Abstract
In vitro and animal model studies are of great interest for selecting new phytochemicals, including polyphenols with antioxidative properties, as candidates for antidiabetic drugs. This review provides evidence from a critical literature data analysis on the effects of plant extract supplementation in diabetes mellitus management. We considered and meta-analyzed the efficacy of oral supplementation of plant extracts in animal model studies and examined physiological and oxidative stress parameters. Finally, 23 articles were included in the meta-analysis, revealing three plants with experimentally confirmed in vivo and in vitro antidiabetic properties: Gymnema montanum, Momordica charantia and Moringa oleifera. The following parameter changes resulted from an investigation of the supplementation: reduced oxidative stress, decreased insulin resistance, increased insulin release, reduced adiposity, and a modulatory effect on glycolysis and gluconeogenesis, as well as attenuation of diabetes-associated weight loss, reduced fasting blood glucose and lowered oxidative status. A comparison of Gymnema montanum versus Glybenclamide revealed the superiority of extracts over drug administration in some aspects. Although the analyzed extracts are promising candidates for antidiabetic treatment, there is much inconsistent data in the literature. Therefore, ultimate references for using these compounds in the prevention of diabetes are currently not applicable.
1. Introduction
According to the WHO, diabetes mellitus (DM) is one of the most widespread chronic diseases, and the number of cases is rising rapidly. The number of affected patients in 2014 reached 422 million, an almost two-fold increase compared to 1980 [1]. Current estimations predict that diabetic patients will reach 578 million by 2030 and 700 million by 2045 [2].
Oxidative stress (OS) is one of the leading causes of the development of diabetes and its complications [3]. Although organisms have an integrated antioxidant defense system to block the negative impact of reactive oxygen species (ROS), diabetes can cause this system to fail. Hence, supplementation with exogenous plant-derived antioxidants might possess capacities to avert oxidative stress-induced diseases.
Recently, there have been numerous studies in which plant extracts were used to treat various diseases with traditional medicines [4]. It is estimated that nearly a quarter of all modern medicines are derived from natural products [5]. Among the renowned antioxidant properties of several plants––including green tea, cinnamon, curcumin, grape seeds, and many berries––several new species used in traditional medicine have been documented. In vitro and animal model studies reflect an interest in selecting new phytochemical resources that possess antioxidative properties as candidates for drugs in antidiabetic approaches.
On the one hand, our long-term interest as a team is focused on investigating the molecular etiology of DM, in particular gestational diabetes mellitus (GDM) [6]; on the other hand, we are investigating the potential application of phytochemicals with antioxidant/antidiabetic activity obtained from the defatted seeds of Oenothera paradoxa. During our search for new sources of phytochemicals applicable to the management of DM, as revealed in animal model studies, we recently performed a meta-analysis to verify the efficacy of oral supplementation with plant extracts. We selected Gymnema montanum, Momordica charantia and Moringa oleifera since the well-documented data for DM-induced rats revealed their potential contribution to diabetes management and provoked more detailed studies.
1.1. Gymnema montanum Effect on Diabetes
Gymnema montanum (G. montanum, GM) is an endemic, woody climbing shrub found mainly in Africa and India. Leaves of GM have medical applications, andthey have a long history of use in India’s Ayurvedic medicine as an antidiabetic drug, diuretic, and digestive stimulant [7].
Currently, extracts from Gymnema leaves have found application in metabolic syndrome, weight loss, and cough.
Ananthan et al. studied the effect of the ethanolic extract from the leaves of Gymnema montanum on lipid-peroxidation-induced oxidative stress in experimental diabetes animal models [8]. Male Winstar rats were used, and diabetes was induced with alloxan monohydrate. One group of rats was treated with the plant extract, and the second with the antidiabetic drug Glibenclamide. The plasma levels of thiobarbituric acid reactive substances (TBARS) and hydroperoxides were estimated.
Furthermore, glutathione (GSH) and Vitamin C were investigated [9]. The studies confirmed increased lipid peroxidation in the plasma of diabetic rats. The study by Ananthan et al. showed that the plasma level of peroxides in rats fed with Gymnema montanum extract tended to be near the average. Further studies showed that the investigated plant also had an impact on the appropriate level of natural antioxidants such as Vitamin C. Several studies reported decreased levels of Vitamin C and Vitamin E among diabetic patients [10,11]. However, supplementation with Gymnema montanum extract increased these crucial antioxidant vitamins [12].
Ananthan et al. [8] also concluded that the administration of the analyzed extract to the diabetic rats caused increased GSH. The authors suggested that this increase activates enzymes needed for the appropriate action of GSH, such as glutathione peroxidase and glutathione S-transferase. They also hypothesized that the antidiabetic effect of Gymnema montanum extract and its ability to decrease the blood glucose level could be due to a strengthened insulin release. Furthermore, the efficacy of the plant depends on the degree of β-cell destruction. Another essential feature of Gymnema montanum connected with the antioxidative properties of this plant is its ability to inhibit lipid peroxidation
Diabetes is a disorder with many complications, one of which is abnormalities in the lipid profile. A disturbances in lipids metabolism after glucose impairment is one of the significant pathogenesis factors. It is connected mainly with the enhanced use of fatty acids from adipose tissue, and second with the increase of free fatty acids in the blood [13]. Impairments in lipids metabolism are connected with insulin deficiency and could cause hypercholesterolemia and hypertriglyceridemia [14]. Furthermore, there are pieces of evidence reporting that diabetes could change the lipid profile, which can impact cellular processes such as ion permeability and receptor interactions [15,16,17]. Ramkumar et al. studied the effects of Gymnema montanum on the fatty-acid composition and lipid-profile level in diabetic rats. They also used Glibenclamide as a reference drug [18]. In the alloxan-induced diabetic rats, the levels of glucose and lipids such as total cholesterol (TC), low-density lipoprotein (LDL) and triglycerides (TG), both in serum and in selected tissues, were increased. However, after administering Gymnema montanum extract, a significant decrease in blood glucose and lipid content was observed. The same study carried out using Glibenclamide showed that the modulatory effect of Gymnema montanum was better than the traditionally used drug. Worth underlining is that treating non-diabetes rats with the same concentration of GM extract did not cause any adverse effects. The authors postulated two potential antihyperlipidemic mechanisms. One was the insulin stimulatory effect of GM on β-cells, and the second was the insulin-mimetic activity of GM, which improved the movement of glucose from plasma to peripheral tissues. Since no adverse effects among the non-diabetic rats was observed, the first of the proposed mechanisms was probably more likely.
It was observed [19] in diabetic rats that the level of monounsaturated fatty acids, especially oleic acid, increased significantly, while polyunsaturated fatty-acid (PUFA) levels mainly linolenic and arachidonic acids, decreased. This could have been associated with the susceptibility of PUFAs to the action of free radicals due to the presence of double bonds. The synthetic pathway of the PUFAs requires two essential steps: desaturation and elongation. Diabetes lowers the rate-limiting desaturation step, especially the activity of δ-6-desaturase, which is the enzyme responsible for conversion of linoleic acid to γ-linolenic acid and α-linolenic acid to stearidonic acid [20]. It is known that PUFAs decrease thrombosis and atherosclerosis, and in this way they lower the probability of cardiovascular disorders [21]. The Gymnema montanum extract supplementation reverses changes in lipid content and helps maintain the proper composition of tissue fatty acids.
The extract studied by Ramkumar et al. was analyzed by gas chromatography coupled with mass spectrometry, and it was found that it contained 11.57% w/w of carvacrol, 6.77% of erythritol,4.58% of gallic acid and 3.09% of quercetin [21].
It is postulated that phytochemicals present in GM extract, particularly gallic acid, resveratrol and quercetin, possess the antioxidative, antidiabetic and antihyperlipidemic properties [22] that play a pivotal role in lowering blood glucose in diabetic patients and in improving the action of insulin.
1.2. Momordica charantia Effect on Diabetes
Momordica charantia (M. charantia, MC), a plant belonging to the Cucurbitaceae family, is commonly known as a bitter gourd, balsam pear, bitter melon, or Karela. All plant parts have a bitter taste, including the fruit. India, Japan, Singapore, Vietnam, Amazon, East Africa, Brazil, Malaya, China, Thailand, Colombia, Cuba, Ghana, Haiti, India, Mexico, New Zealand, Nicaragua, Panama, the Middle East, Central and South America are the regions where MC is cultivated. At maturation, the fruit of M. charantia can be used as a dietary food, and because of its multiple beneficial activities, has also been used as a herbal medicine [23].
In ancient history, the seed and fruit were used as medication for diabetes. The other fractions of M. charantia––roots, leaves and even vines––have been used in folk medicine to treat other diseases like diarrhea, toothache and furuncle. Therefore, this plant is the subject of many ongoing studies investigating its potential in preventing and treatingseveral diseases. Each year, more and more papers reveal the plausible effects of supplementation with M. charantia, thereby strongly indicating that this plant possesses various pharmacological functions: antidiabetic, anthelmintic, abortifacient, antimalarial, antimutagenic, antilipolytic, antifertility, hepatoprotective, anti-inflammatory, contraceptive and laxative, anti-ulcerogenic, antioxidative and immune-modulatory [24].
A more comprehensive application of M. charantia in multiple areas of medicine is still restricted due to adverse effects observed in many studies. Some of these are hypoglycemic coma in children, and toxicity or even death in laboratory animals [23].
Extracts of this plant consist of a broad-spectral resource of phytoconstituents. These compounds are usually divided into the following main groups: carbohydrates, polysaccharides, proteins and peptides, lipids, terpenoids, saponins, phenolics and sterols. Despite these main groups, compounds such as unsaturated fatty acids, alkaloids, amino acids, minerals and vitamins are also contained in M. charantia. The distribution of specific compounds varies among different plant parts [25].
Gallic acid, protocatechuic acid, gentisic acid, (+)-catechin, vanillic acid, syringic acid, (−)-epicatechin, p-coumaric acid, benzoic acid, sinapinic acid, o-coumaric acid, chlorogenic acid, t-cinnamic acid and t-ferulic acid are the most abundant flavonoids and phenolic compounds in M. charantia. Their concentration varies in the range of 1.77 ± 0.72% [26], and the fruit is considered to be the major source of phenolic components. It has been proven that an increase in flavonoid concentration is linearly correlated with an increase in antioxidant capacity of M. charantia, probably due to the fact that flavonoids are one of the most effective free radical scavengers and antioxidants [27].
Quinic acid and catechin were determined to be the most abundant flavonoids along with gallic acid, gentisic acid, chlorogenic acid, and epicatechin. Different phenolic acid constituents revealed different distributions among various plant parts. An investigation of extracts derived from different plant parts also revealed the presence of protocatechuic acid, p-coumaric acid, syringic acid, vanillic acid and benzoic acid, tannic acid, ferulic acid, and caffeic acid [28].
Ethanol extract from the dried powder of Momordica charantia was evaluated by Arafatet al. as an antidiabetic treatment in alloxan-induced diabetes rats [29]. Alloxan administration caused hyperglycemia in the tested animals. The HPLC quantitative analysis of the extract showed a high amount of ellagic acid (307.78 mg/100g of dry extract) and myricetin (180.6 mg/100g of dry extract). The concentration of gallic acid was also relatively high—extract supplementation to the diabetic rats’ effects in serum glucose decreased. Several shreds of evidence reported that ellagic acid and myricetin might prevent hepatic steatosis and fibrosis in diabetic patients by improving Nrf2 and CPT1 as well as decreasing protein levels of NF-κB [30,31]. These results were also confirmed by studies carried out by Arafat [29]. Momordica charantia supplementation of diabetic rats prevented mononuclear cell infiltration and decreased myeloperoxidase activity.
Furthermore, this effect may be facilitated partly by inhibiting oxidative stress and inflammation in the liver by the phenolic components of the MC extract. Some researchers indicated that MC extract enhanced insulin secretion by stimulating β-cells [32,33]. On the other hand, Mahomoodally et al. [34] suggested that the administration of MC extract in lab animals reduced glucose absorption from gut walls and stimulated glucose uptake and use in adipose muscle tissue.
It is known that inflammation and oxidative stress are the main factors responsible for liver damage in diabetic patients [35]. Arafat et al. [28] observed decreased concentration in all antioxidant enzymes in alloxan-induced diabetic rats. However, animals treated with the Momordica charantia ethanolic extract significantly enhanced all antioxidant parameters, including improvement of the GSH concentration, SOD and CAT activities. M. charantia polyphenols possess anti-inflammatory and antioxidative properties that play a crucial role in the inhibition of DM and the progression of its complications, mainly due to the inhibition of the accumulation of advanced glycation end-products (AGE’s), which were inhibited significantly by tannins as identified in the extracts. In addition to reducing AGE’s accumulation, tannins also take part in inflammatory state regression and insulin resistance reduction. As a result of the latter two, micro- and macrovascular complications of DM decreased; thus, the analyzed extracts revealed the potential for preventing vascular complications [36]. The correlation between the phenolic acid concentration of M. charantia fruit extract and antiglycation activity was assessed and found to be linearly interconnected with increased suppression of the glycation process [37].
Based on the abovementioned activities of particular chemical components of M. charantia extract and broad literature data, it can be reasserted that, in correlation with its composition, the MC extracts possess the following general activities: antidiabetic, antioxidant, antiviral, antimicrobial, anthelmintic, abortifacient, antimalarial, antimutagenic, antilipolytic, antifertility, hepatoprotective, anti-inflammatory, antitumor, hypolipidemic, immunomodulatory, and wound healing.
1.3. Moringa oleifera Effects on Diabetes
Moringa oleifera (M. oleifera, MO) Lam is a plant that belongs to the Moringaceae family and naturally occurs widely in many tropical and subtropical areas [38,39]. This plant originates from the western and sub-Himalayan tracts, India, Pakistan, Asia Minor, Africa and Arabia [40,41]. It is well-known as the “drumstick tree” or “the horseradish tree” based on the taste of ground root preparations and the ben oil tree from seed-derived oils [41]. Diverse parts of M. oleifera (leaves, fruits, flowers, and roots) are commonly used as food, nutraceuticals, traditional medicine, water sanitization, and biofuel production due to their rich source of many vital nutrients bioactive compounds [42].
Mounting evidence reports that M. oleifera parts, especially the leaves, have nutritional properties or can be used in diet supplementation [43]. Using M. oleifera extract in food products has improved overall nutritional quality, sensory properties and shelf life. The use of leaves, seed and flower powder is well known in various food applications, such as in fortifying amala (stiff dough), ogi (maize gruel), bread, biscuits, yoghurt, cheese, and soups [42].
Moringa in non-traditional medicine is known for treating many diseases, including diabetes, cancer, cardiovascular, neurological, gastroenterological, and inflammatory disorders. Moringa oleifera leaves are the most commonly used part of this plant and contain beta-carotene, vitamins B, C, E, minerals, polyphenols [44,45,46], oxidase, catalase, alkaloids, glucosinolates, isothiocyanates, tannins, and saponins [38,47]. In Moringa seeds, niazimicin and niazirin and a rhamnosyl benzyl carbamate, rhamnosyl benzyl isothiocyanate, and various derivatives of β-sitosterol were identified. This plant’s stems, roots, and other morphological parts are not well researched, unlike the leaves and seeds; therefore, the data on the composition is relatively limited [47].
The biological activity of M. oleifera is usually linked to the presence of phenolic compounds in the leaves, seeds, stems, and roots of the plant. Nevertheless, most of the nutraceutical properties of this plant are associated with glucosinolates and isothiocyanates [48,49].
Moringa oleifera contains high amounts of aromatic glucosinolates, both in the leaves and roots. The main glucosinolate is glucomoringin (a rhamnopyranosylbenzylglucosinolate), whereas moringin is a group representative of isothiocyanate. Anti-inflammatory and antioxidant activity of isothiocyanates has been reported due to their capacity to activate detoxification enzymes [50]. The polyphenol content of Moringa oleifera depends on the plant part. The dried leaves of Moringa oleifera contain many polyphenols, including flavonoids and phenolic acids that are their principal compounds [41]. Their concentrations range from 2090 to 12,200 mg GAE/100 g of DW (or 1600 to 3400 mgTAE/100 g of DW). These amounts are more significant than those found in fruit and vegetables. The observed flavonoids are mainly quercetin and kaempferol, isorhamnetin, and their glycosidic forms [42,44,51], while the phenolic acids are mainly gallic, chlorogenic, ellagic, and ferulic acids [52].
Based on the available literature, about 20 pharmacological properties can be attributed to this plant [53]. It is evident that various Moringa extracts can have hypoglycemic effects in different in vitro and in vivo models [48,54,55]. The antihyperglycemic effect of various aqueous-ethanol extracts of Moringa leaves (95, 75, 50 and 25% v/v and 100% water) was examined in STZ-induced diabetic rats. The most active extracts were further subjected to five liquid–liquid fractionations (hexane, chloroform, ethyl acetate, butanol, and water) and were evaluated for their antihyperglycemic activities. Among all the extracts tested, 95% (v/v) ethanol extract at 1000 mg/kg and butanol fraction at 500 mg/kg reduced blood glucose concentration acutely in the diabetic rats [56,57]. Bamagous et al. demonstrated that ethyl acetate extract of Moringa leaves at a dose of 200 mg/kg administrated to streptozotocin(STZ)-induced diabetic rats significantly decreased their blood glucose and glycosylated hemoglobin (HbA1c) levels compared to the control group [58]. Similar results were obtained in other studies using Moringa leaf powder at 50 mg/day. Administration of this extract to alloxan-induced diabetic rats for eight weeks triggered a significant decrease in blood glucose concentration compared with untreated diabetic rats [59].
Considering these results, the preferred doses of Moringa leaves used in most of these studies were 100, 200, and 300 mg/kg body weight. The long-term effect of Moringa leaves on glycemia in animals was evident. Additionally, no antagonistic effect of Moringa leaves intake was found in these long-term studies. However, long-term animal studies are still limited.
A few studies on the hypoglycemic properties of M. oleifera extracts in humans are available. In type 2 diabetic patients (40–58 years), consuming a standard calorie-restricted diet (1200–1800 kcal) and taking sulfonylurea medication, led to a significant reduction in postprandial blood glucose levels from 210 mg/dL to 191, 174, and 150 mg/dL, respectively after the first, second, and third month of supplementation. Moreover, in only the T2DM group, HbA1c significantly decreased from an initial value of 7.81 to 7.4 after three months of supplementation [60]. Other results exhibited a significant reduction of both fasting (28%) and postprandial (26%) blood glucose concentrations in the diabetic subjects after 40 days of Moringa leaf (8 g) intake, while no changes were noticed in the control group. Fombang et al. revealed that Moringa leaves tea at 200 and 400 mL caused an overall decrease of 17% and 19%, respectively, in the glycemia of human subjects. The explanation of the Moringa tea effect was that it has a strong antioxidant potential which may enhance its antihyperglycemic effect [61].
Recently, there were numerous reports on Moringa leaf extract as a food additive. The antidiabetic effect of Moringa leaf powder (20 g) incorporated into a traditional meal versus control meal was shown. After consuming the meal containing Moringa leaf powder, a significant decrease in blood glucose concentration at 90, 120, and 150 min in diabetic subjects compared to the control meal was detected. It had no effect on the blood glucose concentration of the healthy subjects [62].
The aforementioned human studies emphasize the antidiabetic potential of Moringa leaves, which were administered in different forms: capsule, tea, and food. The following doses of 7 and 8 g (as leaf powder), 200 and 400 mL (as tea), 20 g (as leaf powder incorporated into a traditional meal), and 5% w/w (as leaf powder incorporated into cookies) were used. However, these studies are limited in number because of different study designs and short sample sizes and durations [61,62].
The antidiabetic activity of Moringa oleifera may encompass various mechanisms of action, including the stimulation of insulin secretion, inhibition of α-amylase and α-glucosidase activities, decrease of gluconeogenesis in the liver, increase of glucose uptake in the muscles and liver, inhibition of glucose uptake from the intestine, and antioxidative properties. The antidiabetic activity of this plant may be the result of alleviating insulin resistance, either by neutralizing oxidative stress or by attenuating inflammation. The evidence regarding this antidiabetic plant and its phytochemicals acting directly on insulin activation signaling is very limited in the literature. The antidiabetic properties of Moringa leaves are attributed to the presence of small bioactive molecules: (4-hydroxyphenylacetonitrite (1), fluoropyrazine (2), methyl-4-hydroxybenzoate (3), vanillin (4), 4-α-L-rhamnopyranosylbenzylisothiocyanate (5), and 3,4-dihydroxy benzonitrile (6), as well as phenolics and flavonoids such as gallic acid (7) and rutin (8).
Hafizur et al. revealed that compounds (1)–(4) exhibited a significant glucose-dependent insulin release at a stimulatory glucose concentration of 16.7 mM, with a concomitant dose-dependent release of insulin at 200 μM. It has been suggested that possible mechanisms coupled to insulin secretion include the role of protein kinase A-mediated insulin secretion from pancreatic β-cells [63]. In the study by Attakpa et al. on diabetic mice, Moringa leaf extract improved insulin sensitivity by stimulating the insulin-dependent Akt pathway and upregulating glucose transporter GLUT4 expression in the muscles [64]. Furthermore, in diabetic rats consuming Moringa leaf extract, improved glycogen synthase activities, glycogen contents, and glucose uptake in the liver and muscles were detected [65].
It is believed that MO may act as an antidiabetic agent by reducing glucose levels. The underlying mechanism of this action is linked to quercetin, which can act as an inhibitor of GLUT2 [66]. No effect on GLUT5 or SGLT1 was noticed [67]. Moreover, quercetin may activate adenosine monophosphate-activated protein kinase (AMPK) to increase glucose uptake through stimulation of GLUT4 in skeletal muscle and to decrease the production of glucose through downregulation of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase(G6Pase) in the liver [68].
According to Waterman, after consumption of isothiocyanate-rich Moringa leaf extract, decreased plasma insulin, insulin resistance, and liver gluconeogenesis were detected in mice fed a very high-fat diet [69]. In different research, the intake of Moringa leaves caused a decrease in gastric emptying in GK rats, and this hypoglycemic effect could be linked to the presence of quercetin-3-glucoside and fibre content in the leaves [70].
In the literature, research results demonstrated the effect of Moringa leaf extract on the inhibition of α-amylase and α-glucosidase enzymes involved in the digestion of sugars in the intestine [71,72], however, the results regarding the effect of Moringa leaves on insulin secretion are inconsistent. Some authors showed the positive effect of those extracts [58,65], whereas some studies demonstrated no effect [72]. Another possible mechanism for the hypoglycemic properties of the aqueous extract from Moringa leaves is the decreasing expression of pyruvate carboxylase enzyme in the liver and regenerating damaged pancreatic β-cells and hepatocytes through its antioxidant activities [73].
It is worth noting that polyphenols from an MO aqueous leaf extract may inhibit protein oxidation, formation of AGEs, and protein cross-linking in glycation reactions [74]. The ability of polyphenols to scavenge free radicals derived from glycoxidation processes may explain their protection against protein glycation [75]. Phytochemicals from MO extract exhibited antioxidative properties and prevented the oxidation of lipids, thereby showing hypolipidemic and anti-atherosclerosis activity [38,76].
Sierra-Campos determined the effects of Moringa oleifera leaf extract on rat paraoxonase 1 (rPON1) and catalase (rCAT) activities in alloxan-induced diabetic rats. These extracts may probably activate both rPON1 and rCAT due to the influence of the specific flavonoids on the enzyme structure. The molecular blind docking analysis showed that rPON1 might have two binding sites for flavonoids. Moreover, flavonoids maybe bound at four sites in rCAT [77].
In diabetic rats treated with M. oleifera, a significant decrease of serum NFkβ levels and the upregulation of BCL-2 in both the kidney and liver were observed. Additionally, interleukin levels in the kidney (IL-18) and liver (IL-1α, IL-18) decreased [78].
Beyond the abovementioned activity, others have been revealed. Methanolic extract of Moringa oleifera leaves at adose of 200 mg/kg per body weight, inhibited by approximately 64% cell growth in Ehrlich ascites carcinoma cells in Swiss albino mice and caused upregulation of the pro-apoptotic gene Bax and tumor suppressor gene p53. It also downregulated the anti-apoptotic gene Bcl-2, thus indicating the extract’s anticancer activity [79]. Silver nanoparticles (Ag-NPs) biologically synthesized from Moringa oleifera leaf extract possess a significantly enhanced antibacterial activity against selected pathogenic bacterial strains, which strongly indicates that M. oleifera could be a potential source of Ag-NPs for successful use as an antibacterial agent in the pharmaceutical and cosmetic industries [80]. M. oleifera plays a pivotal role in the anti-inflammation and antioxidant processes of the kidney as revealed in conducted by Akter et al.’s [81] review of the literature, which emphasized the pharmacological and therapeutic potential of M. oleifera, as well as prospects of Moringa-based effective drug development beneficial for humans.
General characteristics of the content and properties of all three described above extracts are individually presented in Table 1.

Table 1.
M. charantia, M. oleifera and G. montanum and their characteristics.
The following analysis was aimed to systematically evaluate the literature and indicate potent plant candidates for a restricted meta-analysis. We applied Cochrane guidelines to investigate the efficacy of oral supplementation of subjected plant origin extracts in diabetes mellitus management in animal model studies. The initial literature search revealed about 300 original papers indicating three potent plant extracts for further analysis, of which only 23 articles were finally included in the restricted meta-analysis, revealing the experimentally confirmed in vivo and in vitro antidiabetic properties of Gymnema montanum (9 articles), Momordica charantia (7 articles) and Moringa oleifera (7 articles). In the meta-analysis protocol, data on physiological and oxidative stress parameters extracted from the original papers were examined and statistically analyzed. After any needed proper adjustments using RevMan 5.4. The categories of the analyzed parameters and observed tendencies of changes are presented in Table 2.

Table 2.
Distribution and changes of analyzed parameters in meta-analysis.
2. Meta-Analysis
2.1. Literature Search, Data Screening and Extraction Protocol
A comprehensive search of PubMed, MEDLINE, the Cochrane Library and Google Scholar databases was performed to classify studies reporting oral supplementation with various plant-derived extracts in rats with induced DM. Two independent investigators conducted the screening procedure, in which studies published after the year 2000 were considered suitable for analysis.
All steps of the meta-analysis were performed with high precision according to Cochrane guidelines for the systematic review of interventions [101]. The initial search procedure with quotation constructed as follow: “Plant extracts [AND] rats [AND] Diabetes mellitus” revealed 1982 papers, the screening of which led to the identification of numerous extracts that were considered potentially relevant.
Further steps consisted of a detailed screening based on the following inclusion criteria:
- (1)
- original paper from an interventional experiment conducted on the suitable animal model (induced diabetic rats);
- (2)
- experiment design involving the intervention group and control group either with or without a standard antidiabetic drug intervention;
- (3)
- compatibility in the range of analyzed parameters and the manner of results presentation.
To fulfill the Cochrane criteria, two independent investigators working in parallel extracted the following characteristics from full-text papers, according to a standardized data extraction protocol: the surname of the lead author, year of publication, size of experimental/control/drug groups, study duration, study design, inclusion and exclusion criteria for each experiment measured and analyzed outcomes (primary and secondary).
After this step, the 300 original papers were determined suitable and relevant for further analysis, revealing three mentioned previously plant extracts as potent candidates for meta-analysis. Finally, only 23 met the inclusion criteria and were further meta-analyzed.The workflow of the screening procedure is presented in Figure 1.
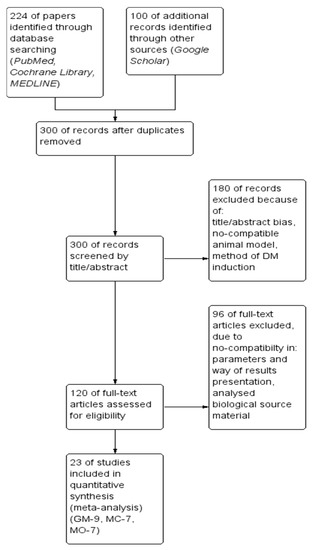
Figure 1.
The flow diagram of the study selection procedure with numerical data about the inclusion and exclusion protocol.
The continuous data, after adequate transformation, were presented with the use of the same scales. The analyses were performed using the Mean Difference (MD). Furthermore, the results were presented with 95% confidential intervals (CI). No dichotomous data were analyzed. Presented values for all analyzed parameters were defined in the endpoint of the intervention termination day and presented as mean values with standard deviation (SD). In studies using more than one dose of extracts, the most effective one (as determined by authors of publications) was applied for this meta-analysis. The comparison was conducted with the random-effect model, while heterogeneity was assessed with χ2 and I2 tests. All extracted data were analyzed using the statistical software package—RevMan 5.4 (Review Manager, Copenhagen, Denmark: the Nordic Cochrane Centre). Forest plots were used to visualize the final meta-analysis results. A p-value < 0.05 was considered statistically significant in all analyses.
The total number of rats included in all experiments was 409 (intervention-treated: 175, control: 186, drug-treated: 48). The distribution of the number of rats in particular groups were as follows: (1) intervention groups: G. montanum–60, M. charantia–48, M. oleifera–67; (2) control groups: G. montanum–71, M. charantia–48, M. oleifera–67; (3) drug-treated groups: G. montanum–48.
Considering the physiological efficacy parameters, the following number of papers reported the particular selected parameters: glycemia, 20 articles (G. montanum–9, M. charantia–5, M. oleifera–6);insulinemia, 14 articles (G. montanum–9, M. charantia–3, M. oleifera–2);body weight, 10 articles (G. montanum–4, M. charantia–3, M. oleifera–3);food intake, 4 articles (G. montanum); glucose uptake by diaphragm, 3 articles (M. oleifera). In the case of oxidative stress, the following number of papers reported the selected parameters: TBARS, 2 articles (G. montanum); hydroperoxides, 2 articles (G. montanum); SOD, 3 articles (M. oleifera); and CAT, 3 articles (M. oleifera).
2.2. The Results for Physiological Parameters Analysis
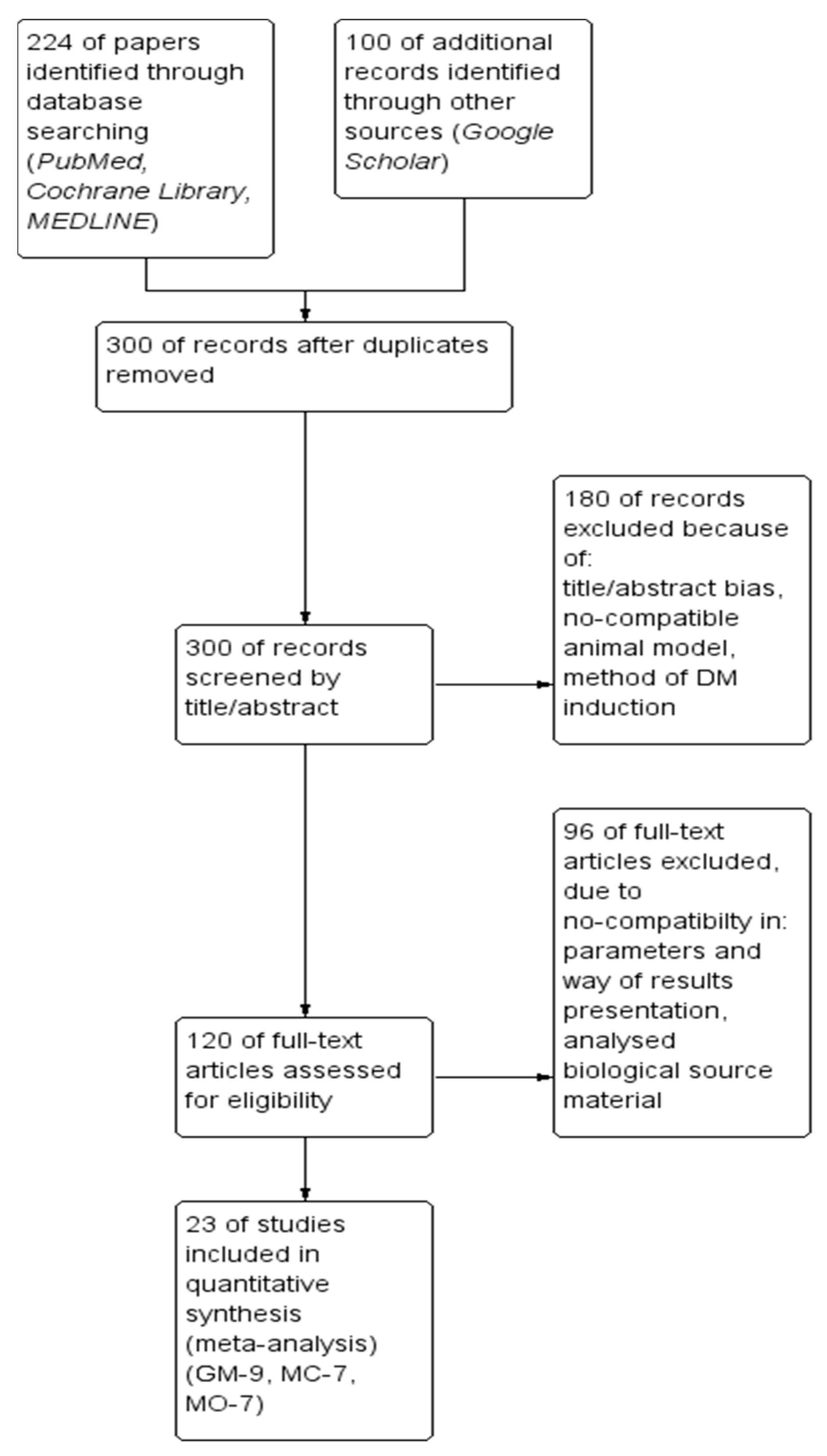
Reduction of glycemia was observed in three cases indicating the benefits of all three extracts for supplementation in glycemic control as pooled MD, respectively, for G. montanum: −204.98 (95% CI [−231.18, −178.78]; p<0.00001) (see Figure 2); and M. charantia: −121.68 (95% CI [−152.50, −90.86]; p < 0.00001)]. The results for M. oleifera were inconsistent and should be interpreted with caution. Extract supplementation also revealed more robust hypoglycemic activity in comparison with the antidiabetic drug Glybenclamide in the case of G. montanum extracts, pooled MD: −57.71 (95% CI [−86.39, −29.03], p < 0.0001), indicating the effects of supplementation might be superior compared with this drug.
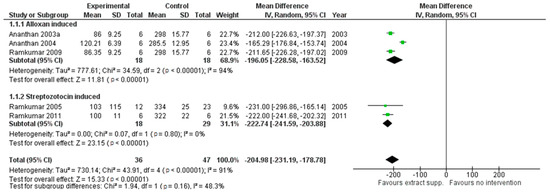
Figure 2.
The results of G. montanum supplementation on glycemic levels in serum of induced diabetic rats (Forest plot).
Insulin levels increased in serum obtained from rats supplemented with G. montanum and M. charantia. The pooled MD were, respectively, G. montanum: 7.99 (95% CI [0.66, 15.32], p = 0.03);and M. charantia: 2.20 (95% CI [1.08, 3.32], p = 0.0001), indicating the positive impact on the secretory function of the pancreas. In the case of M. oleifera, the results were not precise due to the differences among the subgroups. Therefore, such results might be misleading.
Glybenclamide supplementation showed a more visible insulin increase when compared with G. montanum in insulin increasing effect (pooled MD: −50.26 (95% CI [−108.06, 7.84], p = 0.09)).
Striking changes in the body weight of rats was observed in the group supplemented with G. montanum, indicating the probable high calorific value of the analyzed extracts and its potential influence on animal metabolism mediated by the insulin increase. The pooled MD was 63.27(95% CI [54.96, 71.57], p < 0.00001), and the rest of the results did not show consistent effects of supplementation.
Reduction in food intake was observed in rats supplemented with G. montanum (pooled MD: −53.32 (95% CI [−108.62, 1.97], p = 0.06)), and when compared with the Glybenclamide (pooled MD: −2.61 (95% CI [−4.46, −0,76], p = 0.006)).
Other results for physiological parameters show inconsistency and should be interpreted with precautions.
2.3. The Results of Parameters Related to Oxidative Status Analysis
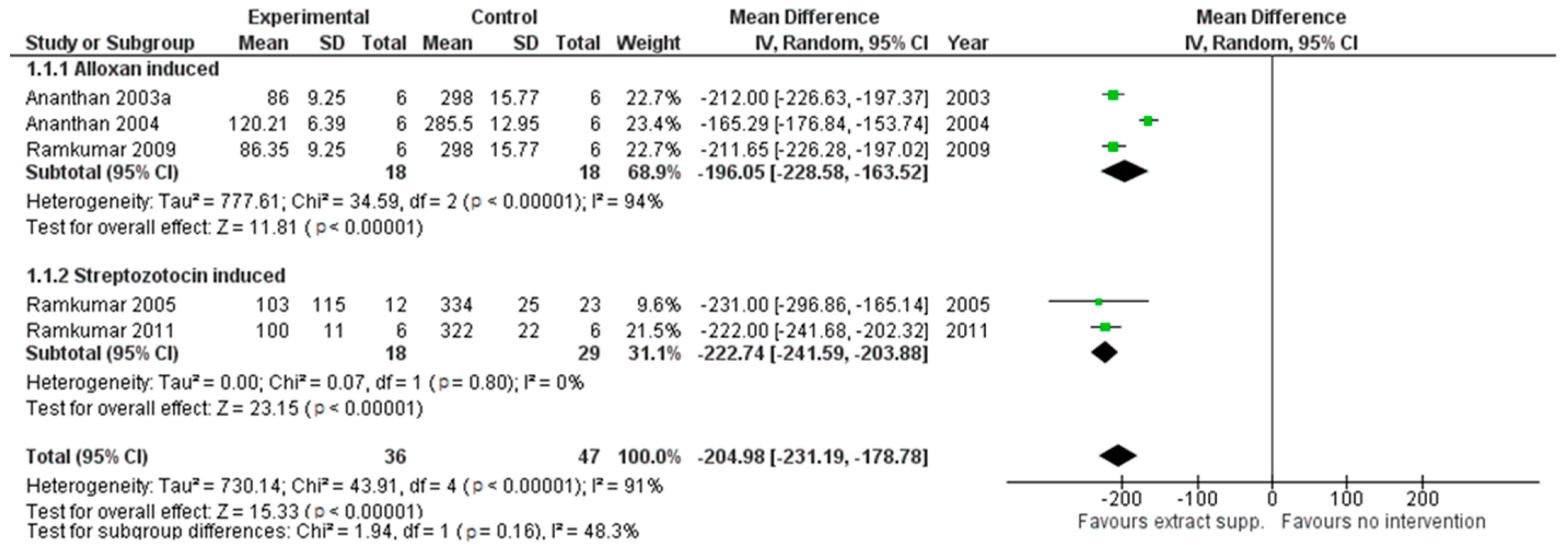
G. montanum extracts exhibited a lower impact on the level of TBARS (pooled MD: −0.71 (95% CI [−1.15, −0.27], p = 0.002)) (see Figure 3) and hydroperoxides (pooled MD: −17.33 (95% CI [−22.9, −11.75], p < 0.00001)) in rat plasma, revealing the positive influence on the oxidative status of animals.
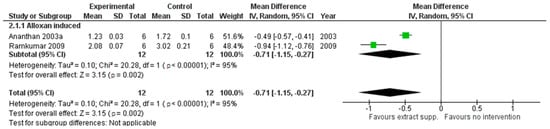
Figure 3.
An animal model trial evaluated G. montanum supplementation on TBARS levels in serum of induced diabetic rats (Forest plot).
In comparison with the antidiabetic drug Glybenclamide, G. montanum also displayed decreasing effect on hydroperoxide serum level (pooled MD: −4.95 (95% CI [−8.47, −1.44], p = 0.006)), while the results of the TBARS analysis were inconsistent and should be interpreted with caution. As a result of supplementation with M. oleifera extracts, reduced SOD activitywas observed (pooled MD: −132.23 (95% CI [−187.45, −77.00], p < 0.00001)), which corresponded with the reduction in oxidative stress.
Results not mentioned in the above section contained several inconsistencies, and conclusions based on them might cause incorrect outcomes.
More detailed data and results not presented above could be found in Supplementary Material (see the Supplementary Material Section).
3. Discussion
The therapeutic potential of plant-derived antioxidants, mostly polyphenolic compounds, is strongly evident in large studies in which plant extracts are applied to support the treatment of various diseases, including civilization, chronic diseases pertaining to obesity, diabetes mellitus or the heart [5,6,102,103,104,105,106]. Additionally, growing evidence indicates a clear link between the consumption of several plants and the prevention or treatment of chronic diseases like diabetes mellitus (DM). Thus, a healthy diet supplemented with isolated phytochemicals known for their beneficial effects can modify the risk of developing such chronic diseases [104].
Our long-term interests have focused on the molecular etiology of DM for several years and moved towards the potential application of phytochemicals with antioxidant/antidiabetic activity in diabetes treatment. Furthermore, because numerous recent studies were conducted using animal models supplemented with antioxidant/antidiabetic phytochemicals, we presented the evidence and results from a critical literature analysis followed by a strictly tailored meta-analysis of such studies.
A comprehensive search of scientific databases revealed studies reporting oral supplementation with various plant-derived extracts in rats with induced DM. The meta-analysis revealed the contribution of oral plant extract supplementation, which may trigger more detailed studies on the application of those extracts in DM management and the evaluation of the molecular basis of their action.
The final analysis was focused on antidiabetic, hypoglycemic and antioxidative activity of three plant extracts: Gymnema montanum, Momordica charantia, and Moringa oleifera in model rats with induced diabetes.
G. montanum and M. charantia displayed hypoglycemic, antidiabetic and antioxidative activity when supplemented to diabetic rats. Other plants belonging to the Apocynaceae family, such as G. sylvestre, and many members of the Cucurbitaceae family (C. indica, B. hispida, and M. charantia itself) are now postulated to be potent plants with antidiabetic activity [107]. Moreover, both G. montanum and M. charantia increased the insulin level, suggesting an impact on the secretory activity of β cells of the pancreas, which was confirmed by Navarrete et al. [108]. The beneficial impact of G. montanum and M. charantia on hepatic function and insulin secretion was displayed in glucose homeostasis parameters indicating their hypoglycemic activity [109].
The hypoglycemic, antihyperlipidemic and insulin increasing activity of G. montanum might be attributable to gymnenic acids, similar to the case of dihydroxygymnenic triacetate isolated from G. sylvestre, another representative of the Apocynaceae family [110].
The oxidative status of the rats treated with G. montanum extracts improved; therefore, the applied treatment was effective in oxidative stress (OS) reduction. G. montanum supplementation reduced TBARS and hydroperoxides levels significantly, resulting in a decrease in OS. Comparing G. montanum extract supplementation and Glybenclamide treatment gave precise results only in the case of reduced hydroperoxides produced in animals supplemented with the extract. In the case of the TBARS level, the authors did not observe any significant differences. An increase in the hexokinase activity, glucose-6-phosphate dehydrogenase and glycogen content was observed in some studies, indicating the favorable effect of G. montanum on glucose homeostasis [94]. As reported by Ramkumar et al., the activity of main hepatic parameters and enzymes involved in glucose metabolism indicated the positive influence of G. montanum on liver function.
Considering that diabetic hyperglycemia results from defects in insulin secretion and action, the above activities made extracts from G. montanum and M. charantia promising drug candidates because ofthe molecular activity of the phytochemicals included in these extracts. In summary, supplementation with the plant extracts selected for this analysis revealed their hypoglycemic activity particularly related to hepatic function improvement in glucose homeostasis, increased insulin level and the insulin sensitivity of peripheral tissues.
In some studies, animals treated with the plant extracts (particularly G. montanum) gained weight. It may be attributable to the high caloric value of the extract and its impact on the metabolism of rats, but it might also be interpreted as a secondary outcome of an increased insulin level, which displayed a key role in lipid metabolism. However, the induction of DM is commonly followed by weight loss in induced rats, mainly due to the catabolism of fats and proteins caused by insulin deficiency [111]. Thus, it can be postulated that the insulin-increasing activity due to supplementation with plant extracts played a decisive dual role: on the one hand, hypoglycemic; on the other hand, weight loss prevention. These aspects are of particular value when designing further trials in which a caloric value and a sugars-and-lipids pattern in the plant extracts would also be a vital parameter to consider.
In the meta-analysis, we also assessed the effects of selected plant extracts on glycemic control in animal models compared to treatment with the well-known glucose-lowering drug Glybenclamide. This oral hypoglycemic agent is a second-generation sulfonylurea derivative that increases insulin secretion, probably by interacting with sulfonylurea receptors on beta cells or by interfering with ATP-sensitive potassium channels on pancreatic beta cells. The comparison of the activity of the extracts with Glybenclamide demonstrated the favorable influence of the G. montanum extracts on the glucose level, precisely its superior hypoglycemic activity. Additional studies are needed to reveal the molecular mechanisms underlying the hypoglycemic activity of G. montanum that are different from those already described above.
Additionally, in rats supplemented with G. montanum, a reduction in food intake was observed compared to those treated with an oral drug. This fact might be probably related to the high caloric value and the high dietary fibre content of extracts. In the case of Gymnema montanum, the literature data are limited; therefore, more studies on the bioactivity of this plant, as well as its composition, are needed [92].
The analysis of the activity of antioxidant enzymes (CAT, SOD) provided additional information about the impact of supplementation on the oxidative status in rats. The M. oleifera extracts reduced the activity of SOD in the treated rats, lowering the oxidative stress by decreasing free-radical concentration. Some discrepancies were observed in the analysis of the CAT activity and should be interpreted with caution. However, the observed increase in CAT activity agreed with the previously reported data [99]. The M. oleifera extracts are rich in phytochemicals (polyphenols, vitamins, flavonoids, tannins, saponins and alkaloids) that might have been responsible for OS reduction [36,111].
Therefore, it may be assumed that the plant extracts from Gymnema montanum, Momordica charantia, and Moringa oleifera supplemented to rats that had induced diabetes revealed a positive influence on the oxidative status and are very potent candidates for diabetic management, accordingly to results obtained from analyzed cases.
The analysis was based on a search of the recent literature (after the year 2000) from medical databases, providing much reliable data. Two independent researchers carefully evaluated and conducted the review protocol, data extraction, and further citation, and all of those were processed according to the Cochrane guidelines [101]. The results were reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [111]. The analyzed parameters are vital factors reflecting the changes in physiology and function of DM-induced rats and reflect the OS status of subjects. Since our analysis was focused on critical physiological and biochemical parameters reflecting the outcomes of the intervention, the results provide precise data for drawing conclusions based on them.
Comparing different studies on plant extracts’ medical/supplementary potential is often hampered or impossible since there are significant distinctions between studies. These involve the final content of phytochemicals resulting from different extraction procedures (solvents, conditions, storage and analysis), and different criteria of diabetes recognition and experimental protocols, which display numerous variances, including study groups and controls, intervention duration, administered doses, analyzed parameters or other factors. Finally, there are many imprecisions in how results are reported. Therefore, the restricted and detailed protocol for meta-analysis allowed drawing conclusions based upon numerous independent studies. That is what we did to provide relevant and reliable results that constituted the basis for the final analysis and why we only took 23 out of 300 primarily selected papers.
Several points of criticism should be remembered when evaluating the health effects of supplementation with phytochemicals like polyphenols, which, as far as has been established, consist of a group of representative compounds (approximately 10,000) having broad diversity in structure and physiological role.
It is challenging to conclude their short- and long-term health effects from experimental and interventional data. It also has to be remembered that the biological activity of all phytochemicals is robustly dependable on many other factors such as their bioavailability in organisms and the degree of the chemical treatment during the extraction protocol, which can alter the former and further activity in the organism. The role of the recipient’s microbiota in the metabolism of phytochemicals is to alterthe activity of supplemented compounds by producing different metabotypes, which can display different activities and affect physiological functions in an alternating manner. All of the above factors cause high inter-individual differences in the biological response to supplementation. Awareness of the fact that the analyzed effect of supplementation of animals with phytochemicals was the result of multiple synergistic/antagonistic interactions of multiple dietary components, environmental factors and individual properties of subjects is a crucial factor that needs to be considered when drawing conclusions from such data. Additionally, the possible risk of the low adherence of the experimental protocol to real-life conditions, in which polyphenol consumption might be completely different, can complicate the analysis. Moreover, the restricted selection of data from trials with plant extracts conducted in chemical laboratories raises considerations about the health benefits emerging from a diet naturally enriched by polyphenols and other phytochemicals and the different synergistic effects of various components might be observed.
Despite these limitations, the results of this meta-analysis strongly encourage further studies to evaluate the impact of plant-derived extracts on diabetic patients’ physiological and oxidative status parameters. Further study to investigate the activity of these analyzed extracts in humans is strongly recommended since there is no direct way to extrapolate results obtained from animals to humans due to their complexity. There is always a risk of severe adverse events and toxicity from such supplementation in humans. It is crucial to indicate the differences between the animal model of diabetes and the physiology of human patients with this disease, and this constitutes a critical limitation at the entry point of study design and on further data analysis. The abovementioned results are promising and may trigger the further development of experimental and clinical approaches to investigate the application of these plant extracts in more advanced and detailed studies.
4. Conclusions
The presented meta-analysis confirmed that extracts of Gymnema montanum, Momordica charantia and Moringa oleifera represent a promising and attractive source of phytochemicals with proven antidiabetic and antioxidant activity in rat models of diabetes. They increase pancreatic insulin and insulin sensitivity in peripheral tissues, reduce insulin resistance and hepatic gluconeogenesis, and have a modulatory effect on glycolysis, gluconeogenesis and antihyperlipidemic properties. All three extracts reduced oxidative stress and revealed antiperoxidative features to protect β-cells against ROS. They are, therefore, good candidates for the management and treatment of diabetes in mammals, especially humans. Moreover, all three plants have been widely used in traditional medicine.
This study revealed that the application of stringent inclusion/exclusion criteria (23 out of 300 papers) displayed a lack of generally accepted standards in a description of experiments with plant extracts (a type of extraction, analysis of extract, the concentration of different phytochemicals) and experimental protocols, making a direct comparison and analysis more demanding. However, considering all the strengths and limitations, this meta-analysis is a reliable source of data and might constitute an inducement for further physiological and mechanistic studies.
This review would shed light on how plant-based drugs could potentially be a beneficial agents in treating of aging, oxidative stress and hyperglycemia-associated abnormalities.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cimb44020049/s1.
Author Contributions
Conceptualization: M.K. and M.B.-M.; methodology: M.K.; software: M.K.; validation: M.K., M.B.-M. and I.B.-P.; formal analysis: L.A.W.; investigation: M.K.; resources: M.K., M.B.-M. and I.B.-P.; data curation: M.K. and M.B.-M.; writing—original: M.K., M.B.-M. and I.B.-P.; draft preparation: M.K., M.B.-M. and I.B.-P.; writing—review and editing: M.K., M.B.-M., I.B.-P. and L.A.W.; visualization: M.K.; supervision: M.B.-M. and L.A.W.; project administration: M.K.; funding acquisition: M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported grant from the Medical University of Lodz (Project no. 502-03/0-160-01/502-04-034 to M.K.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO Global Reports on Diabetes. 2016. Available online: https://www.who.int/diabetes/publications/grd-2016/en/ (accessed on 11 January 2022).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from The International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnefont-Rousselot, D.; Bastard, J.P.; Jaudon, M.C.; Delattre, J. Consequences of The Diabetic Status on the Oxidant/Antioxidant Balance. Diabetes Metab. 2000, 26, 163–176. [Google Scholar] [PubMed]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular Mechanisms Underlying Curcumin-Mediated Therapeutic Effects in Type 2 Diabetes and Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, S.; Andrica, F.; Serban, M.C.; Timar, R. Polyphenols-Rich Natural Products for the Treatment of Diabetes. Curr. Med. Chem. 2015, 22, 14–22. [Google Scholar] [CrossRef]
- Vajravelu, E.B.P. Rare and Endemic Species Collected after Fifty Years or More from South India. In An Assessment of Threatened Plants of India. In Proceedings of The Seminar Onrare and Endemic Species Re-Collected after Fifty Years or More from Southindia; Jain, S.K., Raw, R.R., Eds.; Botanical Survey Of India: Howrah, India, 1983; Volume 14. [Google Scholar]
- Ananthan, R.; Baskar, C.; Narmathabai, V.; Pari, L.; Latha, M.; Ramkumar, K.M. Antidiabetic Effect of Gymnema Montanum Leaves: Effect on Lipid Peroxidation Induced Oxidative Stress in Experimental Diabetes. Pharmacol. Res. 2003, 48, 551–556. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Pari, L.; Saravanan, G. Effect of Phaseolus Vulgaris on Circulatory Antioxidants and Lipids in Rats with Streptozotocin-Induced Diabetes. J. Med. Food 2002, 5, 97–103. [Google Scholar] [CrossRef]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant Effects of Vitamins in Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Diabetol. Metab. Syndr. 2018, 10, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Christie-David, D.J.; Girgis, C.M.; Gunton, J.E. Effects of Vitamins C and D in Type 2 Diabetes Mellitus. Nutr. Diet. Suppl. 2015, 7, 21–28. [Google Scholar]
- Garg, M.C.; Bansal, D.D. Protective Antioxidant Effect of Vitamin C and Vitamin E in Streptozotocin-Induced Diabetic Rats. Indian J. Exp. Biol. 2000, 38, 101–104. [Google Scholar]
- Frei, B. Ascorbic Acid Protects Lipids in Human Plasma and Low-Density Lipoprotein Against Oxidative Damage. Am. J. Clin. Nutr. 1991, 54, 1113S–1118S. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Shulman, G.I. Free Fatty Acids in Obesity and Type 2 Diabetes: Defining Their Role in The Development of Insulin Resistance and Beta-Cell Dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. 3), 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jaiprakash, R.; Rani, M.A.; Venkataraman, B.V.; Andrade, C. Effect of Felodipine on Serum Lipid Profile in Short Term Streptozotocin Diabetes in Rats. Indian J. Exp. Biol. 1993, 31, 283–284. [Google Scholar] [PubMed]
- Wang, L.; Folsom, A.R.; Zheng, Z.J.; Pankow, J.S.; Eckfeldt, J.H. Investigators, AS Plasma Fatty Acid Composition and Incidence of Diabetes in Middle-Aged Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.A.; Pari, L. Combined N-Benzoyl-D-Phenylalanine and Metformin Treatment Reverse Changes in the Fatty Acid Composition of Streptozotocin Diabetic Rats. J. Basic Clin. Physiol. Pharmacol. 2006, 17, 17–28. [Google Scholar] [CrossRef]
- Sochor, M.; Zaher Baquer, N.; Mclean, P. Glucose Over and Under Utilization in Diabetes: Comparative Studies on the Changes in Activities of Enzymes of Glucose Metabolism in Rat Kidney and Liver. Mol. Phys. 1985, 7, 51–68. [Google Scholar]
- Ramkumar, K.M.; Vijayakumar, R.S.; Ponmanickam, P.; Velayuthaprabhu, S.; Archunan, G.; Rajaguru, P. Antihyperlipidaemic Effect of Gymnema Montanum: A Study on Lipid Profile and Fatty Acid Composition in Experimental Diabetes. Basic Clin. Pharmacol. Toxicol. 2008, 103, 538–545. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A. Effect of Antioxidants on Nerve And vascular Dysfunctions in Experimental Diabetes. Diabetes Res. Clin. Pract. 1999, 45, 137–146. [Google Scholar] [CrossRef]
- Demaison, L.S.J.; Moreau, D.; Grynberg, A. Influence of the Phospholipid N-6/N-3 PUFA Ratio on the Mitochondrial Oxidative Metabolism Before and after Myocardial Ischemia. Biochem. Biophys. Acta 1994, 1227, 53–59. [Google Scholar]
- López-Vélez, M.; Martínez-Martínez, F.; Del Valle-Ribes, C. The Study of Phenolic Compounds as Natural Antioxidants in Wine. Crit. Rev. Food Sci. Nutr. 2003, 43, 233–244. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.P. Pharmacological Actions and Potential Uses of Momordica Charantia: A Review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica Charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [Green Version]
- Raman, A.; Lau, C. Anti-Diabetic Properties and Phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine 1996, 2, 349–362. [Google Scholar] [CrossRef]
- Patel, S.; Patel, T.; Parmar, K.; Bhatt, Y.; Patel, Y.; Patel, N.M. Isolation, Characterization and Antimicrobial Activity of Charantin from Momordica Charantialinn. Fruit. Int. J. Drug Dev. Res. 2010, 2, 629–634. [Google Scholar]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant Properties and Quantitative UPLC-MS Analysis of Phenolic Compounds from Extracts of Fenugreek (Trigonella Foenum-Graecum) Seeds and Bitter Melon (Momordica charantia) Fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Horax, R.; Hettiarachchy, N.; Islam, S. Total Phenolic Contents And Phenolic Acid Constituents in 4 Varieties Of Bitter Melons (Momordica Charantia) and Antioxidant Activities of Their Extracts. J. Food Sci. 2005, 70, C275–C280. [Google Scholar] [CrossRef]
- Arafat, S.Y.; Nayeem, M.; Jahan, S.; Karim, Z.; Reza, H.M.; Md Hemayet, H.; Shohel, M.; Md Ashraful, A. Ellagic Acid-Rich momordica Charantia Fruit Pulp Supplementation Prevented Oxidative Stress, Fibrosis and Inflammation in Liver Ofalloxan-Induced Diabetic Rats. Orient Pharm. Exp. Med. 2016, 16, 267–278. [Google Scholar] [CrossRef]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic Acid Attenuates High-Carbohydrate, High-Fat Diet-Induced Metabolic Syndrome in Rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Kandasamy, N.; Ashokkumar, N. Protective Effect of Bioflavonoid Myricetin Enhances Carbohydrate Metabolic Enzymes and Insulin Signaling Molecules in Streptozotocin-Cadmium induced Diabetic Nephrotoxic Rats. Toxicol. Appl. Pharmacol. 2014, 279, 173–185. [Google Scholar] [CrossRef]
- Ahmed, I.; Adeghate, E.; Cummings, E.; Sharma, A.K.; Singh, J. Beneficial Effects and Mechanism of Action of Momordica Charantia Juice in The Treatment of Streptozotocin-induced Diabetes Mellitus In Rat. Mol. Cell Biochem. 2004, 261, 63–70. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, M. Regeneration Of Beta Cells in islets of langerhans of the pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (Bitter Gourd) Fruits. Indian J. Exp. Biol. 2007, 45, 1055–1062. [Google Scholar] [PubMed]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Effect of exogenous atp on Momordica charantia Linn. (Cucurbitaceae) induced inhibition of D-Glucose, L-Tyrosine and Fluid Transport Across Rat Everted Intestinal Sacs In Vitro. J. Ethnopharmacol. 2007, 110, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Wlazlo, N.; Beijers, H.J.; Schoon, E.J.; Sauerwein, H.P.; Stehouwer, C.D.; Bravenboer, B. High Prevalence Of Diabetes Mellitus in Patientswith Liver Cirrhosi. Diabet. Med. 2010, 27, 1308–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Farah, N.; Bukhari, S.A.; Ali, M.; Naqvi, S.A.; Mahmood, S. Phenolic acid profiling and antiglycation studies of leaf and fruit extracts of tyrosine primed Momordica charantia seeds for possible treatment of diabetes mellitus. Pak. J. Pharm. Sci. 2018, 31, 2667–2672. [Google Scholar]
- Vargas-Sanchez, K.; Garay-Jaramillo, E.; Gonzalez-Reyes, R.E. Effects of Moringa oleiferaon Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef] [Green Version]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, the nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef]
- Glover-Amengor, M.; Aryeetey, R.; Afari, E.; Nyarko, A. Micronutrient composition and acceptability of Moringa oleifera leaf-fortified dishes by children in Ada-East district, Ghana. Food Sci. Nutr. 2017, 5, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manguro, L.O.; Lemmen, P. Phenolics of Moringa oleifera leaves. Nat. Prod. Res. 2007, 21, 56–68. [Google Scholar] [CrossRef]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Lorenzo, J.M.; Afolayan, A.J.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, N.A.; Gaytán-Martínez, M.; de la Luz Reyes-Vega, M.; Loarca-Piña, G. Glucosinolates and Isothiocyanates from Moringa oleifera: Chemical and Biological Approaches. Plant Foods Hum. Nutr. 2020, 75, 447–457. [Google Scholar] [CrossRef]
- Fahey, J.W.; Olson, M.E.; Stephenson, K.K.; Wade, K.L.; Chodur, G.M.; Odee, D.; Nouman, W.; Massiah, M.; Alt, J.; Egner, P.A.; et al. The Diversity of Chemoprotective Glucosinolates in Moringaceae (Moringa spp.). Sci. Rep. 2018, 8, 7994. [Google Scholar] [CrossRef] [Green Version]
- Abdul lRazis, A.F.; Konsue, N.; Ioannides, C. Isothiocyanates and Xenobiotic Detoxification. Mol. Nutr. Food Res. 2018, 62, e1700916. [Google Scholar] [CrossRef]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional Characterization and Phenolic Profiling of Moringa oleifera Leaves Grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.A.; Castro-Lopez, C.; Rojas, R.; Sanchez-Alejo, E.J.; Ngangyo-Heya, M.; Martinez-Avila, G.C.G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Forster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R.; Mewis, I. Ecotype variability in growth and secondary metabolite profile in Moringa oleifera: Impact of sulfur and water availability. J. Agric. Food Chem. 2015, 63, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, G.; De Petrocellis, L.; Schiano Moriello, A.; Bertoli, S.; Leone, A.; Battezzati, A.; Mazzini, S.; Bassoli, A.; Moringin, A. Stable Isothiocyanate from Moringa oleifera, Activates the Somatosensory and Pain Receptor TRPA1 Channel In Vitro. Molecules 2020, 25, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, J.; Khan, I.; Blundell, R. Moringa oleifera and glycemic control: A review of current evidence and possible mechanisms. Phytother. Res. 2019, 33, 2841–2848. [Google Scholar] [CrossRef]
- Irfan, H.M.; Asmawi, A.Z.; Khan, N.A.K.; Sadikun, A.; Mordi, M.N. Anti-diabetic activity-guided screening of aqueous-ethanol Moringaoleifera extracts and fractions: Identification of marker compounds. Trop. J. Pharm. Res. 2017, 16, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Bamagous, G.A.; Al Ghanidi, S.S.; Ibrahim, I.A.A.; Mahfoz, A.M.; Afify, M.A.; Alsugoor, M.H.M.; Shammah, A.A.; Arulselvan, P.; Rengarajan, T. Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators. Asian Pac. J. Trop. Biomed. 2018, 8, 320–327. [Google Scholar] [CrossRef]
- Villarruel-Lopez, A.; de la Mora, D.A.L.; Vazquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuno, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127. [Google Scholar] [CrossRef] [Green Version]
- Arun Giridhari, V.; Malathi, D.; Geetha, K. Anti-diabetic property of drumstick (Moringa oleifera) leaf tablets. Int. J. Health Nutr. 2011, 2, 1–5. [Google Scholar]
- Fombang, E.N.; Saa, R.W. Antihyperglycemic activity of Moringa oleifera Lam leaf functional tea in rat models and human subjects. Food Nutr. Sci. 2016, 7, 1021–1032. [Google Scholar]
- Taweerutchana, R.; Lumlerdkij, N.; Vannasaeng, S.; Akarasereenont, P.; Sriwijitkamol, A. Effect of Moringa oleifera Leaf Capsules on Glycemic Control in Therapy-Naive Type 2 Diabetes Patients: A Randomized Placebo-Controlled Study. Evid. Based Complement. Altern. Med. 2017, 2017, 6581390. [Google Scholar] [CrossRef] [Green Version]
- Hafizur, R.M.; Maryam, K.; Hameed, A.; Zaheer, L.; Bano, S.; Sumbul, S.; Sana, A.; Saleem, R.; Naz, S.; Waraich, R.S.; et al. Insulin-releasing effect of some pure compounds from Moringa oleifera on mice islets. Med. Chem. Res. 2018, 27, 1408–1418. [Google Scholar] [CrossRef]
- Attakpa, E.; Sangaré, M.; Béhanzin, G.; Ategbo, J.; Seri, B.; Khan, N.A. Moringa oleifera Lam. stimulates activation of the insulin-dependent Akt pathway. Antidiabetic effect in diet-induced obesity (DIO) mouse model. Folia Biol. 2017, 63, 42–51. [Google Scholar]
- Olayaki, L.A.; Irekpita, J.E.; Yakubu, M.T.; Ojo, O.O. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [PubMed] [Green Version]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Ndong, M.; Uehara, M.; Katsumata, S.; Suzuki, K. Effects of Oral Administration of Moringa oleifera Lam on Glucose Tolerance in Goto-Kakizaki and Wistar Rats. J. Clin. Biochem. Nutr. 2007, 40, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Leone, A.; Bertoli, S.; Di Lello, S.; Bassoli, A.; Ravasenghi, S.; Borgonovo, G.; Forlani, F.; Battezzati, A. Effect of Moringaoleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps. Nutrients 2018, 10, 1494. [Google Scholar] [CrossRef] [Green Version]
- Azad, S.B.; Ansari, P.; Azam, S.; Hossain, S.M.; Shahid, M.I.; Hasan, M.; Hannan, J.M.A. Anti-hyperglycaemic activity of Moringa oleifera is partly mediated by carbohydrase inhibition and glucose fibre binding. Biosci. Rep. 2017, 37, BSR20170059. [Google Scholar] [CrossRef] [Green Version]
- Abd El Latif, A.; El Bialy Bel, S.; Mahboub, H.D.; AbdEldaim, M.A. Moringa oleifera leaf extract ameliorates alloxan-induced diabetes in rats by regeneration of beta cells and reduction of pyruvate carboxylase expression. Biochem. Cell Biol. 2014, 92, 413–419. [Google Scholar] [CrossRef]
- Nunthanawanich, P.; Sompong, W.; Sirikwanpong, S.; Makynen, K.; Adisakwattana, S.; Dahlan, W.; Ngamukote, S. Moringa Oleifera Aqueous Leaf Extract Inhibits Reducing Monosaccharide-Induced Protein Glycation And Oxidation Of Bovine Serum Albumin. Springerplus 2016, 5, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Kinetics Of Glycoxidation Of Bovine Serum Albumin By Methylglyoxal And Glyoxal And Its Prevention By Various Compounds. Molecules 2014, 19, 4880–4896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumark, P.; Khunawat, P.; Sanvarinda, Y.; Phornchirasilp, S.; Morales, N.P.; Phivthong-Ngam, L.; Ratanachamnong, P.; Srisawat, S.; Pongrapeeporn, K.-U.S. The In Vitro And Ex Vivo Antioxidant Properties, Hypolipidaemic And Antiatherosclerotic Activities Of Water Extract Of Moringa Oleifera Lam. Leaves. J. Ethnopharmacol. 2008, 116, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Campos, E.; Valdez-Solana, M.; Avitia-Dominguez, C.; Campos-Almazan, M.; Flores-Molina, I.; Garcia-Arenas, G.; Tellez-Valencia, A. Effects of Moringaoleifera Leaf Extract on Diabetes-Induced Alterations in Paraoxonase 1 and Catalase in Rats Analyzed through Progress Kinetic and Blind Docking. Antioxidants 2020, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.; Aboua, G.; Omodanisi, E. Effects of Moringaoleifera on oxidative stress, apoptotic and inflammatory biomarkers in the streptozotocin-induced diabetic animal model. S. Afr. J. Bot. 2020, 129, 354–365. [Google Scholar] [CrossRef]
- Das, P.K.; Asha, S.Y.; Siddika, A.; Siddika, A.; Tareq, A.R.M.; Islam, F.; Khanam, J.A.; Rakib, A. Methanolic extract of Moringa oleifera leaves mediates anticancer activities through inhibiting NF-𝜅B and enhancing ROS in Ehrlich ascites carcinoma cells in mice. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 161–170. [Google Scholar] [CrossRef]
- Islam, A.; Manda, C.; Habib, A. Antibacterial potential of synthesized silver nanoparticles from leaf extract of Moringa oleifera. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 67–73. [Google Scholar] [CrossRef]
- Akter, T.; Rahman, M.A.; Moni, A.; Apu, M.A.I.; Fariha, A.; Hannan, M.A.; Uddin, M.J. Prospects for Protective Potential of Moringa oleifera against Kidney Diseases. Plants 2021, 10, 2818. [Google Scholar] [CrossRef]
- Ma, L.; Yu, A.H.; Sun, L.L.; Gao, W.; Zhang, M.M.; Su, Y.L.; Liu, H.; Ji, T. Two new bi desmosine triterpenoid saponins from the seeds of Momordica charantia L. Molecules 2014, 19, 2238–2246. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Z.; Zamhuri, K.F.; Yaacob, A.; Siong, C.H.; Selvarajah, M.; Ismail, A.; Nazrul Hakim, M. In vitro antidiabetic activities and chemical analysis of polypeptide-k and oil isolated from seeds of Momordica charantia (bitter gourd). Molecules 2012, 17, 9631–9640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klomann, S.D.; Mueller, A.S.; Pallauf, J.; Krawinkel, M.B. Antidiabetic effects of bitter gourd extracts in insulin-resistant db/db mice. Br. J. Nutr. 2010, 104, 1613–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garau, C.; Cummings, E.; Phoenix, D.; Singh, J. Beneficial effect and mechanism of action of Momordica charantia in the treatment of diabetes mellitus: A mini-review. Int. J. Diabetes Metab. 2003, 11, 46–55. [Google Scholar]
- Ananthan, R.; Latha, M.; Pari, L.; Ramkumar, K.M.; Baskar, C.G.; Bai, V.N. Effect of Gymnemamontanumon blood glucose, plasma insulin, and carbohydrate metabolic enzymes in alloxan-induced diabetic rats. J. Med. Food 2003, 6, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.Y.; Hsu, C.; Chao, C.Y.; Wein, Y.S.; Kuo, Y.H.; Huang, C.J. Fractionation and identification of 9c, 11t, 13t-conjugated linolenic acid as an activator of PPARalpha in bitter gourd (Momordica charantia L.). J. Biomed. Sci. 2006, 13, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhang, M.; Liu, J.; Zhang, Y.; Zhang, R.-F.; Wei, Z.-C.; Ti, H.-H.; Liu, L.; Qiu, M.-H. Comparison of the content, antioxidant activity, andα-glucosidase inhibitory effect of polysaccharides from Momordica charantia L. species. Mod. Food Sci. 2014, 30, 102–108. [Google Scholar]
- Ramkumar, K.M.; Latha, M.; Venkateswaran, S.; Pari, L.; Ananthan, R.; Bai, V.N. Modulatory effect of Gymnema montanum leaf extract on brain antioxidant status and lipid peroxidation in diabetic rats. J. Med. Food 2004, 7, 366–371. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Rajaguru, P.; Latha, M.; Ananthan, R. Effect of Gymnema montanum leaves on red blood cell resistance to oxidative stress in experimental diabetes. Cell Biol. Toxicol. 2008, 24, 233–241. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Lee, A.S.; Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T.; Kang, K.P.; Lee, S.; Kim, W.; Park, S.K.; Lee, N.H.; et al. Gymnema montanum H. protects against alloxan-induced oxidative stress and apoptosis in pancreatic beta-cells. Cell. Physiol. Biochem. 2009, 24, 429–440. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Manjula, C.; Sankar, L.; Suriyanarayanan, S.; Rajaguru, P. Potential in vitro antioxidant and protective effects of Gymnema montanum H. on alloxan-induced oxidative damage in pancreatic beta-cells, HIT-T15. Food Chem. Toxicol. 2009, 47, 2246–2256. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.M.; Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Sujatha, J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 3390–3394. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839. [Google Scholar]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Brilhante, R.S.N.; Sales, J.A.; Pereira, V.S.; Castelo-Branco, D.; Cordeiro, R.A.; de Souza Sampaio, C.M.; de Araújo Neto Paiva, M.; Santos, J.; Sidrim, J.J.C.; Rocha, M.F.G. Research advances on the multiple uses of Moringaoleifera: A sustainable alternative for socially neglected population. Asian Pac. J. Trop. Med. 2017, 10, 621–630. [Google Scholar] [CrossRef]
- Teixeira, E.M.; Carvalho, M.R.; Neves, V.A.; Silva, M.A.; Arantes-Pereira, L. Chemical characteristics and fractionation of proteins from Moringaoleifera Lam. leaves. Food Chem. 2014, 147, 51–54. [Google Scholar] [CrossRef]
- Adepoju-Bello, A.; Jolayemi, O.; Ehianeta, T.; Ayoola, G. Preliminary phytochemical screening, antioxidant and antihyperglycaemic activity of Moringa oleifera leaf extracts. Pak. J. Pharm. Sci. 2017, 30, 2217–2222. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Burzynska-Pedziwiatr, I.; Bukowiecka-Matusiak, M.; Wojcik, M.; Machala, W.; Bienkiewicz, M.; Spolnik, G.; Danikiewicz, W.; Wozniak, L.A. Dual stimulus-dependent effect of Oenothera paradoxa extract on the respiratory burst in human leukocytes: Suppressing for Escherichia coli and phorbol myristate acetate (PMA) and stimulating for formyl-methionyl-leucyl-phenylalanine (fMLP). Oxidative Med. Cell. Longev. 2014, 2014, 764367. [Google Scholar] [CrossRef] [Green Version]
- Bukowiecka-Matusiak, M.; Turek, I.A.; Woźniak, L.A. Natural phenolic antioxidants and their synthetic derivatives. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 4047–4061. [Google Scholar]
- Wozniak, L.A.; Cypryk, K.; Wojcik, M. Molecular mechanisms of diabetes prevention by structurally diverse antioxidants. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Bagchi, D., Sreejayan, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 315–330. [Google Scholar]
- Wojcik, M.; Krawczyk, M.; Wozniak, L.A. Antidiabetic Activity of Curcumin: Insight into Its Mechanisms of Action; Bagchi, D., Nair, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 385–401. [Google Scholar]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [Green Version]
- Patil, R.; Patil, R.; Ahirwar, B.; Ahirwar, D. Current status of Indian medicinal plants with antidiabetic potential: A review. Asian Pac. J. Trop. Biomed. 2011, 1, S291–S298. [Google Scholar] [CrossRef]
- Cortez-Navarrete, M.; Martınez-Abundis, E.; Pérez-Rubio, K.G.; Gonzalez-Ortiz, M.; Mendez-del Villar, M. Momordica charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. J. Med. Food 2018, 21, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Tuan, N.Q.; Park, M.H.; Quan, K.T.; Oh, J.; Heo, K.S.; Na, M.; Myung, C.S. Cucurbitane Triterpenoids fromthe Fruits of Momordica Charantia Improve Insulin Sensitivity and Glucose Homeostasis in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2018, 62, e1700769. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Eliza, J.; Mohamed, F.K.A. A novel dihydroxygymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J. Ethnopharmacol. 2009, 126, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).