Stimulatory Effects of Extracellular Vesicles Derived from Leuconostoc holzapfelii That Exists in Human Scalp on Hair Growth in Human Follicle Dermal Papilla Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Separation and Isolation of Microorganisms
2.2. 16S rRNA, pheS and atpA Gene Sequence and Phylogenetic Analysis
2.3. Preparation of Probiotic Bacteria
2.4. Isolation of Extracellular Vesicles
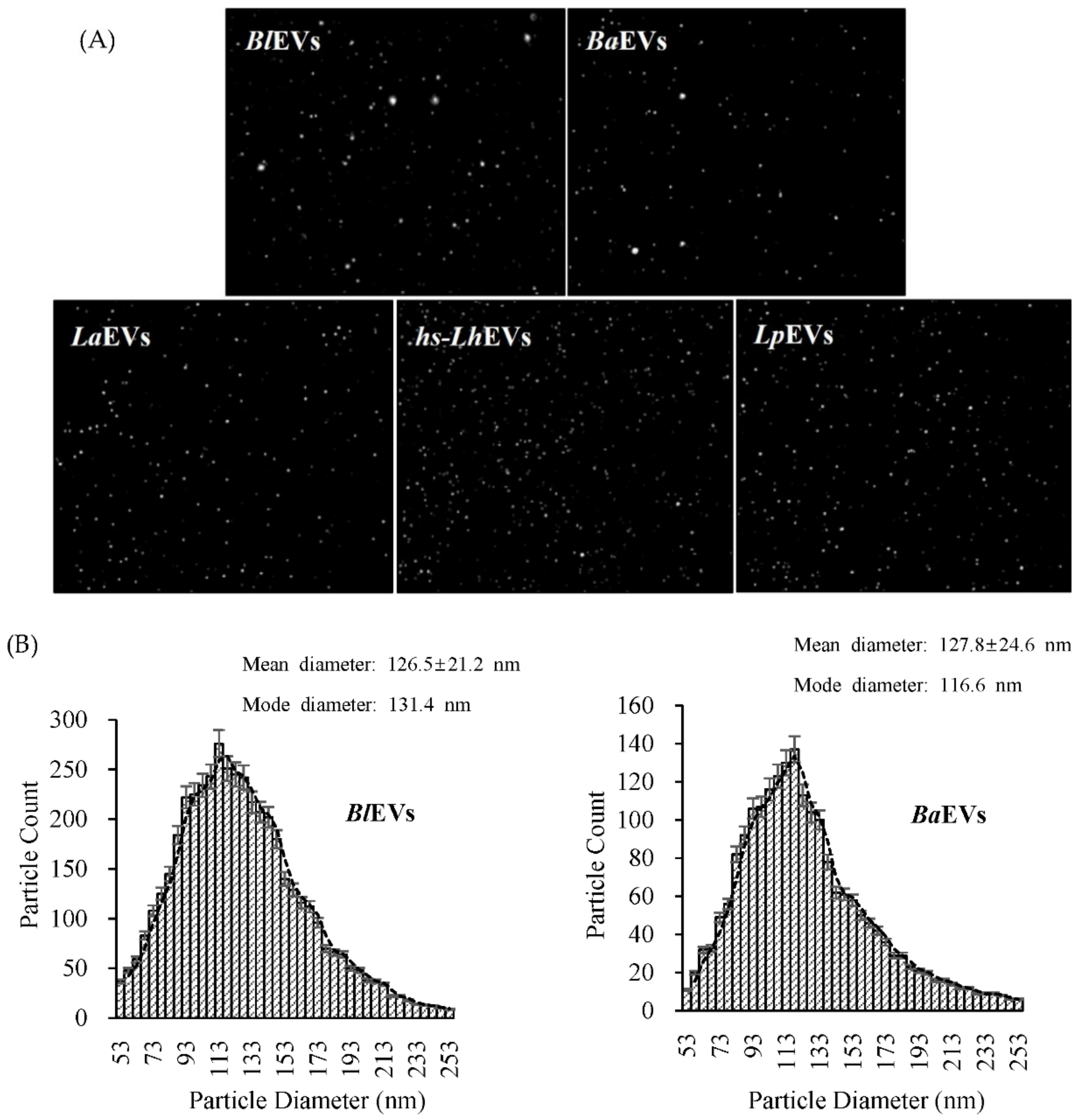
2.5. Nanoparticle Tracking Analysis (NTA)
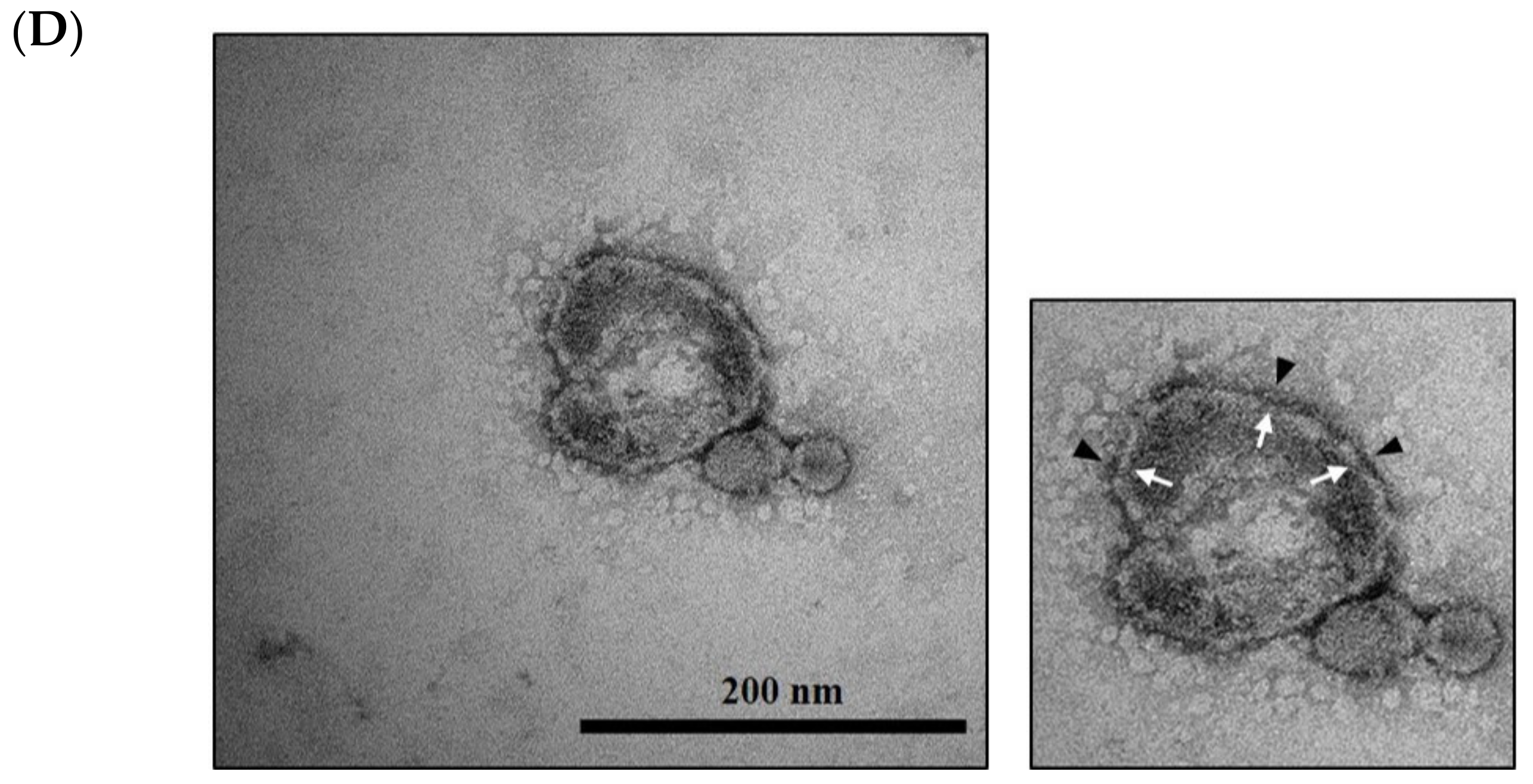
2.6. Cryo-TEM Analysis
2.7. Cell Culture and Proliferation Assay
2.8. Cell Viability Assay
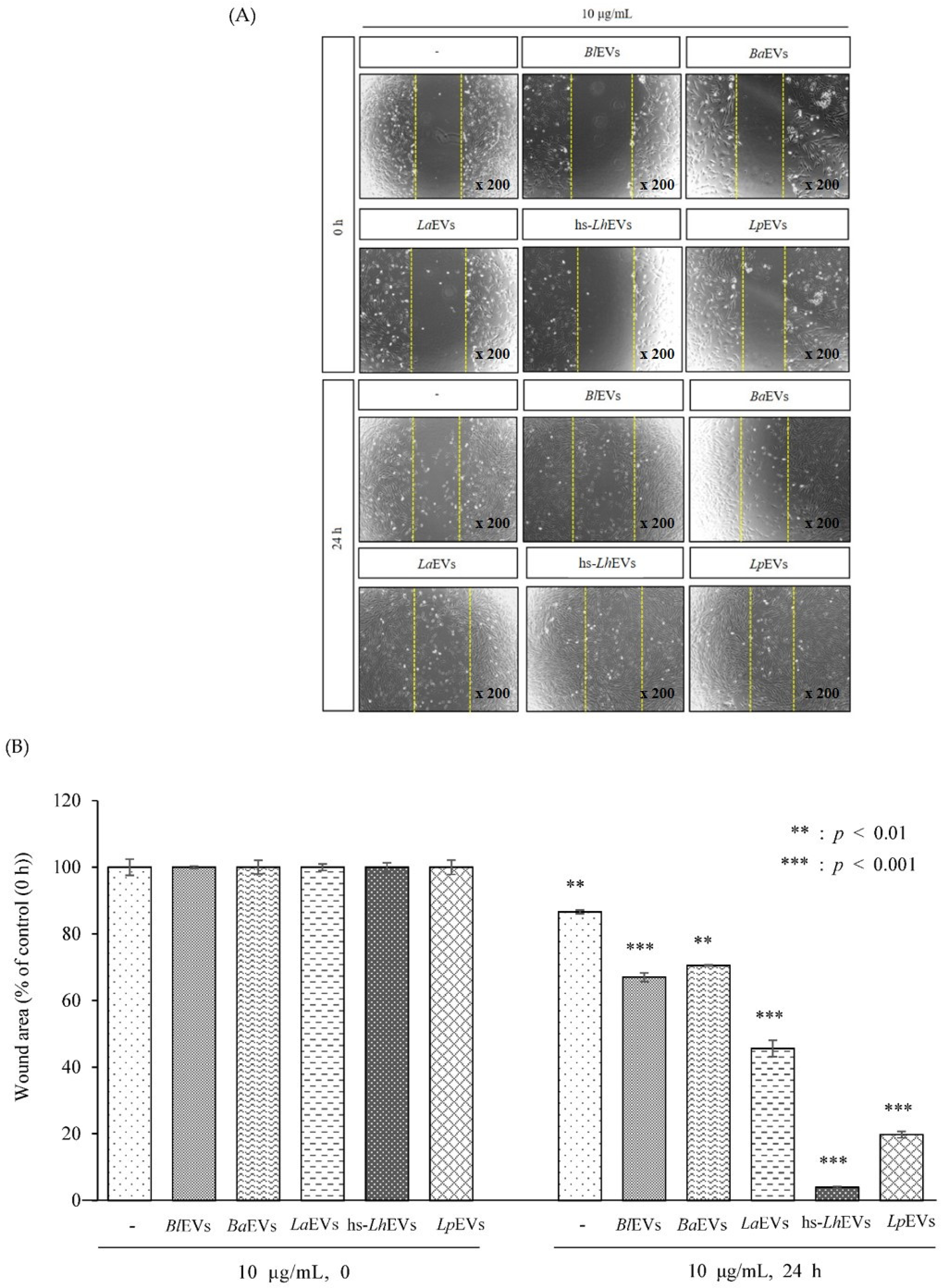
2.9. Wound Healing Assay
2.10. Propidium Iodide (P.I.) Staining and Cell Cycle Analysis
2.11. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.12. Statistical Analysis
3. Results
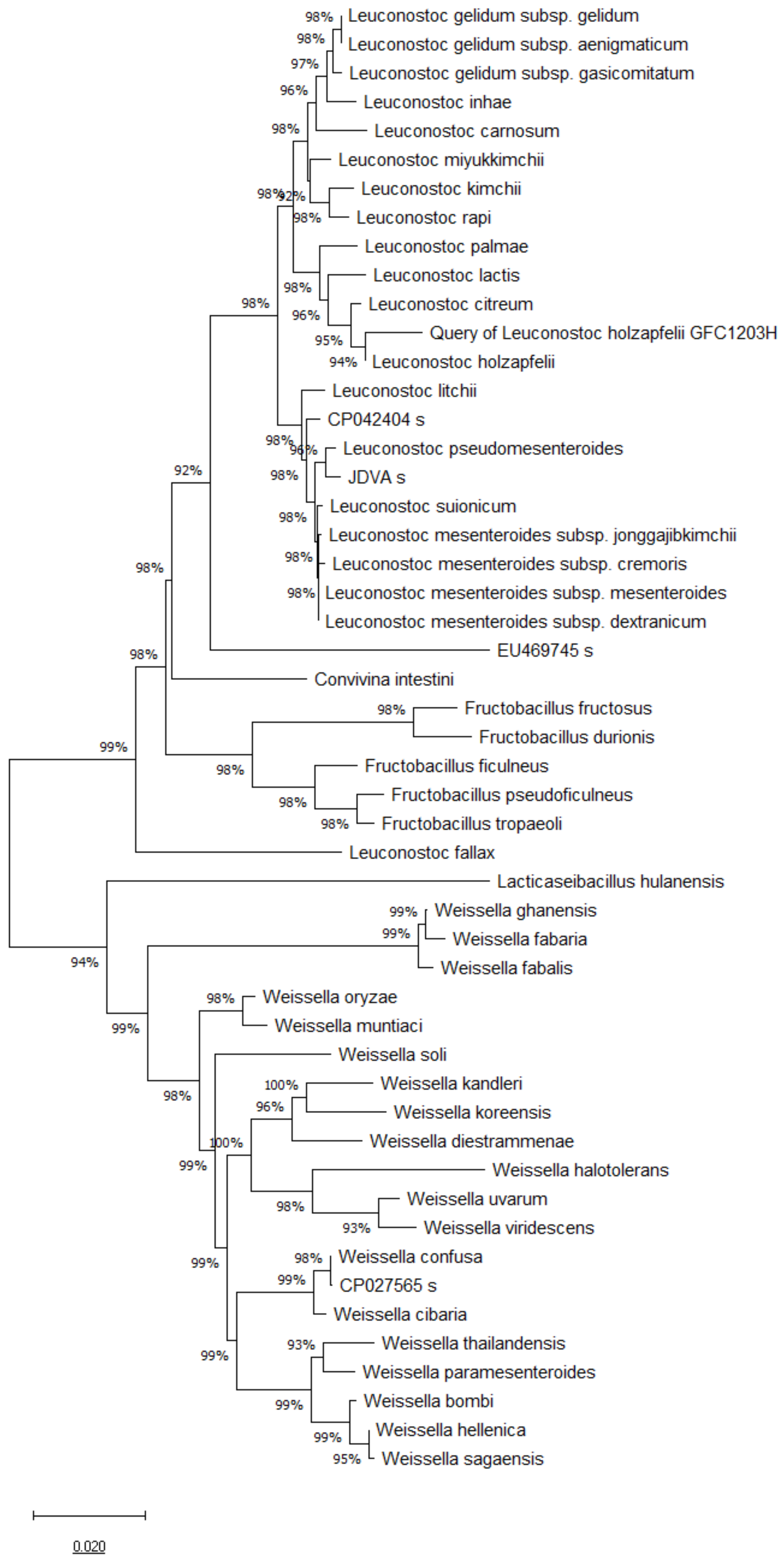
3.1. 16S rRNA, pheS and atpA Gene Sequence and Phylogenetic Analysis
3.2. Probiotic Bacteria Voluntarily Produce Extracellular Vesicles
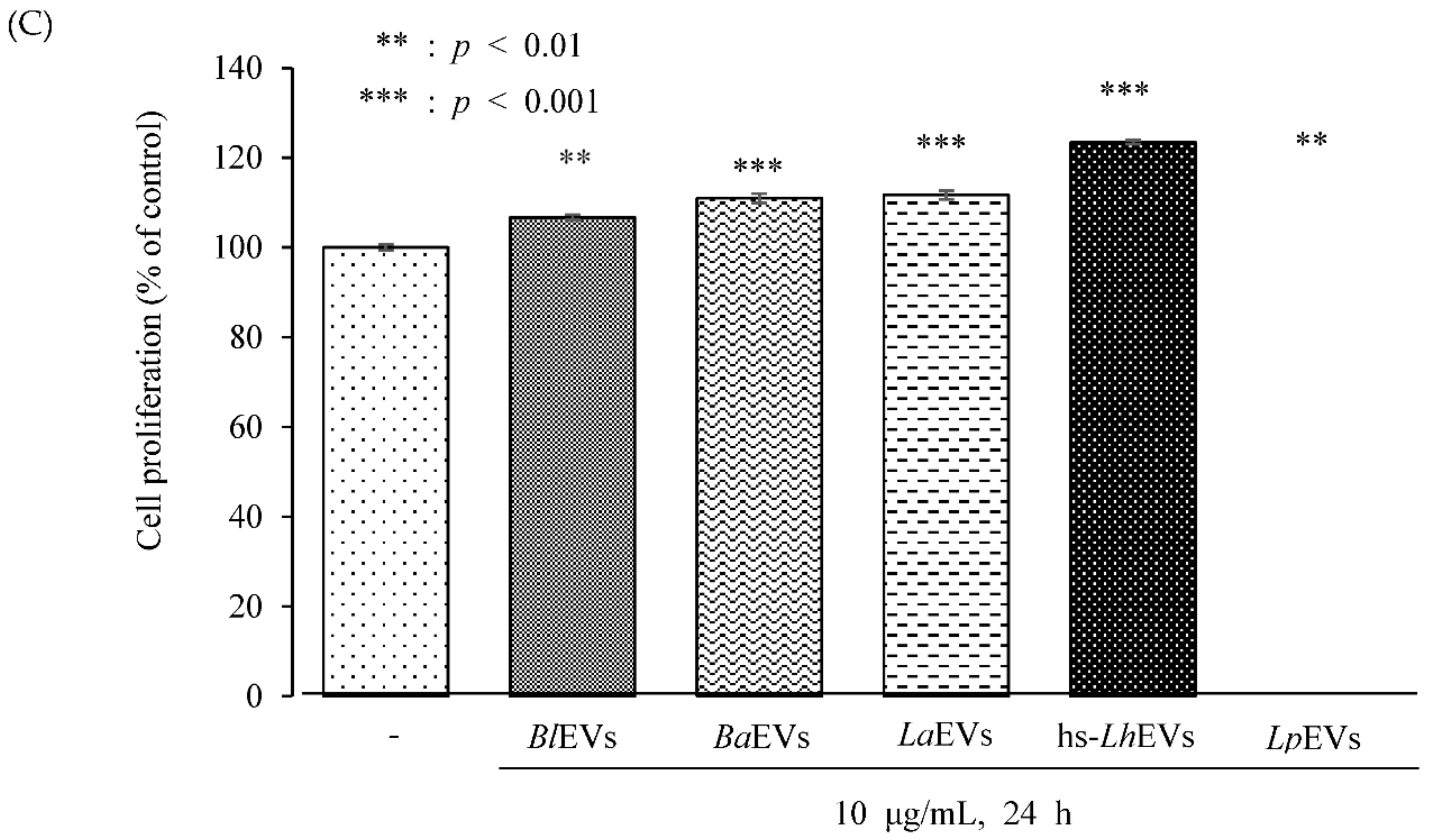
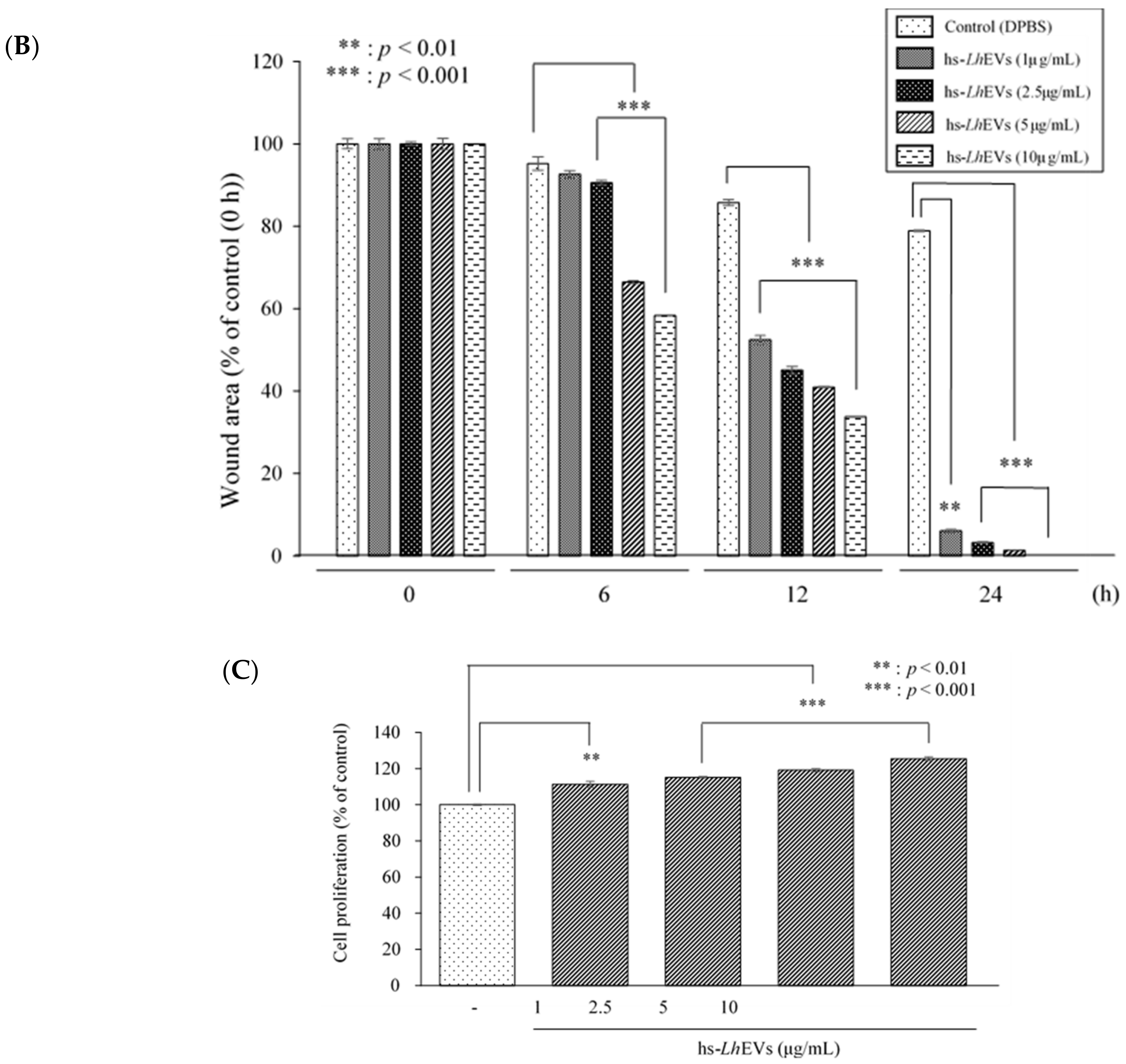
3.3. Probiotic Bacteria-EVs Induce HFDP Cell Migration and Proliferations
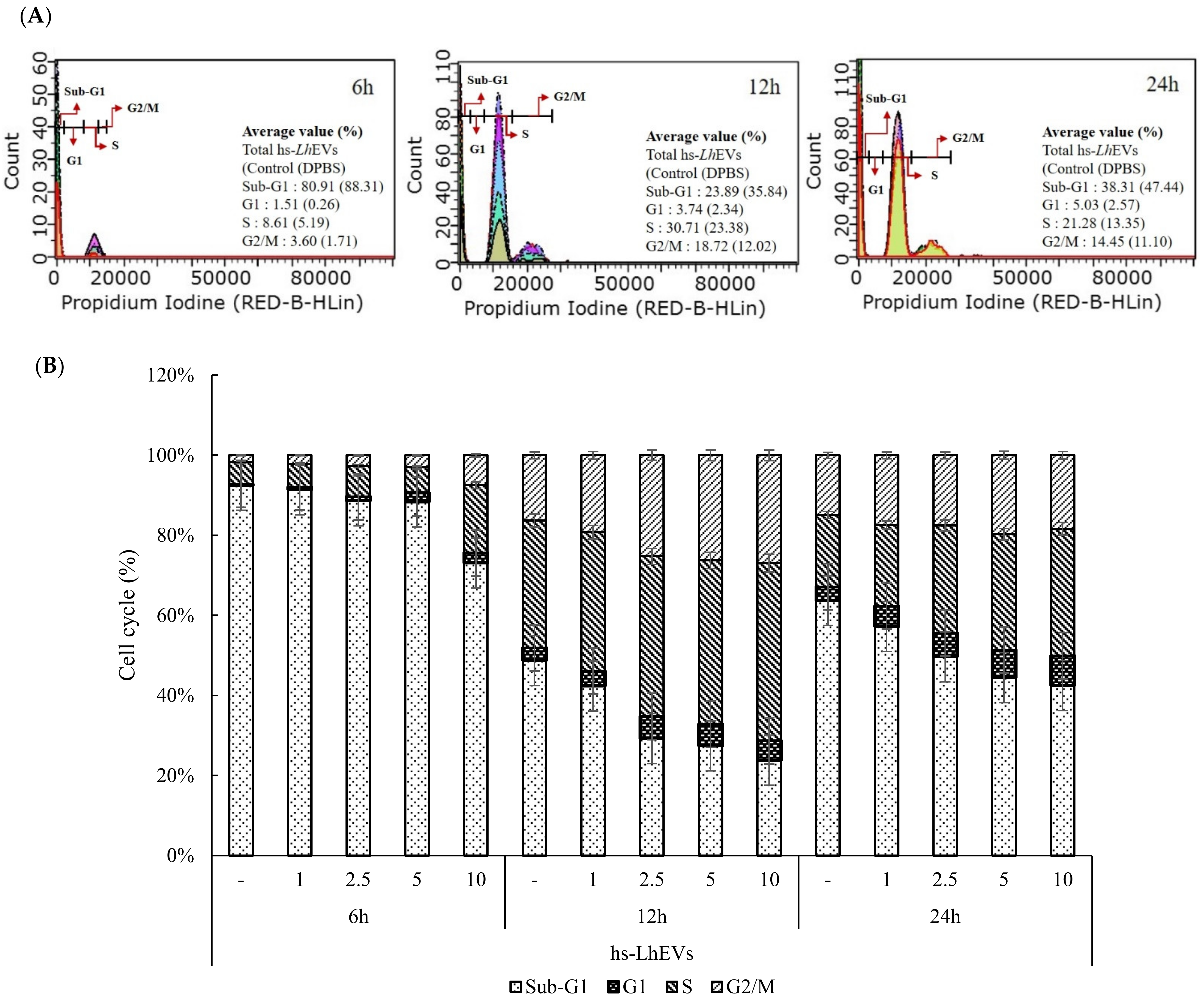
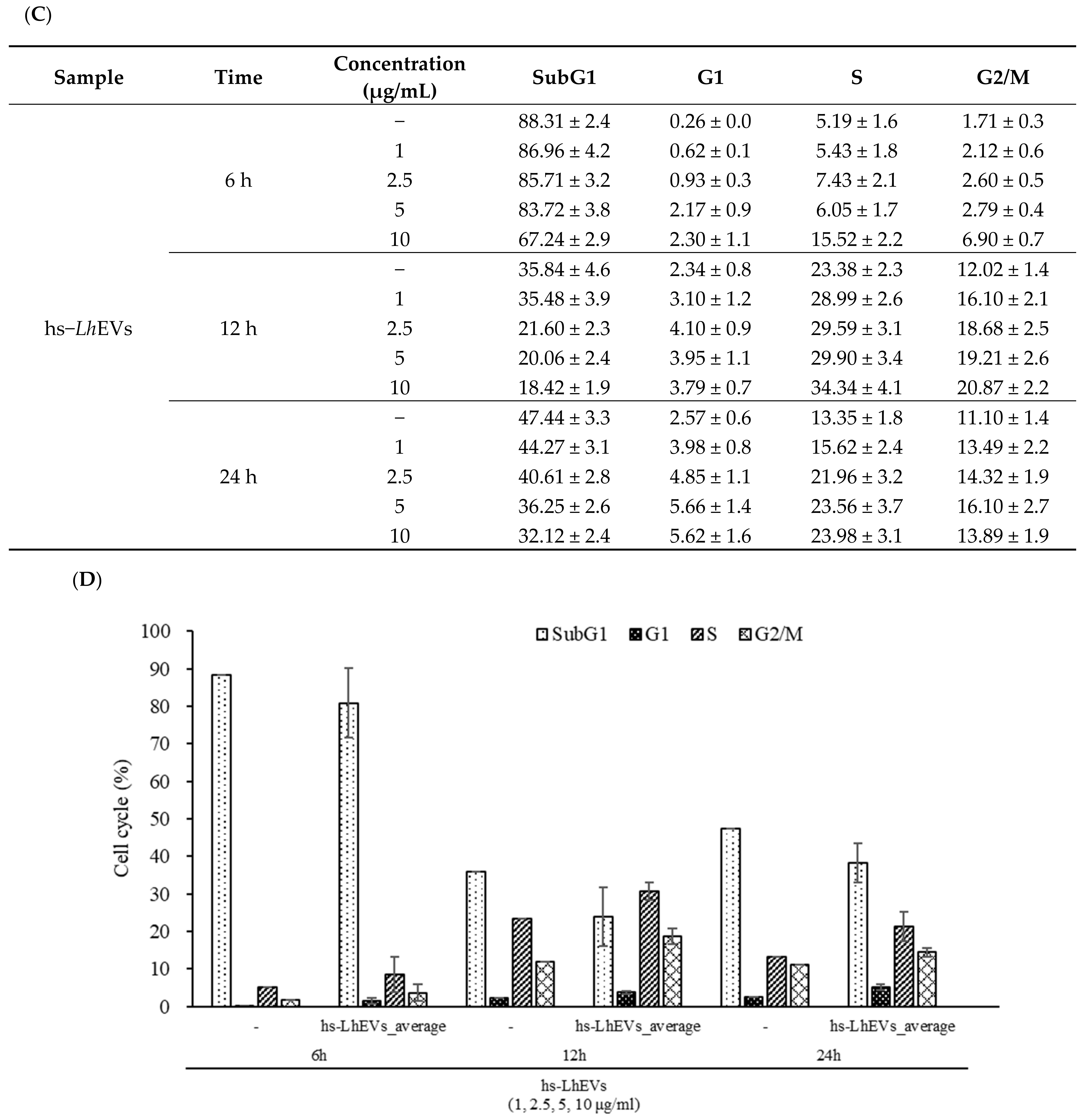
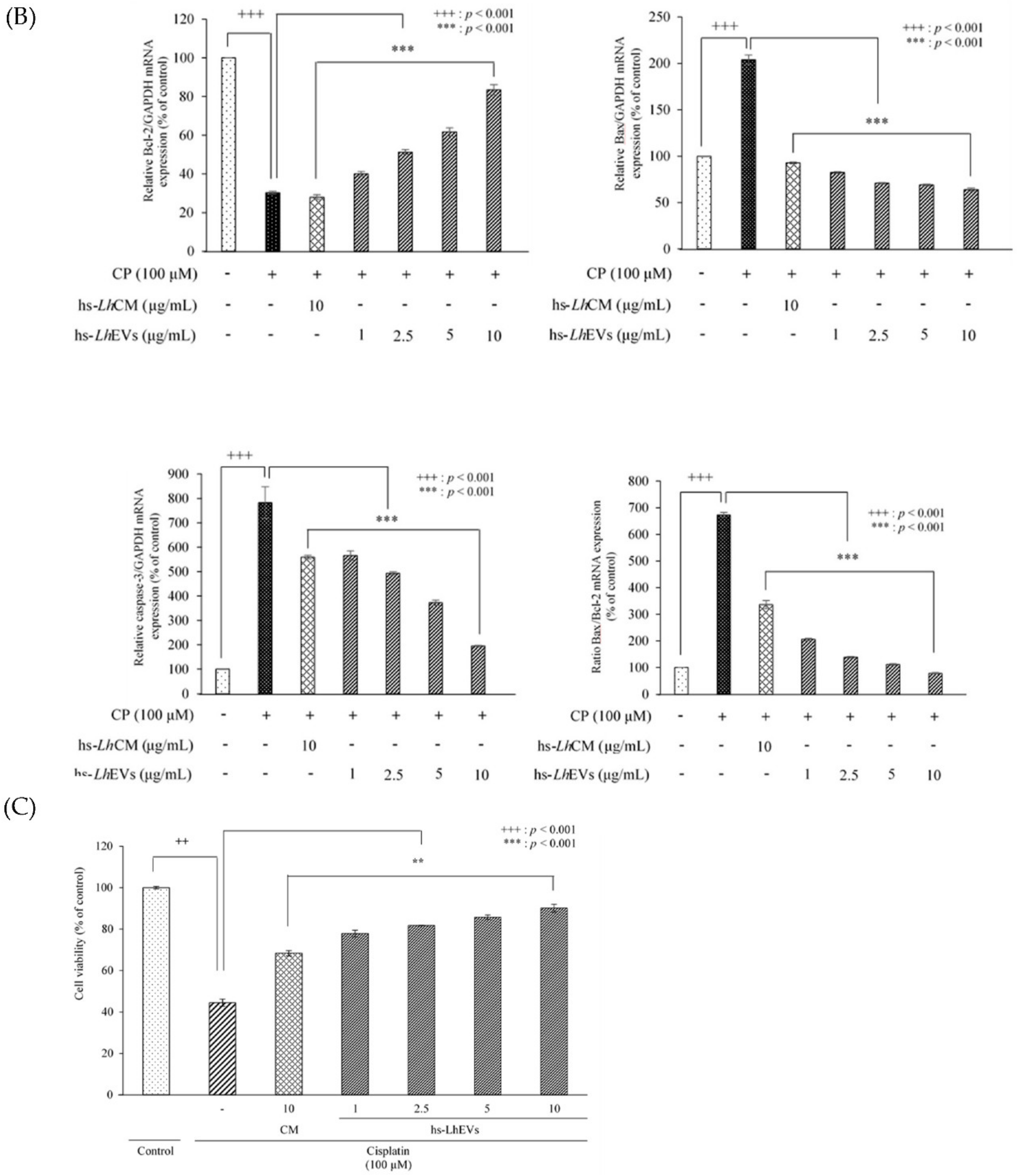
3.4. hs-LhEVs Inhibit Apoptosis and Induce Division via Control Cell Cycle in HFDP Cells
3.5. hs-LhEV Treatment Regulates Apoptosis-Associated mRNA Expression in Cisplatin-Induced HFDP Cells
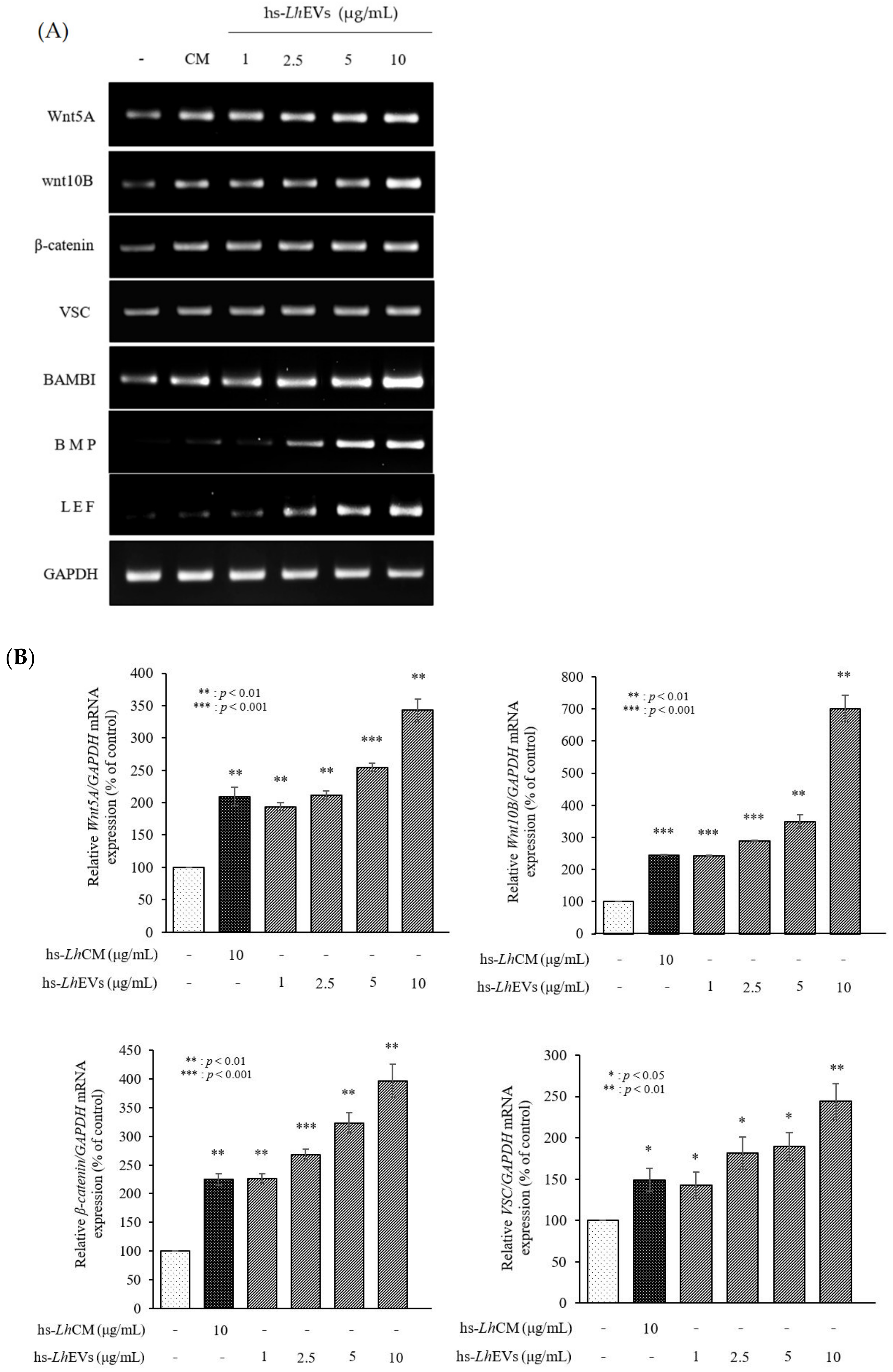
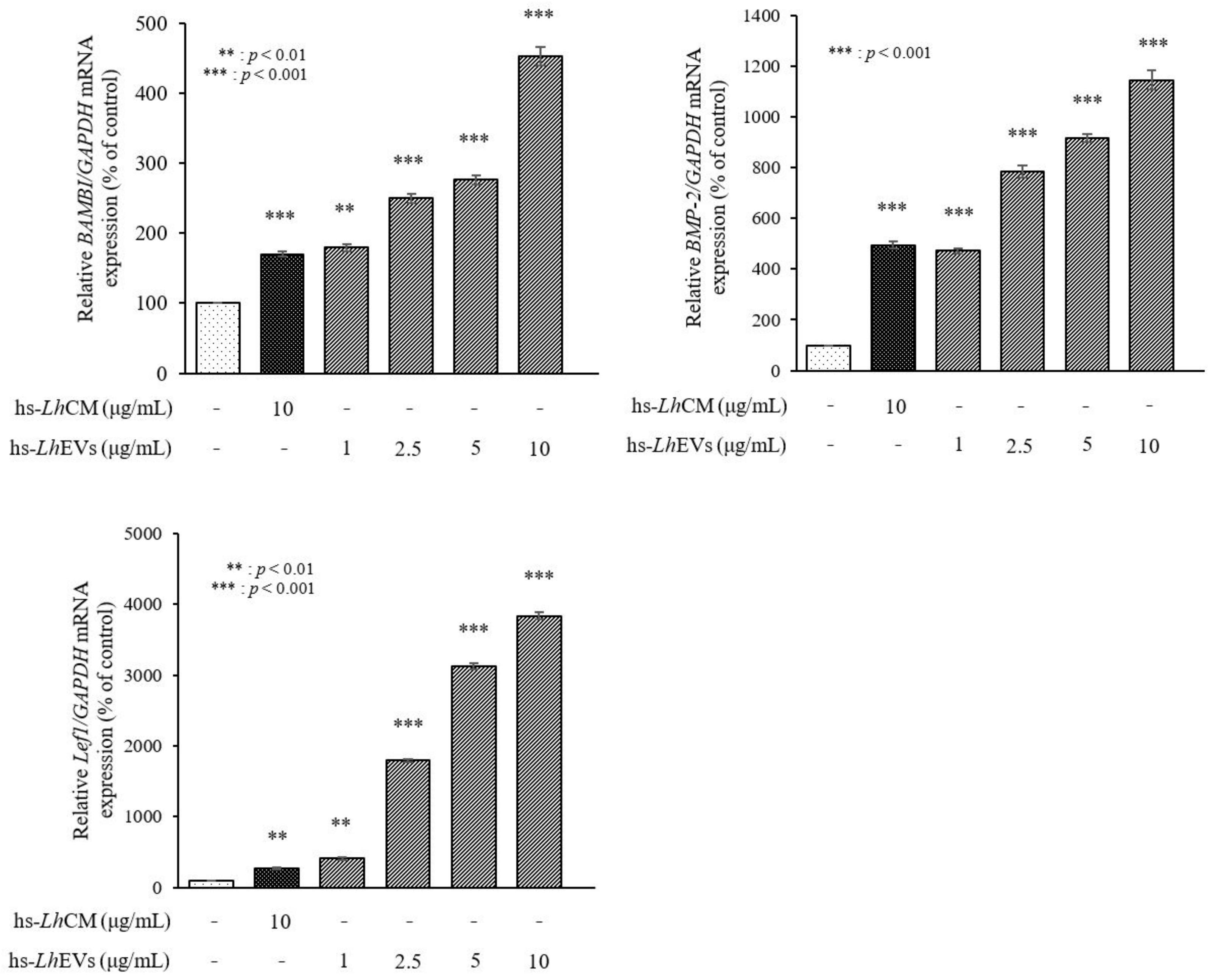
3.6. hs-LhEV Treatment Induces Hair-Inductive-Associated mRNA Activity in HFDP Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EVs | Extracellular vesicles |
| PB-EVs | Probiotic bacteria derived-extracellular vesicles |
| hs-Lh | Human scalp-derived Leuconostoc holzapfelii |
| hs-LhCM | Conditioned medium of Leuconostoc holzapfelii in human scalp |
| hs-LhEVs | Extracellular vesicles derived from Leuconostoc holzapfelii in human scalp |
| BAMBI | Activin membrane-bound inhibitor homolog |
| BMP2 | Bone morphogenetic protein 2 |
| Lef1 | Lymphoid enhancer-binding factor |
| VSC | Versican |
References
- Stenn, K.S. Molecular insights into the hair follicle and its pathology: A review of recent developments. Int. J. Dermatol. 2003, 42, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Nutbrown, M.; MacDonald Hull, S.P.; Baker, T.G.; Cunliffe, W.J.; Randall, V.A. Ultrastructural abnormalities in the dermal papillae of both lesional and clinically normal follicles from alopecia areata scalps. Br. J. Dermatol. 1996, 135, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hao, H.; Xia, L.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Han, Q.; Zhao, Y.; Liu, H.; et al. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci. Rep. 2014, 4, 5432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 2002, 118, 216–225. [Google Scholar] [CrossRef]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. Wnt signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Schlake, T.; Beibel, M.; Weger, N.; Boehm, T. Major shifts in genomic activity accompany progression through different stages of the hair cycle. Gene Expr. Patterns 2004, 4, 141–152. [Google Scholar] [CrossRef]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Krause, K.; Foitzik, K. Biology of the hair follicle: The basics. Semin. Cutan. Med. Surg. 2006, 25, 2–10. [Google Scholar] [CrossRef]
- Muller-Rover, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [Green Version]
- McElwee, K.J.; Shapiro, J.S. Promising therapies for treating and/or preventing androgenic alopecia. Ski. Ther. Lett. 2012, 17, 1–4. [Google Scholar]
- Lodi, G.; Sannino, M.; Cannarozzo, G.; Giudice, A.; Duca, E.D.; Tamburi, F.; Bennardo, L.; Nistico, S.P. Blue lights treatments have been proposed as additional methods of hair loss treatment. Lasers Med. Sci. 2021, 36, 1719–1723. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Glaser, R.L.; Dimitrakakis, C.; Messenger, A.G. Improvement in scalp hair growth in androgen-deficient women treated with testosterone: A questionnaire study. Br. J. Dermatol. 2012, 166, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Irwig, M.S.; Kolukula, S. Persistent sexual side effects of finasteride for male pattern hair loss. J. Sex. Med. 2011, 8, 1747–1753. [Google Scholar] [CrossRef]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [Green Version]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/BAX: A rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar]
- Oltvai, Z.N.; Korsmeyer, S.J. Checkpoints of dueling dimers foil death wishes. Cell 1994, 79, 189–192. [Google Scholar] [CrossRef]
- Wang, L.; Chanvorachote, P.; Toledo, D.; Stehlik, C.; Mercer, R.R.; Castranova, V.; Rojanasakul, Y. Peroxide is a key mediator of Bcl-2 down-regulation and apoptosis induction by cisplatin in human lung cancer cells. Mol. Pharmacol. 2008, 73, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.S.; Leon, M.D.; Devarajan, P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J. Am. Soc. Nephrol. 2002, 13, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Botchkarev, V.A. Molecular mechanisms of chemotherapy-induced hair loss. J. Investig. Derm. Symp. Proc. 2003, 8, 72–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apisarnthanarax, N.; Duvic, M. Dermatologic Complications of Cancer Chemotherapy. In Holland-Frei Cancer Medicine, 5th ed.; Bast, R.C., Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Holland, J.F., Frei, E., Eds.; BC Decker: Hamilton, ON, Canada, 2000; pp. 2271–2278. [Google Scholar]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.R.; Millar, S.E.; Cotsarelis, G. Wnt dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.J.; Dedhar, S.; Coetzee, G.A.; Nelson, C.C. Interaction of nuclear receptors with the Wnt/β-Catenin/Tcf signaling axis: Wnt you like to know? Endocr. Rev. 2005, 26, 898–915. [Google Scholar] [CrossRef]
- Van der Pol, E.; Coumans, F.A.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, L.; Yu, H.; McKenzie, A.J.; Franklin, J.L.; Patton, J.G.; Liu, Q.; Weaver, A.M. Quantitative Proteomic Analysis of Small and Large Extracellular Vesicles (EVs) Reveals Enrichment of Adhesion Proteins in Small EVs. J. Proteome Res. 2019, 18, 947–959. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Yuan, A.; Farber, E.L.; Rapoport, A.L.; Tejada, D.; Deniskin, R.; Akhmedov, N.B.; Farber, D.B. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS ONE 2009, 4, e4722. [Google Scholar] [CrossRef] [Green Version]
- Lässer, C.; Alikhani, V.S.; Ekström, K.; Eldh, M.; Paredes, P.T.; Bossios, A.; Sjöstrand, M.; Gabrielsson, S.; Lötvall, J.; Valadi, H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J. Transl. Med. 2011, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Van Niel, G.; D’Angelo, G.; Rapaso, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- De Vrese, M.; Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 2008, 111, 1–66. [Google Scholar]
- Kaci, G.; Lakhdari, O.; Dore, J.; Ehrlich, S.D.; Renault, P.; Blottière, H.M.; Delorme, C. Inhibition of the NF-kappa B pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl. Environ. Microbiol. 2011, 77, 4681–4684. [Google Scholar] [CrossRef] [Green Version]
- Kainulainen, V.; Tang, Y.; Spillmann, T.; Kilpinen, S.; Reunanen, J.; Saris, P.E.J.; Satokari, R. The canine isolate Lactobacillus acidophilus LAB20 adheres to intestinal epithelium and attenuates LPS-induced IL-8 secretion of enterocytes in vitro. BMC Microbiol. 2015, 15, 4. [Google Scholar] [CrossRef] [Green Version]
- Woo, Y.M.; Kim, O.J.; Jo, E.S.; Jo, M.Y.; Ahn, M.Y.; Lee, Y.H.; LI, C.R.; Lee, S.H.; Choi, J.S.; Ha, J.M.; et al. The effect of Lactobacillus plantarum hydrolysates promoting VEGF production on vascular growth and hair growth of C57BL/6 mice. J. Anal. Sci. Technol. 2019, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.J.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef]
- McBroom, A.J.; Kuehn, M.J. Release of outer membrane vesicles by gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef]
- Rivera, J.; Nakouzi, R.J.; Nakouzi, A.S.; Frases, S.; Nicola, A.; Casadevall, A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. USA 2010, 107, 19002–19007. [Google Scholar] [CrossRef] [Green Version]
- Mittelbrunn, M.; Sánchez-Madrid, F. Intercellular Communication: Diverse Structures for Exchange of Genetic Information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Choi, S.J.; Choi, H.I.; Choi, J.P.; Park, H.K.; Kim, E.K.; Kim, M.J.; Moon, B.S.; Min, T.K.; Rho, M.A.; et al. Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles. Allergy Asthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem. Biophys. Res. Commun. 2018, 500, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. The Neutral Theory of Molecular Evolution; Cambridge University Press: Cambridge, UK, 1983; p. 367. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kumar, S.; Tamura, K.; Jakobsen, I.B.; Nei, M. MEGA2: Molecular Evolutionary Genetics Analysis software. Bioinformatics 2001, 17, 1244–1245. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Joseph, F. Confidence Limits on Phylogenies: An Approach using the Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar]
- Rajendran, R.L.; Gangadaran, P.; Seo, C.H.; Kwack, M.H.; Oh, J.M.; Lee, H.W.; Gopal, A.; Sung, Y.K.; Jeong, S.Y.; Lee, S.W.; et al. Macrophage-Derived Extracellular Vesicle Promotes Hair Growth. Cells 2020, 9, 856. [Google Scholar] [CrossRef] [Green Version]
- Susan, E. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar]
- Johnson, E.O.; Charchandi, A.; Babis, G.C.; Soucacos, P.N. Apoptosis in osteoarthritis: Morphology, mechanisms, and potential means for therapeutic intervention. J. Surg. Orthop. Adv. 2008, 17, 147–152. [Google Scholar]
- Reed, J.C. Double identity for proteins of the Bcl-2 family. Nature 1997, 387, 773–776. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Nimmannit, U.; Chanvorachote, P.; Leonard, S.S.; Pongrakhananon, V.; Wang, L.; Rojanasakul, Y. Hydroxyl radical mediates cisplatin-induced apoptosis in human hair follicle dermal papilla cells and keratinocytes through Bcl-2-dependent mechanism. Apoptosis 2011, 16, 769–782. [Google Scholar] [CrossRef] [Green Version]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Shaikh Qureshi, W.M.; Filip, S.; Mokry, J. Signaling Involved in Hair Follicle Morphogenesis and Development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, M.; Dai, X. Biological characteristics and probiotic effect of Leuconostoc lactis strain isolated from the intestine of black porgy fish. Braz. J. Microbiol. 2013, 44, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, R.; Higuchi, H.; Yoshida, K.; Yonejima, Y.; Hisa, K.; Utsuyama, M.; Osawa, K.; Hirokawa, K. Effects of chocolate containing Leuconostoc mesenteroides strain NTM048 on immune function: A randomized, double-blind, placebo-controlled trial. Immun. Ageing 2018, 15, 29. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Jiang, X.; Zhang, H.; Mehmood, K.; Zhang, L.; Jiang, J.; Waqas, M.; Iqbal, M.; Li, J. Probiotic Potential of Leuconostoc pseudomesente-roides and Lactobacillus Strains Isolated from Yaks. Front. Microbiol. 2018, 9, 2987. [Google Scholar] [CrossRef] [PubMed]
- Brandi, J.; Cheri, S.; Manfredi, M.; Carlo, S.D.; Vanella, V.V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti infammatory, anti pathogenic and proteomic efects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef] [PubMed]
- Maione, A.G.; Brudno, Y.; Stojadinovic, O.; Park, L.K.; Smith, A.; Tellechea, A.; Leal, E.C.; Kearney, C.J.; Veves, A.; Canic, M.T.; et al. Three-dimensional human tissue models that incorporate diabetic foot ulcer-derived fibroblasts mimic in vivo features of chronic wounds. Tissue Eng. Part C Methods 2015, 21, 499–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vince, J.E.; Nardo, D.D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1b Activation. Cell Rep. 2018, 25, 2339–2353. [Google Scholar] [CrossRef] [Green Version]
- Anastas, J.N.; Moon, R.T. WNT signaling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef]




| Gene | Direction | Primer Sequences (5′-3′) | Length of Expected Fragment (bp) |
|---|---|---|---|
| Wnt5A | Sence | 5′-TCCACCTTCCTCTTCACACTGA-3′ | 65 |
| Anti-sence | 5′-CGTGGCCAGCATCACATC-3′ | ||
| Wnt10B | Sence | 5′-CTTTTCAGCCCTTTGCTCTGAT-3′ | 87 |
| Anti-sence | 5′-CCCCTAAAGCTGTTTCCAGGTA-3′ | ||
| BAMBI | Sence | 5′-CTCCCGTTTGCACTACAGCTT-3′ | 58 |
| Anti-sence | 5′-CTTTGCAACCTGCCCCTTT-3′ | ||
| BMP2 | Sence | 5′-GAGGTCCTGAGCGAGTTCGA-3′ | 57 |
| Anti-sence | 5′-TCTCTGTTTCAGGCCGAACA-3′ | ||
| LEF1 | Sence | 5′-CCCGATGACGGAAAGCAT-3′ | 58 |
| Anti-sence | 5′-TCGAGTAGGAGGGTCCCTTGT-3′ | ||
| VCAN | Sence | 5′-GGCAATCTATTTACCAGGACCTGAT-3′ | 103 |
| Anti-sence | 5′-TGGCACACAGGTGCATACGT-3′ | ||
| β-catenin | Sence | 5′-CTGCTGTTTTGTTCCGAATGTC-3′ | 99 |
| Anti-sence | 5′-CCATTGGCTCTGTTCTGAAGAGA-3′ | ||
| BCL-2 | Sence | 5′-TCCCTCGCTGCACAAATACTC-3′ | 72 |
| Anti-sence | 5′-ACGACCCGATGGCCATAGA-3′ | ||
| BAX | Sence | 5′-CTGCAGAGGATGATTGCCG-3′ | 63 |
| Anti-sence | 5′-TGCCACTCGGAAAAAGACCT-3′ | ||
| Caspase-3 | Sence | 5′-TGGTTCATCCAGTCGCTTTG-3′ | 101 |
| Anti-sence | 5′-CATTCTGTTGCCACCTTTCG-3′ | ||
| GAPDH | Sence | 5′-TGGAAATCCCATCACCATCTTC-3′ | 56 |
| Anti-sence | 5′-CGCCCCACTTGATTTTGG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, Y.C.; Ahn, B.H.; Min, J.W.; Lee, K.R.; Park, S.H.; Kang, H.C. Stimulatory Effects of Extracellular Vesicles Derived from Leuconostoc holzapfelii That Exists in Human Scalp on Hair Growth in Human Follicle Dermal Papilla Cells. Curr. Issues Mol. Biol. 2022, 44, 845-866. https://doi.org/10.3390/cimb44020058
Yoon YC, Ahn BH, Min JW, Lee KR, Park SH, Kang HC. Stimulatory Effects of Extracellular Vesicles Derived from Leuconostoc holzapfelii That Exists in Human Scalp on Hair Growth in Human Follicle Dermal Papilla Cells. Current Issues in Molecular Biology. 2022; 44(2):845-866. https://doi.org/10.3390/cimb44020058
Chicago/Turabian StyleYoon, Yeo Cho, Beom Hee Ahn, Jin Woo Min, Kyung Real Lee, Sang Hoon Park, and Hee Cheol Kang. 2022. "Stimulatory Effects of Extracellular Vesicles Derived from Leuconostoc holzapfelii That Exists in Human Scalp on Hair Growth in Human Follicle Dermal Papilla Cells" Current Issues in Molecular Biology 44, no. 2: 845-866. https://doi.org/10.3390/cimb44020058
APA StyleYoon, Y. C., Ahn, B. H., Min, J. W., Lee, K. R., Park, S. H., & Kang, H. C. (2022). Stimulatory Effects of Extracellular Vesicles Derived from Leuconostoc holzapfelii That Exists in Human Scalp on Hair Growth in Human Follicle Dermal Papilla Cells. Current Issues in Molecular Biology, 44(2), 845-866. https://doi.org/10.3390/cimb44020058





