Dysregulation of miR-21-5p, miR-93-5p, miR-200c-3p and miR-205-5p in Oral Squamous Cell Carcinoma: A Potential Biomarkers Panel?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. RNA Extraction
2.3. Evaluation of miRNAs by Real Time Quantitative PCR
2.4. Evaluation of Long Non-Coding RNAs and Gene Expressions by Real Time Quantitative PCR
2.5. Evaluation by Immunohistochemistry of KI67, TP53 and BCL2
3. Results
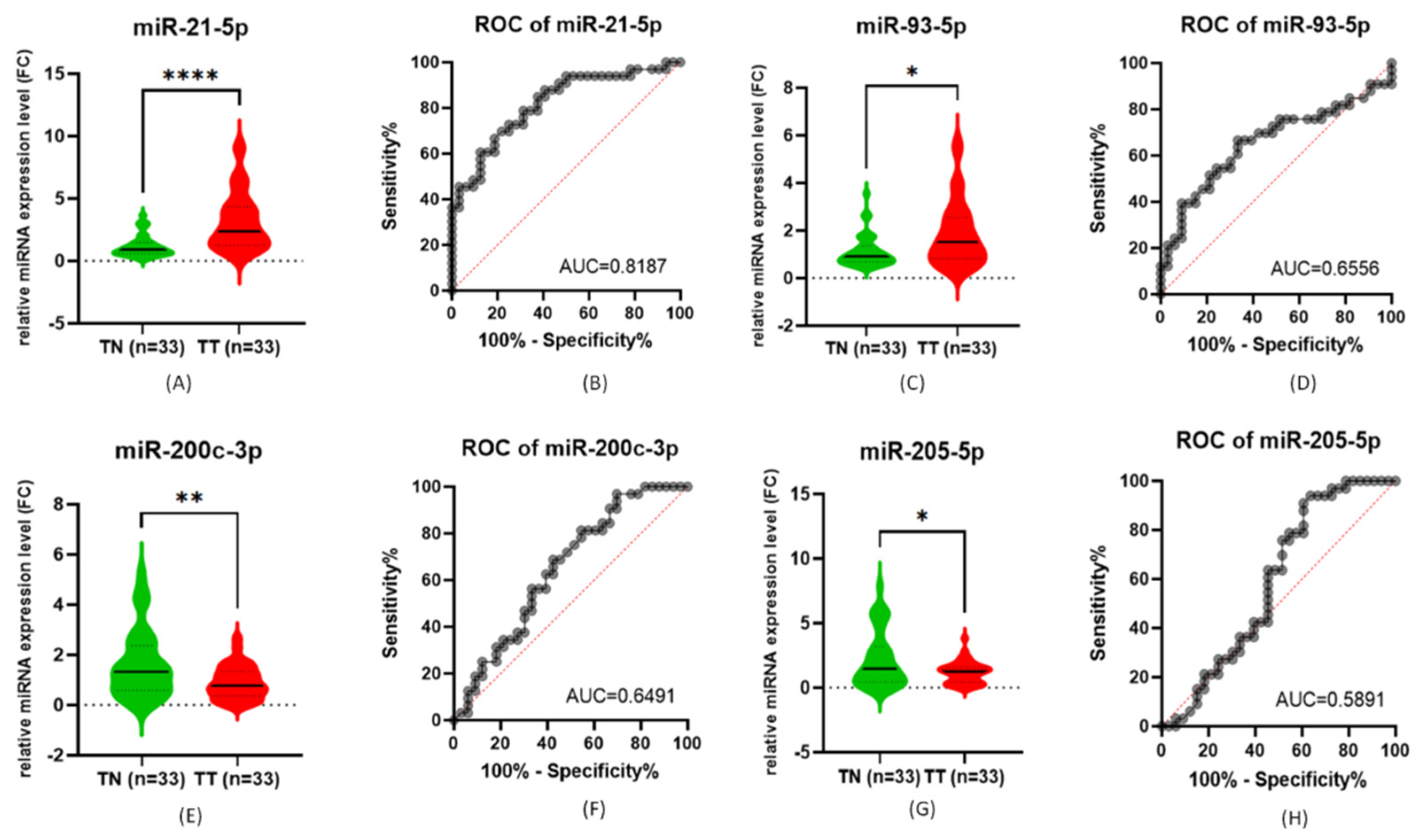
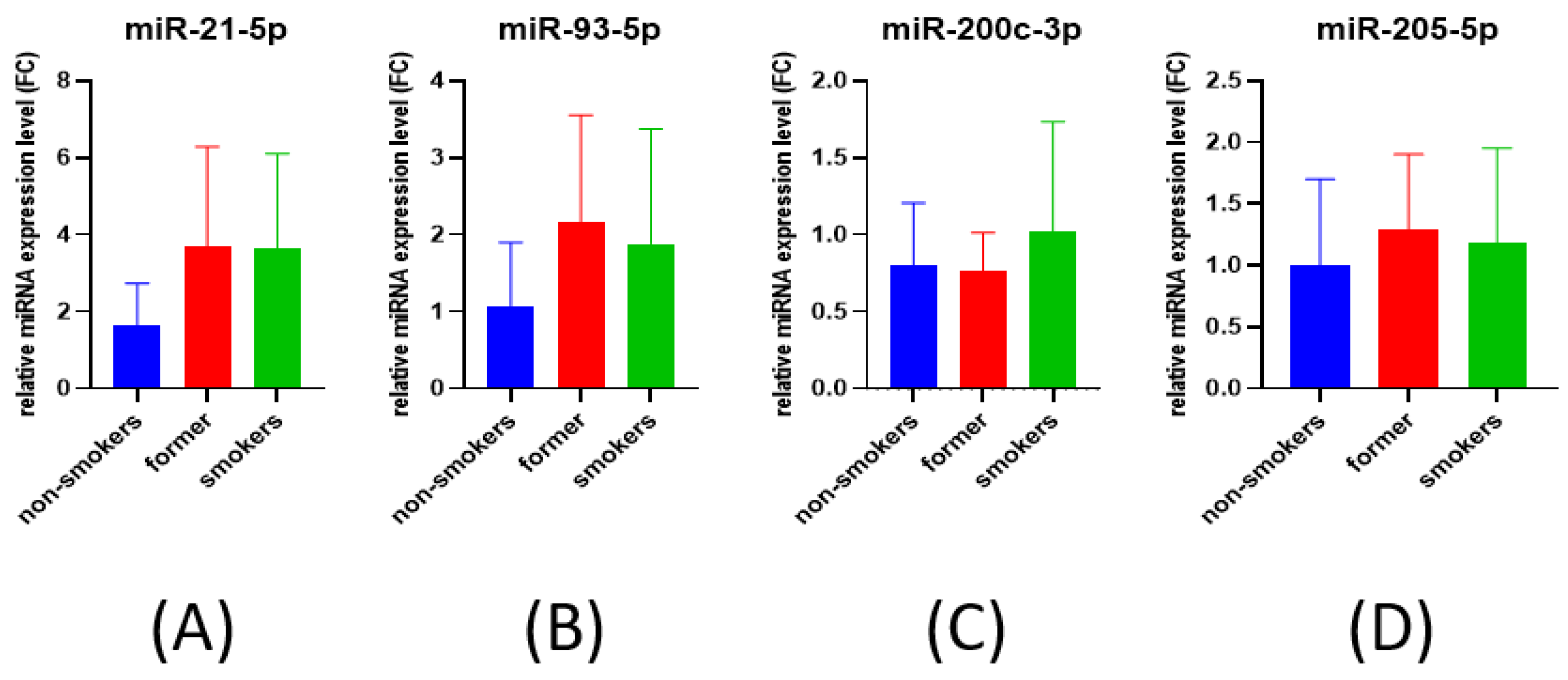
3.1. qRT-PCR for miRNA Expression Evaluation
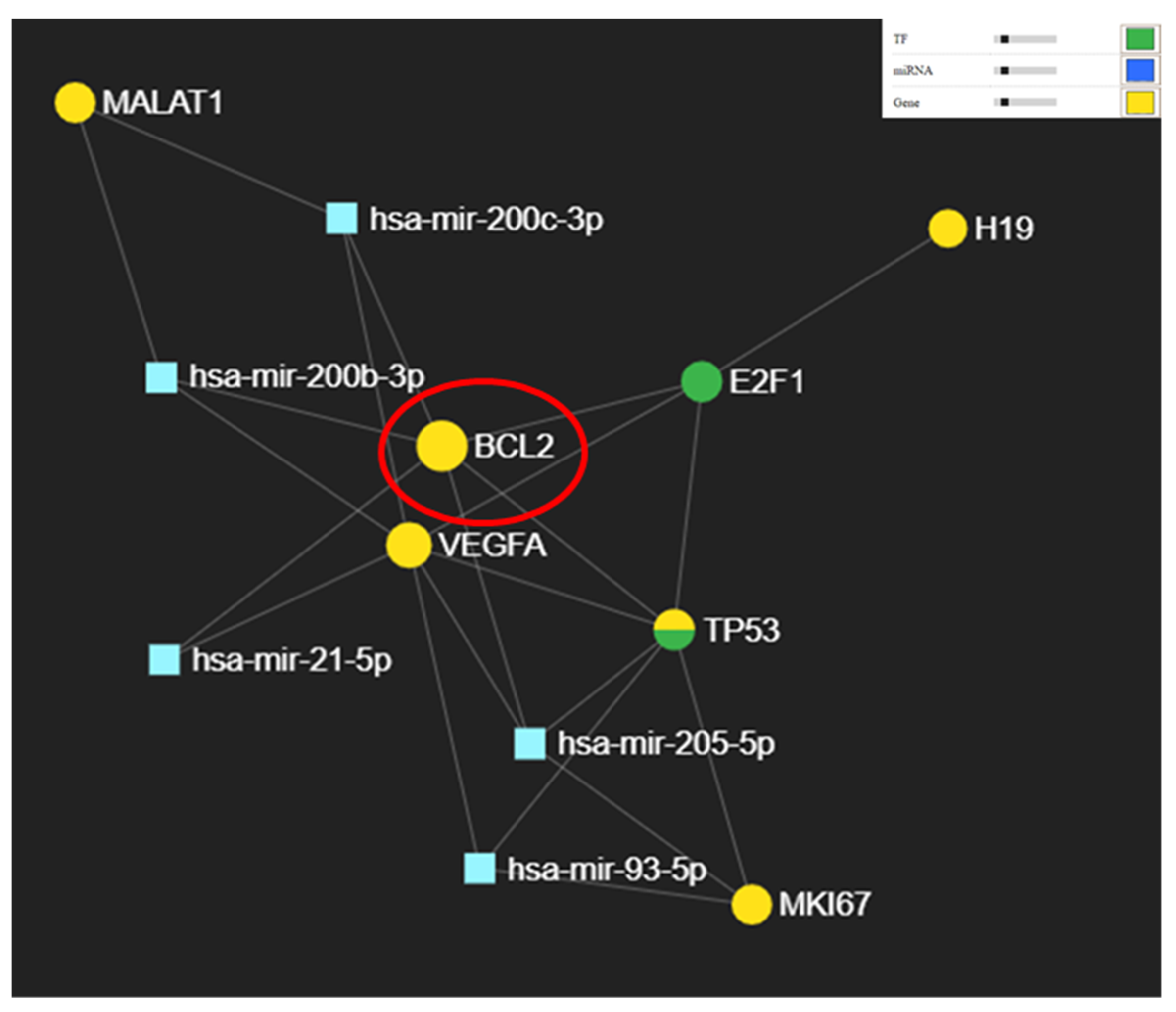
3.2. Potential Targets for miR-21-5p, miR-93-5p, miR-200c-3p, and miR-205-5p
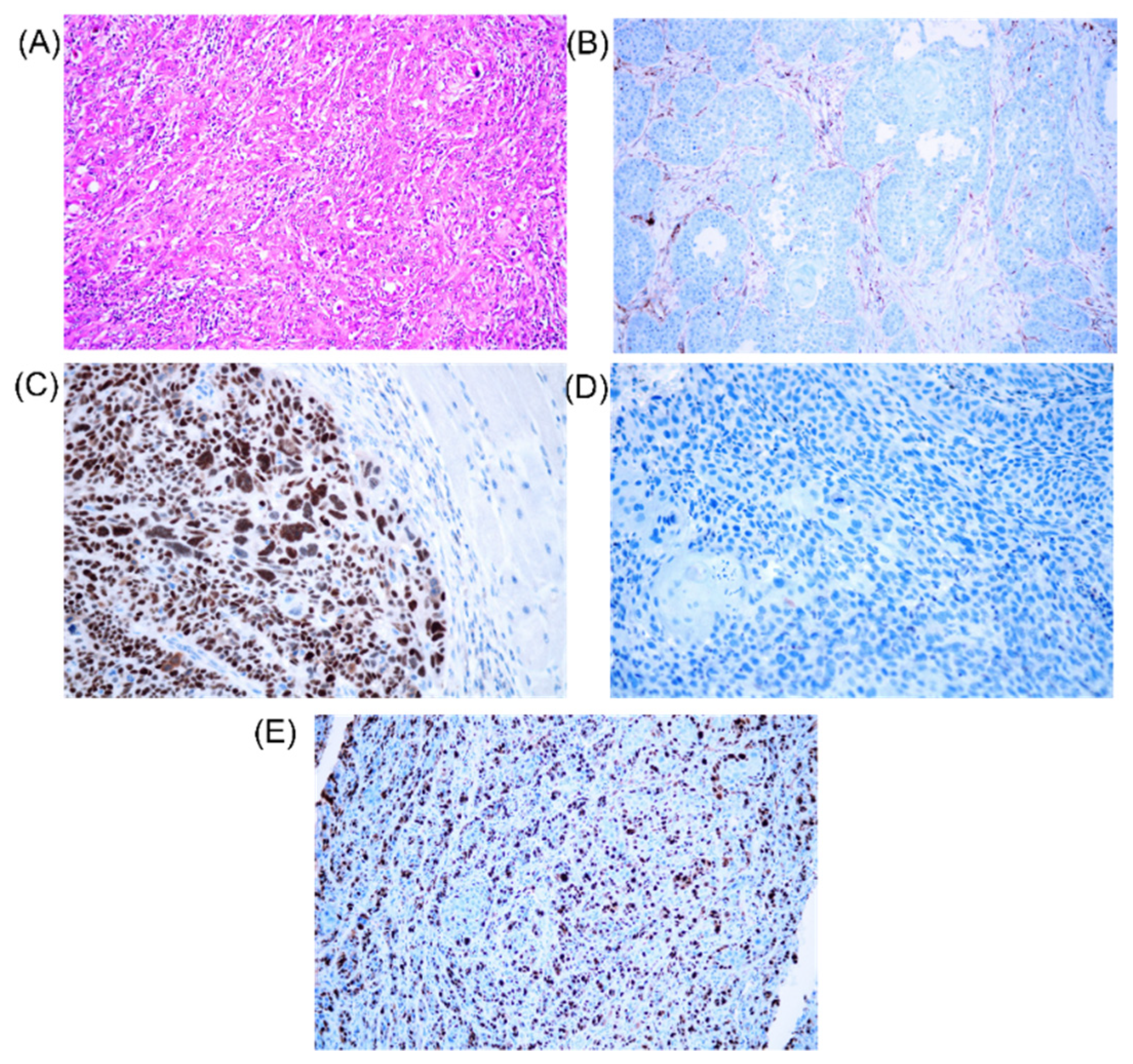
3.3. Evaluation by Immunohistochemistry of KI67, TP53 and BCL2 Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, X.; Zhu, X.-W.; Yang, D.-C.; Chen, R.; Jiang, Y.; Xu, T. Long non-coding RNAs in Oral squamous cell carcinoma: Biologic function, mechanisms and clinical implications. Mol. Cancer 2019, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, B.V.; Uhlmann, L.; Hoffmann, J.; Freier, K.; Thiele, O.C. The localization and risk factors of squamous cell carcinoma in the oral cavity: A retrospective study of 1501 cases. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2018, 46, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.; Pandey, R.; Singh, R.; Gothalwal, R.; Kumar, A. Expression of H19 long non-coding RNA is down-regulated in oral squamous cell carcinoma. J. Biosci. 2020, 45, 145. [Google Scholar] [CrossRef]
- Jain, D.; Dravid, C.; Singla, A.; Kumari, S.; Grover, R.K. Comparison of the Seventh and Eighth Editions of the American Joint Committee on Cancer pT and pN Classifications as Predictors of Survival in Patients With Oral Squamous Cell Carcinoma. Am. J. Clin. Pathol. 2019, 151, 292–301. [Google Scholar] [CrossRef]
- Manikandan, M.; Deva Magendhra Rao, A.K.; Arunkumar, G.; Manickavasagam, M.; Rajkumar, K.S.; Rajaraman, R.; Munirajan, A.K. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol. Cancer 2016, 15, 28. [Google Scholar] [CrossRef] [Green Version]
- Osan, C.; Chira, S.; Nutu, A.M.; Braicu, C.; Baciut, M.; Korban, S.S.; Berindan-Neagoe, I. The Connection between MicroRNAs and Oral Cancer Pathogenesis: Emerging Biomarkers in Oral Cancer Management. Genes 2021, 12, 1989. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Cojocneanu-Petric, R.; Berindan-Neagoe, I.; Campian, R.S. Novel technologies for oral squamous carcinoma biomarkers in diagnostics and prognostics. Acta Odontol. Scand. 2015, 73, 161–168. [Google Scholar] [CrossRef]
- Irimie, A.I.; Braicu, C.; Sonea, L.; Zimta, A.A.; Cojocneanu-Petric, R.; Tonchev, K.; Mehterov, N.; Diudea, D.; Buduru, S.; Berindan-Neagoe, I. A Looking-Glass of Non-coding RNAs in oral cancer. Int. J. Mol. Sci. 2017, 18, 2620. [Google Scholar] [CrossRef] [Green Version]
- Jurj, A.; Zanoaga, O.; Braicu, C.; Lazar, V.; Tomuleasa, C.; Irimie, A.; Berindan-Neagoe, I. A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells. Cancers 2020, 12, 298. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.-C.; Hsia, S.-M.; Wang, T.-H.; Li, P.-J.; Tseng, Y.-H.; Chiu, K.-C.; Tu, H.-F.; Shih, Y.-H.; Shieh, T.-M. The relation between NEAT1 expression level and survival rate in patients with oral squamous cell carcinoma. J. Dent. Sci. 2022, 17, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cao, J.; Peng, X. LINC01234 facilitates growth and invasiveness of oral squamous cell carcinoma through regulating the miR-637/NUPR1 axis. Biomed. Pharmacother. 2019, 120, 109507. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Dann, B.; Pruteanu, L.L.; Oerton, E.; Sharma, N.; Berindan-Neagoe, I.; Módos, D.; Bender, A. Developments in toxicogenomics: Understanding and predicting compound-induced toxicity from gene expression data. Mol. Omics 2018, 14, 218–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalfert, D.; Pesta, M.; Kulda, V.; Topolcan, O.; Ryska, A.; Celakovsky, P.; Laco, J.; Ludvikova, M. MicroRNA profile in site-specific head and neck squamous cell cancer. Anticancer Res. 2015, 35, 2455–2463. [Google Scholar] [PubMed]
- Ghosh, R.D.; Pattatheyil, A.; Roychoudhury, S. Functional Landscape of Dysregulated MicroRNAs in Oral Squamous Cell Carcinoma: Clinical Implications. Front. Oncol. 2020, 10, 619. [Google Scholar] [CrossRef]
- Liu, X.; Shang, W.; Zheng, F. Long non-coding RNA NEAT1 promotes migration and invasion of oral squamous cell carcinoma cells by sponging microRNA-365. Exp. Ther. Med. 2018, 16, 2243–2250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Z.; Cao, H.C.; Huang, D.L.; Wu, Q.; Chen, X.F.; Wan, J.; Zhang, W. MicroRNA-200c plays an oncogenic role in nasopharyngeal carcinoma by targeting PTEN. Tumour. Biol. 2017, 39, 1010428317703655. [Google Scholar] [CrossRef] [Green Version]
- Kawakubo-Yasukochi, T.; Morioka, M.; Hazekawa, M.; Yasukochi, A.; Nishinakagawa, T.; Ono, K.; Kawano, S.; Nakamura, S.; Nakashima, M. miR-200c-3p spreads invasive capacity in human oral squamous cell carcinoma microenvironment. Mol. Carcinog. 2018, 57, 295–302. [Google Scholar] [CrossRef]
- Rajan, C.; Roshan, V.G.D.; Khan, I.; Manasa, V.G.; Himal, I.; Kattoor, J.; Thomas, S.; Kondaiah, P.; Kannan, S. MiRNA expression profiling and emergence of new prognostic signature for oral squamous cell carcinoma. Sci. Rep. 2021, 11, 7298. [Google Scholar] [CrossRef]
- Aali, M.; Mesgarzadeh, A.H.; Najjary, S.; Abdolahi, H.M.; Kojabad, A.B.; Baradaran, B. Evaluating the role of microRNAs alterations in oral squamous cell carcinoma. Gene 2020, 757, 144936. [Google Scholar] [CrossRef]
- Liu, K.Y.P.; Zhu, S.Y.; Brooks, D.; Bowlby, R.; Durham, J.S.; Ma, Y.; Moore, R.A.; Mungall, A.J.; Jones, S.; Poh, C.F. Tumor microRNA profile and prognostic value for lymph node metastasis in oral squamous cell carcinoma patients. Oncotarget 2020, 11, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Greither, T.; Vorwerk, F.; Kappler, M.; Bache, M.; Taubert, H.; Kuhnt, T.; Hey, J.; Eckert, A.W. Salivary miR-93 and miR-200a as post-radiotherapy biomarkers in head and neck squamous cell carcinoma. Oncol. Rep. 2017, 38, 1268–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, H.H.; Tseng, Y.K.; You, J.J.; Kang, B.H.; Wang, T.H.; Yang, C.M.; Chen, H.C.; Liou, H.H.; Liu, P.F.; Ger, L.P.; et al. Next-generation Sequencing for microRNA Profiling: MicroRNA-21-3p Promotes Oral Cancer Metastasis. Anticancer Res. 2017, 37, 1059–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priya, N.S.; Nayak, R.; Bhat, K.; Kugaji, M.; Vijayalakshmi, K.; Rao, K. Site specificity and expression profile of miR-21 in oral squamous cell carcinoma. J. Oral. Maxillofac. Pathol. 2021, 25, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Chamorro Petronacci, C.M.; García García, A.; Padín Iruegas, E.; Rivas Mundiña, B.; Lorenzo Pouso, A.I.; Pérez Sayáns, M. Identification of Prognosis Associated microRNAs in HNSCC Subtypes Based on TCGA Dataset. Medicina 2020, 56, 535. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-H.; Tsao, C.-J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irimie-Aghiorghiesei, A.I.; Pop-Bica, C.; Pintea, S.; Braicu, C.; Cojocneanu, R.; Zimța, A.-A.; Gulei, D.; Slabý, O.; Berindan-Neagoe, I. Prognostic Value of MiR-21: An Updated Meta-Analysis in Head and Neck Squamous Cell Carcinoma (HNSCC). J. Clin. Med. 2019, 8, 2041. [Google Scholar] [CrossRef] [Green Version]
- Dioguardi, M.; Caloro, G.A.; Laino, L.; Alovisi, M.; Sovereto, D.; Crincoli, V.; Aiuto, R.; Coccia, E.; Troiano, G.; Lo Muzio, L. Circulating miR-21 as a Potential Biomarker for the Diagnosis of Oral Cancer: A Systematic Review with Meta-Analysis. Cancers 2020, 12, 936. [Google Scholar] [CrossRef] [Green Version]
- Menderico Junior, G.M.; Theodoro, T.R.; Pasini, F.S.; de Menezes Ishikawa, M.; Santos, N.S.S.; de Mello, E.S.; da Silva Pinhal, M.A.; Moyses, R.A.; Kulcsar, M.A.V.; Dedivitis, R.A.; et al. MicroRNA-mediated extracellular matrix remodeling in squamous cell carcinoma of the oral cavity. Head Neck 2021, 43, 2364–2376. [Google Scholar] [CrossRef]
- Matos, L.L.; Menderico Junior, G.M.; Theodoro, T.R.; Pasini, F.S.; Ishikawa, M.M.; Ribeiro, A.A.B.; de Mello, E.S.; Pinhal, M.; Moyses, R.A.; Kulcsar, M.A.V.; et al. Cancer-associated fibroblast regulation by microRNAs promotes invasion of oral squamous cell carcinoma. Oral Oncol. 2020, 110, 104909. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Liu, C.; Li, G.; Lu, S.; Jing, Q.; Chen, X.; Ma, H.; Zhang, D.; Wang, Y.; et al. miR-93-5p enhances migration and invasion by targeting RGMB in squamous cell carcinoma of the head and neck. J. Cancer 2020, 11, 3871–3881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Ren, S.; Su, Z.; Liu, C.; Deng, T.; Huang, D.; Tian, Y.; Qiu, Y.; Liu, Y. Increased expression of miR-93 is associated with poor prognosis in head and neck squamous cell carcinoma. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 3949–3956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeljic, K.; Jovanovic, I.; Jovanovic, J.; Magic, Z.; Stankovic, A.; Supic, G. MicroRNA meta-signature of oral cancer: Evidence from a meta-analysis. Upsala J. Med. Sci. 2018, 123, 43–49. [Google Scholar] [CrossRef]
- Yap, T.; Seers, C.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; McCullough, M. Non-invasive screening of a microRNA-based dysregulation signature in oral cancer and oral potentially malignant disorders. Oral Oncol. 2019, 96, 113–120. [Google Scholar] [CrossRef]
- Diaz-Riascos, Z.V.; Ginesta, M.M.; Fabregat, J.; Serrano, T.; Busquets, J.; Buscail, L.; Cordelier, P.; Capellá, G. Expression and Role of MicroRNAs from the miR-200 Family in the Tumor Formation and Metastatic Propensity of Pancreatic Cancer. Mol. Ther. Nucleic Acids 2019, 17, 491–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunkumar, G.; Deva Magendhra Rao, A.K.; Manikandan, M.; Prasanna Srinivasa Rao, H.; Subbiah, S.; Ilangovan, R.; Murugan, A.K.; Munirajan, A.K. Dysregulation of miR-200 family microRNAs and epithelial-mesenchymal transition markers in oral squamous cell carcinoma. Oncol. Lett. 2018, 15, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, K.; Wei, P.; Hu, Y.; Peng, Y.; Fang, X.; He, G.; Wu, L.; Chao, M.; Wang, J. Correlation between miR-200 Family Overexpression and Cancer Prognosis. Dis. Markers 2018, 2018, 6071826. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Hasegawa, S.; Uchida, F.; Terabe, T.; Ishibashi Kanno, N.; Kato, K.; Yamagata, K.; Sakai, S.; Kawashiri, S.; Sato, H.; et al. MicroRNA-205-5p suppresses the invasiveness of oral squamous cell carcinoma by inhibiting TIMP-2 expression. Int. J. Oncol. 2018, 52, 841–850. [Google Scholar] [CrossRef]
- Arya, V.; Singh, S.; Daniel, M.J. Clinicopathological correlation of Bcl-2 oncoprotein expression in oral precancer and cancer. J. Oral Biol. Craniofacial Res. 2016, 6, 18–23. [Google Scholar] [CrossRef] [Green Version]




| Fresh Frozen Tissue | ||
|---|---|---|
| Characteristics | Cases (n = 33) | Percentage % |
| Age | ||
| ≤60 | 11 | 33.33 |
| >61 | 22 | 66.67 |
| NA | 0 | 0.00 |
| Sex | ||
| Females | 7 | 21.21 |
| Males | 26 | 78.79 |
| LNM status | ||
| Negative | 16 | 48.48 |
| Positive | 14 | 42.42 |
| NA | 3 | 9.09 |
| TNM status | ||
| T1 | 8 | 24.24 |
| T2 | 6 | 18.18 |
| T3 | 6 | 18.18 |
| T4 | 11 | 33.33 |
| NA | 2 | 6.06 |
| Smoking | ||
| Yes | 24 | 72.73 |
| No | 5 | 15.15 |
| Former | 3 | 9.09 |
| NA | 1 | 3.03 |
| Differentiation | ||
| Well | 11 | 33.33 |
| moderate | 18 | 54.55 |
| Poor | 3 | 9.09 |
| NA | 1 | 3.03 |
| Location | ||
| Head | 3 | 9.09 |
| Tongue | 6 | 18.18 |
| Tonsils | 1 | 3.03 |
| Lip | 5 | 15.15 |
| Mandible/maxilla | 6 | 18.18 |
| Oral cavity | 9 | 27.27 |
| Gum | 1 | 3.03 |
| Palate | 2 | 6.06 |
| miRNA | Assay Code | Mature Sequence |
|---|---|---|
| RNU48 | 0001006 | GATGACCCCAGGTAACTCTGAGTGTGTCGCTGATGCCATCACCGCAGCGCTCTGACC |
| U6 | 001973 | GTGCTCGCTTCGGCAGCACATATACTAAAATTGGAACGATACAGAGAAGATTAGCATGGCCCCTGCGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTT |
| miR-21-5p | 000397 | UAGCUUAUCAGACUGAUGUUGA |
| miR-93-5p | 001090 | CAAAGUGCUGUUCGUGCAGGUAG |
| miR-200c-3p | 002300 | UAAUACUGCCGGGUAAUGAUGGA |
| miR-205-5p | 000509 | UCCUUCAUUCCACCGGAGUCUG |
| Gene | Primer Sequence |
|---|---|
| B2M | F:TTACTTCCTCCACGGAGTCG |
| R:TGAGCTGGGTAGCACCATTT | |
| GAPDH | F:ATCTTCCAGGAGCGAGATCCC |
| R: TGAGTCCTTCCAAGATACCAA | |
| H19 | F:CACCCCCACTGAAAAAGATGAG |
| R:CCTCCATGATGCTGCTTACATG | |
| MALAT1 | F:TGTCCTTATAGGCTGGCCATT |
| R:AACTGCAGAGTTTGAGTGGTTTT |
| Gene | Primer Sequence |
|---|---|
| BCL2 | F:AGTACCTGAACCGGCACC |
| R:GCCGTACAGTTCCACAAAGG |
| No Sample | PI (KI67) | TP53% | Bcl2% |
|---|---|---|---|
| 1 | 30 | <1 (mt) | 0 |
| 2 | 5 | <1 (mt) | 5 |
| 3 | 25 | <1 (mt) | 35 |
| 4 | 15 | <1 (mt) | 10 |
| 5 | 25 | <1 (mt) | 0 |
| 6 | 35 | 80 (mt) | 0 |
| 7 | 15 | <1 (mt) | 10 |
| 8 | 25 | 60 (wt) | 20 |
| 9 | 5 | 80 (mt) | 0 |
| 10 | 50 | <1 (mt) | 30 |
| 11 | 60 | <1 (mt) | 10 |
| 12 | 55 | <1 (mt) | 15 |
| 13 | 40 | 65 (wt) | 5 |
| 14 | 60 | 90 (mt) | 5 |
| 15 | 40 | <1 (mt) | 5 |
| 16 | 10 | 25 (wt) | 15 |
| 17 | 35 | 95 (mt) | 1 |
| 18 | 25 | 25 (wt) | 0 |
| 19 | 20 | <1 (mt) | 5 |
| 20 | 7 | 15 (wt) | 0 |
| 21 | 10 | 15 (wt) | 15 |
| 22 | 15 | 60 (wt) | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghiorghiesei, O.; Zanoaga, O.; Raduly, L.; Aghiorghiesei, A.I.; Chiroi, P.; Trif, A.; Buiga, R.; Budisan, L.; Lucaciu, O.; Pop, L.A.; et al. Dysregulation of miR-21-5p, miR-93-5p, miR-200c-3p and miR-205-5p in Oral Squamous Cell Carcinoma: A Potential Biomarkers Panel? Curr. Issues Mol. Biol. 2022, 44, 1754-1767. https://doi.org/10.3390/cimb44040121
Aghiorghiesei O, Zanoaga O, Raduly L, Aghiorghiesei AI, Chiroi P, Trif A, Buiga R, Budisan L, Lucaciu O, Pop LA, et al. Dysregulation of miR-21-5p, miR-93-5p, miR-200c-3p and miR-205-5p in Oral Squamous Cell Carcinoma: A Potential Biomarkers Panel? Current Issues in Molecular Biology. 2022; 44(4):1754-1767. https://doi.org/10.3390/cimb44040121
Chicago/Turabian StyleAghiorghiesei, Ovidiu, Oana Zanoaga, Lajos Raduly, Alexandra Iulia Aghiorghiesei, Paul Chiroi, Andrada Trif, Rares Buiga, Liviuta Budisan, Ondine Lucaciu, Laura Ancuta Pop, and et al. 2022. "Dysregulation of miR-21-5p, miR-93-5p, miR-200c-3p and miR-205-5p in Oral Squamous Cell Carcinoma: A Potential Biomarkers Panel?" Current Issues in Molecular Biology 44, no. 4: 1754-1767. https://doi.org/10.3390/cimb44040121
APA StyleAghiorghiesei, O., Zanoaga, O., Raduly, L., Aghiorghiesei, A. I., Chiroi, P., Trif, A., Buiga, R., Budisan, L., Lucaciu, O., Pop, L. A., Braicu, C., Campian, R., & Berindan-Neagoe, I. (2022). Dysregulation of miR-21-5p, miR-93-5p, miR-200c-3p and miR-205-5p in Oral Squamous Cell Carcinoma: A Potential Biomarkers Panel? Current Issues in Molecular Biology, 44(4), 1754-1767. https://doi.org/10.3390/cimb44040121












