A Possible Novel Protective Effect of Piceatannol against Isoproterenol (ISO)-Induced Histopathological, Histochemical, and Immunohistochemical Changes in Male Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Chemicals
2.3. Animals
2.4. Experimental Design
2.5. Histological Techniques
2.6. Histopathological Examination
2.7. Histochemical Examination
2.8. Immunohistochemical Examination
2.9. Morphometric Study
2.10. Statistical Analysis
3. Results
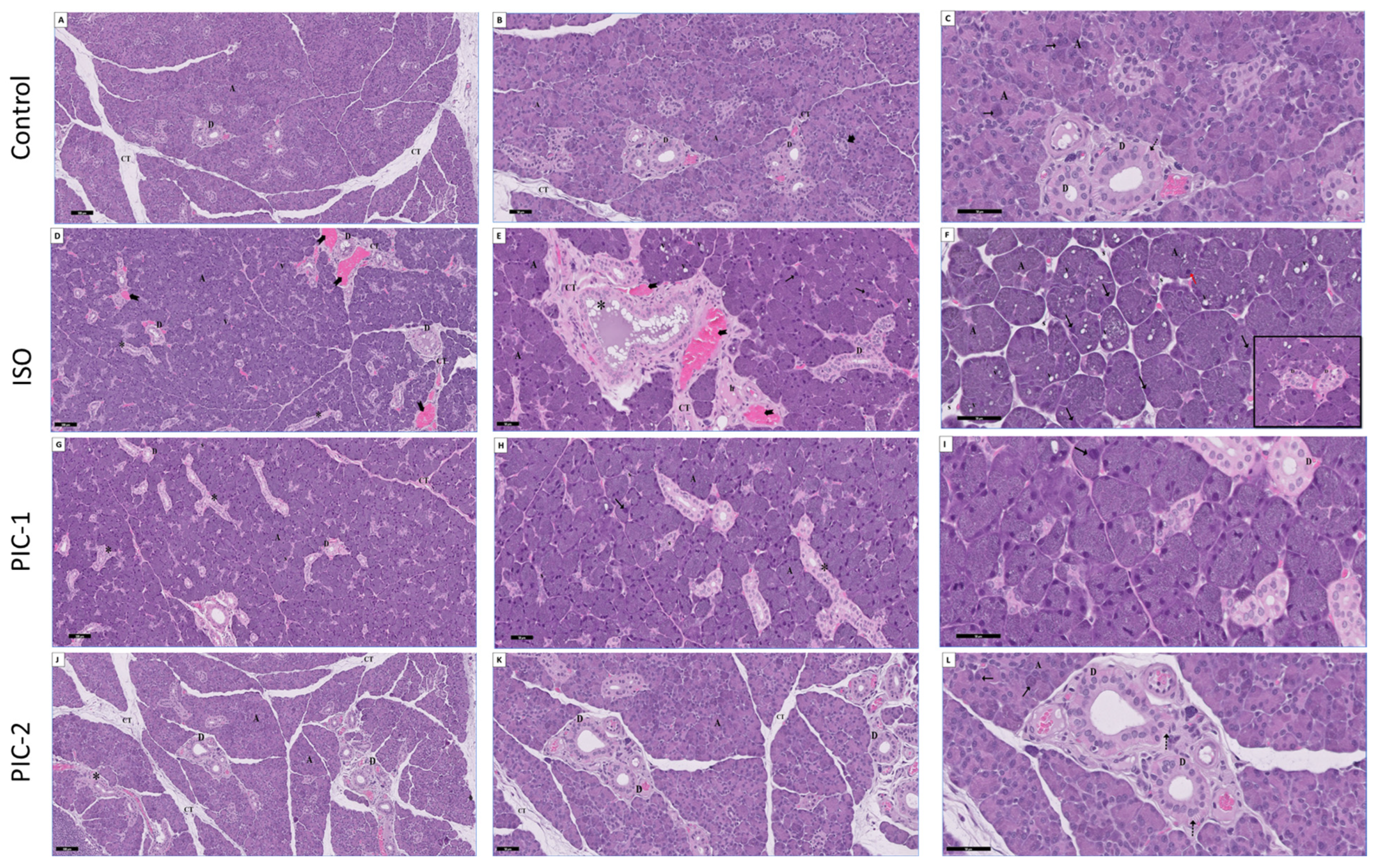
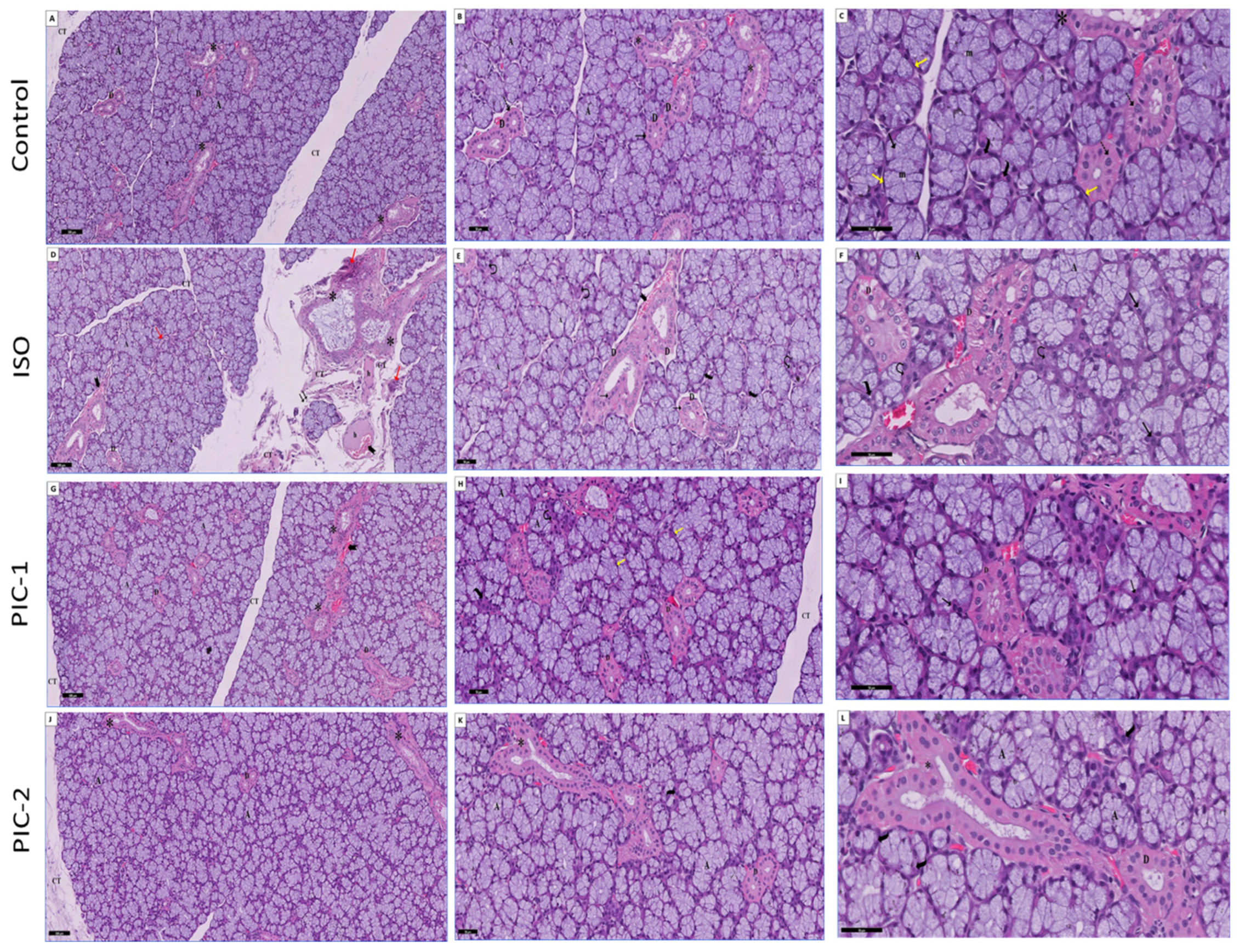
3.1. Histopathological Results
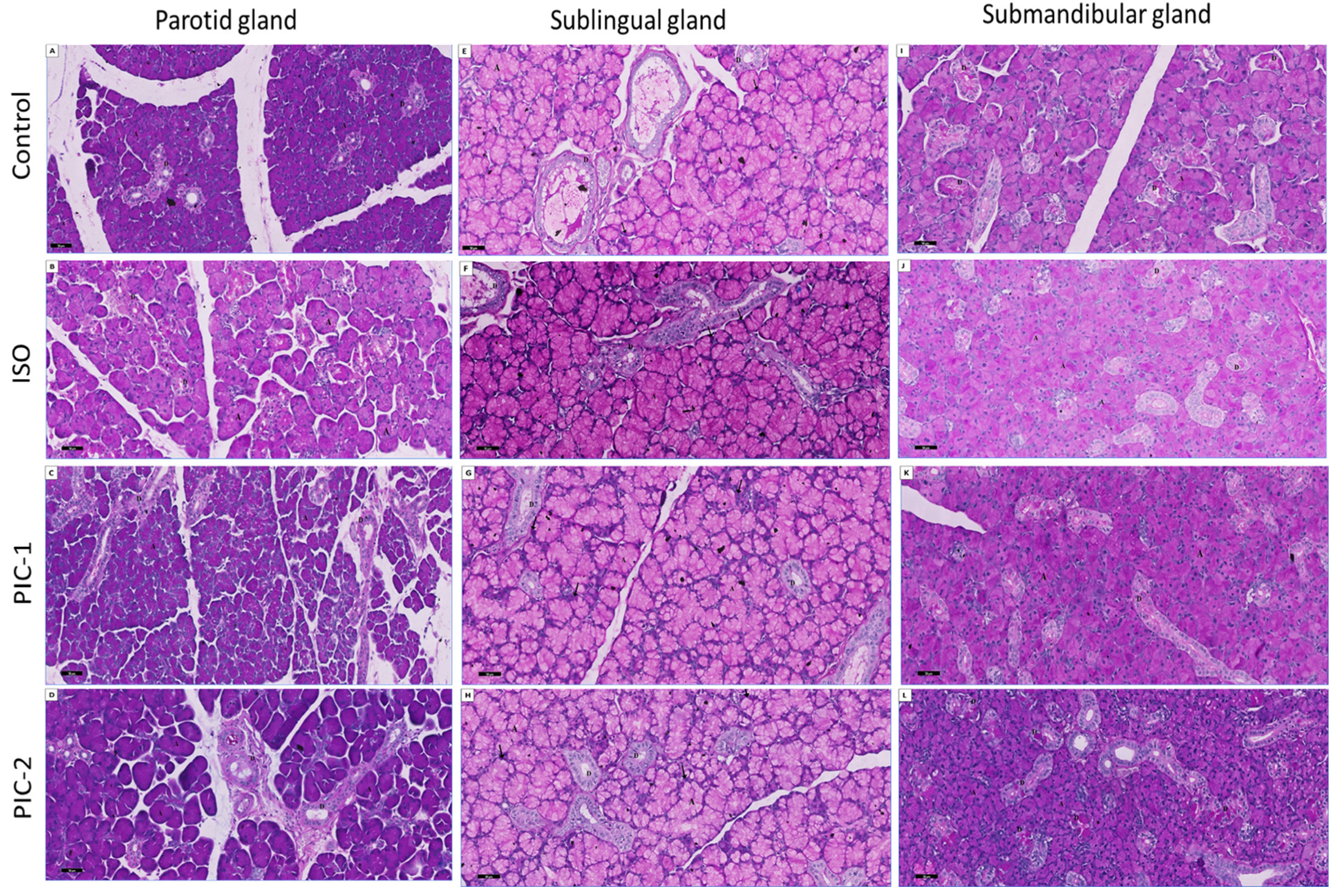
3.1.1. Examination of Hematoxylin and Eosin-Stained Sections
3.1.2. Examination of Masson Trichrome-Stained Sections
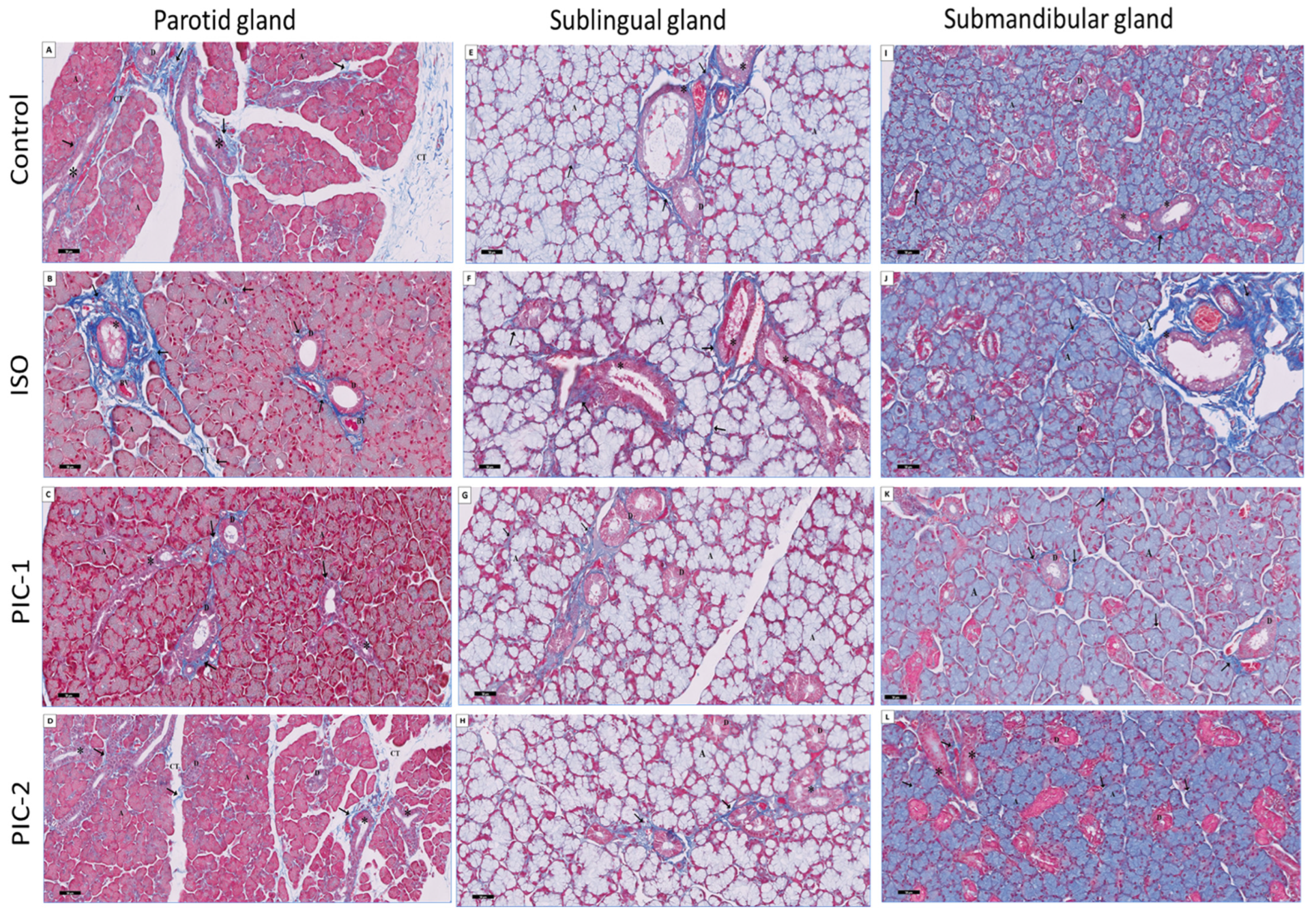
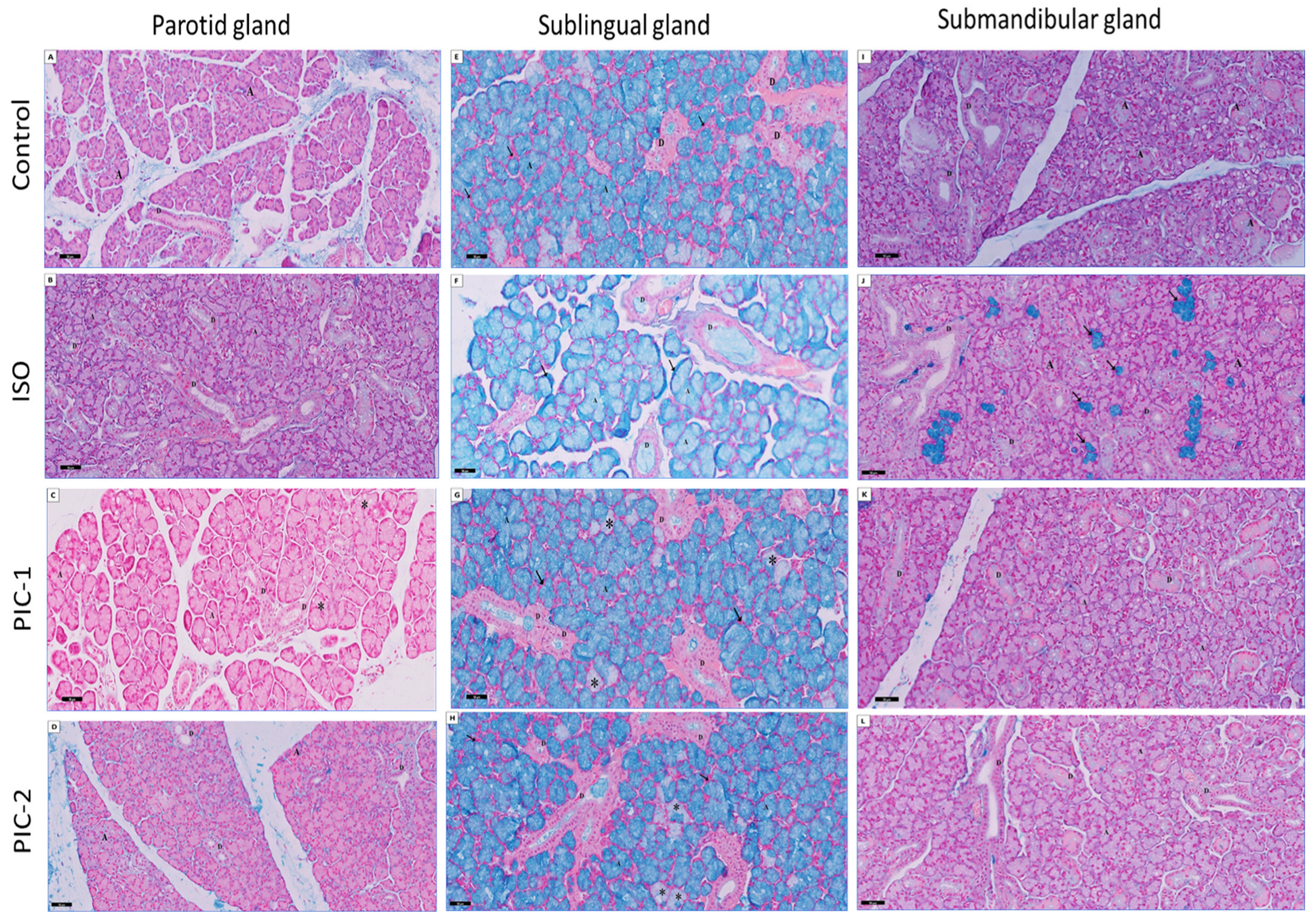
3.2. Histochemical Results
3.2.1. Examination of Alcian Blue Stain-Stained Sections
3.2.2. Examination of PAS-Stained Sections
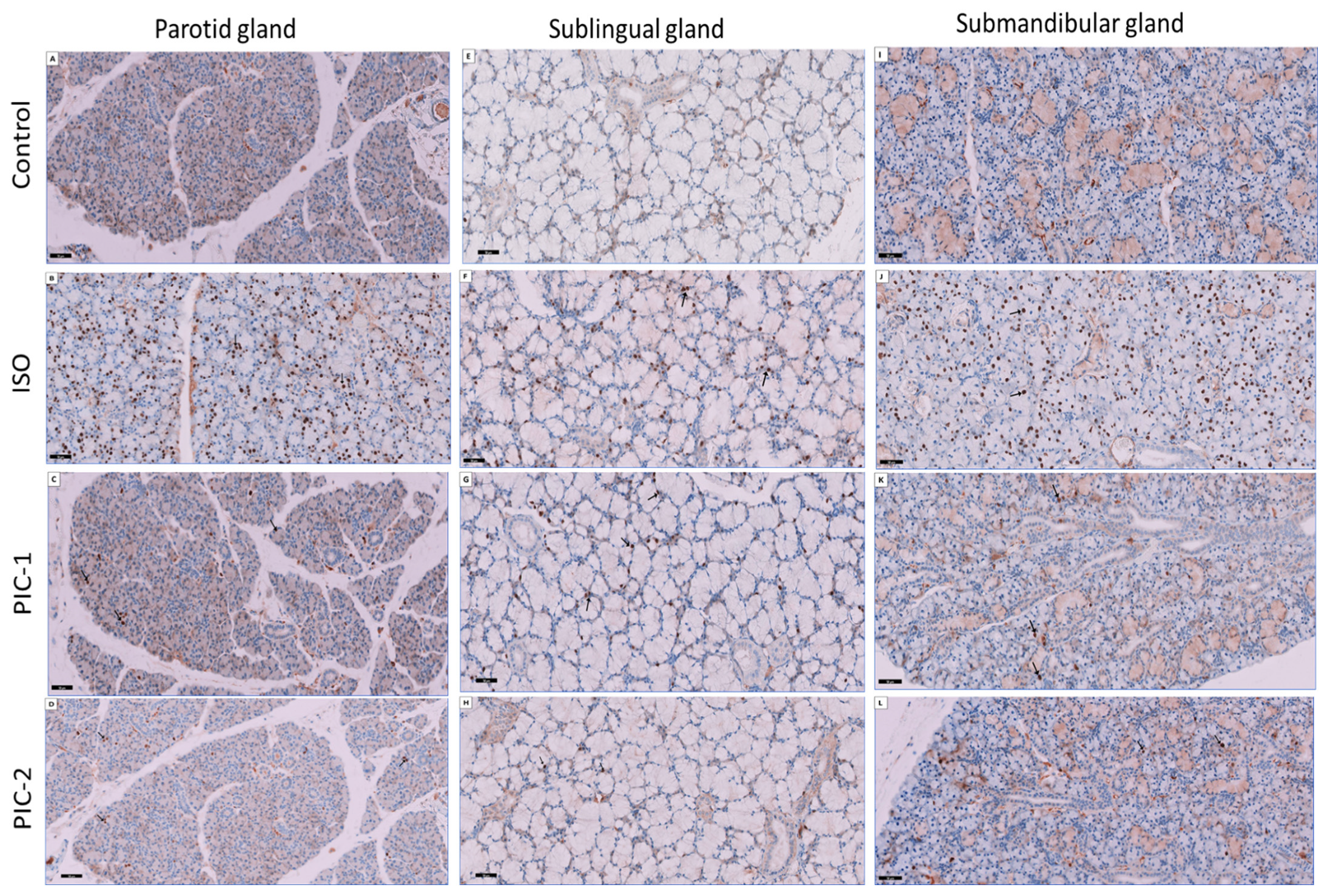
3.3. Immunohistochemical Results
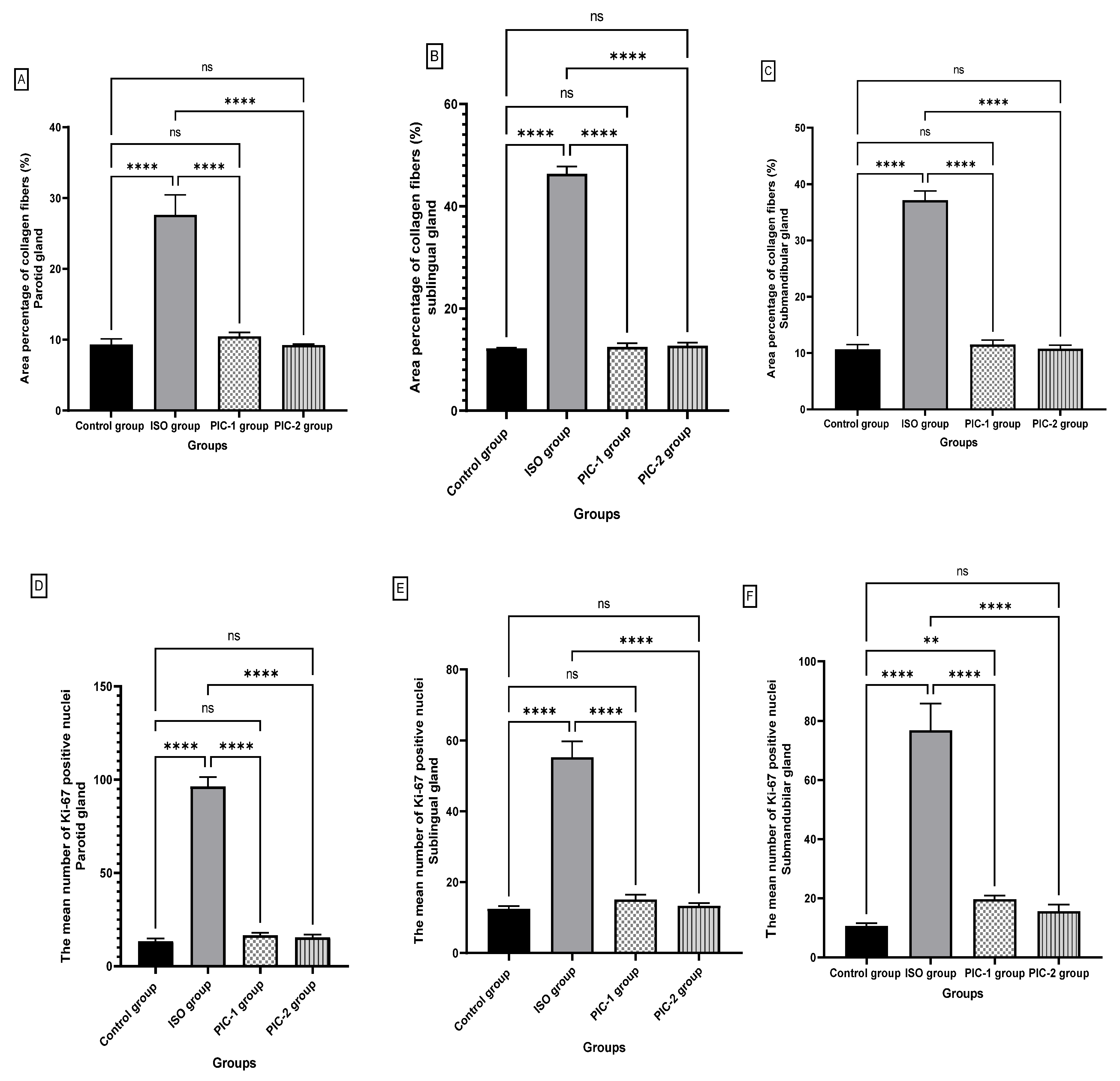
4. Morphometric and Statistical Results
4.1. Area %age of Collagen Fibers
4.2. Ki-67 Immunoexpression
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Punj, A. Secretions of human salivary gland. In Salivary Glands-New Approaches in Diagnostics and Treatment; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Tanasiewicz, M.; Hildebrandt, T.; Obersztyn, I. Xerostomia of various etiologies: A review of the litera-ture. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2016, 25, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Glick, M. Burket’s Oral Medicine, 12th ed.; BC Decker: Hamilton, ON, USA, 2015. [Google Scholar]
- Ngo, D.Y.J.; Thomson, W.M. An Update on the Lived Experience of Dry Mouth in Sjögren’s Syndrome Patients. Front. Oral Health 2021, 2, 767568. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.M.L.; Soerensen, C.; Proctor, G.; Carpenter, G. Salivary functions in mastication, taste and textural perception, swallowing and initial digestion. Oral Dis. 2018, 24, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther. Clin. Risk Manag. 2014, 11, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smidt, D.; Torpet, L.A.; Nauntofte, B.; Heegaard, K.M.; Pedersen, A.M.L. Associations between labial and whole salivary flow rates, systemic diseases and medications in a sample of older people. Commun. Dent. Oral Epidemiol. 2010, 38, 422–435. [Google Scholar] [CrossRef]
- Fleming, M.; Craigs, C.L.; Bennett, M.I. Palliative care assessment of dry mouth: What matters most to patients with advanced disease? Support Care Cancer 2020, 28, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Tiisanoja, A.; Syrjälä, A.-M.; Kullaa, A.; Ylöstalo, P. Anticholinergic burden and dry mouth in middle-aged people. JDR Clin. Transl. Res. 2020, 5, 62–70. [Google Scholar] [CrossRef]
- Lee, K.A.; Park, J.-C.; Park, Y.K. Nutrient intakes and medication use in elderly individuals with and without dry mouths. Nutr. Res. Pract. 2020, 14, 143–151. [Google Scholar] [CrossRef]
- Pedersen, A.M.L. Diseases causing oral dryness. In Dry Mouth; Carpenter, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 7–31. [Google Scholar] [CrossRef]
- Fathi, Y.; Hoseini, E.G.; Atoof, F.; Mottaghi, R. Xerostomia (dry mouth) in patients with COVID-19: A case series. Futur. Virol. 2021, 16, 315–319. [Google Scholar] [CrossRef]
- Scully Cbe, C. Drug effects on salivary glands: Dry mouth. Oral Dis. 2003, 9, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Abou Elkhier, M.; Elmeadawy, S.; Salama, N. Effect of salbutamol on the parotid salivary gland of rats: Ultrastructural study. Egypt Dent. J. 2018, 64, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Copik, A.; Baldys, A.; Nguyen, K.; Sahdeo, S.; Ho, H.; Kosaka, A.; Dietrich, P.J.; Fitch, B.; Raymond, J.R.; Ford, A.P.D.W.; et al. Isoproterenol acts as a biased agonist of the alpha-1A-adrenoceptor that selectively activates the MAPK/ERK pathway. PLoS ONE 2015, 10, e0115701. [Google Scholar] [CrossRef]
- Susa, T.; Sawai, N.; Aoki, T.; Iizuka-Kogo, A.; Kogo, H.; Negishi, A.; Yokoo, S.; Takata, K.; Matsuzaki, T. Effects of repeated administration of pilocarpine and isoproterenol on aquaporin-5 expression in rat salivary glands. Acta Histochem. ET Cytochem. 2013, 46, 187–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alterman, A.; Mathison, R.; Coronel, C.E.; Stroppa, M.M.; Finkelberg, A.B.; Gallará, R.V. Functional and proteomic analysis of submandibular saliva in rats exposed to chronic stress by immobilization or constant light. Arch. Oral Biol. 2012, 57, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.P.; Sweitzer, N.K.; Indik, J.H.; Acharya, D.; William, P. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020, 29, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Kochav, S.M.; Coromilas, E.; Nalbandian, A.; Ranard, L.S.; Gupta, A.; Chung, M.K.; Gopinathannair, R.; Biviano, A.B.; Garan, H.; Wan, E.Y. Cardiac arrhythmias in COVID-19 infection. Circ. Arrhythmia Electrophysiol. 2020, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef]
- Holmberg, K.V.; Hoffman, M.P. Anatomy, biogenesis and regeneration of salivary glands. Saliva Secret Funct. 2014, 24, 1–13. [Google Scholar]
- Keihanian, F.; Moohebati, M.; Saeidinia, A.; Mohajeri, S.A.; Madaeni, S. Therapeutic effects of medicinal plants on isoproterenol-induced heart failure in rats. Biomed. Pharmacother. 2021, 134, 111101. [Google Scholar] [CrossRef]
- World Health Organization (WHO). A Vision for Primary Health Care in the 21st Century: Towards Universal Health Coverage and the Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.; Kim, K.-H. The therapeutic potential of piceatannol, a natural stilbene, in metabolic diseases: A review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setoguchi, Y.; Oritani, Y.; Ito, R.; Inagaki, H.; Maruki-Uchida, H.; Ichiyanagi, T.; Ito, T. Absorption and metabolism of piceatannol in rats. J. Agric. Food Chem. 2014, 62, 2541–2548. [Google Scholar] [CrossRef]
- Eid, B.G.; Abdel-Naim, A.B. Piceatannol attenuates testosterone-induced benign prostatic hyperplasia in rats by modulation of Nrf2/HO-1/NFκB axis. Front Pharmacol. 2020, 11, 614897. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Ahmed, O.A.A.; Asfour, H.Z.; Aldawsari, H.M.; Algandaby, M.M.; Eid, B.G.; Abdel-Naim, A.B.; Awan, Z.A.; Alghaith, A.F.; et al. Piceatannol-loaded emulsomes exhibit enhanced cytostatic and apoptotic activities in colon cancer cells. Antioxidants 2020, 9, 419. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Xu, L.; Yang, X.; Cai, J.; Ma, J.; Cheng, H.; Zhao, K.; Yang, L.; Cao, Y.; Qin, Q.; Zhang, C.; et al. Resveratrol attenuates radiation-induced salivary gland dysfunction in mice. Laryngoscope 2013, 123, E23–E29. [Google Scholar] [CrossRef]
- Inoue, H.; Kishimoto, A.; Ushikoshi, R.; Hasaka, A.; Takahashi, A.; Ryo, K.; Muramatsu, T.; Ide, F.; Mishima, K.; Saito, I. Resveratrol improves salivary dysfunction in a non-obese diabetic (NOD) mouse model of Sjögren’s syndrome. J. Clin. Biochem. Nutr. 2016, 59, 107–112. [Google Scholar] [CrossRef]
- Allushi, B.; Bagavant, H.; Papinska, J.; Deshmukh, U.S. Hyperglycemia and salivary gland dysfunction in the non-obese diabetic mouse: Caveats for preclinical studies in Sjögren’s syndrome. Sci. Rep. 2019, 9, 17969. [Google Scholar] [CrossRef]
- Cano, I.P.; Dionisio, T.J.; Cestari, T.M.; Calvo, A.; Ishikiriama, B.L.C.; de Faria, F.A.C.; Siqueira, W.L.; Santos, C.F. Losartan and isoproterenol promote alterations in the local renin-angiotensin system of rat salivary glands. PLoS ONE 2019, 14, e0217030. [Google Scholar] [CrossRef] [PubMed]
- Wahdan, S.A.; Azab, S.S.; Elsherbiny, D.A.; El-Demerdash, E. Piceatannol ameliorates cisplatin-induced his-tological and biochemical alterations in rats kidney. IJPPS 2017, 9, 305–311. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Culling, C.F.A.; Allison, R.T.; Barr, W.T. Haematoxylin and its counterstain. In Cellular Pathology Technique, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1985; pp. 111–152. [Google Scholar]
- Kiernan, J.A. Histological and histochemical methods: Theory and practice. Shock 1999, 12, 479. [Google Scholar]
- Bussari, S.; Ganvir, S.M.; Sarode, M.; Jeergal, P.A.; Deshmukh, A.; Srivastava, H. Immunohistochemical detection of proliferative marker Ki-67 in benign and malignant salivary gland tumors. J. Contemp. Dent. Pract. 2018, 19, 375–383. [Google Scholar] [CrossRef]
- Fedirko, N.V.; Kruglikov, I.A.; Kopach, O.V.; Vats, J.A.; Kostyuk, P.G.; Voitenko, N.V. Changes in functioning of rat submandibular salivary gland under streptozotocin-induced diabetes are associated with altera-tions of Ca2+ signaling and Ca2+ transporting pumps. Biochim Biophys Acta Mol. Basis Dis. 2006, 1762, 294–303. [Google Scholar] [CrossRef] [Green Version]
- Al-Refai, A.S.; Khaleel, A.K.; Ali, S. The effect of green tea extract on submandibular salivary gland of methotrexate treated albino rats: Immunohistochemical study. J. Cytol. Histol. 2014, 5, 1. [Google Scholar]
- Ogawa, M.; Oshima, M.; Imamura, A.; Sekine, Y.; Ishida, K.; Yamashita, K.; Nakajima, K.; Hirayama, M.; Tachikawa, T.; Tsuji, T. Functional salivary gland regeneration by transplantation of a bioengineered organ germ. Nat. Commun. 2013, 4, 2498. [Google Scholar] [CrossRef]
- Porcheri, C.; Mitsiadis, T.A. Physiology, pathology and regeneration of salivary glands. Cells 2019, 8, 976. [Google Scholar] [CrossRef] [Green Version]
- Amano, O.; Mizobe, K.; Bando, Y.; Sakiyama, K. Anatomy and histology of rodent and human major salivary glands—Overview of the Japan salivary gland society-sponsored workshop. Acta Histochem Cytochem. 2012, 45, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, J.C.; Grisius, M.; Massey, W. Salivary Hypofunction and Xerostomia: Diagnosis and Treatment. Dent. Clin. N. Am. 2005, 49, 309–326. [Google Scholar] [CrossRef]
- Turner, M.; Jahangiri, L.; Ship, J.A. Hyposalivation, xerostomia and the complete denture: A systematic review. J. Am. Dent. Assoc. 2008, 139, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Rius, J.; Brunet-Llobet, L.; Lahor-Soler, E.; Farré, M. Salivary secretory disorders, inducing drugs, and clinical management. Int. J. Med. Sci. 2015, 12, 811–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hand, A.R.; Ho, B. Mitosis and hypertrophy of intercalated duct cells and endothelial cells in the iso-proterenol-treated rat parotid gland. J. Dent. Res. 1985, 64, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.G.; Tan, J.; Gorr, S.-U.; Darling, D.S. Isoproterenol increases sorting of parotid gland cargo proteins to the basolateral pathway. Am. J. Physiol. Physiol. 2007, 293, C558–C565. [Google Scholar] [CrossRef]
- Ten Hagen, K.G.; Balys, M.M.; Tabak, L.A.; Melvin, J.E. Analysis of isoproterenol-induced changes in parotid gland gene expression. Physiol. Genomics 2002, 8, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Chisholm, D.M.; Adi, M.M. Cell proliferation and apoptosis in isoprenaline-induced sialosis in the rat submandibular glands. Int. J. Exp. Pathol. 1995, 76, 263–269. [Google Scholar]
- Melvin, J.E.; Nguyen, H.-V.; Nehrke, K.; Schreiner, C.M.; Hagen, K.G.T.; Scott, W. Targeted disruption of the Nhe1 gene fails to inhibit β1-adrenergic receptor-induced parotid gland hypertrophy. Am. J. Physiol. Liver Physiol. 2001, 280, G694–G700. [Google Scholar] [CrossRef]
- Simson, J.A.V. Evidence of cell damage in rat salivary glands after isoproterenol. Anat. Rec. 1972, 173, 437–451. [Google Scholar] [CrossRef]
- Leal, S.C.; Toledo, O.A.; de Bezerra, A.C.B. Morphological alterations of the parotid gland of rats main-tained on a liquid diet. Braz. Dent. J. 2003, 14, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Kuntsal, L.; Firat, D.; Sirin, Y. Prevention of liquid-diet-induced damages on submandibular glands by selenium supplementation in rats. Tohoku J. Exp. Med. 2003, 201, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Amal, A.E.B. Histological and ultrastructural evaluation of the protective effect of ginseng on gam-ma-irradiated rats’ salivary glands. Nat. Sci. 2013, 11, 114–121. [Google Scholar]
- Al Ankily, M.; Shamel, M.; Bakr, M.M. Epidermal growth factor improves the ultrastructure of sub-mandibular salivary glands of streptozotocin induced diabetic rats-a qualitative study. Int. J. Med. Dent. Sci. 2020, 9, 1803–1810. [Google Scholar]
- Kassab, A.A.; Tawfik, S.M. Effect of a caffeinated energy drink and its withdrawal on the submandibular salivary gland of adult male albino rats: A histological and immunohistochemical study. Egypt. J. Histol. 2018, 41, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Elsharkawy, G.E.Z.; Alhazzazi, T.Y. The effect of the commonly used antidepressant drug amitriptyline (TCAs) on the salivary glands. J. Dent. Oral Disord. Ther. 2016, 4, 1–5. [Google Scholar]
- Evcimen, N.D.; King, G.L. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar]
- Moubarak, R. The effect of hypercholesterolemia on the rat parotid salivary gland (histopathological and immunohistochemical study). Cairo Dent. J. 2008, 24, 19–28. [Google Scholar]
- Thangaiyan, R.; Arjunan, S.; Govindasamy, K.; Khan, H.A.; Alhomida, A.S.; Prasad, N.R. Galangin attenuates isoproterenol-induced inflammation and fibrosis in the cardiac tissue of albino wistar rats. Front. Pharmacol. 2020, 11, 585163. [Google Scholar] [CrossRef]
- El-Kordy, E.A.; Alanazi, A.D.; Ali, S.S.; Makhlouf, M.M.M.; Rabah, S.O. Histological, histochemical and uitra-structural changes in the submandibular gland of starved young male cats. J. Cytol Histol. 2014, 5, 1. [Google Scholar]
- Al Okaili, A.G.; Sedeeq, B.I.; Hazeem, M.I. Histological changes of the submandibular salivary gland of mice maintained on a liquid diet. Tikrit J. Pure Sci. 2009, 14, 22–25. [Google Scholar]
- El-Nozahy, A.A.; Smail, M.I.A. The response of rat submandibular salivary gland to plant protein diet: Biological and histochemical study. Int. J. Health Sci. 2013, 7, 309–315. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Regulation of cell secretion: The integrated action of cyclic AMP and calcium. In Cyclic Nucleotides; Springer: Berlin, Germany, 1982; pp. 227–270. [Google Scholar] [CrossRef]
- Albers, T.; Sabbatini, M.E. Cyclic Nucleotides as Mediators of Acinar and Ductal Function. Pancreapedia Exocrine Pancreas Knowl Base. Pancreapedia Exocrine Pancreas Knowl. Base 2020, 1–28. [Google Scholar] [CrossRef]
- Selye, H.; Veilleux, R.; Cantin, M. Excessive stimulation of salivary gland growth by isoproterenol. Science 1961, 133, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Schneyer, C.A. Salivary gland changes after isoproterenol-induced enlargement. Am. J. Physiol. Content 1962, 203, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Thoungseabyoun, W.; Tachow, A.; Pakkarato, S.; Rawangwong, A.; Krongyut, S.; Sakaew, W.; Kondo, H.; Hipkaeo, W. Immunohistochemical localization of cannabinoid receptor 1 (CB1) in the submandibular gland of mice under normal conditions and when stimulated by isoproterenol or carbachol. Arch. Oral Biol. 2017, 81, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, O.; Sato, Y.-I.; Sakai, Y.; Yamashina, S. Intercalated duct cells in the rat parotid gland may behave as tissue stem cells. Anat. Sci. Int. 2009, 84, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Elhawary, Y.; Metwally, E.; Mohammed, M.A.; Grawish, M. The effect of salbutamol on the parotid salivary gland of albino rats (immunohistochemical study). Res. J. Med. Sci. 2013, 7, 13–19. [Google Scholar] [CrossRef]
- Elsaied, H.A. Histological and immunohistochemical study of selenium regenerative effect on submandibular and sublingual glands of aging rats. Egypt Dent. J. 2019, 65, 3413–3426. [Google Scholar] [CrossRef] [Green Version]
- Kukreja, A.; Wadhwa, N.; Tiwari, A. Therapeutic Role of Resveratrol and Piceatannol in Disease Prevention. J. Blood Disord. Transfus. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Jung, W.K.; Park, S.-B.; Ryu, H.Y.; Kim, Y.H.; Kim, J. Polydatin alleviates diabetes-induced hyposalivation through anti-glycation activity in db/db mouse. Pharmaceutics 2021, 14, 51. [Google Scholar] [CrossRef]
- Şimşek, G.; Gürocak, S.; Karadaǧ, N.; Karabulut, A.B.; Demirtaş, E.; Karataş, E.; Pepele, E. Protective effects of resveratrol on salivary gland damage induced by total body irradiation in rats. Laryngoscope 2012, 122, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y. Long-term oral administration of piceatannol (3,5,3′,4′-tetrahydroxystilbene) attenuates colon tumor growth induced by azoxymethane plus dextran sulfate sodium in C57BL/6J mice. Nutr. Cancer 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, S.A.; Mugri, M.H.; Elamin, N.M.H.; Kamil, M.A.; Osman, H.; Eid, B.G.; Shaik, R.A.; Shaker, S.S.; Alrafiah, A. A Possible Novel Protective Effect of Piceatannol against Isoproterenol (ISO)-Induced Histopathological, Histochemical, and Immunohistochemical Changes in Male Wistar Rats. Curr. Issues Mol. Biol. 2022, 44, 2505-2528. https://doi.org/10.3390/cimb44060171
Alghamdi SA, Mugri MH, Elamin NMH, Kamil MA, Osman H, Eid BG, Shaik RA, Shaker SS, Alrafiah A. A Possible Novel Protective Effect of Piceatannol against Isoproterenol (ISO)-Induced Histopathological, Histochemical, and Immunohistochemical Changes in Male Wistar Rats. Current Issues in Molecular Biology. 2022; 44(6):2505-2528. https://doi.org/10.3390/cimb44060171
Chicago/Turabian StyleAlghamdi, Samar A., Maryam H. Mugri, Nahid M. H. Elamin, Mona Awad Kamil, Hind Osman, Basma G. Eid, Rasheed A. Shaik, Soad S. Shaker, and Aziza Alrafiah. 2022. "A Possible Novel Protective Effect of Piceatannol against Isoproterenol (ISO)-Induced Histopathological, Histochemical, and Immunohistochemical Changes in Male Wistar Rats" Current Issues in Molecular Biology 44, no. 6: 2505-2528. https://doi.org/10.3390/cimb44060171
APA StyleAlghamdi, S. A., Mugri, M. H., Elamin, N. M. H., Kamil, M. A., Osman, H., Eid, B. G., Shaik, R. A., Shaker, S. S., & Alrafiah, A. (2022). A Possible Novel Protective Effect of Piceatannol against Isoproterenol (ISO)-Induced Histopathological, Histochemical, and Immunohistochemical Changes in Male Wistar Rats. Current Issues in Molecular Biology, 44(6), 2505-2528. https://doi.org/10.3390/cimb44060171





