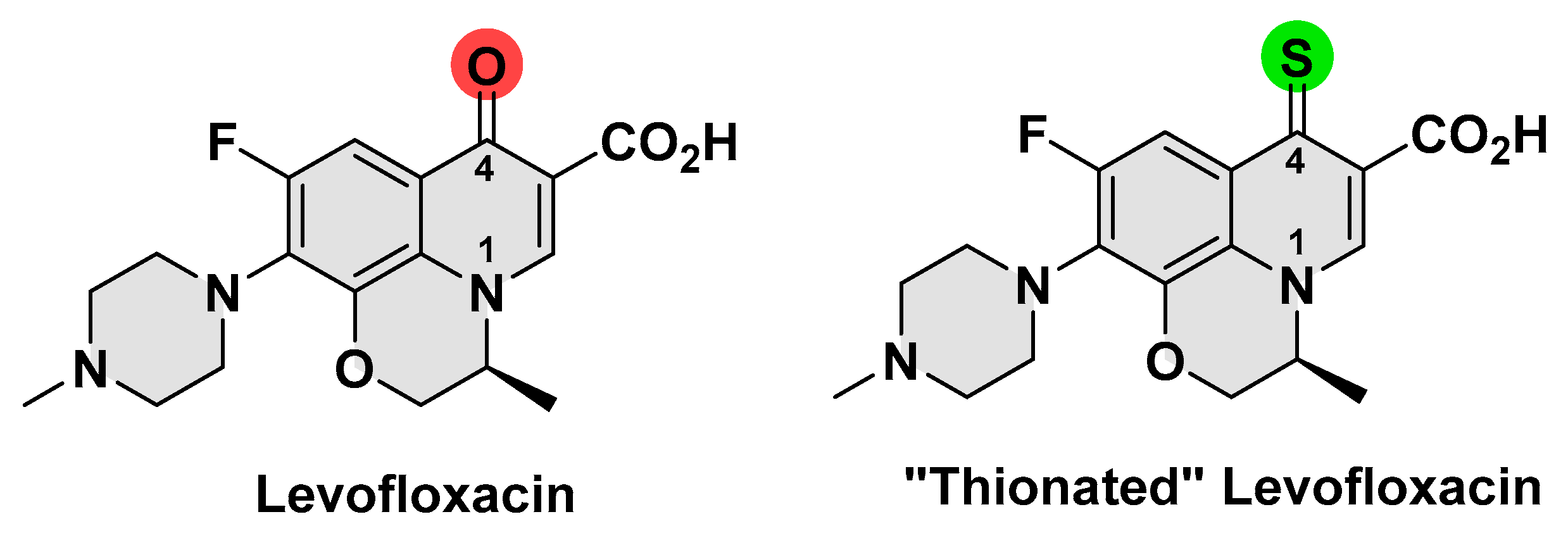
3.1. Synthesis and Characterization of Thionated Levofloxacin
3.2. Antimicrobial Activity
3.2.1. Well Diffusion Method
3.2.2. Dilution Method
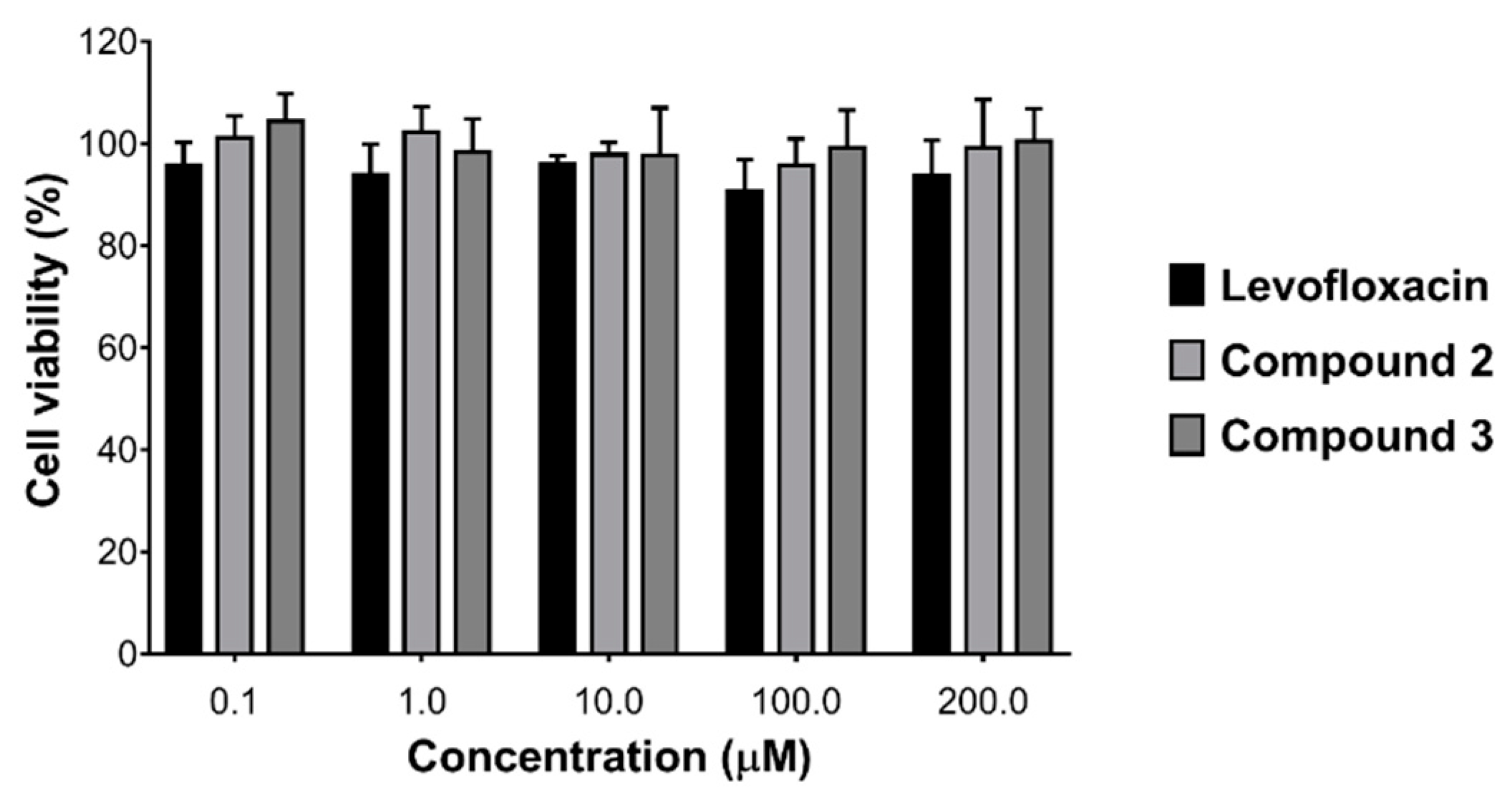
3.3. Biocompatibility and Cytotoxicity Assays
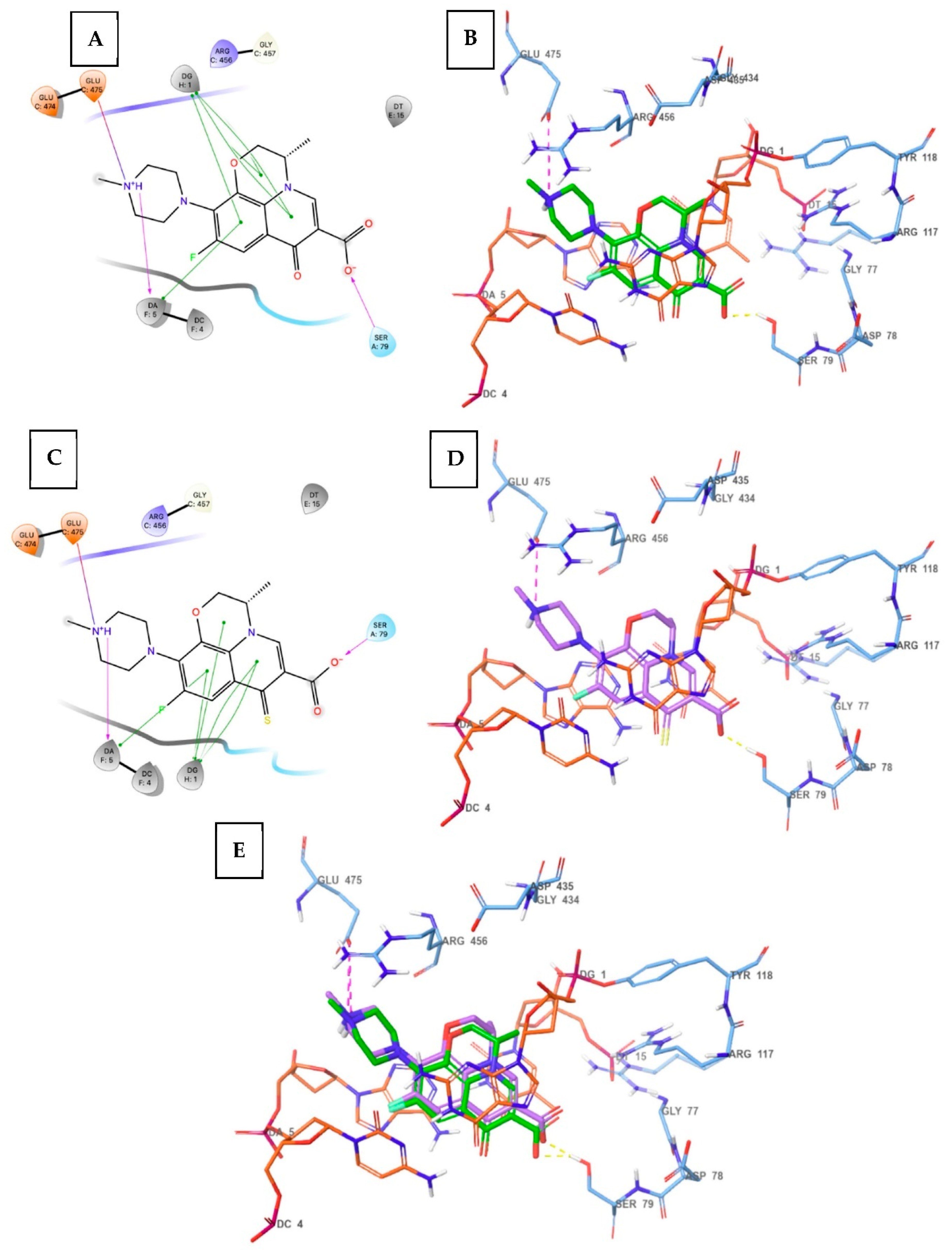
3.4. Molecular Docking
3.5. Spectroscopic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mugnaini, C.; Pasquini, S.; Corelli, F. The 4-quinolone-3-carboxylic acid motif as a multivalent scaffold in medicinal chemistry. Curr. Med. Chem. 2009, 16, 1746–1767. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G.; Dowell, S.F.; Mandell, L.A.; File, T.M.; Musher, D.M.; Fine, M.J. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2000, 31, 347–382. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, J.S.; Hooper, D.C. Treatment of genitourinary tract infections with fluoroquinolones: Activity in vitro, pharmacokinetics, and clinical efficacy in urinary tract infections and prostatitis. Antimicrob. Agents Chemother. 1989, 33, 1655–1661. [Google Scholar] [CrossRef][Green Version]
- Hooper, D.C. Clinical applications of quinolones. Biochim. Biophys. Acta 1998, 1400, 45–61. [Google Scholar] [CrossRef]
- Malik, B.A.; Singh, G.; Mir, T. 1998 management recommendations for sexually transmitted diseases. JK Pract. 1999, 6, 84–91. [Google Scholar]
- Hooper, D.C. New uses for new and old quinolones and the challenge of resistance. Clin. Infect. Dis. 2000, 30, 243–254. [Google Scholar] [CrossRef]
- Drlica, K.; Hiasa, H.; Kerns, R.; Malik, M.; Mustaev, A.; Zhao, X. Quinolones: Action and resistance updated. Curr. Top Med. Chem. 2009, 9, 981–998. [Google Scholar] [CrossRef]
- Jacoby, G.A. Mechanisms of resistance to quinolones. Clin. Infect. Dis. 2005, 41, S120–S126. [Google Scholar] [CrossRef]
- Andriole, V.T. The quinolones: Past, present, and future. Clin. Infect. Dis. 2005, 41, S113–S119. [Google Scholar] [CrossRef]
- Kocsis, B.; Domokos, J.; Szabo, D. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Med. Chem. Comm. 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, T.H.; Bark, K.M. Physicochemical properties of quinolone antibiotics in various environments. Eur. J. Med. Chem. 2002, 37, 443–460. [Google Scholar] [CrossRef]
- Khan, M.N.; Ali, W.; Shah, Z.; Idrees, M.; Gulab, H.; Adnan, A. Validated Spectrofluorimetric Method for the Determination of Moxifloxacin in Its Pure Form, Pharmaceutical Preparations, and Biological Samples. Anal. Sci. 2020, 36, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Măciucă, A.M.; Munteanu, A.C.; Uivarosi, V. Quinolone Complexes with Lanthanide Ions: An Insight into their Analytical Applications and Biological Activity. Molecules 2020, 25, 1347. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.C.; Servilha, R.O.; De Oliveira, E.F.; Lavarda, F.C.; Ximenes, V.F.; da Silva-Filho, L.C. Theoretical-Experimental Photophysical Investigations of the Solvent Effect on the Properties of Green- and Blue-Light-Emitting Quinoline Derivatives. J. Fluoresc. 2017, 27, 1709–1720. [Google Scholar] [CrossRef]
- Azanza, J.R.; Sádaba, B.; Quetglas, E.G.; Escolar, M. Levofloxacin. Rev. Med. Univ. Navarra. 1998, 42, 220–225. [Google Scholar]
- Wimer, S.M.; Schoonover, L.; Garrison, M.W. Levofloxacin: A therapeutic review. Clin. Ther. 1998, 20, 1049–1070. [Google Scholar] [CrossRef]
- Jang, W.H.; Yoo, D.H.; Park, S.W. Prevalence of and Risk Factors for Levofloxacin-Resistant E. coli Isolated from Outpatients with Urinary Tract Infection. Korean J. Urol. 2011, 52, 554–559. [Google Scholar] [CrossRef]
- Fleming, G.T.; McCarthy, D.M.; Colombet, N.; Patching, J.W. The effect of levofloxacin concentration on the development and maintenance of antibiotic-resistant clones of Escherichia coli in chemostat culture. J. Ind. Microbiol. Biotechnol. 2002, 29, 155–162. [Google Scholar] [CrossRef]
- Wang, C.H.; Yu, C.M.; Hsu, S.T.; Wu, R.X. Levofloxacin-resistant Stenotrophomonas maltophilia: Risk factors and antibiotic susceptibility patterns in hospitalized patients. J. Hosp. Infect. 2020, 104, 46–52. [Google Scholar] [CrossRef]
- Marangon, F.B.; Miller, D.; Muallem, M.S.; Romano, A.C.; Alfonso, E.C. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. Am. J. Ophthalmol. 2004, 37, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Endimiani, A.; Brigante, G.; Bettaccini, A.A.; Luzzaro, F.; Grossi, P.; Toniolo, A.Q. Failure of levofloxacin treatment in community-acquired pneumococcal pneumonia. BMC Infect. Dis. 2005, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, Y.; Bao, Z.; Lin, D.; Liu, H.; Yu, A.; Lei, L.; Li, X.; Xu, X. Thermosensitive glycol chitosan-based hydrogel as a topical ocular drug delivery system for enhanced ocular bioavailability. Int. J. Pharm. 2019, 570, 118688. [Google Scholar] [CrossRef]
- Derbali, R.M.; Aoun, V.; Moussa, G.; Frei, G.; Tehrani, S.F.; Del’Orto, J.C.; Hildgen, P.; Roullin, V.G.; Chain, J.L. Tailored Nanocarriers for the Pulmonary Delivery of Levofloxacin against Pseudomonas aeruginosa: A Comparative Study. Mol. Pharm. 2019, 16, 1906–1916. [Google Scholar] [CrossRef]
- Legnani, L.; Toma, L.; Caramella, P.; Chiacchio, M.A.; Giofrè, S.; Delso, I.; Tejero, T.; Merino, P. Computational Mechanistic Study of Thionation of Carbonyl Compounds with Lawesson’s Reagent. J. Org. Chem. 2016, 81, 7733–7740. [Google Scholar] [CrossRef] [PubMed]
- Sissi, C.; Palumbo, M. The quinolone family: From antibacterial to anticancer agents. Curr. Med. Chem. Anti-Cancer Agents 2003, 3, 439–450. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Haroon, R.A.; Bardaweel, S.K.; Hajjo, R.; Sweidan, K. N-Phenyl-6-Chloro-4-Hydroxy-2-Quinolone-3-CarboxAmides: Molecular Docking, Synthesis, and Biological Investigation as Anticancer Agents. Molecules 2020, 26, 73. [Google Scholar] [CrossRef]
- De Freitas Paulo, T.; Duhayon, C.; De França Lopes, L.G.; Silva Sousa, E.H.; Chauvin, R.; Bernardes-Génisson, V. Further Insights into the Oxidative Pathway of Thiocarbonyl-Type Antitubercular Prodrugs: Ethionamide, Thioacetazone, and Isoxyl. Chem. Res. Toxicol. 2021, 34, 1879–1889. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, J. In vitro biotransformation studies of 2-oxo-clopidogrel: Multiple thiolactone ring-opening pathways further attenuate prodrug activation. Chem. Res. Toxicol. 2013, 26, 179–190. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Loredo, A.; Cole, C.; Xiao, H. Single-atom replacement as a general approach towards visible-light/near-infrared heavy-atom-free photosensitizers for photodynamic therapy. Chem. Sci. 2020, 11, 6701–6708. [Google Scholar] [CrossRef]
- Bauer, A. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Gedam, V.V.; Punyapwar, S.V.; Raut, P.A.; Chahande, A.D. Rapid In Vitro Proliferation of Tinospora Cordifolia Callus Biomass from Stem and Leaf with Phytochemical and Antimicrobial Assessment. In Waste Valorisation and Recycling; Springer: Berlin/Heidelberg, Germany, 2019; pp. 421–431. [Google Scholar]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Veselkov, D.A.; Laponogov, I.; Pan, X.S.; Selvarajah, J.; Skamrova, G.B.; Branstrom, A.; Narasimhan, J.; Prasad, J.V.; Fisher, L.M.; Sanderson, M.R. Structure of a quinolone-stabilized cleavage complex of topoisomerase IV from Klebsiella pneumoniae and comparison with a related Streptococcus pneumoniae complex. Acta Crystallogr. D Struct. Biol. 2016, 72, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Murray, P.R.; Baron, E.J. Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Nguyen, S.T.; Ding, X.; Butler, M.M.; Tashjian, T.F.; Peet, N.P.; Bowlin, T.L. Preparation and antibacterial evaluation of decarboxylated fluoroquinolones. Bioorg. Med. Chem. Lett. 2011, 21, 5961–5963. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wu, P.J.; Chung, K.Y.; Chen, Y.A.; Li, E.Y.; Chou, P.T. Room-temperature phosphorescence from small organic systems containing a thiocarbonyl moiety. Phys. Chem. Chem. Phys. 2017, 19, 8896–8901. [Google Scholar] [CrossRef] [PubMed]


| Compound | B. spizizenii | S. aureus | E. coli | P. aeruginosa | P. mirabilis |
|---|---|---|---|---|---|
| 1 | 19 | 12 | 19 | NZ | 20 |
| 2 | 20 | 11 | 20 | NZ | 22 |
| 3 | 26 | 21 | 23 | 13 | 25 |
| Levofloxacin | 35 | 32 | 26 | 29 | 29 |
| DMSO | NZ | NZ | NZ | NZ | NZ |
| Compound | B. spizizenii | S. aureus | E. coli | P. aeruginosa | P. mirabilis | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 1 | 3.9 | 23.4 | 125 | 500 | 7.8 | 125 | NT | NT | 15.6 | 250 |
| 2 | 3.9 | 21.4 | 250 | >500 | 7.8 | 125 | NT | NT | 15.6 | 250 |
| 3 | 1.9 | 3.9 | 62.5 | 250 | 1.9 | 15.6 | 31.25 | 125 | 7.8 | 31.25 |
| Levofloxacin | 0.06 | 0.4 | 0.9 | 3.9 | 0.24 | 3.9 | 0.24 | 1.9 | 0.48 | 7.8 |
| Compound | IC50 (µM) at 96 h | |
|---|---|---|
| A549 | H1299 | |
| 3 | 180 ± 20 | 173.3 ± 16.7 |
| Levofloxacin | >200 | >200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.I.M.; Abul-Futouh, H.; Bourghli, L.M.S.; Abu-Sini, M.; Sunoqrot, S.; Ikhmais, B.; Jha, V.; Sarayrah, Q.; Abulebdah, D.H.; Ismail, W.H. Design and Synthesis of Thionated Levofloxacin: Insights into a New Generation of Quinolones with Potential Therapeutic and Analytical Applications. Curr. Issues Mol. Biol. 2022, 44, 4626-4638. https://doi.org/10.3390/cimb44100316
Ibrahim AIM, Abul-Futouh H, Bourghli LMS, Abu-Sini M, Sunoqrot S, Ikhmais B, Jha V, Sarayrah Q, Abulebdah DH, Ismail WH. Design and Synthesis of Thionated Levofloxacin: Insights into a New Generation of Quinolones with Potential Therapeutic and Analytical Applications. Current Issues in Molecular Biology. 2022; 44(10):4626-4638. https://doi.org/10.3390/cimb44100316
Chicago/Turabian StyleIbrahim, Ali I. M., Hassan Abul-Futouh, Laurance M. S. Bourghli, Mohammad Abu-Sini, Suhair Sunoqrot, Balqis Ikhmais, Vibhu Jha, Qusai Sarayrah, Dina H. Abulebdah, and Worood H. Ismail. 2022. "Design and Synthesis of Thionated Levofloxacin: Insights into a New Generation of Quinolones with Potential Therapeutic and Analytical Applications" Current Issues in Molecular Biology 44, no. 10: 4626-4638. https://doi.org/10.3390/cimb44100316
APA StyleIbrahim, A. I. M., Abul-Futouh, H., Bourghli, L. M. S., Abu-Sini, M., Sunoqrot, S., Ikhmais, B., Jha, V., Sarayrah, Q., Abulebdah, D. H., & Ismail, W. H. (2022). Design and Synthesis of Thionated Levofloxacin: Insights into a New Generation of Quinolones with Potential Therapeutic and Analytical Applications. Current Issues in Molecular Biology, 44(10), 4626-4638. https://doi.org/10.3390/cimb44100316









