Proteomic Changes to the Updated Discovery of Engineered Insulin and Its Analogs: Pros and Cons
Abstract
1. Introduction
2. Background of Insulin Preparations
3. Insulin Preparations Having a Lesser Period of Action
Regular Insulin
4. Insulin Preparations Having Quick Action
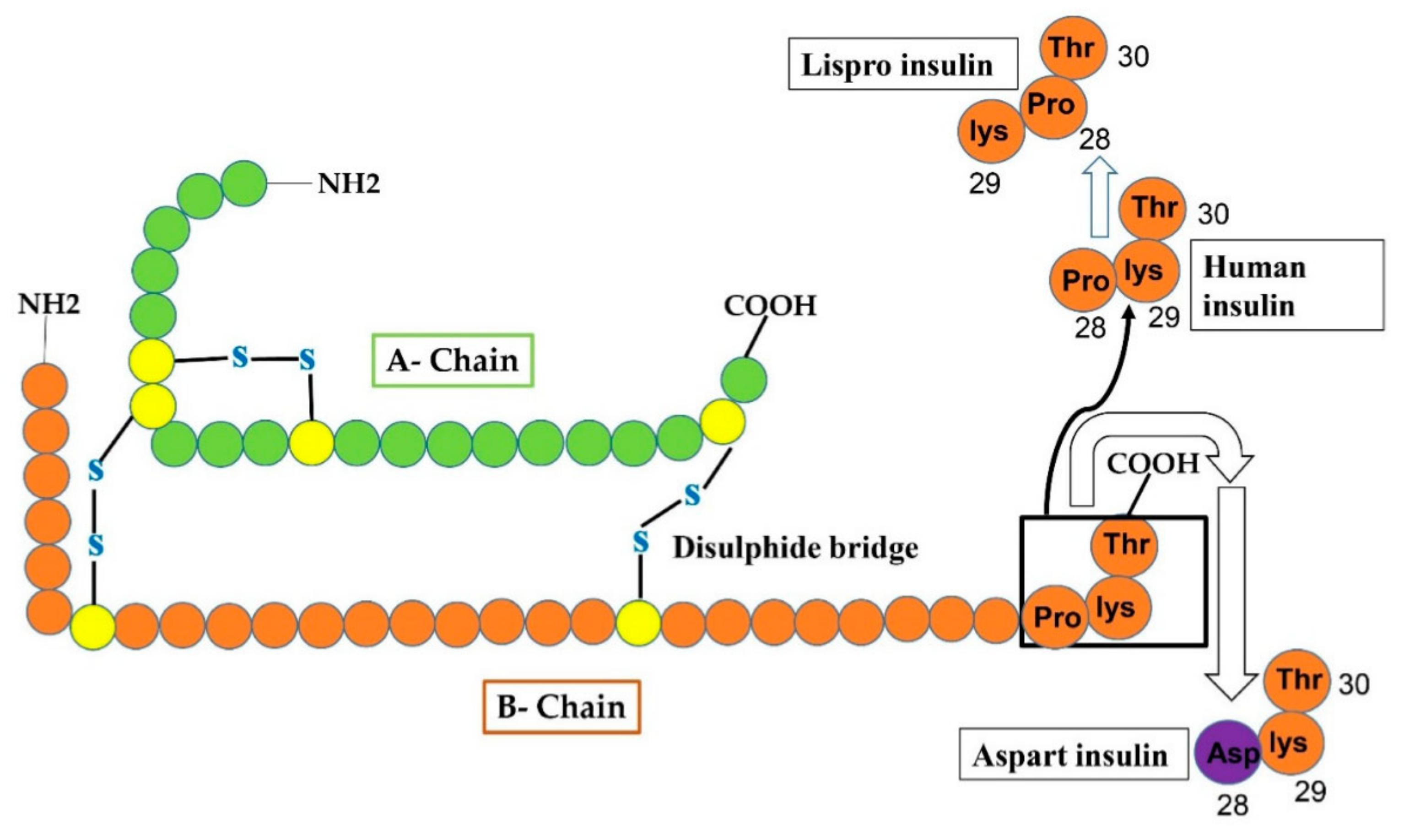
4.1. Lispro Insulin
4.2. Insulin Aspart
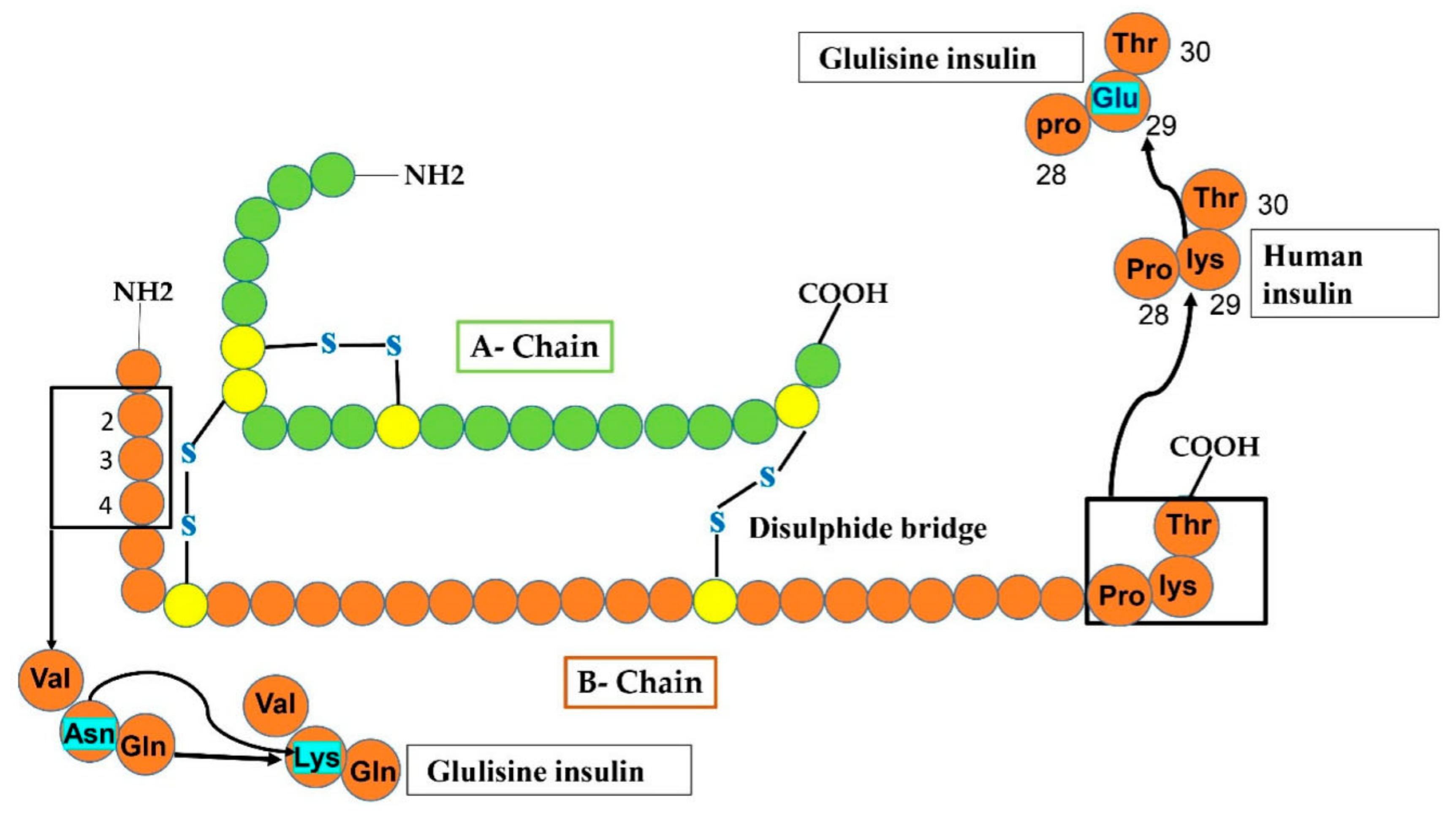
4.3. Insulin Glulisine
5. Insulin Preparations Having Long Term-Action
5.1. Insulin Glargine
5.2. Insulin Glargine U300 (Gla-300)
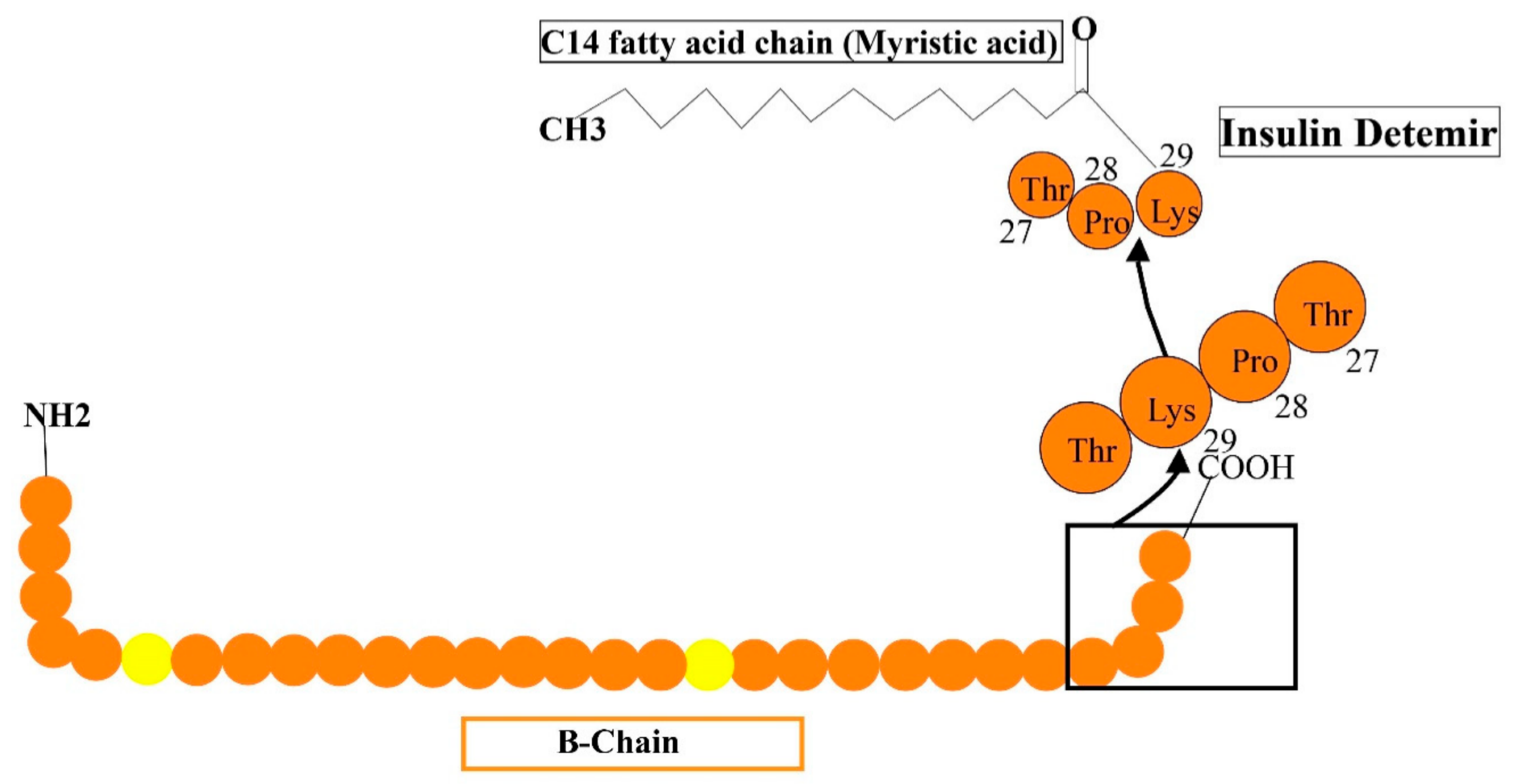
5.3. Insulin Detemir
5.4. Insulin Degludec
6. Fusion of Insulin Preparations (Two-Phase Insulins)
7. Positive and Negative Impacts of Insulin and Its Analogs Usage against TIDM
7.1. Influences of Fast-Acting Insulins in Comparison to the Human Regular Insulin
7.2. Influences of Long-Lasting Insulin Preparations
- Detemir insulin in comparison to NPH: The outcomes obtained after treatment with detemir or NPH insulins reveal the identical extent of HbA1c/FPS (fasting plasma sugar) [80,81,82,83,84], since detemir is correlated with the lesser possibility of hypoglycemia [81,83,84,85,86], and involving in night-time hypoglycemic evidence [80,81,82,84,85,86,87]. Analysis for two years observed somewhat lesser HbA1c extent through detemir therapy than NPH insulin, with additionally lesser FPG, primarily observed in an analysis of six months [85]. The weight reduction was usually seen after detemir therapy compared with the NPH insulin, an important feature of detemir insulin in individuals with type-I diabetes [80,82,83,84,85,86,87]. Some reports present administration of detemir for two times regularly more widespread than the subjection of once- regular detemir [88].
- Glargine-100 compared to Glargine-300: Glargine 300 shows similar glycemic control outcomes just like glargine-100, yet with rare nighttime hypoglycemic episodes. The Edition IV findings revealed comparable glycemic control through Gla-300 and Gla-100, but in the earlier eight weeks of therapy, the nighttime or acute hypoglycemic incidences were lesser and attained less weight with a variation of −0.6 kg through Gla-300 treatment [89]. However, after expanding six-months of Edition IV testing observed similar glucose control in both therapy categories and the same hypoglycemic evidence with Gla-100 and Gla-300 [90].
- Degludec insulin in comparison to glargine insulin: In many findings, the comparable decline in hemoglobin A1c [91,92,93,94,95] and gain of weight [93,94] was observed in subjects treated with Degludec in contrast to subjects who were treated with Gla-100. Anyhow, it was declared that treatment with Degludec showed identical [93,95] or lesser throughout hypoglycemic events and lesser or rare night-time hypoglycemic events [91,92,94,95] in comparison with the Gla-100.
- d.
- Degludec insulin in comparison to the Detemir insulin: Certain face-to-face efforts have been made to compare the impacts of insulin degludec and detemir. Certain analysis declared that the outcome of both degludec and detemir in decreasing HbA1c was similar within 26 and 52 weeks [104,105,106]. In one analysis during this duration, the decline in FPG was substantially higher with degludec treatment [105] but not at 52 weeks in an alternative study [107]. Furthermore, it was declared that there was also substantially rare evidence of nighttime hypoglycemia per subject-year through degludec therapy in comparison with detemir. Yet, the comparable rate of entire accustomed episodes of hypoglycemia per subject-year during 26 and 52 weeks were obtained for both preparations [104,105,106,107].
7.3. Monitoring of Hemoglobin (Hb) A1c through Insulin Analogs
7.4. Consequences of Insulin Preparations Regarding Special Considerations
7.4.1. Children and Teenagers
7.4.2. Pregnant Women
7.4.3. Aged People
8. Side Effects of Using Insulin Analogs (Negative Aspects)
9. Some Obstacles Faced by Patients with Diabetes through Insulin Therapy
10. The Introduction of Other Methods for Insulin Delivery to Fight against These Challenges
11. Upcoming Directions Concerning Insulin Therapy
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Polonsky, K.; Given, B.; Van Cauter, E. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J. Clin. Investig. 1988, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Owerbach, D.; Bell, G.I.; Rutter, W.J.; Brown, J.A.; Shows, T.B. The insulin gene is located on the short arm of chromosome 11 in humans. Diabetes 1981, 30, 267–270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walker, M.D.; Edlund, T.; Boulet, A.M.; Rutter, W.J. Cell-specific expression controlled by the 5′-flanking region of insulin and chymotrypsin genes. Nature 1983, 306, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Rouille, Y.; Gong, Q.; Martin, S.; Carroll, R.; Chan, S. The role of prohormone convertases in insulin biosynthesis: Evidence for inherited defects in their action in man and experimental animals. Diabetes Metab. 1996, 22, 94–104. [Google Scholar]
- Liu, M.; Ramos-Castañeda, J.; Arvan, P. Role of the connecting peptide in insulin biosynthesis. J. Biol. Chem. 2003, 278, 14798–14805. [Google Scholar] [CrossRef]
- Campbell, R.K.; Campbell, L.K.; White, J.R. Insulin lispro: Its role in the treatment of diabetes mellitus. Ann. Pharmacother. 1996, 30, 1263–1271. [Google Scholar] [CrossRef]
- Holleman, F.; Hoekstra, J.B. Insulin lispro. N. Engl. J. Med. 1997, 337, 176–183. [Google Scholar] [CrossRef]
- Fukumoto, H.; Seino, S.; Imura, H.; Seino, Y.; Eddy, R.L.; Fukushima, Y.; Byers, M.G.; Shows, T.B.; Bell, G.I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc. Natl. Acad. Sci. USA 1988, 85, 5434–5438. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; pp. 905–911. [Google Scholar]
- Ginter, E.; Simko, V. Global prevalence and future of diabetes mellitus. In Diabetes; Springer: New York, NY, USA, 2013; pp. 35–41. [Google Scholar]
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Group, I.D.A. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2011. Diabetes Res. Clin. Pract. 2013, 100, 277–279. [Google Scholar]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Porte, D. β-cells in type II diabetes mellitus. Diabetes 1991, 40, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Nosek, L.; Roepstorff, C.; Chenji, S.; Klein, O.; Haahr, H. Distinct prandial and basal glucose-lowering effects of insulin degludec/insulin aspart (IDegAsp) at steady state in subjects with type 1 diabetes mellitus. Diabetes Ther. 2014, 5, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Donner, T.; Sarkar, S. Insulin–Pharmacology, Therapeutic Regimens, and Principles of Intensive Insulin Therapy; Endotext. MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Mathieu, C.; Gillard, P.; Benhalima, K. Insulin analogues in type 1 diabetes mellitus: Getting better all the time. Nat. Rev. Endocrinol. 2017, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, M.A.; Dhayalan, B.; Rege, N.; Chatterjee, D.; Weiss, M.A. ‘Smart’ insulin-delivery technologies and intrinsic glucose-responsive insulin analogues. Diabetologia 2021, 64, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, M.A.; Dhayalan, B.; Chen, Y.-S.; Chatterjee, D.; Varas, N.; Weiss, M.A. Structural principles of insulin formulation and analog design: A century of innovation. Mol. Metab. 2021, 52, 101325. [Google Scholar] [CrossRef]
- Kurtzhals, P.; Nishimura, E.; Haahr, H.; Høeg-Jensen, T.; Johansson, E.; Madsen, P.; Sturis, J.; Kjeldsen, T. Commemorating insulin’s centennial: Engineering insulin pharmacology towards physiology. Trends Pharmacol. Sci. 2021, 42, 620–639. [Google Scholar] [CrossRef]
- Home, P. The evolution of insulin therapy. Diabetes Res. Clin. Pract. 2021, 175, 108816. [Google Scholar] [CrossRef]
- Mathieu, C.; Martens, P.-J.; Vangoitsenhoven, R. One hundred years of insulin therapy. Nat. Rev. Endocrinol. 2021, 17, 715–725. [Google Scholar] [CrossRef]
- Rodbard, H.W.; Rodbard, D. Biosynthetic human insulin and insulin analogs. Am. J. Ther. 2020, 27, e42–e51. [Google Scholar] [CrossRef]
- Vajo, Z.; Fawcett, J.; Duckworth, W.C. Recombinant DNA technology in the treatment of diabetes: Insulin analogs. Endocrin. Rev. 2001, 22, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Taneja, G.; Kumar, A.; Sahu, M.; Sharma, G.; Kumar, A.; Sardana, S.; Deep, A. Insulin analogs: Glimpse on contemporary facts and future prospective. Life Sci. 2019, 219, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mosekilde, E.; Jensen, K.S.; Binder, C.; Pramming, S.; Thorsteinsson, B. Modeling absorption kinetics of subcutaneous injected soluble insulin. J. Pharm. Biopharm. 1989, 17, 67–87. [Google Scholar] [CrossRef]
- Heise, T.; Pieber, T. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes. Metab 2007, 9, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Tildesley, H.; Chiasson, J.-L.; Tsui, E.; Strack, T. Insulin lispro in CSII: Results of a double-blind crossover study. Diabetes 1997, 46, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Howey, D.C.; Bowsher, R.R.; Brunelle, R.; Woodworth, J.R. A Rapidly absorbed analogue of human insulin. Diabetes 1994, 43, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Burge, M.R.; Castillo, K.R.; Schade, D.S. Meal composition is a determinant of lispro-induced hypoglycemia in IDDM. Diabetes Care 1997, 20, 152–155. [Google Scholar] [CrossRef]
- Rys, P.; Pankiewicz, O.; Łach, K.; Kwaskowski, A.; Skrzekowska-Baran, I.; Malecki, M. Efficacy and safety comparison of rapid-acting insulin aspart and regular human insulin in the treatment of type 1 and type 2 diabetes mellitus: A systematic review. Diabetes Metab. 2011, 37, 190–200. [Google Scholar] [CrossRef]
- Quianzon, C.C.; Cheikh, I. History of insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 18701. [Google Scholar] [CrossRef]
- Sanlioglu, A.D.; Altunbas, H.A.; Balci, M.K.; Griffith, T.S.; Sanlioglu, S. Clinical utility of insulin and insulin analogs. Islets 2013, 5, 67–78. [Google Scholar] [CrossRef]
- Home, P. The pharmacokinetics and pharmacodynamics of rapid-acting insulin analogues and their clinical consequences. Diabetes Obes. Metab. 2012, 14, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Barriocanal, L.; Lindholm, A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur. J. Clin. Pharmacol. 1991, 55, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Homko, C.; Deluzio, A.; Jimenez, C.; Kolaczynski, J.W.; Boden, G. Comparison of insulin aspart and lispro: Pharmacokinetic and metabolic effects. Diabetes Care 2003, 26, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Plank, J.; Wutte, A.; Brunner, G.; Siebenhofer, A.; Semlitsch, B.; Sommer, R.; Hirschberger, S.; Pieber, T.R. A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care 2002, 25, 2053–2057. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P.; PLoSker, G.L. Insulin Glulisine. Drugs 2009, 69, 1035–1057. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.P.; Stickelmeyer, M.P.; Flora, D.B.; Chance, R.E.; Frank, B.H.; DeFelippis, M.R. Self-association properties of monomeric insulin analogs under formulation conditions. Pharm. Res. 1998, 15, 1434–1441. [Google Scholar] [CrossRef]
- Zoete, V.; Meuwly, M.; Karplus, M. Study of the insulin dimerization: Binding free energy calculations and per-residue free energy decomposition. Proteins Struct. Funct. Bioinform. 2005, 61, 79–93. [Google Scholar] [CrossRef]
- Brunner, G.; Sendlhofer, G.; Wutte, A.; Ellmerer, M.; Søgaard, B.; Siebenhofer, A.; Hirschberger, S.; Krejs, G.; Pieber, T. Pharmacokinetic and pharmacodynamic properties of long-acting insulin analogue NN304 in comparison to NPH insulin in humans. Exp. Clin. Endocrinol. 2000, 108, 100–105. [Google Scholar] [CrossRef]
- Heise, T.; Nosek, L.; Rønn, B.B.; Endahl, L.; Heinemann, L.; Kapitza, C.; Draeger, E. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 2004, 53, 1614–1620. [Google Scholar] [CrossRef]
- Porcellati, F.; Bolli, G.B.; Fanelli, C.G. Pharmacokinetics and pharmacodynamics of basal insulins. Diabetes Technol. Therapeut. 2011, 13, S-15–S-24. [Google Scholar] [CrossRef]
- Rosenstock, J.; Fonseca, V.; McGill, J.; Riddle, M.; Hallé, J.-P.; Hramiak, I.; Johnston, P.; Davis, M. Similar progression of diabetic retinopathy with insulin glargine and neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes: A long-term, randomised, open-label study. Diabetologia 2009, 52, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Investigators, O.T. Rationale, design, and baseline characteristics for a large international trial of cardiovascular disease prevention in people with dysglycemia: The ORIGIN Trial (Outcome Reduction with an Initial Glargine Intervention). Am. Heart J. 2008, 155, 26.e1–26.e13. [Google Scholar]
- Bolli, G.; Consoli, A.; Giaccari, A. Early insulin treatment in type 2 diabetes: ORIGINal sin or valuable choice as ORIGINal treatment? An open debate on the ORIGIN study results. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Bohn, B.; Bramlage, P.; Wagner, C.; Kaltheuner, M.; Welp, R.; Sziegoleit, S.; Zimmermann, A.; Reuter, H.M.; Hummel, M.; Gloyer, J. Which patients from routine care use the new insulin analogue glargine U300 compared to patients with glargine U100: A multicenter analysis of 14,123 patients with insulin glargine from die diabetes registries DPV and DIVE. Wien. Med. Wochenschr. (1946) 2017, 168, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Hurren, K.M.; O’Neill, J.L. Pharmacodynamic and pharmacokinetic evaluation of insulin glargine U300 for the treatment of type 1 diabetes. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.; White, J.R., Jr. New insulin glargine 300 U/mL for the treatment of type 1 and type 2 diabetes mellitus. Ann. Pharmacother. 2015, 49, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Munshi, M.N.; Gill, J.; Chao, J.; Nikonova, E.; Patel, M. Insulin glargine 300 U/mL is associated with less weight gain while maintaining glycemic control and low risk of hypoglycemia compared with insulin glargine 100 U/mL in an aging population with type 2 diabetes. Endocr. Pract. 2018, 24, 143–149. [Google Scholar] [CrossRef]
- Ritzel, R.; Roussel, R.; Giaccari, A.; Vora, J.; Brulle-Wohlhueter, C.; Yki-Järvinen, H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs. glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 541–548. [Google Scholar] [CrossRef]
- Hermansen, K.; Davies, M.; Derezinski, T.; Ravn, G.M.; Clauson, P.; Home, P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006, 29, 1269–1274. [Google Scholar] [CrossRef]
- Haahr, H.; Heise, T. A review of the pharmacological properties of insulin degludec and their clinical relevance. Clin. Pharmacoki. 2014, 53, 787–800. [Google Scholar] [CrossRef]
- Korsatko, S.; Deller, S.; Koehler, G.; Mader, J.K.; Neubauer, K.; Adrian, C.L.; Thomsen, H.; Haahr, H.; Pieber, T.R. A comparison of the steady-state pharmacokinetic and pharmacodynamic profiles of 100 and 200 U/mL formulations of ultra-long-acting insulin degludec. Clin. Drug Investig. 2013, 33, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Radermecker, R.; Scheen, A. Biphasic insulin aspart (NovoMix 50). Rev. Med. Liege 2008, 63, 688–692. [Google Scholar] [PubMed]
- Vaag, A.; Lund, S.S. Insulin initiation in patients with type 2 diabetes mellitus: Treatment guidelines, clinical evidence and patterns of use of basal vs premixed insulin analogues. Eur. J. Endocrinol. 2012, 166, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Raedler, L.A. Tresiba (insulin Degludec injection) and Ryzodeg 70/30 (insulin Degludec and insulin Aspart injection): Two new insulin analogs for glycemic control in diabetes mellitus. Am. Health Drug Benefits 2016, 9, 144. [Google Scholar] [PubMed]
- Deacon, C.; Lebovitz, H.E. Comparative review of dipeptidyl peptidase-4 inhibitors and sulphonylureas. Diabetes Obes. Metab. 2016, 18, 333–347. [Google Scholar] [CrossRef]
- Dudkowski, C.; Tsai, M.; Liu, J.; Zhao, Z.; Schmidt, E.; Xie, J. The pharmacokinetics and pharmacodynamics of alogliptin in children, adolescents, and adults with type 2 diabetes mellitus. Eur. J. Clin. Pharmacol. 2017, 73, 279–288. [Google Scholar] [CrossRef]
- Egan, A.G.; Blind, E.; Dunder, K.; de Graeff, P.A.; Hummer, B.T.; Bourcier, T.; Rosebraugh, C. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N. Engl. J. Med. 2014, 370, 794–797. [Google Scholar] [CrossRef]
- Binder, C. Absorption of Injected Insulin: A Clinical-Pharmacological Study. Acta Pharmacol. Toxicol. 1969, 27, 1–83. [Google Scholar] [CrossRef]
- Owens, D.R. Human Insulin: Clinical Pharmacological Studies in Normal Man; Springer Science & Business Media: Berlin, Germany, 1986. [Google Scholar]
- Houlden, R. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada: Introduction. Can. J. Diabetes 2018, 42, S162–S167. [Google Scholar] [CrossRef]
- Laranjeira, F.O.; de Andrade, K.R.; Figueiredo, A.C.; Silva, E.N.; Pereira, M.G. Long-acting insulin analogues for type 1 diabetes: An overview of systematic reviews and meta-analysis of randomized controlled trials. PLoS ONE 2018, 13, e0194801. [Google Scholar] [CrossRef]
- Tricco, A.C.; Ashoor, H.M.; Antony, J.; Beyene, J.; Veroniki, A.A.; Isaranuwatchai, W.; Harrington, A.; Wilson, C.; Tsouros, S.; Soobiah, C. Safety, effectiveness, and cost effectiveness of long acting versus intermediate acting insulin for patients with type 1 diabetes: Systematic review and network meta-analysis. BMJ 2014, 349, g5459. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Ahmad, F.; Lal, A.; Yu, C.; Bai, Z.; Bennett, H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: A meta-analysis. Can. Med. Assoc. J. 2009, 180, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Renner, R.; Pfützner, A.; Trautmann, M.; Harzer, O.; Sauter, K.; Landgraf, R. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Results of a multicenter trial. German Humalog-CSII Study Group. Diabetes Care 1999, 22, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Muggeo, M.; Cattin, L.; Arcangeli, A.; Pozzilli, P.; Provenzano, V.; Francesconi, A.; Calatola, P.; Santeusanio, F. Incidence of severe nocturnal hypoglycemia in patients with type 1 diabetes treated with insulin lispro or regular human insulin in addition to basal insulin glargine. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 519–526. [Google Scholar] [CrossRef]
- Holleman, F.; Schmitt, H.; Rottiers, R.; Rees, A.; Symanowski, S.; Anderson, J.H.; Group, B.-U.I.L.S. Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. Diabetes Care 1997, 20, 1827–1832. [Google Scholar] [CrossRef]
- Heller, S.R.; Amiel, S.A.; Mansell, P. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. UK Lispro Study Group. Diabetes Care 1999, 22, 1607–1611. [Google Scholar] [CrossRef]
- Home, P.; Lindholm, A.; Riis, A.f.; Group, E.I.A.S. Insulin aspart vs. human insulin in the management of long-term blood glucose control in type 1 diabetes mellitus: A randomized controlled trial. Diabet. Med. 2000, 17, 762–770. [Google Scholar] [CrossRef]
- Raskin, P.; Guthrie, R.A.; Leiter, L.; Riis, A.; Jovanovic, L. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000, 23, 583–588. [Google Scholar] [CrossRef]
- Tamás, G.; Marre, M.; Astorga, R.; Dedov, I.; Jacobsen, J.; Lindholm, A.; Group, I.A.S. Glycaemic control in type 1 diabetic patients using optimised insulin aspart or human insulin in a randomised multinational study. Diabetes Res. Clin. Pract. 2001, 54, 105–114. [Google Scholar] [CrossRef]
- Home, P.; Hallgren, P.; Usadel, K.; Sane, T.; Faber, J.; Grill, V.; Friberg, H. Pre-meal insulin aspart compared with pre-meal soluble human insulin in type 1 diabetes. Diabetes Res. Clin. Pract. 2006, 71, 131–139. [Google Scholar] [CrossRef]
- DeVries, J.; Lindholm, A.; Jacobsen, J.; Heine, R.; Home, P.; Group, T.C.I.A.S. A randomized trial of insulin aspart with intensified basal NPH insulin supplementation in people with Type 1 diabetes. Diabet. Med. 2003, 20, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Rosenstock, J.; Ways, K. Optimized basal-bolus insulin regimens in type 1 diabetes: Insulin glulisine versus regular human insulin in combination with basal insulin glargine. Endorine Pract. 2005, 11, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, M.; Prager, R.; Robinson, A.; Busch, K.; Ellis, G.; Souhami, E.; Van Leendert, R. Efficacy and safety of insulin glulisine in patients with type 1 diabetes. Horm. Metab. Res. 2005, 37, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, K.; Sukumar, N.; Rafnsson, S.B.; Saravanan, P. Efficacy and safety of rapid-acting insulin analogs in special populations with type 1 diabetes or gestational diabetes: Systematic review and meta-analysis. Diabetes Ther. 2018, 9, 891–917. [Google Scholar] [CrossRef] [PubMed]
- Van Bon, A.C.; Bode, B.W.; Sert-Langeron, C.; DeVries, J.H.; Charpentier, G. Insulin glulisine compared to insulin aspart and to insulin lispro administered by continuous subcutaneous insulin infusion in patients with type 1 diabetes: A randomized controlled trial. Diabetes Technol. Ther. 2011, 13, 607–614. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw, I.; Vague, P.; Selam, J.L.; Skeie, S.; Lang, H.; Draeger, E.; Elte, J. Insulin detemir used in basal-bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes. Metab. 2005, 7, 73–82. [Google Scholar] [CrossRef]
- Kølendorf, K.; Ross, G.P.; Pavlic-Renar, I.; Perriello, G.; Philotheou, A.; Jendle, J.; Gall, M.A.; Heller, S. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in type 1 diabetes. Diabet. Med. 2006, 23, 729–735. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Simpson, R.; Hylleberg, B.; Draeger, E.; Bolinder, J. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin. Therapeut. 2004, 26, 724–736. [Google Scholar] [CrossRef]
- Standl, E.; Lang, H.; Roberts, A. The 12-month efficacy and safety of insulin detemir and NPH insulin in basal-bolus therapy for the treatment of type 1 diabetes. Diabetes Technol. Therapeut. 2004, 6, 579–588. [Google Scholar] [CrossRef]
- Vague, P.; Selam, J.-L.; Skeie, S.; De Leeuw, I.; Elte, J.W.; Haahr, H.; Kristensen, A.; Draeger, E. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care 2003, 26, 590–596. [Google Scholar] [CrossRef]
- Bartley, P.; Bogoev, M.; Larsen, J.; Philotheou, A. Long-term efficacy and safety of insulin detemir compared to neutral protamine Hagedorn insulin in patients with Type 1 diabetes using a treat-to-target basal–bolus regimen with insulin aspart at meals: A 2-year, randomized, controlled trial. Diabet. Med. 2008, 25, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, K.; Fontaine, P.; Kukolja, K.; Peterkova, V.; Leth, G.; Gall, M.-A. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004, 47, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Bartley, P.; Russell-Jones, D.; Hanaire-Broutin, H.; Heeg, J.-E.; Abrams, P.; Landin-Olsson, M.; Hylleberg, B.; Lang, H.; Draeger, E. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: A randomized clinical trial. Diabetes Care 2004, 27, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, H.; Jacques, R.; Gardner, K.; Amiel, S.; Mansell, P. Twice-rather than once-daily basal insulin is associated with better glycaemic control in type 1 diabetes mellitus 12 months after skills-based structured education in insulin self-management. Diabet. Med. 2015, 32, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Home, P.D.; Bergenstal, R.M.; Bolli, G.B.; Ziemen, M.; Rojeski, M.; Espinasse, M.; Riddle, M.C. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabtes: A randomized, phase 3a, open-label clinical trial (EDITION 4). Diabetes Care 2015, 38, 2217–2225. [Google Scholar] [CrossRef]
- Home, P.D.; Bergenstal, R.M.; Bolli, G.B.; Ziemen, M.; Rojeski, M.; Espinasse, M.; Riddle, M.C. Glycaemic control and hypoglycaemia during 12 months of randomized treatment with insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 1 diabetes (EDITION 4). Diabetes Obes. Metab. 2018, 20, 121–128. [Google Scholar] [CrossRef]
- Birkeland, K.I.; Home, P.D.; Wendisch, U.; Ratner, R.E.; Johansen, T.; Endahl, L.A.; Lyby, K.; Jendle, J.H.; Roberts, A.P.; DeVries, J.H. Insulin degludec in type 1 diabetes: A randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care 2011, 34, 661–665. [Google Scholar] [CrossRef]
- Bode, B.; Buse, J.; Fisher, M.; Garg, S.; Marre, M.; Merker, L.; Renard, E.; Russell-Jones, D.; Hansen, C.; Rana, A.J.D.m. Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal–bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN® Basal–Bolus Type 1): 2-year results of a randomized clinical trial. Diabet. Med. 2013, 30, 1293–1297. [Google Scholar] [CrossRef]
- Gough, S.; Harris, S.; Woo, V.; Davies, M. Insulin degludec: Overview of a novel ultra long-acting basal insulin. Diabetes Obes. Metab. 2013, 15, 301–309. [Google Scholar] [CrossRef]
- Lane, W.; Bailey, T.S.; Gerety, G.; Gumprecht, J.; Philis-Tsimikas, A.; Hansen, C.T.; Nielsen, T.S.; Warren, M. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: The SWITCH 1 randomized clinical trial. JAMA 2017, 318, 33–44. [Google Scholar] [CrossRef]
- Mathieu, C.; Hollander, P.; Miranda-Palma, B.; Cooper, J.; Franek, E.; Russell-Jones, D.; Larsen, J.; Tamer, S.C.; Bain, S.C.; Investigators, N.-T. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): A 26-week randomized, treat-to-target trial with a 26-week extension. J. Clin. Endocrinol. Metab. 2013, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Vora, J.; Cariou, B.; Evans, M.; Gross, J.L.; Harris, S.; Landstedt-Hallin, L.; Mithal, A.; Rodriguez, M.R.; Meneghini, L. Clinical use of insulin degludec. Diabetes Res. Clin. Pract. 2015, 109, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J.; King, A.B.; Del Prato, S.; Sreenan, S.; Balci, M.K.; Muñoz-Torres, M.; Rosenstock, J.; Endahl, L.A.; Francisco, A.M.O.; Hollander, P.J.T.L. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): A phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012, 379, 1498–1507. [Google Scholar] [CrossRef]
- Zinman, B.; Fulcher, G.; Rao, P.V.; Thomas, N.; Endahl, L.A.; Johansen, T.; Lindh, R.; Lewin, A.; Rosenstock, J.; Pinget, M.J.T.L. Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes: A 16-week, randomised, open-label, phase 2 trial. Lancet 2011, 377, 924–931. [Google Scholar] [CrossRef]
- Jonassen, I.; Havelund, S.; Hoeg-Jensen, T.; Steensgaard, D.B.; Wahlund, P.-O.; Ribel, U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm. Res. 2012, 29, 2104–2114. [Google Scholar] [CrossRef] [PubMed]
- Wakil, A.; Atkin, S.L.J.E.o.o.b.t. Insulin degludec–a new-generation basal insulin. Expert Opin. Biol. Ther. 2012, 12, 539–542. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Bailey, C.J.; Barnett, A.H. Insulin degludec: A new ultra-longacting insulin. Lancet 2012, 379, 1465–1467. [Google Scholar] [CrossRef]
- Traynor, K. Insulin degludec products approved for diabetes. Am. J. Health Syst. Pharm. 2015, 72, 1834. [Google Scholar] [CrossRef]
- Davies, M.; Sasaki, T.; Gross, J.; Bantwal, G.; Ono, Y.; Nishida, T.; Tojjar, D.; Seino, H.J.D. Comparison of insulin degludec with insulin detemir in type 1 diabetes: A 1-year treat-to-target trial. Diabetes Obes. Metab. 2016, 18, 96–99. [Google Scholar] [CrossRef]
- Davies, M.J.; Gross, J.; Ono, Y.; Sasaki, T.; Bantwal, G.; Gall, M.; Niemeyer, M.; Seino, H.; BEGIN BB T1 study group. Efficacy and safety of insulin degludec given as part of basal–bolus treatment with mealtime insulin aspart in type 1 diabetes: A 26-week randomized, open-label, treat-to-target non-inferiority trial. Diabetes Obes. Metab. 2014, 16, 922–930. [Google Scholar] [CrossRef]
- Hirsch, I.B.; Bode, B.; Courreges, J.-P.; Dykiel, P.; Franek, E.; Hermansen, K.; King, A.; Mersebach, H.; Davies, M. Insulin degludec/insulin aspart administered once daily at any meal, with insulin aspart at other meals versus a standard basal-bolus regimen in patients with type 1 diabetes: A 26-week, phase 3, randomized, open-label, treat-to-target trial. Diabetes Care 2012, 35, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.; Franek, E.; Mersebach, H.; Bardtrum, L.; Hermansen, K. Safety and efficacy of insulin degludec/insulin aspart with bolus mealtime insulin aspart compared with standard basal–bolus treatment in people with Type 1 diabetes: 1–year results from a randomized clinical trial (BOOST® T1). Diabet. Med. 2017, 34, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.; Luzio, S.; Dunseath, G.; Pauvaday, V.; Mustafa, N.; Owens, D. Relationship between HbA1c and indices of glucose tolerance derived from a standardized meal test in newly diagnosed treatment naive subjects with type 2 diabetes. Diabet. Med. 2006, 23, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Woerle, H.J.; Neumann, C.; Zschau, S.; Tenner, S.; Irsigler, A.; Schirra, J.; Gerich, J.E.; Göke, B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res. Clin. Pract. 2007, 77, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Horvath, K.; Jeitler, K.; Berghold, A.; Ebrahim, S.H.; Gratzer, T.W.; Plank, J.; Kaiser, T.; Pieber, T.R.; Siebenhofer, A. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2007, 2, CD005613. [Google Scholar] [CrossRef] [PubMed]
- Plank, J.; Siebenhofer, A.; Berghold, A.; Jeitler, K.; Horvath, K.; Mrak, P.; Pieber, T. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch. Intern. Med. 2005, 165, 1337–1344. [Google Scholar] [CrossRef]
- Koivisto, V.A.; Tuominen, J.A.; Ebeling, P.J.D.C. Lispro Mix25 insulin as premeal therapy in type 2 diabetic patients. Diabetes Care 1999, 22, 459–462. [Google Scholar] [CrossRef]
- Roach, P.; Yue, L.; Arora, V. Improved postprandial glycemic control during treatment with Humalog Mix25, a novel protamine-based insulin lispro formulation. Humalog Mix25 Study Group. Diabetes Care 1999, 22, 1258–1261. [Google Scholar] [CrossRef]
- Yamada, S.; Watanabe, M.; Kitaoka, A.; Shiono, K.; Atsuda, K.; Tsukamoto, Y.; Kawana, Y.; Irie, J. Switching from premixed human insulin to premixed insulin lispro: A prospective study comparing the effects on glucose control and quality of life. Int. Med. 2007, 46, 1513–1517. [Google Scholar] [CrossRef]
- Boehm, B.; Home, P.; Behrend, C.; Kamp, N.; Lindholm, A. Premixed insulin aspart 30 vs. premixed human insulin 30/70 twice daily: A randomized trial in type 1 and type 2 diabetic patients. Diabet. Med. 2002, 19, 393–399. [Google Scholar] [CrossRef]
- Mortensen, H.; Kocova, M.; Teng, L.Y.; Keiding, J.; Bruckner, I.; Philotheou, A. Biphasic insulin aspart vs. human insulin in adolescents with type 1 diabetes on multiple daily insulin injections. Pediatr. Diabetes 2006, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Schmoelzer, I.; De Campo, A.; Pressl, H.; Stelzl, H.; Dittrich, P.; Oettl, K.; Wascher, T. Biphasic insulin aspart compared to biphasic human insulin reduces postprandial hyperlipidemia in patients with type 2 diabetes. Exp. Clin. Endocrinol. 2005, 113, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Reiterer, E.; Ong, K.; Regan, F.; Salzano, G.; Acerini, C.; Dunger, D. A randomized cross-over trial to identify the optimal use of insulin glargine in prepubertal children using a three-times daily insulin regimen. Diabet. Med. 2007, 24, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Schober, E.; Schoenle, E.; Van Dyk, J.; Wernicke-Panten, K. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2002, 15, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.; Schoenle, E.; Gucev, Z.; Mordhorst, L.; Gall, M.A.; Ludvigsson, J. Insulin detemir compared with NPH insulin in children and adolescents with type 1 diabetes. Diabet. Med. 2007, 24, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, E.; Swan, K.L.; Tamborlane, W.V.; Sherr, J.L.; Martin, M.; Weinzimer, S.A. The alteration of aspart insulin pharmacodynamics when mixed with detemir insulin. Diabetes Care 2012, 35, 690–692. [Google Scholar] [CrossRef]
- Cengiz, E.; Tamborlane, W.V.; Martin-Fredericksen, M.; Dziura, J.; Weinzimer, S.A. Early pharmacokinetic and pharmacodynamic effects of mixing lispro with glargine insulin: Results of glucose clamp studies in youth with type 1 diabetes. Diabetes Care 2010, 33, 1009–1012. [Google Scholar] [CrossRef]
- Mathiesen, E.R.; Hod, M.; Ivanisevic, M.; Garcia, S.D.; Brøndsted, L.; Jovanovič, L.; Damm, P.; McCance, D.R. Maternal efficacy and safety outcomes in a randomized, controlled trial comparing insulin detemir with NPH insulin in 310 pregnant women with type 1 diabetes. Diabetes Care 2012, 35, 2012–2017. [Google Scholar] [CrossRef]
- Hod, M.; Mathiesen, E.R.; Jovanovič, L.; McCance, D.R.; Ivanisevic, M.; Durán-Garcia, S.; Brøndsted, L.; Nazeri, A.; Damm, P. A randomized trial comparing perinatal outcomes using insulin detemir or neutral protamine Hagedorn in type 1 diabetes. J. Matern.-Fetal Neonatal Med. 2014, 27, 7–13. [Google Scholar] [CrossRef]
- Pantalone, K.M.; Faiman, C.; Olansky, L. Insulin glargine use during pregnancy. Endocr. Pract. 2011, 17, 448–455. [Google Scholar] [CrossRef]
- McElduff, A.; Moses, R.G. Insulin therapy in pregnancy. Endocrinol. Metab. Clin. 2012, 41, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Pandya, N.; DiGenio, A.; Gao, L.; Patel, M. Efficacy and safety of insulin glargine compared to other interventions in younger and older adults: A pooled analysis of nine open-label, randomized controlled trials in patients with type 2 diabetes. Drugs Aging 2013, 30, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Malek, R.; Gonzalez-Galvez, G.; El Naggar, N.; Shah, S.; Prusty, V.; Litwak, L. Safety and Effectiveness of Insulin Detemir in Different Age-Groups in the A 1 chieve Study. Diabetes Ther. 2013, 4, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Kuroe, A.; Taniuguchi, A.; Fukushima, M.; Nakai, Y.; Ohgushi, M.; Ohya, M.; Seino, Y. Early and late onset side effects of short-acting insulin analogue in seven Japanese diabetic patients. Diabetes Res. Clin. Pract. 2007, 77, 412–413. [Google Scholar] [CrossRef]
- Heller, S.; Colagiuri, S.; Vaaler, S.; Wolffenbuttel, B.; Koelendorf, K.; Friberg, H.; Windfeld, K.; Lindholm, A. Hypoglycaemia with insulin aspart: A double-blind, randomised, crossover trial in subjects with Type 1 diabetes. Diabet. Med. 2004, 21, 769–775. [Google Scholar] [CrossRef]
- Simpson, D.; McCormack, P.L.; Keating, G.M.; Lyseng-Williamson, K.A. Insulin Lispro. Drugs 2007, 67, 407–434. [Google Scholar] [CrossRef]
- Chantelau, E.; Lee, D.; Hemmann, D.; Zipfel, U.; Echterhoff, S. What makes insulin injections painful? Br. Med. J. 1991, 303, 26. [Google Scholar] [CrossRef]
- Atlan-Gepner, C.; Bongrand, P.; Farnarier, C.; Xerri, L.; Choux, R.; Gauthier, J.-F.; Brue, T.; Vague, P.; Grob, J.-J.; Vialettes, B. Insulin-Induced Lipoatrophy in Type I Diabetes: A possible tumor necrosis factor-α-mediated dedifferentiation of adipocytes. Diabetes Care 1996, 19, 1283–1285. [Google Scholar] [CrossRef]
- Gallwitz, B.; Westrup, D.; Schmeisl, G. Significance of insulin analogues in the treatment of people with type 2 diabetes. Dtsch. Med. Wochenschr. 2014, 139, 2199–2203. [Google Scholar]
- Grunberger, G. Insulin analogs—Are they worth it? Yes! Diabetes Care 2014, 37, 1767–1770. [Google Scholar] [CrossRef]
- Davidson, M.B. Insulin analogs—Is there a compelling case to use them? No! Diabetes Care 2014, 37, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Laffel, L. American Diabetes Association Transitions Working GroupDiabetes care for emerging adults: Recommendations for transition from pediatric to adult diabetes care systems: A position statement of the American diabetes association, with representation by the American College of osteopathic family physicians, the American Academy of pediatrics, the American association of clinical endocrinologists, the American osteopathic association, the centers for disease control and prevention, children with diabetes, the endocrine Society, the International Society for pediatric and adolescent diabetes, juvenile diabetes research Foundation international, the National diabetes education program, and the pediatric endocrine Society (formerly Lawson Wilkins pediatric endocrine Society). Diabetes Care 2011, 34, 2477–2485. [Google Scholar] [PubMed]
- Silverstein, J.; Klingensmith, G.; Copeland, K.; Plotnick, L.; Kaufman, F.; Laffel, L.; Deeb, L.; Grey, M.; Anderson, B.; Holzmeister, L.A. Care of children and adolescents with type 1 diabetes: A statement of the American Diabetes Association. Diabetes Care 2005, 28, 186–212. [Google Scholar] [CrossRef] [PubMed]
- Crasto, W.; Jarvis, J.; Khunti, K.; Davies, M. New insulins and new insulin regimens: A review of their role in improving glycaemic control in patients with diabetes. Postgrad. Med. J. 2009, 85, 257–267. [Google Scholar] [CrossRef]
- Home, P.; Riddle, M.; Cefalu, W.T.; Bailey, C.J.; Bretzel, R.G.; Del Prato, S.; Leroith, D.; Schernthaner, G.; van Gaal, L.; Raz, I. Insulin therapy in people with type 2 diabetes: Opportunities and challenges? Diabetes Care 2014, 37, 1499–1508. [Google Scholar] [CrossRef]
- Knutsen, P.G.; Voelker, C.Q.; Nikkel, C.C. Clinical insights into a new, disposable insulin delivery device. Diabetes Spectr. 2015, 28, 209–213. [Google Scholar] [CrossRef]
- American Diabetes Association. 7. Diabetes technology: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43, S77–S88. [Google Scholar] [CrossRef]
- BD. Diabetes Products. BD Diabetes Prod. 2019. Available online: https://www.bd.com/en-uk/products/diabetes/diabetes-products (accessed on 15 June 2021).
- Kesavadev, J.; Saboo, B.; Krishna, M.B.; Krishnan, G.J.D.T. Evolution of insulin delivery devices: From syringes, pens, and pumps to DIY artificial pancreas. Diabetes Ther. 2020, 11, 1251–1269. [Google Scholar] [CrossRef]
- Fry, A. Insulin delivery device technology 2012: Where are we after 90 years? J. Diabetes Sci. Technol. 2012, 6, 947–953. [Google Scholar] [CrossRef]
- Shaw, K.F.; Valdez, C.A. Development and implementation of a U-500 regular insulin program in a federally qualified health center. Clin. Diabetes 2017, 35, 162–167. [Google Scholar] [CrossRef][Green Version]
- Shah, R.B.; Patel, M.; Maahs, D.M.; Shah, V.N. Insulin delivery methods: Past, present and future. Int. J. Pharm. Res. 2016, 6, 1. [Google Scholar] [CrossRef]
- Zambanini, A.; Newson, R.B.; Maisey, M.; Feher, M.D. Injection related anxiety in insulin-treated diabetes. Diabetes Res. Clin. Pract. 1999, 46, 239–246. [Google Scholar] [CrossRef]
- Fu, A.Z.; Qiu, Y.; Radican, L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr. Med. Res. Opin. 2009, 25, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-W.; Sonaje, K.; Liao, Z.-X.; Hsu, L.-W.; Chuang, E.-Y. pH-responsive nanoparticles shelled with chitosan for oral delivery of insulin: From mechanism to therapeutic applications. Acc. Chem. Res. 2012, 45, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.; Jacques, Y. Oral insulin and buccal insulin: A critical reappraisal. J. Diabetes Sci. Technol. 2009, 3, 568–584. [Google Scholar] [CrossRef] [PubMed]
- Hancu, N.; Czupryniak, L.; Genestin, E.; Sourij, H. A Pan-European and Canadian prospective survey to evaluate patient satisfaction with the SoloSTAR insulin injection device in type 1 and type 2 diabetes. J. Diabetes Sci. Technol. 2011, 5, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Halberg, I.B.; Lyby, K.; Wassermann, K.; Heise, T.; Zijlstra, E.; Plum-Mörschel, L. Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: A randomised, double-blind, phase 2 trial. Lancet Diabetes Endocrinol. 2019, 7, 179–188. [Google Scholar] [CrossRef]
- Akbari, V.; Hendijani, F.; Feizi, A.; Varshosaz, J.; Fakhari, Z.; Morshedi, S.; Mostafavi, S. Efficacy and safety of oral insulin compared to subcutaneous insulin: A systematic review and meta-analysis. J. Endocrinol. Investig. 2016, 39, 215–225. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanif, N.; Wu, H.; Xu, P.; Li, Y.; Bibi, A.; Zulfiqar, A.; Iqbal, M.Z.; Tahir, M.; Zhang, X.; Ali, A. Proteomic Changes to the Updated Discovery of Engineered Insulin and Its Analogs: Pros and Cons. Curr. Issues Mol. Biol. 2022, 44, 867-888. https://doi.org/10.3390/cimb44020059
Hanif N, Wu H, Xu P, Li Y, Bibi A, Zulfiqar A, Iqbal MZ, Tahir M, Zhang X, Ali A. Proteomic Changes to the Updated Discovery of Engineered Insulin and Its Analogs: Pros and Cons. Current Issues in Molecular Biology. 2022; 44(2):867-888. https://doi.org/10.3390/cimb44020059
Chicago/Turabian StyleHanif, Naeema, Hezhou Wu, Peizhou Xu, Yun Li, Amir Bibi, Asma Zulfiqar, Muhammad Zafar Iqbal, Muhammad Tahir, Xiangyang Zhang, and Asif Ali. 2022. "Proteomic Changes to the Updated Discovery of Engineered Insulin and Its Analogs: Pros and Cons" Current Issues in Molecular Biology 44, no. 2: 867-888. https://doi.org/10.3390/cimb44020059
APA StyleHanif, N., Wu, H., Xu, P., Li, Y., Bibi, A., Zulfiqar, A., Iqbal, M. Z., Tahir, M., Zhang, X., & Ali, A. (2022). Proteomic Changes to the Updated Discovery of Engineered Insulin and Its Analogs: Pros and Cons. Current Issues in Molecular Biology, 44(2), 867-888. https://doi.org/10.3390/cimb44020059







